Abstract
Some human herpesviruses (HHV) are etiological contributors to a wide range of malignant diseases. These HHV express latent membrane proteins (LMPs), which are type III membrane proteins consistently exposed at the cell surface in these malignancies. These LMPs have relatively large cytoplasmic domains but only short extracellular loops connecting transmembrane segments that are accessible at the surface of infected cells, but they do not elicit antibodies in the course of natural infection and tumorigenesis. We report here that conformational peptides mimicking two adjacent loops of the Epstein-Barr virus (EBV) LMP1 (2LS peptides) induce high-affinity antibodies with remarkable antitumor activities in mice. In active immunization experiments, LMP1-targeting 2LS vaccine conferred tumor protection in BALB/c mice. Moreover, this tumor protection is dependent upon a humoral anti-2LS immune response as demonstrated in DO11.10 (TCR-OVA) mice challenged with LMP1-expressing tumor and in SCID mice xenografted with human EBV-positive lymphoma cells. These data provide a proof of concept for 2LS immunization against short external loops of viral LMPs. This approach might possibly be extended to other infectious agents expressing type III membrane proteins.
After the primary infection, some viruses, especially human herpesviruses (HHV) such as Epstein-Barr virus (EBV), cytomegalovirus, Kaposi's sarcoma herpesvirus (HHV8), varicella-zoster virus, and herpes simplex virus, persist lifelong in all infected individuals, most often in an asymptomatic latent form. However, in the long term, some HHV can be involved in the emergence of malignant diseases in a small subset of infected individuals. EBV-associated lymphomas and carcinomas (22, 37), HHV8-associated Kaposi's sarcomas (30), and human cytomegalovirus-associated glioblastomas (24) are examples of beta- and gammaherpesvirus-related human malignancies. All these malignancy-associated viruses encode type III membrane proteins which are expressed during the latent state of infection and thus can be called latent membrane proteins (LMPs). These viral LMPs (vLMPs), or “multipass” membrane proteins, appeared to be necessary for virus-driven host cell survival and/or transforming activity (1, 3, 28, 31). They are regarded by some authors as evolutionary mimics of cellular chemokine/cytokine receptors, and, like cellular receptors, they recruit numerous cytoplasmic adaptors. The several transmembrane domains of these vLMPs seem to mimic activated cellular chemokine/cytokine receptor structures and to function with versatile signaling devices, reprogramming cellular signaling networks to modulate cellular function after infection. They contribute prominently to virus survival in latently infected individuals and to virus-related human pathologies, including cancer (8, 14, 19, 34, 36). Despite expressing vLMP antigens at their membrane surface, these latently infected cells are very poor in initiating effective immune responses in infected individuals, thus facilitating viral persistence in humans (2, 17, 38). One reason for this poor immunogenicity may be the constitutive cell signaling property reported for these vLMPs in latently infected cells (3, 16, 35, 38). Consequently, unnecessary overexpression and large extracellular domains for ligand binding may facilitate vLMP immune escape (3, 35, 38). Thus, a major therapeutic approach involved the discovery of naturally active compounds or pharmacological agents that specifically block viral receptor functioning (12, 35). Compounds emerged from high-throughput screening of synthetic chemical libraries, but we still lack specific agents for vLMPs, as they cross-react with cellular chemokine/cytokine receptors and cellular signaling pathways (35). Functional antibodies (Abs) recognizing membrane proteins for anticancer therapies have recently emerged, but there are very few of these and they resulted mostly from serendipity rather than from a systematic design strategy (5). To date, LMPs as a target for a virus-specific immunotherapeutic Ab strategy have not been explored extensively. Some studies have been conducted with purified full-length LMPs from EBV, a gammaherpesvirus, but these studies failed to produce or detect Abs recognizing LMP extracellular domains (10, 20, 29). One reason for this poor immunogenicity could be the too-short extracellular structure of these LMPs, which could explain the failure of latently infected individuals to produce cytolytic Abs (21). To test this hypothesis, we used as an LMP model the EBV-encoded oncoprotein LMP1 which mimics a constitutively active tumor necrosis factor receptor-like molecule and is expressed during EBV latent infection (16). This LMP1 expression was observed in most EBV-carrying malignancies (16, 22, 37), therefore causing EBV to be classified as a class I human carcinogenic agent (11). Here, we report an original humoral approach, because Abs have unlimited diversity and are often exquisitely specific and readily produced. Indeed, to overcome the too-short extracellular size of LMP, we hypothesized that synthesis of a peptide mimicking several extracellular loops of LMP would be a successful general strategy for the development of Abs with the high-pressure liquid chromatography affinity necessary for neutralizing and cytolytic effectiveness, as described previously (4, 33a). We argue here that this new process (D. Tranchand Bunel, 28 January 2003, French patent application FR0300943; D. Tranchand Bunel, 28 July 2005, U.S. Patent Office, US069140) was rewarding, as by vaccinating mice with peptides that covered two adjacent extracellular loops of LMP1 (2LS peptides), we obtained the production of neutralizing and cytolytic high-affinity Abs. Moreover, these Abs induced by 2LS peptide vaccination appeared to confer protection of mice against the development of tumors expressing LMP1.
MATERIALS AND METHODS
Peptide synthesis and coupling.
All peptides were assembled by stepwise 9-fluorenylmethoxy carbonyl (Fmoc)/tert-butyl solid-phase synthesis (6) using Rink amide resin (Applied Biosystems, Foster City, CA) and O-(7-azabenzotriazol-1-yl)-N,N,N′,N′-tetramethyl-uronium-hexafluoroborate (HATU) (Sigma-Aldrich, Steinheim, Germany) activation as described previously (23). Briefly, side chain-protected Fmoc-l-amino acids (Novabiochem, San Diego, CA) and HATU were introduced in a twofold excess in N-methylpyrrolidone with a fourfold excess of diisopropylethylamine. Each reaction completion was confirmed with the ninhydrin test. Occasionally, a second coupling was needed to ensure reaction completion. The N-α Fmoc group was deprotected with 20% piperidine in dimethylformamide (one treatment for 5 min followed by another for 15 min). After final Fmoc deprotection and acetylation, the side chain-protecting groups and peptide were removed from the resin with a conventional trifluoroacetic acid-phenol-triisopropylsilane-water mixture (90:3:2:5, vol/wt/vol/vol) for 2 h. Peptides were precipitated and extensively washed with ice-cold diethyl ether before being dissolved in 5% acetic acid and lyophilized. Purification of synthetic peptides to >95% was carried out by reversed-phase high-performance chromatography on a preparative Nucleosil C18 column (5 mm by 250 mm, 100 Å; Macherey Nagel, Düren, Germany). The homogeneity of all peptides was checked by single-peak observation on analytical reversed-phase high-performance chromatography and by expected molecular mass on positive-matrix-assisted laser desorption ionization-time-of-flight mass spectrometry (Voyager-DE STR; Applied Biosystems, La Jolla, CA). The characteristics of synthesized peptides are presented in Table 1. P1L1-2 and P2L2-3 peptides were site-specifically conjugated to Imject-maleimide-activated keyhole limpet hemocyanin (KLH) (PerBio, France) according to the manufacturer's instructions. For experiments conducted with DO11.10 (TCR-OVA) mice, peptides were coupled to Imject-maleimide-activated ovalbumin (OVA) (PerBio, France). The molar ratio of peptides coupled to KLH or OVA carrier was determined by acid hydrolysis of carrier alone and carrier-coupled peptides; the molar ratios varied from 11:1 to 14:1 for KLH and from 6:1 to 9:1 for OVA.
TABLE 1.
2LS sequences synthesized and mass spectrometric characteristics
| EBV protein and 2LS constructa | Synthesized sequence | Mass (Da) |
|
|---|---|---|---|
| Expected | Exptlb | ||
| LMP1 | |||
| P1L1-2 | MSDWTGGALCLWNLHGQAL | 2,071.9 | 2,072.7 |
| P1L2-3 | LWNLHGQALCLYLQQNWWT | 2,386.8 | 2,387.7 |
| P1L1-3 | MSDWTGGALCLYLQQNWWT | 2,272.6 | 2,273.4 |
| LMP2 | |||
| P2L2-3 | TWRIEDPPFNSLCVDAVLQLSPL | 2,613.0 | 2,613.9 |
2LS constructs were derived from two EBV latent membrane proteins, LMP1 and LMP2. In this study, the P2L2-3 peptide from the EBV LMP2 protein was used only to immunize control mice in in vivo experiments.
Mass spectrometric measurements were carried out using a Voyager-DE STR matrix-assisted laser desorption ionization-time-of-flight mass spectrometer (Applied Biosystems).
Induction of anti-2LS responses: assessment of immunogenicity and Ab isotypes.
For quantitative assessment of peptide immunogenicity, groups of female BALB/c mice, 6 to 8 weeks old, were injected subcutaneously (s.c.) in the base of the tail at days 0, 14, 28, and 42 with 50 μl (40 μg) of KLH-conjugated PIL1-2 or P1L2-3 peptide. For s.c. inoculations (100 μl per dose), KLH-conjugated peptides were formulated as an emulsion in an equal volume of complete Freund's adjuvant for the primary injection and incomplete Freund's adjuvant for the following inoculations. Sera were prepared from blood taken at 1 week after each injection.
Enzyme-linked immunosorbent assays (ELISAs) were conducted on serum samples as described previously (33) using P1L1-2, P1L2-3, or P1L1-3 peptide as the coating antigen. Briefly, 96-well microtiter plates (Nunc, Costar) were coated with target or irrelevant peptide (10 μg/ml) in 50 mM NaHCO3 (pH 9.6). Sera were incubated in the coated plates for 60 min at 20°C. Bound Ab was revealed with goat anti-mouse peroxidase conjugate (Sigma) (1 h at room temperature). Finally, plates were developed in the dark for 30 min using orthophenylenediamine/H2O2 (Sigma) as the substrate, and reactions were stopped using sulfuric acid. Optical densities were measured at 492 nm. The titers of Abs (immune sera) are expressed as the reciprocal of the highest dilution of serum to achieve an optical density of 0.4, which represents approximately five times the background binding of normal mouse serum (NMS). The isotypes of Abs specific for P1L1-2 peptide present in sera diluted 1/150 were determined by ELISA using rabbit antisera directed against mouse immunoglobulin G1 (IgG1), IgG2a, IgG2b, or IgG3 (ICN Pharmaceuticals, Costa Mesa, CA) according to the manufacturer's instructions.
Determination of association constants by competitive ELISA.
Intrinsic association constants of anti-2LS Abs in immune sera were determined using a method called competitive ELISA (33) as described by Friguet et al. (9). It was performed on pools of immune sera collected from groups of five mice 1 week after each dose of P1L1-2 vaccine. Initial capture reactions were performed in liquid phase: samples of pooled immune sera at constant concentrations (1/100) were incubated with the target peptide at increasing concentrations (16 serial dilutions from 10−10 M to 2 × 10−6 M) at 4°C for 18 h in phosphate-buffered saline (PBS) with 2% bovine serum albumin and 0.2% Tween. Subsequently, concentrations of Abs remaining unbound after capture reactions were assessed by ELISA as described above for serum analysis. The resulting absorbance (A) was referred to as A0 (absorbance corresponding to the initial concentration of Abs in the serum sample not subjected to capture). For each pool of sera, A/A0 values were plotted against a0 (the peptide concentration in the capture reaction), resulting in a sigmoidal curve. Finally, sigmoidal binding curves were linearized by A0/(A0 − A) = f(1/a0) transformation in order to allow calculation of the slopes of initial curve segments corresponding to the mean avidity of Abs elicited by the peptide antigens.
Cell lines and animal models.
Human embryonic kidney 293 (HEK293) cells, Sp2/O mouse myeloma cells, and DG75 (an EBV-negative human Burkitt's lymphoma cell line) were grown in RPMI 1640 (Gibco-Invitrogen) supplemented with 2 mM glutamine, 100 IU/ml penicillin, 100 μg/ml streptomycin, and 10% fetal calf serum. Two types of EBV-transformed cells were used in this study: the classical B95-8 EBV-transformed lymphoblastoid cells (LCLs) derived from peripheral human B cells (32) and the E1 cell line, which is an EBV-transformed human monocytic cell line obtained and characterized in our laboratory (18). Animal experiments were performed on female BALB/c mice (6 to 8 weeks old) purchased from Charles River Laboratories (L'Arbresle, France). TCR-OVA (DO11.10) transgenic mice on the BALB/c background generated a restricted T-cell repertoire (OVA specific) and were generously provided by Dennis Loh. TCR-OVA and female SCID mice on the BALB/c background are bred in our institution (Institut Pasteur, Lille, France). These experiments were conducted according to the guidelines of the Ethics Committee of the Pasteur Institute (Lille, France).
Plasmid construction and obtainment of stably transfected cell lines.
The pSV-HA-LMP1 vector was obtained by cloning the LMP1 cDNA from the B95-8 EBV strain in a pSG5-derived expression plasmid as previously described (18). The LMP1 sequence from the pSV-HA-LMP1 vector was subcloned into the HindIII and BamHI restriction sites of the autoreplicative pREP4 plasmid (Invitrogen) to generate the pREP4-LMP1 vector. The integrity of LMP1 in the pREP4-LMP1 vector was verified by DNA sequencing. HEK cells were transfected with either pSV-HA-LMP1 (LMP1-HEK) or pREP4-LMP1 vector (pLMP1-HEK) using the polyethylenimine reagent ExGen 500 (Euromedex). Sp2/O cells transfected with the autoreplicative pREP4-LMP1 vector using polyethylenimine were subjected to hygromycin treatment (200 μg/ml) for selection of a clone called F2 (pLMP1-Sp2/O) that was used in this study as an LMP1-positive mouse tumor cell line. The pLMP1-HEK clone was similarly selected with hygromycin. HEK and Sp2/O cells were transfected with pREP4 alone as controls.
Cell toxicity assays and FACS analysis.
LCLs (6 ml, 1 × 106 cells/ml) were cultured in six-well plates for 4 to 6 days with increasing concentrations of P1L1-2 Ab or anti-KLH immune serum in the presence of normal rabbit serum or NMS (1/60) as a complement provider. Toxicity was determined by quantification of living cells using trypan blue staining. P1L1-2 Ab binding to living LCLs and E1 cells was determined by fluorescence-activated cell sorter (FACS) analysis. Briefly, LCLs and E1 cells were washed with PBS plus 2% human serum albumin (PBS-H) and resuspended for 30 min at 4°C in PBS-H containing P1L1-2 Ab (1/1,500) or NMS (1/500). After two washes with cold PBS, pellets were resuspended in PBS-H and incubated for 30 min with goat anti-mouse-fluorescein isothiocyanate conjugate as recommended by the manufacturer (Sigma). After two washes, flow cytometry analysis was performed on an EPICS-XL cytometer (Coulter).
Immunoprecipitation and WB.
Cell protein extracts were prepared in lysis buffer (20 mM Tris-HCl [pH 7.4], 50 mM NaCl, 5 mM EDTA, 1% Nonidet P-40, and protease inhibitors). In addition to our anti-2LS Abs, we used commercial Abs directed to cryptic determinants localized in the large C-terminal intracellular domain of LMP1, S12 (BD Pharmingen) and CS1-4 (Dako, France). Lysates were subjected to immunoprecipitation using protein A-Sepharose beads (Amersham-Pharmacia) loaded with P1L1-2 or CS1-4 Abs. Cell protein extracts or immunoprecipitates were run on denaturing, nonreducing 12% polyacrylamide gels. Western blotting (WB) was performed using S12 as an anti-LMP1 Ab and standard ECL System reagents (Amersham-Pharmacia). Some experiments were conducted to immunoprecipitate the target of P1L1-2 Ab on the surface of 500,000 live LCL or F2 cells, which were first incubated with P1L1-2 Abs, washed to eliminate nonreactive Abs, and subjected to lysis and immunoprecipitation as described above.
Immunocytochemistry.
For immunocytochemistry experiments, cells were trypsinized when necessary. One million cells suspended in 100 μl PBS were layered onto glass slides, fixed for 5 min in 1% paraformaldehyde-PBS, and washed with PBS. Endogenous peroxidases were blocked with 4% H2O2. All subsequent incubations were performed in PBS plus 5% skim milk powder without detergent. Cells were first incubated at room temperature for 45 min with mouse P1L1-2 Abs (1/300 dilution) plus normal rabbit serum (1/300 dilution). P2L2-3 Abs were used as controls. Bound primary Abs were subsequently labeled using a standard ABC technique (Extravidin; Sigma) and diaminobenzidine revelation (Sigma).
Tumor-bearing BALB/c mice.
For active immunizations, groups of six BALB/c or DO11.10 (TCR-OVA) mice were immunized with 2LS peptides P1L1-2 and P2L2-3 coupled to KLH and OVA, respectively, in association with Freund's adjuvant as described for induction of anti-2LS Abs. Two weeks after the last immunization, mice were challenged with F2, a cell line selected from pREP4-LMP1-transfected SP2/O cells as described above. A total of 1 × 106 or 6 × 106 F2 (SP2/O-LMP1) cells were injected s.c. in a volume of 200 μl of PBS in the posterior neck area of TCR-OVA or wild-type BALB/c mice, respectively, and animals were then followed for surface tumor evolution for up to 150 days. Mice were euthanized when tumors reached a size of 200 mm2 or became ulcerated. If individual mice within a group were euthanized, the last measurement was carried over to subsequent points in the group to calculate mean areas.
For passive Ab transfer (serotherapy), P1L1-2 and P2L2-3 hyperimmune sera were prepared from BALB/c mice bled 1 week after three courses of 2LS peptide vaccination performed at 14-day intervals. Groups of 10 SCID mice were inoculated s.c. at day 0 with 3 million human EBV-positive E1 cells. Intraperitoneal (i.p.) injections of P1L1-2, P2L2-3, or irrelevant control mouse hyperimmune serum (40 μl in a 1/5 dilution in PBS) were administered at days 3 and 5 into SCID mice when a tumor became palpable, and the mice were followed for survival for 70 days. Survival curves were plotted by the Kaplan-Meier method.
Statistical analysis.
The paired Student t test was used for determining significance of the results.
RESULTS
2LS peptide constructs elicit high-affinity Ab production.
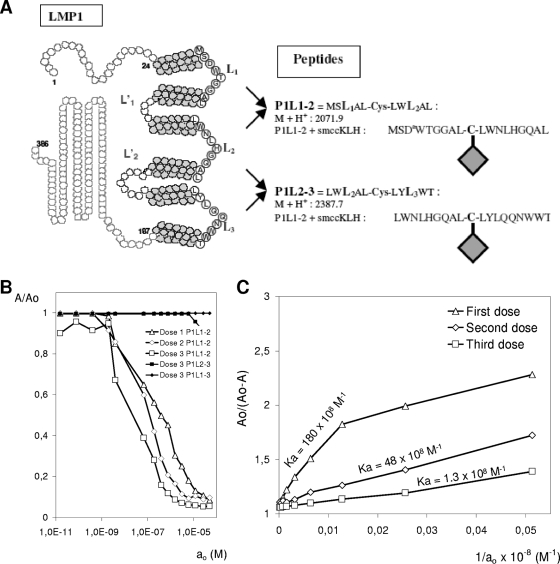
Sequence analysis assigned LMP1 as a type III integral membrane protein with six transmembrane helices connected by three short extracellular loops and two intracellular loops, with both the N- and C-terminal domains having an intracellular orientation (Fig. 1A). Transmembrane domain-predicting programs were used to precisely map the loops connecting transmembrane domains of LMP1, indicating a probable compact structure in the lipid bilayer with very short loops that comprise no more than six to eight residues (http://www.expasy.org/cgi-bin/protscale.pl). These results are in accordance with previous experimental data showing EBV LMP1 to cluster into small patches in the plasma membrane and be oriented as shown in Fig. 1A (15, 16). These data suggested that loops were in close proximity in the LMP1 structure and might be mimicked by a single peptide sequence combining two adjacent external loops (2LS peptide), as shown in Fig. 1A.
FIG. 1.
Design of 2LS conformational epitopes and characteristics of P1L1-2 Abs. (A) Topology prediction for LMP1, indicating 2LS peptide construction strategy and the region targeted by the Abs. The Cys residue located centrally in each peptide is used to combine residues from two adjacent external loops (circled letters) and to branch the resulting construct to KLH or OVA. (B) Determination of P1L1-2 Ab specificity by competitive ELISA at various concentrations (ao, 10−10 to 10−6 M) of P1L1-2, P1L2-3, and P1L1-3 peptides. Sera were collected from BALB/c mice (five per group) a week after each P1L1-2 vaccine dose and tested at a 1/150 dilution for binding to peptides. At equilibrium, the mixture was analyzed by direct ELISA with the three peptides as solid-phase antigen for only the third vaccine dose. (C) The displacement curves obtained in panel B by fitting A/Ao to ao were linearized by A/(Ao − A) to 1/ao transformation. Ab affinity is proportional to the slopes of initial curve segments and increases with the number of doses of vaccines in a representative P1L1-2-vaccinated mouse group. The slope for each curve, corresponding to Ab affinity for collected sera, is indicated. Results are representative of four independent experiments.
Peptides mimicking extracellular LMP1 loops (P1L1-2 and P1L2-3) were synthesized and coupled to activated KLH as a carrier protein before being administered s.c. into BALB/c mice. In previous attempts by various groups, numerous protocols of immunizations with purified full-length LMP or linear loop peptides failed to yield an Ab response to extracellular domains (10, 20, 29). The 2LS constructs described here were shown to be immunogenic by their capacity to induce peptide-specific Ab responses (Table 2). Using the 2LS approach, anti-loop Ab production was obtained in 100% of immunized mice, with the highest Ab response occurring with P1L1-2 after four injections. Therefore, P1L1-2 was selected for subsequent experiments reported in this study. Qualitative properties of P1L1-2 Ab were analyzed by displacement curve study against different 2LS peptides and by affinity measurements by the method of Friguet et al. (9), which agreed well with affinities calculated by analysis of kinetic biosensor data. P1L1-2 Ab did not cross-react with P1L2-3 or P1L1-3 peptides, which both showed partial peptide sequence identity with P1L1-2 (Fig. 1B and Table 1). As depicted in Fig. 1C, the intrinsic association constant increased with the number of vaccine doses, and three doses of vaccine were sufficient to obtain P1L1-2 Abs with strikingly high affinities (intrinsic association constant of 1.4 108 M−1), which were comparable and even superior to those obtained with monoclonal antipeptide Abs (2.4 × 106 M−1 to 3.1 × 108 M−1) currently used in tumor biology research (4).
TABLE 2.
Anti-2LS peptide antibody titers following the first, second, and third doses of vaccines
| Inoculum and ELISAa | Mean (± SEM) anti-P1L1-2 or P1L2-3 Ab titer (log10)b after dose: |
||
|---|---|---|---|
| 1 | 2 | 3 | |
| P1L1-2-KLH | |||
| P1L1-2 | 2.66 ± 0.10 | 3.66 ± 0.17 | 4.43 ± 0.16 |
| P1L2-3 | <1 | <1 | <1 |
| P1L2-3-KLH | |||
| P1L1-2 | <1 | <1 | <1 |
| P1L2-3 | 1.17 ± 0.15 | 2.85 ± 0.31 | 3.25 ± 0.26 |
| KLH | |||
| 2LS peptides | <1 | <1 | <1 |
Inocula were administered in complete Freund's adjuvant for the primary inoculation and in incomplete Freund's adjuvant for each boost. The dose of each peptide was 20 nmol, and all inocula were administered by the s.c. route. Serum samples were taken a week following each dose of vaccine. Pooled serum samples were tested in ELISA using P1L1-2 and P1L2-3 peptides as the coating antigens.
Titers represent the geometric means for groups of five female BALB/c mice bled a week following each dose of vaccine.
The P1L1-2 vaccine elicits specific and in vitro functional Abs.
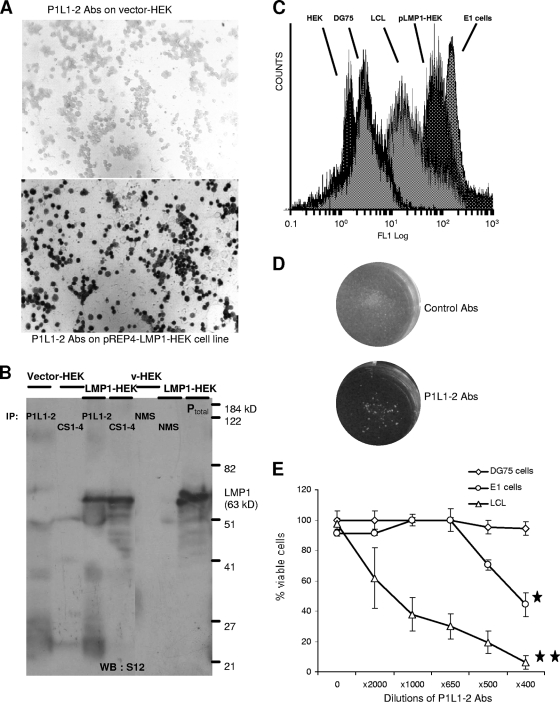
We then investigated whether P1L1-2 Ab could recognize the native full-length LMP1 protein. As shown in Fig. 2A, specific cell surface binding of P1L1-2 Ab was detected by immunocytochemistry on an LMP1-positive HEK cell line (pLMP1-HEK) which was stably transfected with pREP4-LMP1 vector but not on cells transfected with the control pREP4 vector (HEK). WB analysis was also performed using cellular extracts of LMP1-overexpressing cells (LMP1-HEK; HEK293 cells transfected with pSV-HA-LMP1 vector) (18). No signal was detected with P1L1-2 Ab (data not shown), in contrast to the case for two monoclonal Abs, S12 and CS1-4, that bind cryptic determinants in the intracellular C-terminal domain of LMP1 (29). However, to reveal P1L1-2 Ab binding to LMP1, immunoprecipitation experiments were conducted on LMP1-overexpressing HEK cell extracts and followed by WB analysis using the S12 Ab. Figure 2B shows that LMP1 was equally well immunoprecipitated by the monoclonal Ab CS1-4 and the P1L1-2 Abs and reveals a major band at 63 kDa, an apparent molecular mass identical to the migration of LMP1 from LMP1-overepressing HEK total cell extracts (Ptotal in Fig. 2B). In contrast, immunoprecipitation with NMS pulldown (Fig. 2B) or with anti-KLH mouse serum (data not shown) did not reveal any LMP1 band from HEK and LMP1-HEK cell extracts. These data suggested that the binding of P1L1-2 Ab to LMP1 was inhibited by denaturation of antigen inherent to the WB technique but not by solubilization in mild detergent as used for immunoprecipitation (NP-40 extracts) (Fig. 2B). This let thus suppose that the epitope recognized on LMP1 by PIL1-2 Abs is conformational, as observed for other functional Abs such as rituximab, the antitetraspan CD20 (27).
FIG. 2.
P1L1-2 Abs recognizes native LMP1 and presents functional in vitro activities. (A) To test binding of P1L1-2 Abs to native LMP1, HEK293 cells were stably transfected with pREP4-LMP1 vector (pLMP1-HEK). As revealed by staining, P1L1-2 Abs reacted with all pLMP1-HEK cells but not with control vector-transfected HEK cells (magnification, ×400). (B) To confirm specific binding of P1L1-2 Abs to wild-type LMP1 sequence, cell lysates of pSV-HA-LMP1-transfected HEK293 cells (LMP1-HEK) or vector-HEK293 cells (vector-HEK) were subjected to immunoprecipitation (IP) with P1L1-2 Abs and the commercial CS1-4 Ab, a monoclonal Ab directed to a cytoplasmic cryptic domain of LMP1 or NMS. Immunoprecipitates and total cell extracts of LMP1-HEK (Ptotal) were tested for the presence of LMP1 by immunoblotting using S12, a monoclonal anti-LMP1 with characteristics similar to those of CS1-4. No immunoprecipitate was observed with either extracts from vector-HEK, used as negative control, or NMS. (C) Binding of P1L1-2 Abs to live cell lines resulting from EBV-driven (LCLs and E1 cells) or pREP4-LMP1-induced (LMP1-HEK) LMP1 expression was monitored by cytofluorimetric analysis. Binding of P1L1-2 abs was shown on LCL, E1, and pLMP1-HEK cell lines, which expressed natural or transfected endogenous LMP1, but not on DG75, an EBV-negative Burkitt's lymphoma line, or on a pREP4-transfected HEK cell line. (D) Growth inhibition and cell aggregation of LCL cells incubated with anti-P1L1-2 Abs for 4 days. Cell aggregation was not observed with control mouse serum (upper), whereas metabolic arrest and cell aggregation were visualized without magnification for cells treated with anti-P1L1-2 Abs (lower). (E) LCL, E1, and DG75 cell viabilities were assessed after 4 days of culture with P1L1-2 Abs from P1L1-2-KLH-immunized mice at the indicated dilutions in the presence of a large excess of NMS (1/60) with active complement as described in the text. Data are means ± standard errors of the means from three independent experiments. *, significantly different from control serum (*, P < 0.5; **, P < 0.001 [Student's t test]).
In a second step, the reactivity of P1L1-2 Ab was tested on living cell lines showing either stably LMP1-transfected expression or natural EBV-driven LMP1 expression (Fig. 2C). Specific cell surface binding of P1L1-2 Ab was detected by indirect fluorescence and FACS analysis on the intact live B95-8-transformed LCL (31), on the EBV-transformed monocytic E1 cell line (18), and on a stably pREP4-LMP1-transfected HEK cell line (pLMP1-HEK). No fluorescence was observed on DG75, a B-cell line from human Burkitt's lymphoma which is EBV negative, and on HEK cells transfected with pREP4 alone (Fig. 2C). As expected, a heterogeneous distribution of fluorescence was observed with LCLs, in agreement with previous studies reporting heterogeneous LMP1 expression (ranging from 1- to 1,000-fold) in the same population of LCLs. In contrast, a tight fluorescence peak is observed with pLMP1-HEK, which is in agreement with the homogeneous immunocytochemical P1L1-2 Ab stainings of HEK and SP2/0 cell lines transfected with the pREP4-LMP1 plasmid (Fig. 2A and 3B).
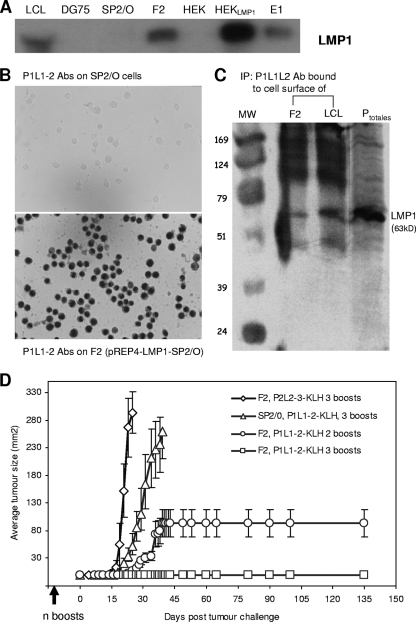
FIG. 3.
P1L1-2 vaccine induces Abs targeting cell surface LMP1 and protection of BALB/c mice from tumors. (A) LMP1 expression was assessed in LCLs, E1 cells, and the F2 cell line, obtained by selection of pREP4-LMP1-transfected Sp2/O cells. Equimolar amounts of cell lysates were tested for LMP1 expression by immunoblot analysis using S12, a monoclonal Ab reacting against a cytoplasmic tail of LMP1. The pSV-HA-LMP1-transfected HEK293 cells (HEKLMP1) overexpressing LMP1 were used as positive control. DG75 is an EBV-negative Burkitt's lymphoma line, and Sp2/O and HEK were transfected with the vectors alone. (B) Extracellular binding of P1L1-2 Abs to LMP1 was revealed by F2 cell staining. Sp2/O cells transfected with vector alone were used as a control (magnification, ×400). (C) To demonstrate that LMP1 was the target of P1L1-2 Abs at the surface of stained cells, live F2 cells and LCLs were incubated with P1L1-2 Abs, washed, and subjected to lysis, and bound Abs were immunoprecipitated (IP) as described in the text. LMP1 molecules from cell surface were detected in immunoprecipitates by immunoblot analysis using S12. HEKLMP1 cell extracts (Ptotal) were used to locate the 63-kDa LMP1 band in the immunoblot. (D) BALB/c mice (six per group) were immunized s.c. three and four times, 2 weeks apart, with the P2L2-3-KLH or P1L1-2-KLH vaccine. Two weeks after the final immunization, mice were challenged with 6 × 106 SP2/O or F2 tumor cells s.c. and followed for surface tumor growth with a caliper (mm2). Mice were euthanized when tumors reached a size of 200 mm2 or become ulcerated. If individual mice within a group were euthanized, the final measurement was carried over to subsequent time points. All control mice immunized with P2L2-3-KLH or P1L1-2-KLH vaccine were euthanized, respectively, by day 19 or 33. Mouse groups treated with the high (three boosts) or low (two boosts) P1L1-2 vaccination protocol were completely or partially protected from F2 tumor development. All mice which did not develop tumor by day 30 remained tumor free beyond 150 days. Error bars represent standard errors of the means. Results are representative of at least three independent experiments.
The functional effects of P1L1-2 Ab surface binding were subsequently investigated on LCLs and E1 cells. We first observed that incubation of LCLs with anti-P1L1-2 Abs for 4 days blocked LCL proliferation. Massive cell aggregates and the absence of pH modification reflecting metabolic arrest were obvious, whereas control mouse serum did not affect LCL proliferation (Fig. 2D). These data suggested that LMP1 cross-linking with anti-P1L1-2 Abs was disturbing antiapoptotic LMP1 signaling in LCLs and influenced cell growth. This hypothesis was confirmed by cell cycle analysis using propidium iodide staining and flow cytometry (data not shown). A high percentage of LCL cells treated for 24 h with anti-P1L1-2 Abs were primarily in the sub-G1 stage, reflecting an increase in DNA fragmentation indicative of apoptosis, whereas LCLs incubated with NMS were in the proliferating stage, particularly in G1 and S phases (data not shown). When combined with a source of complement (1/60 crude mouse or rabbit serum), P1L1-2 Ab induced rapid LCL and E1 cell lysis in a dose-dependent manner, with 50% inhibitory concentrations at dilutions of 1/1,500 and 1/500, respectively, and no effect on DG75, an EBV-negative Burkitt's lymphoma cell line, was observed at any dilution (Fig. 2E). The lower E1 cell sensitivity to P1L1-2 Abs could be explained by the fact that E1 cells express a lower level of cell surface LMP1 than LCLs as revealed by immunocytochemistry (data not shown). Altogether these data confirm that P1L1-2 Abs specifically react with conformational LMP1 epitopes accessible on the surface of live EBV-transformed cells. Importantly, these P1L1-2 Abs induced significant cytostatic and cytolytic effects on LMP1-expressing cells.
Preventive 2LS vaccination induces tumor protection.
Our next aim was to determine whether 2LS peptides would be useful tools for vaccination against LMP1-positive malignancies in vivo. We thus designed a murine model based on s.c. injections of genetically modified Sp2/O myeloma cells into BALB/c mice. Target SP2/O cells were stably transfected with pREP4-LMP1 vector (pLMP1-SP2/O), which was previously used as a substitute for the natural and malignant EBV type II latency observed in Hodgkin's disease or nasopharyngeal carcinoma (NPC) (22, 37). Figure 3A compares LMP1 expression on different cell lines used in this study and shows that the F2 cell line, selected from pREP4-LMP1-transfected Sp2/O cells, expresses native LMP1 at a level comparable to that observed in LCLs or E1 cells but lower than that in overexpressing LMP1-HEK cells (HEKLMP1; HEK cells transfected by pSV-HA-LMP1).
At this stage, we addressed the question of the cell surface expression of LMP1 on F2 cells. As shown in Fig. 3B, a marked labeling was revealed by P1L1-2 Abs on fixed F2 cells but not on Sp2/O cells transfected with pREP4 alone. LMP2-specific P2L2-3 Abs could not stain either F2 or SP2/O cells (data not shown). Taking advantage of P1L1-2 Ab cell surface binding, we demonstrated by immunoprecipitation that the native LMP1, expressed on the surface of the cytoplasmic membrane of live F2 cells and LCLs, was the target of P1L1-2 Abs (Fig. 3C). To explore the effect of preventive P1L1-2 vaccination, groups of six BALB/c mice were immunized in different regimens with the P1L1-2 peptide or irrelevant P2L2-3 peptide coupled to KLH and challenged with 6 × 106 SP2/O or autologous F2 cells (pLMP1-SP2/O myeloma cells). Mice were euthanized when tumors reached a size of 200 mm2 or became ulcerated. If individual mice within a group were euthanized, the final measurement was carried over to subsequent time points. We found that tumor protection could be achieved by P1L1-2 vaccine in a dose-dependent manner in BALB/c mice (Fig. 3D). Interestingly, a control group immunized with P1L1-2 peptide and receiving Sp2/O tumors developed delayed tumor growth compared to the F2-injected group vaccinated with the irrelevant P2L2-3 peptide. This control group vaccinated with P2L2-3 peptide developed measurable and rapidly growing tumors within 5 days and required euthanasia by day 19 after inoculation. It seems that transfection of Sp2/O cells with LMP1 induced more aggressive tumors in BALB/c mice. In contrast, in all mice injected with F2 tumors and immunized with P1L1-2 vaccine, the tumor growth appeared to be significantly delayed at least to 23 days. Importantly, in mice (a group of six) immunized with four injections of P1L1-2-KLH (high-immunization protocol), the tumor development was totally abolished. In the case of three immunizations (low-immunization protocol), antitumor activity appeared to be more heterogeneous. Indeed, tumor growth was completely prevented in four out of six mice, and of the two others, one developed outgrowth tumor from 23 days and the second from 27 days after F2 tumor challenge (Fig. 3D).
Curative 2LS vaccination induces partial antitumor responses.
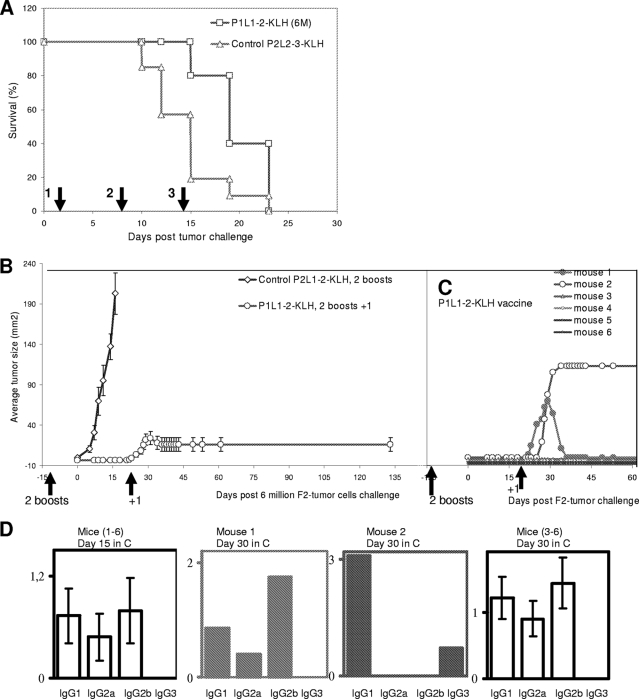
In the next step, we intended to investigate whether the P1L1-2 vaccine could be used for curative vaccination. Simultaneous P1L1-2 vaccination and F2 tumor inoculation into naïve wild-type BALB/c mice failed to protect animals against tumor development (Fig. 4A), certainly because of the in vivo strong aggressiveness of pLMP1-Sp2/O tumors compared to Sp2/O tumors (Fig. 3D) and also because the time was too short for mounting an efficient immune response. Thus, it was not possible to consider a protocol of isolated curative vaccination in this model. Therefore, we decided to combine curative with preventive vaccination using the low-immunization protocol presented in Fig. 3D (three immunizations). The results from one representative experiment of this protocol are depicted in Fig. 4B and C. Groups of six mice were subjected to preventive vaccination with only two vaccine boosts of P1L1-2-KLH or of P2L2-3-KLH for the control group. Animals were challenged with 6 × 106 F2 cells 2 weeks after the final boost. At day 23 of tumor challenge, all mice were then injected with a new P1L1-2 vaccine dose. Out of six mice, four remained tumor free beyond 150 days, one developed a fast-growing tumor (124 mm2) (mouse 2 in Fig. 4C) and underwent euthanasia by day 35, and the last presented, after a growth phase, total disappearance of the tumor. Indeed, in this mouse (mouse 1 in Fig. 4C), after an initial growth, the tumor started to regress 7 days after the curative boost (87 mm2), completely disappeared in 10 days, and did not reappear to beyond 150 days. Therefore, although protection was not achieved in all cases of use of this preventive/curative protocol, encouraging results were obtained by P1L1-2 immunizations in rejection of LMP1-positive tumor cells.
FIG. 4.
P1L1-2 vaccine can induce a partial curative response in BALB/c mice. Groups of six BALB/c mice were challenged with 6 × 106 LMP1-expressing F2 tumor cells and immunized with an irrelevant P2L2-3-KLH control vaccine or the anti-LMP1 P1L1-2-KLH vaccine. Mice were followed for surface tumor growth with a caliper (mm2) and euthanized when tumors reached a size of 200 mm2 or become ulcerated. If individual mice within a group were euthanized, the final measurement was carried over to subsequent time points. (A) Therapeutic vaccination did not protect against tumor challenge. During F2 tumor challenge, two groups of six mice were injected three times with P2L2-3-KLH or P1L1-2-KLH vaccine as indicated by the arrows. All control and anti-LMP1-treated mice were euthanized by day 23. (B) Therapeutic vaccination of preimmunized mice partially protects against tumor challenge. Two groups of six mice were injected with P2L2-3-KLH or P1L1-2-KLH vaccine and received their second boost 2 weeks before F2 tumor challenge (low-vaccination protocol). At day 23, mice received a therapeutic P1L1-2-KLH vaccine dose (arrows, +1). (C) Individual tumor growth in the P1L1-2-KLH-vaccinated mice represented in panel B. Two mice developed low or high tumor progression. The mouse with low tumor progression regressed (mouse 1), and that with high tumor progression had to be euthanized by day 35 (mouse 2). The mice which controlled tumor (mice 1 and 3 to 6) remained tumor free beyond 150 days. (D) Isotype profiles and serum levels of P1L1-2 Abs in the experiment represented in panels B and C. Circulating IgG isotypes were determined at day 15 before tumor appearance for each mouse (mice 1 to 6) and at day 30 for mice with tumor regression (mouse 1) or tumor development (mouse 2). Data are expressed as mean ± standard error of the mean for six mice per group. Results are representative of three independent experiments.
In order to understand the heterogeneity in antitumor survival observed in this preventive/curative vaccination, additional analysis was done to investigate the isotype profile of circulating Abs in each mouse receiving P1L1-2 vaccines. Figure 4D shows collected results corresponding to the representative experiment shown in Fig. 4B and C. At day 15 after tumor challenge, all mice were tumor free and each produced P1L1-2 Abs with predominantly IgG2b and IgG1 isotypes and also IgG2a (mice 1 to 6 in Fig. 4D). At day 30, a similar profile was observed in the four mice which did not develop tumors and in the mice which cured their tumors after an additional curative P1L1-2 boost (mouse 1 in Fig. 4D). Interestingly, in mice which did not control their tumor growth, a complete absence of IgG2a and IgG2b but an appearance of IgG3 production was observed at day 30 (mouse 2 in Fig. 4D and data not shown). Although the influence of isotype distribution on the activity of antitumor Abs is not well understood, it is noteworthy that IgG2b and IgG2a have been shown to be essential for tumor lysis in another murine system (26). In addition, it is known that IgG1 and IgG3 have neither cellular (Ab-dependent cellular cytotoxicity) nor complement (complement-dependent cytotoxicity) activating functions (25). The exact role of tumor cells in the disappearance of the protective IgG2b and IgG2a responses is not clear, but these two isotypes disappeared in mice not controlling LMP1-positive tumors. The antitumor activity of P1L1-2 Abs observed in these in vivo experiments may be correlated to the in vitro complement data presented in Fig. 2D showing a role of P1L1-2 Abs in the induction of complement-mediated cytotoxicity of LMP1-expressing cells.
The anti-LMP1 response in tumor-bearing mice is dependent on P1L1-2 vaccination.
Subsequently, we sought to determine whether tumor rejection was induced exclusively by the P1L1-2 vaccine or also involved an additional contribution of endogenous LMP1 by performing a secondary challenge. BALB/c mice that eradicated F2 tumors after P1L1-2 immunizations were once more challenged with 6 × 106 F2 (SP2/0-LMP1) cells at 150 days after the initial tumor challenge. There is no evidence of immune protection against this secondary tumor challenge, as all mice developed massive tumors, although with a longer average delay compared to the mice that were not previously immunized (data not shown). This longer delay (30 days) before tumor appearance was observed in some of these previously immunized mice and could be explained by the presence of residual levels of P1L1-2 Abs, which did not exceed 5% of the maximal anti-P1L1-2 Ab responses (data not shown). These results support the notion that tumor rejection needs high levels of circulating anti-P1L1-2 Abs, as was the case during the first challenge after the third course of P1L1-2 vaccine, and is not mediated by the presentation of endogenous tumor-transfected LMP1 to T-cell receptor or immunological memory. This notion is consistent with observations reported in human patients bearing LMP1-positive tumors that failed to produce anti-LMP1 Abs or T cells (21). This suggested that the vaccinations with P1L1-2 peptide induced the circulation of P1L1-2 Abs with anti-LMP1 activity and specificity and induced protection against LMP1-positive tumors.
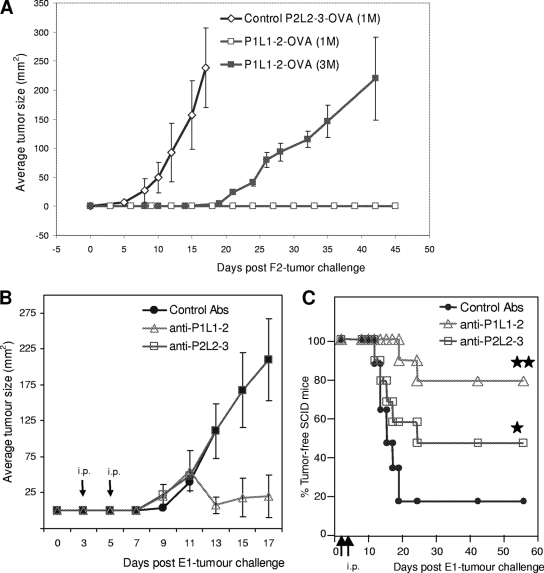
2LS vaccine-induced tumor protection is dependent upon a humoral immune response.
The role of Abs in P1L1-2 vaccine-induced therapeutic Abs and tumor protection was next investigated using two animal models: DO11.10 and SCID mice. T-cell receptor transgenic mice (DO11.10) generated a “quasi” OVA-specific T-cell population (TCR-OVA), monoclonal at more than 97%, with, as a consequence, a poor response to in vivo immunization, although B-cell development is unaffected in these TCR-OVA mice. The number of F2 cells necessary to induce tumors in TCR-OVA mice was lower than that for wild-type mice, perhaps due to minor allogeneic differences in this BALB/c strain. As for wild-type BALB/c, two boosts with P1L1-2-OVA vaccine before F2 tumor challenge provided total protection of TCR-OVA mice, in comparison with control P2L2-3-OVA immunizations (Fig. 5A). The development of tumors could be achieved by increasing the number of F2 tumor cells injected threefold, but even under these conditions, the growth was delayed compared to that for control mice receiving the lower cell inoculum. As TCR-OVA mice lacked a complete T-cell repertoire, this suggests a requirement for B cells and P1L1-2 Abs in tumor protection. To ascertain the involvement of Abs in tumor protection, we therefore investigated whether immune serum transfers would be sufficient to protect SCID animals against tumor development. SCID mice lack functional recombinase required for VDJ rearrangements generating B-cell and T-cell receptors and, as a result, have specific defects in B and T cells. In addition, the SCID model allows xeno-engraftment with human tumors, therefore validating a human therapeutic strategy. We thus chose for this study an EBV-transformed human monocytic cell line which coexpresses LMP1 and LMP2A from the endogenous EBV genome (type II latency) (18) and mimics several severe human malignancies (22, 37). SCID mice were inoculated with 3 × 106 E1 cells, followed by two i.p. injections of immune sera, as described in Materials and Methods, at days 3 and 5. We found that tumor protection can be achieved by passive transfer of P1L1-2 and P2L2-3 Abs into xenografted SCID mice (Fig. 5B and C). Indeed, serotherapy (a term usually used for “passive transfer”) with P1L1-2 Abs delayed tumor development (Fig. 5B) and, compared to results for control animals, increased survival of treated mice by 60% (Fig. 5C). The lower survival efficiency of P2L2-3 Ab (30%) could be explained by the fact that E1 cells express lower level of cell surface LMP2A compared to LMP1 as revealed by immunocytochemistry (data not shown). The success of P1L1-2 vaccine-induced tumor protection in TCR-OVA mice combined with the protection of xenografted SCID mice by passive transfer of 2LS Abs strongly suggested that immune Abs are the primary mechanism of tumor protection. The occasional lack of P1L1-2 vaccine-induced tumor protection in 2LS preventive/curative experiments (Fig. 4A) could be directly linked to the switch to IgG1 and IgG3 isotypes (Fig. 4D), suggesting a critical role of IgG2a and IgG2b in 2LS vaccine-induced tumor protection. Even if this wrong deviation in isotype needs to be elucidated before going further, these experiments confirm on the whole that 2LS targeting of LMP1 and LMP2 led to the production of specific therapeutic Abs that might be necessary for the treatment of human malignancies.
FIG. 5.
P1L1-2 vaccine induces P1L1-2 Abs that protect from tumors in TCR-OVA mice and SCID mice xenografted with human E1 cells. (A) Groups of six TCR-OVA mice received s.c., 2 week apart, two doses of P1L1-2-OVA or P2L2-3-OVA vaccine. Five days after the final immunization, mice were challenged with 1 or 3 million F2 tumor cells (1 M, 3 M) s.c. and were followed for surface tumor growth with a caliper (mm2). P1L1-2-immunized TCR-OVA T-cell-deficient mice were protected from tumor challenge compared to P2L2-3-immunized controls (P < 0.0001). A threefold increase in tumor cell number was necessary to observe tumor development in P1L1-2-immunized TCR-OVA mice. (B and C) Mean tumor size evolution (B) and survival of SCID mice (C) (10/group) challenged with EBV-infected human E1 cells and subsequent i.p. injections by passive Ab transfers with P1L1-2, P2L2-3, or KLH control immune sera. SCID mice were treated at days 3 and 5 after tumor challenge as indicated by the arrows, at a moment where tumors became palpable. Only 20% of control animals survived at day 60 (not requiring euthanasia), whereas treatment with P2L2-3 Ab led to 50% survival, and mice treated with P1L1-2 Ab survived up to 80%. (*, P2L2-3 Abs versus control [P < 0.5]; **, P1L1-2 Abs versus control [P < 0.001]). Error bars represent standard errors of the means. Results are representative of at least three independent experiments.
DISCUSSION
For the first time, we showed that it was possible to produce Abs with high affinities against the extracellular loops of EBV LMP1. This breakthrough was achieved by the use of conformational 2LS peptides mimicking the structure of short amino acid sequences contained in two adjacent loops. The data demonstrate that these 2LS peptides are highly immunogenic, eliciting Abs showing high affinity and specificity toward native protein, as demonstrated in immunoprecipitation and cytofluorometry experiments. Amazingly, a vaccination approach based on immunization showed notable antitumor effects against LMP1-expressing tumors in different protocols and mouse models. It will be very important to know whether a similar approach can be extended to patients carrying EBV-associated malignancies. We first sought to determine whether our peptide constructs mimicking extracellular LMP1 loops would be recognized by sera of EBV-infected individuals. Neither sera from individuals currently infected with EBV (n = 30) or infected with EBV in the past (n = 45) nor sera from patients with NPC (n = 28) reacted with synthesized P1L1-2 or P1L2-3 peptides, suggesting that no Ab with these specificities was produced as a result of physiological immune responses in latently EBV-infected humans (data not shown). We infer that the too-short external loops make LMP1 invisible to humoral responses, which would explain virus persistence during the entire life of the infected host. In other words, external loops of LMP1 are antigenic although not immunogenic in (all) EBV-carrying patients (21). Therefore, the 2LS peptide vaccination strategy is predicted to be useful to elicit cytotoxic Abs against LMP1-infected cells that would not arise spontaneously in latently EBV-infected individuals, thus targeting all infected cells expressing vLMP1. 2LS active immunization with curative intent can be considered in patients suffering from EBV-associated Hodgkin's disease, NPC, or some gastric cancers where a large number of malignant cells consistently express LMP1 and/or LMP2 (22, 37). 2LS immunization could be easily combined with classical therapeutic means such as radiotherapy and chemotherapy. It might provide an interesting alternative to cell-based immunotherapy, which is negatively affected by local immunomodulation and major histocompatibility complex class I/II downregulation in the tumor microenvironment, as described previously (17, 21, 38). Furthermore, 2LS vaccination might be considered for prophylaxis in individuals at high risk of NPC, as identified by serological screening (7). On the other hand, LMP1 external loops appeared to be recognized by our artificial Abs. Therefore, serotherapy with Abs elicited by 2LS immunization could be considered for immunocompromised patients suffering from EBV-associated posttransplant lymphomas, though vaccination before transplantation could become a reference in the future. More generally, our findings provide a new rationale for the design of active or passive therapeutic vaccines targeting type III membrane proteins devoid of large extracellular domains, where only short extracellular loops are accessible at the cell surface. It may be worthwhile to compare passive immunotherapy with 2LS Abs to active 2LS vaccination of human malignancies. Until now, most functional Abs recognizing membrane proteins used for anticancer therapies resulted mostly from serendipity rather than a systematic design strategy. As a consequence, injection of therapeutic Abs, either monoclonal or polyclonal, was used instead of induction of therapeutic Abs by vaccination, and passive transfer of the desired Ab specificity was the rule in antitumor therapy (5). It becomes apparent that active immunotherapy with 2LS vaccine would be easier to apply at a low cost to large patient populations in developing countries than serotherapy or passive immunotherapy with therapeutic Abs, which are expensive and difficult to produce for humans in large quantities. Our data also suggest that clinical trials of active immunization against human EBV pathologies should attempt to generate Abs of the IgG1 subtype, the human analog of murine IgG2a/IgG2b. The well known B95-8 strain has been used as a prototype sequence in our experiments, but different EBV strains or variants have been described and show important sequence modifications in transmembrane and intracellular domains of LMP1 that are a limitation for the design of T-cell epitopes in therapeutic cellular strategies. Rare strains show amino acid substitutions in LMP1 loops; for example, the Asian CAO isolate shows one substitution (D/N) in the first L1 loop of LMP1. Obviously, our approach could be applied to a large number of viral proteins. Among gammaherpesviruses, HHV8, which is a major etiological agent of Kaposi's sarcoma, encodes one protein similar to LMP2 (30). Other herpesviruses involved in various types of diseases also encode type III membrane proteins with short extracellular loops, for example, the herpes simplex virus ECRF3 protein or the human cytomegalovirus US28 protein (14, 36). Beyond herpesviruses, the 2LS targeting approach is potentially applicable for targeting of type III membrane proteins of other infectious agents, such as bacterial and fungal porins, which display the same type of extracellular loop pattern (13), but also for self-proteins such as the voltage- and ligand-gated ion channels, ABC transporters, and chemokine and neurotransmitter receptors. Some of these proteins have been described to be upregulated in cancer cells and should provide the foundation for related 2LS Ab strategies. We hope that our 2LS results will give ideas to others for wider adoption of a peptide strategy to rapidly develop therapeutic vaccines as novel anticancer or infectious agents.
Acknowledgments
We thank Jean Coll for providing E1 cells and Irène Joab for providing human sera from NPC patients. We acknowledge H. Drobecq for peptide mass analysis.
Footnotes
Published ahead of print on 2 September 2009.
REFERENCES
- 1.Beisser, P. S., L. Laurent, J. L. Virelizier, and S. Michelson. 2001. Human cytomegalovirus chemokine receptor gene US28 is transcribed in latently infected THP-1 monocytes. J. Virol. 75:5949-5957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bernstein, D. I. 2001. Potential for immunotherapy in the treatment of herpesvirus infections. Herpes 8:8-11. [PubMed] [Google Scholar]
- 3.Brinkmann, M. M., and T. F. Schulz. 2006. Regulation of intracellular signalling by the terminal membrane proteins of members of the Gammaherpesvirinae. J. Gen. Virol. 87:1047-1074. [DOI] [PubMed] [Google Scholar]
- 4.Burton, D. R. 2002. Antibodies, viruses and vaccines. Nat. Rev. 2:706-713. [DOI] [PubMed] [Google Scholar]
- 5.Carter, P. J. 2006. Potent antibody therapeutics by design. Nat. Rev. Immunol. 6:343-357. [DOI] [PubMed] [Google Scholar]
- 6.Chan, W., and P. D. White. 2000. Fmoc solid phase peptide synthesis, p. 9-40. In B. D. Hames (ed.), Peptide synthesis: a practical approach. Oxford University Press, Oxford, United Kingdom.
- 7.Chien, Y. C., J. Y. Chen, M. Y. Liu, H. I. Yang, M. M. Hsu, C. J. Chen, and C. S. Yang. 2001. Serologic markers of Epstein-Barr virus infection and nasopharyngeal carcinoma in Taiwanese men. N. Engl. J. Med. 345:1877-1882. [DOI] [PubMed] [Google Scholar]
- 8.Couty, J. P., and M. C. Gershengorn. 2005. G-protein-coupled receptors encoded by human herpesviruses. Trends Pharmacol. Sci. 26:405-411. [DOI] [PubMed] [Google Scholar]
- 9.Friguet, B., B. F. Chaffotte, L. Djavadi-Ohaniance, and M. E. Goldberg. 1995. Under proper experimental conditions the solid-phase antigen does not disrupt the liquid phase equilibrium when measuring dissociation constants by competition ELISA. J. Immunol. Methods 182:145-150. [DOI] [PubMed] [Google Scholar]
- 10.Fruehling, S., S. K. Lee, R. Herrold, B. Frech, G. Laux, E. Kremmer, F. A. Grasser, and R. Longnecker. 1996. Identification of latent membrane protein 2A (LMP2A) domains essential for the LMP2A dominant-negative effect on B-lymphocyte surface immunoglobulin signal transduction. J. Virol. 70:6216-6226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.International Agency for Research on Cancer. 1997. Epstein-Barr virus and Kaposi's sarcoma herpesvirus/human herpesvirus 8. IARC Monogr. Eval. Carcinog. Risks Hum. 70:1-492. [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson, Z., M. Schwarz, C. A. Power, T. N. Wells, and A. E. Proudfoot. 2005. Multi-faceted strategies to combat disease by interference with the chemokine system. Trends Immunol. 26:268-274. [DOI] [PubMed] [Google Scholar]
- 13.Klein, M., A. Kotz, K. Bernardo, and M. Kronke. 2003. Detection of Chlamydia pneumoniae-specific antibodies binding to the VD2 and VD3 regions of the major outer membrane protein. J. Clin. Microbiol. 41:1957-1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Landolfo, S., M. Gariglio, G. Gribaudo, and D. Lembo. 2003. The human cytomegalovirus. Pharmacol. Ther. 98:269-297. [DOI] [PubMed] [Google Scholar]
- 15.Liebowitz, D., D. Wang, and E. Kieff. 1986. Orientation and patching of the latent infection membrane protein encoded by Epstein-Barr virus. J. Virol. 58:233-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mann, K. P., D. Staunton, and D. A. Thorley-Lawson. 1985. Epstein-Barr virus-encoded protein found in plasma membranes of transformed cells. J. Virol. 55:710-720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marshall, N. A., M. A. Vickers, and R. N. Barker. 2003. Regulatory T cells secreting IL-10 dominate the immune response to EBV latent membrane protein 1. J. Immunol. 170:6183-6189. [DOI] [PubMed] [Google Scholar]
- 18.Masy, E., E. Adriaenssens, C. Montpellier, et al. 2002. Human monocytic cell lines transformed in vitro by Epstein-Barr virus display a type II latency and LMP-1-dependent proliferation. J. Virol. 76:6460-6472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McLean, K. A., P. J. Holst, L. Martini, T. W. Schwartz, and M. M. Rosenkilde. 2004. Similar activation of signal transduction pathways by the herpesvirus-encoded chemokine receptors US28 and ORF74. Virology 325:241-251. [DOI] [PubMed] [Google Scholar]
- 20.Meij, P., M. B. Vervoort, C. J. Meijer, E. Bloemena, and J. M. Middeldorp. 2000. Production monitoring and purification of EBV encoded latent membrane protein 1 expressed and secreted by recombinant baculovirus infected insect cells. J. Virol. Methods 90:193-204. [DOI] [PubMed] [Google Scholar]
- 21.Meij, P., A. Leen, A. B. Rickinson, S. Verkoeijen, M. B. Vervoort, E. Bloemena, and J. M. Middeldorp. 2002. Identification and prevalence of CD8(+) T-cell responses directed against Epstein-Barr virus-encoded latent membrane protein 1 and latent membrane protein 2. Int. J. Cancer 99:93-99. [DOI] [PubMed] [Google Scholar]
- 22.Middeldorp, J. M., A. A. Brink, A. J. van den Brule, and C. J. Meijer. 2003. Pathogenic roles for Epstein-Barr virus (EBV) gene products in EBV-associated proliferative disorders. Crit. Rev. Oncol. Hematol. 45:1-36. [DOI] [PubMed] [Google Scholar]
- 23.Miranda, L. P., and P. F. Alewood. 1999. Accelerated chemical synthesis of peptides and small proteins. Proc. Natl. Acad. Sci. USA 96:1181-1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mitchell, D. A., W. Xie, R. Schmittling, C. Learn, A. Friedman, R. E. McLendon, and J. H. Sampson. 2008. Sensitive detection of human cytomegalovirus in tumours and peripheral blood of patients diagnosed with glioblastoma. Neurol. Oncol. 10:10-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nanni, P., L. Landuzzi, G. Nicoletti, et al. 2004. Immunoprevention of mammary carcinoma in HER-2/neu transgenic mice is IFN-gamma and B cell dependent. J. Immunol. 173:2288-2296. [DOI] [PubMed] [Google Scholar]
- 26.Nimmerjahn, F., and J. V. Ravetch. 2005. Divergent immunoglobulin G subclass activity through selective Fc receptor binding. Science 310:1510-1512. [DOI] [PubMed] [Google Scholar]
- 27.Polyak, M. J., and J. P. Deans. 2002. Alanine-170 and proline-172 are critical determinants for extracellular CD20 epitopes; heterogeneity in the fine specificity of CD20 monoclonal antibodies is defined by additional requirements imposed by both amino acid sequence and quaternary structure. Blood 99:3256-3262. [DOI] [PubMed] [Google Scholar]
- 28.Rosenkilde, M. M., and T. N. Kledal. 2006. Targeting herpesvirus reliance of the chemokine system. Curr. Drug Targets 7:103-118. [DOI] [PubMed] [Google Scholar]
- 29.Rowe, M., H. S. Evans, L. S. Young, K. Hennessy, E. Kieff, and A. B. Rickinson. 1987. Monoclonal antibodies to the latent membrane protein of Epstein-Barr virus reveal heterogeneity of the protein and inducible expression in virus-transformed cells. J. Gen. Virol. 68:1575-1586. [DOI] [PubMed] [Google Scholar]
- 30.Sharp, T. V., H. W. Wang, A. Koumi, D. Hollyman, Y. Endo, H. Ye, M. Q. Du, and C. Boshoff. 2002. K15 protein of Kaposi's sarcoma-associated herpesvirus is latently expressed and binds to HAX-1, a protein with antiapoptotic function. J. Virol. 76:802-816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sodhi, A., S. Montaner, and J. S. Gutkind. 2004. Viral hijacking of G-protein-coupled-receptor signalling networks. Nat. Rev. Mol. Cell Biol. 5:998-1012. [DOI] [PubMed] [Google Scholar]
- 32.Thorley-Lawson, D. A., L. Chess, and J. L. Strominger. 1977. Suppression of in vitro Epstein-Barr virus infection. A new role for adult human T lymphocytes. J. Exp. Med. 146:495-508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tranchand Bunel, D., H. Gras-Masse, B. Bourez, L. Dedecker, and C. Auriault. 1999. Evaluation of an Epstein-Barr virus (EBV) immunoglobulin M enzyme-linked immunosorbent assay using a synthetic convergent peptide library, or mixotope, for diagnosis of primary EBV infection. J. Clin. Microbiol. 37:2366-2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33a.Tranchand Bunel, D. 2004. Abstr. 11th Int. EBV Symp., Regensburg, Germany, 20 to 25 September 2004, abstr. 15.05.
- 34.Vischer, H. F., R. Leurs, and M. J. Smit. 2006. HCMV-encoded G-protein-coupled receptors as constitutively active modulators of cellular signalling networks. Trends Pharmacol. Sci. 27:56-63. [DOI] [PubMed] [Google Scholar]
- 35.Vischer, H. F., J. W. Hulshof, I. J. de Esch, M. J. Smit, and R. Leurs. 2006. Virus-encoded G-protein-coupled receptors: constitutively active (dys)regulators of cell function and their potential as drug target. Ernst. Schering Found. Symp. Proc. 2:187-209. [DOI] [PubMed] [Google Scholar]
- 36.Wong, M. M., and N. E. Fish. 2003. Chemokines: attractive mediators of the immune response. Semin. Immunol. 15:5-14. [DOI] [PubMed] [Google Scholar]
- 37.Young, L. S., and A. B. Rickinson. 2004. Epstein-Barr virus: 40 years on. Nat. Rev. Cancer 4:757-768. [DOI] [PubMed] [Google Scholar]
- 38.Zou, W. 2005. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat. Rev. Cancer 5:263-274. [DOI] [PubMed] [Google Scholar]