Abstract
Plummer, Gordon (Baylor University College of Medicine, Houston, Tex.), and Brian Lewis. Thermoinactivation of herpes simplex virus and cytomegalovirus. J. Bacteriol. 89:671–674. 1965.—The stability of herpes simplex virus and of human cytomegalovirus at 4, 22, 36, and 50 C was studied. A plateau or lag phase, during which no loss of viability was detected, was a constant feature of the inactivation curves of herpesvirus and cytomegalovirus at 36 and 22 C and of herpesvirus at 4 C. Unlike herpesvirus, cytomegalovirus was repeatedly found to be less stable at 4 C (and at 10 C) than at room temperature (22 C). Extracellular herpesvirus harvested from the supernatant culture fluid seemed to be more stable than intracellular virus obtained by sonic disintegration of the infected cells. Serum had a stabilizing effect at 36 C on both intracellular and extracellular virus.
Full text
PDF
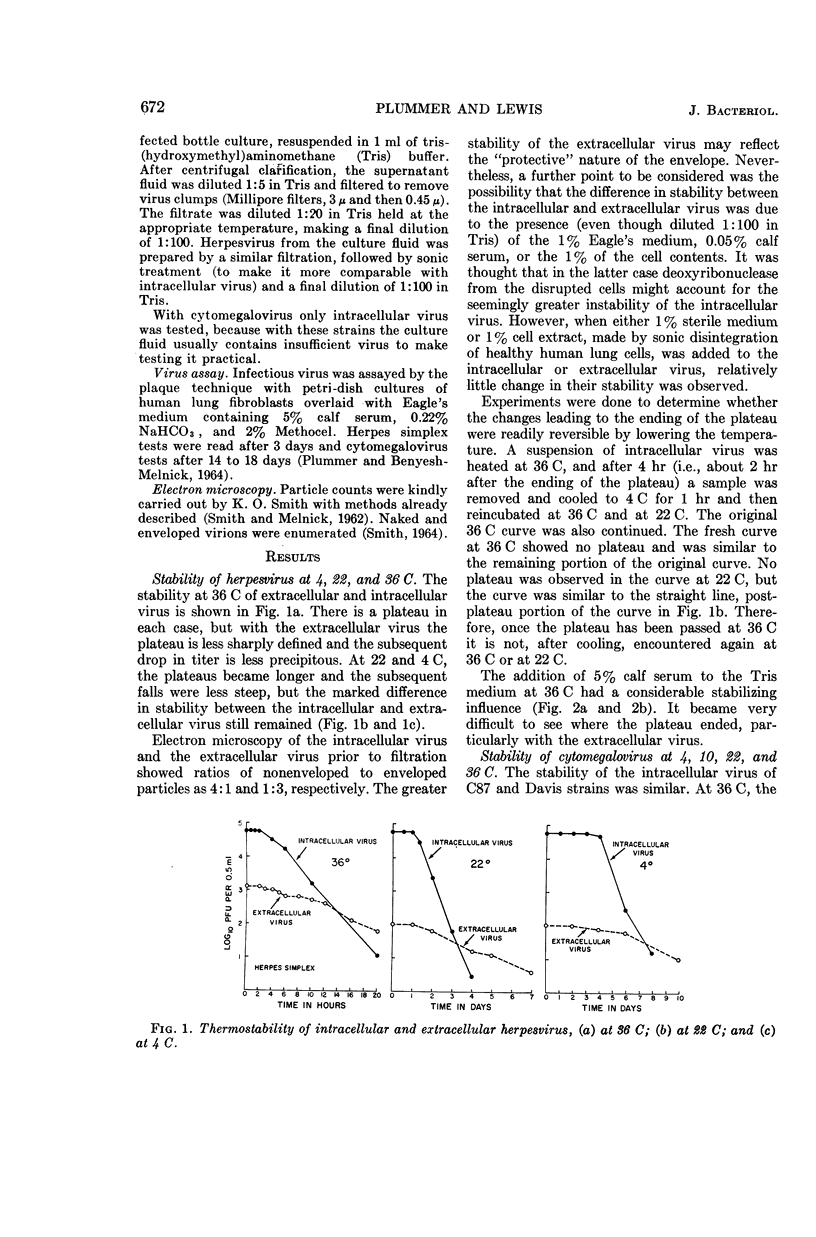
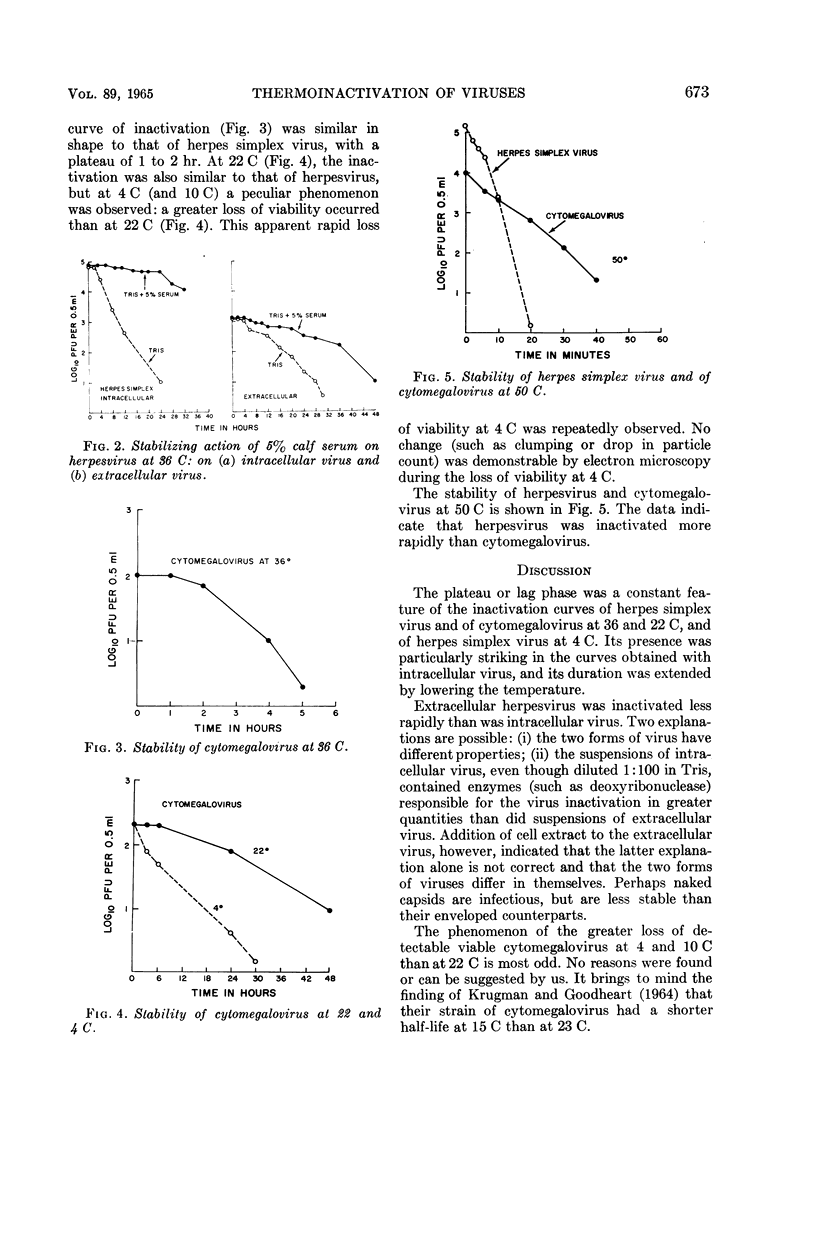

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BACHRACH H. L., BREESE S. S., Jr, CALLIS J. J., HESS W. R., PATTY R. E. Inactivation of foot-and-mouth disease virus by pH and temperature changes and by formaldehyde. Proc Soc Exp Biol Med. 1957 May;95(1):147–152. doi: 10.3181/00379727-95-23148. [DOI] [PubMed] [Google Scholar]
- HOGGAN M. D., ROIZMAN B. The effect of the temperature of incubation on the formation and release of herpes simplex virus in infected FL cells. Virology. 1959 Aug;8:508–524. doi: 10.1016/0042-6822(59)90052-2. [DOI] [PubMed] [Google Scholar]
- KAPLAN A. S. A study of the herpes simplex virus-rabbit kidney cell system by the plaque technique. Virology. 1957 Dec;4(3):435–457. doi: 10.1016/0042-6822(57)90078-8. [DOI] [PubMed] [Google Scholar]
- KAPLAN C. The heat inactivation of vaccinia virus. J Gen Microbiol. 1958 Feb;18(1):58–63. doi: 10.1099/00221287-18-1-58. [DOI] [PubMed] [Google Scholar]
- KRUGMAN R. D., GOODHEART C. R. HUMAN CYTOMEGALOVIRUS. THERMAL INACTIVATION. Virology. 1964 Jun;23:290–291. doi: 10.1016/0042-6822(64)90300-9. [DOI] [PubMed] [Google Scholar]
- PLUMMER G., BENYESH-MELNICK M. A PLAQUE REDUCTION NEUTRALIZATION TEST FOR HUMAN CYTOMEGALOVIRUS. Proc Soc Exp Biol Med. 1964 Oct;117:145–150. doi: 10.3181/00379727-117-29520. [DOI] [PubMed] [Google Scholar]
- PLUMMER G. SEROLOGICAL COMPARISON OF THE HERPES VIRUSES. Br J Exp Pathol. 1964 Apr;45:135–141. [PMC free article] [PubMed] [Google Scholar]
- SMITH K. O., MELNICK J. L. A method for staining virus particles and identifying their nucleic acid type in the electron microscope. Virology. 1962 Jul;17:480–490. doi: 10.1016/0042-6822(62)90143-5. [DOI] [PubMed] [Google Scholar]
- SMITH K. O. RELATIONSHIP BETWEEN THE ENVELOPE AND THE INFECTIVITY OF HERPES SIMPLEX VIRUS. Proc Soc Exp Biol Med. 1964 Mar;115:814–816. doi: 10.3181/00379727-115-29045. [DOI] [PubMed] [Google Scholar]
- WALLIS C., MELNICK J. L. THERMOSENSITIVITY OF POLIOVIRUS. J Bacteriol. 1963 Sep;86:499–504. doi: 10.1128/jb.86.3.499-504.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOUNGNER J. S. Thermal inactivation studies with different strains of poliovirus. J Immunol. 1957 Apr;78(4):282–290. [PubMed] [Google Scholar]


