Abstract
Co-application of the convulsant 4-aminopyridine (4-AP) and the GABAB receptor antagonist CGP 55845 to adult guinea pig hippocampal slices elicits giant GABA-mediated postsynaptic potentials (GPSPs) and epileptiform discharges. Here we tested the effects of the group I metabotropic glutamate receptor (mGluR) subtype-selective antagonists LY 367385 (mGlu1, 100 µM), MPEP (mGlu5, 10 µM), and MTEP (mGlu5, 500 nM) on this synchronous activity. Electrophysiological field recordings were performed in the CA3 region of hippocampal slices from adult guinea pigs. The mGlu5 receptor antagonists increased GPSP rate, but the mGlu1 receptor antagonist did not. This ability of mGlu5 receptor antagonists to increase the rate of GPSPs indicates that enough endogenous glutamate is released under these conditions to activate group I mGluR; nevertheless, co-application of a mGlu1 receptor antagonist (LY 367385 or JNJ 16259685) and MPEP did not decrease pre-existing epileptiform activity. Furthermore, co-application of LY 367385 and MPEP did not prevent the emergence of epileptiform activity. When ionotropic glutamate receptor (iGluR) antagonists were present, neither MPEP nor the group I mGluR agonist DHPG changed GPSP rate, suggesting that pyramidal cell-to-interneuron iGluR-mediated synaptic connections are involved in the rate change mechanism. In contrast to the lack of effect of group I mGluR antagonists on epileptiform activity in the 4-AP/CGP 55845 model, group I mGluR antagonists blocked the emergence of longer epileptiform events and decreased the overall amount of synchronous activity in the GABAA antagonist/4-AP model. In conclusion, in the 4-AP/CGP 55845 model, enough glutamate was released to activate group I mGluRs and affect GPSP rate via mGlu5 receptors; however, this group I mGluR activation was not required for the generation of the epileptiform activity.
Introduction
Group I metabotropic glutamate receptor (mGluR) activation has been implicated as a mediator of epileptiform activity in several different models both in vitro (Arvanov et al., 1995; Burke and Hablitz, 1995; Taylor et al., 1995; Merlin and Wong, 1997; Martín et al., 2001; Lee et al., 2002) and in vivo (Tizzano et al., 1995; Camón et al., 1998; Thomsen and Dalby, 1998; Chapman et al., 1999; Chapman et al., 2000; Smolders et al., 2004; Yan et al. 2005). In some of these models group I mGluR antagonists block the emergence of the epileptiform activity but do not block established epileptiform activity (Arvanov et al., 1995; Martín et al., 2001). Both the mGlu1 and mGlu5 subtypes of group I mGluR have been shown to play a role in the generation of epileptiform activity (Chapman et al., 1999; Chapman et al., 2000; Merlin, 2002; Smolders et al., 2004), with possible differential roles in induction and maintenance of the activity (Merlin, 2002). These experiments and others have generated interest in group I mGluRs as possible targets for antiepileptic and even antiepileptogenic drug development (Merlin, 2002; Moldrich et al., 2003; Wong et al., 2005). In addition, these experiments bring to mind the question of whether group I mGluR activation is required for the induction and/or maintenance of all epileptiform activity. With this question in mind, here we test the ability of group I mGluR antagonists to block or prevent the emergence of epileptiform activity in the 4-aminopyridine (4-AP)/CGP 55845 model, which has some relevant features not shared by other in vitro models.
In the presence of the convulsant 4-AP and the GABAB receptor antagonist CGP 55845, giant GABA-mediated postsynaptic potentials (GPSPs) occur rhythmically every 15–30 s, and each GPSP is directly followed by an ionotropic glutamate-dependent epileptiform discharge (Kantrowitz et al., 2005). The 4-AP/CGP 55845 model is of particular interest because it shares features with tissue taken from epileptic humans: 1) In contrast to many other in vitro epilepsy models, the epileptiform activity is dependent upon intact GABAA receptor function, which may also be true of epileptiform activity in tissue taken from epileptics (Babb et al., 1989; Kohling et al., 1998; Cohen et al., 2002); 2) Brain tissue from epileptic humans has been reported to lack GABAB receptor function (Deisz, 1999); 3) Brain tissue taken from patients with intractable epilepsy shows evidence of depolarizing GABA responses in pyramidal cells generated by synchronous interneuron activity (Cohen et al., 2002). Likewise, in the 4-AP /CGP 55845 model, the GPSPs are caused by the synchronous firing of a group of GABAergic interneurons (Michelson and Wong, 1991), and each GPSP in the pyramidal cell has a prominent depolarizing GABA component (Kantrowitz et al., 2005). Rodent data indicate that it is the depolarizing component of the GPSP that triggers the discharge that follows (Kantrowitz et al., 2005; see also Avoli et al., 1996).
Because we expect a condition of increased endogenous glutamate release in the presence of 4-AP and CGP 55845, we expect mGluRs to be activated and to possibly play a role in the generation or modulation of the synchronous activity. Here we find evidence of group I mGluR activation in 4-AP/CGP 55845 and investigate its role in the generation of the epileptiform activity and its influence upon GPSP rate.
Methods
Slice preparation and electrophysiology
The experiments were done in adult guinea pig hippocampal slices. The animal protocol was approved by the Animal Care and Use Committee at SUNY Downstate Medical Center and is in compliance with international guidelines. Guinea pigs (220–350 g) were anesthetized with halothane and decapitated with a guillotine. 300 µm transverse slices of hippocampus were cut using a vibratome. Cutting solution was the same as the extracellular recording solution listed below except CaCl2 was 0.5 mM and MgCl2 was 8 mM. Slices were transferred to a holding chamber and held at 31.5°C for 1 hour and at room temperature thereafter as described in Kantrowitz et al. (2005). After one hour or longer in the holding chamber, slices were transferred to the recording chamber where they were maintained at an interface between continuously perfusing oxygenated solution and humidified 95% O2 / 5% CO2 gas at 34°C.
The extracellular recording solution (which was also the solution in the holding chamber) for all the experiments except the ones using gabazine contained (in mM) 125 NaCl, 25 NaHCO3, 2.5 KCl, 1.6 MgCl2, 2.0 CaCl2, 11 d-glucose, as used previously (e.g., Kantrowitz et al., 2005). The extracellular recording solution for the experiments using gabazine was the same except the KCl concentration was 5.0 mM instead of 2.5 mM. 5.0 mM is the KCl concentration used by Merlin and Wong (1997) and Lee et al. (2002) in experiments using GABAA antagonist to elicit synchronous activity in hippocampal slices. GABAA antagonist does not elicit synchronous activity in 2.5 mM KCl in adult guinea pig hippocampal slices (A. Salah, V. Chen, K. L. Perkins, personal observation).
Extracellular field potential recordings were performed with a glass electrode (0.3 – 2 MΩ) filled with 150 mM NaCl. The recording electrode was placed at the CA3 stratum lacunosum-moleculare (SLM)-stratum radiatum (SR) border or in the CA3 pyramidal cell body layer. Preliminary dual-electrode experiments demonstrated that the field potentials occur simultaneously but with a different polarity at the two sites. Our electrode placement was chosen to give the largest signal to noise ratio, which was most often at the SLM-SR border.
Drugs
Drugs used to elicit epileptiform activity in the majority of experiments were 4-aminopyridine (4-AP, 50 µM) and the GABAB receptor antagonist (2S)-3-[[(1S)-1-(3,4-dichlorophenyl)ethyl]amino-2-hydroxypropyl](phenylmethyl) phosphinic acid (CGP 55845, 1–2 µM; Davies et al., 1993). 4-AP is pro-convulsant because it increases transmitter release (Buckle and Haas, 1982), apparently due to block of certain voltage-gated potassium channels on synaptic terminals (Storm, 1988; Coetzee et al., 1999). In adult guinea pigs, co-application of 4-AP and CGP 55845 is preferable to 4-AP alone (Rutecki et al., 1987) for the study of epileptiform activity because 4-AP alone often elicits only GPSPs (Kantrowitz et al., 2005). In a separate set of experiments epileptiform activity was induced by applying the GABAA antagonist gabazine (6 µM) along with 4-AP (50 µM).
Group I mGluR antagonists used were (S)-(+)-α-amino-4-carboxy-2-methylbenzeneacetic acid (LY 367385, 100 µM), which preferentially blocks the mGlu1 receptor subtype; 3,4-dihydro-2H-pyrano[2,3-b]quinolin-7-yl)-(cis-4-methoxycyclohexyl)-methanone (JNJ 16259685, 100 nM, dissolved in DMSO), a non-competitive antagonist which also preferentially blocks mGlu1 receptor-mediated effects; 2-methyl-6-(phenylethynyl)pyridine hydrochloride (MPEP, 10 µM), which preferentially blocks the mGlu5 receptor subtype; and 3-((2-methyl-1,3-thiazol-4-yl)ethynyl)pyridine hydrochloride (MTEP, 500 nM), which also preferentially blocks the mGlu5 receptor subtype. These particular mGluR antagonists at these concentrations were chosen for their potency and specificity at group I metabotropic glutamate receptors. Often LY 367385 and MPEP were applied together in order to completely block group I mGluRs; in those cases they will be referred to as LY/MPEP. In one experiment the group I mGluR agonist (S)-3,5-dihydroxyphenylglycine (DHPG, 50 µM) was used. When noted, the AMPA/kainate ionotropic glutamate receptor antagonist 2,3-dioxo-6-nitro-1,2,3,4,-tetrahydrobenzo[f]quinoxaline-7-sulfonamide disodium salt (NBQX, 10 µM ) and/or the NMDA ionotropic glutamate receptor antagonist d-(−)-2-amino-5-phosphonopentanoic acid (d-AP5, 50 µM) were added to the recording solution. The MTEP was purchased from Ascent Scientific (Weston, United Kingdom). The other glutamate receptor antagonists and agonist and the CGP 55845 were purchased from Tocris Cookson (Ellisville, MO). Other chemicals were purchased from Sigma-Aldrich (St. Louis, MO).
When 4-AP/CGP 55845 were added to the recording solution alone or simultaneous with other drugs, the brain slice was exposed for 1 hour, during which the GPSP rate stabilized, before the addition of a new drug. When a new drug was added, the activity was measured for 10–20 min after waiting a period of 30–40 min to insure complete drug wash in. When washing out a drug, the drug was washed for 30–40 minutes, and then the activity was measured for a period of 10–20 minutes.
Data analysis
A GPSP alone was not considered to be epileptiform activity. A GPSP followed by a discharge was considered to be epileptiform activity. When a discharge followed a GPSP, it was made up of one or more individual deflections in the field potential recording, each of which corresponds to a burst in the pyramidal cells (Kantrowitz et al., 2005). In a departure from our earlier paper (Kantrowitz et al., 2005), each of these individual deflections following the GPSP will be termed a discharge deflection (DD). Three measures were used to analyze the 4-AP/CGP 55845 data: the number of GPSPs per 10-min period, the number of DDs per GPSP (zeros were included in computing the mean), and the total number of DDs per 10-min period. The number of DDs per 10-min period was used as the measure of the magnitude of epileptiform activity.
When recording activity in gabazine or gabazine/4-AP, there were no GPSPs. We did not attempt to distinguish between what we considered to be epileptiform activity and what we considered to be simply synchronous activity; i.e., we did not employ a threshold length above which events were considered to be epileptiform. All synchronous events were measured from the initial peak of the first deflection to the final peak of the last deflection as indicated in Fig. 5A and included in the measure of total time spent in synchronous activity.
Values are reported as mean ± SD. n values in the text refer to the number of slices (or to the number of pairs of slices in the paired-slice experiments). In no case were more than 2 slices from the same animal used (or more than 2 pairs of slices in the paired-slice experiments), and in no case were two slices used simultaneously. Where percent change is reported, the percent change was calculated for each recording individually, and then the mean of the changes was calculated. Statistics were performed on raw data rather than on the percent change data. Unless otherwise noted, each slice served as its own control. For experiments in which there were three conditions (control, drug, wash) the nonparametric Friedman 2-way analysis of variance test was used initially to test for a difference among the groups; P < 0.05 was considered a significant difference. For cases in which the Friedman test showed no significant difference, the Friedman P value is shown in the text. For experiments in which there were only two groups or in which the Friedman test had shown a significant difference, P values were calculated using the Wilcoxon signed ranks test, which is the nonparametric equivalent of the paired t-test. P < 0.05 was considered significant. P values reported in the text are Wilcoxon values unless noted as Friedman. When determining whether the number of DDs per GPSP changed within a single slice before and after drug or was different between control and LY/MPEP slices in a single pair (e.g., “DDs/GPSP decreased in 3 out of 7 slices”), the t-test was used and the n was the number of GPSPs in ten minutes (approximately 30). In these cases, exact P values are not reported; P > 0.05 is reported as no change. Graphs were made using SigmaPlot 9.01 (Systat Software, Inc., Point Richmond, CA). Statistics were performed using SPSS 15 software (Chicago, IL).
Results
Group I mGluR antagonists did not decrease ongoing epileptiform activity in the 4-AP/CGP 55845 model but did increase GPSP rate
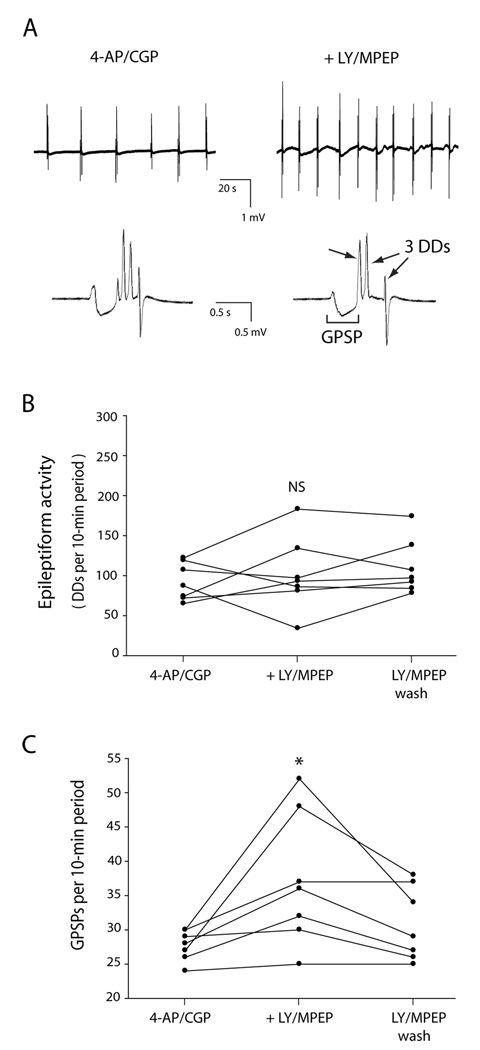
Exposure of slices to 4-AP/CGP 55845 elicited epileptiform activity as described previously (Kantrowitz et al., 2005). An epileptiform event consisted of a GPSP followed by one or more DDs and was 500 ms to 2 s long (Fig. 1A). In order to investigate the effect of complete block of group I mGluRs on ongoing epileptiform activity, slices were exposed first to 4-AP/CGP 55845 and then to LY 367385 and MPEP (LY/MPEP) followed by washout of the LY/MPEP. LY/MPEP did not change the level of epileptiform activity, as measured by the total number of DDs per 10-min period (13 ± 49% change; P = 0.6, Friedman test, n = 7; Fig. 1B). In addition, there was no consistent change in the mean number of DDs per GPSP (−7 ± 49% change; P = 0.2, Friedman test; DDs/GPSP decreased with LY/MPEP in 3 out of 7 slices, increased in 3/7 and stayed the same in 1/7). However, the mean number of GPSPs per 10-minute period increased with the addition of LY/MPEP (33 ± 30% increase; P = 0.018, n = 7; Fig. 1C), and then fell with washout of LY/MPEP (P = 0.043 drug vs. wash, n = 7; Fig. 1C). Wash was not significantly different from control (P = 0.1). We also tested co-application of the non-competitive mGlu1 receptor antagonist JNJ 16259685 with MPEP. Slices were exposed first to 4-AP/CGP 55845 and then to JNJ 16259685 and MPEP (JNJ/MPEP) followed by washout of the JNJ/MPEP. JNJ/MPEP did not change the level of epileptiform activity, as measured by the total number of DDs per 10-min period (18 ± 28 % change; P = 0.4, Friedman test, n = 5). In addition, there was no consistent change in the mean number of DDs per GPSP (0.7 ± 35 % change; P = 0.9, Friedman test, n=5). However, the mean number of GPSPs per 10-minute period increased with the addition of JNJ/MPEP (29 ± 10% increase; P = 0.043, n = 5), and then fell with washout of JNJ/MPEP (P = 0.043 drug vs. wash, n = 5). Wash was not significantly different from control (P = 0.1).
Fig. 1. Co-application of the group I mGluR antagonists LY 367385 (LY) and MPEP did not change the amount of epileptiform activity but did increase the rate of giant GABA-mediated postsynaptic potentials (GPSPs). Slices were exposed first to 4-AP/CGP 55845 and then additionally to LY/MPEP, followed by washout of the LY/MPEP.
A: Extracellular voltage traces recorded from same slice before (left) and after (right) exposure to LY/MPEP. Electrode was placed at stratum lacunosum-moleculare /stratum radiatum border in CA3. Top row: In this example, 6 GPSPs with accompanying discharge deflections (DDs) occurred in a 2-min period before LY/MPEP, and 10 occurred in a 2-min period after LY/MPEP. Bottom row: Single epileptiform events are expanded to reveal a GPSP followed by 4 DDs before LY/MPEP (left) and a GPSP followed by 3 DDs after LY/MPEP (right). The traces in the bottom row were recorded from the same slice as the traces in the top row. B: The epileptiform activity, as measured by total number of DDs per 10-min period, did not consistently change with the addition of LY/MPEP. C: The number of GPSPs per 10-min period increased after addition of LY/MPEP, and decreased with washout of LY/MPEP. * indicates statistically significantly different from control; NS indicates not statistically significantly different from control.
MPEP and MTEP, but not LY 367385 and not d-AP5, increased GPSP rate
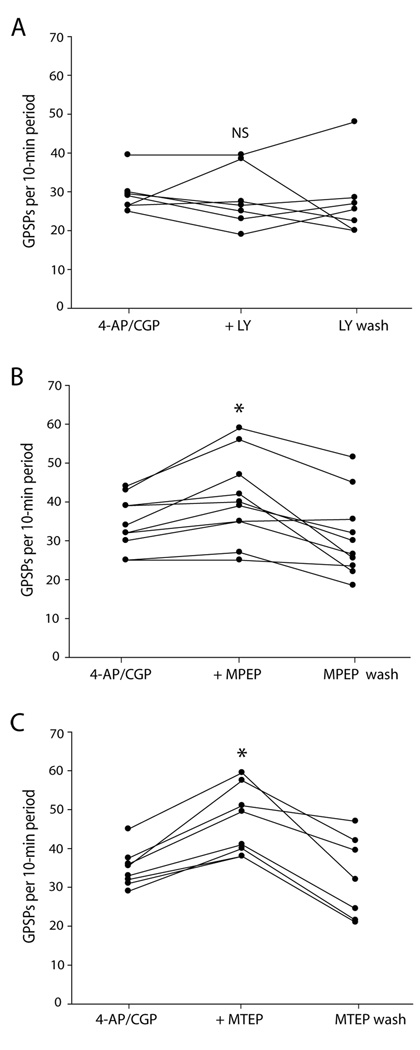
LY 367385 selectively blocks the mGlu1 receptor subtype of group I mGluRs (Clark et al., 1997); whereas MPEP selectively blocks the mGlu5 receptor subtype (Gasparini et al., 1999). We did experiments to separately test their effects on GPSP rate. Application of LY 367385 did not affect the rate of ongoing GPSPs (−3 ± 24 % change; P = 0.5, Friedman test, n = 7; Fig. 2A). Application of MPEP increased the rate of ongoing GPSPs (17 ± 13 % change; P = 0.008, n = 10; Fig. 2B), which then decreased with washout of the MPEP (P = 0.007, drug vs. wash, n = 10; Fig. 2B). Wash was not significantly different from control (P = 0.2).
Fig. 2. mGlu5 receptor antagonists increased GPSP rate.
A: Application of the selective mGlu1 receptor antagonist LY 367385 (100 µM) did not affect the rate of ongoing GPSPs. B: Application of the selective mGlu5 receptor antagonist MPEP (10 µM) increased the rate of ongoing GPSPs, which then decreased with washout of the MPEP. C: Application of the potent and selective mGlu5 receptor antagonist MTEP (500 nM) increased the rate of GPSPs, which then decreased with washout of the MTEP. * indicates statistically significantly different from control; NS indicates not statistically significantly different from control.
Since MPEP has been reported to have some antagonist activity at NMDA receptors at 20 µM in cell culture (O’Leary et al., 2000; however, contrast that to its lack of effect on NMDA-mediated potentials at 10 µM in hippocampal slices, Francesconi et al., 2004), we next tested whether the increase in the rate of GPSPs may have been caused by MPEP acting at NMDA receptors rather than at mGlu5 receptors. Application of d-AP5 did not increase the rate of GPSPs and instead actually decreased the rate of GPSPs (−16 ± 9 % change; P = 0.018, n = 7). (d-AP5 did not block the epileptiform discharges.) See further investigation of iGluR antagonist effect on GPSP rate below.
In order to confirm that MPEP was acting via mGlu5 receptors, we repeated the MPEP experiment substituting the potent and specific mGlu5 receptor antagonist MTEP (Anderson et al., 2002). MTEP increased the rate of GPSPs (34 ± 14 % change; P = 0.012, n = 8; Fig. 2C), which then decreased with washout of the MTEP (P = 0.018, drug vs. wash, n = 7; Fig. 2C). Wash was not significantly different from control (P = 0.4 ).
We also measured DDs per 10-min period in these single-antagonist experiments. None of the three antagonists significantly changed the number of DDs per 10-minute period: LY 367385 (−2 ± 20 % change; P = 1.0, n = 7); MPEP (−16 ± 33 % change; P = 0.1, n = 10; MTEP (−5 ± 45 % change; P = 0.5, n = 8).
In the presence of iGluR antagonists, MPEP did not increase GPSP rate and group I mGluR agonist did not decrease GPSP rate
We tested whether MPEP would also increase GPSP rate in the presence of the iGluR antagonists d-AP5 and NBQX. As reported previously (Kantrowitz et al., 2005), block of ionotropic glutamate receptors (iGluRs) blocked the epileptiform activity but did not block the GPSPs (Fig. 3A).
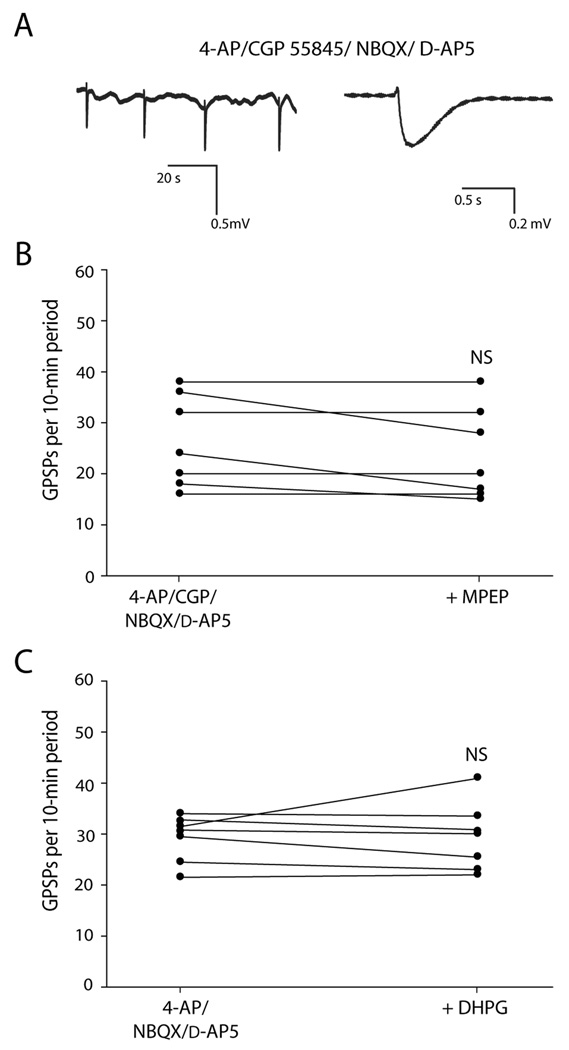
Fig. 3. In the presence of ionotropic glutamate receptor (iGluR) antagonists, mGlu5 receptor antagonist did not increase GPSP rate, and group I mGluR agonist did not decrease GPSP rate.
A: In the presence of 4-AP/CGP 55845A and the iGluR antagonists NBQX and d-AP5, GPSPs still occurred rhythmically (left), but each GPSP was not followed by an epileptiform discharge (expanded trace of second GPSP, right). B: In the presence of NBQX and d-AP5, the number of GPSPs per 10-min period did not significantly change after addition of mGlu5 receptor antagonist MPEP (10 µM). C: In the presence of NBQX and d-AP5, the number of GPSPs per 10-min period did not significantly change after addition of the group I mGluR agonist DHPG (50 µM). NS indicates not statistically significant.
Slices were exposed to 4-AP, CGP 55845, NBQX, and d-AP5 for one hour, and then MPEP was added. Addition of MPEP clearly did not increase GPSP rate (−10 ± 13% change, P = 0.1, n = 7; Fig. 3B). Since epileptiform activity is blocked in iGluR antagonists, less glutamate would be released and presumably there would thus be less activation of group I mGluRs. It was unclear, then, whether MPEP failed to increase GPSP rate because there was very little mGlu5 receptor activation to block, or whether it was because the MPEP effect on rate is accomplished via ionotropic glutamatergic synaptic activity.
In order to address this question, we applied the group I mGluR agonist DHPG in the presence of iGluR antagonists. In this scenario, the group I mGluRs would be well-activated despite the presence of iGluR antagonists. Since mGluR antagonists normally increase GPSP rate, we would expect mGluR agonist to decrease GPSP rate if the mGluR rate mechanism works independent of ionotropic glutamatergic synaptic transmission. Following a 1-hr exposure to 4-AP and the iGluR antagonists d-AP5 and NBQX, DHPG was added to the recording solution. Application of DHPG did not affect the rate of ongoing GPSPs (0 ± 14 % change; P = 0.4, n = 7; Fig. 3C).
iGluR antagonists themselves decreased GPSP rate
The above experiments indicate that intact ionotropic glutamatergic synaptic transmission is required in order for mGlu5 receptor antagonists to increase GPSP rate. The hypothesis is that mGlu5 receptor antagonist is enhancing the ability of pyramidal cells to trigger the synchronous GABAergic events. When recording in whole-cell voltage-clamp mode with ionotropic glutamatergic transmission intact, it is apparent that some giant GABA-mediated postsynaptic currents (GPSCs, the voltage-clamp equivalent of GPSPs) are directly preceded by large, ionotropic glutamate-mediated inward synaptic currents and some are not (Perkins, personal observation; also compare Fig. 1A, which has no downward deflection preceding the GPSP to Fig. 4A, which has a downward deflection directly preceding the GPSP). The GPSPs that are directly preceded by synaptic glutamatergic events may be triggered by those events. If so, we would expect the rate of GPSPs to decrease when iGluR are blocked. Slices were exposed first to 4-AP/CGP 55845 for 1 hr and then additionally to the iGluR antagonists NBQX and d-AP5. As reported previously, the NBQX/d-AP5 blocked the discharge deflections but not the GPSPs (Kantrowitz et al., 2005, see also Fig. 3A). The NBQX/d-AP5 also blocked the iGluR-mediated field potentials that had directly preceded some of the GPSPs. The NBQX/D-AP5 reduced the number of GPSPs per 10-min period by 25 ± 15 % (P = 0.018, n= 7), suggesting that a subset of GPSPs may normally be triggered by an iGluR-mediated synaptic event.
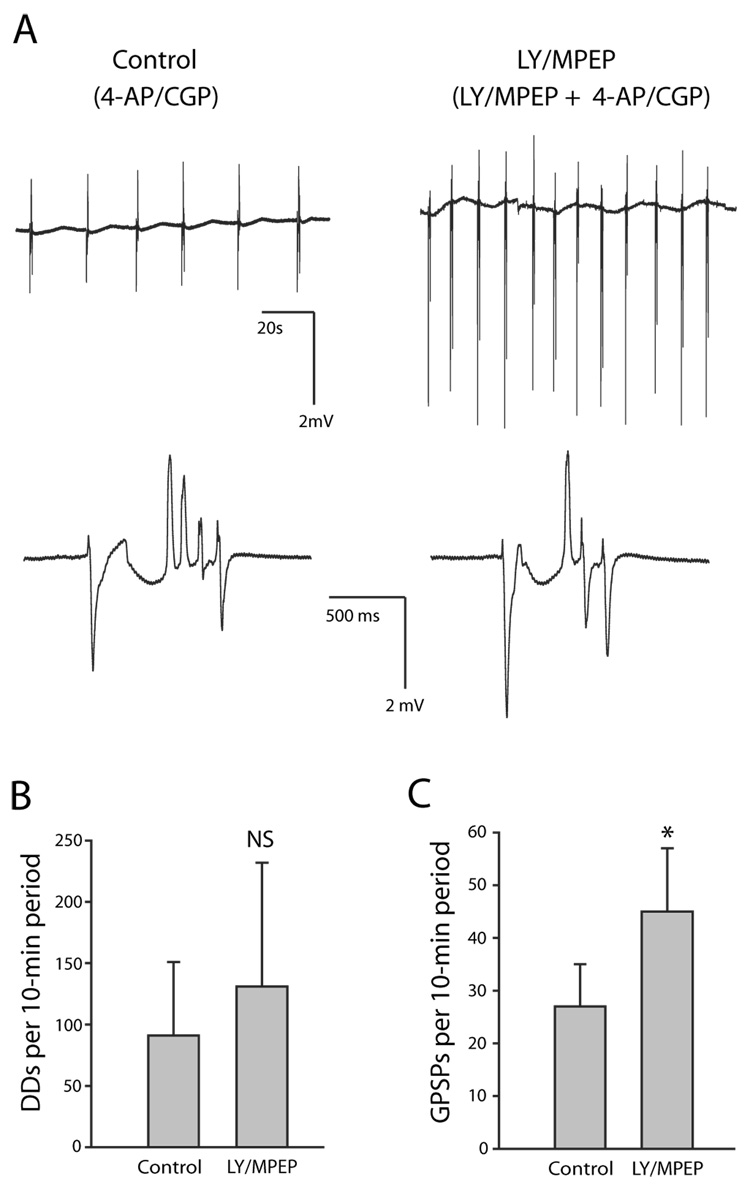
Fig. 4. Group I mGluR antagonists LY 367385 and MPEP did not prevent emergence of epileptiform activity. Slices from the same animal were paired (n = 7 pairs). The control group of slices was exposed only to 4-AP/CGP 55845. The experimental group of slices was exposed first to LY/MPEP alone for 30 min and then to 4-AP/CGP 55845 in the continued presence of LY/MPEP.
A: Extracellular voltage traces recorded from control slice exposed only to 4-AP/CGP 55845 (left) and slice exposed to LY/MPEP and 4-AP/CGP 55845 (right). Electrode was placed at stratum lacunosum-moleculare/stratum radiatum border in CA3. Top row: In this example, 6 GPSPs with accompanying discharge deflections (DDs) occurred in a 2-min period in the slice not exposed to LY/MPEP, and 12 occurred in a 2-min period in the slice exposed to LY/MPEP. Bottom row: Single epileptiform events (from a different pair of slices) are expanded to reveal a GPSP followed by 4 DDs in the slice not exposed to LY/MPEP (left) and a GPSP followed by 3 DDs in the slice exposed to LY/MPEP (right). While the average number of discharge deflections (DDs)/ GPSP was lower in the LY/MPEP slice in this particular pair, overall there was no significant difference in DDs/ GPSP between the control and experimental groups (LY/MPEP < control in 3/7 pairs, LY/MPEP > control in 2/7 pairs, LY/MPEP ≈ control in 2/7 pairs). B: No significant difference in epileptiform activity as measured by total number of DDs per 10-min period in control group compared to group exposed to LY/MPEP + 4-AP/CGP 55845. C: There were more GPSPs per 10-min period in the slices exposed to LY/MPEP. Error bars are SD. * indicates statistically significant; NS indicates not statistically significant.
LY/MPEP did not prevent the emergence of epileptiform activity induced by 4-AP/CGP 55845
Other groups have found that mGluR antagonists may have no effect on ongoing epileptiform activity and yet block the emergence of epileptiform activity (Arvanov et al., 1995; Martín et al., 2001). In order to test whether group I mGluR activation would block the emergence of epileptiform activity in the 4-AP/CGP 55845 model, paired slices from the same animal were used to compare epileptiform activity in the control group, which was exposed only to 4-AP/CGP 55845, with that in the experimental group, which was exposed first to LY/MPEP and then additionally to 4-AP/CGP 55845. Pre-exposure of the experimental-group slices to the group I mGluR antagonists for 30 min insured that there was no group I mGluR activation upon exposure to 4-AP/CGP 55845. The experiments were done alternating between control protocol first and experimental protocol first. All comparisons in these paired-slice experiments used n = 7 pairs. Epileptiform activity was generated in both protocols (Fig. 4A). There was no difference in epileptiform activity between the two groups as measured by the total number of DDs per 10-min period (P = 0.4, Fig. 4B). There also was no difference between the two groups in the number of DDs per GPSP (P = 0.5; control mean 3.2 ± 2.3; LY/MPEP mean 3.0 ± 2.6). There was, however, a significantly greater number of GPSPs per 10-min period in the slices exposed to LY/MPEP (P = 0.028; Fig. 4C), in confirmation of the above experiments.
Pre-exposure to LY/MPEP blocked the emergence of longer epileptiform events in the GABAA antagonist/ 4-AP model
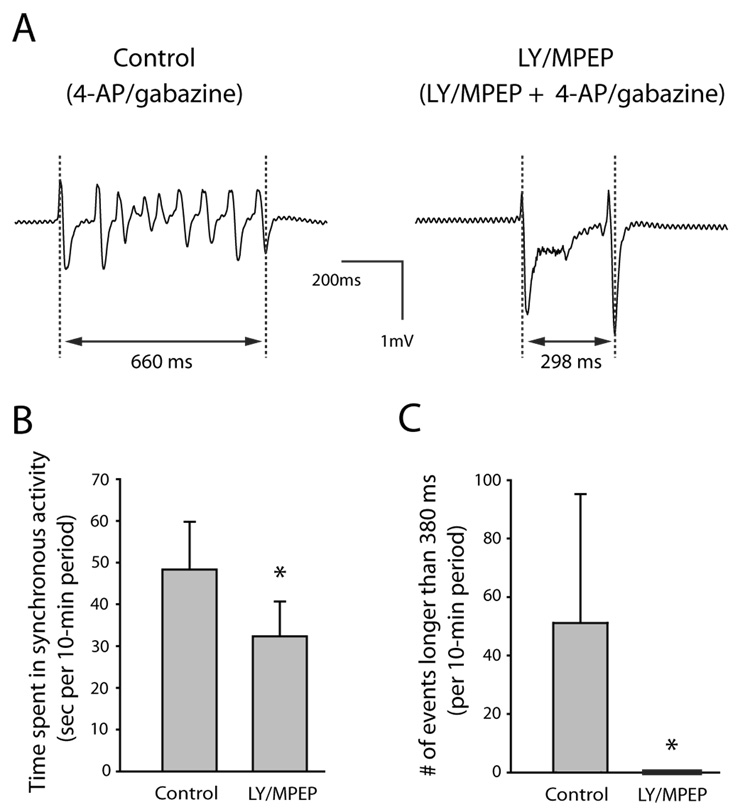
Group I mGluR antagonists have prevented the emergence of epileptiform activity in vitro or protected against the onset of seizures in vivo in several different models (see discussion). In order to test if group I mGluR antagonists could prevent the emergence of epileptiform activity in our adult guinea pig hippocampal slices using a model other than the 4-AP/CGP model, we tested the effect of LY/MPEP using the 4-AP/GABAA antagonist model. Paired slices from the same animal were used to compare epileptiform activity in the control group, which was exposed only to the GABAA antagonist gabazine and 4-AP (gabazine/4-AP) with that in the experimental group, which was exposed first to LY/MPEP for 30–40 minutes and then additionally to gabazine/4-AP (Fig. 5). The experiments were done alternating between control protocol first and experimental protocol first. All comparisons in these paired-slice experiments used n = 7 pairs. Slices exposed to LY/MPEP had less synchronous activity; total synchronous activity in ten minutes averaged 48.3 ± 11.5 s in slices that were not exposed to LY/MPEP and 32.3 ± 8.3 s in slices that were exposed to LY/MPEP (P = 0.028; Fig. 5B). We found no significant difference when comparing mean length of single synchronous events without LY/MPEP (376 ms ± 240 ms) to with LY/MPEP 201 ± 57 ms (P=0.091); however, we noted the presence of a group of longer synchronous events in the slices not exposed to LY/MPEP (Fig. 5C). GABAA receptor antagonist alone (without 4-AP) produces spontaneous synchronous events (e.g., Merlin and Wong, 1997; Lee et al., 2002). In separate slices we recorded synchronous events in gabazine alone. The mean length of synchronous events in gabazine alone was 337 ± 43 ms (n = 4 slices). The mean length plus one SD was therefore 380 ms. We then analyzed the data to see how many synchronous events in the gabazine + 4-AP paired-slice experiments were longer than 380 ms. There were 0 synchronous events longer than 380 ms in a 10-min period in the slices exposed to LY/MPEP and a mean of 51 ± 44 synchronous events longer than 380 ms in a 10-min period in the slices not exposed to LY/MPEP. The difference was significant (P = 0.019).
Fig. 5. Group I mGluR antagonists LY 367385 and MPEP reduced epileptiform activity induced by co-application of 4-AP and the GABAA antagonist gabazine. Slices from the same animal were paired (n = 7 pairs). The control group of slices was exposed only to 4-AP/gabazine. The experimental group of slices was first exposed to LY/MPEP alone for 30–40 min and then to 4-AP/gabazine in the continued presence of LY/MPEP.
A: Extracellular voltage traces showing a single epileptiform event recorded from control slice exposed only to 4-AP/gabazine (left) and experimental slice exposed to LY/MPEP and 4-AP/gabazine (right). Electrode was placed at stratum lacunosum-moleculare /stratum radiatum border in CA3. Each synchronous event was measured from the first peak of the initial deflection to the final peak of the last deflection as indicated in the figure. B: The slices exposed to LY/MPEP spent significantly less time in synchronous activity than control slices. In both control and experimental slices, all synchronous events occurring in the 10-minute period between 30 and 40 minutes of 4-AP/gabazine exposure were measured and the lengths were added together as a measure of time spent in synchronous activity per 10-min period. C: There were significantly more synchronous events longer than 380 ms in the control slices than in those exposed to LY/MPEP. Error bars are SD. * indicates statistically significant.
Discussion
The data presented here show that enough glutamate was released in the presence of 4-AP/CGP 55845 to activate group I mGluRs and affect GPSP rate, but that this activation was not involved in the generation of the epileptiform activity in this model. Group I mGluR antagonists neither reduced ongoing epileptiform activity nor prevented the emergence of epileptiform activity in the 4-AP/CGP 55845 model. In contrast, group I mGluR antagonists did decrease epileptiform activity in the GABAA antagonist/4-AP model.
Group I mGluR and epileptiform activity
Other groups have also shown that group I mGluR antagonists reduce epileptiform activity in in vitro models other than the 4-AP/CGP 55845 model. For example, group I mGluR antagonists block persistent epileptiform activity induced by the group I mGluR agonist DHPG in guinea pig hippocampal slices (Merlin and Wong, 1997). Prior to us, Lee et al. (2002) showed that group I mGluR antagonists decrease epileptiform activity induced by 4-AP and GABAA receptor antagonist in the CA3 region of mouse hippocampal slices. Experiments using 4-AP in rat amygdala (Arvanov et al., 1995) and using 4-AP/low Mg++ in juvenile CA1 rat hippocampus (Martín et al., 2001) showed that while the group I and II mGluR antagonist MCPG did not affect pre-existing ictal-like activity, pre-application of MCPG did prevent the emergence of ictal-like epileptiform activity. Despite the existence of these three 4-AP examples in which group I mGluR antagonists inhibit epileptiform activity, our 4-AP/CGP 55845 experiments indicate that using 4-AP in the induction of the in vitro epileptiform activity is not sufficient to confer group I mGluR antagonist sensitivity upon the epileptiform activity.
We hypothesize that different mechanisms of generation of the epileptiform activity in the different models accounts for why group I mGluR antagonists suppress or prevent the emergence of epileptiform activity in some models and not in others. Both the triggering mechanism and the mechanism which sustains a discharge may differ among the different models. For example, whereas in the 4-AP/CGP 55845 model the depolarizing GABA component of the GPSP triggers the onset of each epileptiform discharge (Kantrowitz et al., 2005), the same could not be true in the 4-AP/GABAA antagonist model because GABAA-mediated potentials are blocked. Although neither Arvanov et al. (1995) nor Martín et al. (2001) was routinely blocking GABAA-mediated synaptic transmission (Martín et al. did block GABAA receptors in a few experiments to show that GABAA activation was not required for the epileptiform activity), the ictal-like epileptiform activity in these reports does not appear to be initiated by a GPSP and thus likely involves different mechanisms than the epileptiform activity recorded in our 4-AP/CGP experiments. In addition, in the 4-AP/CGP 55845 model, the depolarizing GABA input probably also helps to sustain the string of ionotropic glutamate-mediated discharge deflections by providing an underlying depolarization of the pyramidal cell that lasts the same length of time as an individual epileptiform event (Kantrowitz et al., 2005); whereas in some other models, in particular the model of persistent epileptiform activity induced by DHPG (Merlin and Wong, 1997), group I mGluR activation may provide this underlying depolarization, possibly through activation of ImGluR(V) (Wong et al. 2004).
Group I mGluR antagonists have also shown the ability to protect against some types of seizures in in vivo models. Group I mGluR antagonists protect against the onset of sound-induced seizures in mice genetically prone to audiogenic seizures: mGlu1 and mGlu5 receptor antagonists protect against sound-induced generalized motor seizures in DBA/2 mice (Chapman et al., 1999, 2000); mGlu1 receptor antagonists protect against sound-induced seizures in genetically epilepsy-prone rats (Chapman et al. 1999); and mGlu5 receptor antagonist protects against sound-induced seizures in a mouse model of Fragile X syndrome (Yan et al., 2005). Group I mGluR antagonists also prevent the onset of seizures induced by intrahippocampal injection of the muscarinic agonist pilocarpine (Smolders et al., 2004). There have been conflicting results when testing the ability of mGlu1 receptor antagonists to protect against seizures induced by the GABAA antagonist pentylenetetrazole in mice, which may be due to the different antagonist employed: AIDA was protective (Thomsen and Dalby, 1998) and LY 456236 was not (Shannon et al., 2005). Reports that group I mGluR antagonists are efficacious at protecting against seizures in the 6-Hz electroshock model of partial seizures and the amygdala kindling model of temporal lobe epilepsy (Barton et al., 2003; Shannon et al., 2005) have been disputed by Löscher et al. (2006) who contend that the doses used in the initial studies were much higher than that needed to saturate group I mGluRs, which may mean the agents were acting via some other mechanism.
It may be the case that only epileptiform activity that was initially caused via overactivity in a specific set of pathways will be shown to be sensitive to group I mGluR antagonists. Interestingly, there is now an mGluR theory of Fragile X syndrome, which hypothesizes that lack of the fragile X mental retardation protein (FMRP) causes a hyperactive response to mGlu5 receptor activation (Bear, 2005), possibly accounting for many of the symptoms of Fragile X syndrome, including the audiogenic seizures (Chuang et al., 2005; Yan et al., 2005). The pilocarpine-induced seizures, which were also sensitive to group I mGluR antagonists (Smolders et al., 2004), may share some of the same pathways as seizures induced by overly stimulating group I mGluR. Muscarinic receptor activation and group I mGluR activation share some effector pathways (discussed in Chuang et al. 2002), and carbachol-induced and DHPG-induced epileptiform activities recorded from hippocampal slices look indistinguishable (Chuang et al. 2002).
Group I mGluR and GPSP rate
In contrast to a lack of effect of group I mGluR antagonists on epileptiform activity in our 4-AP/CGP experiments, the mGlu5 receptor antagonists did consistently increase the rate of GPSPs. Since MPEP and MTEP increased the rate of GPSPs, it follows, then, that the endogenous activation of mGlu5 receptors in 4-AP causes the GPSPs to occur at a lower rate than if the mGlu5 receptors had not been activated. There are several mechanisms by which activation of mGlu5 receptors could decrease GPSP rate. It is known that mGlu5 receptors exist on some hippocampal interneurons (Romano et al., 1995; Luján et al., 1996; van Hooft et al., 2000) and on CA3 pyramidal cells (Romano et al., 1995; Luján et al., 1996). Even though the generation of GPSPs is primarily an interneuron network phenomenon (Michelson and Wong, 1991), and iGluR activation was not required for the generation of spontaneous GPSPs, iGluR antagonists did decrease GPSP rate, indicating that some portion of the GPSPs are probably triggered by a synchronous iGluR-mediated input, as suggested by the electrophysiological recordings. Since mGlu5 receptor antagonist did not increase the rate of GPSPs in the presence of iGluR antagonists, and group I mGluR agonist did not decrease the rate of GPSPs in the presence of iGluR antagonists, the data suggest that mGlu5 receptor antagonist normally works via the pyramidal cell-to-interneuron ionotropic glutamatergic synapse to increase GPSP rate. Among other possibilities, the mGlu5 receptor antagonist may be 1) increasing the synchronicity of pyramidal cell activity so that the interneuron network receives stronger excitatory inputs that are thus more likely to trigger a GPSP, 2) increasing the sensitivity of the interneurons to excitatory input, so that the same excitatory input is more likely to trigger a GPSP, or 3) shortening the network refractory period that follows the generation of a GPSP (Perreault and Avoli, 1992; Perkins, 2002) so that the interneuron network is capable of responding sooner to an excitatory input. It is unclear which target of mGlu5 receptor activation would be involved in the rate change. Group I mGluR activation can cause a decrease in potassium conductance by inhibiting various potassium channels (e.g., Charpak et al., 1990; Chemin et al., 2003), can turn on the non-inactivating inward current ImGluR(V) (Chuang et al., 2000), and can potentially have a myriad of other effects (Hermans and Challiss, 2001).
Of additional interest is that the mGlu5 receptor antagonists increased GPSP rate while the mGlu1 receptor antagonist had no effect on GPSP rate. One possible reason for differential effects would be if mGlu5 but not mGlu1 receptors were present at the locus of action. Both mGlu1 and mGlu5 receptors are present on CA3 pyramidal cells (Luján et al., 1996); however, some hippocampal interneurons express mGlu5 receptors and not mGlu1 receptors (van Hooft et al., 2000). A locus of action at CA3 interneurons expressing exclusively the mGlu5 subtype of group I mGluR would thus be one explanation. Alternatively, activation of the two receptor subtypes may exert different effects despite being co-expressed at the locus of action. Differential effects of mGlu5 and mGlu1 receptor activation have been reported in the presence of co-expression of the two group I mGluR subtypes in hippocampal pyramidal cells (Mannaioni et al., 2001) and in several other cell types (reviewed by Valenti et al., 2002). These differential effects could be due to the different C-terminal domains of the receptor subtypes, which give them different phosphorylation sites and protein-binding sites (Joly et al., 1995; Tateyama and Kubo, 2007) or to the single amino acid residue difference in the G protein-coupling domains of the two receptor subtypes (Kawabata et al., 1996; Dale et al., 2002).
In conclusion, whereas we showed here that enough glutamate was released in the presence of 4-AP/CGP 55845 to activate mGlu5 receptors and affect GPSP rate, we also showed that group I mGluR antagonism did not decrease ongoing epileptiform activity in the 4-AP/CGP 55845 model. In addition, in contrast to the 4-AP/GABAA antagonist model, group I mGluR antagonists did not inhibit the emergence of epileptiform activity in the 4-AP/CGP 55845 model. These data indicate that while group I mGluR activation plays a role in the generation of epileptiform activity in some epilepsy models, it is not a universal requirement for the generation of epileptiform activity. Instead, the combined presence of 1) a synchronous GABA-mediated depolarization to provide both the trigger for each epileptiform event and an underlying depolarization to sustain the event and 2) synchronous iGluR-mediated inputs to provide phasic depolarizations within an event seems to be sufficient for the generation of epileptiform activity in the 4-AP/CGP 55845 model.
Acknowledgements
The authors thank L. Merlin, S. Young, J. Lopez, and R. Bianchi for helpful discussions, V. Chen for participation in preliminary experiments, and J. Weedon for help with statistics.
Grants
This project was sponsored by National Institute of Neurological Disorders and Stroke Grant NS-047435 to K. L. Perkins.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson JJ, Rao SP, Rowe B, Giracello DR, Holtz G, Chapman DF, Tehrani L, Bradbury MJ, Cosford ND, Varney MA. [3H]Methoxymethyl-3-[(2-methyl-1,3-thiazol-4-yl)ethynyl]pyridine binding to metabotropic glutamate receptor subtype 5 in rodent brain: in vitro and in vivo characterization. The Journal of Pharmacology and Experimental Therapeutics. 2002;303:1044–1051. doi: 10.1124/jpet.102.040618. [DOI] [PubMed] [Google Scholar]
- Arvanov VL, Holmes KH, Keele NB, Shinnick-Gallagher P. The functional role of metabotropic glutamate receptors in epileptiform activity induced by 4-aminopyridine in the rat amygdala slice. Brain Research. 1995;669:140–144. doi: 10.1016/0006-8993(94)01243-b. [DOI] [PubMed] [Google Scholar]
- Avoli M, Barbarosie M, Lücke A, Nagao T, Lopantsev V, Köhling R. Synchronous GABA-mediated potentials and epileptiform discharges in the rat limbic system in vitro. Journal of Neuroscience. 1996;16:3912–3924. doi: 10.1523/JNEUROSCI.16-12-03912.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babb TL, Pretorius JK, Kupfer WR, Crandall PH. Glutamate decarboxylase-immunoreactive neurons are preserved in human epileptic hippocampus. Journal of Neuroscience. 1989;9:2562–2574. doi: 10.1523/JNEUROSCI.09-07-02562.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton ME, Peters SC, Shannon HE. Comparison of the effect of glutamate receptor modulators in the 6 Hz and maximal electroshock seizure models. Epilepsy Research. 2003;56:17–26. doi: 10.1016/j.eplepsyres.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Bear MF. Therapeutic implications of the mGluR theory of fragile X mental retardation. Genes, Brain, and Behavior. 2005;4:393–398. doi: 10.1111/j.1601-183X.2005.00135.x. [DOI] [PubMed] [Google Scholar]
- Buckle PJ, Haas HL. Enhancement of synaptic transmission by 4-aminopyridine in hippocampal slices of the rat. Journal of Physiology. 1982;326:109–122. doi: 10.1113/jphysiol.1982.sp014180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke JP, Hablitz JJ. Modulation of epileptiform activity by metabotropic glutamate receptors in immature rat neocortex. Journal of Neurophysiology. 1995;73:205–217. doi: 10.1152/jn.1995.73.1.205. [DOI] [PubMed] [Google Scholar]
- Camón L, Vives P, de Vera N, Martínez E. Seizures and neuronal damage induced in the rat by activation of group I metabotropic glutamate receptors with their selective agonist 3,5-dihydroxyphenylglycine. Journal of Neuroscience Research. 1998;51:339–548. doi: 10.1002/(SICI)1097-4547(19980201)51:3<339::AID-JNR7>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Chapman AG, Yip PK, Yap JS, Quinn LP, Tang E, Harris JR, Meldrum BS. Anticonvulsant actions of LY 367385 ((+)-2-methyl-4-carboxyphenylglycine) and AIDA ((RS)-1-aminoindan-1,5-dicarboxylic acid) European Journal of Pharmacology. 1999;368:17–24. doi: 10.1016/s0014-2999(99)00014-x. [DOI] [PubMed] [Google Scholar]
- Chapman AG, Nanan K, Williams M, Meldrum BS. Anticonvulsant activity of two metabotropic glutamate group I antagonists selective for the mGlu5 receptor: 2-methyl-6-(phenylethynyl)-pyridine (MPEP), and (E)-6-methyl-2-styryl-pyridine (SIB 1893) Neuropharmacology. 2000;39:1567–1574. doi: 10.1016/s0028-3908(99)00242-7. [DOI] [PubMed] [Google Scholar]
- Charpak S, Gahwiler BH, Do KQ, Knopfel T. Potassium conductances in hippocampal neurons blocked by excitatory amino-acid transmitters. Nature. 1990;347:765–767. doi: 10.1038/347765a0. [DOI] [PubMed] [Google Scholar]
- Chemin J, Girard C, Duprat F, Lesage F, Romey G, Lazdunski M. Mechanisms underlying excitatory effects of group I metabotropic glutamate receptors via inhibition of 2P domain K+ channels. The EMBO Journal. 2003;22:5403–5411. doi: 10.1093/emboj/cdg528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang SC, Bianchi R, Wong RKS. Group I mGluR activation turns on a voltage-gated inward current in hippocampal pyramidal cells. Journal of Neurophysiology. 2000;83:2844–2853. doi: 10.1152/jn.2000.83.5.2844. [DOI] [PubMed] [Google Scholar]
- Chuang SC, Zhao W, Bauchwitz R, Yan Q, Bianchi R, Wong RK. Prolonged epileptiform discharges induced by altered group I metabotropic glutamate receptor-mediated synaptic responses in hippocampal slices of a fragile X mouse model. Journal of Neuroscience. 2005;25:8048–8055. doi: 10.1523/JNEUROSCI.1777-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang SC, Zhao W, Young SR, Conquet F, Bianchi R, Wong RKS. Activation of group I mGluRs elicits different responses in murine CA1 and CA3 pyramidal cells. Journal of Physiology. 2002;541:113–121. doi: 10.1113/jphysiol.2001.013309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark BP, Baker SR, Goldsworthy J, Harris JR, Kingston AE. (+)-2-Methyl-4-carboxyphenylglycine ( LY367385) selectively antagonises metabotropic glutamate mGluR1 receptors. Bioorganic & Medicinal Chemistry Letters. 1997;7:2777–2780. [Google Scholar]
- Coetzee WA, Amarillo Y, Chiu J, Chow A, Lau D, McCormack T, Moreno H, Nadal MS, Ozaita A, Poutney D, Saganich M, Vega-Saenz de Miera E, Rudy B. Molecular diversity of K+ channels. Annals of the New York Academy Sciences. 1999;68:233–285. doi: 10.1111/j.1749-6632.1999.tb11293.x. [DOI] [PubMed] [Google Scholar]
- Cohen I, Navarro V, Clemenceau S, Baulac M, Miles R. On the origin of interictal activity in human temporal lobe epilepsy in vitro. Science. 2002;298:1418–1421. doi: 10.1126/science.1076510. [DOI] [PubMed] [Google Scholar]
- Dale LB, Babwah AV, Ferguson SSG. Mechanisms of metabotropic glutamate receptor desensitization: role in the patterning of effector enzyme activation. Neurochemistry International. 2002;41:319–326. doi: 10.1016/s0197-0186(02)00073-6. [DOI] [PubMed] [Google Scholar]
- Davies CH, Pozza MF, Collingridge GL. CGP 55845A: a potent antagonist of GABAB receptors in the CA1 region of rat hippocampus. Neuropharmacology. 1993;32:1071–1073. doi: 10.1016/0028-3908(93)90073-c. [DOI] [PubMed] [Google Scholar]
- Deisz RA. GABAB receptor-mediated effects in human and rat neocortical neurones in vitro. Neuropharmacology. 1999;38:1755–1766. doi: 10.1016/s0028-3908(99)00136-7. [DOI] [PubMed] [Google Scholar]
- Francesconi W, Cammalleri M, Sanna PP. The metabotropic glutamate receptor 5 is necessary for late-phase long-term potentiation in the hippocampal CA1 region. Brain Research. 2004;1022:12–18. doi: 10.1016/j.brainres.2004.06.060. [DOI] [PubMed] [Google Scholar]
- Gasparini F, Lingenhöhl K, Stoehr N, Flor PJ, Heinrich M, Vranesic I, Biollaz M, Allgeier H, Heckendorn R, Urwyler S, Varney MA, Johnson EC, Hess SD, Rao SP, Sacaan AI, Santori EM, Veliçelebi G, Kuhn R. 2-Methyl-6-(phenylethynyl)-pyridine (MPEP), a potent, selective and systemically active mGlu5 receptor antagonist. Neuropharmacology. 1999;38:1493–1503. doi: 10.1016/s0028-3908(99)00082-9. [DOI] [PubMed] [Google Scholar]
- Hermans E, Challiss RAJ. Structural, signaling and regulatory properties of the group I metabotropic glutamate receptors: prototypic family C G-protein-coupled receptors. The Biochemical Journal. 2001;359:465–484. doi: 10.1042/0264-6021:3590465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joly C, Gomeza J, Brabet I, Curry K, Bockaert J, Pin JP. Molecular, functional, and pharmacological characterization of the metabotropic glutamate receptor type 5 splice variants: comparison with mGluR1. Journal of Neuroscience. 1995;15:3970–3981. doi: 10.1523/JNEUROSCI.15-05-03970.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantrowitz JT, Francis NN, Salah A, Perkins KL. Synaptic depolarizing GABA response in adults is excitatory and proconvulsive when GABAB receptors are blocked. Journal of Neurophysiology. 2005;93:2656–2667. doi: 10.1152/jn.01026.2004. [DOI] [PubMed] [Google Scholar]
- Kawabata S, Tsutsumi R, Kohara A, Yamaguchi T, Nakanishi S, Okada M. Control of calcium oscillations by phosphorylation of metabotropic glutamate receptors. Nature. 1996;383:89–92. doi: 10.1038/383089a0. [DOI] [PubMed] [Google Scholar]
- Kohling R, Lucke A, Straub H, Speckmann EJ, Tuxhorn I, Wolf P, Pannek H, Oppel F. Spontaneous sharp waves in human neocortical slices excised from epileptic patients. Brain. 1998;121:1073–1087. doi: 10.1093/brain/121.6.1073. [DOI] [PubMed] [Google Scholar]
- Lee AC, Wong RKS, Chuang S-C, Shin H-S, Bianchi R. Role of synaptic metabotropic glutamate receptors in epileptiform discharges in hippocampal slices. Journal of Neurophysiology. 2002;88:1625–1633. doi: 10.1152/jn.2002.88.4.1625. [DOI] [PubMed] [Google Scholar]
- Löscher W, Dekundy A, Nagel J, Danysz W, Parsons CG, Potschka H. mGlu1 and mGlu5 receptor antagonists lack anticonvulsant efficacy in rodent models of difficult-to-treat partial epilepsy. Neuropharmacology. 2006;50:1006–1015. doi: 10.1016/j.neuropharm.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Luján R, Nusser Z, Roberts JD, Shigemoto R, Somogyi P. Perisynaptic location of metabotropic glutamate receptors mGluR1 and mGluR5 on dendrites and dendritic spines in the rat hippocampus. European Journal of Neuroscience. 1996;8:1488–1500. doi: 10.1111/j.1460-9568.1996.tb01611.x. [DOI] [PubMed] [Google Scholar]
- Mannaioni G, Marino MJ, Valenti O, Traynelis SF, Conn PJ. Metabotropic glutamate receptors 1 and 5 differentially regulate CA1 pyramidal cell function. Journal of Neuroscience. 2001;21:5925–5934. doi: 10.1523/JNEUROSCI.21-16-05925.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín ED, Araque A, Buño W. Synaptic regulation of the slow Ca2+-activated K+ current in hippocampal CA1 pyramidal neurons: Implications in epileptogenesis. Journal of Neurophysiology. 2001;86:2878–2886. doi: 10.1152/jn.2001.86.6.2878. [DOI] [PubMed] [Google Scholar]
- Merlin LR, Wong RKS. Role of group I metabotropic glutamate receptors in the patterning of epileptiform activities in vitro. Journal of Neurophysiology. 1997;78:539–544. doi: 10.1152/jn.1997.78.1.539. [DOI] [PubMed] [Google Scholar]
- Merlin LR. Differential roles for mGluR1 and mGluR5 in the persistent prolongation of epileptiform bursts. Journal of Neurophysiology. 2002;87:621–625. doi: 10.1152/jn.00579.2001. [DOI] [PubMed] [Google Scholar]
- Michelson HB, Wong RKS. Excitatory synaptic responses mediated by GABAA receptors in the hippocampus. Science. 1991;253:1420–1423. doi: 10.1126/science.1654594. [DOI] [PubMed] [Google Scholar]
- Moldrich RX, Chapman AG, De Sarro G, Meldrum BS. Glutamate metabotropic receptors as targets for drug therapy in epilepsy. European Journal of Pharmacology. 2003;476:3–16. doi: 10.1016/s0014-2999(03)02149-6. [DOI] [PubMed] [Google Scholar]
- O’Leary DM, Movsesyan V, Vicini S, Faden AI. Selective mGluR5 antagonists MPEP and SIB-1893 decrease NMDA or glutamate-mediated neuronal toxicity through actions that reflect NMDA receptor antagonism. British Journal of Pharmacology. 2000;131:1429–1437. doi: 10.1038/sj.bjp.0703715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KL. GABA application to hippocampal CA3 or CA1 stratum lacunosum-moleculare excites an interneuron network. Journal of Neurophysiology. 2002;87:1404–1414. doi: 10.1152/jn.00430.2001. [DOI] [PubMed] [Google Scholar]
- Perreault P, Avoli M. 4-aminopyridine-induced epileptiform activity and a GABA-mediated long-lasting depolarization in the rat hippocampus. Journal of Neuroscience. 1992;12:104–115. doi: 10.1523/JNEUROSCI.12-01-00104.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano C, Sesma MA, McDonald CT, O'Malley K, Van den Pol AN, Olney JW. Distribution of metabotropic glutamate receptor mGluR5 immunoreactivity in rat brain. The Journal of Comparative Neurology. 1995;355:455–469. doi: 10.1002/cne.903550310. [DOI] [PubMed] [Google Scholar]
- Rutecki PA, Lebeda FL, Johnston D. 4-aminopyridine produces epileptiform activity in hippocampus and enhances synaptic excitation and inhibition. Journal of Neurophysiology. 1987;57:1911–1924. doi: 10.1152/jn.1987.57.6.1911. [DOI] [PubMed] [Google Scholar]
- Shannon HE, Peters SC, Kingston AE. Anticonvulsant effects of LY456236, a selective mGlu1 receptor antagonist. Neuropharmacology. 2005;49:188–195. doi: 10.1016/j.neuropharm.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Smolders I, Lindekens H, Clinckers R, Meurs A, O'Neill MJ, Lodge D, Ebinger G. In vivo modulation of extracellular hippocampal glutamate and GABA levels and limbic seizures by group I and II metabotropic glutamate receptor ligands. Journal of Neurochemistry. 2004;88:1068–1077. doi: 10.1046/j.1471-4159.2003.02251.x. [DOI] [PubMed] [Google Scholar]
- Storm JF. Temporal integration by a slowly inactivating K+ current in hippocampal neurons. Nature. 1988;336:379–381. doi: 10.1038/336379a0. [DOI] [PubMed] [Google Scholar]
- Tateyama M, Kubo Y. Coupling profile of the metabotropic glutamate receptor 1α is regulated by the C-terminal domain. Molecular and Cellular Neurosciences. 2007;34:445–452. doi: 10.1016/j.mcn.2006.11.021. [DOI] [PubMed] [Google Scholar]
- Taylor GW, Merlin LR, Wong RKS. Synchronized oscillations in hippocampal CA3 neurons induced by metabotropic glutamate receptor activation. Journal of Neuroscience. 1995;15:8039–8052. doi: 10.1523/JNEUROSCI.15-12-08039.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen C, Dalby NO. Roles of metabotropic glutamate receptor subtypes in modulation of pentylenetetrazole-induced seizure activity in mice. Neuropharmacology. 1998;37:1465–1473. doi: 10.1016/s0028-3908(98)00138-5. [DOI] [PubMed] [Google Scholar]
- Tizzano JP, Griffey KI, Schoepp DD. Induction or protection of limbic seizures in mice by mGluR subtype selective agonists. Neuropharmacology. 1995;34:1063–1067. doi: 10.1016/0028-3908(95)00083-i. [DOI] [PubMed] [Google Scholar]
- Valenti O, Conn PJ, Marino MJ. Distinct physiological roles of the Gq-coupled metabotropic glutamate receptors co-expressed in the same neuronal populations. Journal of Cellular Physiology. 2002;191:125–137. doi: 10.1002/jcp.10081. [DOI] [PubMed] [Google Scholar]
- van Hooft JA, Giuffrida R, Blatow M, Monyer H. Differential expression of group I metabotropic glutamate receptors in functionally distinct hippocampal interneurons. Journal of Neuroscience. 2000;20:3544–3551. doi: 10.1523/JNEUROSCI.20-10-03544.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong RK, Bianchi R, Chuang SC, Merlin LR. Group I mGluR-induced epileptogenesis: distinct and overlapping roles of mGluR1 and mGluR5 and implications for antiepileptic drug design. Epilepsy Currents. 2005;5:63–68. doi: 10.1111/j.1535-7597.2005.05207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong RK, Chuang SC, Bianchi R. Plasticity mechanisms underlying mGluR-induced epileptogenesis. Advances in Experimental Medicine and Biology. 2004;548:69–75. doi: 10.1007/978-1-4757-6376-8_5. [DOI] [PubMed] [Google Scholar]
- Yan QJ, Rammal M, Tranfaglia M, Bauchwitz RP. Suppression of two major fragile X syndrome mouse model phenotypes by the mGluR5 antagonist MPEP. Neuropharmacology. 2005;49:1053–1066. doi: 10.1016/j.neuropharm.2005.06.004. [DOI] [PubMed] [Google Scholar]