Abstract
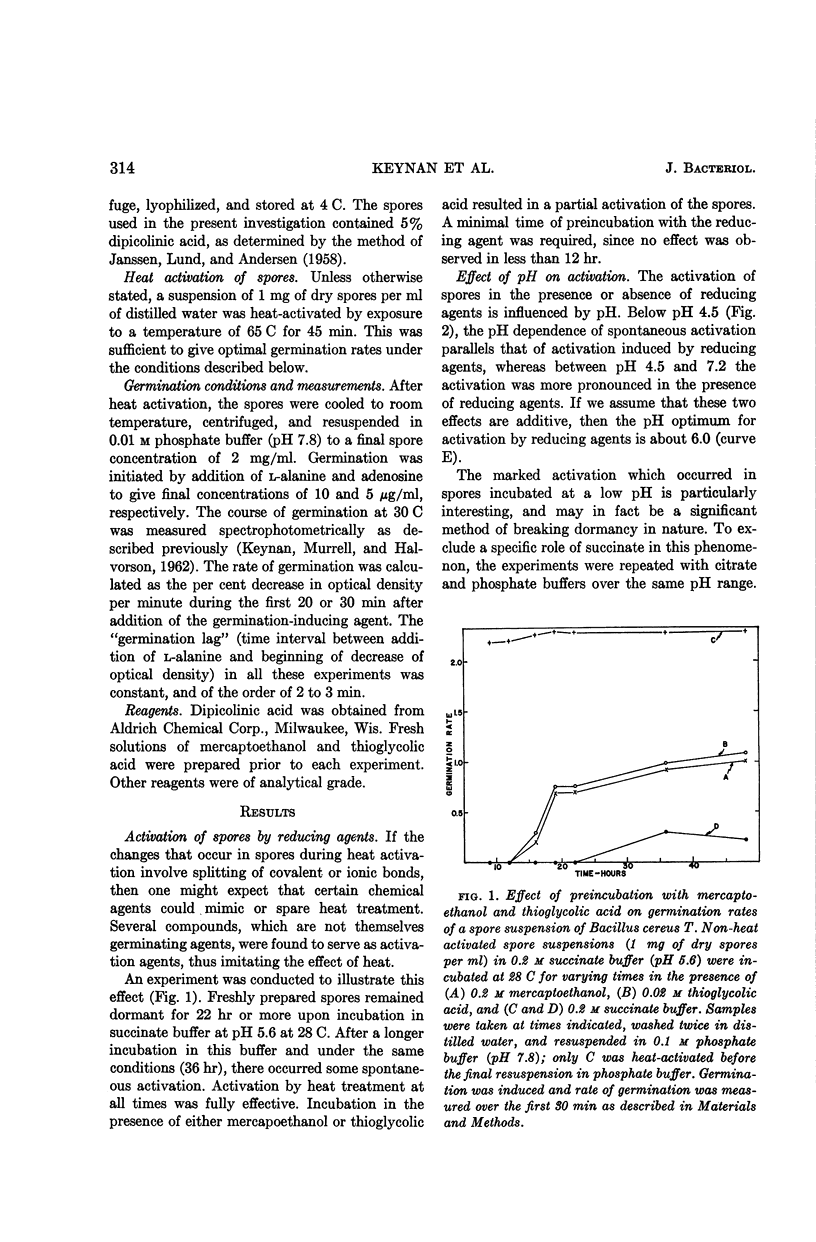
A. Keynan (Israel Institute of Biological Research, Ness Ziona, Israel), Z. Evenchik, H. O. Halvorson, and J. W. Hastings. Studies on the activation of bacterial endospores. J. Bacteriol. 88:313–318. 1964.—Heat activation of bacterial endospores was imitated by suspending spores in reducing agents (mercaptoethanol or thioglycolate) or in a pH less than 4.5. Urea (6 m) had no effect on spores. In addition to the well-known activation at 65 C for 45 min, spores were also activated by exposure to 34 C for 48 hr. The activation by heat and by reducing agents was reversible; the reverse reaction was temperature-dependent. No reversion occurred at −20 C, whereas at 28 C the spores reversed to their original dormant state within 72 hr.
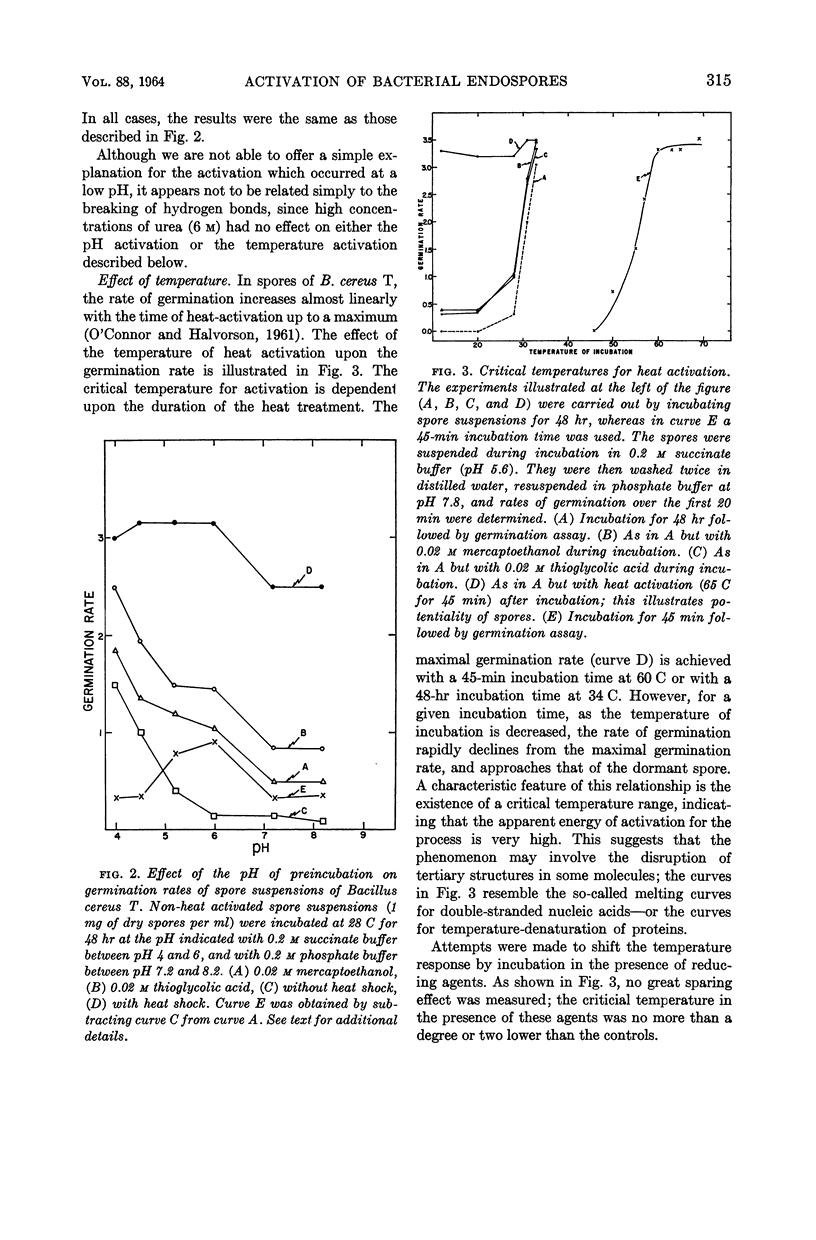
It is suggested that the heat-activation phenomenon could be explained by assuming that heat or reducing agents change the tertiary structure of a protein responsible for the maintenance of the dormant state by reducing the disulfide linkages which stabilize the protein in a specific configuration. The partial denaturation of this protein is reversible by reoxidation of the reduced disulfide bonds.
Full text
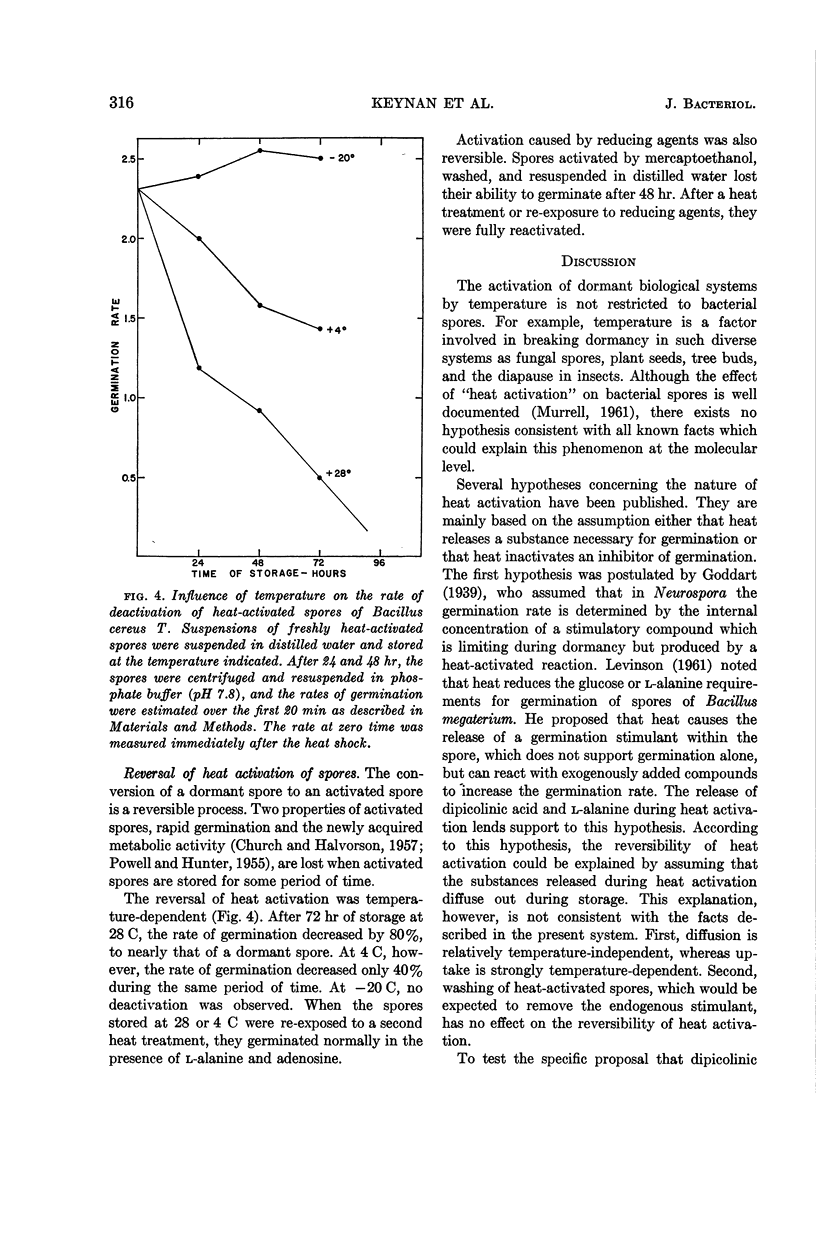
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CHURCH B. D., HALVORSON H. Intermediate metabolism of aerobic spores. I. Activation of glucose oxidation in spores of Bacillus cereus var terminalis. J Bacteriol. 1957 Apr;73(4):470–476. doi: 10.1128/jb.73.4.470-476.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans F. R., Curran H. R. The accelerating effect of sublethal heat on spore germination in mesophilic aerobic bacteria. J Bacteriol. 1943 Dec;46(6):513–523. doi: 10.1128/jb.46.6.513-523.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JANSSEN F. W., LUND A. J., ANDERSON L. E. Colorimetric assay for dipicolinic acid in bacterial spores. Science. 1958 Jan 3;127(3288):26–27. doi: 10.1126/science.127.3288.26. [DOI] [PubMed] [Google Scholar]
- KEYNAN A., MURRELL W. G., HALVORSON H. O. Germination properties of spores with low dipicolinic acid content. J Bacteriol. 1962 Feb;83:395–399. doi: 10.1128/jb.83.2.395-399.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVINSON H. S., HYATT M. T. Some effects of heat and ionizing radiation on spores of Bacillus megaterium. J Bacteriol. 1960 Oct;80:441–451. doi: 10.1128/jb.80.4.441-451.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W. H., Ordal Z. J. REVERSIBLE ACTIVATION FOR GERMINATION AND SUBSEQUENT CHANGES IN BACTERIAL SPORES. J Bacteriol. 1963 Jan;85(1):207–217. doi: 10.1128/jb.85.1.207-217.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'CONNOR R. J., HALVORSON H. O. L-Alanine dehydrogenase: a mechanism controlling the specificity of amino acid-induced germination of Bacillus cereus spores. J Bacteriol. 1961 Nov;82:706–713. doi: 10.1128/jb.82.5.706-713.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POWELL J. F., HUNTER J. R. Spore germination in the genus bacillus: the modification of germination requirements as a result of preheating. J Gen Microbiol. 1955 Aug;13(1):59–67. doi: 10.1099/00221287-13-1-59. [DOI] [PubMed] [Google Scholar]
- VINTER V. Spores of microorganisms. IX. Gradual development of the resistant structure of bacterial endospores. Folia Microbiol (Praha) 1962 Mar;7:115–120. doi: 10.1007/BF02927234. [DOI] [PubMed] [Google Scholar]


