Abstract
Phosphodiesterases (PDEs) are important regulators of signal transduction processes. While much is known about the function of cyclic GMP specific PDEs in the retina, much less is known about the closely related, cyclic AMP specific PDEs. The purpose of the present study is to characterize and localize PDE4 within the adult rat retina. We have used western blotting, RT-PCR, and immunohistochemistry together with retrograde labeling to determine the presence and location of each PDE4 subtype. Western blot analysis revealed that multiple isoforms of PDE4A, B, and D subtypes are present within the retina, whereas the PDE4C subtype was absent. These data were confirmed by RT-PCR. Using immunohistochemistry we show that all 3 PDE4s are abundantly expressed within the retina where they all colocalize with retrograde-labeled retinal ganglion cells, as well as bipolar cells, horizontal cells, and cholinergic amacrine cells, whereas Müller cells lack PDE4 expression. Uniquely, PDE4B was expressed by the inner and outer segments of rod photoreceptors as well as their terminals within the outer plexiform layer. Collectively, our results demonstrate that PDE4s are abundantly expressed throughout the rodent retina and this study provides the framework for further functional studies.
Keywords: cAMP, Rolipram, PDE4, PKA, immunohistochemistry, retrograde
Cyclic nucleotide second messengers are vital for signaling within the retina. The importance of cGMP signaling is well established within the first stages of visual transduction, however less is known about cAMP signaling. Many events that take place in the retina both developmentally and in the adult retina are dependent upon the second messenger cAMP. For example, cAMP plays a role in synaptogenesis (Firth et al., 2005, Stellwagen et al., 1999), post-synaptic transmission (Blazynski, 1987, Brown and Makman, 1972, Osborne, 1991, Reppert et al., 1995, Van Buskirk and Dowling, 1981), and electrical communication between retinal cells (Urschel et al., 2006). This indicates the need for tight regulation of this ubiquitous second messenger.
To better understand the roles of cAMP within the retina we must focus on the cellular sites of cAMP regulation which include production and hydrolysis. Production of cAMP is solely accomplished by the activity of adenylate cyclases, whereas hydrolysis requires the activity of phosphodiesterases. Adenylate cyclases consist of over 9 functionally different isoforms which have been localized to discrete layers within the retina (Abdel-Majid et al., 2002). The next step towards a more complete understanding of the regulation of cAMP is identification of the cAMP-phosphodiesterases and their relative distributions.
Phosphodiesterases belong to a superfamily of 11 known genes all of which hydrolyze cyclic nucleotide second messengers cAMP, cGMP, or both (Lugnier, 2006). These 11 families are divided based on their substrate specificity, location within different tissues, and their molecular structure (Charbonneau et al., 1986, Conti, 2000, Mehats et al., 2002). PDE6, a well established retinal PDE is cGMP specific and restricted to photoreceptors where it plays an integral and well-established role in phototransduction, whereby it facilitates cGMP hydrolysis following light stimulation (Fung et al., 1981, Miki et al., 1973, Wheeler and Bitensky, 1977, Zhang and Cote, 2005). Similarly, PDE1 a dual specific PDE activated by calmodulin (Beavo et al., 2007) was found in abundance within the retina (Santone et al., 2006). However PDE4, the most abundant PDE expressed in the CNS (Bolger et al., 1994), is less-well studied in the retina (Borisy et al., 1993).
PDE4 is characterized as being both cAMP specific (Marchmont and Houslay, 1980) and uniquely inhibited by the drug rolipram (Wachtel, 1982). Comprised of 4 genes (A,B,C,D) each of which consist of about 4-6 splice variants make PDE4s one of the largest families of PDEs (Bolger, 1994, Swinnen et al., 1989). Each isoform, of which there are approximately 20, contains 2-3 highly conserved regions. These consist of a catalytic region conserved among all cAMP-PDEs, where cAMP hydrolysis occurs, and 1 to 2 upstream so-called conserved regions UCR1 and/or UCR2 conserved only between the PDE4 genes (Bolger et al., 1993, Charbonneau et al., 1986, Jin et al., 1992). Long, short, super-short, and dead-short form classification is based on the inclusion / exclusion of the UCR1 and the relative size of the UCR2 (Conti et al., 1995, Houslay and Adams, 2003, Houslay et al., 2007). The UCRs allow for activation / inhibition through phosphorylation by different kinases. Activation of the long forms are mediated by cAMP dependent protein kinase-A (PKA) (MacKenzie et al., 2002, Sette and Conti, 1996) which provides a mechanism for negative feedback on the level of cAMP (Oki et al., 2000). Additionally, extracellular regulated kinase (ERK1/2) also activates short forms and provides the only known means of inhibition to long forms (Hoffmann et al., 1999, MacKenzie et al., 2000).
Existing knowledge related to the distribution of PDE4s in the retina is based on the use of radioactive rolipram to study binding sites (0). Such studies demonstrated that rolipram binding sites were concentrated within the inner retina indicating that PDE4 was largely restricted to the inner retina (Borisy et al., 1993). However, it should be noted that this method did not provide any specificity as to which PDE4 genes were present or give their precise cellular locations. Increasing interest in the roles played by PDE4s in cAMP signaling, led us to investigate the presence and location of all 4 PDE4 genes (A-D), within the rat retina. The results establish for the first time that 3 of the 4 known PDE4 subtypes are present in the adult rat retina as well as determine their specific location with the aid of known retinal cell markers. Portions of these results have previously been reported in abstract form (Whitaker and Cooper, 2007).
Materials and Methods
Tissue Preparation
For western blot and PCR analyses, adult male (250g) Long Evan’s hooded rats (Harlan, Indianapolis, IN) were euthanized with sodium pentobarbital (100mg/kg, i.p.) and decapitated. Each eye was enucleated, retinas were removed, quickly flash frozen in liquid nitrogen, and stored in -80°C for future use. For immunohistochemistry deeply anesthetized rats (sodium pentobarbital, 100mg/kg, i.p.) were transcardially perfused first with calcium-free Tyrodes solution, followed by 4% paraformaldehyde (PFA) diluted in 0.1M phosphate buffer (PB), pH 7.4, and post-fixed overnight at 4°C in 4% PFA. Whole eyes were cryo-protected in 30% sucrose in PB 3-5 days. Vertical sections were cut at 14μm on a freezing cryostat and subsequently stored in -80°C. The treatment and care of all animals used were carried out in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals and was approved by the institutional animal care and use committee (IACUC) at the University of Louisville.
Retrograde Labeling
For identification of retinal ganglion cells retrograde labeling from the superior colliculus was used. Rats were anesthetized with a cocktail of ketamine (37.5 mg/kg, i.m.) and xylazine (5 mg/kg, i.m.) then mounted on a stereotaxic apparatus. Before incision the scalp area was shaved, and cleansed with 70% ethyl alcohol followed by Betadine™ surgical scrub. Following incision, bilateral holes were drilled over the superior colliculus (SC) measured from bregma using known brain coordinates (Paxinos and Watson, 2005) and 4-5μl of 1% cholera toxin B-subunit (CTB) (Sigma, St Louis, MO) was injected bilaterally using a 10μl Hamilton syringe (Hamilton Co., Reno, NV) (Rivera and Lugo, 1998, Zhang and Diamond, 2006). Animals were given a 7-day recovery period to allow for retrograde transport, then anesthetized with pentobarbital, perfused, and processed for vertical sections, as described above. Sections through the superior colliculus were processed and visualized for CTB to confirm location of injection site.
Primary Antibodies
The primary antibodies, source, species, hosts and dilutions used for immunoflourescence are listed in Table 1. All PDE4 antibodies were produced in rabbit against synthetic peptides as follows PDE4A (CTPGRWGSGGDPA), PDE4B (ETDIDIATEDKSPIDTC), PDE4C (PDPGDLPLDNQRTC), and PDE4D (CTQDSESTEIPLDEQVEE) and are specific for all isoforms within each subtype (Farooqui et al., 2001). Specificity of these specific PDE4 antibodies were previously confirmed in studies using PDE4 knockouts where an absence of immuno-labeled bands was demonstrated in western blots (Fujita et al., 2007).
Table 1.
List of primary antibodies, source, species, hosts, and dilutions used for immunoflourescence microscopy
| Antibody | Source | Catalog /Clone No. | Host | Dilution |
|---|---|---|---|---|
| PDE4A | FabGennix Inc, Frisco, TX | PD4-112AP Lot: kqc.K553Ig |
Rabbit | 1:100 |
| PDE4B | FabGennix Inc | PD4-201AP Lot: k5185Ig.02 |
Rabbit | 1:150 |
| PDE4C | FabGennix Inc | PD4-301AP Lot: A.84.PB3 |
Rabbit | 1:100 |
| PDE4D | FabGennix Inc | PD4-401AP Lot: Agk21-Ig/197.2.25-03 |
Rabbit | 1:150 |
| Calbindin | Sigma, St. Louis, MO | C9848; clone CB-955 | Mouse | 1:500 |
| Chx10 | Exalpha Biologicals, Inc, Watertown, MA | X1180P | Sheep | 1:500 |
| Choline Acetyltransferase (ChAT) |
Chemicon, Temecula, CA | AB144P | Goat | 1:200 |
| PKCα | Upstate, Lake Placid, NY | 05-154; clone M4 | Mouse | 1:500 |
| Glutamine Synthetase |
Chemicon | MAB302; clone GS-6 | Mouse | 1:1000 |
| GLAST | Chemicon | AB1782 | Guinea Pig | 1:1000 |
| VGLUT1 | Chemicon | AB5905 | Guinea Pig | 1:1000 |
| Rhodopsin | Sigma | R5403; clone 1D4 | Mouse | 1:1000 |
| CTB | List Biological Laboratories, Inc, Campbell, CA | 703 | Goat | 1:1000 |
Several antibodies were used to differentiate various cells within the retina. Antibody to calbindin labels all horizontal cells of the rodent retina (Peichl and Gonzalez-Soriano, 1994, Rabie et al., 1985). The antibody used here was raised against a protein from purified bovine kidney calbindin-D-28K and recognizes a ~28kDa band on immunoblots which is abolished with preadsorption (Gargini et al., 2007). Antibody to rhodopsin specifically labels rod outer segments (Fekete and Barnstable, 1983, Heitzmann, 1972). The antibody used in this study was raised against the 9 amino acid C-terminal known as the 1D4 epitope of bovine rhodopsin and recognizes a ~40kDa band in western blot (manufacturer’s product information). Antibody to choline acetyltransferase (ChAT) specifically labels cholinergic amacrine cells (Haverkamp and Wassle, 2000, Voigt, 1986). The antibody used in this study was raised against a protein derived from a human placental enzyme which detects a ~68kDa protein (Brunelli et al., 2005). Specificity was determined using preadsorption which abolished immunostaining (Motts et al., 2008).
Rod bipolar cells can be differentiated with antibodies targeted at protein kinase Cα (PKCα) (Greferath et al., 1990). Monoclonal PKCα antibody was raised against a protein purified from rabbit brain cytosol (Jaken and Kiley, 1987) and detects a ~82kDa protein (Leach et al., 1988) which is present in rod bipolar cells and also some dopaminergic amacrine cells (Negishi et al., 1988). Chx10 a homeobox containing transcription factor is expressed by retinal progenitor cells in early development, but in the mature retina, it is expressed in all bipolar cells (Elshatory et al., 2007, Liu et al., 1994). Antibody was raised against a recombinant protein consisting of amino acids 1 to 131 of the N-terminal domain of human Chx10 protein detects a ~46kDa protein in rat retina (manufacturer’s product information). Antibody labeling coincides with previous in situ hyrbridization data (Liu et al., 1994) and immunohistochemistry (Elshatory et al., 2007, Mojumder et al., 2007). Antibody to vesicular glutamate transporter 1 (VGlut1) was used to selectively label ribbon synapses of photoreceptor terminals in the outer plexiform layer (OPL) and bipolar cell synaptic terminals in the inner plexiform layer IPL (Johnson et al., 2003, Mimura et al., 2002, Sherry et al., 2003). Vesicular glutamate transporter 1 (VGlut1) antibody was raised against a synthetic peptide (GATHSTVQPPRPPPPVRDY) located at the C-terminal end of rat VGlut1 protein sequence and detects a 60kDa protein in western blots of brain homogenate (Melone et al., 2005). Specificity as determined after preadsorption of antibody produced no immunoreactivity (Johnson et al., 2003).
Müller cells also comprise a large portion of the retina. Antibodies to glutamine synthetase (GS) (Riepe and Norenburg, 1977) and to L-glutamate/L-aspartate transporter (GLAST, also termed EAAT1 - excitatory amino acid transporter) (Lehre et al., 1997, Rauen et al., 1996) specifically label both the cytosolic and membrane compartments of Müller cells, respectively (Derouiche and Rauen, 1995, Ding and Weinberg, 2007). Glutamine synthetase antibody was raised against purified protein from sheep brain and detects a ~45kDa band in western blots (manufacture’s data sheet). GLAST antibody was raised against a synthetic peptide from the C-terminal of rat GLAST sequence (QLIAQDNEPEKPVADSETKM) (manufacture’s data sheet) and detects a ~65kDa band in western blots of primary cultured astrocytes (Vermeiren et al., 2005). Immunostaining for GLAST was abolished after preadsorption (Douyard et al., 2007). Additionally, to distinguish between the rod and cone photoreceptors, the fluorsecien isothyocynate (FITC) conjugated peanut agglutinin (PNA) lectin from Arachis hypogaea, was used to bind glycoproteins located specifically on the inner and outer segments and synaptic pedicles of cones, (Blanks and Johnson, 1984, Johnson et al., 1986).
Western Blot Analysis
Isolated retinas were sonicated for 5 seconds x 2 in ice-cold RIPA buffer (50mM Tris-HCl, 150mM NaCl, 1% Igepal, 0.5% sodium deoxycholate, 0.1% SDS, pH 8.0, Sigma) along with a phosphatase and protease inhibitor cocktail (1:100, FabGennix, Frisco, TX) then centrifuged at 4°C at 14000 r.p.m. for 30 min. The supernatant was collected and protein concentration was assessed using a BCA kit (Pierce Biotechnology Inc., Rockford, IL). 60μg of protein was separated on a 4-15% SDS-PAGE gradient gel, transferred to PVDF membranes (Milipore, Billerica, MA), and blocked for 1hr at room temperature with 5% fat-free dry milk dissolved in tris-buffered saline [NaCl - 0.138 M, KCl - 0.0027 M, pH 8.0] plus 0.1% tween-20 (TBST) (Sigma). Primary antibodies rabbit anti-PDE4A, B, C, D were diluted (1:5000 PDE4A; 1:2500 PDE4B; 1:1000 PDE4C; 1:10000 PDE4D, FabGennix) in 5% bovine serum albumin and TBST overnight at 4°C. Following overnight incubation membranes were repeatedly rinsed with TBST, followed by addition of horse radish peroxidase conjugated donkey anti-rabbit secondary (1:10000, Jackson Immuno, West Grove, PA) in 5% milk TBST for 1 hr at room temperature. This was followed by several more rinses before visualization with an ECL visualization kit (Pierce Biotechnology Inc.) and exposure on radiographic film. Relative molecular weights of bands were determined by comparison with protein standards as well as through inference from the reported molecular weights of the known PDE4 isoforms.
RT-PCR and Real Time RT-PCR
Retina samples were quickly thawed and homogenized in 1 ml of QIAzol Lysis Reagent (Qiagen, Valencia, CA) using a PolyTron homogenizer. Total RNA was extracted from retinas with the RNeasy Lipid Tissue Mini Kit (Qiagen) according to manufacturer’s directions. Yield and purity of RNA was estimated using a NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies, Wilmington, DE) and agarose gel electrophoresis, respectively. A total of 10μg of RNA was reverse transcribed using the high-capacity cDNA archiving kit (Applied Biosystems, Foster, CA) with random primers, in accordance with the manufacturer’s specifications.
For RT-PCR experiments, 50μl reactions were used with 10ng of cDNA, 10mM primers see table 2, and TITANIUM™ Taq PCR kit (Clontech, Mountain View, CA) as per manufacturer’s instructions. PCR conditions used were 95°C for 1min, (Denaturing 95°C 30sec > Annealing 57°C 1min > Extending 72°C 1min) for 40 cycles, and lastly 72°C for 7min on a MJ Research 225 Tetrad Thermal Cycler (Global Medical Instrumentation, Inc., Ramsey, MN). PCR products were run on an agarose gel where candidate bands were cut out and purified with QIAquick Gel Extraction Kit (Qiagen). Authenticity of each band was confirmed using direct sequencing on a CEQ™ 8000 Genetic Analysis System (Beckman Coulter, Inc., Fullerton, CA) and queried against NCBI’s Blastn (Altschul et al., 1997). Primer sequences for PDE4A, PDE4B, and PDE4D were used as reported in a prior study (Kostic et al., 1997) and PDE4C was generated with Primer3 online software (Rozen and Skaletsky, 2000).
Table2.
List of primer sequences used for RT-PCR experiments
| Gene | Forward Primer 5′ -3′ | Reverse Primer 5′ -3′ | Fragment Size (bp) |
|---|---|---|---|
| PDE4A | GCGGGACCTAGCTGAAGAAATTCC | CAGGGTGAGTCCACATCGTGG | 233 |
| PDE4B | CAGCTCATGACCCAGATAAGTGG | GTCTGCACAAGTGTACCATGTTGCG | 787 |
| PDE4C | CATGATTGTCACACCCTTCG | TCCCCATTTGTTGGTGTCTT | 544 |
| PDE4D | CCCTCTTGACTGTTATCATGCACACC | GATCCTACATCATGTATTGCACTGGC | 262 |
Probes used for TaqMan quantitative RT-PCR were selected for PDE4A (Rn00565354_m1), PDE4B (Rn00566785_m1), and PDE4D (Rn00566798_m1) from the pre-validated Assays-On-Demand (Applied Biosystems) library. For real-time PCR experiments, 50μl reactions were used with a final concentration of 10ng of cDNA, 900nM of primers, 250nM of probes, and TaqMan Universal PCR Master Mix (Applied Biosystems) and ran for 40 cycles. Data was collected and analyzed with sequence detection software (7000 Sequence Detection System, ABI Prism). Relative expression levels of the target genes were analyzed according to the 2-ΔΔCt method (Livak and Schmittgen, 2001). Tissue was normalized against 18s expression and Ct values were expressed as fold change. Experiments were performed in duplicate for each gene with 6 independent biological replicates. Ct values were compared with a one-way ANOVA and Tukey’s post hoc tests where applicable using SPSS v.13.0 (SPSS, Chicago, IL) statistical software. Levene’s test of homogeneity of variances revealed no significant difference in biological replicates.
Immunohistochemistry
Frozen sections of retina were allowed to unfreeze and dry until a pap-pen smear could be placed around pieces of tissue, then rehydrated in tris-buffered saline pH=7.2 (TBS). Slides were blocked for 1 hr at room temperature with 10% normal donkey serum (NDS) (Jackson Immuno) in TBS and 0.05% Triton X-100 (Tx-TBS). Primary antibodies were incubated overnight at 4°C in 10% NDS. Donkey raised species-specific secondary antibodies conjugated to either fluorosecien isothyocynate (FITC), cyanine (CY2), indocarbocyanine (CY3), and/or indodicarbocyanine (CY5) (1:200, Jackson Immuno) were added for 1 hr at room temperature followed by several TBS rinses. To label nuclei 4′,6-diamidino-2-phynelindole (DAPI) was added (1:1000) for 10 min in TBS during a washing step. Slides were cover-slipped using freshly made Mowiol (Sigma). Immunolabeling of retinal whole mount preparations were processed the same as vertical sections. Control experiments included the omission of primary and/or secondary antibodies or preadsorbtion with antigenic blocking peptide (FabGennix).
Image acquisition and processing
Images of immunofluorescently labeled tissue sections were obtained with the aid of an Olympus laser confocal microscope and digitized with Fluoview 500 software (Mellville, NY). Orthogonal views displaying cross sectional view through the z-axis (optical stack) were constructed using Fluoview 500 software. Adobe photoshop v9.02 (Adobe Systems Inc., San Jose, CA) was used to sharpen images, provide a consistent level of brightness and contrast, and compose the final plates.
Results
Expression and distribution of PDE4
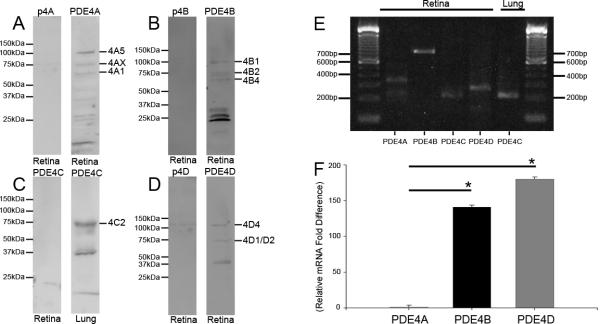
Western blot analysis revealed that PDE4 antibodies detected multiple isoforms within the retina. Antibody to PDE4A detected 3 different bands which represent a long form PDE4A5, a putative short form, PDE4Ax at ~76kDa, and a known super-short form, PDE4A1 (Fig. 1A). Likewise, antibody to PDE4B detected 3 separate isoforms, two long forms, PDE4B1 and PDE4B2, and a short form, PDE4B4 (Fig. 1B). Anti-PDE4D antibody demonstrated the presence of 2 immunolabeled bands which were representative of a known long form, PDE4D4, and a short form, PDE4D1/2 (Fig. 1D). Antibody to PDE4C did not show any immunolabeled bands in western blots of the retina but when lung protein was used as a positive control (Gantner et al., 1997), a known long form, PDE4C2, was detected (Fig 1C). All immunolabeled bands were abolished after preadsorption (Fig. 1A, B, D) including those at ~50kDa or less which are thought to correspond to either newly discovered dead-short forms (Houslay et al., 2007) or PDE4 degradation products (Ahmed et al., 2004).
Figure 1.
PDE4 protein and mRNA expression in the rodent retina. PDE4A (A), PDE4B (B), and PDE4D (D) are represented within the retina with distinct known isoforms which are blocked with preadsorption of the antibody. PDE4C (C) protein was absent from the retina but abundant within lung, positive control tissue. Preadsorbtion with sequence specific blocking peptide p4A for PDE4A (A), p4B for PDE4B (B), and p4D for PDE4D (D) blocked all bands. RT-PCR analysis (E) reveals predicted bands of the proper size for all PDE4s. Interestingly PDE4C which was identified within lung also detects putative PDE4C mRNA within retina as well. Quantification of relative PDE4 mRNA expression (F) reveals that PDE4B and PDE4D are over 150 fold more abundant than PDE4A. Real-time PCR results of PDE4A, PDE4B, and PDE4D are expressed as fold change in mRNA. *p < .001.
RT-PCR (Fig. 1E) was used to confirm the results described above. All PCR products detected for PDE4A, B, and D were sequenced and confirmed as PDE4s with NCBI’s Blastn. While PDE4C was not detected with western blots, a PCR product of the expected size was found to match with PDE4C and a generic PDE domain with 75% confidence. This apparent discrepancy is discussed later. Comparison of relative mRNA expression of PDE4 genes reveal a significant (n = 6, p<.001) difference between Ct values of PDE4A (23.2 ± 1.4) and PDE4B (16.2 ± 1.6). Likewise a significant fold difference (p<.001) was observed between PDE4A and PDE4D (15.9 ± 1.7) expression (Fig. 1F).
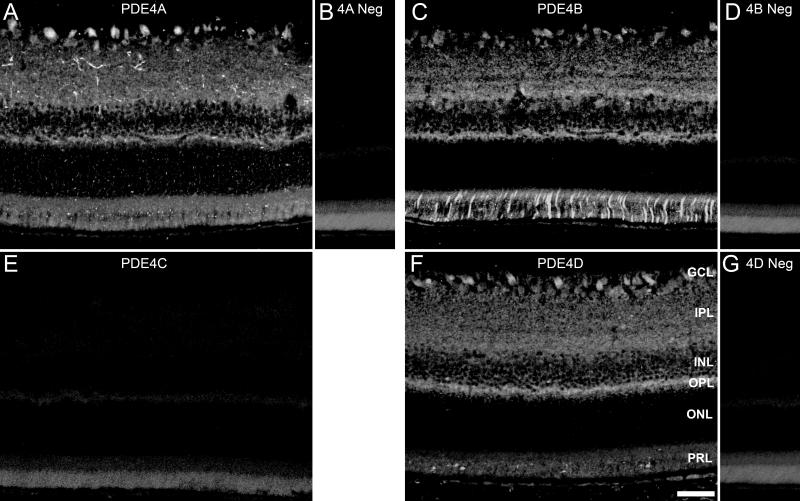
To determine the locations of the PDE4 subtypes we performed immunohistochemical analysis of the PDE4 gene-families, A, B, C and D, in the retina of adult rats (Fig. 2A, C, E, F). Preadsorbed antibodies which served as negative controls (Fig. 2B, D, G) express low levels of background which was similar to the no primary antibody controls (data not shown), and both demonstrate some auto-fluorescence within the photoreceptor inner and outer segment layers of the retina. Immunolabeling for each of the subtypes indicates a presence of these three PDE4s within the retina, primarily the retinal ganglion cell layer (GCL), inner plexiform layer (IPL), inner nuclear layer (INL) and outer plexiform layer (OPL). Whereas the outer nuclear layer (ONL) lacked PDE4 expression, some photoreceptor outer segments were labeled with antibodies to PDE4B. PDE4B alone displayed expression within inner / outer segments (IS/OS). PDE4C was not detected, thereby confirming the results obtained with western blots (Fig. 2E).
Figure 2.
PDE4 expression in the rodent retina. Immunofluorescent images of PDE4A (A), PDE4B (C), PDE4D (F) and preadsorbed p4A (B), p4B (D), and p4D (G) localize primarily to the inner retina, whereas PDE4C (E) immunolabeling was absent. Scale bar = 50μm
PDE4 expression in the photoreceptor terminals
The above results indicate a presence of PDE4s within the OPL. Higher magnification revealed that PDE4A and PDE4D are confined to the inner half of the OPL (Fig. 3A, G), while PDE4B was expressed throughout (Fig 3D). To determine their specific locations, we double-labeled with VGlut1, a marker for photoreceptor terminals (Johnson et al., 2003, Sherry et al., 2003). As seen in Figure 3B, E, H, VGlut1 immunolabeling is limited to the outer border of the OPL at photoreceptor terminals. PDE4A and PDE4D immunolabeling revealed no colocalization with VGlut1 (Fig. 3G, I). PDE4B immunolabeling however, displayed strong colocalization with VGlut1 indicating a presence in photoreceptor terminals (Fig. 3H). PDE4B was the only PDE4 subtype expressed within the photoreceptor outer/inner segment layer. To determine whether this expression was in rods or cones we double labeled with PNA which binds to the outer envelope of cone inner and outer segments (Blanks and Johnson, 1984, Johnson et al., 1986)(Fig. 3K). No colocalizations were present between PDE4B and PNA labeled photoreceptors (Fig. 3L). These results indicate that the PDE4B expression was limited to presumptive rod photoreceptors. To determine the presence of PDE4B within photoreceptor outer segments we double labeled with an antibody to rhodopsin, a protein highly expressed within outer segments (Fig. 3N) (Fekete and Barnstable, 1983, Heitzmann, 1972). Figure 3O demonstrates that PDE4B expression is localized within the outer segments. PDE4B expression appeared to be localized to a potential subset of photoreceptors. To further analyzed the expression of PDE4B and PNA labeling in a whole mount preparation (Fig. 4). Figure 4C demonstrates that PDE4B expression is limited to a subpopulation of photoreceptors which lack PNA. Multiple instances were observed of a close localization between PDE4B and PNA, however these were confirmed to not colocalize through orthogonal views (Fig. 4F). In summary, PDE4B was the only PDE4 subtype located in putative rod photoreceptor outer segments as well as with their synaptic terminals.
Figure 3.
PDE4 expression in the photoreceptors. Double labeling of PDE4A (A), PDE4B (D), and PDE4D (G) with VGlut1 (B, E, H). VGlut1 is restricted to the outer OPL within photoreceptor terminals. In contrast, PDE4A (C) and PDE4D (I) are confined to the inner OPL and fail to colocalize with VGlut1 positive terminals. PDE4B (F) is present throughout the OPL and strongly colocalizes with VGlut1. Labeling with anti-PDE4B labels photoreceptor inner and outer segments (J) labeling with PNA specifically labels cone inner and outer segments (K) whereas double labeling reveals no colocalization (L). Labeling of PDE4B (M), rhodopsin (N), and Dapi nuclear stain (M, N, O) demonstrate PDE4B localization within outer segments (O). Scale bar = 25μm.
Figure 4.
PDE4B expression pattern in the photoreceptors. Double labeling for PDE4B (A, D) and PNA (B, E) within a whole mounted retina. Labeling with PNA specifically labels cone photoreceptors (B, E). PDE4B labeling (A, D) is present within photoreceptors not labeled with PNA (C, F). Orthogonal views (D-F) demonstrates a close approximation of PNA and PDE4B labeling but no colocalization (F). Scale = 50μm.
PDE4 expression in the horizontal cells
We observed the presence of PDE4A, B, and D within the inner portion of the OPL and cell bodies bordering this layer (Fig 5A, G, M), which may correspond to horizontal cells. To test this, double immunolabeling with antibody to PDE4 and calbindin was used. Calbindin labels all horizontal cells of the mammalian retina (Peichl and Gonzalez-Soriano, 1994, Rabie et al., 1985, Rohrenbeck et al., 1987). Figure 5B, H, N indicates the presence of calbindin within the cell bodies and processes of horizontal cells. All 3 PDE4s colocalized with the horizontal cell somata and their processes (Fig. 5C, I, O). We further confirmed these colocalizations using orthogonal views through the z-axis which clearly demonstrate that colocalization were maintained throughout the optical stack.
Figure 5.
PDE4 expression in the horizontal cells. Double labeling for PDE4A (A), PDE4B (G), and PDE4D (M) and calbindin (B, H, N). Calbindin specifically labels horizontal cells and their processes which ramify near the border of the OPL and INL. All 3 PDE4s (C, I, O) colocalize with calbindin positive somata (arrows). This is confirmed with orthogonal views through the z-axis (horizontal line). Scale bar = 8μm for orthogonal views and 25μm for all other figures.
PDE4 expression in the bipolar cells
Distribution of PDE4s within the INL revealed immunolabeled cell bodies anatomically indicative of bipolar cells (Fig. 6A, E, I). To investigate whether PDE4s were located within these cells we co-labeled with an antibody specific for PKCα which detects rod-bipolar cells (Greferath et al., 1990) and Chx10 (Elshatory et al., 2007, Liu et al., 1994) which detects all bipolar cells. We found that all 3 PDE4s colocalized with rod bipolar cells and putative cone bipolar cells which were identified as Chx10 but not PKCα immunolabeled cells (Fig. 6D, H, L). Expression of PDE4s in the IPL was diffuse which made examination of their expression within the terminals of these bipolar cells inconclusive.
Figure 6.
PDE4 expression in the bipolar cells. Double labeling of PDE4A (A), PDE4B (E), and PDE4D (I) with PKCα (B, F, J) and Chx10 (C, G, K). PKCα immunoreactivity is present in rod bipolar cells, whereas Chx10 identifies all bipolar cells. All 3 PDE4s (D, H, L) colocalize with rod bipolar cells which are also Chx10 immunopositive (arrows) and presumptive cone bipolar cells (arrowheads) which are negative for PKCα labeling. Scale bar = 25μm.
PDE4 expression in the amacrine cells
There are many reports of the effects of rolipram, a PDE4 inhibitor, on the cholinergic system (Silvestre et al., 1999) providing evidence of a close interaction, thus we decided to evaluate the expression of PDE4 within retinal cholinergic amacrine cells. ChAT antibody recognizes cholinergic amacrine cells in both the INL and GCL, the latter being displaced amacrine cells (Perry and Walker, 1980, Voigt, 1986) (Fig 7B, E, H). Immunolabeling is also present within separate strata within the IPL corresponding to an inner ON cholinergic level and an outer OFF cholinergic level (Haverkamp and Wassle, 2000, Voigt, 1986). All 3 PDE4s colocalized with ChAT positive amacrine cell bodies of both the INL and GCL layers (Fig. 7F).
Figure 7.
PDE4 expression in the cholinergic amacrine cells. Double labeling of PDE4A (A), PDE4B (D), and PDE4D (G) with ChAT (B, E, H). ChAT labeling detects cholinergic amacrine cells and 2 strata within the IPL, an inner ON cholinergic layer and an outer OFF cholinergic layer. All 3 PDE4s (C, F, I) colocalize with ChAT positive cholinergic cells in the INL (arrows) and displaced amacrine cells (arrowheads). Scale bar = 25μm.
PDE4 expression in the retinal ganglion cells
Over 90% of the rat retinal ganglion cells reportedly terminate within the superior colliculus (Linden and Perry, 1983). Retrograde labeling with cholera toxin subunit-B (CTB) has been successfully used to label these cells as well as their dendrites which terminate within the outer part of the IPL (Zhang and Diamond, 2006). We found that 1wk transport of CTB strongly labeled retinal ganglion cell bodies (Fig. 8B, E, H). Double labeling revealed that all 3 PDE4s are expressed within CTB labeled retinal ganglion cell bodies (Fig. 8C, F, I).
Figure 8.
PDE4 expression in the retinal ganglion cells. Double labeling of PDE4A (A), PDE4B (D), and PDE4D (G) with CTB (B, E, H). Retrograde labeling from the superior colliclus with cholera toxin B subunit was detected within multiple ganglion cells. All 3 PDE4s (C, F, I) colocalized with CTB-labeled retinal ganglion cells (arrows). Scale bar = 25μm.
PDE4 expression in the Müller cells
Müller cells are the most abundant retinal glial cell and they have extensive processes from the outer limiting membrane to the inner limiting membrane (Jeon et al., 1998). To determine whether PDE4 was expressed by Müller cells we used antibody to the L-glutamate/L-aspartate transporter (GLAST) and to glutamine synthetase which identify the membrane and cytoslic components of Müller cells, respectively (Derouiche and Rauen, 1995, Ding and Weinberg, 2007). Representative labeling of PDE4B (Fig. 9A) was similar to PDE4A and PDE4D (data not shown). These data demonstrate that GLAST antibody labeled the membranes of cells within the INL (Fig. 9B). Though we observed some approximation between PDE4s and GLAST (Fig 9C), it seems likely that the anti-GLAST labels the extensive glial membranes which envelope and ramify through the INL. The anti-GLAST and anti-GS co-labeled cells were distinct from the anti-PDE4 labeled cells of the INL (Fig. 8D). In summary, the cell bodies of Müller cells did not contain any PDE4.
Figure 9.
PDE4 expression in the Müller cells. Labeling of PDE4B (A) with GLAST (B, C, D) and GS (D) indicate the absence of PDE4B in Müller cells. While some approximation were found between PDE4B and GLAST (arrowhead), these cells were not positive for GS staining. Likewise cells with both GS and GLAST co-labeling (arrow) do not colocalize with PDE4B. Scale bar = 25μm.
Discussion
Cyclic-AMP concentration in the normal retina is regulated through both production and hydrolysis. Previous studies have begun to investigate one side of this story, the production of cAMP through adenylate cyclases by providing the discrete location of various functionally unique isoforms (Abdel-Majid et al., 2002). The present study focused on the proteins responsible for cAMP degradation and shows for the first time: i) 3 PDE4 subtypes (A, B, and D) as well as their various isoforms are present in the retina and ii) demonstrates their specific cellular distribution. For example, only PDE4B was found in rod photoreceptors. Interestingly all three PDE4s were located in bipolar cells, cholinergic amacrine cells, and retinal ganglion cells. Finally, none of the PDE4s was associated with the retinal Muller cells. Figure 10 summarizes the expression of PDE4A, B, and D.
Figure 10.
Summary of PDE4 expression within the rodent retina. PDE4A, PDE4B, and PDE4D expression within various cell types where G - ganglion cells, Ch cholinergic amacrine cells, M - Müller cells, CB - cone bipolar cells, RB - rod bipolar cells, H - horizontal cells, C - cones, and R - rods.
Why study PDE4s?
With over 11 large families of PDEs each with multiple splice variants it was impossible to investigate all PDEs in one study. We chose the specific PDE4 family for the 3 reasons that separate it from other PDEs. First, PDE4 is thought to be the most abundant PDE expressed throughout the CNS (Bolger et al., 1994). Second, PDE4 is cAMP specific (Marchmont and Houslay, 1980), which is also the case for PDE7 (Bloom and Beavo, 1996, Michaeli et al., 1993) and PDE8 (Fisher et al., 1998, Hayashi et al., 1998, Soderling et al., 1998). Lastly, PDE4, unlike PDE7 and PDE8, can be specifically inhibited by the drug rolipram which will be vital for future functional studies of PDE4 in the retina (Lugnier, 2006, Wachtel, 1982). This is not to say that other PDEs are not present or responsible for the hydrolysis of cAMP, for instance, PDE7 and PDE8 are cAMP specific and both potentially play a role however their existence within the retina is yet to be determined. While some PDEs are cGMP specific, others share a dual specificity for both cyclic nucleotides cAMP and cGMP and might also play a role in hydrolyzing cAMP. For example, PDE1 a dual specific PDE that is stimulated by Ca2+ / calmodulin kinase was found to hydrolyze cAMP with greater affinity than cGMP and was localized primarily to the outer retina (Santone et al., 2006).
PDE4s comprise a large family with 4 known subtypes containing over 20 unique splice variants (Bolger, 1994, Swinnen et al., 1989). The antibodies used for this study detect the subfamily specific C-terminus of each PDE4, thus allowing for localization of all variants present within the retina. We found that PDE4A, B, and D were all present within the retina. In contrast, PDE4C, which is mostly absent from the CNS (Lamontagne et al., 2001, Obernolte et al., 1997), was demonstrably absent from the retina as determined by western blotting and immunohistochemistry. However, RT-PCR revealed a possible PDE4C band present within the retina indicating that we cannot absolutely rule out the limited expression of PDE4C. One possibility for detection of PDE4C in RT-PCR only is due to the sensitivity of this technique, trace amounts possibly in blood cells, could be amplified to a signal detectable by RT-PCR, whereas western blotting or immunohistochemistry were not able to detect trace amounts of protein. This exemplifies the need for using multiple techniques for detection. Another possibility was that the PDE4C sequence which has not been confirmed, shares a high homology with PDE1, a PDE known to be expressed in rodent retina (Santone et al., 2006), as determined by preliminary studies using CLUSTAL W online alignment software (Higgins et al., 1996).
In some cases PDE4A, B, and D were expressed in relatively similar locations of the retina and some might theorize that due to this there might be some redundancy between these subtypes. However, recent studies using subtype specific knockouts have provided evidence that each subfamily expresses distinct phenotypes (Ariga et al., 2004, Conti et al., 2003, Jin et al., 2005, Lehnart et al., 2005). These functional differences may be related to different splice variations which have unique N-terminal domains that bestow both spatial and functional specificity.
Possible role of PDE4s in neurotransmitter release within photoreceptors and outer plexiform layer
Our results indicate that PDE4s are highly expressed in the outer plexiform layer. Uniquely, only the PDE4B gene was expressed within outer segments of putative rod photoreceptors and their terminals within the OPL. Interestingly, PDE4B appeared to label a potential subset of rods or PNA-negative cones. Further studies are needed to address this labeling pattern. Cyclic-AMP levels are known to fluctuate during light / dark cycles (Traverso et al., 2002, Willardson et al., 1996). Within the photoreceptors light induces a decrease of cAMP, purportedly through a negative coupling of dopamine receptors to adenylate cyclases located within the photoreceptors (Cohen et al., 1992). The finding of PDE4 within the photoreceptor terminals is also consistent with previous reports that a PDE4 highly homologous to mammalian PDE4 was expressed within Aplysia synaptic terminals (Park et al., 2005). In addition, we know that adenylate cyclases are highly expressed in neuronal synapses (Mons et al., 1995) as well as within both plexiform layers of the retina (Abdel-Majid et al., 2002).
Emphasis on the importance of controlled cAMP signaling at synapses is evident in studies of long term potentiation within the cerebellum (Linden and Ahn, 1999) and hippocampus (Nguyen and Woo, 2003) where they facilitate transmitter release (Fiumara et al., 2004). In fact, this release is dependent on both PKA (Linden and Ahn, 1999) and the presynaptic protein RIM-1α (Castillo et al., 2002). Part of a superfamily of RIM proteins (Wang et al., 1997, Wang et al., 2000) that facilitate vesicle binding (Kiyonaka et al., 2007), Rim-1α is a target for PKA phosphorylation (Lonart et al., 2003). RIM interacts with presynaptic active zone protein components (CAZ) such as piccolo, where they are known to colocalize at photoreceptor terminals of the OPL (Dick et al., 2001, tom Dieck et al., 2005). Interestingly, recent evidence suggests that the fluctuations of cAMP observed during long term potentiation are due to changes both in transcription and translation of PDE4B (Ahmed and Frey, 2003, Ahmed et al., 2004). Thus PDE4 may play a considerable role in modifying amounts of neurotransmitter release at the synaptic level by precisely altering levels of cAMP.
Possible role of PDE4 within traditional rod and cone pathways
Within the retina cAMP plays a role in normal visual signal transmission through the rod and cone pathways. We observed co-expression of all three PDE4s within PKCα positive rod bipolar cells however the function of cAMP within these cells is unclear (Maguire and Werblin, 1994). In addition to rod bipolar cells, we observed PDE4 expression in cone bipolar cells that were labeled with Chx10, but not positive for PKCα. Recent studies suggest that some cone bipolar cells, which can be identified using PKA, contribute to the rod driven pathway (Mataruga et al., 2007). There is also evidence that cAMP contributes to both OFF and ON cone pathways (Bragadottir and Jarkman, 1995, Maguire and Werblin, 1994).
We also demonstrate that PDE4s are expressed within the cholinergic amacrine cells. In development we know that both GABA and cholinergic inputs mediate retinal wave propagation (Feller et al., 1996, Fischer et al., 1998) which are dependent upon PKA (Dunn et al., 2006, Stellwagen et al., 1999) however less is known about the affect of cAMP signaling on these pathways in the adult retina. In the current study we focused on a subset of cholinergic amacrine cells, however PDE4 was localized to non-cholinergic cell bodies indicating the possibility of expression within other amacrine cells.
Possible role of PDE4s in ganglion cell survival and regeneration
We show that all three PDE4s are expressed in retrogradely labeled retinal ganglion cells. The beneficial use of cAMP in survival of retinal ganglion cells following optic nerve axotomy are still being debated (Monsul et al., 2004, Watanabe et al., 2003). Following such injury decreases in the transcription factor CREB (cAMP response element binding protein) phosphorylation have been observed (Kim and Park, 2005), possibly due to changes in PDE4 abundance and/or activity. On the other hand, use of cAMP in promoting regeneration of axotomized optic nerves has been advantageous (Chierzi et al., 2005, Choi et al., 2003, Monsul et al., 2004, Watanabe et al., 2003). Likewise, inhibition of PDE4 promotes axonal regeneration within the injured spinal cord (Nikulina et al., 2004, Pearse et al., 2004). Thus identification of PDE4s within retinal ganglion cells provides another target to better investigate recovery following injury.
PDE4 in non-neuronal cells of the retina
Müller cells are known to uptake glutamate within the retina (Ehinger and Falck, 1971, White and Neal, 1976). Interestingly, Müller cells have intact cAMP signaling (Kubrusly et al., 2005) whereby addition of a cAMP analogue increases glutamate uptake purportedly through augmenting GLAST (Sakai et al., 2006). In our study we failed to find expression of PDE4 within Müller cell bodies of the retina. While we found some approximation between PDE4s and GLAST, these cells were not glutamine synthetase positive leading us to believe Müller cells either lack PDE4 or have very low expression levels within their somata.
Conclusion
Previous work showing rolipram binding in the rat retina revealed labeling concentrated within the inner retina, mostly the retinal ganglion cell layer and inner plexiform layer (Borisy et al., 1993). Our results coincide with these findings and demonstrate, using double labeling immunohistochemistry, that PDE4 expression is specific to certain cell types. Similar to the localization of adenylate cyclases (Abdel-Majid et al., 2002), we demonstrate that PDE4s are abundantly expressed within the retina. PDE4s are vital to cAMP signaling and this study provides a framework for numerous further functional studies in understanding the role of second messenger signaling within the retina.
Acknowledgements
The authors would like to extend their thanks to Dr. Martha E. Bickford for her generous donation of ChAT antibody used in this study in addition to use of equipment and technical help. We also would like to thank Drs. Xiao M. Xu, Robert F. Lundy, Jr., and Fred J. Roisen for use of equipment and technical help. This work was partially supported by NIH grants R01EY017594, P20RR016481 and P30ES014443.
Abbreviations
- cAMP
cyclic adenosine monophosphate
- cDNA
complimentary deoxynucleic acid
- cGMP
cyclic guanosine monophosphate
- ChAT
choline acetalytransferase
- CNS
central nervous system
- CREB
cyclic adenosine monophosphate response element binding protein
- CTB
cholera toxin B subunit
- Cy2
cyanine
- Cy3
indocarbocyanine
- Cy5
indodicarbocyanine
- DAPI
4′,6-diamidino-2-phynelindole
- dNTP
deoxyribonucleotide triphosphates
- ECL
enhanced chemiluminescence
- ERK
extracellular regulated kinase
- FITC
fluorosecien isothyocynate
- GABA
γ amino butyric acid
- GCL
ganglion cell layer
- GLAST
L-glutamate / L-aspartate transporter
- GS
glutamine synthetase
- HRP
horseradish peroxidase
- INL
inner nuclear layer
- IPL
inner plexiform layer
- IS/OS
inner and outer segments
- kDa
kiloDaltons
- mRNA
messenger ribonucleic acid
- ONL
outer nuclear layer
- OPL
outer plexiform layer
- PDE
phosphodiesterase
- PFA
paraformaldehyde
- PKA
protein kinase A
- PKCα
protein kinase C α-subunit
- PNA
peanut agglutinin
- RT-PCR
reverse transcriptase polymerase chain reaction
- TBST
tris buffered saline and tween-20
- Tx-TBS
triton X-100 and tris buffered saline
- UCR1
upstream conserved region 1
- UCR2
upstream conserved region 2
- VGlut1
vesicular glutamate transporter 1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdel-Majid RM, Tremblay F, Baldridge WH. Localization of adenylyl cyclase proteins in the rodent retina. Brain Res Mol Brain Res. 2002;101:62–70. doi: 10.1016/s0169-328x(02)00163-8. [DOI] [PubMed] [Google Scholar]
- Ahmed T, Frey JU. Expression of the specific type IV phosphodiesterase gene PDE4B3 during different phases of long-term potentiation in single hippocampal slices of rats in vitro. Neuroscience. 2003;117:627–638. doi: 10.1016/s0306-4522(02)00838-2. [DOI] [PubMed] [Google Scholar]
- Ahmed T, Frey S, Frey JU. Regulation of the phosphodiesterase PDE4B3-isotype during long-term potentiation in the area dentata in vivo. Neuroscience. 2004;124:857–867. doi: 10.1016/j.neuroscience.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariga M, Neitzert B, Nakae S, Mottin G, Bertrand C, Pruniaux MP, Jin SL, Conti M. Nonredundant function of phosphodiesterases 4D and 4B in neutrophil recruitment to the site of inflammation. J Immunol. 2004;173:7531–7538. doi: 10.4049/jimmunol.173.12.7531. [DOI] [PubMed] [Google Scholar]
- Beavo J, Francis SH, Houslay MD. Cyclic nucleotide phosphodiesterases in health and disease. CRC Press/Taylor & Francis; Boca Raton: 2007. [Google Scholar]
- Blanks JC, Johnson LV. Specific binding of peanut lectin to a class of retinal photoreceptor cells. A species comparison. Invest Ophthalmol Vis Sci. 1984;25:546–557. [PubMed] [Google Scholar]
- Blazynski C. Adenosine A1 receptor-mediated inhibition of adenylate cyclase in rabbit retina. J Neurosci. 1987;7:2522–2528. [PMC free article] [PubMed] [Google Scholar]
- Bloom TJ, Beavo JA. Identification and tissue-specific expression of PDE7 phosphodiesterase splice variants. Proc Natl Acad Sci U S A. 1996;93:14188–14192. doi: 10.1073/pnas.93.24.14188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolger G, Michaeli T, Martins T, St John T, Steiner B, Rodgers L, Riggs M, Wigler M, Ferguson K. A family of human phosphodiesterases homologous to the dunce learning and memory gene product of Drosophila melanogaster are potential targets for antidepressant drugs. Mol Cell Biol. 1993;13:6558–6571. doi: 10.1128/mcb.13.10.6558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolger GB. Molecular biology of the cyclic AMP-specific cyclic nucleotide phosphodiesterases: a diverse family of regulatory enzymes. Cell Signal. 1994;6:851–859. doi: 10.1016/0898-6568(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Bolger GB, Rodgers L, Riggs M. Differential CNS expression of alternative mRNA isoforms of the mammalian genes encoding cAMP-specific phosphodiesterases. Gene. 1994;149:237–244. doi: 10.1016/0378-1119(94)90155-4. [DOI] [PubMed] [Google Scholar]
- Borisy FF, Hwang PN, Ronnett GV, Snyder SH. High-affinity cAMP phosphodiesterase and adenosine localized in sensory organs. Brain Res. 1993;610:199–207. doi: 10.1016/0006-8993(93)91401-d. [DOI] [PubMed] [Google Scholar]
- Bragadottir R, Jarkman S. A cyclic adenosine monophosphate agonist elevates the b- and c-waves of the rabbit direct-current electroretinogram. Doc Ophthalmol. 1995;90:291–303. doi: 10.1007/BF01203864. [DOI] [PubMed] [Google Scholar]
- Brown JH, Makman MH. Stimulation by dopamine of adenylate cyclase in retinal homogenates and of adenosine-3′:5′-cyclic monophosphate formation in intact retina. Proc Natl Acad Sci U S A. 1972;69:539–543. doi: 10.1073/pnas.69.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunelli G, Spano P, Barlati S, Guarneri B, Barbon A, Bresciani R, Pizzi M. Glutamatergic reinnervation through peripheral nerve graft dictates assembly of glutamatergic synapses at rat skeletal muscle. Proc Natl Acad Sci U S A. 2005;102:8752–8757. doi: 10.1073/pnas.0500530102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo PE, Schoch S, Schmitz F, Sudhof TC, Malenka RC. RIM1alpha is required for presynaptic long-term potentiation. Nature. 2002;415:327–330. doi: 10.1038/415327a. [DOI] [PubMed] [Google Scholar]
- Charbonneau H, Beier N, Walsh KA, Beavo JA. Identification of a conserved domain among cyclic nucleotide phosphodiesterases from diverse species. Proc Natl Acad Sci U S A. 1986;83:9308–9312. doi: 10.1073/pnas.83.24.9308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chierzi S, Ratto GM, Verma P, Fawcett JW. The ability of axons to regenerate their growth cones depends on axonal type and age, and is regulated by calcium, cAMP and ERK. Eur J Neurosci. 2005;21:2051–2062. doi: 10.1111/j.1460-9568.2005.04066.x. [DOI] [PubMed] [Google Scholar]
- Choi JS, Kim JA, Joo CK. Activation of MAPK and CREB by GM1 induces survival of RGCs in the retina with axotomized nerve. Invest Ophthalmol Vis Sci. 2003;44:1747–1752. doi: 10.1167/iovs.01-0886. [DOI] [PubMed] [Google Scholar]
- Cohen AI, Todd RD, Harmon S, O’Malley KL. Photoreceptors of mouse retinas possess D4 receptors coupled to adenylate cyclase. Proc Natl Acad Sci U S A. 1992;89:12093–12097. doi: 10.1073/pnas.89.24.12093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti M. Phosphodiesterases and cyclic nucleotide signaling in endocrine cells. Mol Endocrinol. 2000;14:1317–1327. doi: 10.1210/mend.14.9.0534. [DOI] [PubMed] [Google Scholar]
- Conti M, Nemoz G, Sette C, Vicini E. Recent progress in understanding the hormonal regulation of phosphodiesterases. Endocr Rev. 1995;16:370–389. doi: 10.1210/edrv-16-3-370. [DOI] [PubMed] [Google Scholar]
- Conti M, Richter W, Mehats C, Livera G, Park JY, Jin C. Cyclic AMP-specific PDE4 phosphodiesterases as critical components of cyclic AMP signaling. J Biol Chem. 2003;278:5493–5496. doi: 10.1074/jbc.R200029200. [DOI] [PubMed] [Google Scholar]
- Derouiche A, Rauen T. Coincidence of L-glutamate/L-aspartate transporter (GLAST) and glutamine synthetase (GS) immunoreactions in retinal glia: evidence for coupling of GLAST and GS in transmitter clearance. J Neurosci Res. 1995;42:131–143. doi: 10.1002/jnr.490420115. [DOI] [PubMed] [Google Scholar]
- Dick O, Hack I, Altrock WD, Garner CC, Gundelfinger ED, Brandstatter JH. Localization of the presynaptic cytomatrix protein Piccolo at ribbon and conventional synapses in the rat retina: comparison with Bassoon. J Comp Neurol. 2001;439:224–234. doi: 10.1002/cne.1344. [DOI] [PubMed] [Google Scholar]
- Ding JD, Weinberg RJ. Distribution of soluble guanylyl cyclase in rat retina. J Comp Neurol. 2007;500:734–745. doi: 10.1002/cne.21206. [DOI] [PubMed] [Google Scholar]
- Douyard J, Shen L, Huganir RL, Rubio ME. Differential neuronal and glial expression of GluR1 AMPA receptor subunit and the scaffolding proteins SAP97 and 4.1N during rat cerebellar development. J Comp Neurol. 2007;502:141–156. doi: 10.1002/cne.21294. [DOI] [PubMed] [Google Scholar]
- Dunn TA, Wang CT, Colicos MA, Zaccolo M, DiPilato LM, Zhang J, Tsien RY, Feller MB. Imaging of cAMP levels and protein kinase A activity reveals that retinal waves drive oscillations in second-messenger cascades. J Neurosci. 2006;26:12807–12815. doi: 10.1523/JNEUROSCI.3238-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehinger B, Falck B. Autoradiography of some suspected neurotransmitter substances: GABA glycine, glutamic acid, histamine, dopamine, and L-dopa. Brain Res. 1971;33:157–172. doi: 10.1016/0006-8993(71)90314-3. [DOI] [PubMed] [Google Scholar]
- Elshatory Y, Deng M, Xie X, Gan L. Expression of the LIM-homeodomain protein Isl1 in the developing and mature mouse retina. J Comp Neurol. 2007;503:182–197. doi: 10.1002/cne.21390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farooqui SM, Al-Bagdadi F, Houslay MD, Bolger GB, Stout R, Specian RD, Cherry JA, Conti M, O’Donnell JM. Surgically induced cryptorchidism-related degenerative changes in spermatogonia are associated with loss of cyclic adenosine monophosphate-dependent phosphodiesterases type 4 in abdominal testes of rats. Biol Reprod. 2001;64:1583–1589. doi: 10.1095/biolreprod64.6.1583. [DOI] [PubMed] [Google Scholar]
- Fekete DM, Barnstable CJ. The subcellular localization of rat photoreceptor-specific antigens. J Neurocytol. 1983;12:785–803. doi: 10.1007/BF01258151. [DOI] [PubMed] [Google Scholar]
- Feller MB, Wellis DP, Stellwagen D, Werblin FS, Shatz CJ. Requirement for cholinergic synaptic transmission in the propagation of spontaneous retinal waves. Science. 1996;272:1182–1187. doi: 10.1126/science.272.5265.1182. [DOI] [PubMed] [Google Scholar]
- Firth SI, Wang CT, Feller MB. Retinal waves: mechanisms and function in visual system development. Cell Calcium. 2005;37:425–432. doi: 10.1016/j.ceca.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Fischer KF, Lukasiewicz PD, Wong RO. Age-dependent and cell class-specific modulation of retinal ganglion cell bursting activity by GABA. J Neurosci. 1998;18:3767–3778. doi: 10.1523/JNEUROSCI.18-10-03767.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher DA, Smith JF, Pillar JS, St Denis SH, Cheng JB. Isolation and characterization of PDE8A, a novel human cAMP-specific phosphodiesterase. Biochem Biophys Res Commun. 1998;246:570–577. doi: 10.1006/bbrc.1998.8684. [DOI] [PubMed] [Google Scholar]
- Fiumara F, Giovedi S, Menegon A, Milanese C, Merlo D, Montarolo PG, Valtorta F, Benfenati F, Ghirardi M. Phosphorylation by cAMP-dependent protein kinase is essential for synapsin-induced enhancement of neurotransmitter release in invertebrate neurons. J Cell Sci. 2004;117:5145–5154. doi: 10.1242/jcs.01388. [DOI] [PubMed] [Google Scholar]
- Fujita M, Imaizumi M, D’Sa C, Zoghbi SS, Crescenzo MS, Hong J, Musachio JL, Gee AD, Seidel J, Green MV, Pike VW, Duman RS, Innis RB. In vivo and in vitro measurement of brain phosphodiesterase 4 in rats after antidepressant administration. Synapse. 2007;61:78–86. doi: 10.1002/syn.20347. [DOI] [PubMed] [Google Scholar]
- Fung BK, Hurley JB, Stryer L. Flow of information in the light-triggered cyclic nucleotide cascade of vision. Proc Natl Acad Sci U S A. 1981;78:152–156. doi: 10.1073/pnas.78.1.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gantner F, Tenor H, Gekeler V, Schudt C, Wendel A, Hatzelmann A. Phosphodiesterase profiles of highly purified human peripheral blood leukocyte populations from normal and atopic individuals: a comparative study. J Allergy Clin Immunol. 1997;100:527–535. doi: 10.1016/s0091-6749(97)70146-5. [DOI] [PubMed] [Google Scholar]
- Gargini C, Terzibasi E, Mazzoni F, Strettoi E. Retinal organization in the retinal degeneration 10 (rd10) mutant mouse: a morphological and ERG study. J Comp Neurol. 2007;500:222–238. doi: 10.1002/cne.21144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greferath U, Grunert U, Wassle H. Rod bipolar cells in the mammalian retina show protein kinase C-like immunoreactivity. J Comp Neurol. 1990;301:433–442. doi: 10.1002/cne.903010308. [DOI] [PubMed] [Google Scholar]
- Haverkamp S, Wassle H. Immunocytochemical analysis of the mouse retina. J Comp Neurol. 2000;424:1–23. [PubMed] [Google Scholar]
- Hayashi M, Matsushima K, Ohashi H, Tsunoda H, Murase S, Kawarada Y, Tanaka T. Molecular cloning and characterization of human PDE8B, a novel thyroid-specific isozyme of 3′,5′-cyclic nucleotide phosphodiesterase. Biochem Biophys Res Commun. 1998;250:751–756. doi: 10.1006/bbrc.1998.9379. [DOI] [PubMed] [Google Scholar]
- Heitzmann H. Rhodopsin is the predominant protein of rod outer segment membranes. Nat New Biol. 1972;235:114. doi: 10.1038/newbio235114a0. [DOI] [PubMed] [Google Scholar]
- Higgins DG, Thompson JD, Gibson TJ. Using CLUSTAL for multiple sequence alignments. Methods Enzymol. 1996;266:383–402. doi: 10.1016/s0076-6879(96)66024-8. [DOI] [PubMed] [Google Scholar]
- Hoffmann R, Baillie GS, MacKenzie SJ, Yarwood SJ, Houslay MD. The MAP kinase ERK2 inhibits the cyclic AMP-specific phosphodiesterase HSPDE4D3 by phosphorylating it at Ser579. Embo J. 1999;18:893–903. doi: 10.1093/emboj/18.4.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houslay MD, Adams DR. PDE4 cAMP phosphodiesterases: modular enzymes that orchestrate signalling cross-talk, desensitization and compartmentalization. Biochem J. 2003;370:1–18. doi: 10.1042/BJ20021698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houslay MD, Baillie GS, Maurice DH. cAMP-Specific phosphodiesterase-4 enzymes in the cardiovascular system: a molecular toolbox for generating compartmentalized cAMP signaling. Circ Res. 2007;100:950–966. doi: 10.1161/01.RES.0000261934.56938.38. [DOI] [PubMed] [Google Scholar]
- Jaken S, Kiley SC. Purification and characterization of three types of protein kinase C from rabbit brain cytosol. Proc Natl Acad Sci U S A. 1987;84:4418–4422. doi: 10.1073/pnas.84.13.4418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon CJ, Strettoi E, Masland RH. The major cell populations of the mouse retina. J Neurosci. 1998;18:8936–8946. doi: 10.1523/JNEUROSCI.18-21-08936.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin SL, Lan L, Zoudilova M, Conti M. Specific role of phosphodiesterase 4B in lipopolysaccharide-induced signaling in mouse macrophages. J Immunol. 2005;175:1523–1531. doi: 10.4049/jimmunol.175.3.1523. [DOI] [PubMed] [Google Scholar]
- Jin SL, Swinnen JV, Conti M. Characterization of the structure of a low Km, rolipram-sensitive cAMP phosphodiesterase. Mapping of the catalytic domain. J Biol Chem. 1992;267:18929–18939. [PubMed] [Google Scholar]
- Johnson J, Tian N, Caywood MS, Reimer RJ, Edwards RH, Copenhagen DR. Vesicular neurotransmitter transporter expression in developing postnatal rodent retina: GABA and glycine precede glutamate. J Neurosci. 2003;23:518–529. doi: 10.1523/JNEUROSCI.23-02-00518.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson LV, Hageman GS, Blanks JC. Interphotoreceptor matrix domains ensheath vertebrate cone photoreceptor cells. Invest Ophthalmol Vis Sci. 1986;27:129–135. [PubMed] [Google Scholar]
- Kim HS, Park CK. Retinal ganglion cell death is delayed by activation of retinal intrinsic cell survival program. Brain Res. 2005;1057:17–28. doi: 10.1016/j.brainres.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Kiyonaka S, Wakamori M, Miki T, Uriu Y, Nonaka M, Bito H, Beedle AM, Mori E, Hara Y, De Waard M, Kanagawa M, Itakura M, Takahashi M, Campbell KP, Mori Y. RIM1 confers sustained activity and neurotransmitter vesicle anchoring to presynaptic Ca(2+) channels. Nat Neurosci. 2007;10:691–701. doi: 10.1038/nn1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostic MM, Erdogan S, Rena G, Borchert G, Hoch B, Bartel S, Scotland G, Huston E, Houslay MD, Krause EG. Altered expression of PDE1 and PDE4 cyclic nucleotide phosphodiesterase isoforms in 7-oxo-prostacyclin-preconditioned rat heart. J Mol Cell Cardiol. 1997;29:3135–3146. doi: 10.1006/jmcc.1997.0544. [DOI] [PubMed] [Google Scholar]
- Kubrusly RC, da Cunha MC, Reis RA, Soares H, Ventura AL, Kurtenbach E, de Mello MC, de Mello FG. Expression of functional receptors and transmitter enzymes in cultured Muller cells. Brain Res. 2005;1038:141–149. doi: 10.1016/j.brainres.2005.01.031. [DOI] [PubMed] [Google Scholar]
- Lamontagne S, Meadows E, Luk P, Normandin D, Muise E, Boulet L, Pon DJ, Robichaud A, Robertson GS, Metters KM, Nantel F. Localization of phosphodiesterase-4 isoforms in the medulla and nodose ganglion of the squirrel monkey. Brain Res. 2001;920:84–96. doi: 10.1016/s0006-8993(01)03023-2. [DOI] [PubMed] [Google Scholar]
- Leach KL, Powers EA, McGuire JC, Dong L, Kiley SC, Jaken S. Monoclonal antibodies specific for type 3 protein kinase C recognize distinct domains of protein kinase C and inhibit in vitro functional activity. J Biol Chem. 1988;263:13223–13230. [PubMed] [Google Scholar]
- Lehnart SE, Wehrens XH, Reiken S, Warrier S, Belevych AE, Harvey RD, Richter W, Jin SL, Conti M, Marks AR. Phosphodiesterase 4D deficiency in the ryanodine-receptor complex promotes heart failure and arrhythmias. Cell. 2005;123:25–35. doi: 10.1016/j.cell.2005.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehre KP, Davanger S, Danbolt NC. Localization of the glutamate transporter protein GLAST in rat retina. Brain Res. 1997;744:129–137. doi: 10.1016/s0006-8993(96)01022-0. [DOI] [PubMed] [Google Scholar]
- Linden DJ, Ahn S. Activation of presynaptic cAMP-dependent protein kinase is required for induction of cerebellar long-term potentiation. J Neurosci. 1999;19:10221–10227. doi: 10.1523/JNEUROSCI.19-23-10221.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden R, Perry VH. Massive retinotectal projection in rats. Brain Res. 1983;272:145–149. doi: 10.1016/0006-8993(83)90371-2. [DOI] [PubMed] [Google Scholar]
- Liu IS, Chen JD, Ploder L, Vidgen D, van der Kooy D, Kalnins VI, McInnes RR. Developmental expression of a novel murine homeobox gene (Chx10): evidence for roles in determination of the neuroretina and inner nuclear layer. Neuron. 1994;13:377–393. doi: 10.1016/0896-6273(94)90354-9. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lonart G, Schoch S, Kaeser PS, Larkin CJ, Sudhof TC, Linden DJ. Phosphorylation of RIM1alpha by PKA triggers presynaptic long-term potentiation at cerebellar parallel fiber synapses. Cell. 2003;115:49–60. doi: 10.1016/s0092-8674(03)00727-x. [DOI] [PubMed] [Google Scholar]
- Lugnier C. Cyclic nucleotide phosphodiesterase (PDE) superfamily: a new target for the development of specific therapeutic agents. Pharmacol Ther. 2006;109:366–398. doi: 10.1016/j.pharmthera.2005.07.003. [DOI] [PubMed] [Google Scholar]
- MacKenzie SJ, Baillie GS, McPhee I, Bolger GB, Houslay MD. ERK2 mitogen-activated protein kinase binding, phosphorylation, and regulation of the PDE4D cAMP-specific phosphodiesterases. The involvement of COOH-terminal docking sites and NH2-terminal UCR regions. J Biol Chem. 2000;275:16609–16617. doi: 10.1074/jbc.275.22.16609. [DOI] [PubMed] [Google Scholar]
- MacKenzie SJ, Baillie GS, McPhee I, MacKenzie C, Seamons R, McSorley T, Millen J, Beard MB, van Heeke G, Houslay MD. Long PDE4 cAMP specific phosphodiesterases are activated by protein kinase A-mediated phosphorylation of a single serine residue in Upstream Conserved Region 1 (UCR1) Br J Pharmacol. 2002;136:421–433. doi: 10.1038/sj.bjp.0704743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire G, Werblin F. Dopamine enhances a glutamate-gated ionic current in OFF bipolar cells of the tiger salamander retina. J Neurosci. 1994;14:6094–6101. doi: 10.1523/JNEUROSCI.14-10-06094.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchmont RJ, Houslay MD. A peripheral and an intrinsic enzyme constitute the cyclic AMP phosphodiesterase activity of rat liver plasma membranes. Biochem J. 1980;187:381–392. doi: 10.1042/bj1870381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mataruga A, Kremmer E, Muller F. Type 3a and type 3b OFF cone bipolar cells provide for the alternative rod pathway in the mouse retina. J Comp Neurol. 2007;502:1123–1137. doi: 10.1002/cne.21367. [DOI] [PubMed] [Google Scholar]
- Mehats C, Andersen CB, Filopanti M, Jin SL, Conti M. Cyclic nucleotide phosphodiesterases and their role in endocrine cell signaling. Trends Endocrinol Metab. 2002;13:29–35. doi: 10.1016/s1043-2760(01)00523-9. [DOI] [PubMed] [Google Scholar]
- Melone M, Burette A, Weinberg RJ. Light microscopic identification and immunocytochemical characterization of glutamatergic synapses in brain sections. J Comp Neurol. 2005;492:495–509. doi: 10.1002/cne.20743. [DOI] [PubMed] [Google Scholar]
- Michaeli T, Bloom TJ, Martins T, Loughney K, Ferguson K, Riggs M, Rodgers L, Beavo JA, Wigler M. Isolation and characterization of a previously undetected human cAMP phosphodiesterase by complementation of cAMP phosphodiesterase-deficient Saccharomyces cerevisiae. J Biol Chem. 1993;268:12925–12932. [PubMed] [Google Scholar]
- Miki N, Keirns JJ, Marcus FR, Freeman J, Bitensky MW. Regulation of cyclic nucleotide concentrations in photoreceptors: an ATP-dependent stimulation of cyclic nucleotide phosphodiesterase by light. Proc Natl Acad Sci U S A. 1973;70:3820–3824. doi: 10.1073/pnas.70.12.3820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimura Y, Mogi K, Kawano M, Fukui Y, Takeda J, Nogami H, Hisano S. Differential expression of two distinct vesicular glutamate transporters in the rat retina. Neuroreport. 2002;13:1925–1928. doi: 10.1097/00001756-200210280-00019. [DOI] [PubMed] [Google Scholar]
- Mojumder DK, Frishman LJ, Otteson DC, Sherry DM. Voltage-gated sodium channel alpha-subunits Na(v)1.1, Na(v)1.2, and Na(v)1.6 in the distal mammalian retina. Mol Vis. 2007;13:2163–2182. [PubMed] [Google Scholar]
- Mons N, Harry A, Dubourg P, Premont RT, Iyengar R, Cooper DM. Immunohistochemical localization of adenylyl cyclase in rat brain indicates a highly selective concentration at synapses. Proc Natl Acad Sci U S A. 1995;92:8473–8477. doi: 10.1073/pnas.92.18.8473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monsul NT, Geisendorfer AR, Han PJ, Banik R, Pease ME, Skolasky RL, Jr., Hoffman PN. Intraocular injection of dibutyryl cyclic AMP promotes axon regeneration in rat optic nerve. Exp Neurol. 2004;186:124–133. doi: 10.1016/S0014-4886(03)00311-X. [DOI] [PubMed] [Google Scholar]
- Motts SD, Slusarczyk AS, Sowick CS, Schofield BR. Distribution of cholinergic cells in guinea pig brainstem. Neuroscience. 2008 doi: 10.1016/j.neuroscience.2007.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negishi K, Kato S, Teranishi T. Dopamine cells and rod bipolar cells contain protein kinase C-like immunoreactivity in some vertebrate retinas. Neurosci Lett. 1988;94:247–252. doi: 10.1016/0304-3940(88)90025-0. [DOI] [PubMed] [Google Scholar]
- Nguyen PV, Woo NH. Regulation of hippocampal synaptic plasticity by cyclic AMP-dependent protein kinases. Prog Neurobiol. 2003;71:401–437. doi: 10.1016/j.pneurobio.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Nikulina E, Tidwell JL, Dai HN, Bregman BS, Filbin MT. The phosphodiesterase inhibitor rolipram delivered after a spinal cord lesion promotes axonal regeneration and functional recovery. Proc Natl Acad Sci U S A. 2004;101:8786–8790. doi: 10.1073/pnas.0402595101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obernolte R, Ratzliff J, Baecker PA, Daniels DV, Zuppan P, Jarnagin K, Shelton ER. Multiple splice variants of phosphodiesterase PDE4C cloned from human lung and testis. Biochim Biophys Acta. 1997;1353:287–297. doi: 10.1016/s0167-4781(97)00080-8. [DOI] [PubMed] [Google Scholar]
- Oki N, Takahashi SI, Hidaka H, Conti M. Short term feedback regulation of cAMP in FRTL-5 thyroid cells. Role of PDE4D3 phosphodiesterase activation. J Biol Chem. 2000;275:10831–10837. doi: 10.1074/jbc.275.15.10831. [DOI] [PubMed] [Google Scholar]
- Osborne NN. Inhibition of cAMP production by alpha 2-adrenoceptor stimulation in rabbit retina. Brain Res. 1991;553:84–88. doi: 10.1016/0006-8993(91)90233-l. [DOI] [PubMed] [Google Scholar]
- Park H, Lee JA, Lee C, Kim MJ, Chang DJ, Kim H, Lee SH, Lee YS, Kaang BK. An Aplysia type 4 phosphodiesterase homolog localizes at the presynaptic terminals of Aplysia neuron and regulates synaptic facilitation. J Neurosci. 2005;25:9037–9045. doi: 10.1523/JNEUROSCI.1989-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Elsevier Academic; Burlington, MA: 2005. [Google Scholar]
- Pearse DD, Pereira FC, Marcillo AE, Bates ML, Berrocal YA, Filbin MT, Bunge MB. cAMP and Schwann cells promote axonal growth and functional recovery after spinal cord injury. Nat Med. 2004;10:610–616. doi: 10.1038/nm1056. [DOI] [PubMed] [Google Scholar]
- Peichl L, Gonzalez-Soriano J. Morphological types of horizontal cell in rodent retinae: a comparison of rat, mouse, gerbil, and guinea pig. Vis Neurosci. 1994;11:501–517. doi: 10.1017/s095252380000242x. [DOI] [PubMed] [Google Scholar]
- Perry VH, Walker M. Amacrine cells, displaced amacrine cells and interplexiform cells in the retina of the rat. Proc R Soc Lond B Biol Sci. 1980;208:415–431. doi: 10.1098/rspb.1980.0060. [DOI] [PubMed] [Google Scholar]
- Rabie A, Thomasset M, Parkes CO, Clavel MC. Immunocytochemical detection of 28 000-MW calcium-binding protein in horizontal cells of the rat retina. Cell Tissue Res. 1985;240:493–496. doi: 10.1007/BF00222366. [DOI] [PubMed] [Google Scholar]
- Rauen T, Rothstein JD, Wassle H. Differential expression of three glutamate transporter subtypes in the rat retina. Cell Tissue Res. 1996;286:325–336. doi: 10.1007/s004410050702. [DOI] [PubMed] [Google Scholar]
- Reppert SM, Godson C, Mahle CD, Weaver DR, Slaugenhaupt SA, Gusella JF. Molecular characterization of a second melatonin receptor expressed in human retina and brain: the Mel1b melatonin receptor. Proc Natl Acad Sci U S A. 1995;92:8734–8738. doi: 10.1073/pnas.92.19.8734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riepe RE, Norenburg MD. Muller cell localisation of glutamine synthetase in rat retina. Nature. 1977;268:654–655. doi: 10.1038/268654a0. [DOI] [PubMed] [Google Scholar]
- Rivera N, Lugo N. Four retinal ganglion cell types that project to the superior colliculus in the thirteen-lined ground squirrel (Spermophilus tridecemlineatus) J Comp Neurol. 1998;396:105–120. doi: 10.1002/(sici)1096-9861(19980622)396:1<105::aid-cne8>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Rohrenbeck J, Wassle H, Heizmann CW. Immunocytochemical labelling of horizontal cells in mammalian retina using antibodies against calcium-binding proteins. Neurosci Lett. 1987;77:255–260. doi: 10.1016/0304-3940(87)90508-8. [DOI] [PubMed] [Google Scholar]
- Rozen S, Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol. 2000;132:365–386. doi: 10.1385/1-59259-192-2:365. [DOI] [PubMed] [Google Scholar]
- Sakai T, Yoshitoshi T, Nagai Y, Kitahara K. Increased glutamate uptake and GLAST expression by cyclic AMP in retinal glial cells. Graefes Arch Clin Exp Ophthalmol. 2006;244:359–363. doi: 10.1007/s00417-005-0060-1. [DOI] [PubMed] [Google Scholar]
- Santone R, Giorgi M, Maccarone R, Basso M, Deplano S, Bisti S. Gene expression and protein localization of calmodulin-dependent phosphodiesterase in adult rat retina. J Neurosci Res. 2006;84:1020–1026. doi: 10.1002/jnr.21009. [DOI] [PubMed] [Google Scholar]
- Sette C, Conti M. Phosphorylation and activation of a cAMP-specific phosphodiesterase by the cAMP-dependent protein kinase. Involvement of serine 54 in the enzyme activation. J Biol Chem. 1996;271:16526–16534. doi: 10.1074/jbc.271.28.16526. [DOI] [PubMed] [Google Scholar]
- Sherry DM, Wang MM, Bates J, Frishman LJ. Expression of vesicular glutamate transporter 1 in the mouse retina reveals temporal ordering in development of rod vs. cone and ON vs. OFF circuits. J Comp Neurol. 2003;465:480–498. doi: 10.1002/cne.10838. [DOI] [PubMed] [Google Scholar]
- Silvestre JS, Fernandez AG, Palacios JM. Preliminary evidence for an involvement of the cholinergic system in the sedative effects of rolipram in rats. Pharmacol Biochem Behav. 1999;64:1–5. doi: 10.1016/s0091-3057(98)00243-3. [DOI] [PubMed] [Google Scholar]
- Soderling SH, Bayuga SJ, Beavo JA. Cloning and characterization of a cAMP-specific cyclic nucleotide phosphodiesterase. Proc Natl Acad Sci U S A. 1998;95:8991–8996. doi: 10.1073/pnas.95.15.8991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stellwagen D, Shatz CJ, Feller MB. Dynamics of retinal waves are controlled by cyclic AMP. Neuron. 1999;24:673–685. doi: 10.1016/s0896-6273(00)81121-6. [DOI] [PubMed] [Google Scholar]
- Swinnen JV, Joseph DR, Conti M. Molecular cloning of rat homologues of the Drosophila melanogaster dunce cAMP phosphodiesterase: evidence for a family of genes. Proc Natl Acad Sci U S A. 1989;86:5325–5329. doi: 10.1073/pnas.86.14.5325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- tom Dieck S, Altrock WD, Kessels MM, Qualmann B, Regus H, Brauner D, Fejtova A, Bracko O, Gundelfinger ED, Brandstatter JH. Molecular dissection of the photoreceptor ribbon synapse: physical interaction of Bassoon and RIBEYE is essential for the assembly of the ribbon complex. J Cell Biol. 2005;168:825–836. doi: 10.1083/jcb.200408157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traverso V, Bush RA, Sieving PA, Deretic D. Retinal cAMP levels during the progression of retinal degeneration in rhodopsin P23H and S334ter transgenic rats. Invest Ophthalmol Vis Sci. 2002;43:1655–1661. [PubMed] [Google Scholar]
- Urschel S, Hoher T, Schubert T, Alev C, Sohl G, Worsdorfer P, Asahara T, Dermietzel R, Weiler R, Willecke K. Protein kinase A-mediated phosphorylation of connexin36 in mouse retina results in decreased gap junctional communication between AII amacrine cells. J Biol Chem. 2006;281:33163–33171. doi: 10.1074/jbc.M606396200. [DOI] [PubMed] [Google Scholar]
- Van Buskirk R, Dowling JE. Isolated horizontal cells from carp retina demonstrate dopamine-dependent accumulation of cyclic AMP. Proc Natl Acad Sci U S A. 1981;78:7825–7829. doi: 10.1073/pnas.78.12.7825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeiren C, Najimi M, Maloteaux JM, Hermans E. Molecular and functional characterisation of glutamate transporters in rat cortical astrocytes exposed to a defined combination of growth factors during in vitro differentiation. Neurochem Int. 2005;46:137–147. doi: 10.1016/j.neuint.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Voigt T. Cholinergic amacrine cells in the rat retina. J Comp Neurol. 1986;248:19–35. doi: 10.1002/cne.902480103. [DOI] [PubMed] [Google Scholar]
- Wachtel H. Characteristic behavioural alterations in rats induced by rolipram and other selective adenosine cyclic 3′, 5′-monophosphate phosphodiesterase inhibitors. Psychopharmacology (Berl) 1982;77:309–316. doi: 10.1007/BF00432761. [DOI] [PubMed] [Google Scholar]
- Wang Y, Okamoto M, Schmitz F, Hofmann K, Sudhof TC. Rim is a putative Rab3 effector in regulating synaptic-vesicle fusion. Nature. 1997;388:593–598. doi: 10.1038/41580. [DOI] [PubMed] [Google Scholar]
- Wang Y, Sugita S, Sudhof TC. The RIM/NIM family of neuronal C2 domain proteins. Interactions with Rab3 and a new class of Src homology 3 domain proteins. J Biol Chem. 2000;275:20033–20044. doi: 10.1074/jbc.M909008199. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Tokita Y, Kato M, Fukuda Y. Intravitreal injections of neurotrophic factors and forskolin enhance survival and axonal regeneration of axotomized beta ganglion cells in cat retina. Neuroscience. 2003;116:733–742. doi: 10.1016/s0306-4522(02)00562-6. [DOI] [PubMed] [Google Scholar]
- Wheeler GL, Bitensky MW. A light-activated GTPase in vertebrate photoreceptors: regulation of light-activated cyclic GMP phosphodiesterase. Proc Natl Acad Sci U S A. 1977;74:4238–4242. doi: 10.1073/pnas.74.10.4238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker CM, Cooper NGF. Investigative Ophthalmology & Visual Science. Vol. 48. 2007. Characterization of Phosphodiesterase 4 Within the Adult Rat Retina. E-Abstract 3047. [Google Scholar]
- White RD, Neal MJ. The uptake of L-glutamate by the retina. Brain Res. 1976;111:79–93. doi: 10.1016/0006-8993(76)91050-7. [DOI] [PubMed] [Google Scholar]
- Willardson BM, Wilkins JF, Yoshida T, Bitensky MW. Regulation of phosducin phosphorylation in retinal rods by Ca2+/calmodulin-dependent adenylyl cyclase. Proc Natl Acad Sci U S A. 1996;93:1475–1479. doi: 10.1073/pnas.93.4.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Diamond JS. Distinct perisynaptic and synaptic localization of NMDA and AMPA receptors on ganglion cells in rat retina. J Comp Neurol. 2006;498:810–820. doi: 10.1002/cne.21089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Cote RH. cGMP signaling in vertebrate retinal photoreceptor cells. Front Biosci. 2005;10:1191–1204. doi: 10.2741/1612. [DOI] [PubMed] [Google Scholar]