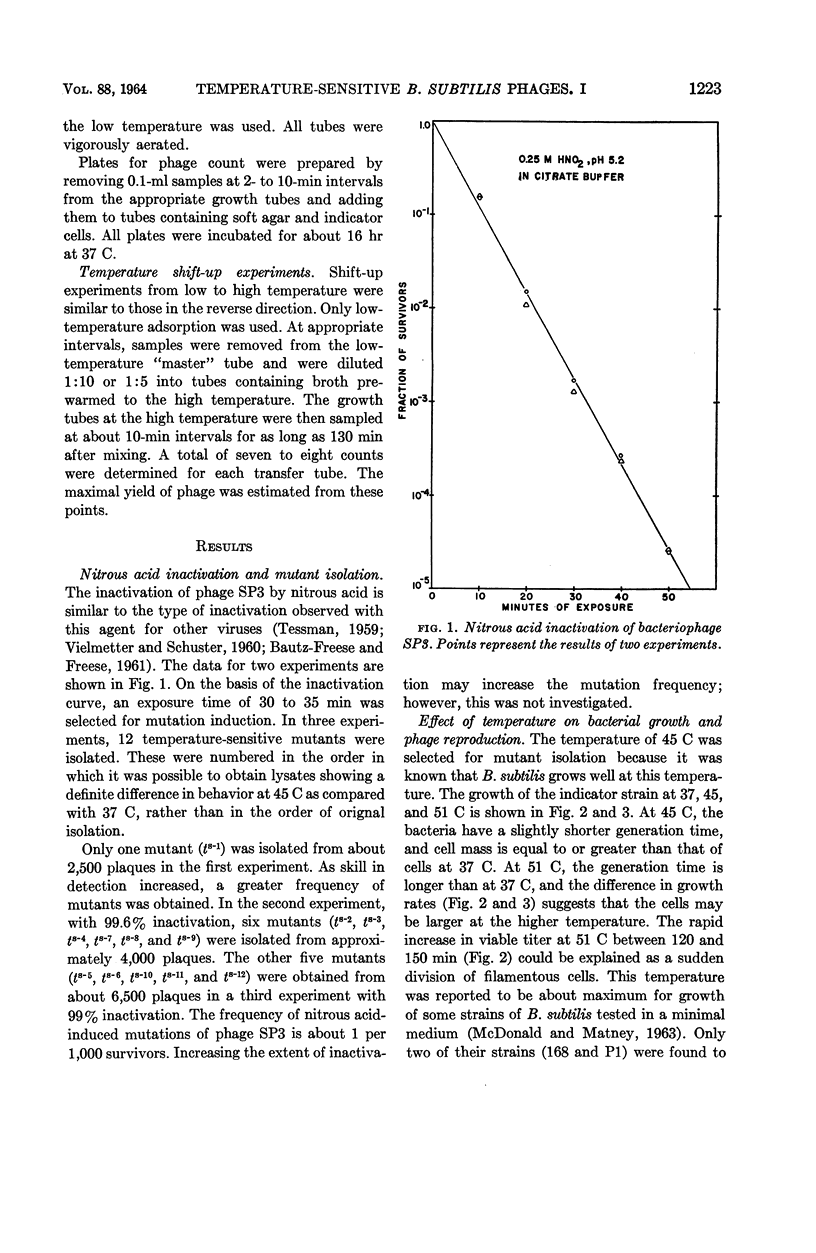
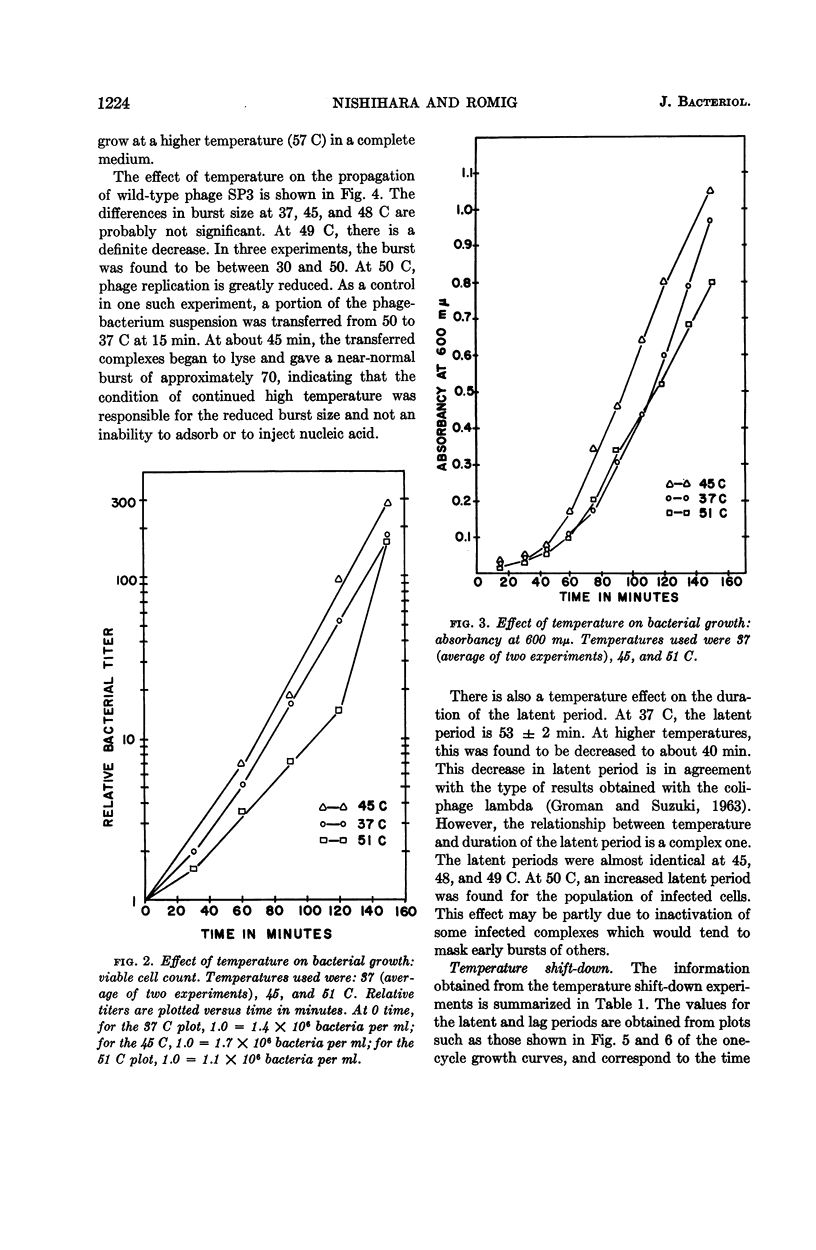
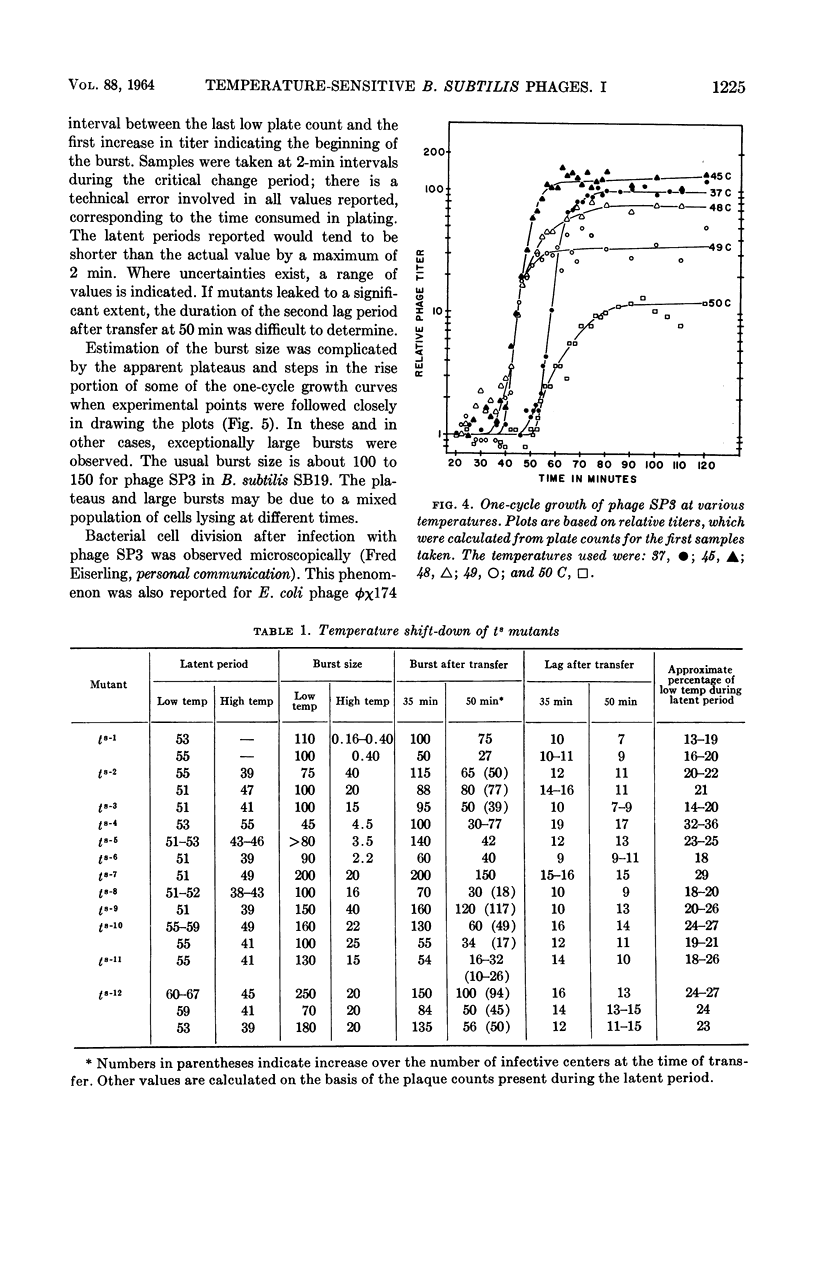
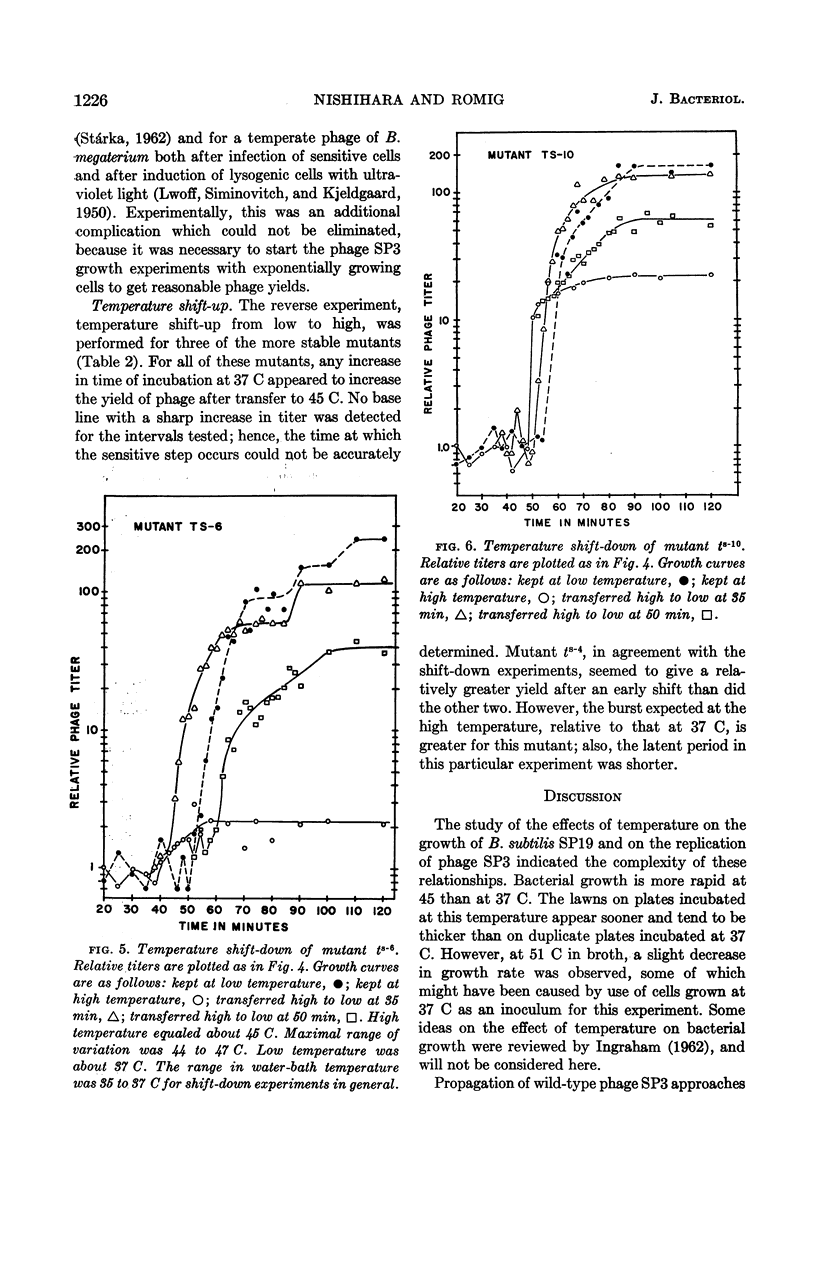
Abstract
Nishihara, Mutsuko (University of California, Los Angeles), and W. R. Romig. Temperature-sensitive mutants of Bacillus subtilis bacteriophage SP3. I. Isolation and characterization. J. Bacteriol. 88:1220–1229. 1964.—Twelve temperature-sensitive mutants of Bacillus subtilis bacteriophage SP3 were isolated from a suspension of the wild type treated with nitrous acid. The mutants were detected because of an impaired ability to replicate in the host cell at 45 C, in contrast to the parental wild type which has essentially the same burst size at temperatures between 37 and 48 C. However, the latent period of the wild-type phages was decreased as growth temperatures were varied from 37 to 49 C. The reduction of burst size at temperatures above 48 C is probably the result of the effect of higher temperatures on the metabolism of the host, because the bacterial growth rate is decreased between 45 and 51 C. A temperature effect on the mutants was studied by infecting cells at 45 C, and then transferring portions of the mixture to 37 C at two time intervals. One transfer was made within the expected 45 C latent period, and the other was made about 10 min after the end of the latent period. In both cases, removal of the phage-bacterium complexes from the inhibitory condition of high temperature did not result in an immediate increase of plaque-forming units. A delay was observed which might correspond to the time necessary to complete the temperature-sensitive synthesis in the infected cells. In experiments with three of the mutants, cells infected at 37 C were transferred to 45 C at various times during the 37 C latent period. The extent of inhibition upon transfer to the high temperature was found to decrease progressively with increasing time of prior incubation at 37 C.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADAMS M. H., LARK G. Mutation to heat resistance in coliphage T51. J Immunol. 1950 Apr;64(4):335–347. [PubMed] [Google Scholar]
- BAUTZ-FREESE E., FREESE E. Induction of reverse mutations and cross reactivation of nitrous acid-treated phage T4. Virology. 1961 Jan;13:19–30. doi: 10.1016/0042-6822(61)90027-7. [DOI] [PubMed] [Google Scholar]
- CAMPBELL A. Sensitive mutants of bacteriophage lambda. Virology. 1961 May;14:22–32. doi: 10.1016/0042-6822(61)90128-3. [DOI] [PubMed] [Google Scholar]
- COHEN S. S. Virus-induced acquisition of metabolic function. Fed Proc. 1961 Jul;20:641–649. [PubMed] [Google Scholar]
- EISERLING F. A., ROMIG W. R. Studies of Bacillus subtilis bacteriophages. Structural characterization by electron microscopy. J Ultrastruct Res. 1962 Jun;6:540–546. doi: 10.1016/s0022-5320(62)80008-2. [DOI] [PubMed] [Google Scholar]
- GROMAN N. B., SUZUKI G. QUANTITATIVE STUDY OF ENDOLYSIN SYNTHESIS DURING REPRODUCTION OF LAMBDA PHAGES. J Bacteriol. 1963 Aug;86:187–194. doi: 10.1128/jb.86.2.187-194.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GROMAN N. B., SUZUKI G. Temperature and lambda phage reproduction. J Bacteriol. 1962 Sep;84:431–437. doi: 10.1128/jb.84.3.431-437.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GROMAN N. B., SUZUKI G. The mechanism of inhibition of lambda phage production at elevated temperatures. Virology. 1961 Jan;13:144–146. doi: 10.1016/0042-6822(61)90045-9. [DOI] [PubMed] [Google Scholar]
- GROMAN N. B. Temperature and the reproduction of lambda-phage mutants. J Bacteriol. 1962 Sep;84:438–445. doi: 10.1128/jb.84.3.438-445.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LURIA S. E. Genetics of bacteriophage. Annu Rev Microbiol. 1962;16:205–240. doi: 10.1146/annurev.mi.16.100162.001225. [DOI] [PubMed] [Google Scholar]
- LWOFF A., SIMINOVITCH L., KJELDGAARD N. Induction de la production de bacteriophages chez une bactérie lysogène. Ann Inst Pasteur (Paris) 1950 Dec;79(6):815–859. [PubMed] [Google Scholar]
- McDonald W. C., Matney T. S. GENETIC TRANSFER OF THE ABILITY TO GROW AT 55 C IN BACILLUS SUBTILIS. J Bacteriol. 1963 Jan;85(1):218–220. doi: 10.1128/jb.85.1.218-220.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NESTER E. W., LEDERBERG J. Linkage of genetic units of Bacillus subtilis in DNA transformation. Proc Natl Acad Sci U S A. 1961 Jan 15;47:52–55. doi: 10.1073/pnas.47.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROMIG W. R., BRODETSKY A. M. Isolation and preliminary characterization of bacteriophages for Bacillus subtilis. J Bacteriol. 1961 Jul;82:135–141. doi: 10.1128/jb.82.1.135-141.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROMIG W. R. Infection of Bacillus subtilis with phenol-extracted bacteriophages. Virology. 1962 Apr;16:452–459. doi: 10.1016/0042-6822(62)90226-x. [DOI] [PubMed] [Google Scholar]
- STARKA J. Cellular division and reproduction of bacteriophage in synchronized cultures of Escherichia coli. J Gen Microbiol. 1962 Sep;29:83–90. doi: 10.1099/00221287-29-1-83. [DOI] [PubMed] [Google Scholar]
- SUSSMAN R., JACOB F. [On a thermosensitive repression system in the Escherichia coli lambda bacteriophage]. C R Hebd Seances Acad Sci. 1962 Feb 19;254:1517–1519. [PubMed] [Google Scholar]


