Abstract
Basic helix-loop-helix (bHLH) proteins constitute a large family of transcriptional regulators in plants. Although they have been shown to play important roles in a wide variety of developmental processes, relatively few have been functionally characterized. Here, we describe the map-based cloning of the Lotus japonicus ROOTHAIRLESS1 (LjRHL1) locus. Deleterious mutations in this locus prevent root hair development, which also aborts root hair-dependent colonization of the host root by nitrogen-fixing bacteria. We show that the LjRHL1 gene encodes a presumed bHLH transcription factor that functions in a nonredundant manner to control root hair development in L. japonicus. Homology search and cross-species complementation experiments defined three members of the Arabidopsis (Arabidopsis thaliana) bHLH protein family, At2g24260, At4g30980, and At5g58010, as functionally equivalent to LjRHL1. Curiously, At2g24260 and At4g30980 mRNA species accumulate independently from the known positive regulators of root hair cell fate, while all three genes act in a partially redundant manner to regulate root hair development in Arabidopsis.
Root hairs constitute tubular extensions of epidermal cells. Formed by the majority of angiosperm plants, they function to increase root surface area, thus facilitating physical anchorage to a substrate while providing a large interface through which nutrients and water are absorbed. Root hairs are also important for plant-microbe interactions, highlighting the key role of these tip-growing cells in biotic and abiotic interactions of the root.
Features of root hair development, such as their exterior position, species-specific patterning, and polarized growth, have made them an attractive model for studying various aspects of cell differentiation and growth in the context of root developmental plasticity. Both endogenous and environmental cues have been implicated in the specification of root hair development, and various proteins and protein complexes have been characterized as positive or negative regulators of root hair differentiation (Ishida et al., 2008).
In Arabidopsis (Arabidopsis thaliana) roots, where files of hair cells alternate with files of nonhair cells, genetic analysis has revealed intricate mechanisms of positional information, epigenetic regulation, and non-cell-autonomous signaling in the specification of epidermal cell fate (Guimil and Dunand, 2006). A protein complex containing the transcriptional regulators WEREWOLF (Lee and Schiefelbein, 1999), GLABRA3 and ENHANCER OF GLABRA3 (GL3/EGL3; Payne et al., 2000; Bernhardt et al., 2003; Zhang et al., 2003), and TRANSPARENT TESTA GLABRA (Galway et al., 1994; Walker et al., 1999), was shown to promote expression of the GL2 gene (Masucci et al., 1996), encoding an HD-ZIP transcription factor, to regulate hairless cell differentiation. Conversely, R3 MYB proteins CAPRICE (CPC; Wada et al., 1997), TRIPTYCHON (TRY; Schellmann et al., 2002), ENHANCER OF TRY AND CPC1 (ETC1; Kirik et al., 2004), and CAPRICE-LIKE MYB3 (CLP3; Tominaga et al., 2008) were identified as positive regulators of hair cell identity. In addition, various networks of transcription factors and other regulatory elements, encompassing different cellular functions, including chromatin remodeling, hormonal signaling, ion fluxes, cell cycle progression, and cytoskeleton rearrangement, were shown to be important for initiation and maintenance of polar root hair growth (Guimil and Dunand, 2006). The availability of a large collection of root hair mutants in Arabidopsis continues to fuel rapid dissection of genetic networks and associated cellular events governing patterning and growth of root hair cells.
Root hair mutants have also been identified from genetic screens of which the primary goal has been the characterization of loci that support the development of root nodule symbiosis in legume plants (Kawaguchi et al., 2002; Karas et al., 2005; Murray et al., 2006) In many legumes, root hairs mediate the initial contact between the legume host and nitrogen-fixing soil bacteria, commonly known as Rhizobium. They actively participate in the recognition of bacterially encoded lipochitin-oligosaccharide signaling molecules known as nodulation or Nod factors and, subsequently, in the colonization of the root by bacteria (Karas et al., 2005), processes that are unknown to Arabidopsis. Prior to or concomitant with the initiation of the infection process at the surface of root hair tips, legume roots respond to bacterial signaling by the initiation of cell division in the root cortex. This leads to the formation of new lateral organs, root nodules, which eventually host the symbiotic bacteria, providing the appropriate conditions for symbiotic nitrogen fixation to occur (Oldroyd and Downie, 2008).
Impairment of root hair development results in defective symbiotic interaction. In the model legume Lotus japonicus, deleterious mutations in the ROOTHAIRLESS1 (LjRHL1) locus prevent root hair formation (Karas et al., 2005). Inoculation of the corresponding Ljrhl1-1 mutant with symbiotic bacteria leads to the initial formation of empty nodule structures, thus uncoupling bacterial colonization of the root from nodule organogenesis (Karas et al., 2005). These features of the Ljrhl1-1 mutant genetic background have been useful in the functional analysis of root nodule organogenesis (Karas et al., 2005; Murray et al., 2007); therefore, we set out to characterize the underlying LjRHL1 locus at the molecular level.
We show here that the deleterious mutations in the LjRHL1 gene, encoding a basic helix-loop-helix (bHLH) transcription factor, were responsible for the lack of root hairs in the L. japonicus Ljrhl1-1 mutant. Among the 162 affiliates of the Arabidopsis bHLH protein family, LjRHL1 showed the highest homology with the members of group XI, which comprises five predicted bHLH transcription factors (Heim et al., 2003). By performing cross-species complementation experiments, we demonstrate functional conservation of L. japonicus LjRHL1 and Arabidopsis At2g24260, At4g30980, and At5g58010 but not At1g03040 and At4g02590 proteins. We demonstrate the partially redundant function of At2g24260, At4g30980, and At5g58010, which provides a plausible explanation of why mutations in these loci do not generate a root hair phenotype in Arabidopsis.
RESULTS
L. japonicus Root-Hairless Mutants
In two independent genetic screens, we have identified a class of ethylmethane sulfonate-induced L. japonicus symbiotic mutant lines, where defective interaction with a natural microsymbiont of L. japonicus, Mezorhizobium loti, was linked with the impairment of root hair development (Kawaguchi et al., 2002; Karas et al., 2005; Murray et al., 2006). Among these mutants, four root-hairless lines were found, and the detailed analysis of their root and symbiotic phenotypes was described (Kawaguchi et al., 2002; Karas et al., 2005). Three of these lines were put into a single complementation group and assigned an allelic designation, Ljrhl1-1, Ljrhl1-2, and Ljrhl1-3 (Karas et al., 2005). However, further analysis of these lines defined them as siblings, carrying the same mutant allele, Ljrhl1-1 (see below). The fourth line, slippery (slp), was derived from a separate screen (Kawaguchi et al., 2002), and its relation to Ljrhl1-1 was unresolved.
Based on the similarity of root hair and symbiotic phenotypes in Ljrhl-1 and slp, we predicted that these lines might carry allelic mutations. The roots of F1 plants, derived from a complementation cross between homozygous Ljrhl1-1 and slp, showed the root-hairless phenotype, thus supporting this prediction (see below). Consequently, we renamed slp as Ljrhl1-2 (Fig. 1).
Figure 1.
Root hair phenotypes of L. japonicus wild-type Gifu (left), Ljrhl1-1 mutant (center), and Ljrhl1-2 mutant (right) lines. Six-day-old seedlings are shown. Note the emerging difference in root length between wild-type and mutant plants.
L. japonicus roots have type 1 hair cell patterning: all or almost all root epidermal cells produce hairs (Karas et al., 2005). The Ljrhl1-1 line remained almost totally hairless when cultivated under various growth conditions (Karas et al., 2005). Root hair formation in Ljrhl1-2 was very low in comparison with the wild-type control, but its frequency varied depending on the growth conditions used. When cultivated in soil, the frequency of root hair formation in Ljrhl1-2 was 62 ± 15 per plant (n = 30), while no root hairs were detected in Ljrhl1-1 under the same growth conditions.
The observed differences in root hair phenotypes between Ljrhl1-1 and Ljrhl1-2 correlated, to some extent, with the severity of their corresponding mutant symbiotic phenotypes. When analyzed in parallel 21 d after inoculation with M. loti carrying the constitutively expressed GUS reporter gene, the Ljrhl1-1 line developed mostly uncolonized nodule primordia, and only a few small nodules colonized by rhizobia were formed, thus confirming our previous data (Karas et al., 2005). In contrast, Ljrhl1-2 formed a limited number of big colonized nodules, although small nodules and uncolonized nodule primordia were as abundant in Ljrhl1-2 as in Ljrhl1-1 (Supplemental Fig. S1). The presence of big nodules at 21 d after inoculation suggested that the root hairs formed by Ljrhl1-2 were able to support limited root colonization by the bacteria in a root hair-dependent manner, similar to the wild-type interaction (Karas et al., 2005).
The initial growth of both mutant lines was as vigorous as that of wild-type plants, and no significant differences, aside from root hair formation and nodulation pattern, were observed. However, unlike Ljrhl1-2, the Ljrhl1-1 line showed increased sensitivity to growth conditions (e.g. watering) and grew less vigorously later during development, which was also reflected in the smaller pod size of this line (data not shown). When grown vertically on the surface of agar plates, both mutants had increased root elongation compared with wild-type plants (Fig. 1; Karas et al., 2005).
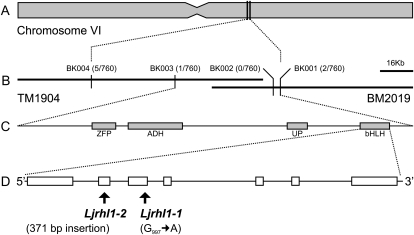
Map-Based Cloning of the LjRHL1/SLP Locus
The initial mapping experiments positioned the Ljrhl1-1 mutation within a 3.2-centimorgan interval on the long arm of L. japonicus chromosome 6, between the microsatellite (simple sequence repeat) markers JM001 and TM0140 (Karas et al., 2005). Fine mapping and subsequent genotyping of 760 Ljrhl1-1 mutants, derived from an F2 segregating population of a cross between the Ljrhl1-1 homozygous line (ecotype Gifu) and a polymorphic wild-type L. japonicus MG-20, enabled us to narrow down this region to 48.8 kb (Fig. 2). This region is flanked by recombination points, as defined by BK001 and BK003 molecular markers, which are located on two overlapping transformation-competent artificial chromosome clones, BM2019 and TM1904, respectively (Fig. 2, A and B). Four genes, encoding a putative zinc finger protein, an aldehyde dehydrogenase, an unknown protein, and a bHLH transcriptional factor, were predicted in this region by a BLAST search of the Arabidopsis genome (Fig. 2C). The PCR amplification and sequencing of all four genes from the Ljrhl1-1 and Ljrhl1-2 mutants and the corresponding wild-type Gifu revealed the presence of mutations in the gene encoding the bHLH transcriptional regulator (accession no. FJ375304) but not in the other three genes. The Ljrhl1-2 line carried a 371-bp insertion in the predicted exon 2 of this gene, while the three Ljrhl1 lines had an identical single base pair substitution of G-997 to A in predicted exon 3 and were, therefore, considered to be siblings (Fig. 2D).
Figure 2.
Map-based cloning of the LjRHL1 locus. A, A schematic of L. japonicus chromosome VI. B, Two overlapping transformation-competent artificial chromosome clones, TM1904 and BM2019, with genetic markers linked to the LjRHL1 locus are indicated. Number of recombinants versus total number of homozygous mutant individuals tested for a given marker is shown in parentheses. C, The region delimited by BK003 and BK001 genetic markers was predicted to contain four genes encoding a presumed zinc finger protein (ZFP), an alcohol dehydrogenase (ADH), unknown protein (UP), and a bHLH domain-containing protein (bHLH). D, Exon-intron structure of the LjRHL1 gene. The positions of genetic lesions in Ljrhl1-1 and Ljrhl1-2 mutant alleles are indicated.
A pair of gene-specific primers that were localized in the first and fourth exons of the predicted bHLH gene was used in genomic PCR amplification (Supplemental Fig. S2A). For both the wild type and Ljrhl1-1, the expected 967-bp genomic fragment was amplified. In contrast, a 1,338-bp fragment was generated when the Ljrhl1-2 genomic DNA was used as a template. Sequencing of all genomic fragments obtained confirmed that they were derived from the bHLH locus and that the 371-bp insertion was indeed present in the Ljrhl1-2 allele.
The same primer pair was used in a reverse transcription (RT)-PCR approach with total RNA derived from uninoculated roots of all three genotypes (Supplemental Fig. S2B). A 447-bp cDNA fragment was amplified from both the wild type and Ljrhl1-1, while several weak bands of higher molecular mass were obtained for the Ljrhl1-2 genetic background. The latter result indicated that alternative forms of the bHLH mRNA were produced due to the presence of the insertion (see below). Together, these results defined the bHLH gene as a viable candidate for the LjRHL1 locus.
To further test this prediction, a full copy cDNA was obtained that corresponded to the bHLH mRNA (see below), and in planta complementation experiments were performed. A binary vector, containing the cauliflower mosaic virus (CaMV) 35S promoter driving expression of a C-terminal translational fusion between GFP and the bHLH cDNAs, was introduced into L. japonicus roots by Agrobacterium rhizogenes-mediated transformation (Murray et al., 2007). The resulting transgenic hairy roots, which emerged from points of inoculation on the hypocotyl of Ljrhl1-1 and Ljrhl1-2 mutant plants, formed root hairs with efficiency comparable to wild-type plants (Supplemental Fig. S3). However, instances where transgenic roots showed patchy root hair formation were also observed. We have attributed this phenotype to the sporadically chimeric nature of hairy root tissues. When transformed with the control vector, containing the CaMV 35S promoter and GFP but lacking the bHLH cDNA, the hairy roots that formed remained hairless, thus recapitulating the phenotype of the original mutant plants (Supplemental Fig. S3).
Cumulatively, based on the data obtained, we concluded that the bHLH gene identified through a map-based cloning approach indeed corresponded to the LjRHL1 locus.
LjRHL1 Encodes a bHLH Transcriptional Factor
Using a combined approach of RT-PCR and 5′- and 3′-RACE, a full copy cDNA of 1,444 nucleotides in length, corresponding to the LjRHL1 mRNA, was reassembled (see “Materials and Methods”). It contained an open reading frame of 1,161 nucleotides encoding a predicted LjRHL1 protein of 40.5 kD. The initiation ATG codon was preceded by a 229-nucleotide-long 5′ untranslated region that contained an in-frame TGA stop codon. At the 3′ end, the TGA stop codon was followed by a 3′ untranslated region of 55 nucleotides. Alignment of the mRNA and the corresponding L. japonicus genomic sequence predicted an LjRHL gene structure of seven exons and six introns (Fig. 2D).
A search for conserved protein domains using BLAST and Pfam algorithms identified the presence of a bHLH motif in the predicted LjRHL1 protein, which was localized between amino acid residues 181 and 236 (Fig. 3). A comparison with a consensuses sequence for plant bHLH domains (Heim et al., 2003) showed that key amino acid residues were present at the conserved positions within the bHLH of LjRHL1 (Fig. 3). Thus, the most critical His-Glu-Arg (H-E-R) residues for amino acid contact with nucleotide bases were present at the conserved amino acid positions 5, 9, and 13, respectively, within the predicted bHLH domain. Furthermore, the hydrophobic residues, presumed to be important for bHLH dimerization and the stability of the resulting DNA-protein dimers (Ferre-D'Amare et al., 1993), such as Leu-23, were also found at the equivalent positions in the bHLH domain of LjRHL1 (Fig. 3).
Figure 3.
Alignment of the predicted LjRHL1 bHLH domain with a consensus sequence for plant bHLH (Heim et al., 2003). Amino acid residues that have been predicted to be important for contact with a nucleotide base (stars), DNA backbone (plus signs), and protein-protein interactions (circles) are indicated, as in Heim et al. (2003).
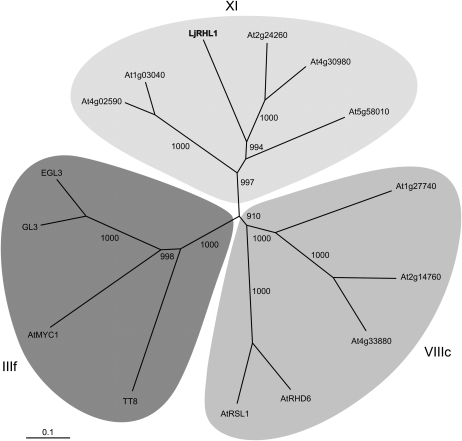
A search for homologous proteins in Arabidopsis identified several significant hits to the predicted bHLH proteins, with the top five encompassing all members of the Arabidopsis bHLH protein subfamily XI (Fig. 4). The amino acid conservation between LjRHL1 and Arabidopsis subfamily XI members, At1g03040, At2g24260, At4g02590, At4g30980, and At5g58010, was mostly restricted to the C-terminal portion of these proteins. This included the bHLH DNA-binding motif as well as additional stretches of amino acid sequence located immediately upstream and downstream and also at some distance downstream from the predicted boundaries of the bHLH domain (Supplemental Fig. S4). This pattern was consistent with the previously reported amino acid conservation between members of the Arabidopsis bHLH subfamily XI (Heim et al., 2003). At2g24260 was the most similar to LjRHL1, displaying 71% identity and 79% similarity within the extended C-terminal region and 98% identity within the bHLH domain. Cumulatively, these data strongly suggested that the LjRHL1 locus encodes a functional bHLH transcriptional factor, a notion that was supported by the observed phenotypes associated with the Ljrhl1-1 and Ljrhl1-2 mutations.
Figure 4.
Phylogenetic analysis of the LjRHL1 protein. Unrooted tree based on an amino acid alignment of full-length sequences of L. japonicus LjRHL1-1 and members of the Arabidopsis bHLH domain protein family belonging to group XI (top) and subgroups IIIf (bottom left) and VIIIc (bottom right). EGL3 and GL3 (subgroup IIIf) and AtRSL1 and AtRHD6 (subgroup VIIIc) were previously shown to be involved in specification of root epidermal cell fate in Arabidopsis (Bernhardt et al., 2003; Heim et al., 2003; Menand et al., 2007).
The point mutation altering nucleotide G-997 to A in exon 3 of the Ljrhl1-1 allele was predicted to replace the amino acid Glu-243 with Lys in the critical H-E-R motif of the bHLH domain (Fig. 2D). Analysis of the 371-bp insertion in the Ljrhl1-2 allele identified this sequence as a member of a group of long terminal repeat retrotransposons defined as terminal-repeat retrotransposons in miniature, or TRIM (Witte et al., 2001). We named this retrotransposon LjTRIM1.
LjTRIM1 has all sequence features characteristic for this class of retrotransposons (Supplemental Fig. S5), including short overall length, 139-bp-long terminal direct repeats, and an internal domain of 88 bp containing a primer binding sequence and a polypurine track. Its insertion at the bottom strand of the LjRHL1 locus led to the typical 5-bp target site direct duplication and resulted in the generation of at least five different species of LjRHL1 mRNA (Supplemental Fig. S3B). These mRNAs (I–V) retained the original LjTRIM1 insertion but differed in splicing patterns of the adjacent introns, 1, 2, and 3. While these introns were correctly spliced in mRNA I, introns 1 and 2 were retained within mRNA II and III, respectively. mRNA IV retained both introns 1 and 2, while intron 3 was spliced normally. Finally, mRNA V retained all three introns. Regardless of the splicing pattern, the premature termination of translation likely occurred in all of these mRNA species due to the presence of a predicted in-frame translation-terminating codon in LjTRIM1 (data not shown).
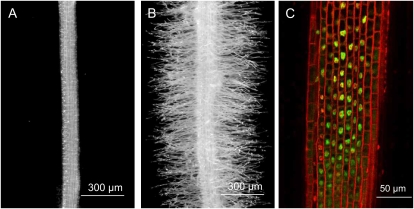
Expression analysis using RT-PCR demonstrated that LjRHL1 mRNA was detectable in all L. japonicus tissues tested (Supplemental Fig. S6). When the GFP-LjRHL1 protein fusion was expressed in transgenic hairy roots under the control of the LjRHL11 promoter, root hair growth in Ljrhl1-1 and Ljrhl1-2 was restored, and the protein was localized in the nucleus of cells in the cell elongation zone of the root (Fig. 5).
Figure 5.
Complementation of the Ljrhl1-2 root-hairless phenotype and localization of the LjRHL1 protein. The Ljrhl1-2 mutant was inoculated with the A. rhizogenes strain AR10, and the resulting transgenic hairy roots carrying either a LjRHL1 promoter-GFP control construct (A) or a chimeric GFP-LjRHL1 gene construct driven by the LjRHL1 promoter (B and C) are shown. The equivalent mature root regions, where fully developed root hairs are normally present, are shown in A and B. C shows the meristematic/elongation zone of the transgenic hairy root showing expression of GFP-LjRHL1 in the nuclei of epidermal cells.
Functional Conservation of L. japonicus LjRHL1 and Arabidopsis bHLH Proteins
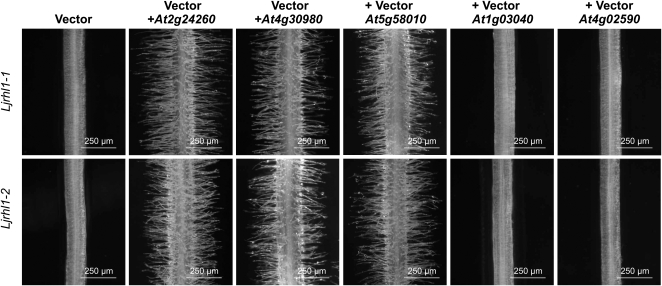
The amino acid sequence conservation between Arabidopsis bHLH proteins of subfamily XI and LjRHL1 prompted us to perform cross-species complementation experiments (Fig. 6). Expression of At1g03040 and At4g02590 under the control of the CaMV 35S promoter in transgenic hairy roots did not rescue the root-hairless phenotype of Ljrhl1-1 and Ljrhl1-2 mutants. In contrast, At2g24260, At4g30980, and At5g58010 complemented the root hair developmental defect of both Ljrh1-1 and Ljrhl1-2 alleleic mutants (Fig. 6). Based on the protein structure similarities and functional complementation results, we renamed the three Arabidopsis genes as LjRHL1-LIKE1 (AtLRL1), AtLRL2, and AtLRL3, respectively. Since our data suggested that these genes might regulate root hair development in Arabidopsis, we set out to test this prediction.
Figure 6.
Cross-species complementation experiments. Under the control of the CaMV 35S promoter, the expression of At2g24260, At4g30980, and At5g5810, but not At1g03040 and At4g02590, complements the root-hairless phenotype of Ljrhl1-1 (top row) and Ljrhl1-2 (bottom row) allelic mutant lines in transgenic hairy roots.
AtLRL1, AtLRL2, and AtLRL3 Genes Regulate Root Hair Development in Arabidopsis
The T-DNA insertion lines corresponding to the three Arabidopsis genes were identified from the Arabidopsis stock center at The Arabidopsis Information Resource and renamed correspondingly as Atlrl1-1 and Atlrl1-2 for the AtLRL1 locus and Atlrl2-1 and Atlrl3-1 for AtLRL2 and AtLRL3, respectively. Their detailed molecular characterization is described in Supplemental Information S1 and Supplemental Figure S7.
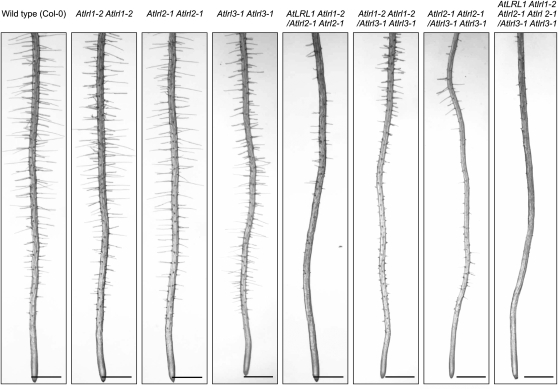
All single locus homozygous T-DNA insertion lines described above showed the wild-type root hair phenotype (Fig. 7). Therefore, genetic crosses were performed to construct the corresponding double mutants. For Atlrl1-1 × Atlrl2-1 and Atlrl1-2 × Atlrl2-1, the double homozygous genotype was not recovered from among all F2 individuals analyzed (see below). However, 20 plants in total that showed significantly diminished root hair development were selected from both segregating populations (Fig. 7; Supplemental Information S1). The initiation of root hairs appeared not significantly affected in these plants, as evidenced by the formation of bulges. However, subsequent root hair elongation was severely reduced and occurred only sporadically, giving rise to a patchy short root hair phenotype (Fig. 7). All of these individuals were homozygous for either Atlrl1 or Atlrl2-1, while they remained heterozygous for the second locus (Supplemental Table S1).
Figure 7.
AtLRL1, AtLRL2, and AtLRL3 act redundantly to positively regulate root hair development in Arabidopsis. A, Roots of 5-d-old homozygous single, double, and triple mutant lines are shown in comparison with the wild type (Col-0). The corresponding genotypes are indicated. Note that for Atlrl1-1 × Atlrl2-1 and Atlrl1-2 × Atlrl2-1 crosses, the phenotype of only one representative progeny, AtLRL1 Atlrl1-2/Atlrl2-1 Atlrl2-1, is shown. Bars = 500 μm.
Double homozygous lines of Atlrl1-2 Atlrl1-2/Atlrl3-1 Atlrl3-1 and Atlrl2-1 Atlrl2-1/Atlrl3-1 Atlrl3-1 genotypes could be recovered and were characterized as having a defective root hair phenotype (Fig. 7). Once again, root hair initiation appeared not affected; however, the subsequent elongation of root hairs was severely diminished in these lines, resulting in a predominantly spiky, short root hair phenotype. A similar phenotype was observed in triple mutant lines, where either the AtLRL1 or AtLRL2 locus remained heterozygous.
Expression of AtLRL3 Requires CPC, CPC-Like MYB, and AtRHD6/AtRSL1 Gene Functions
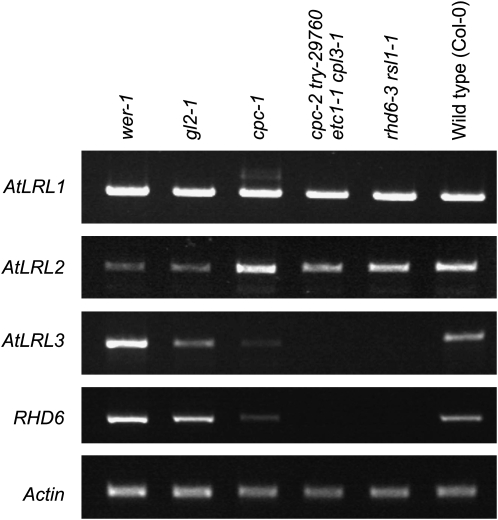
Having established that the AtLRL1, AtLRL2, and AtLRL3 genes positively affect root hair development, we analyzed their expression in roots of three different mutant Arabidopsis genetic backgrounds: cpc1 (Wada et al., 1997), cpc2 try-29760 etc1 cpl3-1 quadruple mutant (Tominaga et al., 2008), and Atrhd6-3 (for root hair defective6) Atrsl1-1 (for rhd six-like1) double mutant (Menand et al., 2007). Since AtRHD6 was shown to be positively regulated by CPC in Arabidopsis (Menand et al., 2007), the analysis of the steady-state level of its corresponding mRNA in the mutant backgrounds listed above was performed in parallel. The AtLRL1 and AtLRL2 mRNAs were detected in all mutant backgrounds tested. In contrast, the AtLRL3 mRNA was present in cpc1 but was absent from both the quadruple and the double mutant lines (Fig. 8).
Figure 8.
RT-PCR expression analysis of At2g24260, At4g30980, At5g58010, and RHD6 in roots of Arabidopsis mutant and wild-type (Col-0) lines (see text for further details). Actin serves as a control. Note that lack of the RHD6 cDNA product in rhd6-3 rsl1-1 is caused by the T-DNA insertion in the RHD6 locus.
DISCUSSION
In this study, we investigated the molecular basis of root hair formation in L. japonicus by analyzing two mutant lines carrying deleterious mutations in the LjRHL1 locus. The main premise of this research was to identify and characterize the underlying defective gene at the molecular level in order to facilitate a more informed use of Ljrhl1-1 and Ljrhl1-2 in experiments that aim at functional dissection of the mechanisms by which legumes accommodate symbiotic bacteria. However, a comparative analysis of root hair development in L. japonicus and Arabidopsis was also explored. Unlike in Arabidopsis, all or almost all of the L. japonicus root epidermal cells produce hairs (Karas et al., 2005); thus, no pattern of root hair and root-hairless cells is formed (Dolan and Costa, 2001).
We showed that the LjRHL1 locus encodes a predicted bHLH transcription factor that is indispensable for root hair formation in L. japonicus. While the presence of LjRHL1 mRNA in all L. japonicus tissues tested suggests that this locus might play a role in other regulatory processes in addition to root hair development, the only consistently discernible pleiotropic effect of Ljrhl1-1 and Ljrhl1-2 allelic mutations observed was a slight increase in the rate of root elongation in comparison with the wild-type genetic background. Lack of a trichome phenotype indicates that LjRHL1 is either not involved or acts redundantly to regulate the development of epidermal cells in aerial tissues.
Localization of the GFP-LjRHL1 chimeric protein in the nuclei of cells encompassing the root elongation and not the root differentiation zone suggested that LjRHL1 functions early in signaling mechanism(s) that specify root hair development in L. japonicus. This expression pattern might also be pertinent to root elongation, since cell growth and differentiation are interlinked and tightly controlled (Guimil and Dunand, 2007). Considering the nature of detected mutations, with Ljrhl1-1 carrying a single base pair substitution and Ljrhl1-2 representing an LjTRIM1 insertion allele, the observation of a slightly stronger mutant root hair phenotype in the former appears somewhat counterintuitive. However, due to its alleged participation in protein complexes, the presence of a mutant LjRHL1 protein (i.e. Ljrhl1-1) might be more deleterious than its total absence, which is the presumed effect of the Ljrhl1-2 mutation; the validity of this assumption, however, remains to be further determined.
The homology search clustered the LjRHL1 protein together with the members of subgroup XI of the Arabidopsis bHLH protein family. Only one out of five members of this subgroup, namely At4g02590, had been functionally classified and is likely involved in regulatory processes associated with pollen tube guidance and/or reception (Pagnussat et al., 2005). Although Heim et al. (2003) suggested possible functional redundancy between At1g03040, At4g02590, At2g24260 (AtLRL1), and At4g30980 (AtLRL2) based on their structural similarities, this has neither been proven nor linked to any particular biological process.
We set out to test whether the three Arabidopsis genes, AtLRL1, AtLRL2, and AtLRL3, which complemented the L. japonicus root-hairless phenotype, also regulate root hair development in Arabidopsis. Our data indicated that this is indeed the case.
The constitutive expression of the corresponding Arabidopsis cDNAs in transgenic hairy roots restored root hair formation in both Ljrhl1-1 and Ljrhl1-2 allelic mutants, demonstrating the functional equivalency, at least in terms of their biochemical properties, between Lotus LjRHL1 and the three predicted Arabidopsis bHLH proteins. Importantly, the analysis of double and triple T-DNA insertion mutants showed that AtLRL1, AtLRL2, and AtLRL3 function in at least a partially redundant manner to positively regulate root hair formation in Arabidopsis. These results are consistent with the corresponding expression profiles, as provided in the Arabidopsis Gene Atlas (http://Arabidopsis.org), where both AtLRL1 and AtLRL3 mRNA are shown to be highly represented in root tissues, including root hair elongation and differentiation zones. AtLRL1, AtLRL2, and AtLRL3 likely mediate the development of Arabidopsis root hair cells by acting downstream from the regulatory complexes that specify pattern formation in the epidermis. However, detailed comparative analyses of spatial-temporal expression patterns in wild-type and mutant Arabidopsis lines will be required to precisely pinpoint their role during the differentiation of trichoblasts.
The inability to recover the Atlrl1/Atlrl2 double homozygote mutant line further suggested that the presence of one of these bHLH proteins is required for viability. Thus, in addition to their role in the root epidermis, the AtLRL1 and AtLRL2 genes likely function in other key developmental mechanisms in Arabidopsis, and this might also be pertinent to AtLRL3, as indicated by the organ-specific expression profile available for this gene (http://Arabidopsis.org). As our research mainly concentrates on the biology of legume plants, we have not pursued these important questions in Arabidopsis. Nevertheless, it is tempting to speculate that, like At4g02590, the AtLRL1, AtLRL2, and AtLRL3 genes are involved in the regulatory pathways relevant to Arabidopsis gametophytic development, although this remains to be further established.
In spite of the apparent defect in root hair development in the double insertion line, our data show that accumulation of AtLRL1 and AtLRL2 transcripts in Arabidopsis roots was not strictly dependent on known positive regulators of root hair cell differentiation/patterning, including CPC, TRY, ETC1, and CLP3. Furthermore, the AtRDH6 and AtRSL1-1 genes, which mediate the development of Arabidopsis root hair cells by acting downstream from the regulatory complexes that specify pattern formation in the epidermis (Menand et al., 2007), were also not required for accumulation of these transcripts. Thus, AtLRL1 and AtLRL2 might be constitutively transcribed in the root epidermis. Furthermore, our data do not rule out the possibility that AtLRL1 and AtLRL2 function in a parallel regulatory pathway(s), independent from the epidermis patterning genes, contributing to root hair initiation and/or being pertinent to root hair elongation in Arabidopsis.
In contrast to AtLRL1 and AtLRL2, the AtLRL3 transcript did not accumulate to any detectable level in either the quadruple or the double Atrhd6-3 Atrsl1-1 mutant background. Thus, AtLRL3 participates in the positive regulation of root hair development in Arabidopsis by acting downstream of the epidermal pattern formation genes and the AtRHD6 and/or AtRSL1 genes.
The presence of networks of redundantly acting bHLH proteins in Arabidopsis likely underscores evolutionary events that led to the expansion of this gene family to its current size of 162 members (Heim et al., 2003; Zhang et al., 2003; Menand et al., 2007). Interestingly, a comparative analysis of Arabidopsis and rice (Oryza sativa) predicted the presence of at least 66 bHLH genes in the genome of the presumed most recent common ancestor of monocots and eudicots (Li et al., 2006). The L. japonicus genome has 118 predicted bHLH domain-encoding genes (Sato et al., 2008), and this number is close to the presumed 91 bHLH transcription factors represented on the Medicago truncatula Gene Chip (Udvardi et al., 2007; Benedito et al., 2008). As the sequenced portion of the L. japonicus genome is postulated to account for approximately 91.3% of the plant gene space (Sato et al., 2008; Szczyglowski and Stougaard, 2008), the total number of bHLH domain-encoding genes (approximately 130 genes) could be lower in L. japonicus than in Arabidopsis and rice. We showed here that at least one member of the L. japonicus bHLH domain protein family, LjRHL1, works in a nonredundant manner to specify root hair development, which significantly contrasts with an apparent redundancy of similar functions in Arabidopsis. Whether the observed redundancy is reflective of differences in the expansion of specific subsets of bHLH genes in Lotus and Arabidopsis genomes in association with the plant-specific inventions, such as a particular type of root hair patterning, remains an interesting subject for future investigations.
MATERIALS AND METHODS
Plant Material and Growth Conditions
Lotus japonicus root hair mutants Ljrhl1-1, -2, and -3 were identified from a screen for genetic suppressors of the L. japonicus Gifu har1-1 hypernodulation phenotype, as described (Karas et al., 2005; Murray et al., 2006). The slp mutant was obtained by screening an independent ethylmethane sulfonate-mutagenized L. japonicus population, as described (Kawaguchi et al., 2002). For Arabidopsis (Arabidopsis thaliana) studies, ecotype Columbia (Col-0) was used as the wild-type reference. The Arabidopsis cpc-2 try-29760 etc1-1 cpl3-1 and Atrhd6-3 Atrsl1-1 mutant lines were kindly provided by Dr. Takuji Wada (Tominaga et al., 2008) and Dr. Liam Dolan (Menand et al., 2007), respectively. All Arabidopsis T-DNA insertion lines were selected from the Arabidopsis Biological Resource Center at The Ohio State University (Alonso et al., 2003), except FLAG_360D12, which was obtained from the French National Institute for Agricultural Research (http://dbsgap.versailles.inra.fr/publiclines; Samson et al., 2002).
All plants were maintained in a growth room under a 16-h/8-h day/night regime unless otherwise stated. L. japonicus plants were subjected to 250 μE s−1 m−2 light intensity at 22°C and were occasionally watered with B&D nutrient solution (Broughton and Dilworth, 1971) containing 0.5 mm KNO3. Arabidopsis plants were subjected to 140 to 180 μE s−1 m−2 light intensity at 22°C.
Evaluation of Root Hair and Symbiotic Phenotypes
L. japonicus seeds were germinated as described previously (Karas et al., 2005), and seedlings were transferred to pots containing a 6:1 mixture of vermiculite and sand and grown for an additional 10 d. The roots of Ljrhl1-1 and slp mutants were scored for the presence of root hairs (n = 30), as described (Karas et al., 2005). To evaluate the root hair phenotype of Arabidopsis, plants were germinated and grown on the surface of vertically positioned agar plates, containing 1× Murashige and Skoog medium, pH 5.8, 1% Suc, and 1% phytagel for 4 d. The roots were inspected, and root hairs that formed were evaluated with respect to their position and were counted within a 1-cm-long region, starting from the root tip (n = 10 for Col-0, Atlrl1-2 Atlrl1-2, and Atlrl2-1 Atlrl2-1; n = 23 for Atlrl1-2 Atlrl1-2/Atlrl2-1 Atlrl2-1).
After growing for the first 7 d under sterile conditions, L. japonicus seedlings were inoculated with the Mezorhizobium loti strain MAFF303099 carrying the constitutively expressed gusA reporter gene (kindly provided by Kazuhiko Saeki; Okazaki et al., 2007). Histochemical analysis of the reporter gene activity was performed 21 d after inoculation. For each genotype, at least 15 roots were stained as in Okazaki et al. (2007) and the number of nodules and nodule primorida was scored as described (Karas et al., 2005).The observations were made using a Nikon SMZ 1500 microscope, and images were captured with a Nikon DMX1200 digital camera.
Identification of Full-Length mRNA and Coding Regions
The LjRHL1/SLP cDNA was amplified by RT-PCR of total RNA derived from L. japonicus roots using coding region-specific primers (HLH_pcDNA_F and HLH_pcDNA_R; see list for all primer sequences below). RACE was subsequently carried out using the First Choice RLM-RACE kit from Ambion, according to the manufacturer's instructions. The full copy LjRHL1/SLP cDNA was reconstituted based on the obtained sequences. An additional set of two LjRHL1/SLP mRNA-specific primers, which were positioned at the extreme 5′ and 3′ ends of the predicted full copy cDNA, was designed (HLH_cDNA_F and HLH_cDNA-R). Using total RNA from L. japonicus roots, RT-PCR was again performed, and the resulting product was entirely sequenced.
The cDNAs corresponding to the coding regions of the Arabidopsis bHLH genes were amplified by RT-PCR (primers used: AT1G03040_F, AT1G03040_R, AT2G24260_F, AT2G24260_R, AT4G30980_F, AT4G30980_R, AT4G02590_F, AT4G02590_R, AT5G58010_F, and AT5G58010_R) and were entirely sequenced for confirmation. Subsequently, these cDNAs were cloned into the pEGAD vector and used in transgenic hairy root experiments (see below).
Expression Analysis
Total RNA from Arabidopsis roots and L. japonicus tissues was isolated and converted into first-strand cDNA as described (Murray et al., 2007).
To evaluate steady-state levels of the LjRHL1/SLP mRNA in different L. japonicus tissues, the following PCR conditions were used: 5 min at 94°C, 35 cycles of 94°C for 30 s, 60°C for 1 min, and 72°C for 30 s, followed by 7 min at 72°C (primers: RHL_E1-4_F and RHL_E1-4_R); the ubiquitin cDNA was amplified (primers: Ubi-F and Ubi-R; see primer list below) using similar PCR conditions except that only 30 cycles were performed.
To perform expression analyses of At2g24260, At4g30980, At5g58010, and RHD6 in roots of mutant/T-DNA insertion and wild-type Arabidopsis lines, the following PCR conditions were used: 5 min at 94°C, 30 or 35 cycles of 94°C for 30 s, 62°C for 1 min, and 72°C for 30 s, followed by 7 min at 72°C (primers: AT2G24260_expF, AT2G24260_expR, AT4G30980_expF, AT4G30980_expR, AT5G58010_expF, and AT5G58010_expR); for actin mRNA, the conditions were 5 min at 94°C, 25 cycles of 94°C for 30 s, 60°C for 30 s, and 72°C for 30 s, followed by 7 min at 72°C (primers: Actin-F and Actin-R; see primer list below).
Transgenic Hairy Roots
The LjRHL1/SLP and Arabidopsis bHLH cDNAs were fused in frame to the C-terminal end of the GFP in the pEGAD vector containing the CaMV 35S promoter (Cutler et al., 2000). For the LjRHL1/SLP protein localization/complementation study, the 35S promoter was replaced by the cognate LjRHL1/SLP promoter (nucleotide positions −3,131 to −223, counting from the predicted LjRHL1/SLP translation initiation site (primers: RHL_PROMTR_F and RHL_PROMTR_R).
The resulting constructs were transferred into the Agrobacterium rhizogenes AR10 strain, and L. japonicus plants were inoculated using the established protocol (Diaz et al., 2005). At least 30 independent plants were transformed for each genotype analyzed. The resulting hairy roots were visually evaluated for the presence of root hairs. For the GFP-LjRHL1/SLP localization study, transgenic hairy roots were counterstained with propidium iodide and GFP fluorescence was visualized and captured using a Leica scanning confocal microscope (TCS SP2; Leica Microsystems).
Primers Used in This Study
Underlined sequences denote restriction site sequences included in the primer. For map-based cloning: BK001_F, 5′-AAATGGGTGCATGTAAAACACA-3′; BK001_R, 5′-TCCGAAATAGACCCCAATGA-3′; BK002_F, 5′-CTGCTGAGTTCCCCATCAAT-3′; BK002_R, 5′-TGGTTAAGGACAAGGGTCAG-3′; BK003_F, 5′-TCAAGTCTTTCAACCTAAGTCGTG-3′; BK003_R, 5′-ATTGCCATGGATTGTGATTG-3′; BK004_F, 5′-GGATTGTAAGGCGAAGTGGA-3′; BK004_R, 5′-CTCATCATTTGTTGGAAGATGG-3′. For amplification of LjRHL1/SLP cDNA: HLH_pcDNA_F, 5′-CCACCCAGATCCAAAATTCA-3′; HLH_pcDNA_R, 5′-CACCCAAACGCTACGTCAT-3′; HLH_CDNA_F, 5′-ATGCAACCCTGTAGCAGAGAA-3′; HLH_CDNA_R, 5′-TACGTCATCACGGTTTCGAC-3′. For RACE: 5′outer race, 5′-TGGTGGTTACGGAACTTGGA-3′; 5′inner race, 5′-TGAGTTGTTGATGATGGTTG-3′; 3′outer race, 5′-AGCCAAGCTAATGGAAGAAGA-3′; 3′inner race, 5′-TCCAAACAATCTCGCCAACCT-3′. For amplification of AT1G03040, AT2G24260, AT4G30980, AT4G02590, and AT5G58010 cDNAs: AT1G03040_F, 5′-GAATTCGAATTCTCCTTCATGGCTAATAACAACAACA-3′; AT1G03040_R, 5′-AAGCTTAAGCTTAAAATCTACGGTGGAGGATTCA-3′; AT2G24260_F, 5′-GAATTCGAATTCAAGAAACCCATCATCATCA-3′; AT2G24260_R, 5′-AAGCTTAAGCTTGCCTAACCCCAAAAGTAAACG-3′; AT4G30980_F, 5′-GAATTCGAATTCGAAGCCATGAACTCCTCGTC-3′; AT4G30980_R, 5′-AAGCTTAAGCTTGTTATCACGGCTTGGAAACG-3′; AT4G02590_F, 5′-GAATTCGAATTCGCTCAAACCATGGCTAGTAACA-3′; AT4G02590_R, 5′-AAGCTTAAGCTTCTACTGTGGAGGATTGTTCTCAGG-3′; AT5G58010_F, 5′-CCCGGGCCCGGGATGGAAAATGGAAATGGAGAAGG-3′; AT5G58010_R, 5′-AAGCTTAAGCTTAGGGAGATTAGCGTTTGACTT-3′. For evaluation of LjRHL1/SLP in L. japonicus tissues and confirmation of the slp mutation: RHL_E1-4_F, 5′-CCGTAACCACCAGATCACCT-3′; RHL_E1-4_R, 5′-TTAGCATTGGGGACCAGTTC-3′; Ubi-F, 5′-TTCACCTTGTGCTCCGTCTTC-3′; Ubi-R, 5′-AACAACAGAACACACAGACAATCC-3′. For evaluation of At2g24260, At4g30980, and At5g58010 in roots of Arabidopsis wild-type and mutant lines: AT2G24260_expF, 5′-AGCAGAAGAAACCCATCATCA-3′; AT2G24260_expR, 5′-AGAAGACGACCTCTCGGTCA-3′; AT4G30980_expF, 5′-TTCTCACTCCCCACCAAAAA-3′; AT4G30980_expR, 5′-CGTATCCGAAAACACGACCT-3′; AT5G58010_expF, 5′-TGTTGGGATCCATCTCTTCC-3′; AT5G58010_expR, 5′-TGCTACAGCACTGGAGATGG-3′; RHD6_EXP_F1, 5′-CATGAGCTACGGCTTCACAA-3′; RHD6_EXP_R1, 5′-TAAAGATTCCATCCCCGTGT-3′; Actin-F, 5′-CCTTACAGAGAGAGGTTACATG-3′; Actin-R, 5′-GACCTTAATCTTCATGCTGCTTGG-3′. For construction of LjRHL1p∷GFP:LjRHL1 plasmids: RHL_PROMTR_F, 5′-TTGATTTGGGTGATCGGATT-3′; RHL_PROMTR_R, 5′-ACCGGTACCGGTATTGATGGGGAACTCAGCAG-3′.
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession number FJ375304.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Nodulation events in wild-type and mutant plants.
Supplemental Figure S2. Genomic and mRNA amplification experiments.
Supplemental Figure S3. Complementation of the root-hairless phenotype.
Supplemental Figure S4. Sequence alignment of the predicted bHLH domain proteins.
Supplemental Figure S5. The DNA sequence of LjTRIM1.
Supplemental Figure S6. Expression of LjRHL1 in L. japonicus.
Supplemental Figure S7. Expression of AtLRL1, AtLRL2, and AtLRL3 mRNAs in Arabidopsis T-DNA insertion lines.
Supplemental Table S1. Genotyping of F2 and F3 progenies.
Supplemental Information S1. Arabidopsis T-DNA insertion lines and genetic analysis of double mutants.
Supplementary Material
Acknowledgments
We thank Alex Molnar for preparation of the figures, Dr. Takuji Wada and Dr. Liam Dolan for providing the Arabidopsis mutant lines, and Dr. Kazuhiko Saeki for providing the M. loti strain. We also thank all members of the Szczyglowski laboratory for providing helpful comments on the manuscript.
This work was supported by the Agriculture and Agri-Food Canada Crop Genomics Initiative and the National Science and Engineering Research Council of Canada (grant nos. R3277A01 to K.S. and PGS–D3 to B.K.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Krzysztof Szczyglowski (szczyglowskik@agr.gc.ca).
The online version of this article contains Web-only data.
Open Access articles can be viewed online without a subscription.
References
- Alonso JM, Stepanova AN, Leisse TJ, Kim CJ, Chen H, Shinn P, Stevenson DK, Zimmerman J, Barajas P, Cheuk R, et al (2003) Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301 653–657 [DOI] [PubMed] [Google Scholar]
- Benedito VA, Torres-Jerez I, Murray JD, Andriankaja A, Allen S, Kakar K, Wandrey M, Verdier J, Zuber H, Ott T, et al (2008) A gene expression atlas of the model legume Medicago truncatula. Plant J 55 504–513 [DOI] [PubMed]
- Bernhardt C, Lee MM, Gonzalez A, Zhang F, Lloyd A, Schiefelbein J (2003) The bHLH genes GLABRA3 (GL3) and ENHANCER OF GLABRA3 (EGL3) specify epidermal cell fate in the Arabidopsis root. Development 130 6431–6439 [DOI] [PubMed] [Google Scholar]
- Broughton WJ, Dilworth MJ (1971) Control of leghaemoglobin synthesis in snake beans. Biochem J 125 1075–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler SR, Ehrhardt DW, Griffitts JS, Somerville CR (2000) Random GFP:cDNA fusions enable visualization of subcellular structures in cells of Arabidopsis at a high frequency. Proc Natl Acad Sci USA 97 3718–3723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz CL, Gronlund M, Schlaman HRM, Spaink HP (2005) Lotus japonicus Handbook. Springer, Dordrecht, The Netherlands
- Dolan L, Costa S (2001) Evolution and genetics of root hair stripes in the root epidermis. J Exp Bot 52 413–417 [DOI] [PubMed] [Google Scholar]
- Ferre-D'Amare AR, Prendergast GC, Ziff EB, Burley SK (1993) Recognition by Max of its cognate DNA through a dimeric b/HLH/Z domain. Nature 363 38–45 [DOI] [PubMed] [Google Scholar]
- Galway ME, Masucci JD, Lloyd AM, Walbot V, Davis RW, Schiefelbein JW (1994) The TTG gene is required to specify epidermal cell fate and cell patterning in the Arabidopsis root. Dev Biol 166 740–754 [DOI] [PubMed] [Google Scholar]
- Guimil S, Dunand C (2006) Patterning of Arabidopsis epidermal cells: epigenetic factors regulate the complex epidermal cell fate pathway. Trends Plant Sci 11 601–609 [DOI] [PubMed] [Google Scholar]
- Guimil S, Dunand C (2007) Cell growth and differentiation in Arabidopsis epidermal cells. J Exp Bot 58 3829–3840 [DOI] [PubMed] [Google Scholar]
- Heim MA, Jakoby M, Werber M, Martin C, Weisshaar B, Bailey PC (2003) The basic helix-loop-helix transcription factor family in plants: a genome-wide study of protein structure and functional diversity. Mol Biol Evol 20 735–747 [DOI] [PubMed] [Google Scholar]
- Ishida T, Kurata T, Okada K, Wada T (2008) A genetic regulatory network in the development of trichomes and root hairs. Annu Rev Plant Biol 59 365–386 [DOI] [PubMed] [Google Scholar]
- Karas B, Murray J, Gorzelak M, Smith A, Sato S, Tabata S, Szczyglowski K (2005) Invasion of Lotus japonicus root hairless 1 by Mesorhizobium loti involves the nodulation factor-dependent induction of root hairs. Plant Physiol 137 1331–1344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi M, Imaizumi-Anraku H, Koiwa H, Niwa S, Ikuta A, Syono K, Akao S (2002) Root, root hair, and symbiotic mutants of the model legume Lotus japonicus. Mol Plant Microbe Interact 15 17–26 [DOI] [PubMed] [Google Scholar]
- Kirik V, Simon M, Huelskamp M, Schiefelbein J (2004) The ENHANCER OF TRY and CPCl gene acts redundantly with TRIPTYCHON and CAPRICE in trichome and root hair cell patterning in Arabidopsis. Dev Biol 268 506–513 [DOI] [PubMed] [Google Scholar]
- Lee MM, Schiefelbein J (1999) WEREWOLF, a MYB-related protein in Arabidopsis, is a position-dependent regulator of epidermal cell patterning. Cell 99 473–483 [DOI] [PubMed] [Google Scholar]
- Li XX, Duan XP, Jiang HX, Sun YJ, Tang YP, Yuan Z, Guo JK, Liang WQ, Chen L, Yin JY, et al (2006) Genome-wide analysis of basic/helix-loop-helix transcription factor family in rice and Arabidopsis. Plant Physiol 141 1167–1184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masucci JD, Rerie WG, Foreman DR, Zhang M, Galway ME, Marks MD, Schiefelbein JW (1996) The homeobox gene GLABRA2 is required for position-dependent cell differentiation in the root epidermis of Arabidopsis thaliana. Development 122 1253–1260 [DOI] [PubMed] [Google Scholar]
- Menand B, Yi KK, Jouannic S, Hoffmann L, Ryan E, Linstead P, Schaefer DG, Dolan L (2007) An ancient mechanism controls the development of cells with a rooting function in land plants. Science 316 1477–1480 [DOI] [PubMed] [Google Scholar]
- Murray J, Karas B, Ross L, Brachmann A, Wagg C, Geil R, Perry J, Nowakowski K, MacGillivary M, Held M, et al (2006) Genetic suppressors of the Lotus japonicus har1-1 hypernodulation phenotype. Mol Plant Microbe Interact 19 1082–1091 [DOI] [PubMed] [Google Scholar]
- Murray JD, Karas BJ, Sato S, Tabata S, Amyot L, Szczyglowski K (2007) A cytokinin perception mutant colonized by Rhizobium in the absence of nodule organogenesis. Science 315 101–104 [DOI] [PubMed] [Google Scholar]
- Okazaki S, Hattori Y, Saeki K (2007) The Mesorhizobium loti purB gene is involved in infection thread formation and nodule development in Lotus japonicus. J Bacteriol 189 8347–8352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldroyd GE, Downie JA (2008) Coordinating nodule morphogenesis with rhizobial infection in legumes. Annu Rev Plant Biol 59 519–546 [DOI] [PubMed] [Google Scholar]
- Pagnussat GC, Yu HJ, Ngo QA, Rajani S, Mayalagu S, Johnson CS, Capron A, Xie LF, Ye D, Sundaresan V (2005) Genetic and molecular identification of genes required for female gametophyte development and function in Arabidopsis. Development 132 603–614 [DOI] [PubMed] [Google Scholar]
- Payne CT, Zhang F, Lloyd AM (2000) GL3 encodes a bHLH protein that regulates trichome development in Arabidopsis through interaction with GL1 and TTG1. Genetics 156 1349–1362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson F, Brunaud V, Balzergue S, Dubreucq B, Lepiniec L, Pelletier G, Caboche M, Lecharny A (2002) FLAGdb/FST: a database of mapped flanking insertion sites (FSTs) of Arabidopsis thaliana T-DNA transformants. Nucleic Acids Res 30 94–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato S, Nakamura Y, Kaneko T, Asamizu E, Kato T, Nakao M, Sasamoto S, Watanabe A, Ono A, Kawashima K, et al (2008) Genome structure of the legume, Lotus japonicus. DNA Res 15 227–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schellmann S, Schnittger A, Kirik V, Wada T, Okada K, Beermann A, Thumfahrt J, Jurgens G, Hulskamp M (2002) TRIPTYCHON and CAPRICE mediate lateral inhibition during trichome and root hair patterning in Arabidopsis. EMBO J 21 5036–5046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczyglowski K, Stougaard J (2008) Lotus genome: pod of gold for legume research. Trends Plant Sci 13 515–517 [DOI] [PubMed] [Google Scholar]
- Tominaga R, Iwata M, Sano R, Inoue K, Okada K, Wada T (2008) Arabidopsis CAPRICE-LIKE MYB 3 (CPL3) controls endoreduplication and flowering development in addition to trichome and root hair formation. Development 135 1335–1345 [DOI] [PubMed] [Google Scholar]
- Udvardi MK, Kakar K, Wandrey M, Montanari O, Murray J, Andriankaja A, Zhang JY, Benedito V, Hofer JM, Chueng F, et al (2007) Legume transcription factors: global regulators of plant development and response to the environment. Plant Physiol 144 538–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada T, Tachibana T, Shimura Y, Okada K (1997) Epidermal cell differentiation in Arabidopsis determined by a Myb homolog, CPC. Science 277 1113–1116 [DOI] [PubMed] [Google Scholar]
- Walker AR, Davison PA, Bolognesi-Winfield AC, James CM, Srinivasan N, Blundell TL, Esch JJ, Marks MD, Gray JC (1999) The TRANSPARENT TESTA GLABRA1 locus, which regulates trichome differentiation and anthocyanin biosynthesis in Arabidopsis, encodes a WD40 repeat protein. Plant Cell 11 1337–1350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte CP, Le QH, Bureau T, Kumar A (2001) Terminal-repeat retrotransposons in miniature (TRIM) are involved in restructuring plant genomes. Proc Natl Acad Sci USA 98 13778–13783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Gonzalez A, Zhao M, Payne CT, Lloyd A (2003) A network of redundant bHLH proteins functions in all TTG1-dependent pathways of Arabidopsis. Development 130 4859–4869 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.