Abstract
Epithelial branching during the process of lung development results in the establishment of distinct functional zones, each of which is characterized by a unique cellular composition and repertoire of local progenitor cells. Significant new insights into cellular and molecular mechanisms of epithelial maintenance that provide insights into the pathophysiology of lung disease have been made in recent years. This review focuses on the complex structure–function relationship in the airway epithelium, how this epithelium is maintained in the normal state and repaired following injury, and how deregulation may contribute to airway disease and cancer.
Keywords: stem cells, lung, disease, neoplasia
Introduction
Lung structure
The epithelium lining the conducting airway and alveolar compartments of the lung play a primary role in gas transport and exchange, respectively. Equally important functions of the epithelium are regulation of host defence and intrinsic reparative capacity following microbial- or xenobiotic-initiated tissue damage. To accomplish these functions, the epithelium of the mammalian lung has evolved a compartmental organization along the proximal–distal axis, distinct regions of which are defined by their unique cellular composition. The normal human tracheal and bronchial epithelium is a pseudostratified epithelium predominantly comprised of basal, ciliated, goblet and serous cells. More distal within intrapulmonary conducting airways is a more simplified columnar epithelium defining the bronchiolar airway epithelium. The bronchiolar epithelium of humans is comprised of ciliated cells, non-ciliated secretory Clara cells and relatively few basal cells and is devoid of goblet cells [1,2]. Conducting airways of the human lung terminate in a transitional zone of respiratory epithelium, referred to as the respiratory bronchiole. Respiratory bronchioles include conducting portions lined by cuboidal bronchiolar epithelium, comprised of Clara and ciliated cells in addition to frequent alveoli that bud from the airway wall.
Despite functional conservation of epithelial compartments between species, their relative size and cellular composition varies. The most dramatic anatomical difference between species is evidenced by the number of intrapulmonary conducting airway branches and the relative abundance of bronchial versus bronchiolar epithelium. Multiple generations of intrapulmonary bronchi observed in humans are limited to one or two generations in mice, in which bronchioles predominate. Moreover, bronchioles in mouse airways make the transition directly into alveolar ducts without the transitional respiratory bronchiolar zone typical of larger mammalian species. Corresponding differences are observed in cell types lining airways of the mouse lung. Basal cells are restricted to the tracheal epithelium, ciliated cell abundance diminishes significantly along the proximal–distal axis, and Clara cells are particularly abundant within bronchiolar airways [3]. Cell types of the alveolar epithelium show minimal differences between species (Figure 1).
Figure 1.
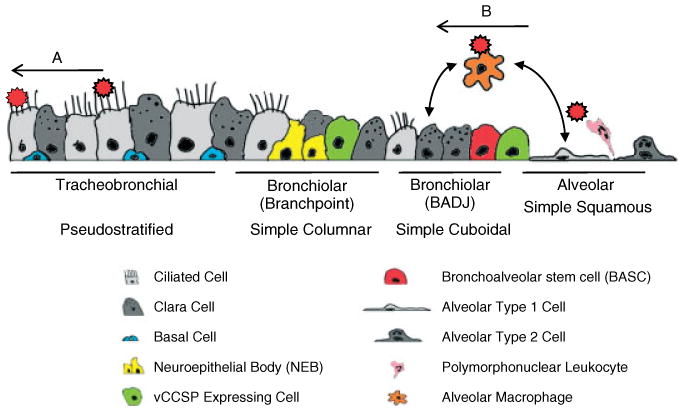
Structure and function of the airway epithelium. The epithelium lining mammalian airways is compartmentalized along the proximal–distal axis into four distinct zones, which can be further subdivided based upon cellular composition, structure and function. The schematic represents changes in cellular composition and function according to airway location in the mouse lung (proximal to the left, distal to the right). The pseudostratified tracheobronchial epithelium is characterized by an abundance of ciliated and basal cells and a lower incidence of non-mucus secretory cells. Secretory cells in proximal airways of larger mammalian species tend to be of more varied phenotype and include serous and goblet cells. Basal cells are less abundant within mouse bronchial airways relative to the bronchi of larger mammalian species. The epithelium of the bronchi is largely composed of columnar ciliated and secretory cells. Secretory cells of both tracheal and bronchial airways in mice share some molecular properties of bronchiolar Clara cells but differ in their mechanism of replacement and responsiveness to airway inflammation. Bronchiolar airways are the most distal conducting airway and are composed of ciliated cells and Clara cells. Other cell types of the conducting airway include rare pulmonary neuroendocrine cells that, when organized into clusters, are commonly referred to as neuroepithelial bodies (NEBs). The conducting airway epithelium of the rodent lung terminates abruptly at the bronchoalveolar duct junction, where airways open into alveolar ducts and clusters of alveoli composed of squamated type I cells and cuboidal type II cells. These cell types are specialized for gas transfer and surfactant production, respectively. Larger mammalian species have a transitional respiratory bronchiole that includes bronchiolar epithelium, with frequent alveolar buds that protrude from the bronchiolar wall (not shown). Epithelial compartmentalization plays a vital role in protecting the lung from microbial infection and inhaled pollutants through two major mechanisms: (a) particles and microbes adsorbed to the viscous mucus layer are cleared through concerted ciliary beating within the subtending periciliary layer (PCL), resulting in effective clearance with minimal need for an immunological response; (b) if mucociliary clearance is overwhelmed or evaded, both innate and adaptive arms of the host immune response are activated. Recent work has identified an epithelial and macrophage signalling network, whereby secreted proteins from airway and alveolar epithelial cells can regulate macrophage activation and inflammatory cell recruitment
The complex anatomical organization of these cellular components is regulated by a highly coordinated and stereotypic developmental programme, as assessed by interrogation of mouse models. Lung development initiates following fate specification within the ventral foregut endoderm and can be defined by Nkx2.1 expression [4]. Recent data indicate that branching occurs through a combination of three geometric branch types in a combination with three stereotypical series [5]. Regulation of cell proliferation and fate are tightly linked to this process, as signalling perturbations impacting these processes lead to a variety of defects in lung branching and specification of epithelial lineages. These signalling events involve reciprocal interactions between epithelial and mesenchymal cell types, resulting in coordinated growth and differentiation. Even though far less is known of signalling axes that contribute to epithelial behaviour in the adult lung, it is clear that the epithelial–mesenchymal signalling axis is retained and its regulation is critical to tissue homeostasis.
Epithelial function: mucociliary clearance and immunoregulation
The complex cellular composition of the airway epithelium provides key defensive properties for the lung (Figure 1). As reviewed extensively by Knowles and Boucher [6], the first line of defence to inhaled particulates, oxidants and biological flora is the airway surface fluid (ASF). The ASF is comprised of the mucus layer, of which the predominant protein components are Muc5ac and Muc5ab, and a subtending periciliary liquid layer (PCL) that provides an unrestricted media for ciliary beating and effective mucus transport. Inhaled microorganisms and environmental stimuli are immediately ensnared in the dense mucus of the ASF, preventing further entry to the more susceptible distal conducting and alveolar compartments. Through precise regulation of PCL height, ciliated cells coordinately regulate mucociliary clearance. Effective mucociliary clearance is estimated to take 6 h, implying that this mechanism alone is not enough to protect from infection [6]. As such, the ASF also includes several anti-microbial proteins that inhibit bacterial proliferation to allow for effective removal through mucociliary clearance without infection, thus minimizing the need for a massive inflammatory response [7].
The distal airway epithelial cells, specifically the Clara cell, may also play a prominent role in providing important immunoregulatory properties. The principle secreted protein product of the Clara cell, Clara cell secretory protein (CCSP, Scgb1a1), is also the predominant protein product of ASF and precipitously declines in chronic lung disease [8–12]. To study the effects of CCSP deficiency on lung function, mice homozygous null for CCSP (CCSP−/−) were generated [13,14]. Exposure of CCSP−/− mice to pulmonary irritants, pollutants and microbes have revealed that Clara cell secretions can regulate the immunoregulatory aspects of the airway epithelium [15–22]. Recent studies in which NF-κB is inhibited or augmented in Clara cells suggests that the LPS elicited inflammatory response is dependent upon signalling in the airway epithelium [23–25]. This process is likely initiated by secretion of paracrine signalling molecules from macrophages such as TNFα [26]. We have more recently demonstrated that the airway epithelium, through Clara cell secretions, regulates crosstalk with lung macrophages [27]. Collectively, these data indicate that airway epithelial specification and effective repair and maintenance are critical for lung homeostasis.
The remainder of this review deals principally with the cellular and molecular mechanisms involved in epithelial maintenance. We place emphasis on defining the putative stem cell hierarchies endogenous to the conducting airway epithelium and their molecular regulators and evaluate their contribution to lung disease.
Defining a stem cell hierarchy
Hierarchical organization of progenitor cells into what is commonly referred to as a ‘stem cell hierarchy’ has classically been described in tissues that display a high rate of cellular turnover, such as the small intestine and skin. Specifically, the intestinal epithelium, as a result of persistent epithelial damage, is continually regenerating, resulting in cellular turnover of approximately 5 days. Tissue stem cells of this hierarchy have been defined using functional assays that interrogate proliferative frequency, self-renewal and differentiation potential [28]. Small numbers of intestinal stem cells are thought to reside at or near the base of the intestinal crypt and give rise to a more abundant population of highly proliferative transit-amplifying cells located more proximally within the crypt [29,30]. Transit-amplifying cells of the intestinal epithelium represent an obligate progenitor cell pool lacking differentiated characteristics and are committed to a high rate of proliferation, necessary for the generation of differentiating progeny [31]. This strategy for rapid tissue replacement is shared by the skin, in which an obligate transit-amplifying progenitor cell that is both abundant and broadly distributed within the epidermis continuously generates differentiating progeny through a stochastic mechanism [32,33]. In the skin, resting tissue stem cells are maintained in a quiescent state and are only activated to proliferate in response to depletion of the transit-amplifying pool, through a process thought to be highly regulated by calcineruin, BMP and NFATc1 [34,35]. The relative quiescence of tissue stem cells in comparison to their more abundant transit-amplifying progeny has become a controversial topic in recent studies investigating intestinal stem cells. However, the property of DNA label retention remains a commonly used assay for the identification and localization of resident tissue stem cells [28].
The epithelium lining pulmonary airways turns over slowly during the normal process of tissue maintenance. Methods used to calculate the rate of epithelial turnover rely on indirect approaches that are based upon measurements of cell proliferation and lineage tagging. Measurements of cell proliferation have principally relied upon stable incorporation of labelled DNA precursors, such as bromodeoxyuridine (BrdU) or [H3]-thymidine, into the DNA of cells traversing S-phase. Using this approach, estimates of the steady-state instantaneous proliferative fraction were determined to be 1.3% in tracheal epithelium [36] and 0.06% in bronchiolar epithelium [37]. Data generated from continuous labelling of the steady-state bronchiolar epithelium further demonstrated that the frequency of bronchiolar epithelium is 1%/day [38]. Assuming a direct relationship between the rate of epithelial cell loss and replacement, these data suggest that epithelial cells have an average life span of 100 days in bronchioles. Direct measurement of ciliated cell half-life as a measure of epithelial turnover, made by Rawlins and Hogan, indicated that this subpopulation of epithelial cells has an estimated half-life of either 6 or 17 months in trachea and bronchioles, respectively [39]. Estimates made using either approach indicate that the epithelium of the trachea turns over more rapidly than that of bronchioles. While both methods are technically flawed, due to the inability to directly assess Clara cell turnover, these studies demonstrate that the frequency with which the airway epithelium turns over is similar to that described for the endocrine pancreas and other foregut-derived tissues [40] but differs significantly from the rapid rate of epithelial turnover observed in tissues derived from hindgut endoderm, such as the small intestine [28]. These data suggest fundamental differences in the regulation of progenitor cells between slowly and rapidly replacing tissue [41,42].
Recent work has called into question the existence of stem cells in slowly renewing tissues [43,44]. As indicated above, the lung epithelium is replaced far more slowly than specialized post-mitotic cell types of either the gut or the epidermis, a property that is reflected in the functional characteristics of airway progenitor cells in resting versus proliferative states. Therefore, it is not surprising that progenitor cell hierarchies of the lung and other slowly renewing tissues do not fit the classical stem cell hierarchies described in tissues of rapid turnover [41]. In fact, Hu and colleagues, through use of in vivo injury models, have recently described the existence of a stem cell capable of renewing the endocrine pancreas, a tissue that until recently was thought to be maintained solely through self-duplication of differentiated β cells [45]. This, and previous work in lung to be discussed in more detail below, illustrate a deviation from the classical stem cell hierarchy marked by lack of an obligate transit-amplifying progenitor cell in the steady state. Rather, slowly renewing tissues such as lung are maintained at steady state by an abundant facultative transit-amplifying progenitor that fulfils characteristics of a differentiated cell type in the quiescent state, yet retains proliferative capacity and the ability to generate daughter cells capable of generating other specialized lineages. Therefore, the endogenous stem cell at steady-state likely remains quiescent. However, studies utilizing in vivo injury models have revealed stem cells that can be functionally distinguished from facultative progenitors, based upon their resistance to environmental stimuli and spatial localization in the conducting airway (Figure 2). These studies are highlighted in detail below.
Figure 2.

Progenitor cell-mediated repair. Using in vivo and in vitro injury models, the airway epithelium has demonstrated remarkable reparative capacity. (A) In the tracheobronchial epithelium, polydocanol or sulphur dioxide injury in mice has revealed the existence of a subpopulation of injury-resistant label-retaining basal cells capable of restoring each cell type of the proximal airway epithelium. Retroviral lineage tracing has also been used to hierarchically organize human bronchial epithelial cells according to their clonogenic and differentiation potentials. Progenitor cells of the bronchiolar epithelium include an abundant pool of Clara cells and naphthalene-resistant cells. Both Clara cells and naphthalene-resistant cells express CCSP, yet only Clara cells are ablated following naphthalene exposure. Naphthalene-resistant CCSP-expressing cells, termed variant CCSP-expressing cells (vCE), localize to neuroepithelial bodies (NEBs) within proximal bronchioles and the bronchoalveolar duct junction (BADJ) of terminal bronchioles. Recent data have described cells with similar properties to vCE cells that can be defined based upon their co-expression of CCSP and proSPC. This cell type has been referred to as a bronchioalveolar stem cell (BASC), based upon the ability of fractionated cells to express markers of airway and alveolar lineages in vitro, with an unknown relationship to the vCE cell previously described. (B) Representation of cell fate for progenitor cell types represented in (A). (C) Possible mechanisms by which injury and repair of the airway epithelium might contribute to tissue remodelling in lung disease. In productive epithelial repair, extracellular matrix (ECM) is dynamically and reversibly regulated, with little neutrophilic inflammation (A). The role of extrinsic cues in proper repair is unknown, but may include direct interaction with subtending remodelled ECM or release of paracrine signalling molecules. (C) In contrast, defective airway epithelial repair resulting from persistent depletion of progenitor cells leads to excessive ECM deposition and neutrophilic inflammation. These findings suggest that airway epithelial reparative capacity can regulate ECM deposition and that defects in epithelial repair recapitulate the tissue remodelling observed in chronic lung diseases
Progenitor cells in airway repair: tracheobronchial epithelium
Studies investigating progenitor cells of the tracheobronchial epithelium have benefited from a combination of in vivo, transplantation and in vitro models to reveal contributions made by distinct cell types of the normal epithelium towards maintenance and renewal. In vivo models have used a variety of agents to injure the epithelium, including detergents such as polydocanol [36], toxic gases such as ozone [46], nitrogen dioxide [46,47] or sulphur dioxide [36], parenterally administered toxicants such as naphthalene [48] and mechanical wounding of the epithelium [49]. Using these models, both basal and non-ciliated secretory cells have been shown to exhibit progenitor potential. In this context, the term ‘progenitor cell’ refers to any proliferative cell defined by stable incorporation of [H3]-thymidine into its nuclear DNA. Cell fractionation and retroviral lineage tracing coupled with functional analysis in reconstituted tracheal grafts has allowed detailed analysis of the differentiation potential and clonogenic capacity of these progenitor cells. These approaches have revealed subpopulations of tracheobronchial basal cells that can be distinguished according to their label retention, differentiation potential and clonogenic capacity [36,50,51]. More recently, related experiments using in vivo lineage-tagging approaches have confirmed results from xenograft experiments and indicate that a subpopulation of cytokeratin 14-expressing cells exhibit both multipotent differentiation potential and significant clonogenic capacity [52,53]. Collectively these data reveal a number of progenitor cell types that contribute to maintenance and repair of the tracheobronchial epithelium, some of which exhibit significant clonogenic potential in vitro or in-tracheal grafts suggestive of the existence of local stem cells.
The observation of a less abundant population of infrequently cycling basal-like cells within intercartilagenous regions of the tracheal epithelium and the ducts of submucosal glands provided the first direct evidence suggesting the existence of stem-like cells within tracheobronchial airways. These cells were found to express high levels of cytokeratin 5 and, when isolated, were greatly enriched for in vitro clone-forming cells. Among the clone-forming cells, approximately 5% had the capacity to generate large clones analogous to highly clonogenic cells observed in vivo and in transplantation experiments [54]. These findings reinforce the concept that basal cells are a heterogeneous population that includes abundant transit-amplifying populations in addition to a rare subset of cells with properties of resident tissue stem cells (Figure 2).
Progenitor cells in airway repair: bronchiolar epithelium
Cell type-selective toxicants have been used extensively to reveal progenitor cell types contributing to epithelial repair. Seminal work by Evans and colleagues used oxidant gases allowing selective injury of ciliated cells to demonstrate that the airway epithelium is repaired through a non-ciliated progenitor [46]. They went on to demonstrate that proliferating Clara cells lose ultrastructural features typical of the quiescent state, giving rise to a cell type that was termed the type A Clara cell. Based upon this analysis, type A Clara cells were distinguished from their mature quiescent counterparts based upon lack of secretory granules and smooth endoplasmic reticulum, and their ability to incorporate labelled DNA precursors. Type A cells contribute to repair by actively proliferating and differentiating into mature Clara cells and terminally differentiated ciliated cells. These studies identified Clara cells as an abundant facultative transit-amplifying progenitor cell with the capacity for bipotential differentiation and suggested that the normally slow rate of epithelial renewal in the steady state could be dramatically increased in the setting of airway injury [47]. Clara cells are analogous to transit-amplifying cells of the gut and epidermis, yet have the important distinction of quiescence and other specialized functions in the steady state, including immunoregulation and xenobiotic metabolism.
The experimental demonstration in airways of an epithelial pool of putative tissue stem cells was made through use of injury models in which the abundant facultative progenitor cell, the Clara cell, was specifically ablated. Clara cells represent one of the principal sites in the lung at which endogenous and xenobiotic lipophilic compounds are metabolized through phase I oxidation reactions, such as those catalysed by cytochrome P450 (CyP450) mono-oxygenases. This function of Clara cells renders them particularly susceptible to chemical injury by xenobiotic chemicals that serve as substrates for members of this class of enzymes, and represents the basis for selective ablation of Clara cells in mice following exposure to naphthalene [55,56]. Use of this injury model in mice allowed identification of two putative stem cell niches in bronchioles based upon the resistance to naphthalene-induced injury and distribution of regenerating epithelial foci containing nascent Clara cells (Figure 2). The first was located predominantly at airway branchpoints found in close proximity to neuroepithelial bodies (NEBs) [48]. NEB-associated regenerative foci harboured two proliferative populations of non-ciliated cells, one expressing Clara cell secretory protein (CCSP) and one expressing the pulmonary neuroendocrine cell (PNEC)-specific marker calcitonin gene-related peptide (CGRP) [37,57,58]. A second microenvironment contributing to replacement of depleted Clara cells was located in the terminal bronchiolar epithelium adjacent to the bronchioalveolar duct junction (BADJ) [48]. Chemically resistant proliferative cells located at the BADJ express the secretory cell marker CCSP and, unlike regenerative foci in more proximal airways, lack a restricted association with PNECs [59].
The critical role played by NEB- and BADJ-associated CCSP-expressing cells in repair of Clara cell-depleted airways was demonstrated using a transgenic mouse model allowing conditional ablation of naphthalene-sensitive and -resistant CCSP-expressing cells. In this model, airway repair was completely abrogated, suggesting that CGRP+ PNECs are not the progenitors of the airway epithelium [38]. These studies clearly identified up to three distinct populations of CCSP-expressing cells that are stably maintained within the bronchiole of normal adult mice. Of these populations, rare NEB- and BADJ-associated CCSP-expressing cells share the property of infrequent proliferation relative to their more differentiated Clara cell counterparts [38,59]. Similarities between rare naphthalene-resistant CCSP-expressing cells and tissue stem cells of other organs form the basis for their designation as a putative bronchiolar stem cell that is sometimes referred to as either the variant Clara cell or variant CCSP-expressing cell [38]. Further distinctions were made between bronchiolar stem and Clara cells, based upon the observation of rare CCSP-expressing cells that co-express the alveolar type 2 cell marker surfactant protein C (SP-C), that these cells were localized to the BADJ, and that they were resistant to naphthalene. Cells with this molecular phenotype were observed in preparations of distal airway/alveolar epithelial cells and could be enriched from this crude cell preparation based upon their unique expression of stem cell antigen-1 (Sca-1) and CD34. The observation that in vitro culture of these cells resulted in the expression of aquaporin 5, a gene commonly associated with type 1 alveolar epithleial cells, led to the conclusion that they harboured the capacity for both bronchiolar and alveolar differentiation and formed the basis for their designation as bronchioalveolar stem cells (BASCs) [60].
At the time of this review, no in vivo data directly demonstrate that a BADJ-associated CCSP-expressing population has the capacity for bronchiolar and alveolar differentiation. As such, the similarity between variant Clara cells and BASCs has not been established. Moreover, recent studies investigating the cell surface phenotype of epithelial cells from mouse conducting airways indicate that, although cell surface Sca-1 allows for discrimination of conducting airway and alveolar epithelial cells, it does not distinguish the local stem cell population residing within distal bronchioles from the more abundant population of Clara cells [61].
Molecular regulation of the lung stem cell hierarchy
Investigation into mechanisms regulating the airway stem cell hierarchy have focused predominantly on genetic manipulation of candidate signalling pathways and molecules with established roles as regulators of lung development or carcinogenesis. These include canonical Wnt signalling through stabilization of β-catenin, signalling through either the MAP kinase or Ras pathways, PTEN, GATA-6 and Bmi1. These studies have typically relied upon knock-out, ectopic expression strategies or the use of conditional gene manipulation approaches that led to altered expression of key regulatory molecules. Ectopic activation of β-catenin signalling, K-Ras signalling or developmental loss of either GATA-6, PTEN, PI3 kinase or p38α MAP kinase, have all been found to result in increases in the abundance of CCSP/Pro-SPC dual immunopositive cells that are believed to represent bronchiolar stem cells [60, 62–68].
Changes in the pool size of bronchiolar progenitor cells have been investigated in many of these models through analysis of the cell surface phenotype described by Kim and co-workers, including Sca-1+/CD34+ cells within the CD45−/CD31− viable fraction of dissociated lung cells [60]. However, even though changes in these signalling pathways result in the expansion of putative stem cells based upon the use of these markers, the absence of a comprehensive molecular phenotype for bronchiolar stem cells that distinguishes them from other airway progenitor cell types suggests that the impact of altered signalling on stem cells per se can not easily be determined. In this context, functional changes in epithelial repair capacity involving use of injury models that are known to result in activation of putative stem cells has the potential to provide a more direct means to interrogate changes in progenitor function.
Roles for progenitor cells in carcinogenesis
Cancers in the lung can be pathologically divided into two categories, based upon the initiating cell type, tumour location and histopathology, and are termed ‘small cell lung cancers’ (SCLCs) and ‘non-small cell lung cancers’ (NSCLCs). NSCLCs can be further divided into squamous cell carcinoma (SCC), adenocarcinoma (AC), and large cell carcinoma (LCC) [69]. As reviewed extensively by Giangreco and Janes, the varying tumour types present is a representation of the complex cellular compartmentalization in the lung [70]. Using a similar strategy to that of Bonnet and Dick [71], Eramo and co-workers recently demonstrated that human lung tumours can be fractionated based upon cell surface expression of CD133+, and demonstrate that CD133+ tumour cells can self-renew and propagate tumours in SCID mice, while CD133− cells do not [72]. These data have been used in support of the popular concept that tumours, much like normal tissues, are maintained by rare cancer stem cells and are the only studies currently to adequately demonstrate that a cancer stem cell may exist in lung.
Despite the lack of unequivocal data demonstrating the existence of lung cancer stem cell, a wide body of work exists studying the molecular mechanisms of lung carcinogenesis in bronchiolar progenitor cells. Many of these studies have stemmed from the finding that an important gene frequently mutated in human lung cancer is K-ras [73]. While there is a large body of evidence that establishes the cause–effect relationship between k-ras and lung cancer, very few data exist that suggest a direct link between k-ras mutations and the cancer-initiating cell or the cancer stem cell. Studies by Jacks and colleagues have demonstrated that Cre-induced activation of a K-rasLSL allele increases the abundance of CCSP/Pro-SPC dual positive bronchiolar cells (or BASCs) that are found at the bronchioalveolar duct junction. Activation of K-ras signalling in this manner also led to the development of adenocarcinomas, with the suggestion of a causal relationship between increased numbers of BASCs and the development of lung cancer [60]. This study has subsequently been followed by several that have attempted to understand alternative molecular mechanisms of tumour initiation, the approach being to manipulate signalling pathways to define those that impact the behaviour of BASCs. Specifically, conditional deletion of PTEN, PI3 kinase or p38α MAP kinase results in increased numbers of BASCs and increased propensity to lung tumour development [64,66,68]. In contrast, deletion of Bmi1 inhibits tumourigenesis through inhibition of BASC expansion [67]. Collectively, these studies have been interpreted to suggest a role for bronchiolar stem cells in the initiation of tumourigenesis. However, these studies either fail to identify the initial target cell within which signalling is disrupted, leading to changes in the behaviour of putative stem cells or other epithelial progenitor cell types of the airway. Therefore, although the concept of a cancer stem cell remains an attractive hypothesis, the relationships between tumour-initiating cells, cancer stem cells and endogenous stem or progenitor cells have yet to be clearly determined in the lung.
Roles for progenitor cells in chronic lung disease
Chronic lung diseases (CLD), such as obliterative bronchiolitis (OB), chronic obstructive pulmonary disease (COPD) and asthma, are characterized by remarkable epithelial and mesenchymal remodelling that includes, but is not limited to, chronic injury to the airway epithelium, decreased abundance of Clara cells, goblet cell hyperplasia, mucus cell metaplasia, subepithelial basement membrane thickening, fibrotic nodules causing stenosis of small airways, airway smooth muscle hypertrophy, and uncontrolled lymphocytic, neutrophilic and monocytic inflammation [74–77]. Accordingly, understanding the aetiology and progression of CLD has been complicated, due to the complex network of cellular crosstalk present in the lung. Defective airway epithelial repair has been proposed as an early event in the initiation of CLD, and chronic defects in reparative capacity and cellular composition may further contribute to disease progression and susceptibility to exacerbation [78]. This implies that normal functions for facultative progenitor and stem cells are compromised. Evidence suggesting that this may be the case comes from analysis of biomarkers of epithelial remodelling that have been used to follow the severity and progression of lung disease. Levels of CCSP/CC16 in airway lining fluid or serum are uniformly reduced in the setting of chronic lung disease, as discussed above, suggesting that epithelial remodelling involves altered differentiation or cell death of this critical bronchiolar progenitor. Increasing evidence from studies in humans and animal models suggests that chronic injury and inflammation inhibits normal epithelial repair and differentiation, leading to remodelling of the entire epithelial mesenchymal trophic unit (EMTU) [77].
Chronic exposure to xenobiotic and biological flora has been postulated to play a causative role in the development and exacerbation of chronic lung disease. As an example, cigarette smoke causes extensive airway epithelial injury, a robust inflammatory response in the lung, and is the principal epigenetic factor contributing to the development of chronic obstructive pulmonary disease [79]. In vitro studies have demonstrated that cigarette smoke can severely blunt epithelial wound repair by decreasing the rate of cell proliferation and migration and altering extracellular matrix remodelling [80]. This observation has been extended to in vivo models in which chronic cigarette smoke exposure in mice was shown to attenuate epithelial repair following naphthalene-induced airway injury [81]. Epithelial cell injury and persistent inflammation that are both associated with cigarette smoking have also been demonstrated to blunt tissue repair in models of epidermal wound healing [82]. These studies indicate that proper epithelial repair can be severely attenuated by environmental challenge.
Mechanisms contributing to tissue remodelling involve changes in the abundance and activity of key matrix remodelling enzymes, leading to excessive depletion of elastin and other structural matrix components critical for maintenance of tissue architecture. Paradoxically, tissue remodelling also includes fibroproliferative responses that lead to inappropriate deposition of matrix molecules. Currently, little is known regarding the cellular crosstalk between injured and repairing epithelium and the underlying mesenchyme. It has been postulated that the EMTU signalling network is essential for proper epithelial repair and that in the context of defective repair this may lead to aberrant and chronic mesenchymal remodelling that includes extensive fibroproliferation, extracellular matrix (EMC) deposition and development of fibrosis. Recent work has identified that ECM production is transiently up-regulated in an in vivo model of productive airway epithelial repair. Specifically, the ECM protein Tenascin C (Tnc) is deposited in the subepithethial mesenchyme, reorganized to the basement membrane subtending regenerating and injured epithelium as repair initiates, and degraded to restore steady-state levels at the return of tissue homeostasis. In contrast, this process is severely deregulated in an in vivo model of defective airway epithelial repair. Irreversible ablation of CCSP-expressing cells using a transgenic suicide gene resulted in chronic ECM deposition subtending the airway epithelium. Surprisingly, defective airway epithelial repair also resulted in overproduction of ECM in the alveolar compartment [83]. This study further supports work demonstrating that defective airway epithelial repair can also lead to secondary effects in the alveolar compartment, including type 2 cell injury [84]. Collectively, these data support an EMTU model in which epithelial repair results in mesenchymal remodelling. This may play a direct role in regulating epithelial proliferation, differentiation and/or migration. After repair is completed, the underlying mesenchyme returns to the steady state. However, in the context of defective epithelial repair or persistent injury, as identified in obliterative bronchiolitis, COPD and cystic fibrosis, the mesenchyme is chronically remodelled, resulting in uncontrolled fibroproliferation, ECM deposition, fibrosis and impaired gas transport/exchange in the airway and alveolar epithelium, respectively (Figure 2C). Further studies are warranted to elucidate the molecular mechanisms regulating this process.
Future directions
Maintenance of the airway epithelium through the action of multiple interdependent regenerative zones suggests that a more thorough understanding of progenitor cell types and their behaviour in normal and diseased states may yield important insights into disease pathophysiology. Examples of basic biological questions that have potential to shed light on mechanisms of normal maintenance, repair and aberrant tissue remodelling include:
Basic studies to define the behaviour of airway progenitor cells in normal and diseased states. Epithelial maintenance is dependent upon a balance between turnover and renewal. If the rate of epithelial turnover is held constant, maintenance is purely a function of progenitor cell self-renewal versus differentiation. There is currently no information in the existing literature that describes the probability with which different progenitor cell types are maintained through self-renewal, or whether this property is impacted by divergence from the steady state, such as might be the case with acute or chronic lung disease. The existence of multiple progenitor cell types that can be hierarchically organized, such as those of the bronchiolar epithelium, increase the complexity of this question. It is possible, for example, that bronchiolar stem cells are maintained through a highly regulated process involving extrinsic signals from the stem cell niche to ensure long-term maintenance of a stem cell pool of a defined size. The likelihood that stem cells are activated to participate in epithelial maintenance would inevitably be related to longevity of the transit-amplifying progenitor cell pool. For example, if TA cell maintenance occurs through a stochastic mechanism, as proposed by Jones and co-workers [32,33], the probability of which is influenced by extrinsic factors such as injury and/or inflammation, the contribution of stem cells towards epithelial maintenance will be strongly influenced by disease. Understanding the basic behaviour of progenitor cells and how this is impacted by disease would provide novel insights into disease pathogenesis and reveal new therapeutic targets that may allow modification of disease outcome.
Improved molecular definition of progenitor cell types and their response to injury and repair. A key component of future studies will be to interrogate the molecular profile of stem cells. Previous studies have identified a broader repertoire of molecular markers for identification and analysis of bronchiolar progenitor cells [85]. Furthermore, bronchioalveolar stem cells (BASCs) were recently isolated and sorted, based upon their Sca-1+CD45−CD31−CD34+ immunophenotype [60]. However, recent cytometric analysis in combination with mouse models suggests these markers do not distinguish putative stem cells from the abundant pool of Clara cells (Teisanu et al, submitted). Previous attempts to isolate airway epithelial stem cells also included enrichment based upon the ability to efflux the DNA dye Hoechst 33 342 dye [86], but recent data indicate that this does not result in significant enrichment [87]. Collectively, these data demonstrate that the use of stem cell properties from other tissues to fractionate airway epithelial stem cells is not a valid approach. Rather, new markers, preferably those that identify a novel cell surface phenotype, will need to be identified, using a combination of techniques including cellular fractionation and microarray analysis.
Development of in vitro culture models to investigate the behaviour and regulation of stem cells. A common functional assessment of stem cell character is determination of self-renewal and differentiation capacity in vitro. Isolated BASCs have been cultured and shown to demonstrate both of these qualities in vitro [60]. However, caveats associated with this assay result preclude appropriate assessment of stem cell behaviour. A more appropriate endpoint would be the coupling of in vitro methods for propagation of progenitor cell types with development of transplantation assays, analogous to those used for functional testing of cells of the haematopoietic lineage to assess the behaviour of these cells in vivo [88,89].
Comparative stem/progenitor cell biology between mammalian species. A major shortfall of current studies is the lack of correlative human data. In order to develop successful cell and molecular therapeutics that translates into the clinic, stem cells in human airway epithelium need to be identified. Comparative studies aimed at revealing similarities and differences in epithelial maintenance between species will provide valuable information relating to the predictive ability of animal models of human disease.
The existence of endogenous lung epithelial stem cells remains a controversial issue. To facilitate a more thorough understanding of progenitor cell behaviour and the putative stem cell hierarchy will require development of novel endpoints to answer the fundamental questions outlined above. These studies will bring the scientific community one step closer to the development of cellular and molecular strategies for the therapeutic treatment of lung disease.
Supplementary Material
Acknowledgments
Supported by NIH Grant Nos ES008964, HL064888 and HL090146.
Footnotes
No conflicts of interest were declared.
Teaching materials: Power Point slides of the figures from this Review may be found in the supporting information.
References
- 1.Boers JE, Ambergen AW, Thunnissen FB. Number and proliferation of basal and parabasal cells in normal human airway epithelium. Am J Respir Crit Care Med. 1998;157:2000–2006. doi: 10.1164/ajrccm.157.6.9707011. [DOI] [PubMed] [Google Scholar]
- 2.Boers JE, Ambergen AW, Thunnissen FB. Number and proliferation of Clara cells in normal human airway epithelium. Am J Respir Crit Care Med. 1999;159:1585–1591. doi: 10.1164/ajrccm.159.5.9806044. [DOI] [PubMed] [Google Scholar]
- 3.Pack RJ, Al-Ugaily LH, Morris G. The cells of the tracheobronchial epithelium of the mouse: a quantitative light and electron microscope study. J Anat. 1981;132:71–84. [PMC free article] [PubMed] [Google Scholar]
- 4.Cardoso WV, Lu J. Regulation of early lung morphogenesis: questions, facts and controversies. Development. 2006;133:1611–1624. doi: 10.1242/dev.02310. [DOI] [PubMed] [Google Scholar]
- 5.Metzger RJ, Klein OD, Martin GR, Krasnow MA. The branching programme of mouse lung development. Nature. 2008;453:745–750. doi: 10.1038/nature07005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Knowles MR, Boucher RC. Mucus clearance as a primary innate defense mechanism for mammalian airways. J Clin Invest. 2002;109:571–577. doi: 10.1172/JCI15217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cole AM, Dewan P, Ganz T. Innate antimicrobial activity of nasal secretions. Infect Immun. 1999;67:3267–3275. doi: 10.1128/iai.67.7.3267-3275.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bernard A, Marchandise FX, Depelchin S, Lauwerys R, Sibille Y. Clara cell protein in serum and bronchoalveolar lavage. Eur Respir J. 1992;5:1231–1238. [PubMed] [Google Scholar]
- 9.Bernard AM, Gonzalez-Lorenzo JM, Siles E, Trujillano G, Lauwerys R. Early decrease of serum Clara cell protein in silica-exposed workers. Eur Respir J. 1994;7:1932–1937. [PubMed] [Google Scholar]
- 10.Robin M, Dong P, Hermans C, Bernard A, Bersten AD, Doyle IR. Serum levels of CC16, SP-A and SP-B reflect tobacco-smoke exposure in asymptomatic subjects. Eur Respir J. 2002;20:1152–1161. doi: 10.1183/09031936.02.02042001. [DOI] [PubMed] [Google Scholar]
- 11.Mattsson J, Remberger M, Andersson O, Sundberg B, Nord M. Decreased serum levels of clara cell secretory protein (CC16) are associated with bronchiolitis obliterans and may permit early diagnosis in patients after allogeneic stem-cell transplantation. Transplantation. 2005;79:1411–1416. doi: 10.1097/01.tp.0000158354.39635.ab. [DOI] [PubMed] [Google Scholar]
- 12.Pilette C, Godding V, Kiss R, Delos M, Verbeken E, Decaestecker C, et al. Reduced epithelial expression of secretory component in small airways correlates with airflow obstruction in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;163:185–194. doi: 10.1164/ajrccm.163.1.9912137. [DOI] [PubMed] [Google Scholar]
- 13.Stripp BR, Lund J, Mango GW, Doyen KC, Johnston C, Hultenby K, et al. Clara cell secretory protein: a determinant of PCB bioaccumulation in mammals. Am J Physiol. 1996;271:L656–664. doi: 10.1152/ajplung.1996.271.4.L656. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Z, Kundu GC, Yuan CJ, Ward JM, Lee EJ, DeMayo F, et al. Severe fibronectin-deposit renal glomerular disease in mice lacking uteroglobin. Science. 1997;276:1408–1412. doi: 10.1126/science.276.5317.1408. [DOI] [PubMed] [Google Scholar]
- 15.Johnston CJ, Mango GW, Finkelstein JN, Stripp BR. Altered pulmonary response to hyperoxia in Clara cell secretory protein deficient mice. Am J Respir Cell Mol Biol. 1997;17:147–155. doi: 10.1165/ajrcmb.17.2.2676. [DOI] [PubMed] [Google Scholar]
- 16.Harrod KS, Mounday AD, Stripp BR, Whitsett JA. Clara cell secretory protein decreases lung inflammation after acute virus infection. Am J Physiol. 1998;275:L924–930. doi: 10.1152/ajplung.1998.275.5.L924. [DOI] [PubMed] [Google Scholar]
- 17.Mango GW, Johnston CJ, Reynolds SD, Finkelstein JN, Plopper CG, Stripp BR. Clara cell secretory protein deficiency increases oxidant stress response in conducting airways. Am J Physiol. 1998;275:L348–356. doi: 10.1152/ajplung.1998.275.2.L348. [DOI] [PubMed] [Google Scholar]
- 18.Johnston CJ, Finkelstein JN, Oberdorster G, Reynolds SD, Stripp BR. Clara cell secretory protein-deficient mice differ from wild-type mice in inflammatory chemokine expression to oxygen and ozone, but not to endotoxin. Exp Lung Res. 1999;25:7–21. doi: 10.1080/019021499270394. [DOI] [PubMed] [Google Scholar]
- 19.Watson TM, Reynolds SD, Mango GW, Boe IM, Lund J, Stripp BR. Altered lung gene expression in CCSP-null mice suggests immunoregulatory roles for Clara cells. Am J Physiol Lung Cell Mol Physiol. 2001;281:L1523–1530. doi: 10.1152/ajplung.2001.281.6.L1523. [DOI] [PubMed] [Google Scholar]
- 20.Hayashida S, Harrod KS, Whitsett JA. Regulation and function of CCSP during pulmonary Pseudomonas aeruginosa infection in vivo. Am J Physiol Lung Cell Mol Physiol. 2000;279:L452–459. doi: 10.1152/ajplung.2000.279.3.L452. [DOI] [PubMed] [Google Scholar]
- 21.Wang SZ, Rosenberger CL, Bao YX, Stark JM, Harrod KS. Clara cell secretory protein modulates lung inflammatory and immune responses to respiratory syncytial virus infection. J Immunol. 2003;171:1051–1060. doi: 10.4049/jimmunol.171.2.1051. [DOI] [PubMed] [Google Scholar]
- 22.Wang SZ, Rosenberger CL, Espindola TM, Barrett EG, Tesfaigzi Y, Bice DE, et al. CCSP modulates airway dysfunction and host responses in an Ova-challenged mouse model. Am J Physiol Lung Cell Mol Physiol. 2001;281:L1303–1311. doi: 10.1152/ajplung.2001.281.5.L1303. [DOI] [PubMed] [Google Scholar]
- 23.Cheng DS, Han W, Chen SM, Sherrill TP, Chont M, Park GY, et al. Airway epithelium controls lung inflammation and injury through the NF-κB pathway. J Immunol. 2007;178:6504–6513. doi: 10.4049/jimmunol.178.10.6504. [DOI] [PubMed] [Google Scholar]
- 24.Sadikot RT, Zeng H, Joo M, Everhart MB, Sherrill TP, Li B, et al. Targeted immunomodulation of the NF-κB pathway in airway epithelium impacts host defense against Pseudomonas aeruginosa. J Immunol. 2006;176:4923–4930. doi: 10.4049/jimmunol.176.8.4923. [DOI] [PubMed] [Google Scholar]
- 25.Elizur A, Adair-Kirk TL, Kelley DG, Griffin GL, de Mello DE, Senior RM. Clara cells impact the pulmonary innate immune response to LPS. Am J Physiol Lung Cell Mol Physiol. 2007;293:L383–392. doi: 10.1152/ajplung.00024.2007. [DOI] [PubMed] [Google Scholar]
- 26.Elizur A, Adair-Kirk TL, Kelley DG, Griffin GL, Demello DE, Senior RM. Tumor necrosis factor-α from macrophages enhances LPS-induced clara cell expression of keratinocyte-derived chemokine. Am J Respir Cell Mol Biol. 2008;38:8–15. doi: 10.1165/rcmb.2007-0203OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reynolds SD, Reynolds PR, Snyder JC, Whyte F, Paavola KJ, Stripp BR. CCSP regulates cross talk between secretory cells and both ciliated cells and macrophages of the conducting airway. Am J Physiol Lung Cell Mol Physiol. 2007;293:L114–123. doi: 10.1152/ajplung.00014.2007. [DOI] [PubMed] [Google Scholar]
- 28.Barker N, van de Wetering M, Clevers H. The intestinal stem cell. Genes Dev. 2008;22:1856–1864. doi: 10.1101/gad.1674008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sangiorgi E, Capecchi MR. Bmi1 is expressed in vivo in intestinal stem cells. Nat Genet. 2008;40:915–920. doi: 10.1038/ng.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 31.Radtke F, Clevers H. Self-renewal and cancer of the gut: two sides of a coin. Science. 2005;307:1904–1909. doi: 10.1126/science.1104815. [DOI] [PubMed] [Google Scholar]
- 32.Clayton E, Doupe DP, Klein AM, Winton DJ, Simons BD, Jones PH. A single type of progenitor cell maintains normal epidermis. Nature. 2007;446:185–189. doi: 10.1038/nature05574. [DOI] [PubMed] [Google Scholar]
- 33.Jones P, Simons BD. Epidermal homeostasis: do committed progenitors work while stem cells sleep? Nat Rev Mol Cell Biol. 2008;9:82–88. doi: 10.1038/nrm2292. [DOI] [PubMed] [Google Scholar]
- 34.Tumbar T, Guasch G, Greco V, Blanpain C, Lowry WE, Rendl M, et al. Defining the epithelial stem cell niche in skin. Science. 2004;303:359–363. doi: 10.1126/science.1092436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Horsley V, Aliprantis AO, Polak L, Glimcher LH, Fuchs E. NFATc1 balances quiescence and proliferation of skin stem cells. Cell. 2008;132:299–310. doi: 10.1016/j.cell.2007.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Borthwick DW, Shahbazian M, Krantz QT, Dorin JR, Randell SH. Evidence for stem-cell niches in the tracheal epithelium. Am J Respir Cell Mol Biol. 2001;24:662–670. doi: 10.1165/ajrcmb.24.6.4217. [DOI] [PubMed] [Google Scholar]
- 37.Reynolds SD, Giangreco A, Power JH, Stripp BR. Neuroepithelial bodies of pulmonary airways serve as a reservoir of progenitor cells capable of epithelial regeneration. Am J Pathol. 2000;156:269–278. doi: 10.1016/S0002-9440(10)64727-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hong KU, Reynolds SD, Giangreco A, Hurley CM, Stripp BR. Clara cell secretory protein-expressing cells of the airway neuroepithelial body microenvironment include a label-retaining subset and are critical for epithelial renewal after progenitor cell depletion. Am J Respir Cell Mol Biol. 2001;24:671–681. doi: 10.1165/ajrcmb.24.6.4498. [DOI] [PubMed] [Google Scholar]
- 39.Rawlins EL, Hogan BL. Ciliated epithelial cell lifespan in the mouse trachea and lung. Am J Physiol Lung Cell Mol Physiol. 2008;295:L231–234. doi: 10.1152/ajplung.90209.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Teta M, Long SY, Wartschow LM, Rankin MM, Kushner JA. Very slow turnover of β-cells in aged adult mice. Diabetes. 2005;54:2557–2567. doi: 10.2337/diabetes.54.9.2557. [DOI] [PubMed] [Google Scholar]
- 41.Stripp BR. Hierarchical organization of lung progenitor cells: is there an adult lung tissue stem cell? Proc Am Thorac Soc. 2008;5:695–698. doi: 10.1513/pats.200801-011AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stripp BR, Reynolds SD. Maintenance and repair of the bronchiolar epithelium. Proc Am Thorac Soc. 2008;5:328–333. doi: 10.1513/pats.200711-167DR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dor Y, Melton DA. How important are adult stem cells for tissue maintenance? Cell Cycle. 2004;3:1104–1106. [PubMed] [Google Scholar]
- 44.Teta M, Rankin MM, Long SY, Stein GM, Kushner JA. Growth and regeneration of adult β cells does not involve specialized progenitors. Dev Cell. 2007;12:817–826. doi: 10.1016/j.devcel.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 45.Xu X, D'Hoker J, Stange G, Bonne S, De Leu N, Xiao X, et al. β cells can be generated from endogenous progenitors in injured adult mouse pancreas. Cell. 2008;132:197–207. doi: 10.1016/j.cell.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 46.Evans MJ, Johnson LV, Stephens RJ, Freeman G. Renewal of the terminal bronchiolar epithelium in the rat following exposure to NO2 or O3. Lab Invest. 1976;35:246–257. [PubMed] [Google Scholar]
- 47.Evans MJ, Cabral-Anderson LJ, Freeman G. Role of the Clara cell in renewal of the bronchiolar epithelium. Lab Invest. 1978;38:648–653. [PubMed] [Google Scholar]
- 48.Stripp BR, Maxson K, Mera R, Singh G. Plasticity of airway cell proliferation and gene expression after acute naphthalene injury. Am J Physiol. 1995;269:L791–799. doi: 10.1152/ajplung.1995.269.6.L791. [DOI] [PubMed] [Google Scholar]
- 49.Shimizu T, Nishihara M, Kawaguchi S, Sakakura Y. Expression of phenotypic markers during regeneration of rat tracheal epithelium following mechanical injury. Am J Respir Cell Mol Biol. 1994;11:85–94. doi: 10.1165/ajrcmb.11.1.7517145. [DOI] [PubMed] [Google Scholar]
- 50.Engelhardt JF, Schlossberg H, Yankaskas JR, Dudus L. Progenitor cells of the adult human airway involved in submucosal gland development. Development. 1995;121:2031–2046. doi: 10.1242/dev.121.7.2031. [DOI] [PubMed] [Google Scholar]
- 51.Borthwick DW, West JD, Keighren MA, Flockhart JH, Innes BA, Dorin JR. Murine submucosal glands are clonally derived and show a cystic fibrosis gene-dependent distribution pattern. Am J Respir Cell Mol Biol. 1999;20:1181–1189. doi: 10.1165/ajrcmb.20.6.3475. [DOI] [PubMed] [Google Scholar]
- 52.Hong KU, Reynolds SD, Watkins S, Fuchs E, Stripp BR. Basal cells are a multipotent progenitor capable of renewing the bronchial epithelium. Am J Pathol. 2004;164:577–588. doi: 10.1016/S0002-9440(10)63147-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hong KU, Reynolds SD, Watkins S, Fuchs E, Stripp BR. In vivo differentiation potential of tracheal basal cells: evidence for multipotent and unipotent subpopulations. Am J Physiol Lung Cell Mol Physiol. 2004;286:L643–649. doi: 10.1152/ajplung.00155.2003. [DOI] [PubMed] [Google Scholar]
- 54.Schoch KG, Lori A, Burns KA, Eldred T, Olsen JC, Randell SH. A subset of mouse tracheal epithelial basal cells generates large colonies in vitro. Am J Physiol Lung Cell Mol Physiol. 2004;286:L631–642. doi: 10.1152/ajplung.00112.2003. [DOI] [PubMed] [Google Scholar]
- 55.Mahvi D, Bank H, Harley R. Morphology of a naphthalene-induced bronchiolar lesion. Am J Pathol. 1977;86:558–572. [PMC free article] [PubMed] [Google Scholar]
- 56.Buckpitt A, Buonarati M, Avey LB, Chang AM, Morin D, Plopper CG. Relationship of cytochrome P450 activity to Clara cell cytotoxicity. II. Comparison of stereoselectivity of naphthalene epoxidation in lung and nasal mucosa of mouse, hamster, rat and rhesus monkey. J Pharmacol Exp Ther. 1992;261:364–372. [PubMed] [Google Scholar]
- 57.Stevens TP, McBride JT, Peake JL, Pinkerton KE, Stripp BR. Cell proliferation contributes to PNEC hyperplasia after acute airway injury. Am J Physiol. 1997;272:L486–493. doi: 10.1152/ajplung.1997.272.3.L486. [DOI] [PubMed] [Google Scholar]
- 58.Reynolds SD, Hong KU, Giangreco A, Mango GW, Guron C, Morimoto Y, et al. Conditional clara cell ablation reveals a self-renewing progenitor function of pulmonary neuroendocrine cells. Am J Physiol Lung Cell Mol Physiol. 2000;278:L1256–1263. doi: 10.1152/ajplung.2000.278.6.L1256. [DOI] [PubMed] [Google Scholar]
- 59.Giangreco A, Reynolds SD, Stripp BR. Terminal bronchioles harbor a unique airway stem cell population that localizes to the bronchoalveolar duct junction. Am J Pathol. 2002;161:173–182. doi: 10.1016/S0002-9440(10)64169-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kim CF, Jackson EL, Woolfenden AE, Lawrence S, Babar I, Vogel S, et al. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell. 2005;121:823–835. doi: 10.1016/j.cell.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 61.Teisanu RM, Lagasse E, Whitesides J, Stripp BR. Prospective isolation of bronchiolar stem cells based upon immunophenotypic and autofluorescence characteristics. Stem Cells. 2008 doi: 10.1634/stemcells.2008-0838. accepted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Reynolds SD, Zemke AC, Giangreco A, Brockway BL, Teisanu RM, Drake JA, et al. Conditional stabilization of beta-catenin expands the pool of lung stem cells. Stem Cells. 2008;26:1337–1346. doi: 10.1634/stemcells.2008-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang Y, Goss AM, Cohen ED, Kadzik R, Lepore JJ, Muthukumaraswamy K, et al. A Gata6–Wnt pathway required for epithelial stem cell development and airway regeneration. Nat Genet. 2008;40:862–870. doi: 10.1038/ng.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ventura JJ, Tenbaum S, Perdiguero E, Huth M, Guerra C, Barbacid M, et al. p38α MAP kinase is essential in lung stem and progenitor cell proliferation and differentiation. Nat Genet. 2007;39:750–758. doi: 10.1038/ng2037. [DOI] [PubMed] [Google Scholar]
- 65.Jackson EL, Willis N, Mercer K, Bronson RT, Crowley D, Montoya R, et al. Analysis of lung tumor initiation and progression using conditional expression of oncogenic K-ras. Genes Dev. 2001;15:3243–3248. doi: 10.1101/gad.943001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yanagi S, Kishimoto H, Kawahara K, Sasaki T, Sasaki M, Nishio M, et al. Pten controls lung morphogenesis, bronchioalveolar stem cells, and onset of lung adenocarcinomas in mice. J Clin Invest. 2007;117:2929–2940. doi: 10.1172/JCI31854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dovey JS, Zacharek SJ, Kim CF, Lees JA. Bmi1 is critical for lung tumorigenesis and bronchioalveolar stem cell expansion. Proc Natl Acad Sci USA. 2008;105:11857–11862. doi: 10.1073/pnas.0803574105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yang Y, Iwanaga K, Raso MG, Wislez M, Hanna AE, Wieder ED, et al. Phosphatidylinositol 3-kinase mediates bronchioalveolar stem cell expansion in mouse models of oncogenic K-ras-induced lung cancer. PLoS ONE. 2008;3:e2220. doi: 10.1371/journal.pone.0002220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Petersen I, Petersen S. Towards a genetic-based classification of human lung cancer. Anal Cell Pathol. 2001;22:111–121. doi: 10.1155/2001/374304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Giangreco A, Groot KR, Janes SM. Lung cancer and lung stem cells: strange bedfellows? Am J Respir Crit Care Med. 2007;175:547–553. doi: 10.1164/rccm.200607-984PP. [DOI] [PubMed] [Google Scholar]
- 71.Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730–737. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- 72.Eramo A, Lotti F, Sette G, Pilozzi E, Biffoni M, Di Virgilio A, et al. Identification and expansion of the tumorigenic lung cancer stem cell population. Cell Death Differ. 2008;15:504–514. doi: 10.1038/sj.cdd.4402283. [DOI] [PubMed] [Google Scholar]
- 73.Johnson L, Mercer K, Greenbaum D, Bronson RT, Crowley D, Tuveson DA, et al. Somatic activation of the K-ras oncogene causes early onset lung cancer in mice. Nature. 2001;410:1111–1116. doi: 10.1038/35074129. [DOI] [PubMed] [Google Scholar]
- 74.Sumi Y, Hamid Q. Airway remodeling in asthma. Allergol Int. 2007;56:341–348. doi: 10.2332/allergolint.R-07-153. [DOI] [PubMed] [Google Scholar]
- 75.Epler GR. Bronchiolitis obliterans organizing pneumonia: definition and clinical features. Chest. 1992;102:S 2–6. doi: 10.1378/chest.102.1_supplement.2s. [DOI] [PubMed] [Google Scholar]
- 76.Hogg JC. Pathophysiology of airflow limitation in chronic obstructive pulmonary disease. Lancet. 2004;364:709–721. doi: 10.1016/S0140-6736(04)16900-6. [DOI] [PubMed] [Google Scholar]
- 77.Boxall C, Holgate ST, Davies DE. The contribution of transforming growth factor-β and epidermal growth factor signalling to airway remodelling in chronic asthma. Eur Respir J. 2006;27:208–229. doi: 10.1183/09031936.06.00130004. [DOI] [PubMed] [Google Scholar]
- 78.Holgate ST, Holloway J, Wilson S, Bucchieri F, Puddicombe S, Davies DE. Epithelial–mesenchymal communication in the pathogenesis of chronic asthma. Proc Am Thorac Soc. 2004;1:93–98. doi: 10.1513/pats.2306034. [DOI] [PubMed] [Google Scholar]
- 79.Chung KF, Adcock IM. Multifaceted mechanisms in COPD: inflammation, immunity, and tissue repair and destruction. Eur Respir J. 2008;31:1334–1356. doi: 10.1183/09031936.00018908. [DOI] [PubMed] [Google Scholar]
- 80.Wang H, Liu X, Umino T, Skold CM, Zhu Y, Kohyama T, et al. Cigarette smoke inhibits human bronchial epithelial cell repair processes. Am J Respir Cell Mol Biol. 2001;25:772–779. doi: 10.1165/ajrcmb.25.6.4458. [DOI] [PubMed] [Google Scholar]
- 81.Van Winkle LS, Evans MJ, Brown CD, Willits NH, Pinkerton KE, Plopper CG. Prior exposure to aged and diluted sidestream cigarette smoke impairs bronchiolar injury and repair. Toxicol Sci. 2001;60:152–164. doi: 10.1093/toxsci/60.1.152. [DOI] [PubMed] [Google Scholar]
- 82.Dovi JV, He LK, DiPietro LA. Accelerated wound closure in neutrophil-depleted mice. J Leukoc Biol. 2003;73:448–455. doi: 10.1189/jlb.0802406. [DOI] [PubMed] [Google Scholar]
- 83.Snyder JC, Zemke AC, Stripp BR. Reparative capacity of airway epithelium impacts deposition and remodeling of extracellular matrix. Am J Respir Cell Mol Biol. 2008 Oct 31; doi: 10.1165/rcmb.2008-0334OC. E-pub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Reynolds SD, Giangreco A, Hong KU, McGrath KE, Ortiz LA, Stripp BR. Airway injury in lung disease pathophysiology: selective depletion of airway stem and progenitor cell pools potentiates lung inflammation and alveolar dysfunction. Am J Physiol Lung Cell Mol Physiol. 2004;287:L1256–1265. doi: 10.1152/ajplung.00203.2004. [DOI] [PubMed] [Google Scholar]
- 85.Zemke AC, Snyder JC, Brockway BL, Drake JA, Reynolds SD, Kaminski N, et al. Molecular staging of epithlieial maturation using secretory cell-specific genes as markers. Am J Respir Cell Mol Biol. 2008 doi: 10.1165/rcmb.2007-0380OC. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Giangreco A, Shen H, Reynolds SD, Stripp BR. Molecular phenotype of airway side population cells. Am J Physiol Lung Cell Mol Physiol. 2004;286:L624–630. doi: 10.1152/ajplung.00149.2003. [DOI] [PubMed] [Google Scholar]
- 87.Reynolds SD, Shen H, Reynolds P, Betsuyaku T, Pilewski J, Gambelli F, et al. Molecular and functional properties of lung side population cells. Am J Physiol Lung Cell Mol Physiol. 2007;292:L972–983. doi: 10.1152/ajplung.00090.2006. [DOI] [PubMed] [Google Scholar]
- 88.Larochelle A, Vormoor J, Hanenberg H, Wang JC, Bhatia M, Lapidot T, et al. Identification of primitive human hematopoietic cells capable of repopulating NOD/SCID mouse bone marrow: implications for gene therapy. Nat Med. 1996;2:1329–1337. doi: 10.1038/nm1296-1329. [DOI] [PubMed] [Google Scholar]
- 89.Bhatia M, Wang JC, Kapp U, Bonnet D, Dick JE. Purification of primitive human hematopoietic cells capable of repopulating immune-deficient mice. Proc Natl Acad Sci USA. 1997;94:5320–5325. doi: 10.1073/pnas.94.10.5320. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


