Abstract
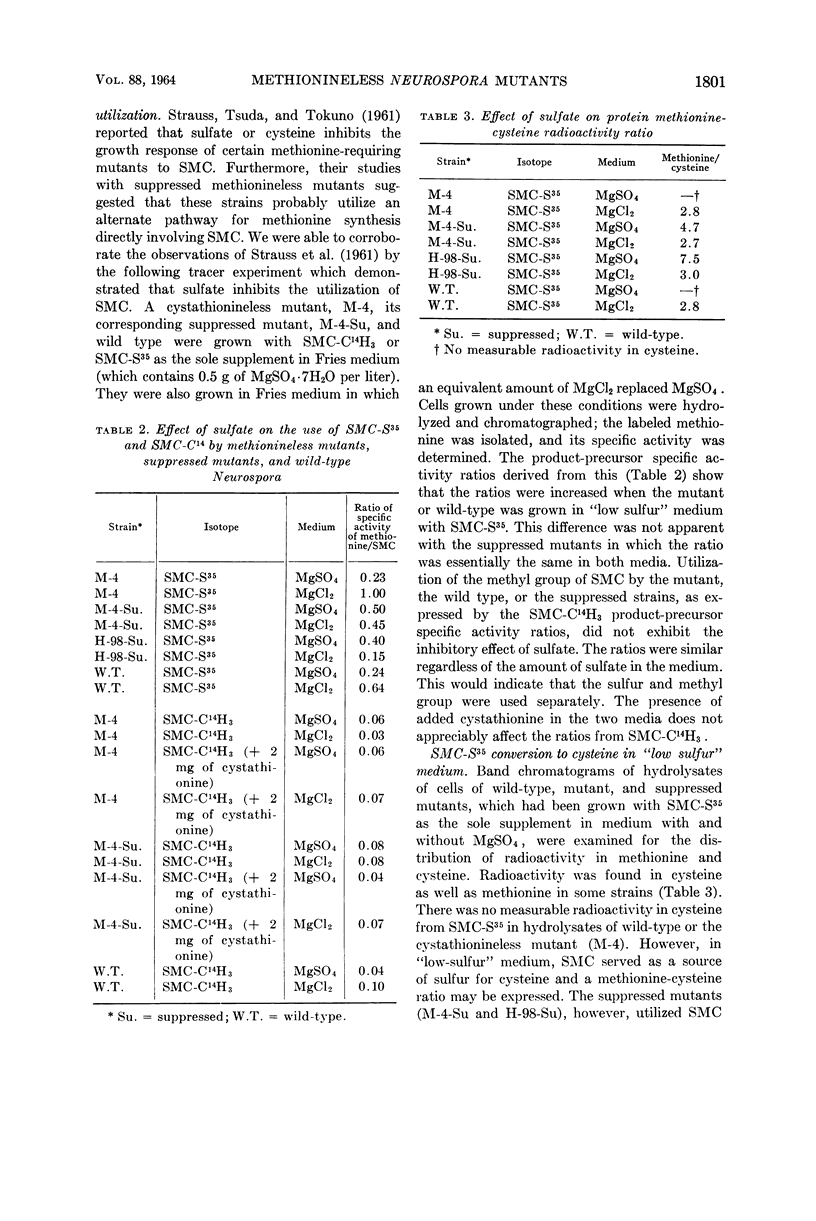
Wiebers, Joyce L. (Purdue University, West Lafayette, Ind.), and Harold R. Garner. Use of S-methylcysteine and cystathionine by methionineless Neurospora mutants. J. Bacteriol. 88:1798–1804. 1964.—Radioactive methionine was found in hydrolysates of various strains of Neurospora crassa when either S-methylcysteine (SMC)-C14H3 or SMC-S35 is the sole addition to minimal medium. Isotope product-precursor specific activity ratios are very similar for the two sources of label. Wild-type and methionineless mutants use sulfur from SMC in the biosynthesis of methionine, but not of cysteine, when grown in regular medium. With a medium nearly free from sulfate, SMC served as a source of sulfur for both cysteine and methionine. Suppressed methionineless mutants incorporated sulfur from SMC into cellular cysteine even in the presence of normal amounts of sulfate. SMC as a possible metabolic precursor of methionine was compared to cystathionine in an experiment with wild-type Neurospora. The four sources of label used were: SMC-C14H3, SMC-S35, cystathionine-U-C14, and cystathionine-S35. In each flask, the organism was offered one of the labeled compounds plus an equivalent amount of the other compound without label. The amount of each compound was sufficient for either to supply its contribution to all of the cellular methionine, if it were successful in competing with endogenous sources. To avoid adaptive breakdown of substrates, the compounds were added continuously at a rate consistent with the amount of growth present. The ratio of specific activity of cellular methionine to precursor was determined for each labeled compound. The results show that SMC sulfur and methyl carbon are used equally well. Cystathionine carbon and sulfur appear to be equally utilized also. A preference for cystathionine is indicated.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DALAL F. R., REGE D. V., SREENIVASAN A. Methionine synthesis in Neurospora crassa. Biochem J. 1961 Nov;81:317–319. doi: 10.1042/bj0810317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FLAVIN M. Microbial transsulfuration: the mechanism of an enzymatic disulfide elimination reaction. J Biol Chem. 1962 Mar;237:768–777. [PubMed] [Google Scholar]
- LIVERMAN J. L., RAGLAND J. B. S-methyl-L-cysteine as a naturally occurring metabolite in Neurospora crassa. Arch Biochem Biophys. 1956 Dec;65(2):574–576. doi: 10.1016/0003-9861(56)90217-x. [DOI] [PubMed] [Google Scholar]
- MONDOVI B., SCIOSCIA-SANTORO A., CAVALLINID Further evidence on the identity of cystathionase and cysteine desulfhydrase. Arch Biochem Biophys. 1963 May;101:363–364. doi: 10.1016/s0003-9861(63)80025-9. [DOI] [PubMed] [Google Scholar]
- MOORE S., STEIN W. H. A modified ninhydrin reagent for the photometric determination of amino acids and related compounds. J Biol Chem. 1954 Dec;211(2):907–913. [PubMed] [Google Scholar]




