Abstract
Background
In spite of advances in the field of lung transplantation, the median survival following lung transplant remains below 5 years. Early rejection is a risk factor for the development of chronic rejection. In animal models of transplant tolerance, regulatory T cells (Tregs) can prevent the establishment of rejection.
Methods
This study was designed to explore the dynamics of Tregs focally and systemically in lung transplant recipients. Sequential surveillance bronchoscopy results were available in 51 patients with at least 4 sequential samples recovered from each patient at defined times post transplant. In 36 individuals, an entire year of BAL was analyzed. In 33 of these individuals an entire year of PBMC specimens were also analyzed. Lung lavage cells were recovered from each bronchoscopy and corresponding blood draw and subjected to polychromatic flow cytometry. The percentage of CD4 lymphocytes which expressed the intracellular transcription factor foxP3 was recorded at each point. At each time point lung biopsy specimens were scored for rejection.
Results
Lung Treg frequency was significantly more variable then blood Treg frequency. Treg frequency in the lung was increased in the aftermath of acute rejection. In contrast, lung Treg frequency declined sequentially in patients demonstrating continued quiescence. Mean BAL Treg level integrated over the first transplant year correlated inversely with the degree of acute cellular rejection. In contrast, blood Treg levels demonstrated no correlation with lung pathology.
Conclusions
Lung Tregs increase in the setting of acute cellular rejection, while declining levels of BAL Tregs correlates with immunologic quiescence.
Keywords: Lung Transplantation, Regulatory T Cells, FoxP3
Introduction
Lung transplant represents the only significant treatment option for many individuals with end-stage lung disease. In spite of refinements in this life saving procedure the survival following lung transplant remains low, with fewer than 50% of patients surviving 5 years. Nevertheless, significant heterogeneity exists with regard to survival with 20% of bilateral lung transplant recipients surviving greater than 10 years(1). Understanding the immunologic basis underlying differences in post transplant survival and freedom from acute rejection represents one of the best avenues for improving the outcomes for all patients.
One of the primary drivers of mortality in lung transplant is chronic rejection, or bronchiolitis obliterans. Repeated lung injury triggered by alloimmunity, infection, reperfusion injury, or gastroesophageal reflux is thought to predispose individuals to develop chronic rejection (2–5). Histologically acute lung injury is often manifest by perivenular and peribronchiolar lymphocytic infiltrates and this histology is termed acute rejection. The mechanisms which influence the risk and degree of repetitive lung injury vs. immunologic quiescence within the lung need to be identified. There are several mechanisms which may play a role in immunologic quiescence in the setting of continued immunosuppression. These include the induction of anergy of potentially alloreactive T cells, deletion of alloreactive T cells via induction therapy, exhaustion of alloreactive T cells, and suppression of T cells by a population of regulatory T cells termed Tregs.
It is clear from animal studies that a minority population of CD4+ cells which express high levels the alpha chain of the IL-2 receptor (CD25) and the intracellular transcription factor foxP3 can provide such suppression and thereby allow for maintenance of the allograft(6). While these cells are active in tolerance induction protocols in animals, their importance in human transplantation is still uncertain. In a small minority of liver and kidney transplant patients who develop spontaneous tolerance it is known that T cell regulation by a subset of CD4 cells is essential to maintaining immunologic quiescence (7) (8). In the present study we quantified the percentage of BAL CD4+ cells which express foxP3 in lung transplant patients. We defined this population of cells as Tregs. We followed this frequency sequentially in the first year post transplant and correlated our findings with the presence or absence of rejection. In parallel with the BAL analysis, we quantified simultaneously the frequency of Tregs in the peripheral circulation.
Methods
Patients
Informed consent was obtained from all patients recruited into this study, under the oversight of our Institutional Review Board. In our institution patients are followed with periodic surveillance bronchoscopy which includes broncho-alveolar lavage and transbronchial biopsy. Patients undergo bronchoscopy at approximately 2 weeks, 4 weeks, 2, 3, 6, 9 and 12 months post-transplant. All patients receive standardized immunosuppression consisting of Tacrolimus, Azathioprine, and Prednisone. We analyzed sequential data on patients who underwent lung transplantation between April 2006 and January 2008, and for whom we had at least 4 sequential surveillance bronchoscopy results. To assess the early correlation of BAL Tregs and rejection we compared the results of biopsies 1–4 in patients with and without rejection. These biopsy and bronchoscopy samples were obtained at similar time points in each patient, and the first 4 bronchoscopy specimens were obtained within the first 6 months after transplant. In this way, the effect of time from transplant on lung immunologic milieu was minimized. Paired t tests were used to assess for differences between Treg frequencies within the same patient. Unpaired t tests were used to assess differences between patients. For longitudinal assessment of Treg frequency and rejection we analyzed patients with an entire year of post transplant follow-up. Correlation between cumulative rejection score and Tregs was calculated by computing the Pearson correlation coefficient. All statistical analysis was performed using GraphPad Prism software (San Diego, CA). To minimize the effect of missing data in the longitudinal analysis we included only patients who had at least 5 BAL specimens and at least 4 pbmc specimens in the first transplant year.
Clinical assessment of rejection
At the time of bronchoscopy 6–10 pieces of alveolated pulmonary parenchyma were biopsied from multiple lobes. Biopsy specimens were immediately fixed in formalin, sectioned and subjected to H&E staining followed by interpretation by a dedicated transplant pulmonary pathologist who was blinded to the flow cytometry data. All biopsies were graded by ISHLT criteria for acute rejection. We considered any A or B score above zero to be indicative of acute cellular rejection.
Recovery of BAL and peripheral blood mononuclear cells
During bronchoscopy, alveolar mononuclear cells were recovered by wedging the bronchoscope in the lingula or right middle lobe. Serial 30 mL aliquots of saline were instilled and aspirated for a total lavage volume of 150–180 mL. In general the return was greater than 100 mL. BAL was immediately centrifuged. Red blood cells (RBC) were removed from the pellet by incubation in High Yield Lysis Buffer for 5 minutes. Cells were washed twice in PBS, and reconstituted in labeling buffer (PBS + 2% BSA). Peripheral blood mononuclear cells were collected by venipunture into 8mL heparin CPT tubes (BD). Cells were recovered according the manufactures protocol. The cellular pellet was lysed of RBC as above and reconstituted in labeling buffer.
Cell Labeling and Flow Cytometery
BAL cells were surfaced stained with monoclonal antibodies to human CD3, CD4, CD8, CD25, and CD127 obtained from BD Pharmingen. Cells were labeled for 30 minutes at 4° C. Following labeling, cells were fixed in Fix/Perm solution (EBioscience). Cells were then labeled with intracellular FoxP3 mAb (EBioscience) overnight at 4° C. Cells were then subjected to flow cytometry using LSRII flow cytometer (BD). All data analysis was performed using FloJo software (Treestar).
Results
Treg BAL frequency declines in the setting of early prolonged quiescence and increases in the aftermath of early rejection
In our center, patients undergo protocol bronchoscopy with BAL at defined time points in the first post transplant year. For our initial analysis, we analyzed the BAL from the first four surveillance bronchoscopies which are performed during the first 6 months after transplantation. A total of 51 patients were analyzed at these early time points. Demographics and incidence of rejection are shown in table 1. FoxP3+ Tregs from BAL came uniformly from the population of CD3+CD4+ cells. These cells were also CD127lo, and generally CD25intermediate to high, although there was considerable overlap between Tregs and FoxP3− CD4+ cells in terms of CD25 expression. Because both Tregs and recently activated naive non-tregs express the Il-2 receptor, we did not consider CD25 to be a reliable marker for Tregs. There was significant variability in the quantity of BAL fluid recovered and in the total number of T cells recovered. A typical BAL yielded between 1–10 million mononuclear cells, of which 1–10% were lymphocytes. We did not detect a significant correlation between absolute lymphocyte counts, percentage of lymphocytes, or BAL recovery and rejection score. Because of the variability in cell recovery, our analysis focused on the frequency of BAL CD4 cells which were positive for foxP3. This Treg ratio allowed us to make comparisons between samples in which there were significant differences in the absolute number of T cells recovered.
Table 1.
| Patient (age, gender) | Diagnosis | Number of Sequential BAL specimens Analyzed First Post Transplant Year | Number of Biopsies with Rejection | Cumulative Rejection Score | Number of PBMC analyzed first post transplant year | Included in 1 year of BAL analysis | Included in 1 year of PBMC analysis |
|---|---|---|---|---|---|---|---|
| 1. 48 M | Emphysema | 9 | 4 | 9 | 5 | Yes | Yes |
| 2. 59 F | Emphysema | 9 | 4 | 6 | 4 | Yes | No |
| 3. 57 M | Hypersensitivity Pneumonitis | 7 | 4 | 9 | 5 | Yes | Yes |
| 4. 38 M | Cystic Fibrosis | 7 | 2 | 4 | 4 | Yes | Yes |
| 5. 56 M | Idiopathic Pulmonary Fibrosis (IPF) | 7 | 4 | 8 | 6 | Yes | Yes |
| 6. 61 M | Emphysema | 5 | 1 | 1 | 3 | Yes | No |
| 7. 66 F | Emphysema | 7 | 0 | 0 | 6 | Yes | Yes |
| 8. 18 M | Cystic Fibrosis | 4 | 4 | 12 | 2 | No | No |
| 9. 22 M | Cystic Fibrosis | 8 | 6 | 11 | 7 | Yes | Yes |
| 10. 54 M | IPF | 8 | 5 | 8 | 6 | Yes | Yes |
| 11. 60 M | Emphysema | 9 | 1 | 1 | 9 | Yes | Yes |
| 12. 31 F | Cystic Fibrosis | 8 | 0 | 0 | 5 | Yes | Yes |
| 13. 18 F | Cystic Fibrosis | 5 | 2 | 4 | 5 | Yes | Yes |
| 14. 30F | Cystic Fibrosis | 6 | 2 | 4 | 5 | Yes | Yes |
| 15. 62M | IPF | 7 | 1 | 3 | 6 | Yes | Yes |
| 16. 40F | IPF | 7 | 3 | 4 | 6 | Yes | Yes |
| 17. 59M | IPF | 4 | 1 | 1 | 4 | No | No |
| 18. 63 M | IPF | 8 | 1 | 1 | 7 | Yes | Yes |
| 19. 60 M | IPF | 6 | 1 | 1 | 6 | Yes | Yes |
| 20. 63 F | IPF | 5 | 1 | 2 | 5 | Yes | Yes |
| 21. 64 M | IPF | 7 | 4 | 7 | 6 | Yes | Yes |
| 22. 60 F | Emphysema | 8 | 2 | 2 | 8 | Yes | Yes |
| 23. 16 M | Bronchiolitis Obliterans | 8 | 1 | 2 | 7 | Yes | Yes |
| 24. 51 F | IPF | 6 | 0 | 0 | 6 | Yes | Yes |
| 25. 48 M | IPF | 6 | 3 | 6 | 6 | Yes | Yes |
| 26. 60 M | Emphysema | 7 | 2 | 5 | 6 | Yes | Yes |
| 27. 61 M | Emphysema | 9 | 4 | 10 | 7 | Yes | Yes |
| 28. 62 M | Emphysema | 9 | 2 | 3 | 9 | Yes | Yes |
| 29. 56 M | Emphysema | 8 | 1 | 1 | 7 | Yes | Yes |
| 30. 46 M | Alpha-1 antitrypsin deficiency | 8 | 3 | 5 | 8 | Yes | Yes |
| 31. 52F | Emphysema | 7 | 2 | 2 | 6 | Yes | Yes |
| 32. 53F | Emphysema | 7 | 1 | 1 | 7 | Yes | Yes |
| 33. 51F | Sarcoidosis | 7 | 2 | 2 | 6 | Yes | Yes |
| 34. 63M | Emphysema | 7 | 0 | 0 | 5 | Yes | Yes |
| 35.54M | IPF | 7 | 1 | 2 | 6 | Yes | Yes |
| 36. 50F | Emphysema | 7 | 2 | 5 | 3 | Yes | No |
| 37. 60F | IPF | 7 | 0 | 0 | 7 | Yes | Yes |
| 38. 60M | IPF | 6 | 1 | 1 | 6 | Yes | Yes |
| 39. 56F | Emphysema | 4 | 0 | 0 | 4 | No | No |
| 40. 58F | IPF | 5 | 0 | 0 | 4 | No | No |
| 41. 59M | Emphysema | 4 | 0 | 0 | 3 | No | No |
| 42. 29F | Hypersensitivity Pneumonitis | 7 | 1 | 2 | 7 | No | No |
| 43. 66M | Emphysema | 6 | 2 | 4 | 6 | No | No |
| 44. 60M | IPF | 8 | 4 | 4 | 8 | No | No |
| 45. 44F | Emphysema | 4 | 0 | 0 | 4 | No | No |
| 46. 57M | AIP | 4 | 0 | 0 | 4 | No | No |
| 47. 61M | IPF | 4 | 2 | 4 | 3 | No | No |
| 48. 61F | Emphysema | 4 | 2 | 3 | 4 | No | No |
| 49. 48M | Emphysema | 4 | 0 | 0 | 4 | No | No |
| 50. 61M | IPF | 4 | 1 | 2 | 4 | No | No |
| 51. 17M | Cystic Fibrosis | 6 | 1 | 1 | 6 | No | No |
Demographics and clinical course of patients analyzed: The first 2 columns show patient age, gender and transplant indication. Also shown are the total number of BAL and PBMC samples analyzed, the total number of any degree of rejection in the first post transplant year and the cumulative rejection score. The final two columns indicate which patients had a year worth of sequential data to be included in the longitudinal analysis (figure 5).
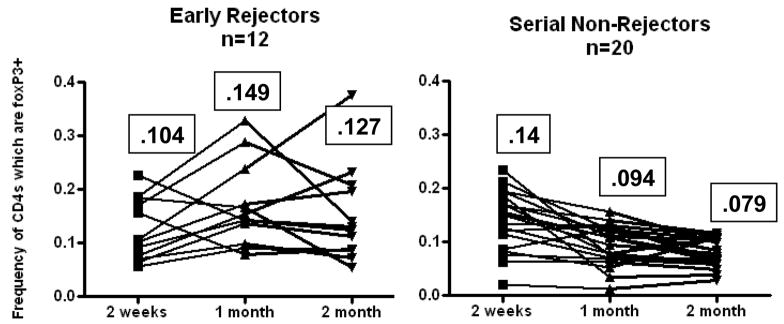
As an initial analysis we compared patients in whom rejection was never observed to patients in who rejection was seen at the first biopsy. Of the 51 patients analyzed there were 14 patients in whom histologic rejection was never seen in the first 4 surveillance biopsies. There were an additional 6 patients in whom histologic rejection was not observed in the early post-transplant period (initial 3 surveillance biopsies). In these 20 patients we observed a decrease in BAL Treg frequency from 14% of total CD4s (range 2–19%) to a frequency of 7.9% of total CD4s (range 1–13%) on subsequent biopsy. In contrast there were 12 patients who demonstrated at least some evidence of histologic rejection on their initial surveillance biopsy. In some of these patients the rejection persisted despite treatment, while in other patients there was eventual quiescence. As shown in figure 1, when analyzed as a group early rejectors had an initial mean Treg BAL frequency of 10.4% (range 7–23%) which increased to 14.9% on subsequent biopsy (range 8–29%). We observed a statistically significant difference in declining BAL Tregs between the 1st and 2nd and 1st and 3rd bronchoscopy in patients who were serial non-rejectors (p <.005). In the 12 patients with early rejection there was near statistical significance with p=.059 between the 1st and 2nd biopsy by 2-tailed paired t-test.
Fig 1. BAL Tregs increase after early rejection and decrease in the setting of back-to-back quiescence.
12 patients demonstrated acute cellular rejection on the first biopsy (left panel). In subsequent biopsies there was a trend toward increased BAL tregs (p=.0586 between 2 weeks and 1 month). 20 patients demonstrated no rejection on the first 3 surveillance biopsies. There was a significant drop in BAL Tregs over that period p<.001 between 2 weeks and 1 month. The mean frequency of each time point is indicated in the boxes.
BAL Tregs increase in the aftermath of untreated rejection
Because the increase in BAL Tregs seen in the aftermath of acute cellular rejection could represent either a direct effect on immune cells from an alloresponse, or a secondary response to changes in baseline immune suppression made in response to an episode of rejection, we analyzed episodes of minimal rejection that did not trigger a change in baseline immunosuppression. In our center, acute cellular rejection is usually treated with a 3 day pulse of high dose corticosteroids. We generally do not change baseline immunosuppression in the face of the first episode of minimal cellular rejection (A1 or B1). We identified 22 cases in our cohort of 51 patients in which minimal cellular rejection did not trigger a change in baseline immunosuppression. As shown in figure 2, in 16 of 22 cases there was an increase in the BAL Tregs in the aftermath of untreated rejection with an average increase of 4.8% (p<.005 by paired 2-tailed t-test) in these 22 cases.
Fig 2. BAL Tregs increase in the aftermath of untreated rejection.
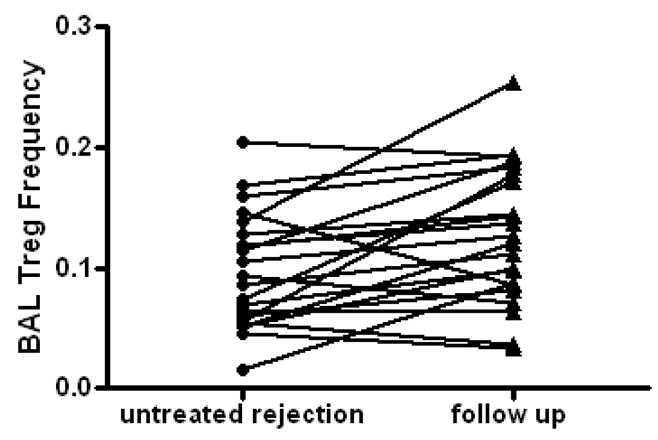
There were 22 instances of rejection which was not treated among the 52 individuals studied. In 16 of these episodes there was a subsequent increase in BAL Tregs recovered on the next bronchoscopy. Overall there was a 3.4% increase in BAL Treg frequency in the aftermath of untreated rejection (p<.005 by paired t-test).
Treg BAL frequency at a single time point does not predict rejection or quiescence in lung transplant patients
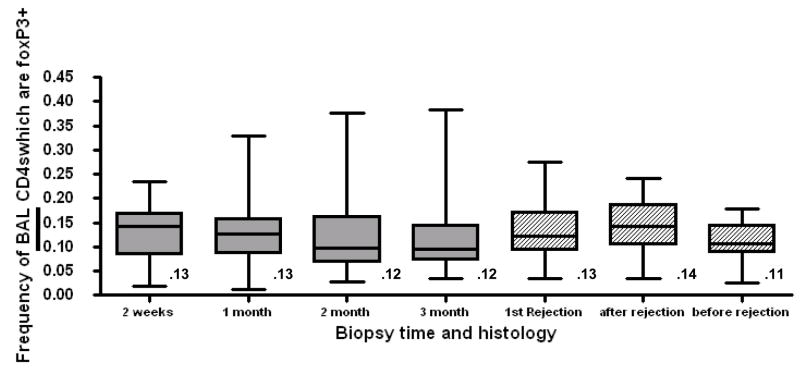
When the population of 51 patients was compared as a group, there were no significant differences in mean and median BAL Treg frequency among the first four surveillance bronchoscopies (Fig 3). When we compared the average BAL Treg frequency in the aftermath of the first episode of rejection to the average BAL Treg frequency to all prior quiescent biopsies there was a statistically significant increase in BAL frequency in the aftermath of rejection (p<.005), but the magnitude of this difference was quite small (14% vs. 11%), and with considerable overlap in the confidence intervals.
Fig 3. Tregs are consistently present in BAL but do not vary significantly with the time post transplant.
Grey boxes show the frequency of BAL Tregs from the first 4 sequential biospies performed at 2 weeks, 1 month, 2 month, and 3month post transplant. Numbers below the box represent the mean Treg frequency. The solid line within the box represents the median frequency. The number above each box indicates mean frequency. P=ns between all time points by 2-tailed t-test. Hatched boxes show Treg frequency in the clinical conditions of the first episode of rejection, the follow-up after rejection and before rejection. P=.0016 between before and after rejection by 2-tailed t-test.
PBMC Treg frequency does not correlate with acute rejection in the first three months post transplant
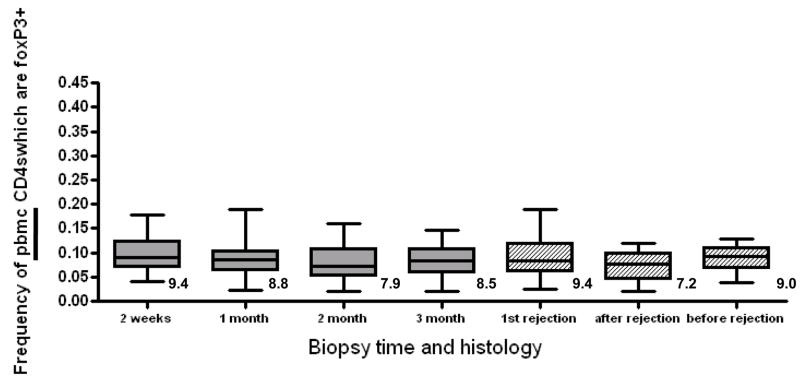
We compared the frequency of pbmc Tregs in our patient cohort, similar to the analysis performed with BAL. For the first four sampling times at 2 weeks, 1 month, 2 months and 3 months we found no significant difference between pbmc Treg frequencies when the cohort was compared as a group (figure 4). There were slightly fewer Tregs in the CD4 pool in pbmc compared with BAL. In contrast to the findings in BAL, we observed no significant difference between quiescent, rejecting and aftermath pbmc Treg frequencies (figure 4).
Fig 4. PBMC Treg frequency does not vary over time or by clinical condition.
Grey boxes show the frequency of pbmc Tregs isolated at the time of the first 4 sequential biospies post transplant. Numbers below the box represent the mean Treg frequency. The solid line within the box represents the median frequency. The number above each box indicates mean frequency. P=ns between all time points by 2-tailed t-test. Hatched boxes show Treg frequency in the clinical conditions of the first episode of rejection, the follow-up after rejection and before rejection. P= 0.20 between before and after rejection by 2-tailed t-test.
BAL Treg frequency but not PBMC Treg frequency correlates with rejection in a longitudinal analysis
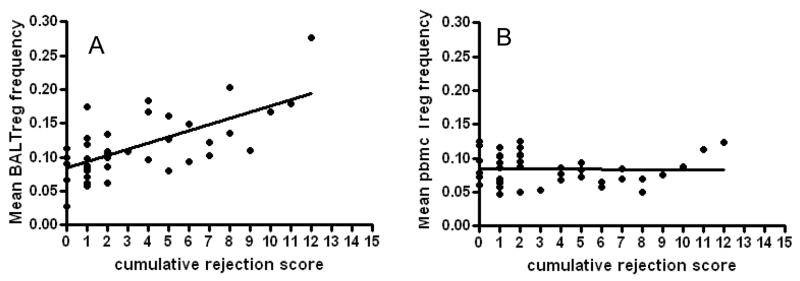
Given the finding that BAL Tregs are increased in the setting of early rejection, and decline in the face of back-to-back quiescence in the first three months post-transplant we sought to determine the relationship between BAL Tregs, pbmc Tregs and overall degree of rejection. Of our cohort of 51 patients, 36 patients had a complete year of follow-up data, and had at least 5 BAL specimens for analysis. 33 of the patients from our cohort had at least 4 pbmc specimens recovered over a year of follow up. We calculated the cumulative rejection score in our patient cohort by adding the total A score and B score from all bronchoscopic biopsies. As shown in figure 5a, there was a high correlation between mean BAL frequency averaged over 1 year and cumulative rejection score. In contrast, PBMC Treg frequency demonstrated no correlation to cumulative rejection score (figure 5b).
Fig 5. BAL but not pbmc Treg frequency correlates with rejection score in a longitudinal assessment.
For all patients with a year of follow-up and at least 5 BAL specimens and at least 4 pbmc specimens the mean Treg frequency was calculated. The cumulative rejection score was calculated as the total A-score and B-score for all transbronchial biopsies performed in the first year. There was a strong correlation between mean BAL treg frequency and rejection score (R2= .44, p<.001). There was no correlation between mean pbmc Treg frequency and rejection score (R2=.0005, p=0.8).
Discussion
The concept of immune regulation has gained considerable enthusiasm in recent years. In animal models of transplantation, Tregs which express FoxP3 can mitigate against rejection and even induce tolerance (9–12). Further, interventions which promote the conversion of non-Tregs into FoxP3+ expression CD4 cells can also promote tolerance (13, 14). In human transplantation, the function of Tregs has been more elusive. Some investigators have shown that a populations of CD4+ cells, both foxP3+ and foxP3− are essential for meta-stable tolerance in the small minority of renal transplant recipients who are able to cease use of immunosuppressive therapy (7). In a more representative renal transplant population of patients using typical immunosuppression, the quantity of urinary foxP3 mRNA correlated with increased risk of acute rejection (15). It is important to note that in patients with histologic rejection, high levels of urinary mRNA did correlate with a good likelihood of subsequent histologic improvement, while patients with rejection and low levels of urinary FoxP3 mRNA had the highest risk for relapsed rejection. Recent data quantifying Treg frequency in histology sections from renal transplant recipients shows that foxP3+ CD4 cells are increased in patients with acute cellular rejection (16). Notably, renal graft survival was decreased in patients having the highest degree of foxP3+ cells by histology.
In clinical lung transplantation, very little is known about the role of regulatory cells on subsequent risk for rejection. Early studies have focused on the frequency of peripheral blood CD4+ cells which contain foxP3+ regulatory cells. For example, high levels of circulating CD4+CD25hi cells correlated with improved outcomes in lung transplant recipients (17). Significant declines in the frequency of foxP3+ Tregs in the peripheral blood has been associated with increasing risk for acute rejection (18). Here we report the most comprehensive analysis to date on the dynamics of Tregs in the BAL fluid and PBMC of lung transplant patients. Because of the protocolized nature of clinical lung transplantation, we are able to capture at relatively defined time points cells from the BAL fluid and subject them to polychromatic flow cytometry. By analyzing simultaneous specimens from the peripheral blood of the same cell population we are able to quantify the frequency of these cells relative to the potential total pool of all circulating Tregs.
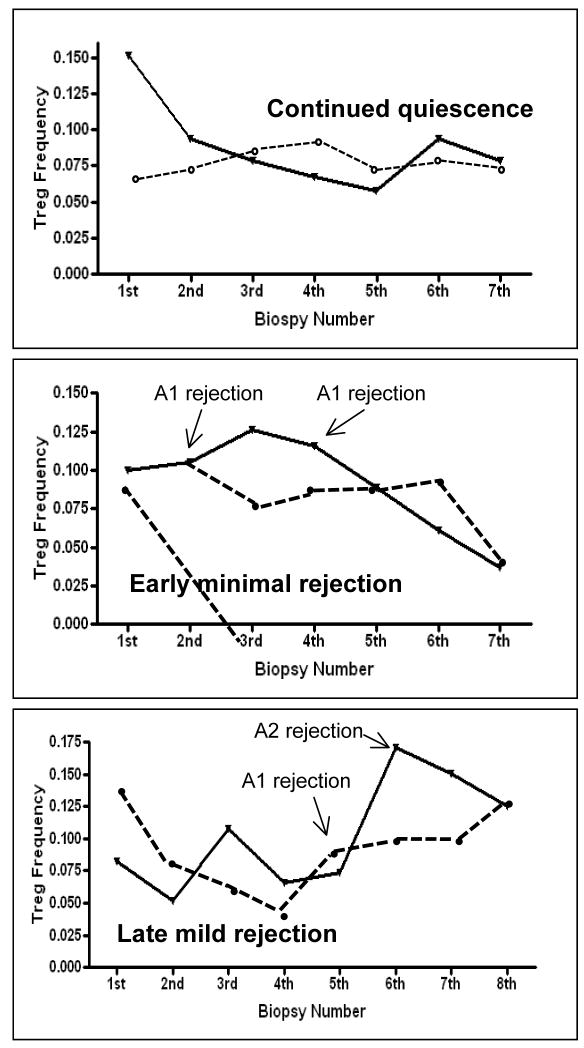
Overall we find that BAL Tregs are highly dynamic and reflective of the intragraft environment. BAL Tregs appear increased in the aftermath of rejection, even in the absence of augmented immune suppression, suggesting that these cells represent an adaptive response to rejection, rather than primary mediators of tolerance. In patients with prolonged continued quiescence there was a significant decline in BAL Treg frequency over time. When integrated over the first transplant year, BAL Tregs were significantly lower in patients with less acute rejection compared to patients with significant composite rejection scores. Hence it is conceivable that serial declining BAL Tregs could represent a biomarker of immunologic quiescence. In contrast to the BAL data, PBMC Tregs are largely consistent over time regardless of clinical situation. The clinical utility of our finding remain uncertain, as different patterns of BAL Treg frequency emerge only when viewed retrospectively. Figure 6 shows the BAL and pbmc trend for several patients with a year of longitudinal data. From these examples it is clear that multiple longitudinal biopsies need to be collected before discernable patterns can be seen.
Fig 6. Longitudinal patterns of BAL and pbmc Tregs in individual patients.
Solid line Indicate BAL Treg frequency and dassed line indicates pbmc Treg frequency. Top panel Shows pattern in individual with no rejection in the first post transplant year. Middle panel Shows pattern in a patient with 2 episodes of early minimal rejection, followed by a Period of prolong quiescence. Bottom panel shows pattern in patient with back-to-back Rejection starting at 6 months post transplant.
There are at least three mechanisms to explain increasing Treg frequencies in patients with more significant rejection: movement of Tregs from the circulation into the transplanted allograft, expansion of Tregs already present in the lung, and conversion of non-Tregs into Tregs –so called induced Tregs (iTregs). One well characterized inducer of iTregs is TGFβ. TGFβ in the proper context can induce the conversion of non-Tregs into iTregs, a process that involves phosphorylation of the intracellular signaling molecule smad3 in lymphocytes. Activation of smad3 signaling appears to be an essential step in formation of iTregs(19). At the same time, TGFβ appears to have a role in airway fibrosis, a hallmark of chronic rejection. In murine lung fibroblasts, TGFβ drives myofibroblast differentiation, an event which leads to deposition of collagen into the extracellular matrix. This effect of TGFβ is also dependent on smad3 signaling (20, 21). This means that lung injury such as rejection may be associated with increases in regulatory cells, which dampen the immune response, but in parallel with increases in similar pathways which activate fibroblasts. Hence one possible scenario present in human lung and renal transplantation is that elevated levels of foxP3+ Tregs are also heralding the presence of signals which lead to organ fibrosis. In our own experience, patients with severe bronchiolitis obliterans demonstrate significantly increased tissue expression of TGFβ compared with patients with stable lung function (Neujahr and Kirk, unpublished observations). Hence it will be vital in future work to assess the correlation of elevated BAL Tregs and subsequent development of BOS.
One of the major limitations of a study of this nature is that it employs phenotypic assessment of cells to infer immunologic mechanisms. It would be virtually impossible to assess suppressive function of recovered BAL T cells with presently available methods in a study of this size. Further it is important to note that we have only characterized the relationship between one particular type of CD4 cells, the foxP3+ CD127lo cell. It is conceivable that one or more populations of foxP3− cells participate in immune regulation. Hence our findings do not refute the possibility that some form of immunologic regulation underlies long term immunologic quiescence in lung transplantation.
Moving forward, the clinical utility of serial immune monitoring in lung transplant patients will likely require simultaneous measurement of several biomarkers. Hence our finding of a relationship between BAL tregs and rejection will need to be combined with serial measurement of other cells to arrive at a robust immunologic “signature” which allows for individualization of care. This so called signature is likely to require assessment of NK cells, neutrophils, macrophages and dendritic cells, cells which can modulate the transformation of non-tregs to treg (22–25), as well as a more comprehensive assessment of T cell phenotypes including armed effector and exhausted T cells.
Abbreviations
- BAL
Bronchoalveolar Lavage
- IPF
Idiopathic Pulmonary Fibrosis
- ISHLT
International Society of Heart and Lung Transplantation
- PBMC
Peripheral Blood Mononuclear Cells
- PBS
Phosphate Buffered Saline
- RBC
Red Blood Cells
- TGFβ
Transforming Growth Factor β
- Tregs
Regulatory T Cells
Footnotes
Funding: This work was supported by the McKelvey Center for Lung Transplantation and Pulmonary Vascular Disease. There are no other commercial associations to disclose
References
- 1.Hertz MI, Aurora P, Boucek MM, et al. Registry of the International Society for Heart and Lung Transplantation: introduction to the 2007 annual reports--100,000 transplants and going strong. J Heart Lung Transplant. 2007;26 (8):763. doi: 10.1016/j.healun.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 2.Lau CL, Palmer SM, Posther KE, et al. Influence of panel-reactive antibodies on posttransplant outcomes in lung transplant recipients. Ann Thorac Surg. 2000;69 (5):1520. doi: 10.1016/s0003-4975(00)01224-8. [DOI] [PubMed] [Google Scholar]
- 3.Khalifah AP, Hachem RR, Chakinala MM, et al. Respiratory viral infections are a distinct risk for bronchiolitis obliterans syndrome and death. Am J Respir Crit Care Med. 2004;170 (2):181. doi: 10.1164/rccm.200310-1359OC. [DOI] [PubMed] [Google Scholar]
- 4.Billings JL, Hertz MI, Savik K, Wendt CH. Respiratory viruses and chronic rejection in lung transplant recipients. J Heart Lung Transplant. 2002;21 (5):559. doi: 10.1016/s1053-2498(01)00405-3. [DOI] [PubMed] [Google Scholar]
- 5.D’Ovidio F, Mura M, Tsang M, et al. Bile acid aspiration and the development of bronchiolitis obliterans after lung transplantation. J Thorac Cardiovasc Surg. 2005;129 (5):1144. doi: 10.1016/j.jtcvs.2004.10.035. [DOI] [PubMed] [Google Scholar]
- 6.Wood KJ, Sakaguchi S. Regulatory T cells in transplantation tolerance. Nat Rev Immunol. 2003;3 (3):199. doi: 10.1038/nri1027. [DOI] [PubMed] [Google Scholar]
- 7.Xu Q, Lee J, Jankowska-Gan E, et al. Human CD4+CD25low adaptive T regulatory cells suppress delayed-type hypersensitivity during transplant tolerance. J Immunol. 2007;178 (6):3983. doi: 10.4049/jimmunol.178.6.3983. [DOI] [PubMed] [Google Scholar]
- 8.Koshiba T, Ito A, Li Y, Wu Y, Takemura M, Sakaguchi S. [Regulatory T cell based transplant tolerance--freedom from immunosuppression] Nippon Rinsho. 2007;65 (3):557. [PubMed] [Google Scholar]
- 9.Neujahr DC, Chen C, Huang X, et al. Accelerated memory cell homeostasis during T cell depletion and approaches to overcome it. J Immunol. 2006;176 (8):4632. doi: 10.4049/jimmunol.176.8.4632. [DOI] [PubMed] [Google Scholar]
- 10.Sanchez-Fueyo A, Sandner S, Habicht A, et al. Specificity of CD4+CD25+ regulatory T cell function in alloimmunity. J Immunol. 2006;176 (1):329. doi: 10.4049/jimmunol.176.1.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kingsley CI, Karim M, Bushell AR, Wood KJ. CD25+CD4+ regulatory T cells prevent graft rejection: CTLA-4- and IL-10-dependent immunoregulation of alloresponses. J Immunol. 2002;168 (3):1080. doi: 10.4049/jimmunol.168.3.1080. [DOI] [PubMed] [Google Scholar]
- 12.Zheng XX, Sanchez-Fueyo A, Sho M, Domenig C, Sayegh MH, Strom TB. Favorably tipping the balance between cytopathic and regulatory T cells to create transplantation tolerance. Immunity. 2003;19 (4):503. doi: 10.1016/s1074-7613(03)00259-0. [DOI] [PubMed] [Google Scholar]
- 13.Chen TC, Cobbold SP, Fairchild PJ, Waldmann H. Generation of anergic and regulatory T cells following prolonged exposure to a harmless antigen. J Immunol. 2004;172 (10):5900. doi: 10.4049/jimmunol.172.10.5900. [DOI] [PubMed] [Google Scholar]
- 14.Fu S, Zhang N, Yopp AC, et al. TGF-beta induces Foxp3 + T-regulatory cells from CD4 + CD25 - precursors. Am J Transplant. 2004;4 (10):1614. doi: 10.1111/j.1600-6143.2004.00566.x. [DOI] [PubMed] [Google Scholar]
- 15.Muthukumar T, Dadhania D, Ding R, et al. Messenger RNA for FOXP3 in the urine of renal-allograft recipients. N Engl J Med. 2005;353 (22):2342. doi: 10.1056/NEJMoa051907. [DOI] [PubMed] [Google Scholar]
- 16.Veronese F, Rotman S, Smith RN, et al. Pathological and clinical correlates of FOXP3+ cells in renal allografts during acute rejection. Am J Transplant. 2007;7 (4):914. doi: 10.1111/j.1600-6143.2006.01704.x. [DOI] [PubMed] [Google Scholar]
- 17.Meloni F, Vitulo P, Bianco AM, et al. Regulatory CD4+CD25+ T cells in the peripheral blood of lung transplant recipients: correlation with transplant outcome. Transplantation. 2004;77 (5):762. doi: 10.1097/01.tp.0000116565.86752.6b. [DOI] [PubMed] [Google Scholar]
- 18.Higuchi T, Mifsud NA, Williams TJ, Snell GI, Kotsimbos TC. Abstract 400: Decreasing regulatory T-cell dynamics early post lung transplant is associated with histopathological acute rejection. International Society for Heart and Lung Transplantation, 27th annual meeting; 2007. [Google Scholar]
- 19.Tone Y, Furuuchi K, Kojima Y, Tykocinski ML, Greene MI, Tone M. Smad3 and NFAT cooperate to induce Foxp3 expression through its enhancer. Nat Immunol. 2008;9 (2):194. doi: 10.1038/ni1549. [DOI] [PubMed] [Google Scholar]
- 20.Ramirez AM, Takagawa S, Sekosan M, Jaffe HA, Varga J, Roman J. Smad3 deficiency ameliorates experimental obliterative bronchiolitis in a heterotopic tracheal transplantation model. Am J Pathol. 2004;165 (4):1223. doi: 10.1016/S0002-9440(10)63382-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ramirez AM, Shen Z, Ritzenthaler JD, Roman J. Myofibroblast transdifferentiation in obliterative bronchiolitis: tgf-beta signaling through smad3-dependent and -independent pathways. Am J Transplant. 2006;6 (9):2080. doi: 10.1111/j.1600-6143.2006.01430.x. [DOI] [PubMed] [Google Scholar]
- 22.Brillard E, Pallandre JR, Chalmers D, et al. Natural killer cells prevent CD28-mediated Foxp3 transcription in CD4+CD25- T lymphocytes. Exp Hematol. 2007;35 (3):416. doi: 10.1016/j.exphem.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 23.Dominitzki S, Fantini MC, Neufert C, et al. Cutting edge: trans-signaling via the soluble IL-6R abrogates the induction of FoxP3 in naive CD4+CD25 T cells. J Immunol. 2007;179 (4):2041. doi: 10.4049/jimmunol.179.4.2041. [DOI] [PubMed] [Google Scholar]
- 24.Fildes JE, Yonan N, Tunstall K, et al. Natural killer cells in peripheral blood and lung tissue are associated with chronic rejection after lung transplantation. J Heart Lung Transplant. 2008;27 (2):203. doi: 10.1016/j.healun.2007.11.571. [DOI] [PubMed] [Google Scholar]
- 25.Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science. 2003;299 (5609):1033. doi: 10.1126/science.1078231. [DOI] [PubMed] [Google Scholar]