Abstract
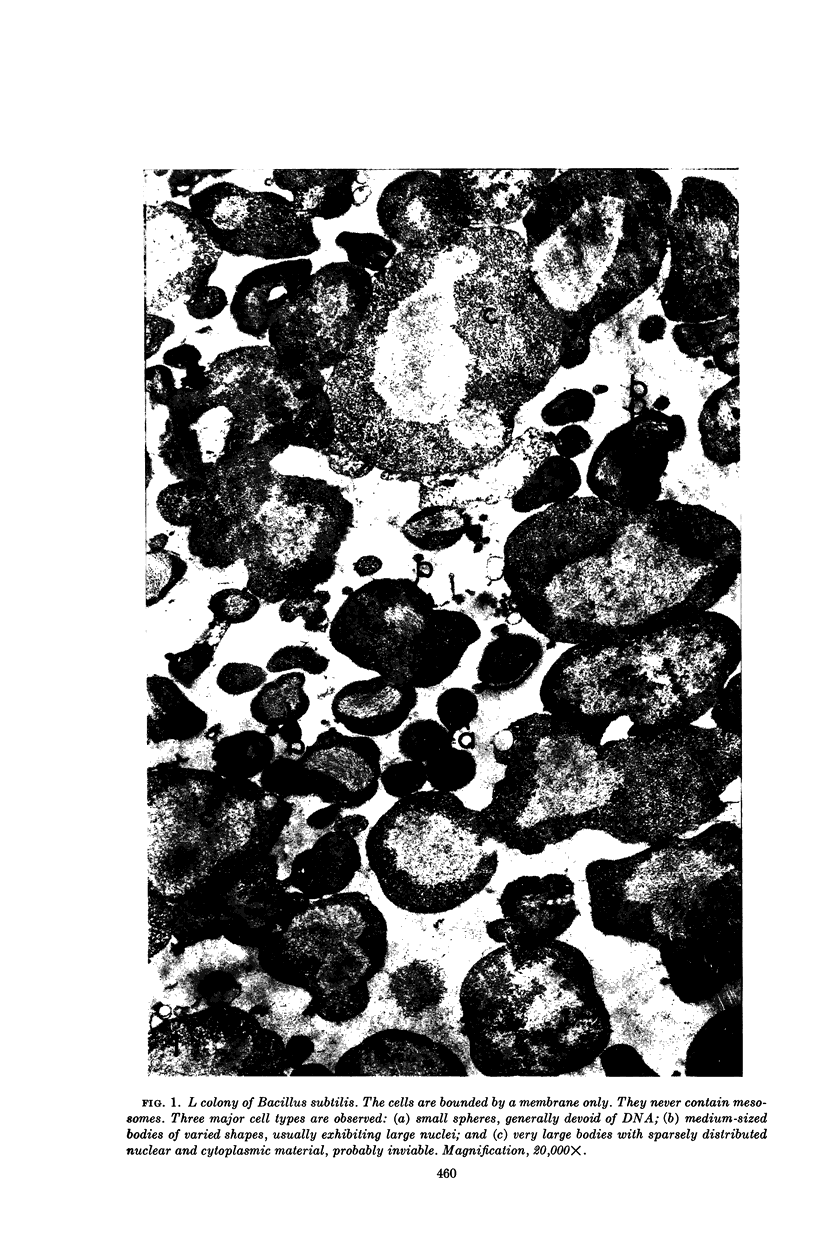
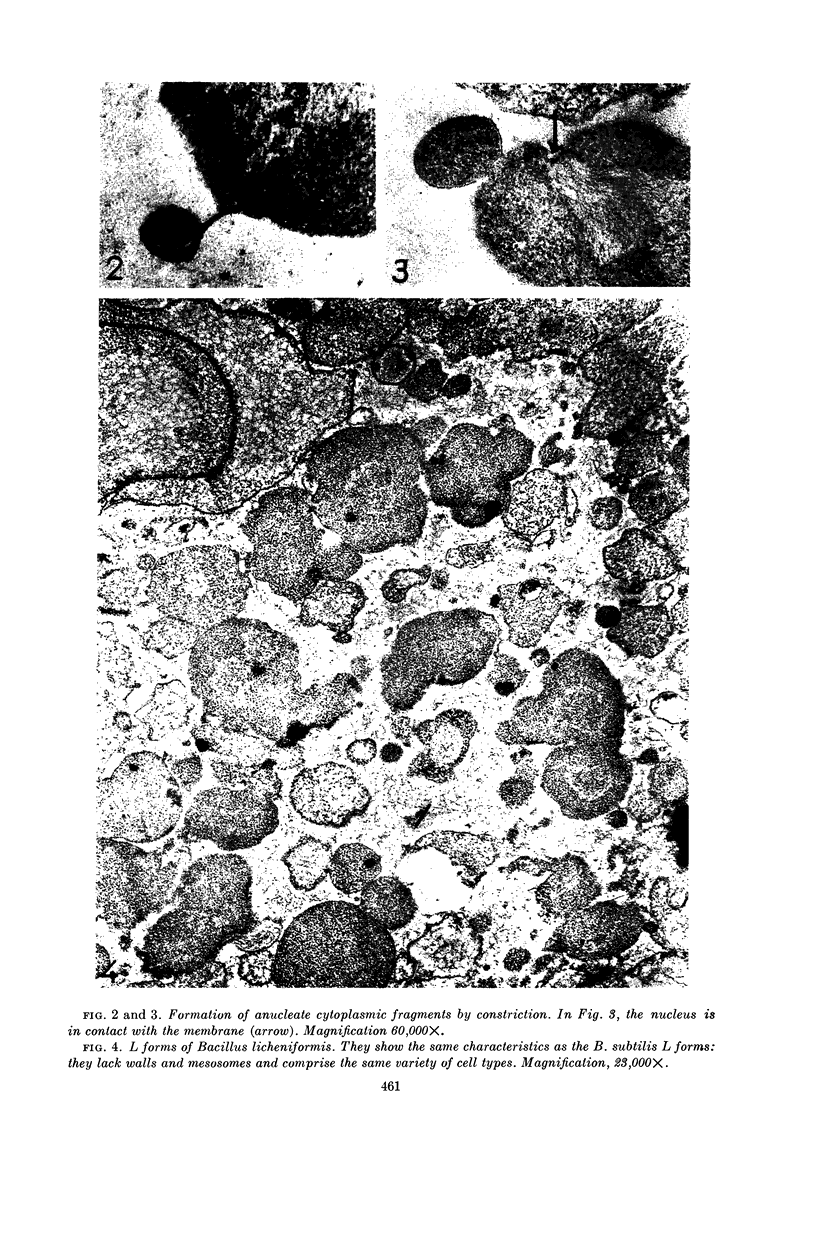
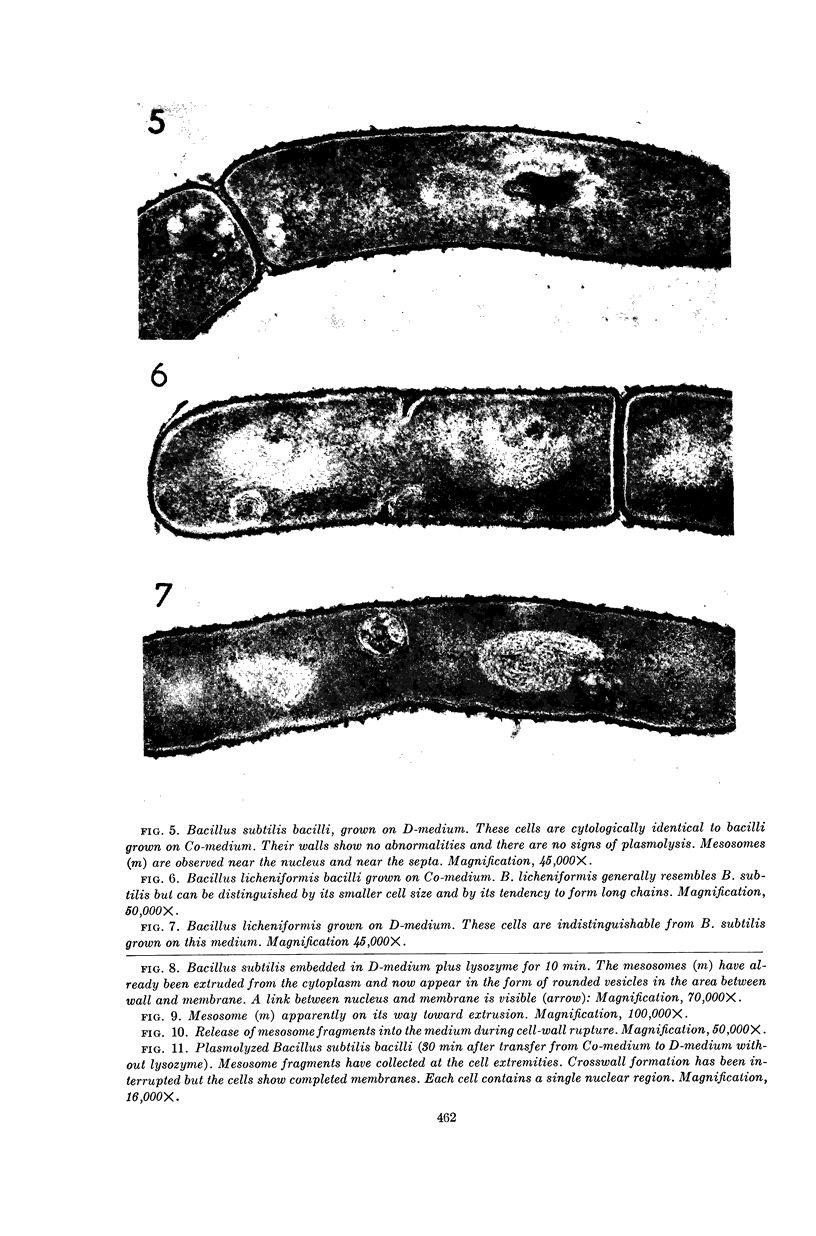
Ryter, Antoinette (Institut Pasteur, Paris, France), and Otto E. Landman. An electron microscope study of the relationship between mesosome loss and the stable L-state (or protoplast state) in Bacillus subtilis. J. Bacteriol. 88:457–467. 1964.—In a prior publication, it was postulated that inability of protoplasts to restart cell-wall synthesis and cell division and the inability of stable mass-conversion L forms to return to the bacillary state were both equivalent and both due to the interruption of a membrane-associated reaction sequence. It was further postulated that this reaction sequence might reside in the mesosome. In the present publication, it is shown by means of electron microscopy of thin sections that protoplasts and L forms do not contain mesosomes. The sequence of events leading to loss of the mesosomes during protoplasting is as follows. Soon after lysozyme addition, the mesosomes are extruded from the cell interior into the space between cell wall and cytoplasmic membrane. Mesosome fragments in the form of small vesicles gather at the poles of the cells and are released, along with intact protoplasts, when the wall fragments. (Sudden shift of bacilli to hypertonic environment also causes extrusion and fragmentation of mesosomes, but this damage is later repaired.) In intact bacilli, mesosomes are in contact with both the peripheral membrane and nuclear material. Upon extrusion of the mesosomes, a direct attachment between nuclear material and cytoplasmic membrane is observed. Deoxyribonucleic acid (DNA)-membrane attachment may play a role in the control of DNA replication. Bacillus subtilis L-colonies consist of irregularly shaped bodies of varying sizes, bounded only by a membrane. Many of the smaller bodies do not contain nuclear material, and many of the large ones appear inviable. Division is accomplished by a disorganized-appearing constriction process. There are no septa.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CHAPMAN G. B., HILLIER J. Electron microscopy of ultra-thin sections of bacteria I. Cellular division in Bacillus cereus. J Bacteriol. 1953 Sep;66(3):362–373. doi: 10.1128/jb.66.3.362-373.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FITZ-JAMES P. C. Participation of the cytoplasmic membrane in the growth and spore fromation of bacilli. J Biophys Biochem Cytol. 1960 Oct;8:507–528. doi: 10.1083/jcb.8.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FITZ-JAMES P. C. Studies on the morphology and nucleic acid content of protoplasts of Bacillus megaterium. J Bacteriol. 1958 Apr;75(4):369–382. doi: 10.1128/jb.75.4.369-382.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GLAUERT A. M. The fine structure of bacteria. Br Med Bull. 1962 Sep;18:245–250. doi: 10.1093/oxfordjournals.bmb.a069988. [DOI] [PubMed] [Google Scholar]
- IMAEDA T., OGURA M. Formation of intracytoplasmic membrane system of mycobacteria related to cell division. J Bacteriol. 1963 Jan;85:150–163. doi: 10.1128/jb.85.1.150-163.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACOB F., BRENNER S. [On the regulation of DNA synthesis in bacteria: the hypothesis of the replicon]. C R Hebd Seances Acad Sci. 1963 Jan 2;256:298–300. [PubMed] [Google Scholar]
- KAYE J. J., CHAPMAN G. B. CYTOLOGICAL ASPECTS OF ANTIMICROBIAL ANTIBIOSIS. III. CYTOLOGICALLY DISTINGUISHABLE STAGES IN ANTIBIOTIC ACTION OF COLISTIN SULFATE ON ESCHERICHIA COLI. J Bacteriol. 1963 Sep;86:536–543. doi: 10.1128/jb.86.3.536-543.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KELLENBERGER E., RYTER A., SECHAUD J. Electron microscope study of DNA-containing plasms. II. Vegetative and mature phage DNA as compared with normal bacterial nucleoids in different physiological states. J Biophys Biochem Cytol. 1958 Nov 25;4(6):671–678. doi: 10.1083/jcb.4.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LANDMAN O. E., HALLE S. ENZYMICALLY AND PHYSICALLY INDUCED INHERITANCE CHANGES IN BACILLUS SUBTILIS. J Mol Biol. 1963 Dec;7:721–738. doi: 10.1016/s0022-2836(63)80119-9. [DOI] [PubMed] [Google Scholar]
- OHYE D. F., MURRELL W. G. Formation and structure of the spore of Bacillus coagulans. J Cell Biol. 1962 Jul;14:111–123. doi: 10.1083/jcb.14.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RYTER A., JACOB F. ETUDE AU MICROSCOPE 'ELECTRONIQUE DES RELATIONS ENTRE M'ESOSOMES ET NOYAUX CHEZ BACILLUS SUBTILIS. C R Hebd Seances Acad Sci. 1963 Nov 13;257:3060–3063. [PubMed] [Google Scholar]
- RYTER A., KELLENBERGER E. L'inclusion au polyester pour l'ultramicrotomie. J Ultrastruct Res. 1958 Dec;2(2):200–214. doi: 10.1016/s0022-5320(58)90018-2. [DOI] [PubMed] [Google Scholar]
- RYTER A., PIECHAUD M. ETUDE AU MICROSCOPE 'ELECTRONIQUE DE QUELQUES SOUCHES DE MORAXELLA. Ann Inst Pasteur (Paris) 1963 Dec;105:1071–1079. [PubMed] [Google Scholar]
- RYTER A., PILLOT J. [Electron microscope study of the external and internal structure of the Reiter treponema]. Ann Inst Pasteur (Paris) 1963 Apr;104:496–501. [PubMed] [Google Scholar]
- Spizizen J. TRANSFORMATION OF BIOCHEMICALLY DEFICIENT STRAINS OF BACILLUS SUBTILIS BY DEOXYRIBONUCLEATE. Proc Natl Acad Sci U S A. 1958 Oct 15;44(10):1072–1078. doi: 10.1073/pnas.44.10.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THORNE C. B. Transduction in Bacillus subtilis. J Bacteriol. 1962 Jan;83:106–111. doi: 10.1128/jb.83.1.106-111.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN ITERSON W. Some features of a remarkable organelle in Bacillus subtilis. J Biophys Biochem Cytol. 1961 Jan;9:183–192. doi: 10.1083/jcb.9.1.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VANDERWINKEL E., MURRAY R. G. [Bacterial intracytoplasmic organelles and the site of oxidation-reduction activity]. J Ultrastruct Res. 1962 Aug;7:185–199. doi: 10.1016/s0022-5320(62)80035-5. [DOI] [PubMed] [Google Scholar]
- WEIBULL C., BECKMAN H. Metabolism of small bodies isolated from a stable Proteus L form. Nature. 1960 Oct 29;188:428–429. doi: 10.1038/188428b0. [DOI] [PubMed] [Google Scholar]