Abstract
Background and Aims
Gastric cancer results from a combination of H. pylori infection, exposure to dietary carcinogens, and predisposing genetic makeup. Because the role of these factors in gastric carcinogenesis cannot be readily determined in humans, the present study examined the role of an oral carcinogen and H. pylori infection in Rhesus monkeys.
Methods
Gastroscopies were performed in 23 monkeys assigned to four groups: controls (C); nitrosating carcinogen ethyl-nitro-nitrosoguanidine (ENNG) administration alone (E); inoculation of a virulent H. pylori strain, alone (H); and ethyl-nitro-nitrosoguanidine in combination with H. pylori (EH). Follow-up gastroscopies and biopsies were performed at 3-month intervals for five years for pathological and molecular studies.
Results
Postinoculation, H and EH groups exhibited persistent infection and antral gastritis. Starting at two- and five-year, respectively, gastric intestinal metaplasia and intraepithelial neoplasia developed in three EH monkeys but in no other groups. Transcriptional analysis of biopsy specimens at five-year revealed group-specific expression profiles, with striking changes in EH monkeys, plus a neoplasia-specific expression profile characterized by changes in multiple cancer-associated genes. Importantly, this neoplastic profile was evident in non-neoplastic mucosa, suggesting that the identified genes may represent markers preceding cancer.
Conclusions
Gastric intraglandular neoplasia is induced in primates when H. pylori infection is associated with consumption of a carcinogen similar to the nitrosamines found in pickled vegetables, suggesting that H. pylori and the carcinogen synergistically induce gastric neoplasia in primates.
Gastric adenocarcinoma is the 4th most frequent cancer worldwide and second in cancer mortality with 700,000 deaths/year.1 Worldwide incidence is 16.2/100,000 persons per year, being extremely variable in different regions and among different ethnic groups.2 Early epidemiological studies among migrant populations demonstrated that being born and raised in regions with high prevalence of gastric cancer drastically increased risk for the disease.3 Concurrent pathology studies demonstrated an excess of intestinal metaplasia in populations at high risk for gastric cancer, which was preceded by gastric inflammation and intestinal metaplasia, thus leading to the prediction of a role for environmental factors.3 After H. pylori discovery,4 retrospective and nested-case epidemiological studies demonstrated that gastric cancer risk is linked to this infection,5 and H. pylori became the first bacterium to be recognized as a type I carcinogen.6 However, only a minority of H. pylori-colonized subjects develop gastric cancer, and host, diet, and other environmental factors likely play co-carcinogenic roles.7, 8
A recent consensus conference concluded that H. pylori infection is a necessary but not sufficient causal factor for gastric adenocarcinoma9 because prospective studies demonstrated that gastric cancer developed in persons infected with H. pylori but not in uninfected persons10 and H. pylori eradication prevented postresection relapse of intraepithelial non-invasive neoplasia (named early gastric carcinoma in Japan).11 Furthermore, successful H. pylori eradication prevented appearance of gastric cancer in patients with no precancerous lesions at enrollment, whereas gastric cancer appeared in 1.2% of subjects remaining infected after treatment.12 Finally, a randomized prospective trial showed that H. pylori eradication therapy reduced, but did not abolish gastric cancer prevalence in high-risk populations, although post-antibiotic eradication of H. pylori was not verified.13
Retrospective epidemiological studies of diet showed increased risk in populations who consumed foods rich in salt and/or dietary carcinogens (e.g. nitrosamines in smoked fish and pickled vegetables).14-16 Conversely, gastric cancer was decreased with high intake of fresh fruits and vegetables.17, 18 In addition, a prospective seven-year study of >500,000 Europeans showed that intake of nitroso compounds was associated with increased risk of gastric cancer.19 There was also a trend towards interaction between H. pylori infection and nitroso compound intake (p =0.09) that was not significant due to high H. pylori seroprevalence.19
These studies illustrate the difficulties encountered when multiple causes of gastric cancer are investigated concurrently in humans. Indeed, maintaining tight control of diet and of H. pylori infection status of patients is virtually impossible in ethical human studies and a molecular understanding of the role of diet and H. pylori infection on the development of gastric cancer has remained elusive.
Rodent models have been extensively used to study H. pylori-induced disease,20 and the Mongolian gerbil has become the animal of choice in studies of gastric carcinogenesis.21 Several investigators have reported that H. pylori colonization alone leads to development of intestinal type gastric adenocarcinoma in Mongolian gerbils.22, 23 In other studies, however, well-differentiated adenocarcinoma were observed only in H. pylori positive gerbils that were also fed a nitrosamine.21 The reason for this discrepancy is currently unknown. Unfortunately, the lack of a sequenced gerbil genome and the relative paucity of gerbil-specific reagents are limiting factors when host responses during carcinogenesis are to be studied.
In contrast, Rhesus monkeys (Macaca mulatta) are closely related to humans from a genetic, histopathologic, immunological, and nutritional standpoint. In addition, free-ranging monkeys are infected by H. pylori,24 peptic ulcer disease was reported in multiple breeding colonies25 and spontaneous gastric cancer was reported in older Rhesus monkeys captured in the wilderness.26, 27
The nitrosamine carcinogen N-Ethyl-N-nitrosoguanidine (ENNG) is a carcinogen similar to nitrosamines found in pickled vegetables, smoked fish and meats consumed by East Asian and East European populations15, 28 and oral administration of ENNG can induce gastric cancer in Rhesus and Cynomolgus monkeys.29 However, these latter studies were performed before it was widely known that colony-bred Rhesus and Cynomolgus monkeys are H. pylori-positive24, 30 and often are also colonized by H. Heilmanni-like bacteria.31 Thus, along with ENNG, H. pylori likely played a role in gastric cancer development in these macaques. In addition, persistent H. pylori infection for 7.5 years without carcinogen administration induced p53 mutations but no gastric cancer in the related Macaca fuscata.32 Together, these studies suggest that both H. pylori and carcinogens are necessary for gastric carcinogenesis in primates, thus explaining why only a fraction of H. pylori-infected humans develop gastric cancer.
To test this hypothesis, 23 purpose-bred Rhesus monkeys were studied over a five-year period. Twelve monkeys were infected with a virulent H. pylori strain isolated from a patient with gastric adenocarcinoma. As schematized in Fig. 1, infected and uninfected monkeys were next divided into two groups each, leading to four experimental groups: (1) controls (C); (2) H. pylori infected (H); (3) uninfected animals given ENNG mixed with food (E); and (4) H. pylori + ENNG (EH). We observed that both H. pylori and carcinogens are necessary to induce gastric cancer in Rhesus monkeys. This is the first experimental study of the individual roles of H. pylori and a carcinogen similar to components of East Asian diets on gastric carcinogenesis in a human-like model and its conclusions may help explain why only a fraction of H. pylori-infected humans develop gastric cancer.
Figure 1.
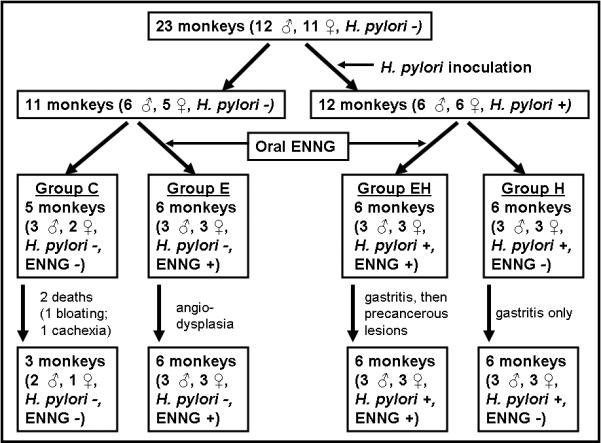
Experimental design, time course and outcome of the study.
Materials and Methods
Animals
Twenty-three purpose-bred Rhesus monkeys were used (See the Supplementary methods section). Studies were approved by AFRRI’s IACUC.
Physical examination, videogastroscopies and mucosal biopsies (See the Supplementary methods section)
All monkeys were monitored daily by veterinary staff. Every three months, they were anesthetized, weighted and videogastroscopies were performed and eight pinch biopsies were taken each from the body and antrum.33
Gastritis was scored on coded slides using the Sydney system34 and a scale of 0-3 (0, intact mucosal lining and essentially no infiltration of the lamina propria; 1, mild mononuclear infiltration in the upper half of the mucosa; 2, moderate mononuclear infiltration throughout the lamina propria; and 3, marked mononuclear infiltration throughout the lamina propria plus polymorphonuclear leukocytes in glands and surface erosions).35 In addition, atrophy was graded on a scale of 0-3 (0: none; 1: mild; 2: moderate; 3: severe).36
After release from quarantine, presence of natural H. pylori infection was verified by gastroduodenal endoscopic examination and culture and histology of mucosal biopsies. The infection was then cured as published35 and H. pylori -negativity was verified (Supplementary methods).
H. pylori culture (See the Supplementary methods section)
Gastric biopsies harvested at the time of each endoscopy were cultured as described.35
H. pylori strain
Strain USU101 was isolated from a patient with gastric adenocarcinoma and was characterized as cagA+ (EPIYA motif ABC), vacA+ (s1, i1, m1) and babA+ using PCR.37-39 Additionally, virulence was verified through measurement of IL8 release in vitro (supplementary methods). USU101 (108 CFU/ml) was sprayed onto the antral mucosa of the H and EH monkeys as described.35
ENNG administration (See the Supplementary methods section)
E and EH monkeys received 30mg of ENNG mixed with food five days a week.
Histopathology and molecular detection of H. pylori by in situ hybridization (See the Supplementary methods section)
Gastric biopsies were fixed, processed and stained or processed for ISH and analyzed as described.40
Molecular detection of H. pylori and Rhesus monkey genes by real-time RT-PCR40 (See the Supplementary methods section)
Expression of H. pylori 16S rRNA and Rhesus monkey IL-1β, IL-8, MGMT, and 18S rRNA genes was determined as described.40
DNA microarray analysis of gene expression
The Rhesus monkey Oligo Microarray Kit (Agilent, Cat #015421 Rhesus monkey) was used for all hybridizations (Supplementary methods).
Statistics
Changes in gene expression over time within animals were compared with a mixed-effects ANOVA model corresponding to a repeated-measures ANOVA model with time as a within-subject factor. Data are reported as mean ± SEM. The Dunnett’s post-hoc test was used to compare the average at each time point to the average at week 0. Fisher’s exact test was used to compare incidence of neoplasia among monkey groups.
Results
Basic health status and body weight were maintained in all groups.
Videogastroscopies, H. pylori Infection Status, and Histopathology
Throughout the study, biopsies harvested from C and E animals remained H. pylori negative whereas H and EH monkeys biopsies were H. pylori positive by culture,35 real-time RT-PCR, and/or in situ hybridization40 following H. pylori inoculation. Initial H. pylori colonization levels were similar in H and EH animals (Fig. 2a). Colonization remained constant in EH animals throughout the study whereas the number of bacteria in the H group steadily increased and was significantly higher than in the EH group at three-year and beyond (p <0.05). The reason for this difference does not appear to be related to histologic gastritis or atrophy as these parameters were not significantly different in the two groups (see below). Instead, it could be related to ENNG-induced alterations of H. pylori receptors in EH animals and/or to modification of the mucosal innate immune response.
Figure 2.
Effect of H. pylori inoculation on H. pylori load (copies/100 ng total RNA) and gastritis scores in the gastric antrum (Mean±SEM). (a) “Preinoc” indicates the uninfected status of the animals at the initiation of the study and is given as a reference. Infected animals were dosed with 108 H. pylori CFU/ml and colonization burden was monitored over time. (b) Gastritis scores were calculated using the updated Sydney System. Note that gastritis scores were similar in the H and EH groups although infectious load was lower in the EH group.
Videogastroscopy revealed that the gastric mucosa remained macroscopically normal in C monkeys throughout the observation period (Fig. 3a). In contrast, starting at one-year, typical erythema and H. pylori-pathognomonic nodularity was observed in H and EH monkeys (Fig. 3b and data not shown), but not in E animals (data not shown).
Figure 3.

Illustration of endoscopic views of the stomach of (a) Control monkey; (b) H. pylori infected monkey with gastroscopic nodularity; and (c) EH animal with a polyp (white arrow) and telangectasias (black arrow).
Histological inflammation was characterized by the presence of lymphoplasmatic and polymorphonuclear leucocytes in most antral and a few corpus biopsies from H and EH, but not in C or E, animals (Fig 4a-4d). In heavily infected H and EH monkeys, lymphocytes and neutrophil polymorphonuclear leucocytes infiltrated gastric glands and formed large lymphoid follicles (Fig 4e), and H. pylori was detected in close association with the immune cells. Calculation of the gastritis Sydney score typically used to evaluate H. pylori-induced inflammation34 showed elevation in the antrum (Fig. 2b) and, to a lesser degree, in the body (data not shown), of the stomach of the H and EH monkeys. The dynamics of gastritis paralleled H. pylori colonization burden (Figure 2a and 2b) and gastritis was significantly correlated with H. pylori density by real-times RT-PCR and in situ hybridization in both groups (P <0.001).
Figure 4.
Histopathology of gastric mucosa in the four groups of monkeys illustrating (a) normal antrum in control monkey at 5-year; (b) antrum from E monkey at 5-year [grade 0 gastritis; hyperchromatic epithelial cells with bigger, more spherical nuclei than in controls, and enlarged lamina propria with pink matrix]; (c) grade 3 gastritis and grade 2 focal atrophy of the antrum in H monkey at 4-year [note intraepithelial lymphocytes]; (d) grade 3 gastritis and grade 1 focal atrophy of the corpus in EH monkey at 3-year; (e) grade 3 gastritis and grade 2 focal atrophy of the corpus in an EH monkey at 3-year [note dilated capillaries in lamina propria]; (f) grade 3 gastritis and grade 1 focal atrophy of the antrum in an EH monkey at 5-year; (g) complete gastric intestinal metaplasia by H&E stain in an EH monkey at 3-year [G: Goblet cells; thin arrows: Paneth cells; arrowheads: absorptive intestinal cells] and (h) by Genta stain [Alcian blue staining of goblet cells]; insert: CDX2-stained nuclei of goblet cells (i) histology of polyp resected at 5-year [low magnification of polyp showing abnormal histology in area delimited by an imaginary line joining the two arrowheads]; and (j) high magnification of region delimited by the box in (i) demonstrating intraepithelial neoplasia with presence of hyperchromatic stratified nuclei (arrowheads) and mitosis (thin arrows).
Starting at two-year of infection plus ENNG administration, antral biopsies from three of the EH monkeys, but none of the C, E, and H animals, showed Type I, complete, small intestinal type, gastric intestinal metaplasia characterized by the presence of Paneth cells, absorptive intestinal cells (Fig. 4g), and goblet cells positive by Alcian blue (Fig. 4h) and CDX2 (Fig 4h, insert). Additionally, gastric spider telangectasias appeared after three years in three EH monkeys (Fig. 3c, black arrows) and in three E animals (data not shown), but in none of the C and H monkeys.
Focal atrophy of the corpus was observed at years three and four in one H monkey (grade 2, not shown) and in two of the EH animals with intraepithelial neoplasia (grade 1, fig. 4d; grade 2, fig. 4e) (see next paragraph). The remaining H and EH monkeys had grade 1 or grade 2 focal atrophy in the antrum but no corpus atrophy. At all other times, atrophy score of all corpus biopsies was 0.
Starting at five-year, the three EH monkeys that displayed intestinal metaplasia exhibited a further step towards gastric cancer. In one male animal, videogastroscopy revealed a flat polyp located 2 mm proximal to the pylorus (Fig. 3c, white arrow). Endoscopic mucosal resection of the polyp41 demonstrated an area of high grade, non-invasive, intraglandular neoplasia (Fig. 4i) with hyperchromatic stratified nuclei (Fig. 4j, arrowheads) and mitoses (Fig. 4j, arrows). Gastric high-grade, non-invasive, intraglandular neoplasia is also referred to as high-grade dysplasia. Importantly, this type of lesion progresses to invasive carcinoma in 60% to 85% of patients.42, 43 In the other two monkeys (one male, one female), there were reactive/regenerative epithelial tubules with cribriform-like pattern, prominent nucleoli, nuclear stratification, piling, and borderline neoplasia (dysplasia).42 Of note, no gastric intestinal metaplasia or neoplasia was observed in any of the C, E or H animals (P <0.015), suggesting that ENNG and H. pylori infection synergistically affect gastric cancer development. It is striking that only the three monkeys that developed neoplasia had complete metaplasia, although it is not considered to harbor the same malignant potential as incomplete, colonic-type intestinal metaplasia. Overall, the pathology observations were not different in male vs. female animals.
Dynamics of expression of genes regulating immune defenses and DNA repair
Molecular mechanisms that control development of H. pylori-induced gastric cancer remain poorly understood. However, it has been hypothesized that H. pylori-induced chronic inflammation results in DNA damage that leads to transformation. Therefore we hypothesized that we might identify differences in expression of specific genes regulating inflammation, immune defense, and DNA damage repair. Thus, we monitored mRNA expression of the inflammatory genes IL-1β and IL-8, which represent cytokines that are intimately involved in the inflammatory response to H. pylori and also play a role in gastric carcinogenesis.44 We also monitored the DNA repair gene O6-methylguanine-DNA methylransferase (MGMT) since it represents the major defense against the cytotoxic and mutagenic O6-alkylguanine produced by ENNG.45 Quantitative real-time TaqMan RT-PCR revealed that expression of each of the genes was decreased following initial antibiotic treatment and H. pylori eradication (Fig. 5a, 5b and 5c, p <0.05). In the C and E groups, expression of these genes remained low for the subsequent five years (Figs 5a, 5b, and 5c). In the H and EH monkeys, H. pylori inoculation initially increased expression of the three genes to levels seen prior to antibiotic treatment. Thereafter, IL-1β and IL-8 expression remained elevated whereas MGMT expression continually decreased at three- and five-year. These results demonstrate that expression of inflammatory genes is persistently elevated in H. pylori-infected macaques, whereas a DNA repair gene that maintains mucosal homeostasis and ensures protection against carcinogenesis is down regulated during later stages of long-term infection.
Figure 5.
Effect of antibiotics and H. pylori on host gene expression. (a-c) “Arrival” denotes initial naturally infected status, i.e. before the monkeys were treated with antibiotics. “Preinoc” indicates the uninfected status of the animals at the initiation of the study and is given as a reference. mRNA expression of IL-1β, IL-8, and MGMT as determined by real-time RT-PCR in C, E, H and EH monkeys (Means ± SEM). Note that, similar to the dynamics of gastritis illustrated in Fig. 2, expression of the three genes decreases after antibiotic treatment and increases at one-year after H. pylori inoculation in EH and H monkeys. However, at later time points, expression of the inflammatory cytokines remains elevated whereas expression of the DNA repair gene MGMT returns to levels similar to those before H. pylori inoculation. In the C and E groups there was no significant difference for IL-1β, IL8, or MGMT expression as compared to the initial levels. (d) Cluster Diagram of the genes showing statistically significant differences in gastric biopsies harvested at 5 years. Letters indicate the relative treatment groups and numbers indicate the particular animal within the indicated group. Red and green colors represent increase or decreased expression, respectively, relative to the reference sample. Note the dendogram shows two major nodes that segregate the majority of C and H samples from E and EH samples. (e) Dendogram showing clustering pattern of C and EH arrays as supervised by the 83-gene neoplastic signature in gastric biopsies harvested at 5 years. Note that all neoplastic samples segregate from non-neoplastic EH and Control samples.
Global transcriptional analysis of antral biopsies
To more comprehensively explore global changes in mucosal gene expression among the four groups of animals, we next conducted transcriptional profiling using RNA harvested from biopsies obtained from macroscopically normal gastric mucosa of C, H, E and EH animals at the five-year time point. Visually normal tissue was used to investigate whether genes that lead to, and drive, carcinogenesis could be identified. DNA microarrays containing probes representing approximately 18,000 Rhesus monkey genes (Agilent) were utilized and detailed protocols are available as supplementary data.46
Hybridized microarrays were analyzed using the Statistical Analysis for Microarrays (SAM) 2.20 algorithm47 and Student’s two-tailed t-tests. Pair-wise comparisons of the H, E and EH arrays to the C arrays were conducted. Analysis revealed that the number of genes showing altered expression was most dramatic in the EH group. Indeed, significant changes in gene expression patterns were such that EH>E>H. Moreover, changes in the EH group were significantly more than the sum of E changes + H changes (Supplementary Tables 1 and 2). This was despite the fact that neither the inflammatory scores nor the expression of IL-1β, IL-8 or MGMT were significantly different between the H and EH groups at this time point (Figs. 2 and 5). A representative selection of genes that showed the most statistically significant changes in expression in each of the groups is provided in Table 1 and a complete list is available as Supplementary Table 1. Additionally, independent verification of changes in expression for a subset of genes is provided as supplementary figures 1 and 2.
Table 1.
Representative selection of genes that showed the most statistically significant changes in expression in each of the groups
| C vs.H. pylori | Gene | Function | Fold Change |
|---|---|---|---|
| Up | SEC22 vesicle trafficking protein-like 2 (SEC22L2) | Early stages of the secretory pathway | 2.3 |
| Prostaglandin-endoperoxide synthase 2 (prostaglandin G/H synthase and cyclooxygenase) (PTGS2) | Major mediator of inflammation and/or a role for prostanoid signaling | 2.3 | |
| Beta-2-microglobulin (B2M) | Associated with the MHC class I heavy chain | 2.2 | |
| Protocadherin alpha 5 (PCDHA5) | Establishment and function of specific cell-cell connections | 2.2 | |
| Sialyltransferase 7D (alpha-N-acetylneuraminyl-2,3-beta-galactosyl-1,3)-N-acetyl galactosaminide alpha-2,6-sialyltransferase) (SIAT7D) | Catalyzes the transfer of sialic acid to galactose-containing substrates | 2.2 | |
| Interleukin 13 (IL13) | Regulates inflammatory and immune responses. | 2.0 | |
| 5-azacytidine induced 2 (AZI2) | Activation of NFKB-dependent gene expression | 2.0 | |
| Down | ATP-binding cassette, sub-family G (WHITE), member 1 (ABCG1) | Macrophage cholesterol and phospholipids transport | 0.5 |
| Heat shock 70kDa protein 4 (HSPA4), | Phosphorylated upon DNA damage | 0.5 | |
| G protein-coupled receptor kinase interactor 1 (GIT1) | Increases the speed of cell migration and rate of formation of protrusions | 0.4 | |
| Activin A receptor, type IB (ACVR1B) | Growth and differentiation factors in the TGF-beta superfamily | 0.4 | |
| Pregnancy specific beta-1-glycoprotein 3 (PSG3) | Belongs to the immunoglobulin superfamily, CEA family. | 0.4 | |
| Putative neuronal cell adhesion molecule (PUNC) | Cell adhesion | 0.4 |
| C vs. ENNG | Gene Name | Function | Fold Change |
|---|---|---|---|
| Up | Cas-Br-M (murine) ecotropic retroviral transforming sequence (CBL) | Oncogene that induces mouse pre-B and pro-B cell lymphomas | 3.4 |
| Nucleoporin 160kDa (NUP160) | Mediates nucleoplasmic transport | 3.0 | |
| Tumor protein, translationally-controlled 1 (TPT1) | Calcium binding and microtubule stabilization | 2.5 | |
| Golgi autoantigen, golgin subfamily a, 2 (GOLGA2) | Golgi auto-antigen involved in maintaining cis-golgi structure | 3.2 | |
| Platelet-derived growth factor receptor, beta polypeptide (PDGFRB) | Mitogen for cells of mesenchymal origin | 3.5 | |
| Exostoses 1 (EXT1) | Ivolved in the chain elongation step of heparan sulfate biosynthesis | 2.8 | |
| CUG triplet repeat, RNA binding protein 1 (CUGBP1) | Regulation of pre-mRNA alternative splicing and mRNA editing and translation | 5.2 | |
| Peroxisomal biogenesis factor 5 (PEX5) | Essential role in peroxisomal protein import | 3.4 | |
| Protein kinase D3 (PRKD3) | Activated by the agonists of G protein-coupled receptors and after B-cell antigen receptor (BCR) engagement | 3.9 | |
| Down | Inositol polyphosphate-5-phosphatase F (INPP5F) | Inositol 1,4,5-trisphosphate (InsP3) 5-phosphatase | 0.3 |
| Core-binding factor, runt domain, alpha subunit 2; translocated to 3 (CBFA2T3) | Putative breast tumor suppressor | 0.3 | |
| Tuberoinfundibular 39 residue protein precursor (TIP39) | Related to parathyroid hormone and is a ligand for PTH receptor-2 | 0.2 | |
| Proteinase 3 (serine proteinase, neutrophil, Wegener granulomatosis autoantigen) (PRTN3) | Degrades elastin, fibronectin, laminin, vitronectin, and collagen types i, iii, and iv | 0.3 | |
| DNA excision repair protein ERCC-8 (Cockayne syndrome WD repeat protein CSA) (LOC713594) | WD repeat protein, which interacts with Cockayne syndrome type B (CSB) protein and with p44 protein. Mutations result in abnormal sensitive to ultraviolet radiation and are defective in the repair of transcriptionally active genes | 0.2 | |
| Triggering receptor expressed on myeloid cells 1 (TREM1) | Ig superfamily receptor for stimulation of monocyte/macrophage- and neutrophil-mediated inflammatory responses | 0.3 |
| C vs EH | Gene Name | Function | Fold Change |
|---|---|---|---|
| Up | Phosphoribosyl pyrophosphate synthetase-associated protein 2 (PRPSAP2) | Catalyzes the formation of phosphoribosylpyrophosphate | 3.7 |
| Glutaredoxin 2 (GLRX2) | Participate in a variety of cellular redox reactions | 3.4 | |
| GTP cyclohydrolase I feedback regulator (GCHFR) | Binds to and mediates tetrahydrobiopterin inhibition of GTP cyclohydrolase I | 3.8 | |
| SMAD, mothers against DPP homolog 4 (Drosophila) (SMAD4) | Collagen Binding and Transcriptional Control | 5.3 | |
| Major histocompatibility complex, class II, DR beta 5 (HLA-DRB5) | Central role in the immune system by presenting peptides derived from extracellular proteins. | 7.0 | |
| SEC22 vesicle trafficking protein-like 2 (SEC22L2) | Acts in the early stages of the secretory pathway | 3.6 | |
| BCL6 co-repressor (BCOR) | Interaction with the corepressor of BCL6, a POZ/zinc finger transcription repressor that is required for germinal center formation and may influence apoptosis | 2.2 | |
| Tripartite motif-containing 22 (TRIM22) | Expression is induced by interferon and may mediate interferon’s effects | 4.0 | |
| Calcium/calmodulin-dependent protein kinase (CaM kinase) II delta (CAMK2D) | Ca(2+)/calmodulin-dependent protein kinase signaling | 3.3 | |
| Phosphoglucomutase 2-like 1 (PGM2L1) | Glycolysis | 4.9 | |
| Leucine-rich repeats and immunoglobulin-like domains 3 (LRIG3) | Regulation of EGFR signaling, and serves as a tumor suppressor gene | 7.0 | |
| Similar to Numb-interacting homolog gene (LOC405753) | Protein transport | 15.8 | |
| Platelet-derived growth factor receptor, beta polypeptide (PDGFRB) | Receptor for members of the platelet-derived growth factor family, which are mitogens for cells of mesenchymal origin | 3.0 | |
| Nicotinamide N-methyltransferase (NNMT) | Responsible for N-methylation | 6.5 | |
| Down | BCL2-like 14 (BCL2L14) | Overexpression induce apoptosis in cells | 0.4 |
| Inositol polyphosphate-5-phosphatase F (INPP5F) | Inositol 1,4,5-trisphosphate (InsP3) 5-phosphatase | 0.4 | |
| Purinergic receptor P2X, ligand-gated ion channel, 7 (P2RX7) | Functions as a ligand-gated ion channel and is responsible for ATP-dependent lysis of macrophages | 0.2 | |
| DNA excision repair protein ERCC-8 (Cockayne syndrome WD repeat protein CSA) (LOC713594) | WD repeat protein, which interacts with Cockayne syndrome type B (CSB) protein and with p44 protein. Mutations result in abnormal sensitive to ultraviolet radiation and are defective in the repair of transcriptionally active genes | 0.2 | |
| Phospholipase D2 (PLD2) | Proposed to function in regulated secretion, cytoskeletal reorganization, transcriptional regulation, and cell cycle control | 0.3 | |
| Otoferlin (OTOF) | Involved in vesicle membrane fusion | 0.3 | |
| Chloride channel 2 (CLCN2) | Ion Transport | 0.4 | |
| Solute carrier family 27, member 1 (SLC27A1) | Fatty Acid Transport | 0.4 | |
| MyoD family inhibitor (MDFI) | Transcription factor that negatively regulates other myogenic family proteins. | 0.5 |
| EH noNeo vs EH Neo | Gene Name | Function | Fold Change |
|---|---|---|---|
| Up | Laeverin (FLJ90650) | Cell surface protein specifically expressed on human embryo-derived extravillous trophoblasts that invades the uterus during placentation | 2.1 |
| Hepatocellular carcinoma-associated antigen HCA557b (LOC151194) | Membrane Associated Unknown Function | 2.3 | |
| Neuronal protein (NP25) | Actin binding tumor suppressor | 2.9 | |
| Epiplakin 1 (EPPK1) | Cytolinker that maintains the integrity of intermediate filaments networks in simple epithelial cells | 3.0 | |
| NPC1 (Niemann-Pick disease, type C1, gene)-like 1 (NPC1L1) | Lipid and lipoprotein metabolism, Hedgehog Receptor activity | 3.0 | |
| Testis nuclear RNA-binding protein (Tenr) | RNA processing and cell differentiation | 3.6 | |
| ATPase, (Na+)/K+ transporting, beta 4 polypeptide (ATP1B4) | Ion Transport | 4.4 | |
| WD repeat domain 76 (WDR76) | WD40 domain, found in proteins that have adaptor/regulatory modules in signal transduction, and affect pre-mRNA processing and cytoskeleton assembly | 5.6 | |
| Down | Cystatin D (CST5) | Protective role against proteinases | 0.2 |
| Proton-dependent dipeptide transporter (PEPT1) | Macrophage oligopeptide transporter | 0.2 | |
| Adrenergic, beta-1-, receptor (ADRB1) | Mediates the effects of the epinephrine and the neurotransmitter norepinephrine | 0.4 | |
| Bone morphogenetic protein 10 (BMP10) | Member of the TGF-beta family of growth factors | 0.4 | |
| Kallikrein 3 (KLK3) | Serine proteases | 0.4 | |
| Homeo box B6 (HOXB6) | Sequence-specific transcription factor that is involved in development. Altered expression is associated with some cases of acute myeloid leukemia and colorectal cancer | 0.5 | |
| Solute carrier family 7 member 6 (SLC7A6) | Amino acid permease | 0.5 | |
| C-reactive protein, pentraxin-related (CRP) | Binds to ligands containing phosphocholine, role in innate immunity | 0.5 | |
| Proteoglycan 2, bone marrow (PRG2) | Constituent of the eosinophil granule involved in antiparasitic defense mechanisms | 0.5 |
Cluster analysis of all 1394 spots (representing 1154 genes) that showed significant differences among all of the analyses revealed distinct patterns of gene expression that segregated samples into two major dendogram nodes: one containing the majority of C and H animals and another containing most E and all EH animals (Fig. 5 d). Within the later node, all but one of the EH animals clustered separately from the E animals. Analysis of the H. pylori infection status of this animal showed that, though infected, the colonization burden was much lower than all other EH animals. Within the second major node of the dendogram, the majority of C and H animals clustered together, while the two E animals that segregated in this node clustered separately (Fig. 5d). These data suggest that the combination of a carcinogen similar to dietary components and H. pylori infection has a synergistic effect on global changes in gene expression, which is likely ultimately responsible for advanced progression to the noninvasive neoplasia identified only in the EH group.
To begin to understand the global pathways affected, we performed ontological classification of genes significantly changed within the different treatment groups. Each of the genes falling into the different ontological classifications, as well as the individual genes identified in our analysis are available on line in Supplementary Tables 1 and 2 and the complete data set is available at http://genomewww5.stanford.edu/. In the H. pylori infected group, there were significant changes in genes associated with apoptosis, cell adhesion, cell communication, cell cycle regulation, cell differentiation, the immune system and inflammation, stress response and signal transduction, each of which has been previously shown to be targeted by H. pylori infection.46, 48, 49
For the E animals that received only ENNG, we observed changes in many of the same pathways targeted by H. pylori infection. However, there was an increase in the number of genes associated with apoptosis, cell communication, cell differentiation and signal transduction. Novel pathways targeted in the E group included genes associated with development of several forms of cancer, adherens and cell junctions and a large number of genes associated with the ubiquitin cycle. Of note, the ontological classification of genes associated with DNA damage repair and metabolism was preferentially targeted in the E group as compared to the H group (Supplementary Table 2). Moreover, increased numbers of DNA damage response genes were targeted in the EH group suggesting that ENNG and H. pylori synergistically induce high levels of DNA damage.
Many of the same ontological groupings altered in the H and E groups were similarly altered in the EH group, but the number of genes found within each classification was dramatically higher in EH monkeys (Supplementary Table 2). Of note, genes associated with development of several different types of cancer, genes associated with the TGFβ signaling pathway and genes from the NFκ B cascade were detected in the EH group.50, 51
Given the fact that three of the EH animals developed intraglandular neoplasia or borderline neoplasia, we sought to determine if a unique signature profile could be identified in the gastric mucosa of these animals as compared to the three EH animals that had not developed neoplasia by five years. Pair-wise comparison of these animals (neoplastic vs non-neoplastic) revealed a total of 83 genes that showed diverse biological functions and were able to cluster neoplastic samples separately from controls and non-neoplastic EH animals (Figure 5e). Ontological classification of these 83 genes revealed that groups of genes associated with apoptosis, cell communication, cell cycle regulation, cell morphogenesis and differentiation and metal ion binding were the most significant classifications affected. Of note, a full 18% of the genes in this neoplastic signature were associated with metal (predominantly zinc) binding and 12% were associated with transcriptional regulation. The large number of factors observed within these groupings suggests a role for these classes of genes in the development of neoplasia in the EH monkeys.
Specifically included only on the neoplasia list (see Supplementary Table 2) were multiple genes known to play a role in cancer.52-56 The Adenomatosis Polyposis Coli (APC) gene and the Murine Double Minute (mdm2, p53 binding protein) oncogene were upregulated whereas the Kallikrein 3 (KLK3) prostate specific antigen (PSA), the apoptosis facilitator bcl2-like 11 gene, the homeo box B6 (HOXB6) gene, and the bone morphogenetic protein 10 (BMP10, a part of the transforming growth factor β superfamily of morphogenetic proteins) were downregulated. 52-56 Also of interest is the up regulation of the epiplakin 1 (EPPK1) gene, which encodes a large multi-domain molecule that links cytoskeletal elements and connects them to junctional complexes.57 Interestingly, changes in the expression of all of these genes was not detected in the H group or in a microarray study of the early effect of H. pylori infection in Rhesus monkeys.58
Discussion
A major finding of the present study was that gastric carcinogenesis, precancerous gastric intestinal metaplasia, and intraepithelial neoplasia appeared in H. pylori-infected monkeys receiving a carcinogen that is present in East Asian diets (EH group) but not if one or both of these factors was absent (groups C, H and E). To our knowledge, this is the first study that provides precise information on the dynamics of gastric carcinogenesis by repeated videogastroscopic examination of the gastric mucosa as well as analysis of the mucosal histopathology over a multiyear observation period at three-month intervals and using rigorous conditions in a primate model. Furthermore, observations were made while implementing tight control of the H. pylori infection status and carcinogenic diet of the animals that would be unethical for long term implementation in humans. Indeed, we were able to detect precancerous lesions and neoplasia with videogastroscopy plus histopathological analysis of the biopsies and to resect a 2 mm diameter polyp at endoscopy using state-of-the-art techniques specifically developed for the diagnosis and treatment of the very same lesions in the clinical environment.59 The pathology of this type of lesion is identical to the early gastric carcinoma observed in humans,60 and is similar to the H. pylori-induced gastric intraepithelial neoplasia reported in mice.61
Targeted monitoring of IL-1β, IL-8 and MGMT expression showed that while levels of both IL-1β and IL-8 increased in H and EH groups, expression of MGMT decreased at the later time points (Fig. 5a-c). The fact that expression of the inflammatory and DNA repair genes were strikingly similar in both groups, suggests that H. pylori alone is responsible for changes in expression in these genes. Since inflammation was similar in the H and EH groups (Fig. 2b) and only animals receiving ENNG developed gastric cancer, DNA damage induced by chronic inflammation was not responsible for neoplastic transformation in EH monkeys. Instead, neoplasia likely occurred due to the fact that the late decrease in DNA repair impacted only EH monkeys because they were the only animals receiving the DNA-damaging carcinogen.
Microarray analysis and ontological classification of genes that showed altered expression revealed that different groups of genes and a significantly larger number of genes were affected in H. pylori-infected animals also receiving ENNG. This fact, combined with appearance of intraepithelial neoplasia only in the EH animals, suggests that ENNG and H. pylori infection synergistically affect the development of gastric cancer by altering the expression of large numbers of genes. Of interest is the large number of metal ion binding genes, and especially Zinc binding genes. Zinc participates in several critical cellular functions, especially DNA repair. Thus, zinc deficiency impairs host protective mechanisms designed to protect against DNA damage, enhances susceptibility to DNA-damaging agents and ultimately increases risk for cancer.62 Furthermore, the binding of zinc with proteins plays an important role in immune efficiency during ageing and in age-related diseases.63
Of particular interest is the fact that we identified a neoplastic expression signature representing 83 genes whose expression was altered only in the three EH monkeys that developed intraepithelial neoplasia. The presence of the cancer associated genes APC, mdm2, PSA, bcl2, HOXB6, and BMP10 is notable since the expression abnormality was characterized in the normal mucosa of the monkeys, i.e. before transformation was visible by histopathology. Thus, the genes may represent useful markers that can be used to improve the understanding, early diagnosis, and prevention of gastric cancer especially when studied in the context of patients with early gastric cancer. The development of diagnostic markers is particularly crucial given recent studies that suggest that, while H. pylori infection is detrimental in some patients, the bacterium may protect against development of esophageal cancer and asthma in other patients.64, 65
In summary, the present primate model of strict and long-term control of mucosal H. pylori infection and intake of a carcinogen similar to dietary components of East Asian foods demonstrates that H. pylori infection and dietary carcinogens synergistically induce neoplastic transformation of gastric epithelial cells. In contrast, long-term H. pylori infection or ENNG alone produced intense gastric mucosal inflammation or chronic dilation of submucosal capillaries, respectively, but no cancer. Gastric neoplasia formation appeared to be preceded by increased expression of cancer-related genes in the apparently normal gastric mucosa. These genes may represent useful tools leading to improvement of the understanding and prevention of gastric cancer.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Drs.Goldstein, Camilli, Cha, and Olsen for comments, procedural assistance, and statistical analysis. The excellent animal husbandry of the members of the AFRRI Veterinary Science Department is gratefully acknowledged. Studies supported by National Institutes of Health grant CA082312 and USUHS grant R083UQ (AD).
The comments and opinions stated in this manuscript are solely those of the authors and in no way reflect those of the Uniformed Services University, the Department of Defense, or the United States Government. All studies were approved by the Institutional Animal Care and Use Committee of the Armed Forces Radiobiology Research Institute and reapproved annually. Supported in part by grant CA82312 of the National Institutes of Health.
Abbreviations
- ENNG
ethyl-nitro-nitrosoguanidine
- C
Controls
- H
H. pylori infected
- E
receiving ENNG
- EH
H. pylori infected receiving ENNG
Footnotes
No conflicts of interest exist
Reference List
- 1.Crew KD, Neugut AI. Epidemiology of gastric cancer. World J Gastroenterol. 2006;12:354–362. doi: 10.3748/wjg.v12.i3.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 3.Haenszel W, Correa P. Developments in the epidemiology of stomach cancer over the past decade. Cancer Res. 1975;35:3452–3459. [PubMed] [Google Scholar]
- 4.Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984;1:1311–1315. doi: 10.1016/s0140-6736(84)91816-6. [DOI] [PubMed] [Google Scholar]
- 5.Parsonnet J, Friedman GD, Vandersteen DP, Chang Y, Vogelman JH, Orentreich N, Sibley RK. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med. 1991;325:1127–1131. doi: 10.1056/NEJM199110173251603. [DOI] [PubMed] [Google Scholar]
- 6.Anonymous . IARC Monograph on the evaluation of carcinogenic risks to humans. Vol. 61. IARC; Lyon: 1994. Schistosomes, liver flukes and Helicobacter pylori; pp. 1–241. [PMC free article] [PubMed] [Google Scholar]
- 7.El-Omar EM, Rabkin CS, Gammon MD, Vaughan TL, Risch HA, Schoenberg JB, Stanford JL, Mayne ST, Goedert J, Blot WJ, Fraumeni JF, Jr., Chow WH. Increased risk of noncardia gastric cancer associated with proinflammatory cytokine gene polymorphisms. Gastroenterology. 2003;124:1193–1201. doi: 10.1016/s0016-5085(03)00157-4. [DOI] [PubMed] [Google Scholar]
- 8.Correa P, Houghton J. Carcinogenesis of Helicobacter pylori. Gastroenterology. 2007;133:659–672. doi: 10.1053/j.gastro.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 9.Fock KM, Talley N, Moayyedi P, Hunt R, Azuma T, Sugano K, Xiao SD, Lam SK, Goh KL, Chiba T, Uemura N, Kim JG, Kim N, Ang TL, Mahachai V, Mitchell H, Rani AA, Liou JM, Vilaichone RK, Sollano J. Asia-Pacific consensus guidelines on gastric cancer prevention. J Gastroenterol Hepatol. 2008;23:351–365. doi: 10.1111/j.1440-1746.2008.05314.x. [DOI] [PubMed] [Google Scholar]
- 10.Uemura N, Okamoto S, Yamamoto S, Matsumara N, Yamaguchi S, Yamakido M, Taniyama K, Sasaki N, Schlemper RS. Helicobacter pylori infection and the development of gastric cancer. New Engl J Med. 2001;345:784–789. doi: 10.1056/NEJMoa001999. [DOI] [PubMed] [Google Scholar]
- 11.Uemura N, Okamoto S. Effect of Helicobacter pylori eradication on subsequent development of cancer after endoscopic resection of early gastric cancer in Japan. Gastroenterol Clin North Am. 2000;29:819–827. doi: 10.1016/s0889-8553(05)70149-7. [DOI] [PubMed] [Google Scholar]
- 12.Wong BC, Lam SK, wong wm, Chen JS, Zheng TT, Feng RE, Lai KC, Hu WH, Yuen ST, Leung SY, Fong DY, Ho J, Ching CK, Chen JS. Helicobacter pylori eradication to prevent gastric cancer in a high-risk region of China: a randomized controlled trial. JAMA. 2004;291:187–194. doi: 10.1001/jama.291.2.187. [DOI] [PubMed] [Google Scholar]
- 13.Fukase K, Kato M, Kikuchi S, Inoue K, Uemura N, Okamoto S, Terao S, Amagai K, Hayashi S, Asaka M. Effect of eradication of Helicobacter pylori on incidence of metachronous gastric carcinoma after endoscopic resection of early gastric cancer: an open-label, randomised controlled trial. Lancet. 2008;372:392–397. doi: 10.1016/S0140-6736(08)61159-9. [DOI] [PubMed] [Google Scholar]
- 14.Weisburger JH, Jones RC. Prevention of formation of important mutagens/carcinogens in the human food chain. Basic Life Sci. 1990;52:105–118. doi: 10.1007/978-1-4615-9561-8_8. [DOI] [PubMed] [Google Scholar]
- 15.Seel DJ, Kawabata T, Nakamura M, Ishibashi T, Hamano M, Mashimo M, Shin SH, Sakamoto K, Jhee EC, Watanabe S. N-nitroso compounds in two nitrosated food products in southwest Korea. Food Chem Toxicol. 1994;32:1117–1123. doi: 10.1016/0278-6915(94)90127-9. [DOI] [PubMed] [Google Scholar]
- 16.Stemmermann GN, Fenoglio-Preiser C. Gastric carcinoma distal to the cardia: a review of the epidemiological pathology of the precusors to a preventable cancer. Pathology. 2002;34:494–503. doi: 10.1016/s0031-3025(17)30697-9. [DOI] [PubMed] [Google Scholar]
- 17.Blot WJ, Li JY, Taylor PR, Guo W, Dawsey S, Wang GQ, et al. Nutrition intervention trials in Linxian, China: supplementation with specific vitamin/mineral combinations, cancer incidence, and disease-specific mortality in the general population. J Natl Cancer Inst. 1993;85:1483–1492. doi: 10.1093/jnci/85.18.1483. [DOI] [PubMed] [Google Scholar]
- 18.Forman D, Burley VJ. Gastric cancer: global pattern of the disease and an overview of environmental risk factors. Best Pract Res Clin Gastroenterol. 2006;20:633–649. doi: 10.1016/j.bpg.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 19.Jakszyn P, Bingham S, Pera G, Agudo A, Luben R, Welch A, Boeing H, Del GG, Palli D, Saieva C, Krogh V, Sacerdote C, Tumino R, Panico S, Berglund G, Siman H, Hallmans G, Sanchez MJ, Larranaga N, Barricarte A, Chirlaque MD, Quiros JR, Key TJ, Allen N, Lund E, Carneiro F, Linseisen J, Nagel G, Overvad K, Tjonneland A, Olsen A, Bueno-de-Mesquita HB, Ocke MO, Peeters PH, Numans ME, Clavel-Chapelon F, Trichopoulou A, Fenger C, Stenling R, Ferrari P, Jenab M, Norat T, Riboli E, Gonzalez CA. Endogenous versus exogenous exposure to N-nitroso compounds and gastric cancer risk in the European Prospective Investigation into Cancer and Nutrition (EPIC-EURGAST) study. Carcinogenesis. 2006;27:1497–1501. doi: 10.1093/carcin/bgl019. [DOI] [PubMed] [Google Scholar]
- 20.Karam SM. Cellular origin of gastric cancer. Ann N Y Acad Sci. 2008;1138:162–168. doi: 10.1196/annals.1414.023. [DOI] [PubMed] [Google Scholar]
- 21.Tsukamoto T, Mizoshita T, Tatematsu M. Animal models of stomach carcinogenesis. Toxicol Pathol. 2007;35:636–648. doi: 10.1080/01926230701420632. [DOI] [PubMed] [Google Scholar]
- 22.Watanabe T, Tada M, Nagai H, Sasaki S, Nakao M. Helicobacter pylori infection induces gastric cancer in Mongolian gerbils. Gastroenterology. 1998;115:642–648. doi: 10.1016/s0016-5085(98)70143-x. [DOI] [PubMed] [Google Scholar]
- 23.Romero-Gallo J, Harris EJ, Krishna U, Washington MK, Perez-Perez GI, Peek RM., Jr. Effect of Helicobacter pylori eradication on gastric carcinogenesis. Lab Invest. 2008;88:328–336. doi: 10.1038/labinvest.3700719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dubois A, Fiala N, Weichbrod RH, Ward GS, Nix M, Mehlman PT, Taub DM, Perez-Perez GI, Blaser MJ. Seroepizootiology of Helicobacter pylori gastric infection in nonhuman primates housed in social environments. J Clinical Microbiol. 1995;33:1492–1495. doi: 10.1128/jcm.33.6.1492-1495.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parker GA, Gilmore CJ, Dubois A. Spontaneous gastric ulcer in a rhesus monkey. Brain Research Bull. 1981;6:445–447. doi: 10.1016/s0361-9230(81)80015-9. [DOI] [PubMed] [Google Scholar]
- 26.Kent SP, Pickering JE. Neoplasms in monkeys (Macaca mulatta): spontaneous and irradiation induced. Cancer. 1958;11:138–147. doi: 10.1002/1097-0142(195801/02)11:1<138::aid-cncr2820110125>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 27.Kimbrough R. Spontaneous malignant gastric tumor in a rhesus monkey (Macaca mulatta) Arch Pathol. 1966;81:343–351. [PubMed] [Google Scholar]
- 28.Domanska K, Kowalski B. Occurrence of volatile N-nitrosamines in Polish processed meat products. Bulletin of the Veterinary Institute in Pulawy. 2000 http://www.cababstractsplus.org/abstracts/Abstract.aspx?AcNo=20043004129. [Google Scholar]
- 29.Ohgaki H, Hasegawa H, Kusama K, Morino K, Matsukura N, Sato S, Maruyama K, Sugimura T. Induction of gastric carcinomas in nonhuman primates by N-Ethyl-N-Nitro-N-Nitrosoguanidine. J Natl Cancer Inst. 1986;77:179–186. [PubMed] [Google Scholar]
- 30.Kim GH, Doi SQ, Mysore JV, Yang M, Reindel J, Dubois A. Characterization of H. pylori isolated from naturally infected cynomolgus monkeys (M. fascicularis) with gastritis. 116 ed. 1999. pp. G650–A150. [Google Scholar]
- 31.Dubois A, Tarnawski A, Newell DG, Fiala N, Dabros W, Strachura J, Krivan H, Heman-Ackah LM. Gastric injury and invasion of parietal cells by spiral bacteria in rhesus monkeys. Are gastritis and hyperchlorhydria infectious diseases? Gastroenterology. 1991;100:884–891. doi: 10.1016/0016-5085(91)90260-r. [DOI] [PubMed] [Google Scholar]
- 32.Oda T, Murakami K, Nishizono A, Kodama M, Nasu M, Fujioka T. Long-term Helicobacter pylori infection in Japanese monkeys induces atrophic gastritis and accumulation of mutations in the p53 tumor suppressor gene. Helicobacter. 2002;7:143–151. doi: 10.1046/j.1523-5378.2002.00074.x. [DOI] [PubMed] [Google Scholar]
- 33.Dixon MF, Genta RM, Yardley JH, Correa P. Histological classification of gastritis and Helicobacter pylori infection: an agreement at last? Helicobacter. 1997;2(Suppl 1):S17–S24. doi: 10.1111/j.1523-5378.1997.06b09.x. The International Workshop on the Histopathology of Gastritis. [DOI] [PubMed] [Google Scholar]
- 34.Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. Am J Surg Pathol. 1996;20:1161–1181. doi: 10.1097/00000478-199610000-00001. International Workshop on the Histopathology of Gastritis, Houston 1994. [DOI] [PubMed] [Google Scholar]
- 35.Dubois A, Berg D, Incecik E, Fiala N, Heman Ackah L, Yang M, Wirth H, Perez-Perez GI, Blaser MJ. Host specificity of Helicobacter pylori strains and host responses in experimentally challenged nonhuman primates. Gastroenterology. 1999;116:90–96. doi: 10.1016/s0016-5085(99)70232-5. [DOI] [PubMed] [Google Scholar]
- 36.Genta RM. Atrophy and atrophic gastritis: one step beyond the Sydney system. Ital J Gastroenterol Hepatol. 1998;30(Suppl 3):S273–S275. [PubMed] [Google Scholar]
- 37.Argent RH, Zhang Y, Atherton JC. Simple method for determination of the number of Helicobacter pylori CagA variable-region EPIYA tyrosine phosphorylation motifs by PCR. J Clin Microbiol. 2005;43:791–795. doi: 10.1128/JCM.43.2.791-795.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rhead JL, Letley DP, Mohammadi M, Hussein N, Mohagheghi MA, Eshagh HM, Atherton JC. A new Helicobacter pylori vacuolating cytotoxin determinant, the intermediate region, is associated with gastric cancer. Gastroenterology. 2007;133:926–936. doi: 10.1053/j.gastro.2007.06.056. [DOI] [PubMed] [Google Scholar]
- 39.Aspholm-Hurtig M, Dailide G, Lahmann M, Kalia A, Ilver D, Roche N, Vikstrom S, Sjostrom R, Linden S, Backstrom A, Lundberg C, Arnqvist A, Mahdavi J, Nilsson UJ, Velapatino B, Gilman RH, Gerhard M, Alarcon T, Lopez-Brea M, Nakazawa T, Fox JG, Correa P, Dominguez-Bello MG, Perez-Perez GI, Blaser MJ, Normark S, Carlstedt I, Oscarson S, Teneberg S, Berg DE, Boren T. Functional adaptation of BabA, the H. pylori ABO blood group antigen binding adhesin. Science. 2004;305:519–522. doi: 10.1126/science.1098801. [DOI] [PubMed] [Google Scholar]
- 40.Liu H, Rahman A, Semino-Mora C, Doi SQ, Dubois A. Specific and sensitive detection of H. pylori in biological specimens by real-time RT-PCR and in situ hybridization. PLoS One. 2008 doi: 10.1371/journal.pone.0002689. http://www.plosone.org/doi/pone.0002689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ahmad NA, Kochman ML, Long WB, Furth EE, Ginsberg GG. Efficacy, safety, and clinical outcomes of endoscopic mucosal resection: a study of 101 cases. Gastrointest Endosc. 2002;55:390–396. doi: 10.1067/mge.2002.121881. [DOI] [PubMed] [Google Scholar]
- 42.Rugge M, Correa P, Dixon MF, Hattori T, Leandro G, Lewin K, Riddell RH, Sipponen P, Watanabe H. Gastric dysplasia: the Padova international classification. Am J Surg Pathol. 2000;24:167–176. doi: 10.1097/00000478-200002000-00001. [DOI] [PubMed] [Google Scholar]
- 43.de Vries AC, Haringsma J, Kuipers EJ. The detection, surveillance and treatment of premalignant gastric lesions related to Helicobacter pylori infection. Helicobacter. 2007;12:1–15. doi: 10.1111/j.1523-5378.2007.00475.x. [DOI] [PubMed] [Google Scholar]
- 44.Correa P, Schneider BG. Etiology of gastric cancer: what is new? Cancer Epidemiol Biomarkers Prev. 2005;14:1865–1868. doi: 10.1158/1055-9965.EPI-05-0029. [DOI] [PubMed] [Google Scholar]
- 45.Kaina B, Christmann M, Naumann S, Roos WP. MGMT: key node in the battle against genotoxicity, carcinogenicity and apoptosis induced by alkylating agents. DNA Repair (Amst) 2007;6:1079–1099. doi: 10.1016/j.dnarep.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 46.Mueller A, Merrell DS, Grimm J, Falkow S. Profiling of microdissected gastric epithelial cells reveals a cell type-specific response to Helicobacter pylori infection. Gastroenterology. 2004;127:1446–1462. doi: 10.1053/j.gastro.2004.08.054. [DOI] [PubMed] [Google Scholar]
- 47.Tusher VG, Tibshirani R, Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci U S A. 2001;98:5116–5121. doi: 10.1073/pnas.091062498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.El-Etr SH, Mueller A, Tompkins LS, Falkow S, Merrell DS. Phosphorylation-independent effects of CagA during interaction between Helicobacter pylori and T84 polarized monolayers. J Infect Dis. 2004;190:1516–1523. doi: 10.1086/424526. [DOI] [PubMed] [Google Scholar]
- 49.Resnick MB, Sabo E, Meitner PA, Kim SS, Cho Y, Kim HK, Tavares R, Moss SF. Global analysis of the human gastric epithelial transcriptome altered by Helicobacter pylori eradication in vivo. Gut. 2006;55:1717–1724. doi: 10.1136/gut.2006.095646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Suzuki M, Shigematsu H, Shames DS, Sunaga N, Takahashi T, Shivapurkar N, Iizasa T, Frenkel EP, Minna JD, Fujisawa T, Gazdar AF. DNA methylation-associated inactivation of TGFbeta-related genes DRM/Gremlin, RUNX3, and HPP1 in human cancers. Br J Cancer. 2005;93:1029–1037. doi: 10.1038/sj.bjc.6602837. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 51.Maeda S, Omata M. Inflammation and cancer: role of nuclear factor-kappaB activation. Cancer Sci. 2008;99:836–842. doi: 10.1111/j.1349-7006.2008.00763.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Morin PJ, Sparks AB, Korinek V, Barker N, Clevers H, Vogelstein B, Kinzler KW. Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science. 1997;275:1787–1790. doi: 10.1126/science.275.5307.1787. [DOI] [PubMed] [Google Scholar]
- 53.Bond GL, Hu W, Levine AJ. MDM2 is a central node in the p53 pathway: 12 years and counting. Curr Cancer Drug Targets. 2005;5:3–8. doi: 10.2174/1568009053332627. [DOI] [PubMed] [Google Scholar]
- 54.Niu Y, Yeh S, Miyamoto H, Li G, Altuwaijri S, Yuan J, Han R, Ma T, Kuo HC, Chang C. Tissue prostate-specific antigen facilitates refractory prostate tumor progression via enhancing ARA70-regulated androgen receptor transactivation. Cancer Res. 2008;68:7110–7119. doi: 10.1158/0008-5472.CAN-07-6507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.De VG, Barba P, Odartchenko N, Givel JC, Freschi G, Bucciarelli G, Magli MC, Boncinelli E, Cillo C. Expression of homeobox-containing genes in primary and metastatic colorectal cancer. Eur J Cancer. 1993;29A:887–893. doi: 10.1016/s0959-8049(05)80432-0. [DOI] [PubMed] [Google Scholar]
- 56.Hardwick JC, Kodach LL, Offerhaus GJ, van den Brink GR. Bone morphogenetic protein signalling in colorectal cancer. Nat Rev Cancer. 2008;8:806–812. doi: 10.1038/nrc2467. [DOI] [PubMed] [Google Scholar]
- 57.Sonnenberg A, Liem RK. Plakins in development and disease. Exp Cell Res. 2007;313:2189–2203. doi: 10.1016/j.yexcr.2007.03.039. [DOI] [PubMed] [Google Scholar]
- 58.Huff JL, Hansen LM, Solnick JV. Gastric transcription profile of Helicobacter pylori infection in the rhesus macaque. Infect Immun. 2004;72:5216–5226. doi: 10.1128/IAI.72.9.5216-5226.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gotoda T. Endoscopic resection for premalignant and malignant lesions of the gastrointestinal tract from the esophagus to the colon. Gastrointest Endosc Clin N Am. 2008;18:435–50. viii. doi: 10.1016/j.giec.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 60.Farinati F, Cardin R, Cassaro M, Bortolami M, Nitti D, Tieppo C, Zaninotto G, Rugge M. Helicobacter pylori, inflammation, oxidative damage and gastric cancer: a morphological, biological and molecular pathway. Eur J Cancer Prev. 2008;17:195–200. doi: 10.1097/CEJ.0b013e3282f0bff5. [DOI] [PubMed] [Google Scholar]
- 61.Rogers AB, Taylor NS, Whary MT, Stefanich ED, Wang TC, Fox JG. Helicobacter pylori but not high salt induces gastric intraepithelial neoplasia in B6129 mice. Cancer Res. 2005;65:10709–10715. doi: 10.1158/0008-5472.CAN-05-1846. [DOI] [PubMed] [Google Scholar]
- 62.Ho E. Zinc deficiency, DNA damage and cancer risk. J Nutr Biochem. 2004;15:572–578. doi: 10.1016/j.jnutbio.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 63.Mocchegiani E, Costarelli L, Giacconi R, Cipriano C, Muti E, Malavolta M. Zinc-binding proteins (metallothionein and alpha-2 macroglobulin) and immunosenescence. Exp Gerontol. 2006;41:1094–1107. doi: 10.1016/j.exger.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 64.Peek RM, Blaser MJ. Helicobacter pylori and gastrointestinal tract adenocarcinomas. Nat Rev Cancer. 2002;2:28–37. doi: 10.1038/nrc703. [DOI] [PubMed] [Google Scholar]
- 65.Chen Y, Blaser MJ. Helicobacter pylori colonization is inversely associated with childhood asthma. J Infect Dis. 2008;198:553–560. doi: 10.1086/590158. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.





