Abstract
The prosurvival transcription factor NF-κB specifically binds promoter DNA to activate target gene expression. NF-κB is regulated through interactions with IκB inhibitor proteins. Active proteolysis of these IκB proteins is, in turn, under the control of the IκB kinase complex (IKK). Together, these three molecules form the NF-κB signaling module. Studies aimed at characterizing the molecular mechanisms of NF-κB, IκB, and IKK in terms of their three-dimensional structures have lead to a greater understanding of this vital transcription factor system.
Structural studies of the NF-κB transcription factor and its regulators I-κB and IKK provide insights into NF-κB dimerization, activation, and DNA binding.
NF-κB is a master transcription factor that responds to diverse cell stimuli by activating the expression of stress response genes. Multiple signals, including cytokines, growth factors, engagement of the T-cell receptor, and bacterial and viral products, induce NF-κB transcriptional activity (Hayden and Ghosh 2008). A point of convergence for the myriad of NF-κB inducing signals is the IκB kinase complex (IKK). Active IKK in turn controls transcription factor NF-κB by regulating proteolysis of the IκB inhibitor protein (Fig. 1). This nexus of three factors, IKK, IκB, and NF-κB, forms the NF-κB signaling module—a molecular relay switch mechanism that is conserved across diverse species (Ghosh et al. 1998; Hoffmann et al. 2006). In this article, we introduce the human NF-κB, IκB, and IKK proteins, and discuss how they function from the perspective of their three-dimensional structures.
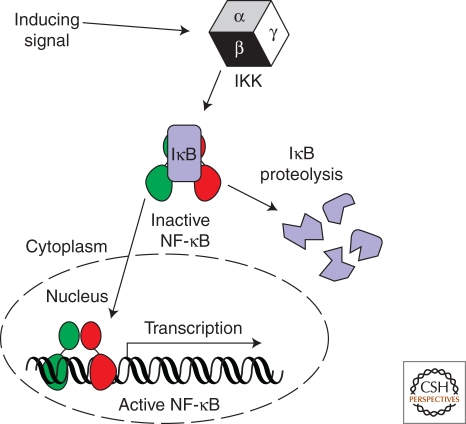
Figure 1.
The NF-κB signaling module. NF-κB exists in the cytoplasm of resting cells by virtue of its noncovalent association with an IκB inhibitor protein. The IκB kinase (IKK) responds to diverse stimuli by catalyzing the phosphorylation-dependent 26 S proteasome-mediated degradation of complex-associated IκB. Active NF-κB accumulates in the nucleus where it binds with DNA sequence specificity in the promoter regions of target genes and activates their transcription.
NF-κB
Introduction to NF-κB
NF-κB was discovered in the laboratory of David Baltimore as a nuclear activity with binding specificity toward a ten-base-pair DNA sequence 5′-GGGACTTTCC-3′ present within the enhancer of the immunoglobin κ light chain gene in mature antibody-producing B cells (Sen and Baltimore 1986). The biochemically purified activity was found to be composed of 50 and 65 kilodalton (kDa) subunits. Cloning of the p50 subunit revealed significant amino-acid sequence homology between its amino-terminal 300 amino acids and the oncogene from the reticuloendotheliosis virus of turkeys and shared by v-Rel and its cellular proto-oncogene c-Rel. This portion of conserved amino acid sequence was termed the Rel homology region (RHR). As is discussed later, the mRNA responsible for producing the p50 subunit was found to encode a longer precursor protein of 105 kDa in size that possesses the entire p50 amino-acid sequence at its amino-terminal end and its own inhibitor within its carboxy-terminal region. Once the cDNA encoding the p65 subunit (also known as RelA) was sequenced, it was also found to contain an amino-terminal RHR. Two additional NF-κB family subunits, RelB and p52 (the processed product of a longer 100-kDa precursor), were also discovered to harbor the conserved RHR within their amino-terminal regions. These five polypeptides, p50, p65/RelA, c-Rel, p52, and RelB, constitute the entire family of NF-κB subunits encoded by the human genome (Fig. 2A).
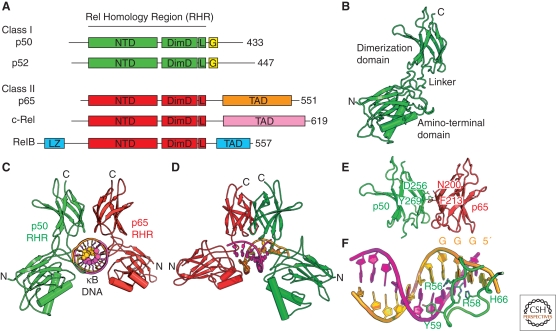
Figure 2.
The NF-κB family. (A) The human genome encodes five polypeptides that assemble in various dimer combinations to form active NF-κB transcription factors. Each of the subunits contains the Rel homology region (RHR) near its amino terminus. The RHR consists of two folded domains, the amino-terminal domain (NTD) and the dimerization domain (DimD), that are joined by a short flexible linker and a carboxy-terminal flexible region that contains the nuclear localization signal (L). Three of the subunits, p65, c-Rel, and RelB, also contain a transcription activation domain (TAD) at their carboxy-terminal ends. RelB contains a predicted leucine zipper motif (LZ) amino-terminal to its RHR. The NF-κB subunits p50 and p52 lack transactivation domains and have glycine-rich regions (G). (B) A ribbon diagram representation of the RHR from p50 in its DNA-bound conformation. (C) The NF-κB p50:p65/RelA heterodimer bound to κB DNA. (D) Another view of the complex. (E) The NF-κB p50:p65/RelA heterodimer dimerization domains with key amino acid side chains labeled. (F) κB DNA from the NF-κB:DNA complex with key base-contacting amino acid residues labeled.
Rel Homology Region Structure
The first glimpse at the structure of the RHR was afforded by the successful determination of two x-ray crystal structures of the NF-κB p50:p50 homodimer in complex with related κB DNA (Ghosh et al. 1995; Müller et al. 1995). The structures uncovered a symmetrical protein: DNA complex structure reminiscent of a butterfly with double-stranded DNA comprising the “body” and two-protein subunit “wings” (Fig. 2B,C). These structures revealed a completely novel DNA binding motif in which one entire 300 amino acid RHR from each p50 subunit in the dimer are involved in contacting one whole turn along the major groove of double-stranded DNA (Baltimore and Beg 1995; Müller et al. 1996).
As revealed by the NF-κB p50:DNA complex structures, the RHR consists of two folded domains. The amino-terminal domain (or Rel-N) is approximately 160–210 amino acids in length and exhibits a variant of the immunoglobulin fold. The carboxy-terminal dimerization domain (referred to as Rel-C) spans roughly 100 amino acids and also adopts an immunoglobulin-like fold. The five RHR-containing NF-κB subunits assemble to form various homo- and heterodimer combinations to form active NF-κB transcription factors. The two domains are joined by a short flexible linker approximately 10 amino acids in length. A carboxy-terminal flexible region in which is embedded the nuclear localization signal terminates the conserved RHR portion of the NF-κB subunits. Besides dimerization, this RHR is responsible for sequence-specific DNA binding, nuclear localization, and interaction with IκB proteins.
Outside of the conserved RHR, three NF-κB subunits, p65/RelA, c-Rel, and RelB, contain a transcription activation domain (TAD) at their extreme carboxy-terminal ends. This region, which is poorly understood in protein structural terms, is responsible for the increase in target gene expression that results from induction of NF-κB and, consequently, NF-κB dimers that possess at least one of these subunits function as activators of transcription. RelB also contains a predicted leucine zipper motif amino-terminal to its RHR. The NF-κB subunits p50 and p52 lack transactivation domains. Rather, their extreme carboxy-terminal ends are rich in glycine. Consequently, NF-κB dimers consisting exclusively of p50 and p52 subunits are capable of nuclear localization and DNA binding but fail to activate target gene expression and, in fact, function both in vitro and in vivo as repressors of transcription (Franzoso et al. 1992).
NF-κB Dimerization
Assembly of individual NF-κB subunits into dimers capable of sequence-specific DNA binding and activating target gene expression is mediated entirely by the dimerization domain. In theory, a total of 15 unique homo- and heterodimers are possible from combinatorial dimerization of the five NF-κB subunits (Hoffmann et al. 2006). Of these, 12 have been identified in vivo. Three that are not known to exist are the RelB:RelB, RelB:c-Rel, and p52:c-Rel. However, of these three, only RelB homodimer fails to exist in vivo, and the status of the other two dimers is uncertain; they could exist under specialized conditions such as the p65/RelA:RelB heterodimer (Marienfeld et al. 2003).
The homo- and heterotropic interactions between NF-κB family subunits follow the central dogma of all protein–protein complexes: A set of amino acid residues from each polypeptide participate in direct contacts with one another, forming the interface, whereas a different set of residues present outside of the interface indirectly affects the stability of the interface by modulating the local environment. One of the most stable NF-κB dimer interfaces is created by the p50:p65/RelA heterodimer (Fig. 2D). The p50:p65/RelA heterodimer assembles with a significantly greater stability than either of the respective p50:p50 or p65/RelA:p65/RelA homodimers (Huang et al. 1997). Differences in the amino acid sequences of p50 and p65/RelA at two positions partially explain the variation in dimerization stability observed in the three dimers. The positions of Tyr-269 and Asp-256 in p50 are occupied by Phe-213 and Asn-200, respectively, in p65. Within the heterodimer, the Asp and Asn approach one another symmetrically at the interface and stabilize the heterodimer through formation of a highly stable hydrogen bond. In contrast, in the homodimers, the juxtaposition of Asp-Asp and Asn-Asn at the interface are detrimental to dimer stability. Similarly, the hydroxyl group on Tyr-269 of p50 hydrogen bonds at the dimer interface, contributing to stabilization of both the p50:p50 homodimer as well as the p50:p65/RelA heterodimer. The substitution of Phe at this position serves to weaken the p65/RelA:p65/RelA homodimer relative to the other two. Taken together, these observations help to explain why the p65/RelA:p65/RelA homodimer forms with lower stability than does the p50:p50 homodimer and why the p50:p65/RelA heterodimer is more stable than both homodimers.
However, direct contact between complementary amino acids at the interface fails to completely explain the observed trends of NF-κB subunit dimerization. Mutation of noninterfacial residues contained within the dimerization domain has been shown to modulate dimerization. For example, changing p65/RelA Cys-216 to Ala affects homodimer formation (Ganchi et al. 1993). The role of noninterfacial amino acid residues in dimerization is most strikingly illustrated in the case of RelB. All interfacial residues in RelB are either identical or homologous to those of other NF-κB subunits. And yet, RelB assembles into a completely unique domain-swapped homodimer (Huang et al. 2005). Domain swapping occurs as a consequence of the destabilization of the folded RelB dimerization domain, suggesting that domain stability is an important determinant of protein–protein interaction. In cells, decreased folding stability in both the amino-terminal and dimerization domains contributes to its degradation by the proteasome, which explains why the RelB homodimer does not exist in vivo (Marienfeld et al. 2001).
Post-translational modification may also play a role in modulating dimerization propensity. One study has shown that RelB forms a dimer with a p65/RelA that is phosphorylated at position Ser-276 (Jacque et al. 2005). This serine is located in a loop within the dimerization domain, projected away from the dimer interface. It is unclear as to how phosphorylation of this serine positively impacts p65/RelA:RelB heterodimer formation. One explanation could be that the increased negative charge may indirectly modulate dimer-forming residues of both p65/RelA and RelB.
NF-κB Recognition of κB DNA
X-ray structures of NF-κB in complex with κB DNA revealed a new mode of DNA recognition wherein a dimer composed of the RHR of two NF-κB subunits intimately contacts double-stranded DNA within the major groove through one complete turn (Ghosh et al. 1995; Müller et al. 1995). NF-κB employs both its amino-terminal and dimerization domains to encircle its target DNA. DNA contacts are mediated by amino acids emanating from loops that connect β-strand elements of secondary protein structure. The p50:p50 homodimer structures revealed that this NF-κB subunit employs its amino acids His-66, Arg-58, and Arg-56 to contact three guanine nucleotide bases at the extreme 5′ ends of its consensus κB DNA. However, X-ray analyses of NF-κB p65/RelA:p65/RelA homodimers bound to κB DNA revealed that, when bound to a canonical 10-base-pair κB DNA, one p65/RelA subunit contacts DNA in an analogous manner as observed in the p50 homodimer structures, whereas the second p65/RelA subunit significantly repositions its entire amino-terminal domain to mediate interactions with the DNA backbone (Chen et al. 1998b). Such a binding mode, which is afforded by flexibility in the short linker region that connects the two structured domains of the RHR, preserves binding affinity at the cost of fewer contacts to DNA bases. A close inspection of the homodimer:DNA complex structures leads to the suggestion that a homodimer of p50 might optimally bind to an 11-base-pair sequence composed of two 5′-GGGPuN half sites bracketing a central A:T base pair. In contrast, the NF-κB p65/RelA homodimer optimally recognizes a nine-base-pair target sequence containing two 5′-GGPuN half sites and a central A:T base pair. Determination of a second X-ray structure of NF-κB p65/RelA homodimer in complex with the κB DNA sequence from the promoter of IL-8 (5′-GGAA T TTCC-3′) confirmed this hypothesis (Chen et al. 2000).
The lessons learned from structural analyses of p50 and p65/RelA homodimers bound to diverse κB DNA sequences suggested that the canonical NF-κB p50:p65/RelA heterodimer might recognize its 10-base-pair target sequence with the p50 subunit binding specifically to a 5′-GGGPyN half site, whereas the p65/RelA subunit binds to a 5′-GGPyN half site separated from the p50 site by one A:T base pair. X-ray structure determination of a p50:p65/RelA RHR heterodimer bound to κB DNA from the original κ light chain gene promoter, which is identical to a κB sequence that is present in the promoter of HIV genome (5′-GGGAC T TTCC-3′), served to confirm this speculation (Chen et al. 1998a). Additional crystal structures of NF-κB p50: p65/RelA heterodimer bound to different κB DNA further support the basic rules of DNA half-site recognition developed from the homodimer:DNA structures (Berkowitz et al. 2002; Escalante et al. 2002).
X-ray crystal structures of several additional NF-κB:DNA complexes have now been determined (Cramer et al. 1997; Cramer et al. 1999; Moorthy et al. 2007; Panne et al. 2007; Fusco et al. 2009). Taken together, these structures suggest a model for how NF-κB dimers recognize κB DNA that contain significant deviations from the consensus sequence. Changes of κB DNA sequence can be of two types. In the first, alteration occurs within the G:C base pairs that occupy positions on the outside of the κB DNA and that are directly contacted by the amino-terminal domain. An example is the altered κB sequence 5′-GGGAC T TTTC-3′ (change from immunoglobin κB sequence is underlined). Because of the loss of an important C:G base pair, the NF-κB p65/RelA subunit conformation when bound to TTCC-3′ is drastically different than to TTTC-3′. The flexible linker region and modular domain architecture within the RHR allows the amino-terminal domain to reposition itself and bind the DNA backbone to accommodate such variations in κB DNA sequence. A second type of κB DNA sequence alteration involves changes within the central five base pairs. Of these, the central A:T is not directly contacted by the protein and the others are recognized nonspecifically through van der Waals contacts by p50 Tyr-59 (Tyr-36 in p65/RelA). The third base pair from the 5′-end or its symmetric pair are less sensitive to change, as illustrated by a comparison of immunoglobin κB and IFN-β κB sequences (5′-GGGAC T TTCC-3′ and 5′-GGGAA T TTCC-3′, respectively). The C:G to A:T base-pair change does not affect DNA recognition by the protein but may influence stability of binding because of the more rigid DNA structure of IFN-β κB sites. In this second case, overall conformations of the protein:DNA complexes are similar, but binding affinity could differ significantly.
Molecular dynamics simulations have revealed intriguing structural transitions of a 20-base-pair DNA containing the κB site of IL-2 promoter (AGAA A TTCC). The central A:T base pair (underlined) undergoes cross-stand stacking and the central A:T base pair flips out of the DNA axis (Mura and McCammon 2008). This dynamic behavior of the DNA suggests a highly complex mechanism of DNA recognition by the NF-κB dimers, in which DNA sequence variations may play a significant role in the recognition process. In general, one can confidently say that the sequences at the center of a κB sequence may profoundly affect the binding affinity and specificity.
IκB
Introduction to IκB
Almost immediately on detecting NF-κB in immune cells, researchers discovered that a latent κB DNA binding activity was present in the cytoplasm of all resting cells and that this pool of NF-κB could be activated by treatment of cell lysates with the weak detergent deoxycholate (Baeuerle and Baltimore 1988a). This suggested that noncovalent interaction with an inhibitor protein was responsible for maintaining NF-κB in an inactive state. Purification of the inhibitor activity led to the cloning of the inhibitory proteins IκBα and IκBβ (Baeuerle and Baltimore 1988b; Thompson et al. 1995). A third IκB gene was later identified by sequence homology in an EST database and was called IκBε (Li and Nabel 1997; Simeonidis et al. 1997; Whiteside et al. 1997). Subsequent experiments demonstrated that it also exhibits NF-κB inhibitory activity.
Classical IκB Sequence and Structure
Both IκBα and IκBβ, as well as the more recently discovered IκBε, contain a central ankyrin repeat domain (ARD) that contains six ankyrin repeats (Fig. 3A). The ankyrin repeat (ANK) is a roughly 33-amino-acid consensus amino acid sequence that appears in multiple copies in numerous proteins (Fig. 3B) (Sedgwick and Smerdon 1999). Ankyrin repeats are part of a greater superfamily of helical repeat motifs, which include HEAT repeats, armadillo repeats, and leucine-rich repeats, and are common to proteins involved in protein–protein interactions (Groves and Barford 1999). At their amino-terminal ends, classical IκB proteins contain a sequence of amino acids that do not adopt a folded structure in solution (Jaffray et al. 1995). Contained within this signal response region are the conserved serine sites of phosphorylation by IKK. Roughly 10 amino acids amino-terminal to this pair of serines reside conserved lysine amino acid sites of poly-ubiquitination. This amino-terminal region of IκBα also contains a functional nuclear export sequence (Johnson et al. 1999; Huang et al. 2000). It is not masked on binding to NF-κB and contributes to the observed cytoplasmic localization of NF-κB:IκBα complexes. Neither IκBβ nor IκBε possess this inherent nuclear export potential. Consequently, stable NF-κB:IκBβ complexes reside stably either in the cytoplasm or the nucleus, whereas IκBε appears to function as a negative feedback regulator of cytoplasmic NF-κB (Malek et al. 2001; Tam and Sen 2001; Kearns et al. 2006). At their carboxy-terminal ends, the three classical IκB proteins contain a short sequence rich in the amino acids proline, glutamic acid, serine, and threonine. This so-called PEST region is common to many proteins that, like IκB, display rapid turnover in cells (Rogers et al. 1986; Pando and Verma 2000). The PEST region of IκBα, however, is also required for its ability to disrupt preformed NF-κB:DNA complexes (Ernst et al. 1995).
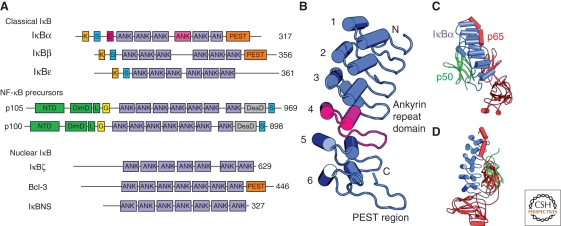
Figure 3.
The family of human IκB proteins. (A) IκB proteins are classified as in text. Classical IκB proteins possess ankyrin repeats (ANK) flanked by an amino-terminal signal response region and carboxy terminal PEST region. The signal response regions contain sites of phosphorylation by IKK (S), ubiquitination (K), and nuclear export (E). The NF-κB precursors serve as IκB proteins as well as the source of the mature p50 and p52 NF-κB subunits. (B) Ribbon diagram of the IκBα structure from the NF-κB:IκBα complex crystal structure. Individual ankyrin repeats are numbered, ANK 4 is colored magenta, and the PEST region is labeled. (C) Ribbon diagram of the NF-κB:IκBα complex. (D) Another view of the complex.
IκB Interactions with NF-κB
The X-ray structure of IκBα in complex with the NF-κB p50:p65/RelA heterodimer was determined independently by two separate laboratories in 1998 (Huxford et al. 1998; Jacobs and Harrison 1998). Both groups relied on a similar strategy of removing the signal response region of IκBα and the amino-terminal domain of the p50 subunit to stabilize the conformationally dynamic complex for cocrystallization (Huxford et al. 2000). The structure reveals how IκBα uses its entire ankyrin repeat-containing domain as well as its carboxy-terminal PEST sequence to mediate an extensive protein–protein interface of roughly 4300 Å2 (Fig. 3C,D). The carboxy-terminal 30 amino acids from the NF-κB p65/RelA subunit RHR, which were disordered in NF-κB:DNA complex structures, adopt an ordered helical structure that contacts the first two ankyrin repeats and forms significant hydrophobic contacts with the amino-terminal face of the IκBα ankyrin repeat stack. This interaction masks the p65/RelA nuclear localization signal. Ankyrin repeats three through five participate in multiple van der Waals contacts with one surface of the p50:p65/RelA heterodimer dimerization domains. The sixth ankyrin repeat and PEST region of IκBα present a vast acidic patch, which opposes the largely positively charged DNA binding surfaces of the p65/RelA amino-terminal domain. As a consequence of this electrostatic interaction, the p65/RelA amino-terminal domain occupies a position relative to the dimerization domain that is rotated roughly 180° and translated 40 Å when compared with its DNA bound structures. The transition of p65/RelA to the conformation observed in the NF-κB:IκB complex does not disrupt the amino-terminal domain structure and is afforded entirely by the flexible linker region that connects the amino-terminal and dimerization domains. The structure of a similar construct of IκBβ bound to the dimerization domain from the NF-κB p65/RelA:p65/RelA homodimer suggests that IκBβ uses a similar strategy in binding to NF-κB, although it relies less on interactions with the p65/RelA amino-terminal domain for complex stability (Malek et al. 2003).
IκBα Dynamics
Protein dynamics, or the rates with which a protein exchanges between quasi-stable folded states, is an extremely important aspect of protein structure and function. Several independent lines of investigation, including thermal and chemical denaturation, computer simulations, NMR spectroscopy, and failed crystallization attempts, have led to the conclusion that the IκBα protein exhibits a high degree of structural dynamics in solution (Huxford et al. 2000; Pando and Verma 2000; Croy et al. 2004; Bergqvist et al. 2006). This runs counter to the data that have emerged from protein engineering studies that clearly show that ankyrin repeat proteins designed after consensus sequences or those that appear in nature are extremely stably folded (Binz et al. 2003; Binz et al. 2004). IκBα has, therefore, evolved as an inherently unstable ankyrin repeat-containing protein. The consequences of this are twofold. First of all, free IκBα is easily degraded in cells. This signal-independent degradation involves the 20S proteasome and the carboxy-terminal PEST of IκBα (Mathes et al. 2008). Moreover, recent computational modeling of the NF-κB pathway through a systems biology approach has confirmed that regulation of NF-κB activation can be controlled by small changes in the rate of degradation of a constitutively expressed free cytoplasmic IκBα (O’Dea et al. 2007). On binding to NF-κB, the dynamic IκBα ankyrin repeat fold becomes stable and the PEST region protein turnover signal sequence is adopted as a DNA-inhibitory functional element (Sue et al. 2008). Degradation of IκBα is shifted from the steady state to a signal-dependent pathway that requires phosphorylation within the flexible amino-terminal signal response region. It is likely that the inherent folding instability of IκBα contributes to it being targeted by the proteasome, whereas NF-κB, which is composed of two stably folded domains and with which IκBα is associated in a complex at subnanomolar dissociation binding constant, remains intact.
Nonclassical IκB Proteins
Through the efforts of investigators attempting to understand regulation of NF-κB, a more diverse family of IκB proteins has emerged. Proteins of the IκB family are all linked by the fact that they contain ankyrin repeats and interact with NF-κB subunits to affect gene expression. The classical IκB proteins, IκBα, IκBβ, and IκBε, have been described. A second class of IκB proteins is represented by the NF-κB precursor proteins p105 and p100 (Basak et al. 2007). These two proteins act both as precursors of NF-κB p50 and p52 subunits, respectively, and as inhibitors of NF-κB (Fig. 3A). The NF-κB precursor proteins are responsible for inhibiting nearly half of the NF-κB in resting cells. However, unlike classical IκB proteins that inhibit NF-κB by forming 1:1 complexes, these NF-κB precursors participate in large multiprotein assemblies, wherein more than one NF-κB dimer can bind to multiple p100 and/or p105 subunits. The assembly of p100 and p105 into larger complexes is mediated by an oligomerization domain located immediately amino-terminal to their ankyrin-repeat domains. This assembly is heterogeneous, i.e., several different NF-κB subunits can be inhibited in a single inhibitory complex (Savinova et al. 2009). Therefore, stimulus-specific degradation of p100 or p105 can in principle release different NF-κB dimers. Together, these dimers exhibit a much broader spectrum of gene regulatory activities (Savinova et al. 2009, Shih et al. 2009). The large heterogeneous NF-κB inhibitory complex assemblies have been dubbed NF-κBsomes to distinguish them from the smaller NF-κB inhibitory complexes formed by IκBα, -β, and -ε.
Together with IκBα and IκBε, the NF-κB precursors are targets of NF-κB-driven transcription. Newly synthesized IκB serve to block NF-κB activity postinduction. This negative feedback regulation of NF-κB by IκB proteins is critical for control of inflammation and other diseases (Hoffmann et al. 2002).
Nuclear IκB Proteins
A third, entirely different class of IκB is represented by the proteins Bcl-3, IκBζ/MAIL, and IκBNS (Fig. 3A). Bcl-3 was cloned as a consequence to its proximity to a breakpoint mutation in some leukemias (Ohno et al. 1990). IκBζ was discovered in a screen of genes that displayed increased expression after induction of immune cells with bacterial lipopolysaccharide (LPS) or the inflammatory cytokine interleukin-1 (IL-1) (Kitamura et al. 2000; Haruta et al. 2001; Yamazaki et al. 2001). IκBNS was identified as a gene that is expressed in T cells during negative selection (Fiorini et al. 2002). Unlike classical IκB, these proteins do not contain amino-terminal signal-dependent phosphorylation sites or carboxy-terminal PEST regions. Their classification as “nuclear IκB” derives from the fact that they contain ankyrin repeats, bind NF-κB subunits, and concentrate within the nucleus when expressed in cells (Michel et al. 2001).
Each of the three nuclear IκB proteins are themselves the products of NF-κB-dependent genes (Eto et al. 2003; Ge et al. 2003; Hirotani et al. 2005). They bind to NF-κB, but, whereas the classical IκB proteins prefer dimers that possess at least one p65/RelA or c-Rel subunit, or nonclassical p105 and p100 binds all NF-κB subunits, nuclear IκBs bind specifically to homodimers of p50 (Hatada et al. 1992; Yamazaki et al. 2001; Trinh et al. 2008). Finally, association of nuclear IκB proteins with nuclear NF-κB can have diverse but important consequences on gene expression (Franzoso et al. 1992; Muta et al. 2003; Hirotani et al. 2005; Motoyama et al. 2005; Riemann et al. 2005). In peritoneal macrophages derived from mice lacking the gene encoding IκBζ, for example, a complete inability to produce the NF-κB-dependent cytokine interleukin-6 (IL-6) in response to LPS treatment was observed (Yamamoto et al. 2004). As IL-6 is a gene that is expressed in a later phase of NF-κB induction, it is apparent that the early induction and nuclear accumulation of IκBζ plays a vital role in the LPS-dependent expression of this pluripotent cytokine.
IKK
Introduction to IKK
In a thrilling conclusion to a search to identify an enzymatic activity that was capable of phosphorylating the two serine amino acids of the signal response region of IκBα, researchers from three labs reported in 1997 the IκB Kinase complex (IKK) (DiDonato et al. 1997; Mercurio et al. 1997; Regnier et al. 1997). This IKK was purified from cytokine-induced HeLa cells and exhibited an apparent molecular mass of 700–900 kDa. Microsequencing revealed two related kinase domain-containing subunits, referred to as IKKα and IKKβ (or alternatively as IKK1 and IKK2, respectively) (DiDonato et al. 1997; Zandi et al. 1997). These exhibit molecular masses of 85 and 87 kDa, respectively, and display 50% identity at the amino-acid level. The IKKα subunit was recognized to be the same protein that was originally cloned in 1995 as CHUK, a kinase with homology to the helix-loop-helix transcription factors, and IKKβ was subsequently identified as a hit in a yeast two-hybrid screen with NIK, a kinase suspected to function upstream of IKK, as bait (Connelly and Marcu 1995; Regnier et al. 1997; Woronicz et al. 1997). A third subunit, known as IKKγ (also known as NEMO), was also identified as a 49 kDa member of the IKK complex (Rothwarf et al. 1998; Yamaoka et al. 1998; Li et al. 1999b; Mercurio et al. 1999).
The function of IKK1/IKKα in cells is somewhat exotic and continues to be elucidated (Hu et al. 2001; Senftleben et al. 2001; Anest et al. 2003; Yamamoto et al. 2003; Sil et al. 2004; Lawrence et al. 2005). However, multiple studies including mouse knockouts have revealed that IKK2/IKKβ is responsible for phosphorylating IκB in response to NF-κB-inducing signals (Li et al. 1999a; Li et al. 1999c; Tanaka et al. 1999). Because of its role as the primary inducer of the NF-κB transcription factor, IKK2/IKKβ plays the critical role in promoting inflammation and cell survival in response to proinflammatory stimuli. The NEMO/IKKγ subunit does not possess any kinase domain or enzymatic activity and instead it acts as an adapter subunit that links the catalytic subunits to receptor proximal signaling molecules. Mouse knockout studies clearly reveal that proper NF-κB signaling through IKK is not possible without this subunit (Makris et al. 2000; Rudolph et al. 2000; Schmidt-Supprian et al. 2000).
IKK Catalytic Subunit Domain Organization
The IKK1/IKKα and IKK2/IKKβ subunits exhibit an uncommon domain organization (Fig. 4A). Their first roughly 300 amino acids contain a clearly recognizable kinase domain. This is followed by a short region that exhibits distant homology to ubiquitin (Ikeda et al. 2007). A central region contains a leucine zipper motif followed by a region with slight homology to the helix-loop-helix transcription factors. This is followed by a serine-rich region. Finally, the carboxy-terminal element of IKKβ has been shown to interact directly with NEMO/IKKγ (May et al. 2000; May et al. 2002). A genome-wide analysis has identified a small clade of proteins with domain organization reminiscent of the catalytic IKK1/IKKα and IKK2/IKKβ subunits (Manning et al. 2002). This kinase subgroup includes the proteins IKKε and TBK1/NAK. Both have been characterized as upstream modulators of IKK activity and are, therefore, both structurally and functionally related to the catalytic IKK subunits (Tojima et al. 2000; Peters and Maniatis 2001).
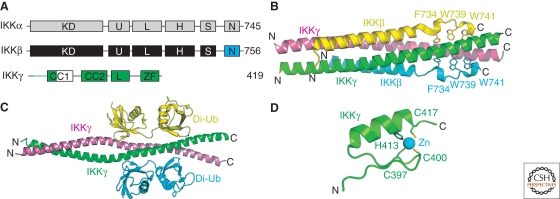
Figure 4.
Subunits of the human IKK complex. (A) Domain organization of IKK subunits. Catalytic subunits contain a kinase domain (KD), ubiquitin-like domain (U), leucine zipper (L), helix-loop-helix (H), serine-rich (S), and NEMO-binding motif (N). The NEMO/IKKγ subunit contains two predicted coiled-coil motifs (CC1 and ‐2), a leucine zipper (L), and a carboxy-terminal zinc-finger (ZF). (B) Ribbon diagram of the IKK2/IKKβ:NEMO/IKKγ complex. Individual polypeptides are labeled as well as some of the conserved hydrophobic amino acid side chains from IKK2/IKKβ. (C) Ribbon diagram of the NEMO/IKKγ:di-ubiquitin complex. (D) The NEMO/IKKγ carboxy-terminal zinc-finger motif structure.
With the exception of the extreme carboxy-terminal NEMO/IKKγ-interacting motif, the structures of IKK1/IKKα and IKK2/IKKβ are unknown. Although it is clear that the kinase domain of IKK2/IKKβ is necessary for catalyzing phospho-transfer, regions of the protein outside of this domain are necessary for directing specificity to the amino-terminal serines of IκBα. Deletion of the leucine zipper and helix-loop-helix has been shown to yield a mutant enzyme that, although it is capable of catalyzing phospho-transfer to IκBα, fails to recognize the amino-terminal serines required for NF-κB activation in response to inflammatory signaling (Shaul et al. 2008).
NEMO/IKKγ Domain Organization
Structural interest in NEMO/IKKγ arises from its lack of a kinase domain or, for that matter, homology to any other protein of known structure. Secondary-structure prediction methods suggest that NEMO/IKKγ is a mostly helical protein with two signature coiled coil (CC) elements and a leucine zipper motif in the middle are flanked by a helical dimerization domain near the amino-terminal end and a Zn-finger motif at the carboxyl terminus. Three-dimensional structures of several fragments of the NEMO/IKKγ subunit either as free polypeptides or bound to ligands have recently been elucidated. These NEMO/IKKγ structures allow one to envision that with the exception of the very ends, NEMO/IKKγ consists of long helices that are punctuated by short unstructured regions. These helical segments wrap around each other forming a long coiled-coil dimer with fraying of the monomers at each end. The carboxy-terminal end contains a Zn-finger motif and the 40-residue long amino terminus is likely to be flexible and unstructured.
The NEMO/IKKγ fragment structures suggest how IKKγ might be involved in cellular signaling. NEMO/IKKγ was previously thought to form a multimer with different segments shown to form dimers, trimers, or tetramers (Agou et al. 2002; Tegethoff et al. 2003; Marienfeld et al. 2006; Drew et al. 2007; Herscovitch et al. 2008). With new structural information, it now appears that previous conclusions might not be accurate. It is not surprising as flexible coiled-coil motifs migrate through the gel filtration beads differently than the stably folded globular proteins of identical mass.
Two Distinct IKK Activation Pathways
The IKK1/IKKα and IKK2/IKKβ subunits are activated through distinct signaling pathways (Senftleben et al. 2001). These pathways are activated by two distinct sets of stimuli. The canonical pathway is triggered by LPS or inflammatory cytokines TNF-α or IL-1 and signals through IKK2/IKKβ to activate p65/RelA and c-Rel dimers through the degradation of IκBα, IκBβ, IκBε, and p105. The noncanonical pathway results from BAFF, LT-β, and CD40 signaling through IKKα, leading to activation of the p52:RelB heterodimer through processing of p100 into p52 (Ghosh and Karin 2002). Activation of both pathways requires interactions of signaling molecules through specific poly-ubiquitin moieties that are covalently linked to some of these molecules. In addition, upstream protein kinases are also essential for activation of both kinases. The major distinction between these two pathways is the involvement of a single upstream kinase, known as NF-κB inducing kinase (NIK), to activate IKKα, whereas multiple kinases can activate IKKβ. IKKα and IKKβ are both present in the same particle in vivo. One puzzling question, however, is whether the different pathways target these two distinct subunits in a single complex or if there is a separate pool of IKKα present in cells that is activated by the noncanonical pathway. For the canonical pathway, however, the IKKβ subunit of the heterodimer must be activated. The role for IKKα in canonical signaling is unclear. The presence of the IκB kinases in multiple signaling pathways suggests that they are capable of participating in diverse signaling complexes throughout the cell.
IKK Complex Oligomerization
Although several combinations of the IKK1/IKKα, IKK2/IKKβ, and NEMO/IKKγ subunits have been proposed to account for the original 700–900 kDa complex, no successful reconstitution from purified components has resulted in an active complex of this size. Therefore, 10 years after isolation of the complex, even the oligomerization state of the IKK complex remains unclear. Given the presence of multiple conserved elements that can potentially mediate homo- and heteromeric interactions between subunits, there exist many possibilities. Early attempts to determine the arrangement of subunits in the complex led to the model that, independent of its NEMO/IKKγ scaffolding protein, IKK1/IKKα and IKK2/IKKβ are capable of forming stable homo- or heterodimers (Zandi et al. 1998). More recent work with human IKK2/IKKβ purified in milligram quantities from recombinant baculovirus-infected sf9 insect cells has shown that the full length subunit purifies as a tetramer. Removal of the carboxy-terminal 100 amino acids containing the serine-rich and NEMO/IKKγ-binding regions results in a dimeric enzyme, whereas removal of the entire carboxy-terminal portion beginning at the leucine zipper renders the kinase monomeric. Interestingly, it was observed that although this monomeric IKK2/IKKβ kinase domain remains catalytically active, it fails to specifically phosphorylate the signal response region of IκBα and instead catalyzes phosphorylation of the PEST (Shaul et al. 2008). The presence of multiple domains linked to flexibility in all three subunits shows why it is difficult to structurally characterize functional IKK complexes.
The Emerging Structure of NEMO/IKKγ
A working structural model of the NEMO/IKKγ subunit has begun to take shape with the recent successful determination of several X-ray and NMR structures of discrete functional portions. The structure of an IKK2/IKKβ polypeptide bound the amino-terminal helical region (residues 44 to 111) of NEMO/IKKγ revealed the complex forms a parallel four-helix bundle where two IKK2/IKKβ peptides associate with the NEMO/IKKγ dimer (Rushe et al. 2008). The IKK2/IKKβ peptides appear to fold on binding to the NEMO/IKKγ-dimer scaffold, making several contacts (Fig. 4B). The affinity of interaction between NEMO/IKKγ and IKK2/IKKβ subcomplex is high (KD is in low nanomolar range) and requires a large array of contacts. However, few of the hydrophobic residues in IKK2/IKKβ that are observed to line the hydrophobic pocket formed by the NEMO/IKKγ dimer are required for complex formation. Although no clear experimental data is available, the IKK:IKKγ complexes might exist as a 2:2 complex, where a dimeric NEMO/IKKγ binds to an IKK dimer. The amino-terminal helical dimerization domain of NEMO/IKKγ interacts with both the IKK1/IKKα and IKK2/IKKβ carboxy-terminal peptides, forming a 1:1:2 (IKK1/IKKα:IKK2/IKKβ:NEMO/IKKγ) IKK complex. This is possibly the basal state of the IKK complex in cells.
Several reports demonstrated an interaction between poly-ubiquitin chains and the CC2-LZ region of NEMO/IKKγ (Lo et al. 2009). Although its was previously thought that the poly-ubiquitin chain is linked through lysine 63 and glycine 76 of ubiquitin, experiments have now shown that linear ubiquitin chains bind to NEMO/IKKγ with 100-fold tighter binding affinity than do K63-linked chains. The X-ray structure of the complex between the CC2-LZ of NEMO/IKKγ and a linear di-ubiquitin motif has been recently elucidated (Rahighi et al. 2009). Two di-ubiquitin motifs are bound to two chains of the LZ motif (Fig. 4C). It is striking that this central region of NEMO/IKKγ also exhibits the elongated coiled-coil motif that was observed in the previously determined IKK2/IKKβ:NEMO/IKKγ complex as well as in the X-ray structure of a complex between the carboxy-terminal helical region of NEMO/IKKγ and the viral protein vFLIP from Kaposi’s sarcoma virus (Bagnéris et al. 2008).
The extreme carboxy-terminal region of NEMO/IKKγ adopts a CCHC-type zinc-finger motif. The solution structure of this region has been determined by multidimensional NMR spectroscopy (Cordier et al. 2008). Its structure adopts the familiar fold of a zinc-finger motif (Fig. 4d). Furthermore, this motif has been characterized as a ubiquitin-binding motif required for NF-κB signaling in response to TNF-α (Cordier et al. 2009).
When taken together, the NEMO/IKKγ substructures suggest that this subunit adopts an elongated helical structure. Two long helices bind one another through extended coiled-coil interactions to form the docking site for a pair of kinase subunits at the amino terminus, binding sites for di-ubiquitin and other proteins throughout the central region, and a pair of zinc-finger ubiquitin binding sites at the carboxyl terminus. This modular arrangement of docking sites for diverse proteins seems appropriate for a subunit that is thought to function principally as a scaffold for inducible signaling. Although significant structural work on IKK complexes remains to be carried out, this oddly elongated NEMO/IKKγ model serves to explain some of the complications associated with early studies that relied primarily on size exclusion chromatography for characterization of complex size and subunit stoichiometry.
ACKNOWLEDGMENTS
T.H. is supported by American Cancer Society grant RSG-08-287-01-GMC. G.G. is supported by National Institutes of Health (NIH) grants (GM085490, AI064326, and NCI141722).
Footnotes
Editors: Louis M. Staudt and Michael Karin
Additional Perspectives on NF-κB available at www.cshperspectives.org
REFERENCES
- Agou F, Ye F, Goffinont S, Courtois G, Yamaoka S, Isräel A, Véron M 2002. NEMO trimerizes through its coiled-coiled C-terminal domain. J Biol Chem 277:17464–17475 [DOI] [PubMed] [Google Scholar]
- Anest V, Hanson JL, Cogswell PC, Steinbrecher KA, Strahl BD, Baldwin AS 2003. A nucleosomal function for IκB kinase-α in NF-κB-dependent gene expression. Nature 423:659–663 [DOI] [PubMed] [Google Scholar]
- Baeuerle PA, Baltimore D 1988a. Activation of DNA-binding activity in an apparently cytoplasmic precursor of the NF-κB transcription factor. Cell 53:211–217 [DOI] [PubMed] [Google Scholar]
- Baeuerle PA, Baltimore D 1988b. IκB: A specific inhibitor of the NF-κB transcription factor. Science 242:540–546 [DOI] [PubMed] [Google Scholar]
- Bagnéris C, Ageichik AV, Cronin N, Wallace B, Collins M, Boshoff C, Waksman G, Barrett T 2008. Crystal structure of a vFlip-IKKγ complex: Insights into viral activation of the IKK signalosome. Mol Cell 30:620–631 [DOI] [PubMed] [Google Scholar]
- Baltimore D, Beg AA 1995. DNA-binding proteins. A butterfly flutters by. Nature 373:287–288 [DOI] [PubMed] [Google Scholar]
- Basak S, Kim H, Kearns JD, Tergaonkar V, O’Dea E, Werner SL, Benedict CA, Ware CF, Ghosh G, Verma IM, Hoffmann A 2007. A fourth IκB protein within the NF-κB signaling module. Cell 128:369–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergqvist S, Croy CH, Kjaergaard M, Huxford T, Ghosh G, Komives EA 2006. Thermodynamics Reveal that Helix Four in the NLS of NF-κB p65 Anchors IκBα, Forming a Very Stable Complex. J Mol Biol 360:421–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkowitz B, Huang DB, Chen-Park FE, Sigler PB, Ghosh G 2002. The x-ray crystal structure of the NF-κB p50:p65 heterodimer bound to the interferon-β κB site. J Biol Chem 277:24694–24700 [DOI] [PubMed] [Google Scholar]
- Binz HK, Amstutz P, Kohl A, Stumpp MT, Briand C, Forrer P, Grütter MG, Plückthun A 2004. High-affinity binders selected from designed ankyrin repeat protein libraries. Nat Biotechnol 22:575–582 [DOI] [PubMed] [Google Scholar]
- Binz HK, Stumpp MT, Forrer P, Amstutz P, Plückthun A 2003. Designing repeat proteins: Well-expressed, soluble and stable proteins from combinatorial libraries of consensus ankyrin repeat proteins. J Mol Biol 332:489–503 [DOI] [PubMed] [Google Scholar]
- Chen YQ, Ghosh S, Ghosh G 1998b. A novel DNA recognition mode by the NF-κB p65 homodimer. Nat Struct Biol 5:67–73 [DOI] [PubMed] [Google Scholar]
- Chen FE, Huang DB, Chen YQ, Ghosh G 1998a. Crystal structure of p50/p65 heterodimer of transcription factor NF-κB bound to DNA Nature 391:410–413 [DOI] [PubMed] [Google Scholar]
- Chen YQ, Sengchanthalangsy LL, Hackett A, Ghosh G 2000. NF-κB p65 (RelA) homodimer uses distinct mechanisms to recognize DNA targets. Structure 8:419–428 [DOI] [PubMed] [Google Scholar]
- Connelly MA, Marcu KB 1995. CHUK, a new member of the helix-loop-helix and leucine zipper families of interacting proteins, contains a serine-threonine kinase catalytic domain. Cell Mol Biol Res 41:537–549 [PubMed] [Google Scholar]
- Cordier F, Grubisha O, Traincard F, Véron M, Delepierre M, Agou F 2009. The zinc finger of NEMO is a functional ubiquitin-binding domain. J Biol Chem 284:2902–2907 [DOI] [PubMed] [Google Scholar]
- Cordier F, Vinolo E, Véron M, Delepierre M, Agou F 2008. Solution structure of NEMO zinc finger and impact of an anhidrotic ectodermal dysplasia with immunodeficiency-related point mutation. J Mol Biol 377:1419–1432 [DOI] [PubMed] [Google Scholar]
- Cramer P, Larson CJ, Verdine GL, Müller CW 1997. Structure of the human NF-κB p52 homodimer-DNA complex at 2.1 Å resolution. EMBO J 16:7078–7090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer P, Varrot A, Barillas-Mury C, Kafatos FC, Müller CW 1999. Structure of the specificity domain of the Dorsal homologue Gambif1 bound to DNA Structure 7:841–852 [DOI] [PubMed] [Google Scholar]
- Croy CH, Bergqvist S, Huxford T, Ghosh G, Komives EA 2004. Biophysical characterization of the free IκBα ankyrin repeat domain in solution. Protein Sci 13:1767–1777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiDonato JA, Hayakawa M, Rothwarf DM, Zandi E, Karin M 1997. A cytokine-responsive IκB kinase that activates the transcription factor NF-κB Nature 388:548–554 [DOI] [PubMed] [Google Scholar]
- Drew D, Shimada E, Huynh K, Bergqvist S, Talwar R, Karin M, Ghosh G 2007. Inhibitor κB kinase β binding by inhibitor κB kinase γ. Biochemistry 46:12482–12490 [DOI] [PubMed] [Google Scholar]
- Ernst MK, Dunn LL, Rice NR 1995. The PEST-like sequence of IκBα is responsible for inhibition of DNA binding but not for cytoplasmic retention of c-Rel or RelA homodimers. Mol Cell Biol 15:872–882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escalante CR, Shen L, Thanos D, Aggarwal AK 2002. Structure of NF-κB p50/p65 heterodimer bound to the PRDII DNA element from the interferon-β promoter. Structure 10:383–391 [DOI] [PubMed] [Google Scholar]
- Eto A, Muta T, Yamazaki S, Takeshige K 2003. Essential roles for NF-κB and a Toll/IL-1 receptor domain-specific signal(s) in the induction of IκBζ. Biochem Biophys Res Commun 301:495–501 [DOI] [PubMed] [Google Scholar]
- Fiorini E, Schmitz I, Marissen WE, Osborn SL, Touma M, Sasada T, Reche PA, Tibaldi EV, Hussey RE, Kruisbeek AM, et al. 2002. Peptide-induced negative selection of thymocytes activates transcription of an NF-κB inhibitor. Mol Cell 9:637–648 [DOI] [PubMed] [Google Scholar]
- Franzoso G, Bours V, Park S, Tomita-Yamaguchi M, Kelly K, Siebenlist U 1992. The candidate oncoprotein Bcl-3 is an antagonist of p50/NF-κB-mediated inhibition. Nature 359:339–342 [DOI] [PubMed] [Google Scholar]
- Fusco AJ, Huang DB, Miller D, Wang VY, Vu D, Ghosh G 2009. NF-κB p52:RelB heterodimer recognizes two classes of κB sites with two distinct modes. EMBO Rep 10:152–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganchi PA, Sun SC, Greene WC, Ballard DW 1993. A novel NF-κB complex containing p65 homodimers: Implications for transcriptional control at the level of subunit dimerization. Mol Cell Biol 13:7826–7835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge B, Li O, Wilder P, Rizzino A, McKeithan TW 2003. NF-κB regulates BCL3 transcription in T lymphocytes through an intronic enhancer. J Immunol 171:4210–4218 [DOI] [PubMed] [Google Scholar]
- Ghosh S, Karin M 2002. Missing pieces in the NF-κB puzzle. Cell 109 Suppl:S81–96 [DOI] [PubMed] [Google Scholar]
- Ghosh S, May MJ, Kopp EB 1998. NF-κB AND REL PROTEINS: Evolutionarily Conserved Mediators of Immune Responses. Annu Rev Immunol 16:225–260 [DOI] [PubMed] [Google Scholar]
- Ghosh G, van Duyne G, Ghosh S, Sigler PB 1995. Structure of NF-κB p50 homodimer bound to a κB site. Nature 373:303–310 [DOI] [PubMed] [Google Scholar]
- Groves MR, Barford D 1999. Topological characteristics of helical repeat proteins. Curr Opin Struct Biol 9:383–389 [DOI] [PubMed] [Google Scholar]
- Haruta H, Kato A, Todokoro K 2001. Isolation of a novel interleukin-1-inducible nuclear protein bearing ankyrin-repeat motifs. J Biol Chem 276:12485–12488 [DOI] [PubMed] [Google Scholar]
- Hatada EN, Nieters A, Wulczyn FG, Naumann M, Meyer R, Nucifora G, McKeithan TW, Scheidereit C 1992. The ankyrin repeat domains of the NF-κB precursor p105 and the protooncogene bcl-3 act as specific inhibitors of NF-κB DNA binding. Proc Natl Acad Sci 89:2489–2493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden MS, Ghosh S 2008. Shared principles in NF-κB signaling. Cell 132:344–362 [DOI] [PubMed] [Google Scholar]
- Herscovitch M, Comb W, Ennis T, Coleman K, Yong S, Armstead B, Kalaitzidis D, Chandani S, Gilmore TD 2008. Intermolecular disulfide bond formation in the NEMO dimer requires Cys54 and Cys347. Biochem Biophys Res Commun 367:103–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirotani T, Lee PY, Kuwata H, Yamamoto M, Matsumoto M, Kawase I, Akira S, Takeda K 2005. The nuclear IκB protein IκBNS selectively inhibits lipopolysaccharide-induced IL-6 production in macrophages of the colonic lamina propria. J Immunol 174:3650–3657 [DOI] [PubMed] [Google Scholar]
- Hoffmann A, Natoli G, Ghosh G 2006. Transcriptional regulation via the NF-κB signaling module. Oncogene 25:6706–6716 [DOI] [PubMed] [Google Scholar]
- Hoffmann A, Levchenko A, Scott ML, Baltimore D 2002. The IκB-NF-κB signaling module: Temporal control and selective gene activation. Science 298:1241–1245 [DOI] [PubMed] [Google Scholar]
- Hu Y, Baud V, Oga T, Kim KI, Yoshida K, Karin M 2001. IKKα controls formation of the epidermis independently of NF-κB Nature 410:710–714 [DOI] [PubMed] [Google Scholar]
- Huang DB, Vu D, Ghosh G 2005. NF-κB RelB forms an intertwined homodimer. Structure 13:1365–1373 [DOI] [PubMed] [Google Scholar]
- Huang DB, Huxford T, Chen YQ, Ghosh G 1997. The role of DNA in the mechanism of NF-κB dimer formation: Crystal structures of the dimerization domains of the p50 and p65 subunits. Structure 5:1427–1436 [DOI] [PubMed] [Google Scholar]
- Huang TT, Kudo N, Yoshida M, Miyamoto S 2000. A nuclear export signal in the N-terminal regulatory domain of IκBα controls cytoplasmic localization of inactive NF-κB/IκBα complexes. Proc Natl Acad Sci 97:1014–1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxford T, Malek S, Ghosh G 2000. Preparation and crystallization of dynamic NF-κB:IκB complexes. J Biol Chem 275:32800–32806 [DOI] [PubMed] [Google Scholar]
- Huxford T, Huang DB, Malek S, Ghosh G 1998. The crystal structure of the IκBα/NF-κB complex reveals mechanisms of NF-κB inactivation. Cell 95:759–770 [DOI] [PubMed] [Google Scholar]
- Ikeda F, Hecker CM, Rozenknop A, Nordmeier RD, Rogov V, Hofmann K, Akira S, Dotsch V, Dikic I 2007. Involvement of the ubiquitin-like domain of TBK1/IKK-i kinases in regulation of IFN-inducible genes. EMBO J 26:3451–3462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs MD, Harrison SC 1998. Structure of an IκBα/NF-κB complex. Cell 95:749–758 [DOI] [PubMed] [Google Scholar]
- Jacque E, Tchenio T, Piton G, Romeo PH, Baud V 2005. RelA repression of RelB activity induces selective gene activation downstream of TNF receptors. Proc Natl Acad Sci 102:14635–14640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffray E, Wood KM, Hay RT 1995. Domain organization of IκBα and sites of interaction with NF-κB p65. Mol Cell Biol 15:2166–2172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson C, Van Antwerp D, Hope TJ 1999. An N-terminal nuclear export signal is required for the nucleocytoplasmic shuttling of IκBα. EMBO J 18:6682–6693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearns JD, Basak S, Werner SL, Huang CS, Hoffmann A 2006. IκBε provides negative feedback to control NF-κB oscillations, signaling dynamics, and inflammatory gene expression. J Cell Biol 173:659–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura H, Kanehira K, Okita K, Morimatsu M, Saito M 2000. MAIL, a novel nuclear IκB protein that potentiates LPS-induced IL-6 production. FEBS Lett 485:53–56 [DOI] [PubMed] [Google Scholar]
- Lawrence T, Bebien M, Liu GY, Nizet V, Karin M 2005. IKKα limits macrophage NF-κB activation and contributes to the resolution of inflammation. Nature 434:1138–1143 [DOI] [PubMed] [Google Scholar]
- Li Z, Nabel GJ 1997. A new member of the IκB protein family, IκBε, inhibits RelA (p65)-mediated NF-κB transcription. Mol Cell Biol 17:6184–6190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z-W, Chu W, Hu Y, Delhase M, Deerinck T, Ellisman M, Johnson R, Karin M 1999c. The IKKβ subunit of IκB Kinase (IKK) is essential for nuclear factor κB activation and prevention of apoptosis. J Exp Med 189:1839–1845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Kang J, Friedman J, Tarassishin L, Ye J, Kovalenko A, Wallach D, Horwitz MS 1999b. Identification of a cell protein (FIP-3) as a modulator of NF-κB activity and as a target of an adenovirus inhibitor of tumor necrosis factor α-induced apoptosis. Proc Natl Acad Sci 96:1042–1047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Van Antwerp D, Mercurio F, Lee KF, Verma IM 1999a. Severe liver degeneration in mice lacking the IκB kinase 2 gene. Science 284:321–325 [DOI] [PubMed] [Google Scholar]
- Lo YC, Lin SC, Rospigliosi CC, Conze DB, Wu CJ, Ashwell JD, Eliezer D, Wu H 2009. Structural basis for recognition of diubiquitins by NEMO Mol Cell 33:602–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris C, Godfrey VL, Krahn-Senftleben G, Takahashi T, Roberts JL, Schwarz T, Feng L, Johnson RS, Karin M 2000. Female mice heterozygous for IKKγ/NEMO deficiencies develop a dermatopathy similar to the human X-linked disorder incontinentia pigmenti. Mol Cell 5:969–979 [DOI] [PubMed] [Google Scholar]
- Malek S, Chen Y, Huxford T, Ghosh G 2001. IκBβ, but not IκBα, functions as a classical cytoplasmic inhibitor of NF-κB dimers by masking both NF-κB nuclear localization sequences in resting cells. J Biol Chem 276:45225–45235 [DOI] [PubMed] [Google Scholar]
- Malek S, Huang DB, Huxford T, Ghosh S, Ghosh G 2003. X-ray crystal structure of an IκBβ:NF-κB p65 homodimer complex. J Biol Chem 278:23094–23100 [DOI] [PubMed] [Google Scholar]
- Manning G, Whyte DB, Martinez R, Hunter T, Sudarsanam S 2002. The protein kinase complement of the human genome. Science 298:1912–1934 [DOI] [PubMed] [Google Scholar]
- Marienfeld RB, Palkowitsch L, Ghosh S 2006. Dimerization of the IκB kinase-binding domain of NEMO is required for tumor necrosis factor α-induced NF-κB activity. Mol Cell Biol 26:9209–9219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marienfeld R, Berberich-Siebelt F, Berberich I, Denk A, Serfling E, Neumann M 2001. Signal-specific and phosphorylation-dependent RelB degradation: A potential mechanism of NF-κB control. Oncogene 20:8142–8147 [DOI] [PubMed] [Google Scholar]
- Marienfeld R, May MJ, Berberich I, Serfling E, Ghosh S, Neumann M 2003. RelB forms transcriptionally inactive complexes with RelA/p65. J Biol Chem 278:19852–19860 [DOI] [PubMed] [Google Scholar]
- Mathes E, O’Dea EL, Hoffmann A, Ghosh G 2008. NF-κB dictates the degradation pathway of IκBα. EMBO J 27:1357–1367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- May MJ, Marienfeld RB, Ghosh S 2002. Characterization of the IκB-kinase NEMO binding domain. J Biol Chem 277:45992–46000 [DOI] [PubMed] [Google Scholar]
- May MJ, D’Acquisto F, Madge LA, Glockner J, Pober JS, Ghosh S 2000. Selective inhibition of NF-κB activation by a peptide that blocks the interaction of NEMO with the IκB kinase complex. Science 289:1550–1554 [DOI] [PubMed] [Google Scholar]
- Mercurio F, Murray BW, Shevchenko A, Bennett BL, Young DB, Li JW, Pascual G, Motiwala A, Zhu H, Mann M, et al. 1999. IκB kinase (IKK)-associated protein 1, a common component of the heterogeneous IKK complex. Mol Cell Biol 19:1526–1538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercurio F, Zhu H, Murray BW, Shevchenko A, Bennett BL, Li J, Young DB, Barbosa M, Mann M, Manning A, et al. 1997. IKK-1 and IKK-2: Cytokine-activated IκB kinases essential for NF-κB activation. Science 278:860–866 [DOI] [PubMed] [Google Scholar]
- Michel F, Soler-Lopez M, Petosa C, Cramer P, Siebenlist U, Müller CW 2001. Crystal structure of the ankyrin repeat domain of Bcl-3: A unique member of the IκB protein family. EMBO J 20:6180–6190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorthy AK, Huang DB, Wang VY, Vu D, Ghosh G 2007. X-ray structure of a NF-κB p50/RelB/DNA complex reveals assembly of multiple dimers on tandem κB sites. J Mol Biol 373:723–734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motoyama M, Yamazaki S, Eto-Kimura A, Takeshige K, Muta T 2005. Positive and negative regulation of nuclear factor-κB-mediated transcription by IκBζ, an inducible nuclear protein. J Biol Chem 280:7444–7451 [DOI] [PubMed] [Google Scholar]
- Müller CW, Rey FA, Harrison SC 1996. Comparison of two different DNA-binding modes of the NF-κB p50 homodimer. Nat Struct Biol 3:224–227 [DOI] [PubMed] [Google Scholar]
- Müller CW, Rey FA, Sodeoka M, Verdine GL, Harrison SC 1995. Structure of the NF-κB p50 homodimer bound to DNA Nature 373:311–317 [DOI] [PubMed] [Google Scholar]
- Mura C, McCammon JA 2008. Molecular dynamics of a κB DNA element: Base flipping via cross-strand intercalative stacking in a microsecond-scale simulation. Nucleic Acids Res 36:4941–4955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muta T, Yamazaki S, Eto A, Motoyama M, Takeshige K 2003. IκBζ, a new anti-inflammatory nuclear protein induced by lipopolysaccharide, is a negative regulator for nuclear factor-κB J Endotoxin Res 9:187–191 [DOI] [PubMed] [Google Scholar]
- O’Dea EL, Barken D, Peralta RQ, Tran KT, Werner SL, Kearns JD, Levchenko A, Hoffmann A 2007. A homeostatic model of IκB metabolism to control constitutive NF-κB activity. Mol Syst Bio 3:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno H, Takimoto G, McKeithan TW 1990. The candidate proto-oncogene bcl-3 is related to genes implicated in cell lineage determination and cell cycle control. Cell 60:991–997 [DOI] [PubMed] [Google Scholar]
- Pando MP, Verma IM 2000. Signal-dependent and -independent Degradation of Free and NF-κB-bound IκBα J Biol Chem 275:21278–21286 [DOI] [PubMed] [Google Scholar]
- Panne D, Maniatis T, Harrison SC 2007. An atomic model of the interferon-β enhanceosome. Cell 129:1111–1123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters RT, Maniatis T 2001. A new family of IKK-related kinases may function as IκB kinase kinases. Biochim Biophys Acta 1471:M57–62 [DOI] [PubMed] [Google Scholar]
- Rahighi S, Ikeda F, Kawasaki M, Akutsu M, Suzuki N, Kato R, Kensche T, Uejima T, Bloor S, Komander D, et al. 2009. Specific recognition of linear ubiquitin chains by NEMO is important for NF-κB activation. Cell 136:1098–1109 [DOI] [PubMed] [Google Scholar]
- Regnier CH, Song HY, Gao X, Goeddel DV, Cao Z, Rothe M 1997. Identification and characterization of an IκB kinase. Cell 90:373–383 [DOI] [PubMed] [Google Scholar]
- Riemann M, Endres R, Liptay S, Pfeffer K, Schmid RM 2005. The IκB protein Bcl-3 negatively regulates transcription of the IL-10 gene in macrophages. J Immunol 175:3560–3568 [DOI] [PubMed] [Google Scholar]
- Rogers S, Wells R, Rechsteiner M 1986. Amino acid sequences common to rapidly degraded proteins: The PEST hypothesis. Science 234:364–368 [DOI] [PubMed] [Google Scholar]
- Rothwarf DM, Zandi E, Natoli G, Karin M 1998. IKKγ is an essential regulatory subunit of the IκB kinase complex. Nature 395:297–300 [DOI] [PubMed] [Google Scholar]
- Rudolph D, Yeh WC, Wakeham A, Rudolph B, Nallainathan D, Potter J, Elia AJ, Mak TW 2000. Severe liver degeneration and lack of NF-κB activation in NEMO/IKKγ-deficient mice. Genes Dev 14:854–862 [PMC free article] [PubMed] [Google Scholar]
- Rushe M, Silvian L, Bixler S, Chen LL, Cheung A, Bowes S, Cuervo H, Berkowitz S, Zheng T, Guckian K, et al. 2008. Structure of a NEMO/IKK-associating domain reveals architecture of the interaction site. Structure 16:798–808 [DOI] [PubMed] [Google Scholar]
- Savinova OV, Hoffmann A, Ghosh G 2009. The NFKB1 and NFKB2 proteins p105 and p100 function as the core of high molecular-weight heterogenous complexes. Mol Cell 12:591–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Supprian M, Bloch W, Courtois G, Addicks K, Isräel A, Rajewsky K, Pasparakis M 2000. NEMO/IKKγ-deficient mice model incontinentia pigmenti. Mol Cell 5:981–992 [DOI] [PubMed] [Google Scholar]
- Sedgwick SG, Smerdon SJ 1999. The ankyrin repeat: A diversity of interactions on a common structural framework. Trends Biochem Sci 24:311–316 [DOI] [PubMed] [Google Scholar]
- Sen R, Baltimore D 1986. Multiple nuclear factors interact with the immunoglobulin enhancer sequences. Cell 46:705–716 [DOI] [PubMed] [Google Scholar]
- Senftleben U, Cao Y, Xiao G, Greten FR, Krahn G, Bonizzi G, Chen Y, Hu Y, Fong A, Sun SC, et al. 2001. Activation by IKKα of a second, evolutionary conserved, NF-κB signaling pathway. Science 293:1495–1499 [DOI] [PubMed] [Google Scholar]
- Shaul JD, Farina A, Huxford T 2008. The human IKKβ subunit kinase domain displays CK2-like phosphorylation specificity. Biochem Biophys Res Commun 374:592–597 [DOI] [PubMed] [Google Scholar]
- Shih VF, Kearns JD, Basak S, Savinova OV, Ghosh G, Hoffmann A 2009. Kinetic control of negative feedback regulators of NF-κB/RelA determines their pathogen- and cytokine-receptor signaling specificity. Proc Natl Acad Sci 106:9619–9624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sil AK, Maeda S, Sano Y, Roop DR, Karin M 2004. IκB kinase-α acts in the epidermis to control skeletal and craniofacial morphogenesis. Nature 428:660–664 [DOI] [PubMed] [Google Scholar]
- Simeonidis S, Liang S, Chen G, Thanos D 1997. Cloning and functional characterization of mouse IκBε. Proc Natl Acad Sci 94:14372–14377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sue SC, Cervantes C, Komives EA, Dyson HJ 2008. Transfer of flexibility between ankyrin repeats in IκBα upon formation of the NF-κB complex. J Mol Biol 380:917–931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam WF, Sen R 2001. IκB family members function by different mechanisms. J Biol Chem 276:7701–7704 [DOI] [PubMed] [Google Scholar]
- Tanaka M, Fuentes ME, Yamaguchi K, Durnin MH, Dalrymple SA, Hardy KL, Goeddel DV 1999. Embryonic lethality, liver degeneration, and impaired NF-κB activation in IKKβ-deficient mice. Immunity 10:421–429 [DOI] [PubMed] [Google Scholar]
- Tegethoff S, Behlke J, Scheidereit C 2003. Tetrameric oligomerization of IκB kinase γ (IKKγ) is obligatory for IKK complex activity and NF-κB activation. Mol Cell Biol 23:2029–2041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JE, Phillips RJ, Erdjument-Bromage H, Tempst P, Ghosh S 1995. IκBβ regulates the persistent response in a biphasic activation of NF-κB Cell 80:573–582 [DOI] [PubMed] [Google Scholar]
- Tojima Y, Fujimoto A, Delhase M, Chen Y, Hatakeyama S, Nakayama K, Kaneko Y, Nimura Y, Motoyama N, Ikeda K, et al. 2000. NAK is an IκB kinase-activating kinase. Nature 404:778–782 [DOI] [PubMed] [Google Scholar]
- Trinh DV, Zhu N, Farhang G, Kim BJ, Huxford T 2008. The nuclear I κB protein IκBζ specifically binds NF-κB p50 homodimers and forms a ternary complex on κB DNA J Mol Biol 379:122–135 [DOI] [PubMed] [Google Scholar]
- Whiteside ST, Epinat JC, Rice NR, Isräel A 1997. IκBε, a novel member of the IκB family, controls RelA and cRel NF-κB activity. EMBO J 16:1413–1426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woronicz JD, Gao X, Cao Z, Rothe M, Goeddel DV 1997. IκB kinase-β: NF-κB activation and complex formation with IκB kinase-a and NIK Science 278:866–869 [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Yamazaki S, Uematsu S, Sato S, Hemmi H, Hoshino K, Kaisho T, Kuwata H, Takeuchi O, Takeshige K, et al. 2004. Regulation of Toll/IL-1-receptor-mediated gene expression by the inducible nuclear protein IκBζ. Nature 430:218–222 [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Verma UN, Prajapati S, Kwak YT, Gaynor RB 2003. Histone H3 phosphorylation by IKKα is critical for cytokine-induced gene expression. Nature 423:655–9 [DOI] [PubMed] [Google Scholar]
- Yamaoka S, Courtois G, Bessia C, Whiteside ST, Weil R, Agou F, Kirk HE, Kay RJ, Isräel A 1998. Complementation cloning of NEMO, a component of the IκB kinase complex essential for NF-κB activation. Cell 93:1231–1240 [DOI] [PubMed] [Google Scholar]
- Yamazaki S, Muta T, Takeshige K 2001. A novel IκB protein, IκB-ζ, induced by proinflammatory stimuli, negatively regulates nuclear factor-κB in the nuclei. J Biol Chem 276:27657–27662 [DOI] [PubMed] [Google Scholar]
- Zandi E, Rothwarf DM, Delhase M, Hayakawa M, Karin M 1997. The IκB kinase complex (IKK) contains two kinase subunits, IKKα and IKKβ, necessary for IκB phosphorylation and NF-κB activation. Cell 91:243–252 [DOI] [PubMed] [Google Scholar]
- Zandi E, Chen Y, Karin M 1998. Direct phosphorylation of IκB by IKKα and IKKβ: Discrimination between free and NF-κB-bound substrate. Science 281:1360–1363 [DOI] [PubMed] [Google Scholar]