Abstract
Telomerase activation is critical for the immortalization of primary human keratinocytes by the high-risk HPV E6 and E7 oncoproteins, and this activation is mediated in part by E6-induction of the hTERT promoter. E6 induces the hTERT promoter via interactions with the cellular ubiquitin ligase, E6AP, and with the c-Myc and NFX-1 proteins, which are resident on the promoter. In the current study we demonstrate that E6 protein interacts directly with the hTERT protein. Correlating with its ability to bind hTERT, E6 also associates with telomeric DNA and with endogenous active telomerase complexes. Most importantly, E6 increases the telomerase activity of human foreskin fibroblasts transduced with the hTERT gene, and this activity is independent of hTERT mRNA expression. Unlike its ability to degrade p53, E6 does not degrade hTERT protein in vitro or in vivo. Our studies of E6/hTERT interactions also reveal that the C-terminal tagged hTERT protein, although incapable of immortalizing fibroblasts, does immortalize keratinocytes in collaboration with the viral E7 protein. Thus, E6 protein mediates telomerase activation by a posttranscriptional mechanism and these findings provide a model for exploring the direct modulation of cell telomerase/telomere function by an oncogenic virus and suggest its potential role in both neoplasia and virus replication.
Keywords: cell immortalization, hTERT, oncoproteins, papillomavirus
The HPV-16 E6 oncoprotein increases cellular telomerase activity, predominantly by inducing transcription of the hTERT gene (1–6). The hTERT protein is the catalytic, rate-limiting subunit of the telomerase enzyme complex and is selectively expressed in a small subset of normal cells (stem cells), tumor tissues, and tumor-derived cell lines (7–10). Interestingly, overexpression of hTERT or c-Myc (which transactivates the hTERT promoter) can substitute for E6 in the immortalization of primary HFKs, indicating that telomerase activation constitutes a major immortalizing function of E6 (11, 12). Our previous studies and those of other laboratories indicate that E6-mediated hTERT transactivation is independent of its ability to degrade p53 or interact with PDZ proteins (11–16). However, hTERT transactivation by E6 is dependent upon its binding the cellular ubiquitin ligase, E6AP (13, 14, 17–20). There appear to be two critical targets for E6/E6AP on the hTERT promoter, Myc (12, 15, 16) and NFX-1 (13, 18, 21), which transactivate and transrepress the promoter respectively. The model for E6 regulation of the hTERT promoter is likely to be quite complex and it is anticipated that there might be additional targets for E6 on this promoter.
In addition to activating telomerase, E6 interacts with a spectrum of cellular proteins that may contribute to HPV oncogenicity. For example, E6 binds proteins that regulate cell differentiation, adhesion, polarity, proliferation, apoptosis, gene transcription, and chromosomal stability (22, 23). E6 interacts with these target proteins via several mechanisms, including two known binding motifs [the LXXLL motif (22–26) and the PDZ domain (22, 23, 27)]. Interestingly, the hTERT protein has three potential LXXLL motifs and this observation lead us to explore a possible direct interaction between the E6 and hTERT proteins. In this study we demonstrate that E6 protein interacts directly with hTERT protein, telomeric DNA, and active telomerase complexes. These direct interactions may not only be important for mediating cell immortalization and progression to cancer, but might also participate in the regulation of HPV replication.
Results
E6 Protein Associates with hTERT Protein In Vitro and In Vivo.
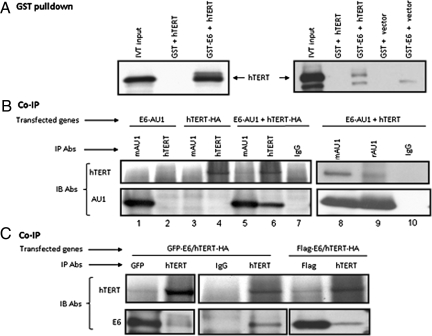
E6 binding to target proteins can be mediated by LXXLL, PDZ, or additional uncharacterized protein domains (22, 23). Interestingly, hTERT protein contains three potential LXXLL motifs located between amino acids 149–153, 447–453, and 836–842 and we hypothesized that E6 might interact with hTERT protein via such domains. To test this possibility, we first performed GST-E6 pull-down experiments with in vitro translated hTERT-HA protein. The results in Fig. 1A Left indicate that GST-E6 was able to bind the hTERT-HA protein, whereas the GST protein alone was not. In repeat assays (Fig. 1A Right), we also included an empty vector in the translation system as a control and verified that the GST-E6 protein did not bring down any protein that comigrated with hTERT. The apparent variability in the efficiency of the GST-E6 pull-down experiments (i.e., Left vs. Right) was due to different ratios of GST-E6 and hTERT. In the Left, we used ≈5-fold more GST-E6 protein relative to hTERT than that in the Right. Although these in vitro results suggest a direct interaction between E6 and hTERT, we cannot dismiss the possibility that other proteins in the translation mix are necessary for mediating this association.
Fig. 1.
HPV E6 interacts with the hTERT protein. (A) E6 and hTERT proteins associate in vitro. The hTERT gene, appended with the HA epitope at its C terminus, was expressed in vitro and reacted with purified GST or GST-E6 protein. The captured products were separated on 4–20% SDS/PAGE and blotted with monoclonal anti-HA antibody (12CA5). pCDNA was used as negative control (Right). (B) E6 (AU1-tagged) and hTERT (HA-tagged or untagged) proteins associate in vivo. Cos cells were transfected with AU1-tagged E6 and/or HA-tagged hTERT (Left, lanes 1–7) or untagged hTERT (Right, lanes 8–10) and 24 h later extracted with RIPA buffer. The lysates were subjected to immunoprecipitation (IP) with IgG, anti-AU1 or anti-hTERT antibodies. The immunoprecipitated proteins were separated with 4–20% SDS/PAGE gel and blotted with anti-hTERT or anti-AU1 antibodies. (C) E6 (GFP- or Flag-tagged) and hTERT proteins associate in vivo. Cos cells were transfected with GFP-E6 (Left) or Flag-E6 (Right) and HA-tagged hTERT. The lysates were immunoprecipitated (IP) with IgG, anti-GFP, anti-Flag, or anti-hTERT antibodies. The immunoprecipitated proteins were separated with 4–20% SDS/PAGE gel and blotted with anti-hTERT antibody (Upper) or anti-GFP and anti-Flag antibody to detect E6 protein.
To verify that the in vitro binding reflected interactions that occurred in the context of the intact cell, we transfected AU1-tagged E6 (E6-AU1) and hTERT-HA into COS cells and performed IP/Western blots using anti-AU1 (for E6) and anti-hTERT or anti-HA (for hTERT). The anti-hTERT antibody does not recognize endogenous TERT protein in monkey COS cells. As anticipated, E6 was precipitated with AU1 antibody, but not with anti-hTERT in E6-AU1 transfected cells (Fig. 1B, lanes 1 and 2). Similarly, hTERT was immunoprecipitated with anti-hTERT, but not AU1 antibody in hTERT-HA transfected cells (Fig. 1B, lanes 3 and 4), thereby establishing the specificity of the immunprecipitation reactions. In cells cotransfected with E6-AU1 and hTERT-HA, we observed that antibody against E6 (AU1) not only immunoprecipitated E6, but also coprecipitated hTERT (Fig. 1B, lane 5). Antibody against hTERT (HA) immunoprecipitated hTERT and coprecipitated E6 (Fig. 1B, lane 6). Thus, E6 and hTERT are associated in cells and the complex can be visualized with antibodies against either of the components. Neither E6 nor hTERT were precipitated with control IgG (Fig. 1B, lane 7). We have also repeated these experiments with HA antibody (for hTERT) and have observed similar results, further verifying the specificity of these interactions. To exclude the possibility that HA tag causes or interferes with E6/hTERT binding, we cotransfected E6-AU1 and untagged hTERT expression vectors. As shown in the right panel of Fig. 1B (lanes 8–10), the rabbit and mouse anti-AU1 antibodies precipitated the E6-AU1 protein and coprecipitated the untagged hTERT protein (lanes 8 and 9). IgG was unable to immunoprecipitate E6-AU1 or coprecipitate hTERT (lane 10). Finally, we repeated these experiments with both GFP- and Flag-tagged E6 proteins (Fig. 1C) and obtained similar results, indicating that the AU1 epitope was not responsible for the interaction between E6 and hTERT.
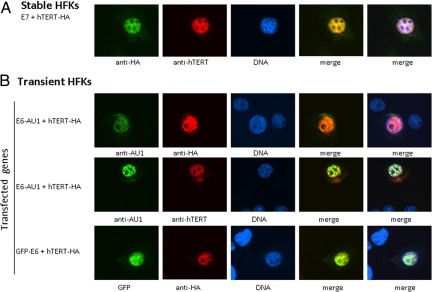
HFK Cells Immortalized with hTERT-HA Demonstrate that Both hTERT and E6 Are Intranuclear.
Primary HFK cells were immortalized with E7/hTERT-HA as indicated in Fig. S1. Although publications have shown the hTERT-HA protein to be defective for immortalizing fibroblasts (28, 29), this protein is clearly capable of cooperating with E7 in the immortalization of HFKs and associating with telomeres (Fig. S2). The resultant HFK cells were costained with anti-hTERT or anti-HA. The HA-tagged hTERT protein was recognized by both anti-HA or anti-hTERT antibodies and localized to the nucleus (Fig. 2A), similar to that observed for untagged hTERT protein (30). To determine whether E6 colocalized with hTERT in HFK cells or whether it altered hTERT localization, we cotransfected HFK cells with hTERT-HA together with either E6-AU1 or GFP-E6. As shown in Fig. 2B, E6-AU1 colocalized with hTERT-HA protein when detected with either anti-HA (Top) or anti-hTERT (Middle) antibodies. Direct immunofluoresence microscopy of cells expressing GFP-E6 verified that GFP-E6 localized with hTERT-HA protein (Bottom).
Fig. 2.
Both E6 and hTERT proteins localize to the nucleus. (A) anti-HA antibody recognizes HA-tagged hTERT protein. Stable HFK line immortalized with E7/hTERT-HA was stained with either anti-HA or anti-hTERT. (B) E6 colocalizes with hTERT in nucleus. AU1 or GFP-tagged E6 and HA-tagged hTERT were transfected human keratinocytes with LipofectAmine 2000 (Invitrogen). E6 was stained with anti-AU1 (Top) or visualized with GFP (Bottom) (green) and hTERT-HA was stained with either anti-HA (Top and Bottom) or anti-hTERT (Middle) (red), DNAs were visualized with Hoechst. Merged staining patterns are shown on the Right.
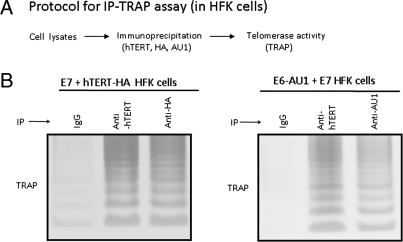
E6 Associates with an Active Telomerase Complex.
The telomerase enzyme is a ribonucleoprotein complex comprised of two core subunits, hTERT protein and a template RNA subunit (hTERC) (31, 32). Because E6 associates with hTERT, we postulated that it might also associate with active telomerase complexes that include hTERT, hTERC, and other accessory proteins (8, 31, 32). We therefore performed immunoprecipation-TRAP assays with HFK cells that were immortalized with E7/hTERT-HA or E6-AU1/E7 oncoproteins (Fig. 3A). The cells were extracted in CHAPS buffer and immunoprecpitated with anti-hTERT antibody, anti-HA, anti-E6 (AU1) antibody, or normal mouse IgG. The immunoprecipitates were then assayed by TRAP to detect telomerase activity. As expected, telomerase activity was detected in both anti-hTERT and anti-HA immunoprecipitates in E7/hTERT-HA transduced HFK cells whereas the IgG treated extracts only revealed background activity (Fig. 3B). More importantly, in HFK cells immortalized with E6-AU1/E7, telomerase activity was not only detected in the hTERT immunoprecipitates, but also in E6 immunoprecipitates (Fig. 3C), indicating that E6 associates with hTERT even when it is present in an active enzymatic complex. Our IP-TRAP assay is obviously very sensitive due its PCR-readout and this therefore allows us to detect low levels of E6/hTERT association in keratinocyte extracts.
Fig. 3.
HPV E6 associates with active telomerase complexes in human cells. (A) An immunoprecipitation and TRAP coupled assay (IP-TRAP). Cells were extracted with CHAPS buffer and immunoprecipitated with IgG, or specific antibody. Then the protein bound beads were assayed by TRAP for telomerase activity. (B) The epitope-tagged hTERT is present in telomerase complexes. HFK cells immortalized with hTERT-HA/E7 were subjected to IP-TRAP assay with IgG, anti-hTERT, or anti-HA antibodies. Active telomerase complexes were able to be precipitated with either HA or hTERT antibodies. (C) E6 protein is present in active telomerase complexes. HFK cells immortalized with HPV16 E6/E7 were immunoprecipitated with IgG, anti-AU1 (for E6) or anti-hTERT antibodies assayed by TRAP.
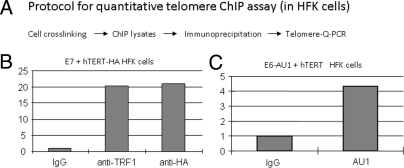
E6 Associates with Telomeres.
While active in vitro telomerase activity (TRAP) can be reconstituted with only three components hTERT, hTERC, and dyskeratin, the intact complex at telomeres is much more complex and highly regulated (8, 31–34). The telomeric complex consists of six proteins referred to collectively as “shelterin” (TRF1, TRF2, POT1, TIN2, TPP1, and RAP1), DNA repair and signaling proteins, and proteins that bind to the G-strand overhang DNA (POT1 and TPP1) (8, 31–34). Based on the above data showing that E6 can associate with active telomerase complexes, we proposed that E6 might also associate with larger protein complexes located at the telomeric ends of chromosomes. We therefore developed a quantitative method (Q-telomere-ChIP) to measure the association of proteins to telomeric DNA sequences. HFK cells immortalized with hTERT-HA/E7 were extracted and immunoprecipitated with IgG, anti-TRF1 (a telomere binding protein) and anti-HA (for hTERT) by using the ChIP-ITTM Express Magnetic Chromatin Immunoprecipitation Kit (Active Motif). ChIP DNAs were subjected to Q-PCR. The signal generated by control IgG was set to 1 (Fig. 4B). The data clearly demonstrated that TRF1 (Fig. 4B) and either HA (Fig. 4B) or Flag-tagged hTERT proteins bound to telomeric DNA sequences. More importantly, when we immunoprecipitated HFK cells (transduced with E6-AU1/hTERT) with AU1 antibody (for E6) (Fig. 4C), we also observed that E6 protein bound to these same sequences, albeit at somewhat lower level (Fig. 4C) than observed for TRF1 (Fig. 4B). Thus, E6 not only interacts with hTERT protein, but it also associates with active telomerase complexes and telomeric DNA sequences.
Fig. 4.
HPV E6 associates with telomeric sequences in human cells. (A) Quantitative telomeric sequence ChIP assay. We developed a ChIP assay to detect the association of E6 with the repeat sequences of chromosomal telomeres. Cells were cross-linked and lysated with ChIP-IT Express Magnetic Chromatin Immunoprecipitation kits (Active Motif). The ChIP lysates were precipitated with IgG or the indicated antibodies. The precipitated DNAs were subjected to real time telomere PCR (53). (B) The epitope-tagged hTERT proteins associate with telomeres. HFK cells transduced with E7 and hTERT-HA were used for the above ChIP assays. Antibody against TRF1 (Telomere repeat binding factor 1) and normal IgG were used for positive and negative controls, respectively. The signal from IgG was set to 1. hTERT-HA associated with telomere sequences in the transduced human cells. (C) E6 associates with telomeres. ChIP lysates from E6-AU1/hTERT transduced cells were subjected to telomere binding assays with AU1 antibody for E6 association.
E6 Does Not Alter the Apparent Stability of hTERT Protein In Vitro or In Vivo.
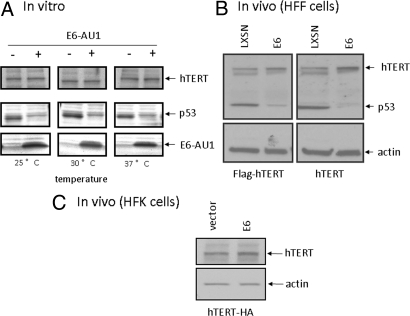
One of the best characterized functions of E6 protein is the degradation of p53 tumor suppressor protein via the E6/E6AP ubiquin ligase and proteosome pathway. This degradation has been verified both in vitro and in vivo (23, 35, 36). E6 also targets other interacting proteins (Ada3, hDlg, MAGI, Scribe) for degradation (22, 23, 37, 38). We therefore asked whether the observed E6/hTERT interactions alter the stability of hTERT protein. Our first study was performed in vitro. E6-AU1 and hTERT-HA protein were synthesized in vitro by using the TNT in vitro transcription and translation kit (Promega). p53 was also synthesized in vitro to serve as a positive control for the in vitro degradation assays. As shown in Fig. 5A, all three proteins were synthesized in vitro and we observed significant degradation of p53 by E6 at 25, 30, or 37 °C for 3 h. However, we did not see any change in the amount of hTERT protein when incubated with E6 protein (Fig. 5A). We also performed these assays at 25, 30, and 37 °C for 30 min, 1 h, 2 h, or 3 h and did not detect degradation of hTERT proteins by E6.
Fig. 5.
E6 does not degrade hTERT protein in vitro or in vivo. (A) E6 does not degrade hTERT protein in vitro. hTERT and p53 proteins were made with TNT IVT kit (Promega) and incubated with either vector or E6 (AU1 tagged) at 25, 30, and 37 °C for 3 h. Products were separated with 4–20% SDS/PAGE gel and blotted with anti-p53 (middle) and hTERT (Top), respectively. E6 protein was visualized with anti-AU1 antibody blotting (Bottom). (B) E6 does not degrade hTERT protein in vivo (HFF). Flag-tagged or untagged hTERT transduced human foreskin fibroblasts were infected with retrovirus vector (pLXSN) or retrovirus expressing E6. After G418 selection, SDS lysates were blotted with anti-hTERT (Top), anti-p53 was used as a positive control for E6-mediated degradation (Middle) and anti-actin was used for internal control (Bottom). (C) E6 does not degrade hTERT protein in vivo (HFK). hTERT-HA was transfected into HFK cells along with either control or E6 expression vector. After 24 h, the cells were lysed with SDS buffer and blotted with anti-hTERT (Top) or anti-actin (Bottom).
We also examined the ability of E6 to degrade hTERT in cells. Stable human foreskin fibroblast lines were transduced with Flag-tagged (Flag-hTERT) or untagged hTERT (in pBABE-puro vector) and then further transduced with pLXSN or pLXSN-E6 retroviruses. After G418 selection, cell lysates were subjected to SDS/PAGE gel and Western blot analysis with anti-hTERT, anti-p53, and anti-β-actin antibodies. p53 protein was significantly decreased in E6-expressing cells compared to control cells. As observed in vitro (Fig. 5A), E6 did not degrade hTERT protein in human fibroblasts (Fig. 5B). Even more convincing, E6 did not alter the amount of hTERT-HA in HFK cells, the natural target for HPV (Fig. 5C). Thus, E6 interacts with hTERT protein in vitro and in vivo, but without detectable degradation. This is not without precedence since E6 can bind some target proteins, such as p300, IRF-3, Myc, and USP15, without promoting degradation (6, 26, 39–41).
E6 Increases Telomerase Activity Independently of Transcription Regulation.
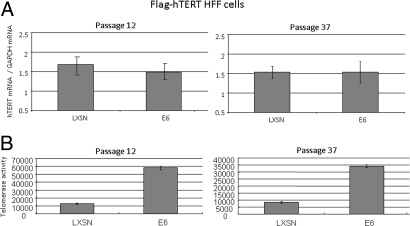
To examine the functional significance of E6/hTERT protein interactions, we performed telomerase assays in the presence and absence of E6. However, in order for us to study the isolated effects of direct E6/hTERT protein interactions, it was necessary for us to use a cell line in which the hTERT gene was nonresponsive to E6. This would thereby allow us to examine telomerase activity independent of endogenous hTERT transcription. We took advantage of our recent finding that E6 fails to induce the hTERT gene or to induce telomerase in human fibroblasts (15). We used human fibroblasts stably transduced with Flag-tagged hTERT (HFF/hTERT). When E6 was transduced into these cells, p53 was significantly decreased (Fig. 5B), indicating that it was functional. More importantly, Fig. 6B illustrates that the telomerase activity increased approximately 3- to 6-fold in the E6-expressing HFF/hTERT cells (compared to HFF/hTERT cells transduced with control retrovirus). We have repeated this protocol several times (>10) with each of three different HFF/hTERT cell lines and have verified enhancement of telomerease activity up to 20-fold. To confirm that E6 was not inducing either the endogenous or exogenous hTERT gene, we measured hTERT mRNA in these cells with real time RT-PCR. The amount of hTERT mRNA was not altered by the expression of E6 (Fig. 6A), indicating that the E6-induced alterations in telomerase activity were not due to changes in hTERT gene expression (either exogenous or endogenous). We conclude that E6 increases cellular telomerase activity not only through transcriptional activation of the hTERT gene, but also by a posttranscriptional mechanism that most likely results from the direct interaction of the E6 and hTERT proteins (Fig. 7).
Fig. 6.
E6 increases telomerase activity independent of hTERT transcription. (A) E6 does not alter hTERT mRNA levels in HFF/hTERT cells. Total RNA was isolated with TRIzol Reagent (Invitrogen) from two separate hTERT-transduced (pBABE vector) HFF cell lines with either pLXSN or E6 retroviruses. Q-RT-PCR (Taqman) was performed to quantify hTERT mRNA. GAPDH was used as an internal control. (B) E6 increases telomerase activity. CHAPS lysates from above cell lines were subjected to real time TRAP assays. These experiments were repeated >10 times in three sets of independent infections, at both early and late times after retrovirus infection.
Fig. 7.
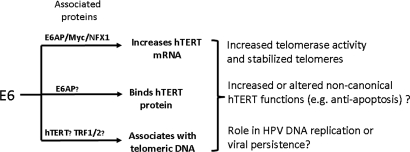
E6 regulation of hTERT function and its possible roles in cell immortalization and HPV biology. Using information from this and previous studies (6, 13–16, 18, 21, 23, 55), we illustrate how HPV E6 can regulate telomerase, telomere function, and hTERT function at multiple levels. The top line summarizes the ability of E6 to transactivate the hTERT promoter via interactions with E6AP/Myc/NFX1–91 and stabilize hTERT mRNA through interactions with NFX1–123. The second and third lines summarize the findings of the current study where E6 has been shown to bind directly to hTERT as well as to telomeric DNA. Hypothetical consequences of these interactions are indicated, including possible alterations in the noncanonical functions of hTERT.
Discussion
Our findings identify a mechanism by which HPV can directly modulate telomerase activity and thereby facilitate cell immortalization. The observation that E6 mediates telomerase activation via two separate but related pathways suggests that telomerase is a critical target for HPV. However, it seems unlikely that the primary intent of increasing hTERT protein and telomerase activity is to immortalize cells and facilitate oncogenic conversion, since tumorigenic cells are nonpermissive for HPV replication. Rather, since hTERT expression is a feature of stem cells, it is possible that E6 mediates the conversion of keratinocytes into a stem-like phenotype such that it can facilitate HPV persistence (or latency) in squamous epithelium. It is also possible that hTERT induction and the resultant increase of telomerase activity might participate in viral DNA replication. For example, telomeric proteins regulate episomal maintenance of the Epstein–Barr virus origin of plasmid replication (42). Currently we do not know whether the hTERT protein is overexpressed or activated in the upper levels of the squamous epithelium where the HPV genome undergoes vegetative DNA synthesis.
In addition to increasing the canonical, telomere-maintenance function of hTERT, it is also possible that E6 increases hTERT protein to mediate noncanonical activities, such as apoptosis blockade (43–45), gene transcription (43, 46, 47), and cell proliferation (43, 45, 46), which could have important roles in the viral life cycle. It is very relevant that hTERT transcriptionally activates cellular gene sets that are very similar to those induced by the Wnt pathway (47) and might reflect its ability to convert primary keratinocytes into a stem-like state (mentioned above).
Our finding that hTERT-HA immortalizes HFK cells in collaboration with E7 was unanticipated. Several reports indicate that hTERT-HA fails to elongate telomeres and immortalize human fibroblasts (28, 29) or HA1 cells (SV40 T/t transformed human embryonic kidney cell line) (48). Indeed, we have verified that hTERT-HA fails to immortalize human fibroblasts (Fig. S1A). However, this same hTERT-HA construct can reproducibly and efficiently immortalize HFK cells in cooperation with E7 (Fig. S1B). Even more interesting, and in contrast to the above cited studies, we also demonstrated that hTERT-HA does associate with telomeres in both early and late passages of HFK cells transduced with E7 and hTERT-HA (Fig. S2). Thus, the hTERT-HA construct is not immortalization-defective in the keratinocyte system. This might be due to cooperative interactions with E7 or to noncanonical, telomere-independent function of hTERT.
In the future it will be important to define whether the E6AP ubiquitin ligase is essential for E6/hTERT interactions, as well as the ability of E6 to associate with telomeric DNA and active telomerase complexes. However, if direct E6/hTERT interactions do indeed play a role in HPV replication or persistence, then it would be anticipated that the low-risk E6 proteins would have a similar activity. Because the low-risk E6 proteins do not bind E6AP, contain a PDZ ligand sequence or bind/activate the hTERT promoter, then these activities would obviously be nonessential for viral replication. On a more general level, it is possible that the transforming proteins of the other small DNA tumor viruses (e.g., SV40 and Adenovirus) might also interact with hTERT protein, because they share with HPV the ability to bind common cellular targets such as p53 and pRb (49–51).
Materials and Methods
Plasmids and Retroviruses.
The following plasmids and corresponding retroviruses were described in refs. 6, 15, 16, 48, and 52: pJS55 vector, pJS55–16E6-AU1, pLXSN vector and pLXSN-16E6, pLXSN-E6-AU1, pJS55-hTERT-HA, pBABE-hTERT, and pBABE-hTERT-HA.
Glutathione S-Transferase (GST) Pull-down Assays.
GST-E6 and GST proteins were expressed in Escherichia coli, induced with IPTG (Sigma), and conjugated to Glutathione-Agarose beads (Sigma). The expression of GST-E6 and GST protein was confirmed by SDS/PAGE and Coomassie Blue staining. HA-tagged hTERT or vector were translated in vitro with TNT T7 Quick Coupled Transcription/Translation System (Promega). The same amount of the IVT proteins were subjected to GST alone or GST-E6 pulldown assays. After electrophoresis with 4–20% of SDS/PAGE, the proteins were transferred to PVDF membranes and visualized with blotting with anti-HA antibody.
In Vitro Degradation Assays.
HA tagged hTERT, p53, and E6 (AU1 tagged) proteins were made in 50 μL volumes using TNT T7 Quick Coupled Transcription/Translation System (Promega). P53 was biotinated during in vitro translation. The degradation assays were performed in 25 mM Tris·HCl (pH 7.5), 100 mM NaCl, and 3 mM DTT at 25, 30, or 37 °C for 30 min, 1 h, 2 h, or 3 h. The reaction mixtures were separated with SDS/PAGE gel and blotted with anti-HA (for hTERT), Transcend HRP-Strepavidin (for p53), or anti-AU1 (for E6).
Cell Culture and Stable Line Selection.
Primary human foreskin keratinocytes (HFKs) and fibroblasts (HFFs) were cultured from neonatal foreskins, as described and maintained in keratinocyte growth media (Invitrogen) supplemented with gentamycin (50 μg/mL) and complete DMEM, respectively. COS cells were maintained in complete DMEM. Retrovirus-infected cells were selected in G418 (50–100 μg/mL) for 5 days or in puromycin (2 μg/mL) for 2–3 days.
Quantitative Telomere Chromatin Immunoprecipitation (ChIP) Assay.
ChIP lysates were made by using ChIP-IT Express Magnetic Chromatin Immunoprecipitation kits (Active Motif). ChIP DNAs were subjected to Q-PCR using primers as below: 5′- CGGTTTGTTTGGGTTTGGGTTTGGGTTTGGGTTT-GGGTT-3′ and 5′- GGCTTGCCTTACCCTTACCCTTACCCTTACCCTTACCCT-3′ for telomere repeats; 5′- TGTGCTGGCCCATCACTTTG-3′ and 5′-ACCAGCCACCACTTTCTGATAGG-3′ for HBG (a single copy gene as control) (53).
Immunoprecipitation and Western Blot.
Cells were lysed after 24 h of transfection with RIPA buffer (50 mM Tris·HCl, pH 7.4, 150 mM NaCL, 1% Nonidet P-40, 0.5% sodium desoxycholate, 0.1% SDS and fresh PMSF, protease inhibitor mixture). Five hundred micrograms of lysate protein was subjected to immunoprecipitation with either anti-hTERT antibody (Rockland) or anti-AU1 antibodies (mouse and rabbit, Convance). The immunoprecipitates were subjected to SDS/PAGE and blotted with an anti-hTERT antibody or anti-AU1 antibody.
Immunoprecipitation and Telomeric Repeat Amplification Protocol (TRAP).
Cells were lysed on ice in CHAPS buffer (0.5% Chaps, 10 mmol/L Tris, pH 7.5, 1 mmol/L MgCl2, 1 mmol/L EGTA, 5 mmol/L β-ME, 10% glycerol, 0.1 mmol/L 4-(2-amino-ethyl)benzene-sulfonyl fluoride hydrochloride (AEBSF). Lysates were immunoprecipitated with anti-hTERT, anti-HA or anit-AU1 (for E6) using milk-blocked protein G beads. The protein complex bound beads were used for a regular TRAP assays as described in ref. 7.
Immunofluoresence Microscopy.
HFKs that were cotransfected with E6-AU1 (or GFP-E6) and hTERT-HA plasmid constructs or stable cell lines expressing E7/hTERT-HA were grown on sterile glass cover slips and fixed in 4% (wt/vol) paraformadehyde and labeled with the primary and secondary antibodies, as below. The following primary antibodies were used: anti-AU1 mouse monoclonal (Covance 1:7000 dilution of ascites), anti-HA (mouse HA11 for HFK stable line, Covance 1:1000 dilution; or rabbit HA11 for transiently transfected HFK, Covance 1:1000 dilution), anti-hTERT (Rockland 1:500 dilution). The secondary antibodies (Alexa Fluor 488 donkey anti-mouse IgG and Alexa Fluor 555 donkey anti-rabbit IgG were from Invitrogen) were used at a concentration of 5 μg/mL. A Zeiss Axioskop microscope and a HAMAMATSU ORCA-ER Digital Camera were used for visualization and microphotography.
Real-Time Quantitative RT-PCR (QRT-PCR).
Real time quantitative RT-PCR for detection of hTERT mRNA and GAPDH was performed as described in refs. 14, 16, and 54.
Real-Time Quantitative TRAP.
Real time quantitative TRAP for detection of telomerase activity was performed as described in refs. 14, 16, and 54.
Supplementary Material
Acknowledgments.
We thank Drs. Robert Weinberg and Liz Blackburn for providing pBABE-hTERT, pBABE-hTERT-HA, pCI-hTERT-HA, and pBABE-Flag-tagged hTERT constructs. We also thank Drs. Alison McBride, Hang Yuan, Gary Disbrow, and Frank Suprynowicz for their advice and suggestions during this study. This work was funded by National Institutes of Health Grants R01CA106440 and R01CA53371 (to R.S.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0906357106/DCSupplemental.
References
- 1.Klingelhutz AJ, Foster SA, McDougall JK. Telomerase activation by the E6 gene product of human papillomavirus type 16. Nature. 1996;380:79–82. doi: 10.1038/380079a0. [DOI] [PubMed] [Google Scholar]
- 2.Gewin L, Galloway DA. E box-dependent activation of telomerase by human papillomavirus type 16 E6 does not require induction of c-myc. J Virol. 2001;75:7198–7201. doi: 10.1128/JVI.75.15.7198-7201.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oh ST, Kyo S, Laimins LA. Telomerase activation by human papillomavirus type 16 E6 protein: Induction of human telomerase reverse transcriptase expression through Myc and GC-rich Sp1 binding sites. J Virol. 2001;75:5559–5566. doi: 10.1128/JVI.75.12.5559-5566.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Veldman T, Horikawa I, Barrett JC, Schlegel R. Transcriptional activation of the telomerase hTERT gene by human papillomavirus type 16 E6 oncoprotein. J Virol. 2001;75:4467–4472. doi: 10.1128/JVI.75.9.4467-4472.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McMurray HR, McCance DJ. Human papillomavirus type 16 E6 activates TERT gene transcription through induction of c-Myc and release of USF-mediated repression. J Virol. 2003;77:9852–9861. doi: 10.1128/JVI.77.18.9852-9861.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Veldman T, Liu X, Yuan H, Schlegel R. Human papillomavirus E6 and Myc proteins associate in vivo and bind to and cooperatively activate the telomerase reverse transcriptase promoter. Proc Natl Acad Sci USA. 2003;100:8211–8216. doi: 10.1073/pnas.1435900100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim NW, et al. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994;266:2011–2015. doi: 10.1126/science.7605428. [DOI] [PubMed] [Google Scholar]
- 8.Belgiovine C, Chiodi I, Mondello C. Telomerase: Cellular immortalization and neoplastic transformation. Multiple functions of a multifaceted complex. Cytogenet Genome Res. 2008;122:255–262. doi: 10.1159/000167811. [DOI] [PubMed] [Google Scholar]
- 9.Cukusic A, Skrobot Vidacek N, Sopta M, Rubelj I. Telomerase regulation at the crossroads of cell fate. Cytogenet Genome Res. 2008;122:263–272. doi: 10.1159/000167812. [DOI] [PubMed] [Google Scholar]
- 10.Harley CB. Telomerase and cancer therapeutics. Nat Rev Cancer. 2008;8:167–179. doi: 10.1038/nrc2275. [DOI] [PubMed] [Google Scholar]
- 11.Kiyono T, et al. Both Rb/p16INK4a inactivation and telomerase activity are required to immortalize human epithelial cells. Nature. 1998;396:84–88. doi: 10.1038/23962. [DOI] [PubMed] [Google Scholar]
- 12.Liu X, et al. Myc and human papillomavirus type 16 E7 genes cooperate to immortalize human keratinocytes. J Virol. 2007;81:12689–12695. doi: 10.1128/JVI.00669-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gewin L, Myers H, Kiyono T, Galloway DA. Identification of a novel telomerase repressor that interacts with the human papillomavirus type-16 E6/E6-AP complex. Genes Dev. 2004;18:2269–2282. doi: 10.1101/gad.1214704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu X, et al. The E6AP ubiquitin ligase is required for transactivation of the hTERT promoter by the human papillomavirus E6 oncoprotein. J Biol Chem. 2005;280:10807–10816. doi: 10.1074/jbc.M410343200. [DOI] [PubMed] [Google Scholar]
- 15.Liu X, et al. Cell-restricted immortalization by human papillomavirus correlates with telomerase activation and engagement of the hTERT promoter by Myc. J Virol. 2008;82:11568–11576. doi: 10.1128/JVI.01318-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu X, et al. HPV E7 contributes to the telomerase activity of immortalized and tumorigenic cells and augments E6-induced hTERT promoter function. Virology. 2008;375:611–623. doi: 10.1016/j.virol.2008.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bedard KM, Underbrink MP, Howie HL, Galloway DA. The E6 oncoproteins from human betapapillomaviruses differentially activate telomerase through an E6AP-dependent mechanism and prolong the lifespan of primary keratinocytes. J Virol. 2008;82:3894–3902. doi: 10.1128/JVI.01818-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu M, et al. NFX1 interacts with mSin3A/histone deacetylase to repress hTERT transcription in keratinocytes. Mol Cell Biol. 2008;28:4819–4828. doi: 10.1128/MCB.01969-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kelley ML, Keiger KE, Lee CJ, Huibregtse JM. The global transcriptional effects of the human papillomavirus E6 protein in cervical carcinoma cell lines are mediated by the E6AP ubiquitin ligase. J Virol. 2005;79:3737–3747. doi: 10.1128/JVI.79.6.3737-3747.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.James MA, Lee JH, Klingelhutz AJ. HPV16–E6 associated hTERT promoter acetylation is E6AP dependent, increased in later passage cells and enhanced by loss of p300. Int J Cancer. 2006;119:1878–1885. doi: 10.1002/ijc.22064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Katzenellenbogen RA, Vliet-Gregg P, Xu M, Galloway DA. NFX1–123 increases hTERT expression and telomerase activity posttranscriptionally in human papillomavirus type 16 E6 keratinocytes. J Virol. 2009;83:6446–6456. doi: 10.1128/JVI.02556-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tungteakkhun SS, Duerksen-Hughes PJ. Cellular binding partners of the human papillomavirus E6 protein. Arch Virol. 2008;153:397–408. doi: 10.1007/s00705-007-0022-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Howie HL, Katzenellenbogen RA, Galloway DA. Papillomavirus E6 proteins. Virology. 2009;384:324–334. doi: 10.1016/j.virol.2008.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen JJ, et al. Identification of an alpha helical motif sufficient for association with papillomavirus E6. J Biol Chem. 1998;273:13537–13544. doi: 10.1074/jbc.273.22.13537. [DOI] [PubMed] [Google Scholar]
- 25.Elston RC, Napthine S, Doorbar J. The identification of a conserved binding motif within human papillomavirus type 16 E6 binding peptides, E6AP and E6BP. J Gen Virol. 1998;79:371–374. doi: 10.1099/0022-1317-79-2-371. [DOI] [PubMed] [Google Scholar]
- 26.Ronco LV, Karpova AY, Vidal M, Howley PM. Human papillomavirus 16 E6 oncoprotein binds to interferon regulatory factor-3 and inhibits its transcriptional activity. Genes Dev. 1998;12:2061–2072. doi: 10.1101/gad.12.13.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kiyono T, et al. Binding of high-risk human papillomavirus E6 oncoproteins to the human homologue of the Drosophila discs large tumor suppressor protein. Proc Natl Acad Sci USA. 1997;94:11612–11616. doi: 10.1073/pnas.94.21.11612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ouellette MM, et al. Telomerase activity does not always imply telomere maintenance. Biochem Biophys Res Commun. 1999;254:795–803. doi: 10.1006/bbrc.1998.0114. [DOI] [PubMed] [Google Scholar]
- 29.Kim M, Xu L, Blackburn EH. Catalytically active human telomerase mutants with allele-specific biological properties. Exp Cell Res. 2003;288:277–287. doi: 10.1016/s0014-4827(03)00217-9. [DOI] [PubMed] [Google Scholar]
- 30.Seimiya H, et al. Involvement of 14–3-3 proteins in nuclear localization of telomerase. EMBO J. 2000;19:2652–2661. doi: 10.1093/emboj/19.11.2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cong YS, Wright WE, Shay JW. Human telomerase and its regulation. Microbiol Mol Biol Rev. 2002;66:407–425. doi: 10.1128/MMBR.66.3.407-425.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shay JW, Wright WE. Telomerase: A target for cancer therapeutics. Cancer Cell. 2002;2:257–265. doi: 10.1016/s1535-6108(02)00159-9. [DOI] [PubMed] [Google Scholar]
- 33.Gonzalez-Suarez I, Gonzalo S. cross-talk between chromatin structure, nuclear compartmentalization, and telomere biology. Cytogenet Genome Res. 2008;122:202–210. doi: 10.1159/000167805. [DOI] [PubMed] [Google Scholar]
- 34.Stern JL, Bryan TM. Telomerase recruitment to telomeres. Cytogenet Genome Res. 2008;122:243–254. doi: 10.1159/000167810. [DOI] [PubMed] [Google Scholar]
- 35.Scheffner M, et al. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell. 1990;63:1129–1136. doi: 10.1016/0092-8674(90)90409-8. [DOI] [PubMed] [Google Scholar]
- 36.Werness BA, Levine AJ, Howley PM. Association of human papillomavirus types 16 and 18 E6 proteins with p53. Science. 1990;248:76–79. doi: 10.1126/science.2157286. [DOI] [PubMed] [Google Scholar]
- 37.Mantovani F, Banks L. The human papillomavirus E6 protein and its contribution to malignant progression. Oncogene. 2001;20:7874–7887. doi: 10.1038/sj.onc.1204869. [DOI] [PubMed] [Google Scholar]
- 38.Munger K, Howley PM. Human papillomavirus immortalization and transformation functions. Virus Res. 2002;89:213–228. doi: 10.1016/s0168-1702(02)00190-9. [DOI] [PubMed] [Google Scholar]
- 39.Patel D, Huang SM, Baglia LA, McCance DJ. The E6 protein of human papillomavirus type 16 binds to and inhibits co-activation by CBP and p300. EMBO J. 1999;18:5061–5072. doi: 10.1093/emboj/18.18.5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zimmermann H, Degenkolbe R, Bernard HU, O'Connor MJ. The human papillomavirus type 16 E6 oncoprotein can down-regulate p53 activity by targeting the transcriptional coactivator CBP/p300. J Virol. 1999;73:6209–6219. doi: 10.1128/jvi.73.8.6209-6219.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vos RM, Altreuter J, White EA, Howley PM. The ubiquitin-specific peptidase USP15 regulates human papillomavirus type 16 E6 protein stability. J Virol. 2009;83:8885–8892. doi: 10.1128/JVI.00605-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Deng Z, et al. Telomeric proteins regulate episomal maintenance of Epstein–Barr virus origin of plasmid replication. Mol Cell. 2002;9:493–503. doi: 10.1016/s1097-2765(02)00476-8. [DOI] [PubMed] [Google Scholar]
- 43.Parkinson EK, Fitchett C, Cereser B. Dissecting the noncanonical functions of telomerase. Cytogenet Genome Res. 2008;122:273–280. doi: 10.1159/000167813. [DOI] [PubMed] [Google Scholar]
- 44.Santos JH, Meyer JN, Van Houten B. Mitochondrial localization of telomerase as a determinant for hydrogen peroxide-induced mitochondrial DNA damage and apoptosis. Hum Mol Genet. 2006;15:1757–1768. doi: 10.1093/hmg/ddl098. [DOI] [PubMed] [Google Scholar]
- 45.Lee J, et al. TERT promotes cellular and organismal survival independently of telomerase activity. Oncogene. 2008;27:3754–3760. doi: 10.1038/sj.onc.1211037. [DOI] [PubMed] [Google Scholar]
- 46.Choi J, et al. TERT promotes epithelial proliferation through transcriptional control of a Myc- and Wnt-related developmental program. PLoS Genet. 2008;4:e10. doi: 10.1371/journal.pgen.0040010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Park JI, et al. Telomerase modulates Wnt signalling by association with target gene chromatin. Nature. 2009;460:66–72. doi: 10.1038/nature08137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Counter CM, et al. Dissociation among in vitro telomerase activity, telomere maintenance, and cellular immortalization. Proc Natl Acad Sci USA. 1998;95:14723–14728. doi: 10.1073/pnas.95.25.14723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Howley PM, Livingston DM. Small DNA tumor viruses: Large contributors to biomedical sciences. Virology. 2009;384:256–259. doi: 10.1016/j.virol.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.DeCaprio JA. How the Rb tumor suppressor structure and function was revealed by the study of Adenovirus and SV40. Virology. 2009;384:274–284. doi: 10.1016/j.virol.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 51.Levine AJ. The common mechanisms of transformation by the small DNA tumor viruses: The inactivation of tumor suppressor gene products: p53. Virology. 2009;384:285–293. doi: 10.1016/j.virol.2008.09.034. [DOI] [PubMed] [Google Scholar]
- 52.Hahn WC, et al. Inhibition of telomerase limits the growth of human cancer cells. Nature Medicine. 1999;5:1164–1170. doi: 10.1038/13495. [DOI] [PubMed] [Google Scholar]
- 53.Cawthon RM. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002;30:e47. doi: 10.1093/nar/30.10.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fu B, Quintero J, Baker CC. Keratinocyte growth conditions modulate telomerase expression, senescence, and immortalization by human papillomavirus type 16 E6 and E7 oncogenes. Cancer Res. 2003;63:7815–7824. [PubMed] [Google Scholar]
- 55.Galloway DA, et al. Regulation of telomerase by human papillomaviruses. Cold Spring Harb Symp Quant Biol. 2005;70:209–215. doi: 10.1101/sqb.2005.70.041. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.