Abstract
Cystic fibrosis (CF) is a fatal genetic disease caused by mutations in the gene encoding the CF transmembrane conductance regulator (CFTR), a protein kinase A (PKA)-activated epithelial anion channel involved in salt and fluid transport in multiple organs, including the lung. Most CF mutations either reduce the number of CFTR channels at the cell surface (e.g., synthesis or processing mutations) or impair channel function (e.g., gating or conductance mutations) or both. There are currently no approved therapies that target CFTR. Here we describe the in vitro pharmacology of VX-770, an orally bioavailable CFTR potentiator in clinical development for the treatment of CF. In recombinant cells VX-770 increased CFTR channel open probability (Po) in both the F508del processing mutation and the G551D gating mutation. VX-770 also increased Cl− secretion in cultured human CF bronchial epithelia (HBE) carrying the G551D gating mutation on one allele and the F508del processing mutation on the other allele by ≈10-fold, to ≈50% of that observed in HBE isolated from individuals without CF. Furthermore, VX-770 reduced excessive Na+ and fluid absorption to prevent dehydration of the apical surface and increased cilia beating in these epithelial cultures. These results support the hypothesis that pharmacological agents that restore or increase CFTR function can rescue epithelial cell function in human CF airway.
Keywords: cystic fibrosis transmembrane conductance regulator (CFTR), epithelial ion transport, epithelial sodium channel (ENaC), human bronchial epithelium (HBE) culture
Drugs that repair the function of proteins that are defective because of gene mutations offer hope for the treatment of genetic diseases such as cystic fibrosis (CF). CF is a fatal genetic disease caused by mutations on both alleles in the gene encoding CFTR (1, 2), a protein kinase A (PKA)-activated epithelial Cl− and HCO3− selective ion channel involved in salt and fluid transport in multiple organs (3–7). Although CF has many clinical manifestations, lung disease is the primary cause of morbidity and mortality (8). One hypothesis to explain the link between defective CFTR and CF lung pathogenesis is that loss of CFTR-mediated Cl− secretion causes airway surface dehydration because of both a decrease in CFTR-mediated Cl− and fluid secretion and a secondary increase in epithelial Na+ channel (ENaC)–mediated Na+ and fluid absorption (9–11). The resulting dehydration of the airway surface is believed to contribute to the deleterious cascade of mucus accumulation, infection, inflammation and destruction that characterizes CF lung disease (12). Current therapies to treat CF lung disease, including mucolytics, antibiotics, and anti-inflammatory agents, treat downstream disease processes that are secondary to the loss of CFTR function. An alternative therapeutic strategy to treat CF is to increase CFTR function by gene therapy or drugs, with the expectation that this would stop the pleiotropic consequences of CFTR mutations and would reduce the severity or slow the progression of the disease.
A number of therapeutic discovery and development efforts are underway to develop drugs that repair the underlying molecular defects in CFTR caused by different CFTR mutations (13, 14). The most common CFTR mutation, F508del, accounts for ≈70% of all CFTR alleles in patients with CF (15). The F508del mutation impairs the intracellular processing and subsequent delivery of CFTR to the cell surface resulting in the loss of CFTR-mediated Cl− and HCO3− secretion (13). Pharmacological agents that increase the cellular processing and delivery of CFTR proteins, such as F508del CFTR, to the cell surface to increase the flow of ions are called CFTR correctors (14, 16, 17). Although less common, CFTR mutations that primarily impair the ability of CFTR at the cell surface to open (e.g., G551D) or conduct Cl− or HCO3− (e.g., R117H) are also found in CF patients (5, 18). Pharmacological agents that increase the flow of ions through activated CFTR present at the cell surface are called CFTR potentiators (14, 17, 19).
Here we describe the in vitro pharmacology of VX-770, the first potent and orally available CFTR potentiator to enter human clinical trials. In a Phase 2 clinical study in CF patients carrying the G551D CFTR mutation, oral administration of VX-770 increased CFTR activity as determined by improvement in biomarkers of CFTR function and improved lung function (20). The results shown here indicate that VX-770 increased the open probability (Po) of the CFTR channel only after its activation by PKA and increased the CFTR-mediated transepithelial current (IT) in cultured human bronchial epithelia (HBE) isolated from the bronchi of CF donor lungs carrying the G551D and/or F508del CFTR mutations. In G551D/F508del HBE, potentiation of CFTR-mediated Cl− secretion by VX-770 reduced excessive ENaC-mediated Na+ and fluid absorption, resulting in an increase in the surface fluid and cilia beat frequency (CBF). These results suggest that pharmacological agents that increase CFTR-mediated Cl− secretion may improve epithelial cell function in CF.
Results
Pharmacological Characterization of VX-770.
We identified VX-770 (Fig. 1A) from a high-throughput screening (HTS) and lead optimization effort to discover CFTR potentiators for clinical development. Briefly, HTS of 228,000 chemically diverse drug-like and lead-like compounds was performed using a cell-based fluorescence membrane potential assay designed to identify CFTR potentiators (17). A lead scaffold was selected for medicinal chemistry optimization based on potency, selectivity, and chemical tractability. Several rounds of analog synthesis led to identification of VX-770. VX-770 was selected for development based on, among other criteria, its ability to potentiate multiple CFTR forms, its in vitro selectivity, and a favorable preclinical pharmacokinetic profile.
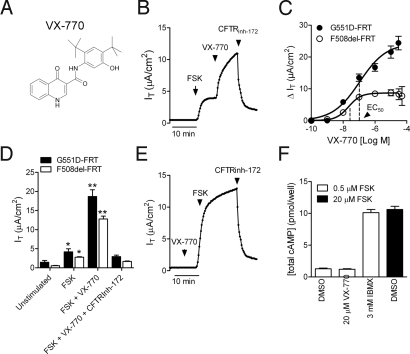
Fig. 1.
VX-770 acted as a potentiator, not an activator, of G551D- and F508del CFTR in recombinant cells. (A) Structure of VX-770 (N-(2,4-di-tert-butyl-5-hydroxyphenyl)-4-oxo-1,4-dihydroquinoline-3-carboxamide)). (B) Representative IT recording from G551D-FRT showing the response to the sequential application of 10 μM forskolin (FSK), 10 μM VX-770, and 20 μM CFTRinh-172. (C) Concentration–response curve of the net increase in forskolin-stimulated IT after sequential application of VX-770 at the concentrations indicated in G551D-FRT (filled circles; n = 12) and temperature-corrected F508del-FRT (open circles; n = 5). (D) Mean (± SEM) of the IT response in G551D- (filled bars; n = 6) and F508del- (open bars; n = 5) FRT under the conditions indicated in B. Single asterisk indicates significant difference relative to unstimulated; double asterisk indicates significant difference relative to forskolin and unstimulated (P < 0.05; one-way ANOVA followed by Tukey's multiple comparison test). (E) Representative IT recording from G551D-FRT showing the response to the sequential application of 10 μM VX-770, 10 μM FSK, and 20 μM CFTRinh-172. (F) Total cAMP (cellular and secreted) was measured after 30 min incubation of FRT cells with 0.5 μM FSK plus DMSO, VX-770, and the nonspecific PDE inhibitor, IBMX (open bars) or with 20 μM FSK alone (filled bar).
The effects of VX-770 on CFTR-mediated Cl− secretion in vitro were assessed in both recombinant cell lines and primary cultures of HBE isolated from the bronchi of CF and non-CF donor lungs. The effect of VX-770 on CFTR-mediated Cl− secretion was initially characterized by measuring the CFTR-mediated IT in Ussing chambers using recombinant Fisher rat thyroid (FRT) cells expressing either human wild-type, G551D, or F508del CFTR. Consistent with the previously identified functional impairment of G551D CFTR (5), the addition of a maximally effective concentration of forskolin (10 μM) caused only a modest increase in the IT (Fig. 1B) compared to wild-type CFTR-FRT cells (6.7 ± 0.9 to 50 ± 1 μA/cm2; n = 7). Sequential addition of VX-770 increased the forskolin-stimulated IT by ≈4-fold with an EC50 of 100 ± 47 nM (n = 12), and this response was blocked by the CFTR inhibitor CFTRinh-172 (21) (Fig. 1 B–D). In G551D-FRT, 10 μM VX-770 alone did not increase the IT (Fig. 1 B vs. E; unstimulated = 0.45 ± 0.03 μA/cm2 vs. 10 μM VX-770 = 0.45 ± 0.04 μA/cm2; n = 3), and VX-770 did not increase cellular cAMP levels in FRT cells (Fig. 1F).
To test whether VX-770 also potentiated F508del CFTR, we incubated F508del-FRT cells at 27 °C overnight to increase the cell surface density of F508del CFTR (13). VX-770 increased the forskolin-stimulated IT in temperature-corrected F508del-FRT cells by ≈6-fold with an EC50 of 25 ± 5 nM (Fig. 1 C and D; n = 5). No response to forskolin and VX-770 addition was observed in FRT cells that did not express CFTR [see supporting information (SI) Fig. S1]. Taken together, the results indicate that VX-770 is a potentiator, not an activator, of G551D- and F508del CFTR–mediated Cl− secretion.
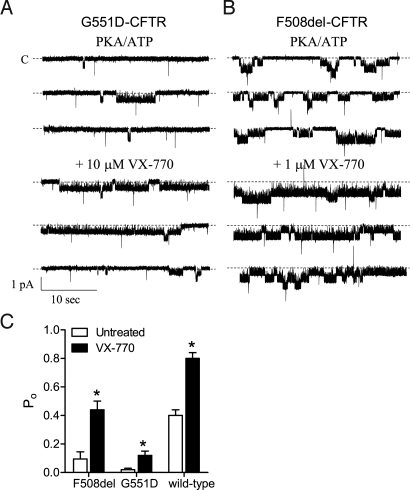
The biophysical basis for CFTR potentiation by VX-770 was investigated by measuring the Po of CFTR in excised membrane patches from recombinant cells expressing G551D-, F508del-, or wild-type CFTR. Before the addition of VX-770, the CFTR channel was exposed to maximally effective concentrations of PKA (75 nM) and ATP (1 mM). Under these conditions, 10 μM VX-770 increased the Po of G551D CFTR by ≈6-fold (Fig. 2 A and C). VX-770 also increased the Po of F508del- and wild-type CFTR by ≈5-fold and ≈2-fold, respectively (Fig. 2 B and C). These data are consistent with the increases in the forskolin-stimulated IT observed with VX-770 in G551D- and F508del-FRT cells (Fig. 1), and suggest that VX-770 acts by increasing CFTR channel gating.
Fig. 2.
VX-770 potentiated the gating activity of CFTR. Representative patch-clamp recording of the single channel current resulting from activation of G551D CFTR in FRT cells (A) and F508del CFTR in NIH 3T3 cells (B) by 1 mM ATP and 75 nM PKA before and during VX-770 application. The maximum effective concentration of VX-770 based on Fig. 1C was used for each CFTR form. All modulators were added to the cytoplasmic surface. Dotted lines indicate closed state. (C) Mean Po of F508del-, G551D-, and wild-type CFTR in the presence of PKA and ATP alone (open bars) and with VX-770 (filled bars). For G551D CFTR, 10 μM VX-770 was added, whereas 1 μM VX-770 was added to F508del- and wild-type CFTR. F508del-NIH 3T3 cells were incubated at 27 °C for 24 h before recording. Asterisks indicate significant difference (P < 0.05; t test; n = 5–6).
Effect of VX-770 in Primary Cultures of HBE Carrying G551D and F508del CFTR Mutations.
To study the effect of VX-770 on CFTR-mediated Cl− secretion in a more physiologically relevant cell system, we used cultures of HBE isolated from the bronchi of CF donor lung tissue. Air-interface cultures of CF HBE exhibit several defects in airway epithelial function that are believed to contribute to the development of CF lung disease, including low Cl− and fluid secretion, excessive Na+ and fluid absorption, and decreased cilia beating secondary to decreased surface fluid (11, 17, 22). In HBE isolated from the bronchi of a single CF donor lung carrying the G551D and F508del CFTR mutations, VX-770 increased the forskolin-stimulated IT with an EC50 of 236 ± 200 nM (n = 16) that was blocked by the inhibitor CFTRinh-172 (Fig. 3 A and B). VX-770 was ≈70-fold more potent than the commonly used CFTR potentiator genistein (23), which had an EC50 of 16 ± 3 μM (n = 3). To calibrate the increase in CFTR function by VX-770, the forskolin-stimulated IT in G551D/F508del HBE was compared to that in HBE isolated from four individuals without CF (non-CF HBE). In G551D/F508del- and non-CF HBE, the peak IT response to 10 μM forskolin was 2.9 ± 0.5 μA/cm2 and 56 ± 6 μA/cm2, respectively. This indicates that the maximum forskolin-stimulated IT in G551D/F508del HBE is equivalent to 5 ± 1% of non-CF HBE (n = 16), consistent with the low CFTR function and severe CF disease observed in individuals carrying both the G551D and F508del mutations (24). VX-770 increased the forskolin-stimulated IT in G551D/F508del HBE by ≈10-fold to 27 ± 2 μA/cm2 (n = 16), which is equivalent to 48 ± 4% of non-CF HBE (Fig. 3B). These data indicate that VX-770 is a potent and efficacious potentiator of CFTR in G551D/F508del HBE.
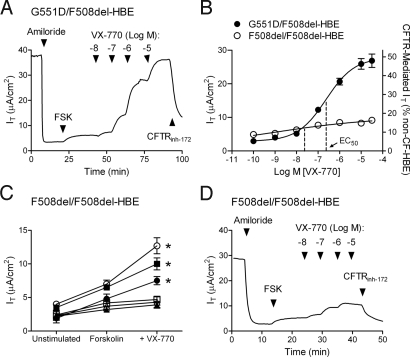
Fig. 3.
VX-770 potentiated CFTR-mediated Cl− secretion in primary cultures of G551D/F508del HBE and F508del HBE. Ussing chamber techniques were used to record the IT resulting from CFTR-mediated Cl− secretion. To isolate the CFTR-mediated IT, a basolateral to apical Cl− gradient was established, 30 μM amiloride was added to block ENaC, and 10 μM (EC99) forskolin (FSK) was applied to activate CFTR. The addition CFTRinh-172 (20 μM) to the apical surface was used to confirm that the forskolin-stimulated IT was caused by CFTR. (A) Representative IT tracing from G551D/F508del HBE. (B) The concentration–response curve for VX-770 in the presence of FSK is shown for G551D/F508del HBE isolated from the bronchi of a single individual (filled circles; n = 16) and F508del HBE isolated from the bronchi of the three individuals that responded to VX-770 (open circles; n = 7–24). Left y axis shows IT responses; right y axis shows IT normalized to the 10 μM FSK-stimulated IT in non-CF HBE (mean ± SEM). Note that the error bars for the F508del HBE were smaller than the symbol. (C) The IT before FSK addition (unstimulated) and during the sequential addition of 10 μM FSK followed by 10 μM VX-770 in F508del HBE isolated from the bronchi of six F508del-homozygous individuals (n = 4–24). (D) Representative IT tracing from F508del HBE isolated from the bronchi represented by the open circles in C.
The effect of VX-770 on F508del CFTR was assessed in cultured HBE isolated from the bronchi of six F508del-homozygous individuals with CF (F508del HBE). In these experiments, there was no attempt to increase the cell surface density of F508del CFTR by low temperature incubation or addition of a CFTR corrector. In the absence of VX-770, a range of forskolin-stimulated IT responses was observed with a mean increase over the unstimulated IT of 2.2 ± 0.9 μA/cm2 (Fig. 3C), equivalent to 4.0 ± 0.6% of non-CF HBE. This is consistent with the low CFTR function and severe CF disease observed in F508del-homozygous individuals (24). The addition of VX-770 caused a significant (P < 0.05) increase in the forskolin-stimulated IT in F508del HBE isolated from three of the six individuals with a mean EC50 of 22 ± 10 nM and a maximum response of 16 ± 4% non-CF HBE (Fig. 3B–D). Thus, VX-770 was able to potentiate CFTR in some F508del HBE cultures, although the magnitude of the effect on the forskolin-stimulated IT was less than in G551D/F508del HBE (Fig. 3B). Similar to the results in FRT cells, the EC50 in F508del HBE was lower than that in G551D/F508del HBE.
Effect of VX-770 on Na+ and Fluid Absorption in G551D/F508del HBE.
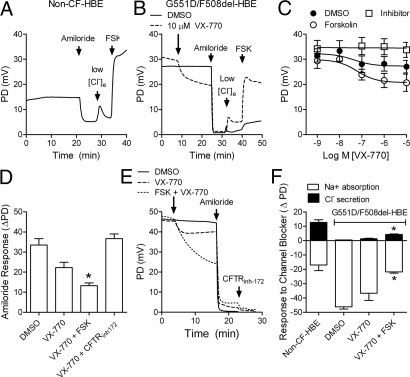
In the CF airway, the loss of CFTR function is believed to cause an increase in ENaC-mediated Na+ absorption as evident in the elevated baseline potential difference (PD) and increased response to amiloride in nasal potential difference (NPD) recordings in CF patients (4). We monitored the baseline PD and amiloride response in cultured G551D/F508del HBE and wild-type HBE under open-circuit recording conditions that resemble the in vivo NPD assays commonly performed in individuals with CF (25). As observed in CF subjects in vivo (23), the in vitro baseline PD was elevated and the amiloride response was larger in G551D/F508del HBE compared with non-CF HBE (Fig. 4 A and B). Addition of VX-770 to G551D/F508del HBE decreased the PD with an IC50 of 43 ± 38 nM (Fig. 4 B and C; n = 6) and decreased the amiloride response (Fig. 4D; n = 6). The effect of VX-770 on the PD and amiloride response were augmented by subsequent addition of forskolin and blocked by prior addition of CFTRinh-172 (Fig. 4 C–D), indicating that these effects were due to CFTR potentiation rather than to direct block of ENaC. This is further supported by the lack of effects of VX-770 on the isolated ENaC-mediated Na+ current in recombinant NIH 3T3 cells expressing the rat α, β, and γ subunits of ENaC (see Fig. S2). To determine the relative contributions of CFTR-mediated Cl− secretion and ENaC-mediated Na+ absorption to the PD in non-CF HBE and G551D/F508del HBE, we monitored the response to amiloride and CFTRinh-172 in the presence or absence of VX-770 with or without forskolin (Fig. 4E). Under these conditions, the increase in the response to CFTRinh-172 correlated with a decrease in the amiloride sensitive PD (Fig. 4F). Together, these results suggest that the increase in CFTR-mediated Cl− secretion by VX-770 caused a secondary decrease in ENaC-mediated ion transport, the latter to levels similar to those in non-CF HBE.
Fig. 4.
VX-770 increased CFTR-mediated Cl− secretion and reduced ENaC-mediated Na+ absorption in G551D/F508del HBE. Representative recording of the PD in non-CF HBE (A) and G551D/F508del HBE (B) obtained with the Ussing chamber technique (open circuit mode) using equimolar Cl− on the basolateral and apical sides. In G551D/F508del HBE, DMSO (vehicle; solid lines) or 10 μM VX-770 (dashed lines) was added to the apical surface before sequential addition of amiloride, low Cl− (5 mM) and 10 μM forskolin (FSK) to the apical surface. (C) Concentration–response curve (mean ± SEM, n = 6) of the PD response to VX-770 addition in the presence of DMSO (filled circles), 10 μM FSK (open circles) or 20 μM CFTRinh-172 (open squares). (D) Net change in PD (ΔPD) after the addition of 30 μM amiloride in the presence of the treatments indicated. (E) Representative PD recording in G551D/F508del HBE showing the response to DMSO (solid line) or 10 μM VX-770 without (dashed line) or with 10 μM FSK (dotted line) followed by sequential addition of 30 μM amiloride and 20 μM CFTRinh-172. (F) The contribution of ENaC and CFTR to the PD in non-CF HBE and G551D/F508del HBE (bar) was determined by measuring the PD change in response to amiloride (open bars) and CFTRinh-172 (filled bars), respectively. All data are from G551D/F508del HBE isolated from the bronchi of a single individual. Asterisks indicate significant difference compared with vehicle-treated controls (ANOVA followed by Tukey's test; mean ± SEM, n = 6).
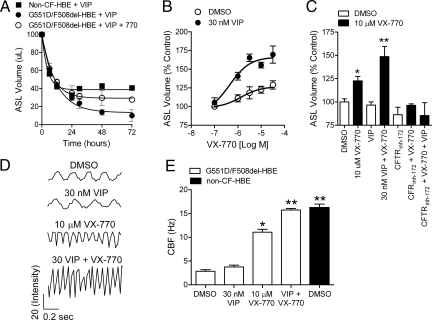
In the CF lung, the decrease in CFTR-mediated Cl− and fluid secretion and the associated increase in ENaC-mediated Na+ and fluid absorption are believed to cause the dehydration of the airway surface (9–11). To determine whether potentiation of CFTR by VX-770 was associated with an increase in the fluid levels on the apical surface of G551D/F508del HBE, we monitored the airway surface liquid (ASL) volume. To do this, we placed 100 μl of fluid on the apical surface and measured the amount remaining after up to 72 h in culture (Fig. 5A). To stimulate PKA-mediated activation of CFTR, 30 nM (≈EC99; see Fig. S3) vasoactive intestinal peptide (VIP) was added to the basolateral surface. VIP was used because it is an abundant natural transmitter in the lung and stimulates CFTR-mediated Cl− and fluid secretion in non-CF but not CF airway epithelium (26, 27). Although the initial rates of decline in the ASL volume were similar, the steady-state level was substantially less in G551D/F508del HBE compared with non-CF HBE (Fig. 5A). In G551D/F508del HBE, the addition of VX-770 increased the ASL volume to approximately half of that observed in non-CF HBE (Fig. 5A). The response to VX-770 was concentration dependent, blocked by CFTRinh-172, and reduced in the absence of VIP (Fig. 5 B and C). These data show that VX-770 increased the amount of fluid on the apical surface of G551D/F508del HBE, indicating less absorption, more secretion, or both.
Fig. 5.
Potentiation of CFTR by VX-770 partially restored fluid regulation and cilia beating in G551D/F508del HBE. (A) The ASL volume in G551D/F508del HBE and wild-type HBE after up to 72 hours' incubation at 37 °C in the presence of 30 nM VIP with or without VX-770. All modulators were added to the basolateral surface. (B) Concentration–response curve (n = 3–9) of the change in ASL volume in G551D/F508del HBE after VX-770 addition at the indicated concentrations in the absence (open circles) or presence (filled circles) of 30 nM VIP. All data were normalized to ASL volume in the vehicle (DMSO)–treated controls and expressed as percent control. (C) Mean (n = 3–9) ASL volume in the absence (open bars) or presence (filled bars) of 10 μM VX-770 and in the presence of 30 nM VIP and/or 20 μM CFTRinh-172. (D) Representative tracings of the light intensity (y axis in relative units) derived from a single region of interest in G551D/F508del HBE monitored 5 days after adding 100 μl of fluid to the apical surface. (E) Mean (± SEM; n = 6) CBF wild-type HBE (filled bars) or G551D/F508del HBE (open bars) after a 5-day treatment with DMSO, 30 nM VIP, 10 μM VX-770, or 30 nM VIP with 10 μM VX-770. Single asterisk indicates significantly different (P < 0.05) from vehicle control in G551D/F508del HBE; double asterisk indicates significantly different (P < 0.05) from vehicle control and VX-770 alone. All data are from G551D/F508del HBE isolated from the bronchi of a single individual.
Dehydration of the airway surface in the CF lung is believed to contribute to the inability of the cilia to extend and beat normally, preventing them from supplying the motive force for mucus transport (11). We next tested whether the increase in the apical fluid level by VX-770 in cultured G551D/F508del HBE was sufficient to increase the CBF, which was significantly reduced compared with that observed in non-CF HBE (Fig. 5 D and E). Treatment of G551D/F508del HBE with 10 μM VX-770 for 5 days increased the CBF compared with vehicle-treated controls, and this response was augmented by VIP (Fig. 5 D and E). In the presence of VIP and VX-770, the CBF in G551D/F508del HBE was similar to that observed for non-CF HBE (Fig. 5E). In addition, a change in the amplitude and duration of the waveform was observed, suggesting that the pattern of cilia beating was also altered by the increase in the apical fluid level (28). These results suggest that the partial restoration of Cl− and Na+ transport by VX-770 in G551D/F508del HBE is associated with an increase in the apical fluid level and an increase in cilia beating, and that these effects are further enhanced by augmenting CFTR activation by the addition of VIP to further stimulate the cAMP/PKA-signaling pathway.
Discussion
We demonstrated in this study that VX-770 is a potentiator of human G551D-, F508del-, and wild-type CFTR Cl− channel function. A CFTR potentiator is a pharmacological agent that increases the flow of ions through activated CFTR channels. For a CFTR potentiator to act, CFTR must be at the cell surface, and the CFTR channel must be activated by endogenous cAMP/PKA signaling pathways such as β-adrenergic, VIP, or adenosine receptor stimulation (9, 26). Thus a potentiator such as VX-770 is expected to work in the background of the normal physiological control over CFTR function by increasing its activity only when and where it is needed. In these in vitro systems, VX-770 fulfilled this definition of a CFTR potentiator in that it increased Cl− secretion only after stimulation of the cAMP/PKA signaling pathway (Fig. 1) and acted by increasing the Po of the CFTR channel (Fig. 2). The ability of VX-770 to increase the Po in excised membrane patches that are removed from the cytosolic signaling pathways suggests that VX-770 acts directly on CFTR to increase its gating activity. The direct action of VX-770 on CFTR is supported by studies indicating that VX-770 did not modulate cAMP/PKA signaling (Fig. 1F). Further studies are required to define the molecular mechanism by which VX-770 potentiates CFTR and to determine whether VX-770 also acts on other defective CFTR forms with other CFTR mutations. Additional studies are also required to determine whether VX-770 potentiates CFTR-mediated HCO3− secretion, a defect that is believed to contribute to abnormal epithelial function in some organs affected in CF, including the lung and pancreas (29).
In vivo studies correlating CFTR activity in CF patients with disease severity suggest that, as a group, individuals exhibiting 10–25% of the CFTR activity observed in non-CF individuals have less severe disease as assessed by age of diagnosis, pulmonary function, and pancreatic function than individuals with no detectible CFTR activity (14, 30–33). The results reported here show that VX-770 increased CFTR-mediated Cl− secretion in G551D/F508del HBE from ≈5% to a maximum level of ≈50% of that measured in non-CF HBE. Based on these in vitro studies, VX-770 might be expected to result in clinical benefit in CF patients carrying the G551D mutation on at least one allele.
VX-770 also increased CFTR-mediated Cl− secretion in cultured HBE isolated from the bronchi of some F508del-homozygous CF individuals to levels above 10% of that observed in non-CF HBE. The reason for the variable CFTR function in cultured HBE derived from different F508del-homozygous donor lungs is not known, but could be due to polyvariant mutant CFTR genes, such as the M470 variant (34), or other genes that affect CFTR levels or activity. Nevertheless, this provocative result suggests that the efficacy of VX-770 in G551D/F508del HBE may be caused by potentiation of both G551D- and F508del CFTR and that consideration should be given to testing VX-770 in F508del-homozygous CF patients. This observation also supports a rationale for assessing the effects of VX-770 in combination with CFTR correctors that are designed to increase the cell surface density of F508del CFTR (17).
In both FRT cells and HBE, the EC50 for VX-770 was lower for F508del CFTR than for G551D CFTR, suggesting that VX-770 may have a higher affinity for F508del CFTR. Other CFTR potentiators, including genistein, are also more potent against F508del CFTR compared with G551D CFTR (19). The lower potency of some CFTR potentiators against G551D CFTR along with homology modeling studies have suggested that these compounds bind at a site near the G551D mutation (35). The ability of VX-770 to potentiate CFTR in some F508del HBE is consistent with previous studies showing the presence of low levels of F508del CFTR function in vitro (17) and in vivo (36).
A long-standing question in the CF field is whether pharmacological agents that directly target and restore the loss in CFTR-mediated Cl− secretion would be sufficient to slow or stop the deleterious cascade of events that lead to disease progression in CF patients, including dehydration of the airway surface and the inability of the airway to clear mucus and microbes (9, 11, 22). In this study, open-circuit recordings using cultured G551D/F508del HBE and non-CF HBE were performed in a way that resembles the in vivo assays of nasal PD that are commonly performed in CF patients (25). As observed in vivo, the baseline PD was elevated and the amiloride response was larger in G551D/F508del HBE compared with that in non-CF HBE. This has been attributed, at least in part, to excessive ENaC-mediated Na+ absorption in CF tissue compared with non-CF tissue (4, 25, 37, 38). Addition of VX-770 decreased the baseline PD and amiloride response in G551D/F508del HBE. Although these PD results do not rule out the possibility that VX-770 may have effects on other channels (39) or proteins, the data may be explained by CFTR potentiation leading to a secondary decrease in ENaC activity. This is supported by the lack of direct inhibition of the rat α-, β-, γ-ENaC channel; however, differences between species and/or subunit stoichiometry between the recombinant and HBE culture models may alter the activity of VX-770 on the isolated human ENaC. In addition, other studies have shown that pharmacological or gene-transfer correction of CFTR function in CF airway cell cultures restored the balance between Cl− secretion and Na+ absorption (40, 41).
Several hypotheses have been proposed to explain the link between the loss in CFTR function and the increase in ENaC function in the CF airway, including changes in cytoplasmic pH (42) and Cl− concentration (43), physical coupling between the two proteins (44), and activation of ENaC by airway surface fluid proteases (45). Our results are consistent with a model proposed by Horisberger (46) whereby the increase in CFTR-mediated Cl− secretion caused by VX-770 would be expected to depolarize the apical membrane and thereby reduce the electrochemical driving force for Na+ absorption through ENaC coexpressed along with CFTR in the apical membrane. This is supported by data suggesting the acute effects of VX-770 on Na+ absorption, the ability of a CFTR inhibitor to block this effect, and the lack of direct ENaC inhibition by VX-770 in cells that do not express CFTR.
The increase in the fluid level on the apical surface of G551D/F508del HBE after several hours of treatment with VX-770 can be explained by an increase in CFTR-mediated Cl− secretion and the concomitant decrease in excessive ENaC-mediated Na+ absorption. Although the amiloride response after addition of VX-770 to G551D/F508del HBE was similar to that in non-CF HBE, the apical fluid level at steady-state was approximately half of that observed in non-CF HBE (Fig. 5A). This may be caused by the incomplete restoration of CFTR-dependent fluid secretory function in these cultures of ciliated airway epithelium. Nevertheless, the increase in apical fluid levels in VX-770–treated G551D/F508del HBE was sufficient to increase cilia beating to levels observed in non-CF HBE. In vivo, we hypothesize that the increase in height of the apical fluid level and frequency of cilia beating would facilitate the mechanical clearance of mucus and microbes, the failure of which is thought to contribute to CF lung pathogenesis (9).
Although VX-770 had some effect on unstimulated HBE, likely because of endogenous levels of cAMP in these cells (47), the maximal pharmacological effect of VX-770 on ion and fluid transport required stimulation of the cAMP/PKA signaling pathway by either forskolin or VIP. It is likely that the situation is more dynamic in vivo, where neuronal pathways and mechanical forces exerted on the airway regulate the epithelial cellular signaling pathways to control the activity of CFTR and other ion transport pathways to regulate fluid transport (48, 49). Because of this, it is possible that the endogenous level of CFTR activation will be higher in vivo. However, the ability of cAMP agonists to augment the effects of VX-770 in vitro raises the question of whether pharmacological activation of CFTR by agents that stimulate the cAMP/PKA-signaling pathway in vivo, such as the β-adrenergic agonists, would enhance any potential clinical benefit of VX-770.
Although transgenic mouse models of CF carrying the G551D or F508del mutation have been developed, we did not test VX-770 in these models because it does not potentiate mouse CFTR expressed in FRT cells (Fig. S4). This difference, however, may be exploited to understand the mechanism of action and binding site of VX-770. Also, because the cell background is the same as that used for expression of the human CFTR forms, these results support the proposed direct action of VX-770 on CFTR.
In conclusion, these in vitro studies demonstrated that VX-770 is a CFTR potentiator that increased CFTR-mediated Cl− transport by increasing the Po of activated CFTR. In G551D/F508del HBE, the increase in CFTR-mediated Cl− secretion by VX-770 was associated with a decrease in amiloride-sensitive Na+ absorption and an increase in the apical fluid height and CBF. The results of these in vitro experiments support the hypothesis that drugs aimed at increasing CFTR function may ameliorate the downstream physiological processes that contribute to CF lung disease.
Materials and Methods
Recombinant and Human Bronchial Epithelial Cell Culture.
FRT cells expressing human G551D CFTR or mouse wild-type CFTR and NIH 3T3 cells expressing human wild-type-, human F508del CFTR or the rat α, β, and γ subunits of ENaC were cultured as previously described (17). HBE were isolated from the bronchi of lungs obtained from non-CF or CF individuals after autopsy or lung transplantation and cultured as previously described (17). For details, see SI Materials and Methods.
Ussing Chamber Recordings.
Ussing chamber techniques using cultured FRT and HBE cells were used to record the IT in the voltage-clamp mode (Vhold = 0 mV) or the PD (PD = Vbasolateral− Vapical) in the open-circuit recording mode (see SI Materials and Methods).
cAMP Measurements.
The total cAMP concentration in FRT cells following test compound application was determined using a cAMP-Screen 96-Well Immunoassay System (see SI Materials and Methods).
Patch-Clamp Recordings.
The single-channel activity of G551D CFTR, wild-type CFTR, and temperature-corrected F508del CFTR was measured using excised inside-out membrane patch recordings as previously described (17). The isolated Na+ current in ENaC-NIH 3T3 cells was recorded using the whole-cell patch clamp recording configuration (for details, see SI Materials and Methods).
Measurement of ASL Volume and CBF.
To monitor changes in the ASL volume and CBF, the apical layer of G551D/F508del HBE was washed twice with absorption buffer followed by addition of 100 μl of absorption buffer to the apical layer. To monitor the ASL volume, the fluid remaining after up to 72 h of incubation with test compounds was aspirated from the apical surface and placed in preweighed tubes. The CBF was monitored as previously described (50) (for details, see SI Materials and Methods).
Statistical Analyses.
Statistical comparisons were made using analysis of variance (ANOVA) followed by Tukey's multiple comparison test or Student's t test. All data are presented as the mean ± SEM.
Supplementary Material
Acknowledgments.
We thank Cystic Fibrosis Foundation Therapeutics Inc. (CFFT) for their generous financial support, as well as Robert Beall, Preston Campbell, John Chabala, Eric Gordon, Diana Wetmore, and Christopher M. Penland for their guidance. We also thank Dr. Joseph W. Pilewski for providing G551D/F508del HBE, Dr. L. J. V. Galietta for providing the G551D-FRT cells, Dr. Michael J. Welsh for providing the NIH 3T3 cells expressing F508del- and wild-type CFTR, and Dr. Mary Lang-Furr for providing the NIH 3T3 cells expressing ENaC. Vertex Pharmaceuticals and CFFT have a financial interest in the discovery and development of therapies for CF.
Footnotes
Conflict of interest statement: F.V.G., S.H., P.D.J.G., B.B., D.C., T.N., A.T., A.S., J.J., A.H., J.Z., J.M., V.A., C.D., J.Y., C.Y., E.R.O., and P.N. are employees of Vertex Pharmaceuticals Incorporated, which is evaluating VX-770 as a potential treatment for cystic fibrosis.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0904709106/DCSupplemental.
References
- 1.Kerem B, et al. Identification of the cystic fibrosis gene: Genetic analysis. Science. 1989;245:1073–1080. doi: 10.1126/science.2570460. [DOI] [PubMed] [Google Scholar]
- 2.Riordan JR, et al. Identification of the cystic fibrosis gene: Cloning and characterization of complementary DNA. Science. 1989;245:1066–1073. doi: 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- 3.Berger HA, et al. Identification and regulation of the cystic fibrosis transmembrane conductance regulator-generated chloride channel. J Clin Invest. 1991;88:1422–1431. doi: 10.1172/JCI115450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Knowles M, Gatzy J, Boucher R. Relative ion permeability of normal and cystic fibrosis nasal epithelium. J Clin Invest. 1983;71:1410–1417. doi: 10.1172/JCI110894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li C, et al. ATPase activity of the cystic fibrosis transmembrane conductance regulator. J Biol Chem. 1996;271:28463–28468. doi: 10.1074/jbc.271.45.28463. [DOI] [PubMed] [Google Scholar]
- 6.Quinton PM. Chloride impermeability in cystic fibrosis. Nature. 1983;301:421–422. doi: 10.1038/301421a0. [DOI] [PubMed] [Google Scholar]
- 7.Poulsen JH, Fischer H, Illek B, Machen TE. Bicarbonate conductance and pH regulatory capability of cystic fibrosis transmembrane conductance regulator. Proc Natl Acad Sci USA. 1994;91:5340–5344. doi: 10.1073/pnas.91.12.5340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosenstein BJ, Cutting GR. The diagnosis of cystic fibrosis: A consensus statement. J Pediatr. 1998;132:589–595. doi: 10.1016/s0022-3476(98)70344-0. [DOI] [PubMed] [Google Scholar]
- 9.Boucher RC. Cystic fibrosis: A disease of vulnerability to airway surface dehydration. Trends Mol Med. 2007;13:231–240. doi: 10.1016/j.molmed.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 10.Joo NS, Irokawa T, Robbins RC, Wine JJ. Hyposecretion, not hyperabsorption, is the basic defect of cystic fibrosis airway glands. J Biol Chem. 2006;281:7392–7398. doi: 10.1074/jbc.M512766200. [DOI] [PubMed] [Google Scholar]
- 11.Matsui H, et al. Evidence for periciliary liquid layer depletion, not abnormal ion composition, in the pathogenesis of cystic fibrosis airways disease. Cell. 1998;95:1005–1015. doi: 10.1016/s0092-8674(00)81724-9. [DOI] [PubMed] [Google Scholar]
- 12.Gibson RL, Burns JL, Ramsey BW. Pathophysiology and management of pulmonary infections in cystic fibrosis. Am J Respir Crit Care Med. 2003;168:918–951. doi: 10.1164/rccm.200304-505SO. [DOI] [PubMed] [Google Scholar]
- 13.Welsh MJ, Smith AE. Molecular mechanisms of CFTR chloride channel dysfunction in cystic fibrosis. Cell. 1993;73:1251–1254. doi: 10.1016/0092-8674(93)90353-r. [DOI] [PubMed] [Google Scholar]
- 14.Van Goor F, Hadida S, Grootenhuis PDJ. Pharmacological rescue of mutant CFTR funciton for the treatment of cystic fibrosis. Topics Med Chem. 2008;3:29. [Google Scholar]
- 15.Bobadilla JL, Macek M, Jr, Fine JP, Farrell PM. Cystic fibrosis: A worldwide analysis of CFTR mutations—correlation with incidence data and application to screening. Hum Mutat. 2002;19:575–606. doi: 10.1002/humu.10041. [DOI] [PubMed] [Google Scholar]
- 16.Pedemonte N, et al. Small-molecule correctors of defective DeltaF508 CFTR cellular processing identified by high-throughput screening. J Clin Invest. 2005;115:2564–2571. doi: 10.1172/JCI24898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Goor F, et al. Rescue of DeltaF508 CFTR trafficking and gating in human cystic fibrosis airway primary cultures by small molecules. Am J Physiol Lung Cell Mol Physiol. 2006;290:L1117–L1130. doi: 10.1152/ajplung.00169.2005. [DOI] [PubMed] [Google Scholar]
- 18.Sheppard DN, et al. Mutations in CFTR associated with mild-disease-form Cl− channels with altered pore properties. Nature. 1993;362:160–164. doi: 10.1038/362160a0. [DOI] [PubMed] [Google Scholar]
- 19.Pedemonte N, et al. Phenylglycine and sulfonamide correctors of defective delta F508 and G551D cystic fibrosis transmembrane conductance regulator chloride-channel gating. Mol Pharmacol. 2005;67:1797–1807. doi: 10.1124/mol.105.010959. [DOI] [PubMed] [Google Scholar]
- 20.Accurso FJ, et al. Interim results of phase 2a study of VX-770 to evaluate safety, pharmacokinetics, and biomarkers of CFTR activity in cystic fibrosis subjects with G551D. Pediatr Pulmonol. 2008;31(Suppl):1. [Google Scholar]
- 21.Ma T, et al. Thiazolidinone CFTR inhibitor identified by high-throughput screening blocks cholera toxin-induced intestinal fluid secretion. J Clin Invest. 2002;110:1651–1658. doi: 10.1172/JCI16112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang C, Finkbeiner WE, Widdicombe JH, McCray PB, Jr, Miller SS. Altered fluid transport across airway epithelium in cystic fibrosis. Science. 1993;262:424–427. doi: 10.1126/science.8211164. [DOI] [PubMed] [Google Scholar]
- 23.Illek B, et al. Defective function of the cystic fibrosis-causing missense mutation G551D is recovered by genistein. Am J Physiol. 1999;277:C833–C839. doi: 10.1152/ajpcell.1999.277.4.C833. [DOI] [PubMed] [Google Scholar]
- 24.Castellani C, et al. Consensus on the use and interpretation of cystic fibrosis mutation analysis in clinical practice. J Cyst Fibros. 2008;7:179–196. doi: 10.1016/j.jcf.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Knowles MR, Paradiso AM, Boucher RC. In vivo nasal potential difference: Techniques and protocols for assessing efficacy of gene transfer in cystic fibrosis. Hum Gene Ther. 1995;6:445–455. doi: 10.1089/hum.1995.6.4-445. [DOI] [PubMed] [Google Scholar]
- 26.Choi JY, et al. Synergistic airway gland mucus secretion in response to vasoactive intestinal peptide and carbachol is lost in cystic fibrosis. J Clin Invest. 2007;117:3118–3127. doi: 10.1172/JCI31992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Derand R, et al. Activation of VPAC1 receptors by VIP and PACAP-27 in human bronchial epithelial cells induces CFTR-dependent chloride secretion. Br J Pharmacol. 2004;141:698–708. doi: 10.1038/sj.bjp.0705597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gheber L, Priel Z. Extraction of cilium beat parameters by the combined application of photoelectric measurements and computer simulation. Biophys J. 1997;72:449–462. doi: 10.1016/S0006-3495(97)78686-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Quinton PM. Cystic fibrosis: Impaired bicarbonate secretion and mucoviscidosis. Lancet. 2008;372:415–417. doi: 10.1016/S0140-6736(08)61162-9. [DOI] [PubMed] [Google Scholar]
- 30.Davis PB, Drumm M, Konstan MW. Cystic fibrosis. Am J Respir Crit Care Med. 1996;154:1229–1256. doi: 10.1164/ajrccm.154.5.8912731. [DOI] [PubMed] [Google Scholar]
- 31.McKone EF, Emerson SS, Edwards KL, Aitken ML. Effect of genotype on phenotype and mortality in cystic fibrosis: A retrospective cohort study. Lancet. 2003;361:1671–1676. doi: 10.1016/S0140-6736(03)13368-5. [DOI] [PubMed] [Google Scholar]
- 32.Noone PG, et al. Lung disease associated with the IVS8 5T allele of the CFTR gene. Am J Respir Crit Care Med. 2000;162:1919–1924. doi: 10.1164/ajrccm.162.5.2003160. [DOI] [PubMed] [Google Scholar]
- 33.Noone PG, et al. Cystic fibrosis gene mutations and pancreatitis risk: Relation to epithelial ion transport and trypsin inhibitor gene mutations. Gastroenterology. 2001;121:1310–1319. doi: 10.1053/gast.2001.29673. [DOI] [PubMed] [Google Scholar]
- 34.Cuppens H, et al. Polyvariant mutant cystic fibrosis transmembrane conductance regulator genes. The polymorphic (Tg)m locus explains the partial penetrance of the T5 polymorphism as a disease mutation. J Clin Invest. 1998;101:487–496. doi: 10.1172/JCI639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moran O, Galietta LJ, Zegarra-Moran O. Binding site of activators of the cystic fibrosis transmembrane conductance regulator in the nucleotide binding domains. Cell Mol Life Sci. 2005;62:446–460. doi: 10.1007/s00018-004-4422-3. [DOI] [PubMed] [Google Scholar]
- 36.Thomas SR, Jaffe A, Geddes DM, Hodson ME, Alton EW. Pulmonary disease severity in men with deltaF508 cystic fibrosis and residual chloride secretion. Lancet. 1999;353:984–985. doi: 10.1016/S0140-6736(98)05447-6. [DOI] [PubMed] [Google Scholar]
- 37.Knowles M, Gatzy J, Boucher R. Increased bioelectric potential difference across respiratory epithelia in cystic fibrosis. N Engl J Med. 1981;305:1489–1495. doi: 10.1056/NEJM198112173052502. [DOI] [PubMed] [Google Scholar]
- 38.Knowles MR, et al. Abnormal ion permeation through cystic fibrosis respiratory epithelium. Science. 1983;221:1067–1070. doi: 10.1126/science.6308769. [DOI] [PubMed] [Google Scholar]
- 39.Bertrand CA, Zhang R, Pilewski JM, Frizzell RA. SLC26A9 is a constitutively active, CFTR-regulated anion conductance in human bronchial epithelia. J Gen Physiol. 2009;133:421–438. doi: 10.1085/jgp.200810097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jiang C, et al. Ability of adenovirus vectors containing different CFTR transcriptional cassettes to correct ion transport defects in CF cells. Am J Physiol. 1996;271:L527–L537. doi: 10.1152/ajplung.1996.271.4.L527. [DOI] [PubMed] [Google Scholar]
- 41.Zabner J, Couture LA, Smith AE, Welsh MJ. Correction of cAMP-stimulated fluid secretion in cystic fibrosis airway epithelia: Efficiency of adenovirus-mediated gene transfer in vitro. Hum Gene Ther. 1994;5:585–593. doi: 10.1089/hum.1994.5.5-585. [DOI] [PubMed] [Google Scholar]
- 42.Reddy MM, Wang XF, Quinton PM. Effect of cytosolic pH on epithelial Na+ channel in normal and cystic fibrosis sweat ducts. J Membr Biol. 2008;225:1–11. doi: 10.1007/s00232-008-9126-4. [DOI] [PubMed] [Google Scholar]
- 43.Briel M, Greger R, Kunzelmann K. Cl− transport by cystic fibrosis transmembrane conductance regulator (CFTR) contributes to the inhibition of epithelial Na+ channels (ENaCs) in Xenopus oocytes co-expressing CFTR and ENaC. J Physiol. 1998;508:825–836. doi: 10.1111/j.1469-7793.1998.825bp.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schreiber R, Hopf A, Mall M, Greger R, Kunzelmann K. The first-nucleotide binding domain of the cystic-fibrosis transmembrane conductance regulator is important for inhibition of the epithelial Na+ channel. Proc Natl Acad Sci USA. 1999;96:5310–5315. doi: 10.1073/pnas.96.9.5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Myerburg M, et al. Airway surface liquid volume regulates ENaC by altering the serine protease-protease inhibitor balance: A mechanism for sodium hyperabsorption in cystic fibrosis. J Biol Chem. 2006;281:27942–27949. doi: 10.1074/jbc.M606449200. [DOI] [PubMed] [Google Scholar]
- 46.Horisberger JD. ENaC CFTR interactions: The role of electrical coupling of ion fluxes explored in an epithelial cell model. Pflugers Arch. 2003;445:522–528. doi: 10.1007/s00424-002-0956-0. [DOI] [PubMed] [Google Scholar]
- 47.Blouquit S, et al. Effects of endothelin-1 on epithelial ion transport in human airways. Am J Respir cell Mol Biol. 2003;29:245–251. doi: 10.1165/rcmb.2002-0104OC. [DOI] [PubMed] [Google Scholar]
- 48.Lazarowski ER, Boucher RC, Harden TK. Constitutive release of ATP and evidence for major contribution of ecto-nucleotide pyrophosphatase and nucleoside diphosphokinase to extracellular nucleotide concentrations. J Biol Chem. 2000;275:31061–31068. doi: 10.1074/jbc.M003255200. [DOI] [PubMed] [Google Scholar]
- 49.Tarran R, Button B, Boucher RC. Regulation of normal and cystic fibrosis airway surface liquid volume by phasic shear stress. Annu Rev Physiol. 2006;68:543–561. doi: 10.1146/annurev.physiol.68.072304.112754. [DOI] [PubMed] [Google Scholar]
- 50.Sisson JH, Stoner JA, Ammons BA, Wyatt TA. All-digital image capture and whole-field analysis of ciliary beat frequency. J Microsc. 2003;211:103–111. doi: 10.1046/j.1365-2818.2003.01209.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.