Abstract
Background
Somatostatin (SS), GH-releasing hormone (GHRH), GH-releasing peptide (GHRP) and the sex-steroid milieu regulate GH secretion.
Objective
To test whether GHRH and GHRP remain effective secretagogues in the face of short-term hypogonadism.
Design
Prospective, randomized double-blind.
Methods
Healthy young men (N = 24) received a GnRH agonist twice 3 weeks apart followed by placebo (N = 13, Pl) or testosterone (N = 11, T) addback. Subjects were then given consecutive i.v. infusions of L-arginine (to restrain SS outflow) and a maximally effective dose of GHRH or GHRP-2 (to test corresponding secretagogue pathways).
Results
GH secretion stimulated by L-arginine/GHRH and by L-arginine/GHRP-2 was unaffected by combined T/E2 depletion. The low T/E2 milieu decreased basal (nonpulsatile) GH secretion (P = 0.038), without altering fasting pulsatile GH secretion or IGF-I or IGFBP-3 concentrations. IGFBP-1 (P < 0.0001) and abdominal visceral fat (AVF, P = 0.017) correlated negatively with fasting basal GH secretion. In contrast, IGF-I (P = 0.0012) and IGFBP-3 (P = 0.015) correlated positively with fasting pulsatile GH secretion. AVF (P = 0.0024) was a negative determinant, and IGF-I a positive determinant (P = 0.018), of GHRH-driven GH pulses. Responses to GHRP-2 were unrelated to any of these factors.
Conclusion
L-arginine/GHRP-2 appears to be an especially robust stimulus of GH secretion, since efficacy is unmodified by profound short-term hypogonadism, a range of AVF estimates, and a spectrum of IGF-I, IGFBP-1 and IGFBP-3 concentrations. Whether robustness also applies to chronic hypogonadism is not known.
Keywords: androgen, fat, human, male, GH, IGF-I, pulsatility, secretion
Introduction
Growth hormone (GH) is secreted predominantly in discrete bursts under the supervision of GH-releasing hormone (GHRH, a 40 and 44 amino-acid peptide synthesized in the arcuate nucleus), GH-releasing peptide (GHRP, of which ghrelin is the endogenous 28 amino-acid prototype produced in the hypothalamus, pituitary gland and stomach), and somatostatin (a 14 amino-acid sequence made in the periventricular nucleus). Pulsatile GH secretion is also regulated strongly by sex-steroid hormones (1), which exert their stimulatory effects to a large degree via SS, GHRH and GHRP (2). Testosterone (T) and its aromatized product, estradiol (E2), determine the amplitude and mass of GH pulses, whereas pathophysiological factors such as age, IGF-I feedback and relative adiposity repress pulsatile GH secretion (3-5). In particular, administration of T to prepubertal children and hypogonadal men enhances GH secretion after infusion of saline, L-arginine or a maximally effective GHRP stimulus (6-9). On the other hand, the degree to which short-term T or E2 deficiency affects combined secretagogue actions in men is far less clear. Whereas two studies inferred that short-term pharmacological T/E2 deprivation does not diminish the efficacy of GHRH infused alone (10;11), how acute hypogonadism affects combined L-arginine/GHRH or L-arginine/GHRP drive is not known. This point is important, inasmuch as Infusion of L-arginine before or with a peptidyl secretagogue has been employed to restrict hypothalamic release of SS (12-15). The motivation for using L-arginine is to permit inferences about the efficacies of GHRH and GHRP in a low-SS milieu (2). Thus, the present study utilizes sequential L-arginine/GHRH vs L-arginine/GHRP-2 infusions to examine their robustness to a low-T/low-E2 milieu in healthy men. Secondarily, we ask whether abdominal visceral-fat mass and prevailing markers of the IGF-I system influence combined-secretagogue effects in this setting.
Methods
Subjects
Volunteers provided written informed consent approved by the Mayo Institutional Review Board and reviewed by the U.S. Food and Drug Administration under investigator-initiated new drug numbers for GHRP-2 and GHRH. Exclusion criteria were exposure to psychotropic or neuroactive drugs within one yr; BMI less than 18 and more than 32.5 kg/m2; anemia (hemoglobin < 12.8 g/dL); drug or alcohol abuse, psychosis, depression, mania or severe anxiety; acute or chronic organ-system disease; use of T, other anabolic steroids or glucocorticoids; endocrinopathy, other than primary thyroidal failure receiving replacement; nightshift work or recent transmeridian travel (exceeding 3 time zones within 7 days of study); acute weight change (loss or gain of > 2 kg in 6 wk); allergy to administered peptides; and unwillingness to provide written informed consent. Each subject had an unremarkable medical history and physical examination and normal screening laboratory tests of hepatic, renal, endocrine, metabolic and hematologic function. The men reported normal sexual development and function.
Protocol
The study design was parallel-cohort, double-blind and prospectively randomized. Twenty-four healthy young men [age 24 ± 0.72 (SEM) yr, BMI 25 ± 0.91 kg/m2] received 2 consecutive im injections of depot leuprolide acetate (3.75 mg im 3 wk apart) to deplete systemic T and E2 concentrations. The synergy between GHRH and GHRP-2 in these subjects was presented earlier (16). Beginning on the day of the second leuprolide injection, volunteers were given either saline (N = 13) or a pharmacological dose of 200 mg T enanthate (N = 11) im weekly for 3 doses (designated as days 0, 7, and 14) double-blind. Secretagogue infusions were scheduled during the time window 10-18 days. Each participant was studied twice in the Clinical-Translational Research Unit (CRU) at least 48 hr apart on separate mornings after a standardized evening meal and subsequent overnight fast.
Volunteers continued their usual level of daily physical activities, except the evening before and on the day of study. Subjects were admitted to the CRU before 1700 hr and stayed overnight. Sleep was not monitored. Room lights were put out at 2230 hr. To limit nutritional confounds, single a constant meal (vegetarian or nonvegetarian) was given in the CRU at 1800 hr the night before study comprising 12 kcal/kg distributed as 50% carbohydrate, 20% protein, and 30% fat. Volunteers then remained fasting, alcohol-abstinent, and caffeine-free overnight until the end of the infusion the next day.
In the CRU, catheters were placed in contralateral forearm veins at 0700 hr to allow simultaneous infusion of secretagogues and blood sampling every 10 min for a total of 6 hr beginning at 0800 hr. Sampling encompassed a 3-hr baseline and 3-hr stimulation interval. Infusions comprised L-arginine 30 gm delivered iv over 30 min, followed immediately by iv bolus of either 1 μg/kg GHRH (GEREF; Serono, Norwalk, MA) or 3 μg/kg GHRP-2 (Takeda Pharmaceuticals, Deerfield, Illinois). The doses of L-arginine and both peptides are maximally stimulatory in adults (17;18). L-arginine was employed to antagonize central somatostatin outflow (12;13).
Blood was also withdrawn at 0800 hr for later assay of serum estradiol (E2), T, LH, FSH, IGF-I, IGFBP-1, IGFBP-3, albumin and SHBG concentrations. Lunch was provided after sampling before discharge from the CRU.
Hormone assays
Serum GH concentrations were determined in duplicate by automated ultrasensitive two-site immunoenzymatic chemiluminescence assay performed on the DxI automated system (Beckman Instruments, Chaska, MN 55318). Interassay CV's were 6.1% at 0.46 μg/L, 4.3% at 3.0 μg/L, 5.0% at 7.2 μg/L and 4.8% at 13.6 μg/L. Intraassay CV's were 4.7% at 0.37 μg/L, 3.5% at 2.5 μg/L and 3.2% at 14.8 μg/L. The lowest detectable GH concentration at 95% confidence is 0.008 μg/L determined by processing a 6-point calibration curve, 5 quality controls and 10 replicates of zero calibrator in multiple assays. The GH standard was recombinant human 22 kDa GH.
E2 and T were measured by tandem liquid-chromatography ion-spray mass spectrometry. For E2, intraassay CV's were 18%, 3.8% and 7.2% at concentrations of 3.6, 40 and 297 pg/mL (multiply by 3.68 for pmol/L). Interassay CV's were 8.1, 4.7, and 4.9% at 16, 31 and 119 pg/mL, respectively. For T, the analytic range is 7-2000 ng/dL (multiply by 0.0347 for nmol/L) for a 0.1 mL volume. Intraassay CV's were 3.3, 2.8, 2.2 and 2.0% at T concentrations of 16, 64, 184 and 927 ng/dL, respectively. Corresponding interassay CV's were 5.1, 3.8, 3.7 and 2.8%. Free and bioavailable T concentrations were calculated as described earlier (19).
IGFBP-1, IGFBP-3 and total IGF-I concentrations were quantified by immunoradiometric assays (Diagnostic Systems Laboratories, Webster, TX) as described (20). Intra- and interassay CV's were 6.1 and 8.4% for IGFBP-1, and 5.8 and 8.5% for IGFBP-3, respectively. Intraassay CV's for IGF-I were 3.4% at 9.4, 3% at 55 and 1.5% at 264 μg/L, and interassay CV's 9% at 64 μg/L and 6.2% at 157 μg/L.
Statistical analysis
An unpaired Student's t test was used to compare age, BMI and AVF as well as baseline fasting hormone concentrations in the 2 groups. Two-way ANOVA (2×2 factorial design) was used to examine the individual and interactive effects of normal vs low T/E2 (2 factors) and secretagogue type (2 factors) on the summed mass of GH secreted in pulses over the 3 hr after secretagogue infusion. Post hoc contrasts were made via Fischer's least-significant difference test (21). Log transformation was used to limit the dispersion of residual variance. Linear regression analysis and Pearson's correlation-coefficient P value were applied to examine the relationship between GH secretion and age, AVF, IGF-I, IGFBP-1 and IGFBP-3 concentrations (Systat, Point Richmond, CA). Bonferroni correction was applied to the 3 IGF-related measures (P ≤ 0.0167). Significant differences were corroborated by the nonparametric rank-sum and Kruskal-Wallis tests (21).
Data are presented as the mean ± SEM. Experiment-wise P < 0.05 was construed as statistically significant.
Statistical power analysis
Data from 18 studies in hypogonadal or normal males (N = 149 subjects total) indicate that parenteral T supplementation increases mean GH concentrations by a weighted-mean effect size (standard-deviate score) of 1.8 (6;8;9;11;22-26). Power analysis assumed that T/E2 depletion exerts an opposite effect of similar relative magnitude. If comparison is made via a one-tailed (based upon the prior hypothesis) unpaired Student's t test at protected P ≤ 0.01, then analysis of data from a total of 24 subjects (approximately 12 in each group) would achieve > 99% power to detect this effect size.
Deconvolution analysis
GH concentration time series were analyzed using a recently developed automated deconvolution method, which was verified mathematically by direct statistical proof and validated empirically by hypothalamo-pituitary sampling and simulated pulsatile time series (27;28). The Matlab-based algorithm first detrends the data and normalizes concentrations to the unit interval [0, 1]. Second, the program creates multiple sets of potential pulse times via an incremental smoothing process (a nonlinear adaptation of the heat-diffusion equation). Third, a maximum-likelihood estimation (MLE) method calculates all secretion and elimination parameters for each of the multiple candidate pulse-time sets. Deconvolution parameters comprise basal secretion (β0), two half-lives (α1, α2), secretory-burst mass (η0, η1), random effects on burst mass (σA), procedural/measurement error (σε), and a 3-parameter flexible Gamma secretory-burst waveform (β1, β2, β3). The fast half-life of GH was represented as 3.5 min constituting 37% of the decay amplitude and the slow half-life as 20.8 min (29). The Akaike information criterion (30) is used to select the optimal pulse-time set from the multiple candidate sets. Other parameters are basal and pulsatile secretion rates (concentration units/3 hr), mass secreted per burst (concentration units), and waveform mode (time delay to maximal secretion after burst onset).
Visceral fat mass
Intraabdominal visceral fat mass was estimated by single-slice abdominal CT scan at L3-L4, exactly as reported (4).
Results
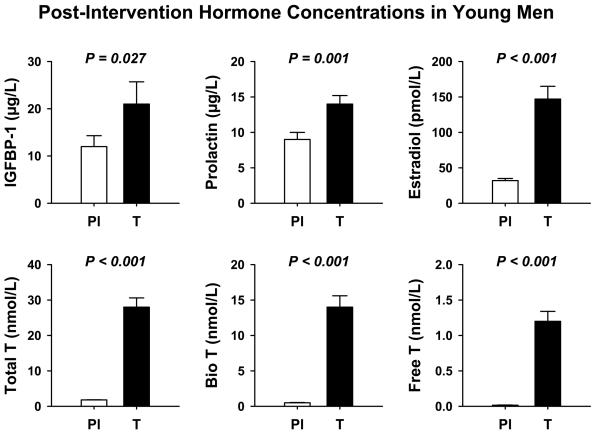
As described earlier and in a Supplemental (Appendix) Table, baseline subject characteristics did not differ in volunteers assigned to the leuprolide/placebo (Pl) and leuprolide/T treatment groups (16). In contrast, post-leuprolide vs post-placebo hormone concentrations differed significantly with respect to: (a) IGFBP-1 (higher in the T addback group, P = 0.027); (b) prolactin (higher in the T group, P = 0.001); (c) FSH (lower in the T group, P < 0.001); and (d) E2, total T, bioavailable T and free T (each higher by P < 0.001 in the T group): Figure 1. The data suggest that eugonadal T concentrations contribute to maintaining IGFBP-1 and prolactin concentrations, and in suppressing FSH concentrations. IGF-I, IGFBP-3, SHBG and LH concentrations were similar in the Pl and T cohorts after leuprolide injection, whereas FSH was lower after leuprolide plus T (0.35 ± 0.11 IU/L) than leuprolide plus Pl (2.3 ± 0.43 IU/L, P = 0.001). Therefore, T potentiated suppression of FSH by leuprolide, without further affecting LH or SHBG. Moreover, IGF-I and IGFBP-3 were not affected.
Figure 1.
Significant hormonal differences in young men given leuprolide plus placebo vs leuprolide plus T addback.
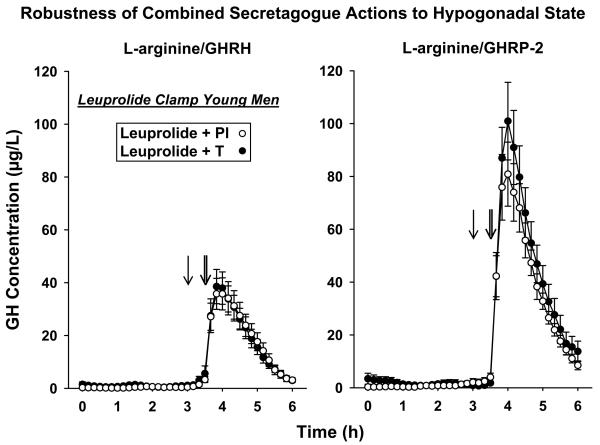
Figure 2 presents (mean ± SEM) GH concentrations sampled every 10 min for 6 h, comprising 3-hr saline-infused and 3-hr secretagogue-stimulated profiles. Data were obtained after leuprolide injection plus Pl vs T addback. The peak and time-course of GH responses were comparable visually in the two cohorts. Unstimulated (pre-secretagogue) mean GH concentrations were 0.61 ± 0.19 (Pl) and 1.3 ± 0.49 μg/L (T) [P = 0.046], and estimated basal (nonpulsatile) GH secretion rates were 2.3 ± 0.52 (Pl) vs 4.0 ± 0.94 μg/L/3 hr (T) [P = 0.038]. Unstimulated fasting pulsatile GH secretion was not affected by the sex-steroid milieu (P = 0.37): Table 1. Thus, short-term T depletion mainly reduced basal GH secretion. In relation to sequential secretagogue-stimulated GH release, two-way ANOVA identified a strong effect of L-arginine/GHRP-2 over L-arginine/GHRP (P < 0.001), but no effect of T vs Pl addback (P = 0.79) and no interaction between secretagogue and sex-steroid milieu (P = 0.49). The effect of L-arginine combined with GHRP-2 on pulsatile GH secretion was 2.0 and 2.7-fold that of L-arginine/GHRH under low T and high T, respectively. Unpaired statistical comparisons of pulsatile GH secretion (μg/L/3 hr) in the Pl vs T-addback cohorts during the separate infusion of L-arginine/GHRH and L-arginine/GHRP-2 confirmed no differences due to T/E2 availability for either secretagogue: Table 1.
Figure 2.
Time profiles of GH concentrations sampled every 10 min for 6 hr fasting in 13 young men given leuprolide and placebo (Pl) and 11 others given leuprolide and T injections. Saline was infused iv for 3 h, then L-arginine for 0.5 hr (single arrows) followed by bolus GHRH or GHRP-2 (double arrows). Data are the mean ± SEM.
Table 1.
Impact of T/E2 Depletion vs Repletion on GH Secretion*
| Endpoint | Leuprolide + Pl (N = 13) | Leuprolide + T (N = 11) | P value |
|---|---|---|---|
| Basal (nonpulsatile) GH secretion | 2.3 ± 0.52 | 4.0 ± 0.94 | 0.019 |
| Unstimulated pulsatile GH secretion | 5.1 ± 2.6 | 4.9 ± 1.6 | P > 0.10 |
| L-arginine/GHRH stimulation | 117 ± 19 | 103 ± 15 | P > 0.10 |
| L-arginine/GHRP-2 stimulation | 235 ± 27 | 273 ± 41 | P > 0.10 |
Units are μg/L/3 h.
Data are the mean ± SEM.
P values were estimated by an unpaired one-tailed Student's t-test under the prior hypothesis that T/E2 depletion lowers GH output.
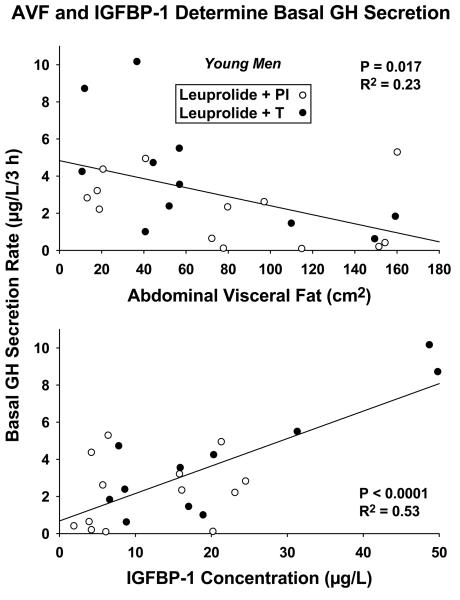
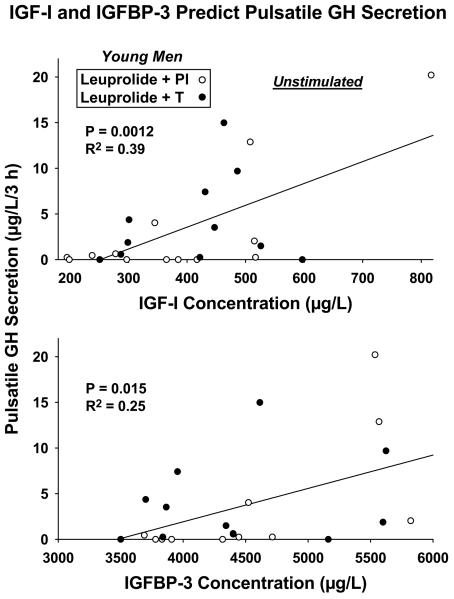
Lack of statistical difference (at good statistical power, here > 95%) between Pl and T-addback responses permitted combining the data (N = 24 subjects) statistically (21). Linear-regression analyses in the combined cohorts revealed that basal (nonpulsatile) GH secretion was inversely related to AVF (R2 = 0.23, P = 0.017) and directly to IGFBP-1 concentrations (R2 = 0.53, P < 0.0001): Figure 3. Fasting unstimulated (saline-infused) pulsatile GH secretion correlated positively with IGF-I (R2 = 0.39, P = 0.0012) and IGFBP-3 (R2 = 0.25, P = 0.015) concentrations: Figure 4. These outcomes point to potentially different modulation of basal vs pulsatile GH secretion in the unstimulated fasting state. These results remained significant at Bonferroni-restricted P ≤ 0.0167 for assessment of the 3 IGF-related measures.
Figure 3.
Regression of unstimulated basal (nonpulsatile) GH secretion rates on CT-estimated abdominal visceral fat (AVF) and serum IGFBP-1 concentrations in 24 young men treated with leuprolide and Pl (open circles) or T (closed circles). Two-tailed P values are given with the correlation coefficients.
Figure 4.
Positive relationships between unstimulated (saline-infused) pulsatile GH secretion and serum IGF-I (top) and IGFBP-3 (bottom) concentrations in 24 young men. Data are presented as described in Figure 3.
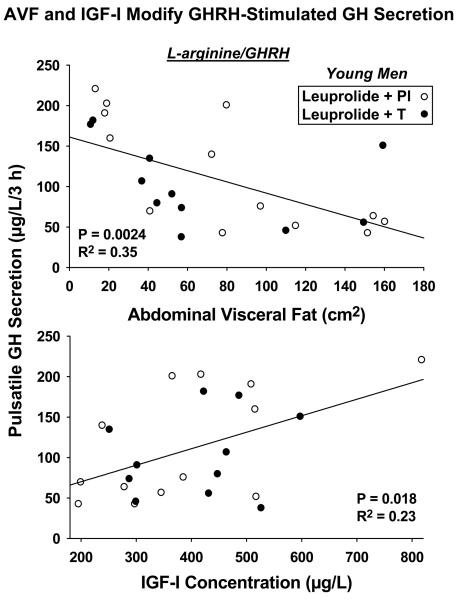
In the setting of secretagogue infusions, regression analysis revealed a markedly negative effect of AVF (R2 = 0.35, P = 0.0024) on L-arginine/GHRH's stimulation of pulsatile GH secretion: Figure 5 (top). In contrast, stimulation by L-arginine/GHRP-2 was not influenced by either age or AVF (both P > 0.10). There was a strong trend toward IGF-I's being a positive statistical determinant of pulsatile GH secretion driven by sequential L-arginine/GHRH infusion (R2 = 0.23, P = 0.018): Figure 5 (bottom). On the other hand, none of IGF-I, IGFBP-1 or IGFBP-3 concentrations, age or AVF correlated with GH responses to L-arginine/GHRP-2. Accordingly, AVF is a major negative determinant of GHRH but not GHRP-2 action in this setting.
Figure 5.
Efficacy of L-arginine/GHRH in stimulating pulsatile GH secretion correlates negatively with AVF (top) and positively with IGF-I concentrations (bottom) (N = 24 men, see Figure 3). This was not true for L-arginine/GHRP-2.
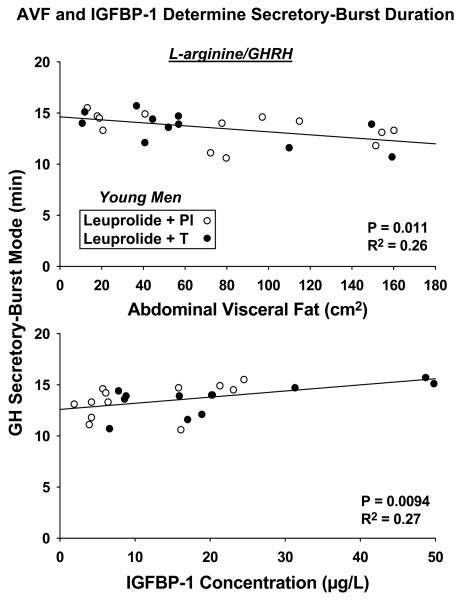
The waveform (time-dependent shape) of GH secretory bursts is estimated as the analytical mode of the secretory burst. Instead of being a measure of mass (amount of GH released), the mode denotes the time in min to attain maximal secretion after objectively estimated burst onset. By linear regression analysis and in the case of L-arginine/GHRH infusion only, AVF (R2 = 0.26, P = 0.011) correlated negatively, and IGFBP-1 positively (R2 = 0.27, P = 0.0094), with GH secretory-burst mode: Figure 6. Thus, the shape (waveform) of GH secretory events is influenced in some manner by AVF and IGF-I, but not only when the secretagogue is GHRH, thereby illustrating a further distinction in the mode of action of GHRH and GHRP-2.
Figure 6.
Impact of AVF (top) and IGFBP-1 concentrations (bottom) on L-arginine/GHRH-stimulated GH secretory-burst waveform. The mode of the waveform is the time delay to maximal GH secretion. No such effects were evident for L-arginine/GHRP-2 stimulation..
Discussion
The present study affirms with high statistical power the a priori hypothesis that GH responses to sequential L-arginine/GHRH and L-arginine/GHRP-2 infusions are resistant to marked short-term reduction of T and E2 concentrations. The outcomes indicate that L-arginine infusion followed by either GHRH or GHRP-2 constitutes an effective secretagogue combination in acutely (within 1 mo) hypogonadal men. On the other hand, only sequential L-arginine/GHRP-2 also stimulated GH secretion independently of AVF and IGF markers. Thus, L-arginine/GHRP-2 may be a useful secretagogue pair under clinical conditions in which hypogonadism is of recent onset and/or possibly overweightness exists.
The gold-standard insulin-tolerance test has some practical limitations, thus prompting evaluation of other powerful GH-releasing agents, such as the combination of L-arginine and GHRP-2 assessed here. For clinical utility, efficacy (maximal response) has been the conventional endpoint. However, complete dose-response estimates would be required to quantity potency and sensitivity as well. Maximal hormone concentrations (height of peak) in general correlate directly with the mass of hormone secreted per burst (31). Thus responses to the secretagogues utilized here yielded consistent inferences for secretory-burst mass (tables and figures) and maximal GH peak height (not shown).
The leuprolide-clamp paradigm also demonstrated that—independently of T/E2 status—unstimulated basal (nonpulsatile) GH secretion was determined positively by IGFBP-1 concentrations and negatively by AVF, whereas unstimulated pulsatile GH secretion was related positively to both IGF-I and IGFBP-3 concentrations. IGF-I concentrations also represented a strongly positive predictor, and AVF a strongly negative predictor, of L-arginine/GHRH efficacy. In contradistinction, none of these factors influenced L-arginine/GHRP-2 stimulation in this cohort of 24 individuals. More studies will be required to assess the generality of this inference in other cohorts.
A parsimonious explanation for the observed nonsteroidal determinants of GHRH efficacy could be that high GHRH receptor-effector function enhances pulsatile GH output, which increases IGF-I and IGFBP-3 concentrations and decreases AVF. This scenario could explain both the positive association between pulsatile GH secretion and IGF-I and IGFBP-3 concentrations, and the negative association between pulsatile GH secretion and AVF. The precise reasons why GHRP does not exhibit the same multiple interdependencies with AVF and IGF-related factors as GHRH are not so clear. A main difference from GHRH is that ghrelin/GHRP exerts hypothalamic effects required for synergism with GHRH (2).
Sex-steroid hormones in women regulate not only the amount of GH secreted in bursts, but also the waveform or time-pattern of GH released within individual bursts (32). We could not detect T/E2 effects on secretory-burst shape in men. The mode represents a shape or time-sensitive estimate of the delay to maximal secretion. This waveform term is independent of the amount (mass) of hormone secreted (33). However, AVF (negatively) and IGFBP-1 (positively) correlated with the time delay to maximal GH secretion under L-arginine/GHRH drive. Analyses using atomic-force microscopy indicate that GH-containing vesicles in pituitary cells must fuse with membrane pores to allow exocytosis (34). How AVF and IGFBP-1 modulate these processes is unknown.
Little is known about the regulation of basal (nonpulsatile) GH secretion (1). In the present study, this measure correlated positively with IGFBP-1 concentrations (R2 = 0.53, P < 0.0001). The precise basis for this new association is not known. One plausible mechanism linking IGFBP-1 and basal GH secretion would be free IGF concentrations, which appear to inhibit GH secretion (35).
Caveats include the relatively small cohort studied (N = 24), possible unknown effects of leuprolide per se, and the need to eventually extend paradigm duration.
In summary, L-arginine/GHRH and L-arginine/GHRP-2 are robust stimulators of GH secretion in a short-term hypogonadal setting. L-arginine/GHRH efficacy is negatively determined by AVF and positively by IGF-I concentrations. No such effects were evident for L-arginine/GHRP-2 stimulation. AVF and IGFBP-1 are significant covariates of the waveform duration of L-arginine/GHRH (but not L-arginine/GHRP-2) induced GH secretory bursts. The present outcomes delineate new distinctions between GHRH and GHRP actions, which may influence the choice of optimal secretagogue in a particular clinical setting.
Supplementary Material
Acknowledgments
We thank Donna Scott for support of manuscript preparation; Ashley Bryant for data analysis and graphics; the Mayo Immunochemical Laboratory for assay assistance; and the Mayo research nursing staff for implementing the protocol. Supported in part via the Center for Translational Science Activities (CTSA) Grant Number 1 UL 1 RR024150 to the Mayo Clinic and Foundation from the National Center for Research Resources (Rockville, MD) and R01 NIA AG19695 from the National Institutes of Health (Bethesda, MD).
Definitions
- basal
nonpulsatile secretion
- baseline
prior to intervention
Footnotes
Conflict of Interest Statement: There is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
References
- 1.Giustina A, Veldhuis JD. Pathophysiology of the neuroregulation of growth hormone secretion in experimental animals and the human. Endocrine Reviews. 1998;19:717–797. doi: 10.1210/edrv.19.6.0353. [DOI] [PubMed] [Google Scholar]
- 2.Veldhuis JD, Roemmich JN, Richmond EJ, Bowers CY. Somatotropic and gonadotropic axes linkages in infancy, childhood, and the puberty-adult transition. Endocrine Reviews. 2006;27:101–140. doi: 10.1210/er.2005-0006. [DOI] [PubMed] [Google Scholar]
- 3.Weltman A, Weltman JY, Hartman ML, Abbott RD, Rogol AD, Evans WS, Veldhuis JD. Relationship between age, percentage body fat, fitness, and 24-hour growth hormone release in healthy young adults: effects of gender. Journal of Clinical Endocrinology Metabolism. 1994;78:543–548. doi: 10.1210/jcem.78.3.8126124. [DOI] [PubMed] [Google Scholar]
- 4.Vahl N, Jorgensen JO, Skjaerback C, Veldhuis JD, Orskov H, Christiansen J. Abdominal adiposity rather than age and sex predicts the mass and patterned regularity of growth hormone secretion in mid-life healthy adults. American Journal of Physiology. 1997;272:E1108–E1116. doi: 10.1152/ajpendo.1997.272.6.E1108. [DOI] [PubMed] [Google Scholar]
- 5.Veldhuis JD, Erickson D, Mielke K, Farhy LS, Keenan DM, Bowers CY. Distinctive inhibitory mechanisms of age and relative visceral adiposity on GH secretion in pre- and postmenopausal women studied under a hypogonadal clamp. Journal of Clinical Endocrinology Metabolism. 2005;90:6006–6013. doi: 10.1210/jc.2005-0854. [DOI] [PubMed] [Google Scholar]
- 6.Chalew SA, Udoff LC, Hanukoglu A, Bistritzer T, Armour KM, Kowarski AA. The effect of testosterone therapy on spontaneous growth hormone secretion in boys with constitutional delay. American Journal of Diseases of Children. 1988;142:1345–1348. doi: 10.1001/archpedi.1988.02150120099049. [DOI] [PubMed] [Google Scholar]
- 7.Loche S, Colao A, Cappa M, Bellone J, Aimaretti G, Farello G, Faedda A, Lombardi G, Deghenghi R, Ghigo E. The growth hormone response to hexarelin in children: reproducibility and effect of sex steroids. Journal of Clinical Endocrinology Metabolism. 1997;82:861–864. doi: 10.1210/jcem.82.3.3795. [DOI] [PubMed] [Google Scholar]
- 8.Martin LG, Clark W, Connor TB. Growth hormone secretion enhanced by androgens. Journal of Clinical Endocrinology Metabolism. 1968;28:425–431. doi: 10.1210/jcem-28-3-425. [DOI] [PubMed] [Google Scholar]
- 9.Giustina A, Scalvini T, Tassi C, Desenzani P, Poiesi C, Wehrenberg WB, Rogol A, Veldhuis JD. Maturation of the regulation of growth hormone secretion in young males with hypogonadotropic hypogonadism pharmacologically exposed to progressive increments in serum testosterone. Journal of Clinical Endocrinology Metabolism. 1997;82:1210–1219. doi: 10.1210/jcem.82.4.3871. [DOI] [PubMed] [Google Scholar]
- 10.Devesa J, Lois N, Arce V, Diaz MJ, Lima L, Tresguerres JA. The role of sexual steroids in the modulation of growth hormone (GH) secretion in humans. Journal of Steroid Biochemistry and Molecular Biology. 1991;40:165–173. doi: 10.1016/0960-0760(91)90179-9. [DOI] [PubMed] [Google Scholar]
- 11.Fryburg DA, Weltman A, Jahn LA, Weltman JY, Samolijik E, Veldhuis JD. Short-term modulation of the androgen milieu alters pulsatile but not exercise or GHRH-stimulated GH secretion in healthy men. Journal of Clinical Endocrinology Metabolism. 1997;82:3710–3719. doi: 10.1210/jcem.82.11.4379. [DOI] [PubMed] [Google Scholar]
- 12.Ghigo E, Arvat E, Valente F, Nicolosi M, Boffano GM, Procopio M, Bellone J, Maccario M, Mazza E, Camanni F. Arginine reinstates the somatotrope responsiveness to intermittent growth hormone-releasing hormone administration in normal adults. Neuroendocrinology. 1991;54:291–294. doi: 10.1159/000125890. [DOI] [PubMed] [Google Scholar]
- 13.Alba-Roth J, Muller OA, Schopohl J, Von Werder K. Arginine stimulates growth hormone secretion by suppressing endogenous somatostatin secretion. Journal of Clinical Endocrinology Metabolism. 1988;67:1186–1189. doi: 10.1210/jcem-67-6-1186. [DOI] [PubMed] [Google Scholar]
- 14.Vale WW, Vaughan J, Yamamoto G, Spiess J, Rivier J. Effects of synthetic human pancreatic (tumor) GH releasing factor and somatostatin, triiodothyronine and dexamethasone on GH secretion in vitro. Endocrinology. 1983;112:1553–1555. doi: 10.1210/endo-112-4-1553. [DOI] [PubMed] [Google Scholar]
- 15.Fairhall KM, Mynett A, Robinson IC. Central effects of growth hormone-releasing hexapeptide (GHRP-6) on growth hormone release are inhibited by central somatostatin action. Journal of Endocrinology. 1995;144:555–560. doi: 10.1677/joe.0.1440555. [DOI] [PubMed] [Google Scholar]
- 16.Veldhuis JD, Bowers CY. Determinants of GH-releasing hormone and GH-releasing peptide synergy in men. American Journal of Physiology - Endocrinology and Metabolism. 2009 doi: 10.1152/ajpendo.91001.2008. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anderson SM, Shah N, Evans WS, Patrie JT, Bowers CY, Veldhuis JD. Short-term estradiol supplementation augments growth hormone (GH) secretory responsiveness to dose-varying GH-releasing peptide infusions in healthy postmenopausal women. Journal of Clinical Endocrinology Metabolism. 2001;86:551–560. doi: 10.1210/jcem.86.2.7240. [DOI] [PubMed] [Google Scholar]
- 18.Veldhuis JD, Evans WS, Bowers CY. Estradiol supplementation enhances submaximal feedforward drive of growth hormone (GH) secretion by recombinant human GH-releasing hormone-1,44-amide in a putatively somatostatin-withdrawn milieu. Journal of Clinical Endocrinology Metabolism. 2003;88:5484–5489. doi: 10.1210/jc.2003-030410. [DOI] [PubMed] [Google Scholar]
- 19.Takahashi PY, Votruba P, Abu-Rub M, Mielke K, Veldhuis JD. Age attenuates testosterone secretion driven by amplitude-varying pulses of recombinant human luteinizing hormone during acute gonadotrope inhibition in healthy men. Journal of Clinical Endocrinology Metabolism. 2007;92:3626–3632. doi: 10.1210/jc.2006-2704. [DOI] [PubMed] [Google Scholar]
- 20.Cosma M, Bailey JN, Miles JM, Bowers CY, Veldhuis JD. Pituitary and/or peripheral estrogen-receptor alpha (ERα) regulates FSH secretion whereas central pathways direct GH and prolactin secretion in postmenopausal women. Journal of Clinical Endocrinology Metabolism. 2008;93:951–958. doi: 10.1210/jc.2007-1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fisher LD, van Belle G. Biostatistics: A Methodology for the Health Sciences. John Wiley & Sons; New York: 1996. Descriptive statistics; pp. 58–74. [Google Scholar]
- 22.Hobbs CJ, Plymate SR, Rosen CJ, Adler RA. Testosterone administration increases insulin-like growth factor-I levels in normal men. Journal of Clinical Endocrinology Metabolism. 1993;77:776–779. doi: 10.1210/jcem.77.3.7690364. [DOI] [PubMed] [Google Scholar]
- 23.Bondanelli M, Ambrosio MR, Margutti A, Franceschetti P, Zatelli MC, degli Uberti EC. Activation of the somatotropic axis by testosterone in adult men: evidence for a role of hypothalamic growth hormone-releasing hormone. Neuroendocrinology. 2003;77:380–387. doi: 10.1159/000071310. [DOI] [PubMed] [Google Scholar]
- 24.Gentili A, Mulligan T, Godschalk M, Clore J, Patrie J, Iranmanesh A, Veldhuis JD. Unequal impact of short-term testosterone repletion on the somatotropic axis of young and older men. Journal of Clinical Endocrinology Metabolism. 2002;87:825–834. doi: 10.1210/jcem.87.2.8222. [DOI] [PubMed] [Google Scholar]
- 25.Veldhuis JD, Keenan DM, Mielke K, Miles JM, Bowers CY. Testosterone supplementation in healthy older men drives GH and IGF-I secretion without potentiating peptidyl secretagogue efficacy. European Journal of Endocrinology. 2005;153:577–586. doi: 10.1530/eje.1.02001. [DOI] [PubMed] [Google Scholar]
- 26.Veldhuis JD, Anderson SM, Iranmanesh A, Bowers CY. Testosterone blunts feedback inhibition of GH secretion by experimentally elevated IGF-I concentrations. Journal of Clinical Endocrinology Metabolism. 2005;90:1613–1617. doi: 10.1210/jc.2004-1303. [DOI] [PubMed] [Google Scholar]
- 27.Chattopadhyay S, Veldhuis JD, Keenan DM. Probabilistic recovery of pulsatile, secretory and kinetic structure: an alternating discrete and continuous schema. Quarterly of Applied Mathematics. 2008;66:401–421. [Google Scholar]
- 28.Keenan DM, Roelfsema F, Biermasz N, Veldhuis JD. Physiological control of pituitary hormone secretory-burst mass, frequency and waveform: a statistical formulation and analysis. American Journal of Physiology. 2003;285:R664–R673. doi: 10.1152/ajpregu.00195.2003. [DOI] [PubMed] [Google Scholar]
- 29.Faria ACS, Veldhuis JD, Thorner MO, Vance ML. Half-time of endogenous growth hormone (GH) disappearance in normal man after stimulation of GH secretion by GH-releasing hormone and suppression with somatostati. Journal of Clinical Endocrinology Metabolism. 1989;68:535–541. doi: 10.1210/jcem-68-3-535. [DOI] [PubMed] [Google Scholar]
- 30.Akaike H. A new look at the statistical model identification. IEEE Transactions on Automatic Control. 1974;19:716–723. [Google Scholar]
- 31.Veldhuis JD, Lassiter AB, Johnson ML. Operating behavior of dual or multiple endocrine pulse generators. American Journal of Physiology. 1990;259:E351–E361. doi: 10.1152/ajpendo.1990.259.3.E351. [DOI] [PubMed] [Google Scholar]
- 32.Veldhuis JD, Keenan DM, Bowers CY. Estimation of the size and shape of GH secretory bursts in healthy women using a physiological estradiol clamp and variable-waveform deconvolution model. American Journal of Physiology - Regulatory, Integrative, and Comparative Physiology. 2007;293:R1013–R1021. doi: 10.1152/ajpregu.00159.2007. [DOI] [PubMed] [Google Scholar]
- 33.Veldhuis JD, Keenan DM, Pincus SM. Motivations and methods for analyzing pulsatile hormone secretion. Endocrine Reviews. 2008;29:823–864. doi: 10.1210/er.2008-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cho SJ, Jeftinija K, Glavaski A, Jeftinija S, Jena BP, Anderson LL. Structure and dynamics of the fusion pores in live GH-secreting cells revealed using atomic force microscopy. Endocrinology. 2002;143:1144–1148. doi: 10.1210/endo.143.3.8773. [DOI] [PubMed] [Google Scholar]
- 35.Chen JW, Hojlund K, Beck-Nielsen H, Sandahl CJ, Orskov H, Frystyk J. Free rather than total circulating insulin-like growth factor-I determines the feedback on growth hormone release in normal subjects. Journal of Clinical Endocrinology Metabolism. 2005;90:366–371. doi: 10.1210/jc.2004-0039. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.