Abstract
We have taken advantage of the strengths of the zebrafish model system to introduce developmental biology and genetics to undergraduates in their second semester of the Introductory Biology course at Emory. We designed a 6-week laboratory module based on research being undertaken by faculty in the department, and incorporated experiments that used current research methods including bioinformatics. Students undertook a range of experiments including direct observation of live wild-type zebrafish at different stages of embryogenesis, whole-mount in situ hybridization of mutant and wild-type embryos, vital dye staining of mutant and wild-type embryos, and pharmacological treatments to perturb normal development. These laboratories engaged the students by providing a hands-on, research-centered experience, while also enhancing their written (worksheets and laboratory reports) and oral (group presentation) communication skills. We describe the proceedings of each lab and the logistics of preparing and running these labs for 400–500 students (120 students taking lab each day), and provide a preliminary assessment of the success of the laboratories data based on student evaluations.
Introduction
Amajor challenge for educators involved in teaching large Introductory Biology courses at colleges and universities is to design labs that try to avoid traditional cookbook approaches. This challenge is part of the wider movement within the science education community that advocates the development of dynamic student-centered learning experiences that engage students in research-oriented learning.1
The Biology Department at Emory University has been working since 2003 to revise its Introductory Biology series to reflect the objectives of this educational movement. One of the aspects of the course revision has been to transform the laboratories into an inquiry-based, research-centered, hands-on learning experience using a problem-solving learning approach. A key aim has been to incorporate modern concepts and techniques in the fields of genetics and bioinformatics.
To communicate to students the nature and excitement of scientific discovery, we decided to base the laboratories on (1) current research being conducted by faculty in the department, (2) experiments that use modern laboratory techniques, (3) experiments that use computational biology methods and bioinformatics, and (4) case studies that make a connection between the laboratory topic and a real-life situation.
Two areas of research strength in the Biology Department are in the fields of developmental biology and genetics. Faculty members use several different model systems in their studies. As a result, it was decided to include a laboratory topic that would take advantage of this strength, and use a vertebrate model system to introduce students to these fields of research.
The zebrafish model system was chosen for experimental and logistical reasons:
(1) zebrafish lay numerous transparent eggs in a controllable and predictable manner;
(2) the embryos develop rapidly along a well-defined embryological staging series that permits observation of embryonic development from fertilization to maturation;
(3) the department has an established zebrafish facility.
Our belief is that when current faculty research and education are integrated in a mutually reinforcing manner, it promotes the development of a student's critical thinking skills, problem-solving abilities, and communication skills that are essential for the future success of our graduates. The intention is that this exposure will excite the students and lead them to get involved in independent research early in their undergraduate careers.
The new introductory laboratories were offered for the first time in the 2005–2006 academic year, and comprise of four different modules over two semesters. Each lab module is intended to compliment and illustrate aspects of the Introductory Biology lecture material that is being taught during that period. In this paper we describe the zebrafish module that we developed for the second semester, as well as a preliminary assessment of the success of this newly devised module based on student evaluations.
Methods and Course Design
Pedagological design
A key consideration in the development of this module was the logistics associated with preparing a laboratory that had a large number of students enrolled. The laboratories run 4 days a week, 2–3 h per day, with approximately a quarter of the students enrolled in the course attending the laboratory each day. The zebrafish module involves six laboratory sessions. A brief outline of the full 6-week module is provided in Table 1. In the following section of this paper, we provide an overview of each session. A detailed description of each laboratory including the materials, methods, solution preparation, and equipment needed is provided in the Supplemental Data for this paper (available online at www.liebertonline.com).
Table 1.
A Timeline for the Zebrafish Laboratory Module
| Week 1 | Lecture: introduction to zebrafish |
| Week 2 | Observation of live zebrafish development |
| Week 3 | Mutation and development I: identifying mutants by comparing patterns of gene expression in wild type and mutants |
| Week 4 | Mutation and development II: staining with vital dye to reveal morphological differences in wild type and mutants |
| Week 5 | Environmental effects on development |
| Week 6 | Lab group presentations |
In the first iteration of the course, the students were asked to draw and record the morphological phenotypes. In subsequent classes in response to student criticisms, Apple iSight cameras were attached to the instructor's dissecting scope to enable students to photograph and record the morphological phenotypes of the different treatments.
Session 1: Introduction to the zebrafish lab module
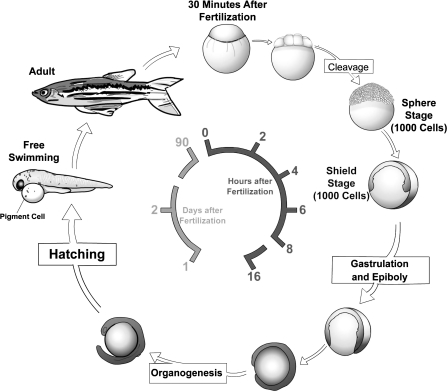
Before the class the students were assigned readings from their textbooks2,3 to acquaint them with a few key concepts in developmental biology, and a review article that describes the history of zebrafish as a model system and its use in biomedical studies.4 The class began with a lecture that reviewed key developmental biology concepts germane to the experiments in the module. We introduced the concept of model organisms, and this led into a discussion of the use of zebrafish as a vertebrate model system. This discussion also covered basic facts about the zebrafish lifecycle (Fig. 1) and animal husbandry. Fish tanks were brought into the class for the students to observe adult fish and to illustrate fish mating and egg collection techniques. We stressed that the experimental techniques covered in the module are used in current biomedical research.
FIG. 1.
Life cycle of the zebrafish. Zebrafish develop rapidly from a one-cell zygote that sits on top of a large yolk cell. Gastrulation begins approximately 6 h post fertilization, hatching at 2 days as a free-swimming larvae. Zebrafish reach sexual maturity around 3 months of age and can live for up to 5 years.
Session 2: Observing zebrafish development
Zebrafish embryos are transparent and develop from an externally fertilized egg. As a result, a student can observe the development of a newly fertilized embryo from a single cell to a free-swimming larvae, in the classroom, in the span of 2–3 days. In this session students observed embryos at cleavage, gastrulation, and organogenesis stages of development.5
The first part of the lab introduced students to the use of stereo (dissecting) microscopes, after which the students undertook a detailed examination and observation of live embryos at four different developmental stages over the 3-h laboratory. Details of how the different stages were precisely timed for the lab are given in the Supplemental Data.
The stages examined were as follows:
(i) Cleavage stages. We provided embryos that were between 0 and 2 h postfertilization (hpf). Each pair of students selected one fertilized embryo and observed it every 15 min over the next 2 h. We taught students to identify the animal and vegetal poles of the embryos and use these landmark features as the basis for their descriptions.
At each 15-min time point we asked students to draw or photograph the embryo both from the lateral and animal pole view and answer the following questions.
(1) How many cells does the embryo have?
(2) If the embryo has undergone cell division, where did the division take place, i.e., what is the plane of division in relation to the embryonic axes?
(3) Are the cell divisions synchronous?
(4) What is the time interval between each cell division?
(5) When looking at the lateral side view of the embryo, are there any obvious differences compared to the previous time point?
(6) When looking from the animal pole, are there any obvious differences compared to the previous time point?
(7) How big is the embryo?
(8) After each division, is the embryo getting bigger?
The purpose of this series of observations was to illustrate the fact that zebrafish are multicellular organisms that arise from a single-cell zygote by cell division. We wanted to emphasize that during early cleavage stages, cell divisions occur in precise planes of orientation and that the cell cycle length is fairly synchronous and of a uniform length of time. However, at mid-blastula stages, cell cycle length changes and becomes asynchronous and nonuniform. Further, we wanted to familiarize students with the important developmental biology concepts of cell division coupled with cell cycle, synchronous and asynchronous cell division, planes of cell division, and the difference between cell division and cell growth.
(ii) Gastrulation stage. We provided embryos at 6–8 hpf so that students could observe the critically important developmental process of gastrulation.
Students selected and observed a single embryo. At 30-min intervals, the students drew or photographed the embryo from a lateral and animal pole view. At each observation time point the students noted the following:
(1) Where the embryo is in relation to the animal and vegetal pole.
(2) When looking down at the embryo from the animal pole, are there any obvious differences compared to the previous time point?
(3) When looking at the lateral side view of the embryo, are there any obvious differences compared to the previous time point?
(4) Record on their drawings/photographs where these differences are and what happens to these differences over the next 2 h.
The observations highlighted the fact that the process of gastrulation requires dramatic cell movement and that the cells must involute to form mesoderm. Students identified the leading edge of the involuting mesoderm, known as the shield in zebrafish, and then measured/noted that cell movements drive the involution forward. Measurements were made using a transparent photocopy of graph paper that could be placed under the Petri dish containing the embryos. In addition, students learned that the process of gastrulation is morphologically the earliest time point at which the anterior–posterior axis of the embryos can be defined. These observations illustrated how the three germ layers, ectoderm, endoderm, and mesoderm, form.
The observations made by the students were designed to reinforce concepts that were being taught in the lectures about gastrulation. Gastrulation is always a difficult concept to illustrate in lectures, so observing the involuting mesoderm helped students visualize this process.
(iii) Organogenesis stage. To illustrate the process of organogenesis, students were given 24 and 48 hpf embryos. By providing embryos at these two ages, we were able to illustrate that different organs and differentiated tissue types form at different embryonic stages and at specific axial locations. The students drew and photographed the embryos, determined the anterior–posterior axes and identified the eyes, ears, heart, intestine, somites, notochord, melanocytes, and iridophores (two types of pigment cell types in zebrafish). They were then asked to determine which germ layer gave rise to each of the organs/tissues they identified.
The examination and identification of the various different structures in these more mature embryos served a second purpose; by becoming familiar with these structures, students would be better able to identify morphological phenotypes in mutants in subsequent laboratory sessions.
All stages were observed during the 3-h laboratory. Each stage was provided in a Petri dish containing embryo media. Students began with the 0–2 hpf embryos, which they had to observe every 15 min for 2 h. After their analysis at 15 min, they immediately began observing the 6–8 hpf embryos, which they had to observe every 30 min. In this way they alternated between the 0–2 and 6–8 hpf embryos. During any free time, they observed the 24 and 48 hpf embryos.
During the laboratory, the instructor displayed a PowerPoint slide of the different developmental stages, and accessed the ZFIN website (http://zfin.org) for developmental stages and movies of early zebrafish development. Live embryos viewed by the instructor's microscope could be projected on the screen, and this combined with the zfin movies enabled the instructor to point to key aspects of zebrafish development.
Session 3: Mutation and development I
In this laboratory, students completed the final steps of a whole-mount in situ hybridization (WISH) on a batch of wild-type and tbx24 mutant embryos using a digoxigenin-labeled riboprobe. The purposes of this laboratory were to introduce the WISH experimental technique as a common method used by developmental biologists to reveal the pattern of tissue specific gene expression, and to introduce the concept of how differences in the pattern of specific gene expression in wild-type and mutant embryos can be used to screen for mutants. Before laboratory, students read “The art and design of genetics screens: zebrafish”6 to familiarize themselves with the types of forward genetics screens that can be undertaken in zebrafish.
WISH
To introduce the students to the WISH technique, the class undertook the final step of this experimental protocol, the color reaction step. Students only undertook this final step as the complete protocol takes 3 days from start to finish.7 While the students carried out the large number of washes required by this technique and developed the color reaction, the instructor lectured on the theory behind in situ hybridization. Topics covered included the method of antisense RNA probe synthesis, detection methods, and how the specificity of a probe can be altered by washing at different stringencies and by hybridizing at different temperatures. In addition, students learned how genetic screens are used to generate mutants which then must be identified, isolated, and analyzed by various methods of screening, and how it is possible to determine the nature of a mutation by the determining the frequency of mutants in a clutch of embryos.
Each pair of students were provided with a clutch of twenty 8–10 somite-stage (12–14 hpf) embryos that had been hybridized with an in situ probe to the transcription factor myoD, to reveal the pattern of somite segmentation.8 For logistical reasons we generated tbx24 morphant embryos and combined 5 of these morphant embryos with 15 wild-type embryos of the same embryonic age. While using morphants is less desirable than using tbx24 mutants obtained from in crosses of heterozygote carriers, logistically this reduced the number of fish stocks we needed to maintain for the class. In addition all the morphants for the class could be generated in a short space of time before the labs instead of needing to continually breed tbx24 heterozygote pairs to obtain sufficient embryos for the class.
Screening for mutants
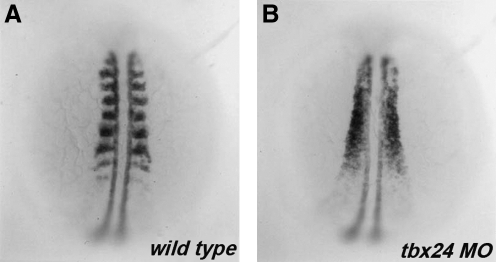
Once the color had developed, students recorded/photographed the pattern of myoD expression in mutant and wild-type siblings (Fig. 2). Subsequently, they separated the embryos into groups based on expression pattern and counted the number in each group to determine the nature of the mutation. A quarter of each clutch had reduced Tbx24 protein expression that is essential for normal somite segmentation.9
FIG. 2.
myoD expression in tbx24 morphants. Dorsal view of 18 hpf wild-type (A) and tbx24 morphant (B) embryos hybridized with a digoxigenin-labeled in situ probe to myoD. Anterior is to the top.
In addition to processing the fixed embryos, the students also observed a clutch of live 8–10 somite embryos, a quarter of which were tbx24 morphants. The purpose was to see if the students could identify the mutant phenotype without the aid of the myod in situ. We only provided a single dish of tbx24 morphants to each section, so only a small number of embryos needed to be injected with the morpholino the day before each class.
To alleviate difficulties that could potentially arise due to students losing embryos, the data/counts from each pair in the class were combined for the whole class. By analyzing this combined data, the class was able to determine that the phenotype was caused by a recessive mutation.
Since bioinformatics has become an important component of any new biology curriculum, we incorporated an exercise in a homework assignment worksheet. Students were given the genomic sequence of tbx24, and then were directed to a series of websites where they first determined the intron–exon structure of the gene, obtained the predicted amino acid sequence, and then, based on the conserved domains in the Tbx24 protein, had to propose its putative function.
Session 4: Mutation and development II
As in the previous laboratory, students were required to identify mutants from a clutch of embryos. This time, however, they screened for mutants using a vital stain, alcian green, which stains cartilage tissue.10 Students had to identify lessen (lsn) mutants and then describe the effect of the mutation on jaw morphology.11 lsn is a mutant in Med24, a component of the Mediator co-transcriptional activation complex. The mutation is homozygous recessive with no heterozygous phenotype. Homozygous mutants exhibit a number of defects in vagal neural crest–derived structures that included the enteric nervous system and posterior gill arch cartilages. These mutants were identified in Dr. Shepherd's lab, and his lab continues to study and characterize them.
Staining with a vital dye—alcian green
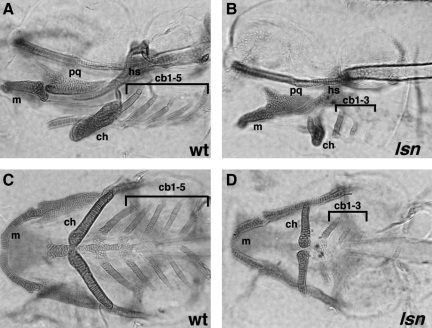
Zebrafish have a defined number of jaw cartilages, and staining 5-day-old embryos with alcian green unambiguously reveals this pattern of cartilages (Fig. 3). Students completed the standard staining protocol for alcian green on a clutch of 5-day-old prefixed embryos, generated from an in-cross of lsn heterozygotes. The staining protocol we used was that described on the zfin K-12 education site (http://www.neuro.uoregon.edu/k12/Larva%20lab%201.html) and is included in full in the Supplemental Data. By examining the clutch of embryos at the end of the staining protocol students were able to screen for and identify the lsn mutants. They photographed/drew the pattern of cartilages in wild type and mutant from a lateral and ventral perspective. Students also observed a clutch of live 5-day-old embryos from a lsn heterozygote in-cross and correlated the morphology of the live lsn mutants with the phenotype they observed in the alcian green–stained embryos.
FIG. 3.
Alcian green staining of pharyngeal cartilages in wildtype (WT) and lessen (lsn) mutants. One hundred and twenty hours postfertilization wild-type (A, C) and lsn mutant (B, D) larvae stained with alcian green shown in lateral (A, B) and ventral (C, D) views. In lsn pharyngeal cartilages, development is abnormal. Notably, the ceratobranchials 4 and 5 are absent in lsn. cb 1–5, ceratobranchial cartilages 1–5; ch, ceratohyal cartilage; hs, hyosymplectic cartilage; m, Meckel's cartilage; pq, palatoquadrate cartilage.
As in the previous laboratory, instructors lectured during breaks in the alcian green staining. Topics covered included a discussion about how jaw cartilage is specified, the role of the neural crest in its formation, and how defects in the specification, migration, or differentiation of neural crest could potentially give rise to the observed jaw phenotype. Also included was a discussion of stem cells, neural crest formation, and how the environment encountered by a cell influences its subsequent development. Again, the instructors reminded the students about how it is possible to determine the inheritance of a mutation by the determining the frequency the mutation arises in a clutch of embryos assuming that the genotype of the parents is known.
The data/counts of the number of wild-type and mutant embryos in each pair's clutch were again combined for the whole class to alleviate difficulties that could potentially arise due to students losing embryos during the processing. By analyzing these combined data, the class were able to determine that the lsn mutation is recessive.
Session 5: Effect of pharmacological agents on zebrafish development
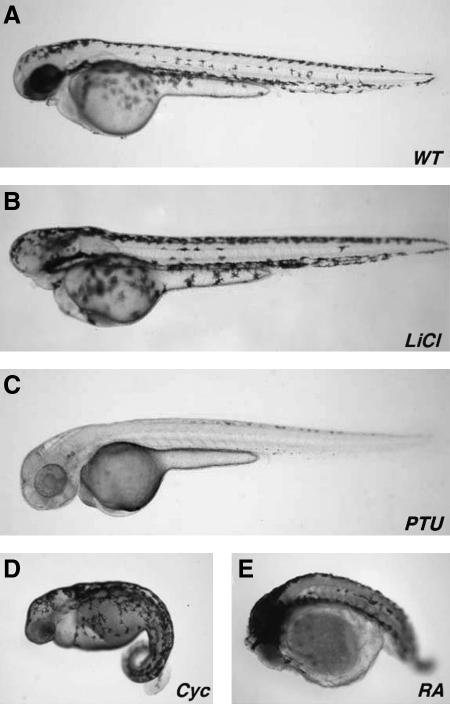
In the final wet laboratory the students were provided with live embryos that had received four different drug treatments, as well as untreated control embryos (Fig. 4). Students were told that these 48 hpf embryos had been treated with lithium chloride,12 cyclopamine,13 phenyl-thiourea,14 or retinoic acid.15 Building on the knowledge base that they had acquired over the previous sessions, the students were first asked to compare the drug-induced phenotypes to untreated controls, and describe the embryological phenotypes with respect to the tissues and anatomical structures that were affected. Second, students had to undertake a literature search for each pharmacological agent, determine which developmental pathway it affected, and then match it to one of the drug-induced phenotypes.
FIG. 4.
Effect of pharmacological agents on zebrafish development. Lateral views of 48 hpf embryos that have been treated with various pharmacological agents: (A) control, (B) lithium chloride, (C) 1-phenyl-2-thiourea, (D) cyclopamine, and (E) retinoic acid. Anterior is to the left.
As a further component of this laboratory, the students were divided into four groups. The members of each group were required to work together over the following week to prepare a 15–20-min class presentation that would describe in detail the morphological phenotype caused by one of the reagents and how the phenotype arose due to the action of the reagent.
Session 6: Class presentations and peer-review process
In the final laboratory session of the course, the four groups in each class gave a 15–20-min presentation describing in detail the morphological phenotype caused by one the reagents in the previous weeks session. By having each group describe the phenotype caused by only one of the reagents, repetitive presentations were avoided.
Each group was expected to prepare a PowerPoint presentation that not only illustrated and described in detail the morphological phenotype caused by the reagent, but also provided information as to its mechanism of action.
The presentations were graded by both the laboratory instructor and the other non-presenting students in the class. The purpose of this was to introduce the students to the concept of peer review.
Assessment and Grading
We used several methods to assess student learning and analysis skills. Upon arrival in class, students submitted a brief pre-laboratory report that contained a summary of the experiment and methods to be undertaken that day. To ensure that students arrived on time, short quizzes that covered old and new material were given in the first 5 min of each laboratory class. The quizzes and prelaboratory reports ensured that students had read the experimental protocol and had familiarized themselves with the material that was to be covered in the laboratory that day.
Participation points were awarded for perfect attendance, prompt arrival to laboratory, and active participation in class and group discussions. Points were also awarded based on their in class peer reviews. At the beginning of the semester, students were placed in groups of four based on a short survey containing questions such as their major, GPA, courses taken in science and biology, laboratory and research experience, problem-based learning experience, and personality. The goal was to put dissimilar students together, so that each one would bring a different strength to the group. For example, we aimed to put a student who had previous research/laboratory experience in each group, to help guide other group members who had had very little laboratory experience.
The students were asked to review their group members as well as themselves in two peer-review evaluations during the semester. Peer review was discussed with students on the first day of laboratory. Students understood that they had a responsibility to their group, to contribute equally to their group assignments and discussions, as well as actively participate in carrying out experiments. We found that in the first peer review given mid-semester, most group members gave each other full points. Interestingly, in the second peer review given at the end of the semester, students began to write about problems with certain group members, especially those who were not contributing to group assignments. Instructors only deducted points for students who had negative peer-review assessments from more than one member of the group.
The two in class peer-evaluation scores were in addition to the peer-evaluation grade that resulted from the final class group presentation. Using peer evaluation, we hoped to train students in the art of giving and receiving constructive criticism.
After each laboratory, students took home worksheets containing questions that assessed their understanding of that day's experiment, as well as reinforced the concepts that were covered (see Supplemental Data for worksheets). Worksheets included basic exercises in calculating how solutions are prepared and reviewed new terminology that was used in laboratory class.
Finally, students were also assessed by their group presentation in week 6 and by multiple-choice questions that were incorporated in the three lecture course exams.
Laboratory Evaluation
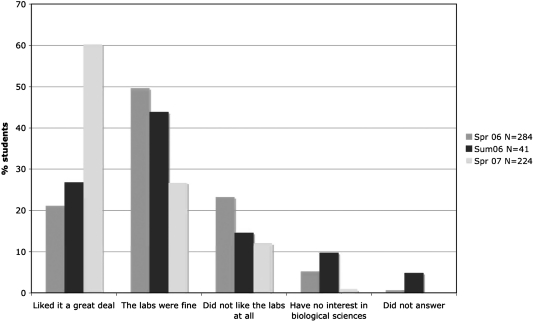
To determine how successful this laboratory module had been from the perspective of the students, we asked them to complete a short survey each time after the course was offered. The first question we asked was, “Did they like the zebrafish lab module?” Based on data collected spring 2006, summer 2006, and spring 2007 a large percent of the students did indeed like the module, and that there was an increase in very positive student evaluation over time, ∼20% in spring 2006, to ∼25% in summer 2006 to 60% in spring 2007 (Fig. 5). We attribute this increase to the laboratory instructors becoming more comfortable and better versed in the material being taught in the laboratory over time.
FIG. 5.
Assessment of whether students liked the labs. Bar graph showing the percentage of students who stated their like/dislike of the whole zebrafish laboratory module during the period spring 2006–spring 2007.
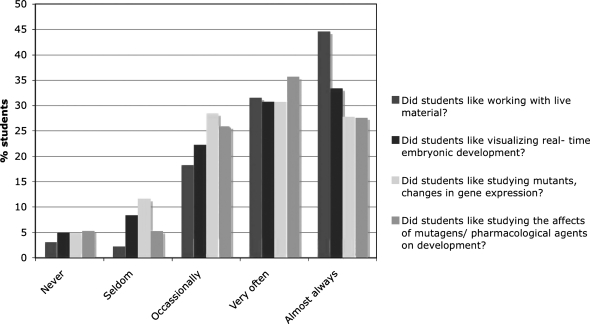
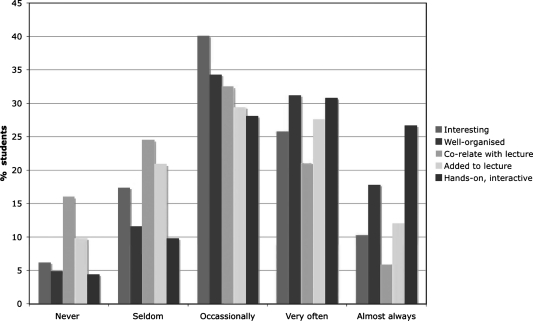
In spring 2007, to obtain a better reflection of students' attitudes toward the lab, we modified the class survey to include specific questions derived from student comments that we had collected over the previous two semesters (see Supplemental Data). These indicate that students liked most of the laboratory exercises, with the majority enjoying working with live material and visualizing development (Fig. 6). Most of the students felt that the labs were hands-on; however, a majority did not feel that the labs correlated well with the lecture course (Fig. 7). We have subsequently attempted to rectify this issue. In spring 2007, 24% of students expressed an interest in taking higher level developmental biology. It is yet to be seen if this actually translates into a larger developmental biology class that currently has an enrollment on average of 30 students per year.
FIG. 6.
Assessment of the specific likes and dislikes of students. Bar graph showing the percentage of students that responded positively or negatively to the question, “Which specific aspects of the Zebrafish Module labs did you like?” during the spring 2007 course.
FIG. 7.
Assessment of the laboratory organization, nature, coordination, and benefit to the lecture course material. Bar graph showing the percentage of students who responded positive or negatively to the questions of whether the labs were well-organized, interesting, hands-on, correlated to the lecture course, and enhanced the lecture material during the spring 2007 course.
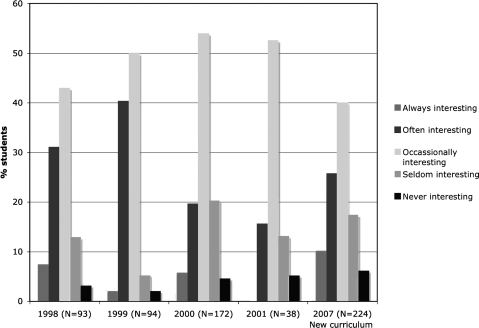
To determine whether the new introductory laboratory curriculum had sparked greater interest in students compared to the more traditional laboratory curriculum used in BIO 142 till 2005, we attempted to do a comparison between students class assessments in the old curriculum and new curriculum. Surveys between the years 2002 and 2005 were unavailable, but data from years 1998 to 2001 were available. These surveys indicated that during this period the majority of students only very often and occasionally found the laboratory exercises interesting (Fig. 8). Comparing the 1998–2001 data to that obtained in 2007, we find that there is a small increase in the percent of students who find the laboratory exercises interesting almost always and very often. However, a majority of students still only describe the laboratory as occasionally interesting.
FIG. 8.
Comparison of the interest of students in the new lab course (2007) versus the previous laboratory course (1998–2001). A bar graph showing the percentage of student responses both positive and negative reflecting their overall interest in the new zebrafish laboratory course (2007) compared to student responses to the previous nonzebrafish lab course (1998–2001).
Challenges Associated with the Course
We encountered several challenges while establishing this laboratory. A key issue related to the selection of laboratory instructors for the course. The selection was based on the research and teaching experience of the individuals who applied for the instructor positions. Most of the instructors were graduate students and postdoctoral fellows who were interested in a teaching career. Unfortunately, very few had specific training in developmental biology, and as a result, it was a challenge to ensure that they were all experienced and familiar enough with the subject material being taught to be able to effectively instruct and direct the laboratories. To overcome this lack of background, the instructors met once a week for a lecture going over the following weeks laboratory. In addition, the instructors were given detailed notes for each laboratory (see Supplemental Data).
Several logistical challenges arose that were associated with coordinating and training the laboratory support staff to be able to breed sufficient pairs of fish, collect embryos, as well as prepare various reagents for the different laboratory sessions. As a result, of the increased amount of work associated with preparing these labs there was an increase in labor costs.
While acknowledging these challenges we believe that this laboratory module can be easily adopted by universities and colleges that already have a zebrafish facility. Smaller colleges that do not have a fish facility may consider using this laboratory series as the basis of a more advanced developmental biology laboratory class. Several of the laboratories could be modified and expanded to make them more challenging and appropriate for juniors and seniors.
Summary and Conclusions
We have devised a new large-scale introductory biology laboratory course that introduces students to the fields of developmental biology and genetics in a laboratory setting. This course has attempted to address some of the issues that previously arose in the department's more traditional cookbook introductory labs. We have utilized the zebrafish model system that lends itself well to this type of laboratory course given the large class size. We believe that our efforts to provide a more relevant and interesting introductory laboratory experience to freshmen and sophomore biology majors have been successful compared with the previous introductory laboratories. This is supported by the data from the student assessments. Further, this course can be viewed as a model for the design of future, more innovative introductory laboratories courses.
Supplementary Material
Acknowledgments
We would like to thank the numerous Biol. 142L TA's and instructors who made this laboratory course possible. We would like to thank Dr. Alex Escobar for his help coordinating the course and for providing us with the student evaluation data. We would like to thank Dr. Robert Esterberg, Robert Russo, Scottye Davis, and Jason Crawley for their hard work helping prepare for these laboratories. We would like to acknowledge the Arthur Vining Davis Foundation for financial support that enabled the Department of Biology to establish the new Introductory Laboratory Series at Emory and research support that made it possible to generate lessen mutant embryos for the labs to IS from NIH (DK067285). We would like to thank Dr. Pat Marsteller for her critical reading of this article.
Disclosure Statement
No competing financial interests exist.
References
- 1.Wood WB. Gentile JM. Education. Teaching in a research context. Science. 2003;302:1510. doi: 10.1126/science.1091803. [DOI] [PubMed] [Google Scholar]
- 2.Freeman S. Biological Science. 2nd. Upper Saddle River, NJ: Pearson Education Inc.; 2005. [Google Scholar]
- 3.Griffiths AJF, et al. An Introduction to Genetic Analysis. 6th. New York: W.H. Freeman and Company; 1996. [Google Scholar]
- 4.Grunwald DJ. Eisen JS. Headwaters of the zebrafish—emergence of a new model vertebrate. Nat Rev Genet. 2002;3:717–724. doi: 10.1038/nrg892. [DOI] [PubMed] [Google Scholar]
- 5.Kimmel CB, et al. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- 6.Patton EE. Zon LI. The art and design of genetic screens: zebrafish. Nat Rev Genet. 2001;2:956–966. doi: 10.1038/35103567. [DOI] [PubMed] [Google Scholar]
- 7.Thisse C. Thisse B. Postlethwait JH. Expression of snail2, a second member of the zebrafish snail family, in cephalic mesendoderm and presumptive neural crest of wild-type and spadetail mutant embryos. Dev Biol. 1995;172:86–99. doi: 10.1006/dbio.1995.0007. [DOI] [PubMed] [Google Scholar]
- 8.Weinberg ES, et al. Developmental regulation of zebrafish MyoD in wild-type, no tail and spadetail embryos. Development. 1996;122:271–280. doi: 10.1242/dev.122.1.271. [DOI] [PubMed] [Google Scholar]
- 9.Nikaido M, et al. Tbx24, encoding a T-box protein, is mutated in the zebrafish somite-segmentation mutant fused somites. Nat Genet. 2002;31:195–199. doi: 10.1038/ng899. [DOI] [PubMed] [Google Scholar]
- 10.Westerfield M. The Zebrafish Book. Eugene, OR: University of Oregon Press; 1993. [Google Scholar]
- 11.Pietsch J, et al. Lessen encodes a zebrafish trap100 required for enteric nervous system development. Development. 2006;133:395–406. doi: 10.1242/dev.02215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stachel SE. Grunwald DJ. Myers PZ. Lithium perturbation and goosecoid expression identify a dorsal specification pathway in the pregastrula zebrafish. Development. 1993;117:1261–1274. doi: 10.1242/dev.117.4.1261. [DOI] [PubMed] [Google Scholar]
- 13.Incardona JP, et al. The teratogenic Veratrum alkaloid cyclopamine inhibits sonic hedgehog signal transduction. Development. 1998;125:3553–3562. doi: 10.1242/dev.125.18.3553. [DOI] [PubMed] [Google Scholar]
- 14.Karlsson J. von Hofsten J. Olsson PE. Generating transparent zebrafish: a refined method to improve detection of gene expression during embryonic development. Marine Biotechnol. 2001;6:522–527. doi: 10.1007/s1012601-0053-4. [DOI] [PubMed] [Google Scholar]
- 15.Marsh-Armstrong N, et al. Retinoic acid is necessary for development of the ventral retina in zebrafish. Proc Natl Acad Sci USA. 1994;91:7286–7290. doi: 10.1073/pnas.91.15.7286. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.