Abstract
Botulinum neurotoxin A (BoNT/A), the most toxic, naturally occurring protein, cleaves synapse-associated protein of 25 kDa and inhibits acetylcholine release from motor nerve endings (MNEs). This leads to paralysis of skeletal muscles. Our study demonstrates that capsaicin protects mouse neuromuscular junctions from the neuroparalytic effects of BoNT/A. Bilateral injection of BoNT/A near the innervation of the Extensor digitorum longus (EDL) muscle of adult Swiss-Webster mice inhibited the toe spread reflex (TSR). However, when capsaicin was coinjected bilaterally, or injected 4 or 8 h before injecting BoNT/A, the TSR remained normal. In animals that were pretreated with capsazepine, capsaicin failed to protect against the neuroparalytic effects of BoNT/A. In vivo analyses demonstrated that capsaicin protected muscle functions and electromygraphic activity from the incapacitating effects of BoNT/A. The twitch response to nerve stimulation was greater for EDL preparations isolated from mice injected with capsaicin before BoNT/A. Capsaicin pretreatment also prevented the inhibitory effects of BoNT/A on end-plate currents. Furthermore, pretreatment of Neuro 2a cells with capsaicin significantly preserved labeling of synaptic vesicles by FM 1-43. This protective effect of capsaicin was observed only in the presence of extracellular Ca2+ and was inhibited by capsazepine. Immunohistochemistry demonstrated that MNEs express transient receptor potential protein of the vanilloid subfamily, TRPV1, the capsaicin receptor. Capsaicin pretreatment, in vitro, reduced nerve stimulation or KCl-induced uptake of BoNT/A into motor nerve endings and cholinergic Neuro 2a cells. These data demonstrate that capsaicin interacts with TRPV1 receptors on MNEs to reduce BoNT/A uptake via a Ca2+-dependent mechanism.
Neurotoxins produced by the anaerobic bacterium Clostridium botulinum are the most potent biological toxins. Although BoNTs are potential weapons for bioterrorism (Arnon et al., 2001), they are increasingly used for treating neurological and cardiovascular diseases, as well as for cosmetic purposes (Schnider et al., 1994; Benedetto, 1999; Tsuboi et al., 2002; Scott, 2004; Bilici et al., 2007; Van Beek et al., 2007; Schulte-Mattler, 2008). These uses provide a potential danger of intoxication with BoNTs.
Active and passive immunization currently protect against and treat poisoning with BoNTs. However, the increasing applicability of BoNTs to treat human diseases makes active immunization less desirable. At the same time, antitoxin is most effective during the early phase of poisoning with BoNTs. Once BoNT enters the intracellular space, antibodies do not have access to the toxin. In this situation, severely poisoned patients depend on artificial ventilation for long periods of time (Souayah et al., 2006). Therefore, it is important to develop effective prophylactic and therapeutic measures for BoNT/A.
The seven serotypes of BoNTs designated A to G (Oguma et al., 1995) are each composed of a heavy (HC) and light (LC) chain linked by a disulfide bond. The primary toxic effect is inhibition of vesicular acetylcholine (ACh) release from motor nerve terminals which leads to muscle paralysis. To achieve this effect, BoNTs undergo a cascade of cellular events (Simpson, 1981) that begin with HC binding to specific extracellular receptors followed by holotoxin endocytosis into the nerve terminal (Yowler et al., 2002; Dong et al., 2006). The HC forms a pore in the endosomal membrane which enables translocation of the LC zinc endoprotease into the cytosol (Montal et al., 1992; Brunger et al., 2007). The LC chain cleaves specific Soluble Nethylmaleimide-sensitive factor Attachment REceptor complex (SNARE) proteins that are essential to exocytosis of ACh-enriched vesicles. BoNT/A LC cleaves synapse-associated protein of 25 kDa (SNAP-25; Blasi et al., 1993) and is the most potent and long-acting BoNT serotype.
In this study we observed that mouse motor nerve terminals and cholinergic Neuro 2a cells express TRPV1, the receptor for capsaicin. Although the function of TRPV1 at cholinergic synapses is unknown, our study revealed that in vivo and in vitro treatment with capsaicin inhibited the effects of BoNT/A on motor nerve endings. This prophylactic action was attributed to inhibition of BoNT/A uptake by capsaicin.
Materials and Methods
Animals.
All animal procedures were approved by the Institutional Animal Care and Use Committee. Adult Swiss-Webster mice were anesthetized with an intraperitoneally injected mixture of ketamine (100 mg/kg) and xylazine (9 mg/kg) before surgical exposure of the peroneal nerve's entry into the EDL muscle. Three microliters of 6.67 pM BoNT/A in HEPES buffer was injected bilaterally into the space surrounding the innervation site of the EDL with a 26-gauge Hamilton syringe. The skin incision was then surgically closed with aseptic technique. In experiments with capsaicin, the drug was either bilaterally coinjected (3 μl of 1 mM stock solution) with BoNT/A or injected 4 or 8 h before BoNT/A. To test for a prophylactic affect of capsaicin on body weight and muscle performance, animals were injected in both hind limbs with either BoNT/A alone or with capsaicin followed by BoNT/A. To test the therapeutic effects of capsaicin, mice were injected with capsaicin 12 h after injection of BoNT/A. At 12 h after BoNT/A, mice showed signs of inhibition of toe spread reflex.
Cell Culture.
Mouse cholinergic neuroblastoma (Neuro 2a) cells were cultured in Dulbecco's modified Eagle's medium-F12 medium, pH 7.4, supplemented with 10% fetal bovine serum and antibiotics. Two groups of Neuro 2a cells were seeded on poly(l-lysine)-coated cover slips. The experimental group was incubated (room temperature) in HEPES-Ringer solution containing 10 μM capsaicin for 15 min and washed. Both groups were then incubated in 10 pM BoNT/A in 40 mM KCl containing HEPES-Ringer solution for 30 min to induce neurotoxin uptake. A portion of the capsaicin-free group was treated with 40 mM KCl in HEPES-Ringer alone as control. After wash with HEPES, the preceding three groups of Neuro 2a cells were incubated for 10 min in 40 mM KCl HEPES containing FM 1-43 (1 μg/ml). Next, the cells were washed twice with HEPES buffer and observed with a LSM-510 confocal microscope, equipped with an argon laser (488 nm) (Carl Zeiss GmbH, Jena, Germany). Images were saved as TIFF files and analyzed with ImageJ software (National Institutes of Health, Bethesda, MD).
Immunoblotting, Confocal Microscopy, and Immunocytochemistry.
EDL, diaphragm, or Triangularis sterni (TS; McArdle et al., 1981) nerve-muscle preparations were dissected from isoflurane-anesthetized Swiss-Webster or yellow fluorescence protein (YFP)-expressing mice. Harvested diaphragm preparations were flash frozen in liquid nitrogen and stored at −80°C until tissue was homogenized in phosphate-buffered saline (PBS) containing 1% Nonidet 40 and complete protease inhibitor cocktail (GE Healthcare, Little Chalfont, Buckinghamshire, UK). Homogenates of Neuro 2a cells were prepared in the same manner. After centrifuging the homogenate at 14,000 rpm for 10 min in the cold, the crude lysate (40 μg) was resolved via SDS-polyacrylamide gel electrophoresis after boiling in 1× Laemmli buffer. Immunoblotting was performed with anti-rabbit TRPV1 antibody (Santa Cruz Biotechnology, Inc., Santa Cruz, CA).
TS or diaphragm nerve-muscle preparations were pinned to a Sylgard-lined Plexiglas chamber and bathed in HEPES-Ringer solution containing 135 mM NaCl, 5 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 5.5 mM dextrose, and 5 mM HEPES. For evaluation of neurotoxin uptake, diaphragm preparations of YFP mice were bathed in 667 pM Alexa Fluor 647-labeled BoNT/A (Alexa Fluor dyes; Invitrogen, Carlsbad, CA) and toxin uptake was initiated in response to nerve stimulation (1 Hz) or exposure to HEPES-Ringer containing 40 mM KCl (osmolarity adjusted by reducing NaCl to 100 mM) as well as 667 pM Alexa Fluor 647-BoNT/A. For immunocytochemistry, nerve-muscle preparations were fixed in 4% formaldehyde PBS (room temperature) for 1 h followed by overnight incubation (4°C) in 100 mM glycine in PBS. Preparations were then permeabilized with 1% Triton X-100 in PBS at 4°C for 6 h followed by three washes with PBS and blocking with 2% bovine serum albumin in PBS overnight. Postsynaptic ACh receptors were labeled by exposing the fixed tissue to 1 ng/ml of Alexa Fluor 488-labeled α-bungarotoxin (α-BnTX) at 4°C for 6 h. The muscle end-plate region was cut out and mounted with vectashield on a slide and kept frozen at −20°C before imaging with a NIKON LSM-410 confocal microscope equipped with argon (488 nm for α-BnTX) and HeNe (647 nm for BoNT/A) lasers. Images were saved and represented as TIFF files.
For the immunohistochemical detection of TRPV1 in hemidiaphragm preparations, freshly isolated tissues were fixed as described above and labeled with anti-rabbit TRPV1 antibody (Santa Cruz Biotechnology Inc.; 1:200 dilution in PBS). The preparations were then washed and stained with fluorescein isothiocyanate-labeled secondary antibody (1:500 dilution in PBS) and subjected to confocal microscopy.
Neuro 2a cells grown on 25-mm round glass coverslips were fixed with 2 ml of 4% paraformaldehyde in PBS at room temperature for 30 min. The cells were washed twice with PBS (2 ml) and treated with 2 ml of 100 mM glycine in PBS for 30 min. After washing twice with PBS, the cells were permeabilized with 300 μl of cold methanol at −20°C for 5 min and washed with 2 ml of PBS. The cells were then blocked with 2% bovine serum albumin for 1 h at 37°C followed by a single wash with PBS and incubation for another 1 h in 300 μl of anti-rabbit TRPV1 antibody in PBS (1:200 dilution). After a single wash in PBS, the cells were incubated for 1 h in the presence of fluorescein isothiocyanate-labeled secondary antibody (1:500 dilution in PBS), washed 3 times with 2 ml of PBS, washed once with distilled water, and mounted with vectashield on a glass slide. The slides were stored at −20°C until viewing with a LSM-510 confocal microscope (Carl Zeiss GmbH) equipped with an argon laser (488 nm) in the Confocal Imaging Facility of the New Jersey Medical School. Images were saved as TIFF files.
In Vivo Study of Muscle Performance.
To evaluate motor strength and coordination mice were first allowed to adapt to a rotating rod (Rotamex-5; Columbus Instruments, Inc., Columbus, OH). After 1 week of training, control mice were capable of 300 s of sustained performance on a rod rotating at 15 rpm. Rotarod performance was tested biweekly after administration of BoNT/A alone or in combination with capsaicin. Mean duration of time on the Rotarod was averaged for three repeated runs separated by 30-min intervals. For each run, mice were kept on the apparatus for no longer than 300 s. Mice were tested in groups of 4 at each time point after BoNT/A. Rotarod testing of mice receiving capsaicin 12 h after BoNT/A injection evaluated capsaicin for a therapeutic effect.
The toe spread reflex was scored from 1 to 5 depending on the number of toes that the mouse could extend when lifted by the tail. The leg strength was scored by evaluating the ability of the mouse to move the legs and grip a rod: 0, no movement toward rod; 1, moves slightly toward rod, but profound weakness prevents gripping the rod to support body weight; 2, supports body weight on the rod with a weak grip; 3, can hold onto rod for at least 30 s; 4, easily reaches for and grips rod to support body weight for at least 1 to 2 min; 5, quickly grasps rod with fast and strong grip that supports body weight for 2 or more min. The waist muscle strength was evaluated and scored based on the ability of the mouse to show movement of the waist when held by its tail: 0, no movement; 1, very slight movement; 2, attempts to lift itself onto a rod; 3, can easily reach for, lifts itself onto, and climbs over a suspended rod; 4, reaches for a rod and holds onto it with greater strength; 5, can easily reach to and hold onto the rod, lifts itself onto the rod with ease. Groups of four mice were scored biweekly, and the scores obtained for individual animals in each group were pooled and averaged.
For electromyography, mice were anesthetized with an intraperitoneal injection of ketamine (100 mg/kg) and xylazine (9 mg/kg). After shaving their abdomen and distal hind limbs, mice were taped prone to a polystyrene foam board. A heated pad maintained body temperature between 32 and 38°C. Stimulating electrodes were 0.7-mm needles insulated with polytef (Dantec sensory needle; Dantec, Skovlunde, Denmark); the cathode was placed close to the sciatic nerve in the proximal thigh, and the anode was placed subcutaneously 1 cm proximally. Motor responses of flexor and extensor muscles were recorded with a ring electrode placed around the animal's hind limb, distal to the site of BoNT/A injection. The reference electrode was a ring electrode placed 1.2 cm distal to the recording electrode. Distance between stimulating and recording electrodes was 1.5 to 2 cm. Both the right and left hind limbs were studied. A ground electrode was placed in the contralateral limb. Muscle nerves were stimulated with monophasic pulses of constant current delivered through a constant current stimulator with fine intensity control (AM 2100). Recordings were made through motor amplifiers (Keypoint; Dantec) connected to a computer for digital storage and off-line analyses. Filter settings were 500 Hz/5 kHz. A threshold of less than 0.7 mA for evoking a motor response indicated that the stimulating electrode was optimally positioned for nerve stimulation. Stimulus intensity was increased until the compound muscle action potential (CMAP) reached maximum amplitude. CMAP amplitude (peak-peak) and distal latency (to negative onset) were then recorded. The electromyograph was recorded a maximum of two times for an individual mouse.
In Vitro Evaluation of Muscle Performance.
EDL nerve-muscle preparations of mice treated with BoNT/A alone or after capsaicin were dissected and mounted in a glass chamber (Radnoti Glass Technology, Inc., Monrovia, CA) filled with oxygenated (95% O2-5% CO2) normal Ringer's solution (pH 7.4, 37°C) containing 135 mM NaCl, 5 mM KCl, 1 mM MgCl2, 2 mM CaCl2, 1 mM Na2HPO4, 15 mM NaHCO3, 5.5 mM glucose. The EDL nerve was drawn into a suction electrode for indirect activation of muscle twitches. One tendon of the muscle was tied to a Grass Force transducer connected to a Digidata 1440A (Molecular Devices, Sunnyvale, CA). This enabled acquisition and analysis with PCLAMP software (Molecular Devices) of muscle mechanical responses to nerve stimulation. Isolated preparations were adjusted to optimal length for force generation and equilibrated for 15 min before stimulation at 0.1 Hz for data acquisition.
In Vitro Electrophysiology.
EDL nerve-muscle preparations were dissected, pinned to a Sylgard-lined Plexiglas chamber, and bathed in HEPES-buffered physiological solution. To activate transmitter release, the muscle nerve was drawn into a suction electrode and stimulated supramaximally. Conventional current clamp or two-electrode voltage clamp (Axoclamp 2B; Molecular Devices) allowed evaluation of stimulus evoked end-plate potentials (EPPs) or currents (EPCs; −75 mV holding potential) at 1, 20, 50, and 70 Hz. For control preparations, 0.75 μM Conotoxin GIIIB was added to the physiological solution to prevent mechanical responses to nerve stimulation.
Chemicals and Drugs.
Sources of toxins were: BoNT/A (Metabiologics Inc., Madison, WI); fluorescently labeled BoNT/A (BBTech, Dartmouth, MA); μ Conotoxin GIIIB (Alamone Labs, Jerusalem, Israel); α-BnTX (Invitrogen). All other chemicals and drugs were obtained from Sigma-Aldrich (St. Louis, MO).
Data Analyses.
Data for all figures were expressed as mean ± S.E.M. Student's t test evaluated the statistical significance of population means. ** and *** indicate p < 0.001 and p <0.0001, respectively.
Results
For the mouse illustrated in the inset photograph of Fig. 1A, capsaicin was injected into the right hind limb at 4 h before bilateral injection of BoNT/A. Within 24 h, the toe spread reflex (TSR) was totally abolished in the left limb, whereas TSR was normal for the capsaicin-preinjected right limb. Figure 1, A to C, summarizes the TSR scores obtained from mice which were injected with BoNT/A either 5 to 10 min before BoNT/A (n = 18) or 4 h (n = 10) or 8 h (n = 4) after an injection of capsaicin into the same hind limb. Although BoNT/A abolished the TSR of all control mice (n = 36), this reflex remained normal for each of the three capsaicin-treated groups (Fig. 1, A–C, solid bars). At times later than 24 h after the BoNT/A injection, TSR remained unaltered for all of the capsaicin-pretreated mice.
Fig. 1.
Capsaicin protects mice from the paralytic effects of BoNT/A. A, the inset illustrates the protective effect of capsaicin pretreatment on the mouse TSR. The histograms of A, B, and C summarize TSR score after injection with BoNT/A alone (n = 16) or after capsaicin pretreatment (n = 32); capsaicin was injected immediately (n = 18; A) or at 4 h (n = 10; B) or 8 h (n = 4; C) before BoNT/A. TSR was scored according to the number (0–5) of toes that the mouse could extend and flex from the midline of the foot. Although BoNT/A abolished the TSR for all BoNT/A alone mice, TSR was normal for all capsaicin-pretreated mice. Data of D, E, and F were derived from mice in which the space surrounding the entry of the peroneal nerve into the EDL of the right hind limb was injected with 3 μl of 1 mM capsaicin solution. Four hours later, 3 μl of 10 pM BoNT/A solution was similarly injected into both hind limbs. D shows that bilateral capsaicin pretreatment prolonged the duration that BoNT/A-injected mice could walk on a rotating rod. Three groups of mice (control, + BoNT/A bilaterally, and + capsaicin (CAP) + BoNT/A bilaterally; four mice in each group) were trained on the Rotarod for 1 week before the experiment. BoNT/A was injected on day 8. The time that each mouse walked on the Rotarod was averaged for three trials at each time point and plotted against the time after beginning the training period. E, summary of the effect of BoNT/A with and without capsaicin pretreatment on the CMAP amplitudes. CMAP amplitude is plotted against time in weeks after BoNT/A. Bars represent the average of normalized values ± S.E.M. F, illustrative records of CMAP for control (a), 4 and 8 weeks after BoNT/A alone (b and d), or BoNT/A after pretreatment with capsaicin (c and e).
Figure 1D illustrates the Rotarod data of control, BoNT/A alone, and capsaicin + BoNT/A-injected mice. To test in vivo muscle function and coordination after BoNT/A, the time mice walked on a rod rotating at 15 rpm was measured. Maximal duration of “walk time” was set to 300 s because all nontreated control mice performed for this duration. Beginning at 24 h after BoNT/A injection, walk time significantly declined below control and reached a minimal value of 127 ± 48 s at 4 days after toxin. The walk time gradually recovered to the control duration at approximately 20 days after BoNT/A. In contrast, mice injected with capsaicin at 4 h before BoNT/A remained equivalent to nontreated controls in walk time. Capsaicin preinjection also reduced the loss of body mass and cessation of grooming behavior associated with the preceding BoNT/A-induced functional abnormalities. Furthermore, assessment of muscle behavior by an investigator blinded to the mouse treatment suggested that capsaicin significantly protected muscle strength and coordination against BoNT/A. That is, motor strength score was significantly less than control at 2 and 3 weeks after BoNT/A alone. In contrast, capsaicin-preinjected mice retained normal motor strength at all times after BoNT/A (Table 1). However, BoNT/A administered at times later than 12 h after capsaicin was fully active in suppressing the preceding indicators of neuromuscular function.
Table 1.
Effect of BoNT/A alone and after capsaicin pretreatment on mouse muscle strength and body weight
Values are the mean ± S.E.M. for 12 to 32 mice.
| Muscle Strength |
Body Weight |
|||||
|---|---|---|---|---|---|---|
| Week 2 | Week 4 | Week 6 | Week 2 | Week 4 | Week 6 | |
| % ± S.E.M. | g ± S.E.M. | |||||
| Control | 100 | 100 | 100 | 30.39 ± 0.44 | 33.44 ± 0.37 | 39.92 ± 0.88 |
| + BoNT/A | 50.36 ± 3.40 | 70.02 ± 6.21 | 90.18 ± 3.68 | 32.66 ± 0.38 | 24.02 ± 0.37 | 33.02 ± 0.12 |
| + Capsaicin; + BoNT/A | 98.04 ± 1.12 | 100 | 100 | 33.08 ± 0.48 | 31.97 ± 0.53 | 38.44 ± 0.78 |
| + Capsaicin | 100 | 100 | 100 | 32.62 ± 0.56 | 33.55 ± 0.51 | 38.39 ± 0.93 |
Analysis of the CMAP amplitude indicated that the preceding protective effect of capsaicin was associated with preservation of neuromuscular physiology in vivo. Although CMAP amplitude declined for both BoNT/A-treated groups, the magnitude of the decline was significantly less for the capsaicin-preinjected mice (Fig. 1D). As illustrated in Fig. 1E, CMAP amplitude was greater at 4 and 8 weeks after BoNT/A for hind limbs pretreated with capsaicin compared with the hind limbs treated with BoNT/A alone. That is, CMAP amplitude was 11.3 ± 2.3 (n = 4), 0.7 ± 0.28 (n = 4), and 4.6 ± 0.92 (n = 4) mV at 4 weeks after BoNT/A for control, BoNT/A, and capsaicin-preinjected, BoNT/A-treated mice, respectively. At 8 weeks after BoNT/A, CMAP amplitude had partially recovered for the BoNT/A-alone and capsaicin-preinjected groups to 2.6 ± 0.79 (n = 4) and 6.7 ± 1.24 (n = 4) mV, respectively.
At 24 h after injection of capsaicin alone muscle strength, CMAP amplitude, and body weight and performance on the Rotarod were equivalent to control.
To evaluate functional neuromuscular transmission, neurally evoked twitches and end-plate electrical signals were evaluated for EDL nerve-muscle preparations isolated from mice injected with BoNT/A alone or at 4 h after capsaicin. The sample records of Fig. 2A are muscle twitches for control, BoNT/A alone, or BoNT/A after capsaicin. As anticipated, the nerve-evoked muscle twitch tension was reduced at 48 h after BoNT/A alone. Preinjection of capsaicin significantly reduced this affect of BoNT/A (Fig. 2B). That is, mean twitch tension was 140 ± 60.0 (n = 4) and 500 ± 100 (n = 4) mg for preparations that received BoNT/A alone and BoNT/A at 4 h after capsaicin, respectively. Thus, although BoNT/A significantly reduced twitch tension after capsaicin, the reduction was less than for preparations treated with BoNT/A alone. In vivo administration of capsaicin alone had no effect on force generation or the amplitude of EPCs (Fig. 2, C and D).
Fig. 2.
Capsaicin protects the neurally evoked twitch of the EDL against BoNT/A. A, representative twitches for control, BoNT/A alone, and BoNT/A plus capsaicin-pretreated mice. B, summary of percentage of normalized twitch tension for EDL muscles treated with BoNT/A alone or after capsaicin. Normalized twitch tension (C) and EPC amplitude (D) for the EDL muscles excised from untreated control mice and mice treated with capsaicin alone. Both twitch tension and EPC amplitude were equivalent to control at 48 h after the injection of capsaicin. Representative EPCs are shown above the bars for mean EPC amplitude (−75 mV holding potential). All bars represent the mean ± S.E.M.
Next, we tested whether capsaicin pretreatment preserves neurotransmitter release which in vivo treatment with BoNT/A alone inhibited. A, B, and C of Fig. 3 illustrate trains (70 Hz) of EPPs for EDL nerve-muscle preparations isolated from control, BoNT/A alone, and capsaicin-preinjected, BoNT/A-treated mice. As anticipated, nerve stimulation failed to produce EPPs for BoNT/A-treated preparations. In contrast, preparations treated with capsaicin before BoNT/A, produced EPPs which, similarly to control, did not fail in response to the 70-Hz nerve stimulation (Fig. 3D).
Fig. 3.
Capsaicin pretreatment preserves ACh release from motor nerve endings against the inhibitory effects of BoNT/A. A, B, and C are representative trains of EPPs recorded at 70 Hz (left traces) and single EPPs at 1 Hz (right traces) for EDL muscles excised from control, + BoNT/A, and + CAP + BoNT/A-treated mice, respectively; preparations are dissected at 48 h after BoNT/A administration. D presents the percentage failure of EPP generation in response to neural stimulation at 70 Hz for the preceding three groups. Percent failure was calculated as 100 × [1 − (the number of EPPs during the 70 Hz train divided by 40)]; 40 is the number of supramaximal stimuli applied to the nerve.
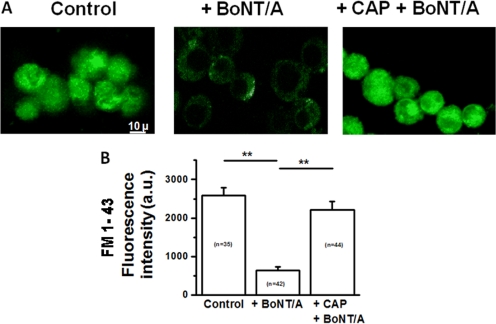
Capsaicin also protected exocytosis-induced uptake of FM 1-43 into Neuro 2a cells from the inhibitory effect of BoNT/A (Fig. 4A). Control Neuro 2a cells were uniformly labeled after a 10-min incubation in 1 μg/ml FM 1-43 in HEPES buffer containing 40 mM KCl. Neuro 2a cells preincubated in HEPES containing 10 pM BoNT/A and 40 mM KCl for 30 min accumulated significantly less FM 1-43 (Fig. 4B). In contrast, BoNT/A no longer reduced FM 1-43 uptake into Neuro 2a cells that were preincubated for 10 min in HEPES containing 10 μM capsaicin before BoNT/A loading. Thus, capsaicin protects the exo- and endocytotic mechanisms of cholinergic Neuro 2a cells against BoNT/A. This protective effect of capsaicin was observed at concentrations as low as 300 nM (Supplemental Fig. 1A).
Fig. 4.
Capsaicin pretreatment of Neuro 2a cells prevents BoNT/A inhibition of 40 mM KCl-stimulated FM 1-43 uptake. A, confocal images of FM 1-43 labeling for control (left), BoNT/A alone (center), and 10 μM capsaicin pretreated (right) Neuro 2a cells. B. Average fluorescence intensity (arbitrary units; a.u.) ± S.E.M for control, BoNT/A alone, and capsaicin pretreated Neuro 2a cells. The number in each bar indicates the number of cells summarized in the mean value.
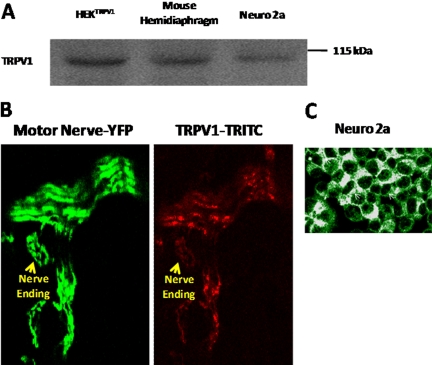
Because capsaicin protected mice from the inhibitory effects of BoNT/A, we tested for expression of the capsaicin receptor, TRPV1 channel protein, in mouse motor neurons. The Western blot of Fig. 5A demonstrates the presence of TRPV1 in HEK 293 cells transiently transfected with the cDNA for this protein, as well as for adult mouse diaphragm muscle and Neuro 2a cells. Figure 5B shows immunocytochemical detection of TRPV1 on a phrenic nerve terminal in the diaphragm muscle of a mouse that expressed YFP in motor neurons. This antibody also detected TRPV1 on the surface of Neuro 2a cells (Fig. 5C).
Fig. 5.
Mouse motor nerve endings and Neuro 2a cells express TRPV1. A is a representative Western blot for TRPV1 probed with anti-rabbit TRPV1 antibody in mouse hemidiaphragm, and Neuro 2a cell lysates as described in Materials and Methods. Lysate of rTRPV1 expressing HEK293 cells was used as control (HEKTRPV1). B and C, respectively, illustrate immunocytochemistry of TRPV1 expression in motor nerve endings (yellow arrows) of YFP mice and Neuro 2a cells (white arrows).
If capsaicin protects the neuroparalytic effects of BoNT/A via TRPV1 activation, then TRPV1 inhibition with the antagonist capsazepine should ablate the protective effects of capsaicin. To test this hypothesis we injected capsazepine (3 μl of 1 mM) into mice 15 min before capsaicin (3 μl of 1 mM) and BoNT/A (3 μl of 6.67 pM). The capsazepine-pretreated mice were unable to spread toes 24 h after injection of capsaicin and BoNT/A similarly to mice injected with BoNT/A alone. In addition, capsazepine treatment antagonized the protective effect of capsaicin on EPCs initiated at stimulus frequencies of 1, 20, 50, and 70 Hz (Figs. 6 and 7). Capsazepine alone did not affect EPC amplitude (Fig. 6, A and B). The average amplitude of the 1st, 10th, 20th, 30th, and 40th EPC in 20, 50, and 70 Hz trains for control mice and mice treated with BoNT/A alone, capsaicin + BoNT/A, and capsazepone + capsaicin + BoNT/A are summarized in Table 2. Capsaicin clearly does not protect stimulus-evoked transmitter release from BoNT/A after pretreatment with capsazepine. Western blot analysis (Fig. 7D) indicates that capsaicin alone or 30 min before BoNT/A does not alter TRPV1 expression.
Fig. 6.
Capsaicin pretreatment preserves ACh release from motor nerve endings against BoNT/A, and capsazepine prevents this effect of capsaicin. A, EPC (1 Hz) recorded from EDL nerve-muscle preparation isolated from control, + capsaicin, + BoNT/A, + capsaicin + BoNT/A, + capsazepine + capsaicin + BoNT/A, and + capsazepine injected mice. B, average EPC amplitudes measured in the six groups of nerve-muscle preparations normalized to untreated controls. Bars are mean ± S.E.M. ***, the differences in mean values are significantly (p < 0.0001) different from control. The numbers of mice and end-plates studied in these experiments are indicated in Table 2.
Fig. 7.
Capsazepine pretreatment antagonizes the protective effect of capsaicin on ACh release at high frequencies. EPCs were measured for EDL nerve-muscle preparations isolated from control, + CAP + BoNT/A, and + capsazepine (CPZ) + CAP + BoNT/A injected mice; stimulus frequencies were 20 (A), 50 (B), and 70 (C) Hz. Numbers of mice and end-plates studied in these experiments are indicated in Table 2. D is a Western blot for TRPV1 probed with anti-rabbit TRPV1 antibody in mouse hemidiaphragm at 90 min after treatment with capsaicin alone or with BoNT/A and capsaicin.
Table 2.
Average end-plate currents measured in EDL nerve-muscle preparations in the control group, groups treated with BoNT/A alone, capsaicin + BoNT/A, and capsazepine + capsaicin + BoNT/A
| Pulse | Control (n = 3, 9) | + BoNT/A (n = 6, 12) | + CAP + BoNT/A (n = 6, 12) | + CPZ + CAP + BoNT/A (n = 4, 12) |
|---|---|---|---|---|
| 20-Hz EPC amplitude (nA) | ||||
| 1 | 170.69 ± 15.47 | 2.69 ± 8.23 | 100.23 ± 8.2 | 3.60 ± 2.52 |
| 10 | 156.86 ± 17.23 | 1.81 ± 3.45 | 113.45 ± 16.12 | 1.82 ± 3.52 |
| 20 | 157.51 ± 17.14 | 0.44 ± 2.28 | 105.52 ± 11.74 | 0.48 ± 2.14 |
| 30 | 163.41 ± 16.63 | 2.16 ± 3.06 | 109.45 ± 5.26 | 0.22 ± 3.14 |
| 40 | 160.61 ± 16.76 | 1.98 ± 3.26 | 107.21 ± 8.45 | 2.03 ± 3.19 |
| 50-Hz EPC amplitude (nA) | ||||
| 1 | 188.44 ± 17.34 | 0.53 ± 3.24 | 136.53 ± 13.22 | 1.22 ± 1.45 |
| 10 | 199.08 ± 10.39 | 2.06 ± 2.14 | 148.23 ± 18.91 | 2.28 ± 1.43 |
| 20 | 175.21 ± 9.31 | 2.08 ± 2.18 | 135.84 ± 25.6 | 1.99 ± 0.91 |
| 30 | 173.42 ± 9.06 | 6.19 ± 2.43 | 128.99 ± 25.82 | 3.41 ± 1.39 |
| 40 | 173.18 ± 8.77 | 0.89 ± 1.56 | 109.92 ± 43.14 | 2.45 ± 1.46 |
| 70-Hz EPC amplitude (nA) | ||||
| 1 | 229.37 ± 13.36 | 0.75 ± 1.33 | 149.34 ± 14.59 | 1.11 ± 2.41 |
| 10 | 248.28 ± 11.99 | 1.64 ± 0.85 | 237.08 ± 48.05 | 1.46 ± 1.52 |
| 20 | 243.03 ± 12.77 | 1.29 ± 1.78 | 130.29 ± 19.28 | 2.02 ± 1.31 |
| 30 | 247.28 ± 11.65 | 2.13 ± 1.18 | 121.19 ± 18.39 | 1.17 ± 1.24 |
| 40 | 246.31 ± 14.57 | 1.31 ± 0.54 | 122.63 ± 18.18 | 0.19 ± 1.46 |
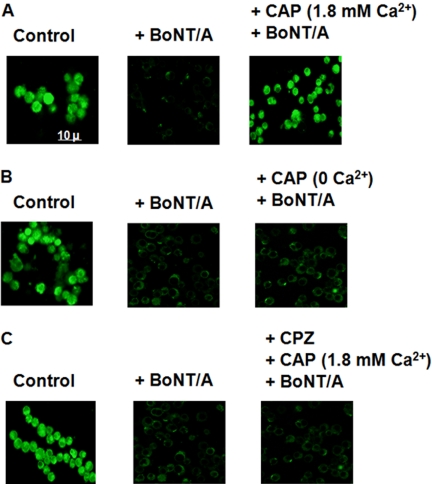
Capsaicin induces Ca2+ influx by activating TRPV1 channels (Vyklicky et al., 2008). If Ca2+ entry via TRPV1 is essential for the protective effects of capsaicin, then this effect should not be observed in the absence of extracellular Ca2+. To test this hypothesis we pretreated Neuro 2a cells with capsaicin in the presence and absence of Ca2+ before exposure to BoNT/A. Figure 8, A and B, shows that BoNT/A reduced uptake of FM 1-43 into Neuro 2a cells pretreated with capsaicin in the absence of extracellular Ca2+. In addition, BoNT/A reduced FM 1-43 uptake into Neuro 2a cells exposed to capsazepine before capsaicin (Fig. 8C). These observations suggest that capsaicin-stimulated Ca2+ influx via TRPV1 is important to the anti-BoNT/A effects of capsaicin.
Fig. 8.
Extracellular Ca2+ is essential for the protective effect of capsaicin, and capsazepine pretreatment prevents this effect of capsaicin. Confocal images of FM 1-43 labeling for control (left), BoNT/A alone (center), and 10 μM CAP-pretreated (right) Neuro 2a cells in the presence of Ca2+ (A) and absence of Ca2+ (B), as well as after pretreatment with 10 μM CPZ before capsaicin (C). Uptake of FM 1-43 was stimulated by coapplication of 40 mM KCl.
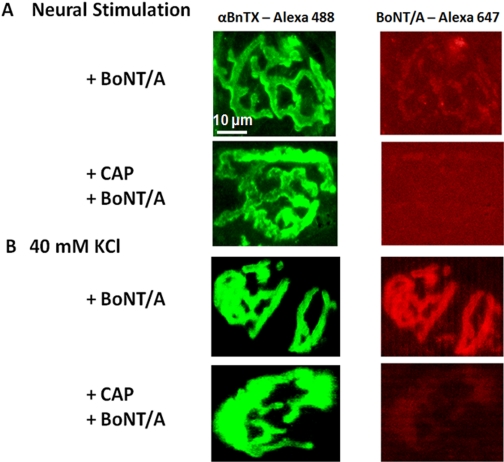
Studies with Alexa Fluor 647-BoNT/A suggested that capsaicin inhibited toxin uptake into cholinergic nerves. The left-hand column of Fig. 9 presents confocal images of motor end-plates in TS nerve-muscle preparations labeled with Alexa Fluor 488 α-BnTX. The right-hand column presents images of corresponding motor nerve endings labeled with Alexa Fluor 647-BoNT/A whose uptake was initiated in response to 1.5 h of 1-Hz nerve stimulation (A; n = 6) or incubation in 40 mM KCl (B; n = 6). These images suggest that a 30-min preincubation in 100 μM capsaicin prevents exocytosis-induced uptake of BoNT/A into motor nerve terminals. Similarly, 100 μM (Fig. 10) or 300 nM (Supplemental Fig. 1B) capsaicin pretreatment reduced BoNT/A uptake into Neuro 2a cells depolarized with 40 mM KCl.
Fig. 9.
Capsaicin pretreatment in vitro reduces BoNT/A uptake into motor nerve endings in response to 90 min of 1 Hz nerve stimulation (A) or incubation in 40 mM KCl (B). The left column illustrates representative fluorescence labeling of Triangularis sterni end-plate ACh receptors with Alexa Fluor 488 α-BnTX. The right column illustrates representative labeling of corresponding motor nerve endings with Alex Fluor 647 BoNT/A. Nerve stimulation and 40 mM KCl induced robust BoNT/A uptake into control motor nerve endings. In contrast, pretreatment with 100 μM capsaicin markedly reduced the amount of BoNT/A uptake.
Fig. 10.
Capsaicin pretreatment inhibits BoNT/A uptake into Neuro 2a cells. Representative fluorescence labeling of Neuro 2a cells with Alexa Fluor 647-BoNT/A for BoNT/A alone (A) and BoNT/A after CAP pretreatment (B). Left, BoNT/A was loaded by incubating cells with Alexa Fluor 647-BoNT/A as described in Materials and Methods. Right, corresponding brightfield images.
The preceding results show that capsaicin protects against BoNT/A. To test for a therapeutic effect, we injected mice with capsaicin at 12 h after BoNT/A when the TSR was clearly inhibited. The following four groups of mice were tested: control, + capsaicin, + BoNT/A, and + BoNT/A + capsaicin (n = 4–8). Figure 11A shows that body weight was consistently greater for mice that received capsaicin at 12 h after BoNT. Furthermore, although CMAP amplitude declined for BoNT/A alone and for BoNT/A + capsaicin-treated groups, a significantly greater degree of recovery was noted for the capsaicin-treated groups at 3 and 7 weeks after BoNT/A. That is, CMAP amplitude at 3 and 7 weeks was 0.3 ± 0.1 mV (n = 4), 2 ± 0.91 mV (n = 4) for BoNT/A-alone mice, compared with 1.2 ± 0.97 mV (n = 4) and 5.9 ± 1.23 (n = 4) for BoNT/A + capsaicin mice. CMAP amplitude was the same for control (7.8 ± 1.18 mV) and mice receiving capsaicin alone (7.6 ± 1.12 mV; n = 4). Figure 11D summarizes the magnitude of CMAP recovery from weeks 1 to 7 for BoNT/A alone and BoNT/A + capsaicin mice and suggests that capsaicin treatment after BoNT/A exposure slightly, but significantly, enhances recovery from BoNT/A-induced neuroparalytic effects.
Fig. 11.
Capsaicin treatment after BoNT/A poisoning improves weight gain and CMAP recovery. A, weight of mice in control (□), capsaicin alone (+ CAP, ■; n = 4), BoNT/A alone (+ BoNT/A, ○; n = 4), and BoNT/A and capsaicin (●, + BoNT/A + CAP; n = 4) groups plotted against day of test. B, CMAP amplitude (percentage of control) for control, capsaicin-alone, BoNT/A-alone, and BoNT/A + capsaicin-treated mice. CMAP amplitudes were normalized to the control values. Bars are the mean CMAP amplitude + S.E.M at various times after BoNT/A administration. C, representative CMAP records for control (a), capsaicin alone (b), BoNT/A alone (c, at 3 weeks; e, at 8 weeks), and capsaicin- and BoNT/A-injected mice (d, at 3 weeks; f, at 7 weeks). D, represents the average fold of increase in CMAP recovery ± S.E.M, from week 1 to week 7, after the injection of capsaicin.
Discussion
The search for antidotes to BoNT/A poisoning is complicated by the multistep process whereby the LC reaches its primary substrate within the motor nerve ending. This complexity undoubtedly contributes to the “disconnect” (Eubanks et al., 2007) between in vitro and in vivo observations that confounds the search for useful BoNT/A antidotes. That is, development of agents that target an apparently well understood step in BoNT/A action in a simplified system may mislead research aimed at antidote development. To minimize this disconnect, we focus in vivo and in vitro experiments on the cholinergic motor nerve ending that is the primary target of BoNT/A. Supportive studies are made of cholinergic Neuro 2a cells.
In this investigation, we examined the action of capsaicin on motor nerve terminal and Neuro 2a cell sensitivity to BoNT/A. The investigation was stimulated by several reports of interactions between capsaicin and clostridial toxins (Mantyh et al., 1996; Meng et al., 2007). Capsaicin is an agonist of the transient receptor potential channel protein of vanilloid subfamily, TRPV1. Mammalian homologues of Drosophila TRP channels are plasma membrane cation permeable channels that interact with a variety of intracellular proteins and macromolecular complexes (Montell, 2005). In this study, Western blotting demonstrated that adult mouse muscle expresses the capsaicin receptor, TRPV1. Furthermore, immunocytochemistry revealed TRPV1 on motor nerve endings and, in agreement with other investigators (Lakshmi and Joshi, 2005), on cholinergic Neuro 2a cells.
Our study did not detect changes of overt neuromuscular behavior, CMAP amplitude, twitch tension, or EPC amplitude of EDL nerve-muscle preparations removed at 24 h after injecting mice with capsaicin. Thus, further study is needed to explore the influence of TRPV1 channels on normal neuromuscular junction function. However, our study clearly demonstrated that capsaicin protects function of the neuromuscular junction against BoNT/A. That is, preinjection of capsaicin protected the TSR and performance on a rotating rod from the paralytic action of BoNT/A. These prophylactic effects of capsaicin were associated with partial protection against BoNT/A-induced inhibition of the neurally evoked CMAP. Thus, TSR and performance on a rotating rod can be normal even when BoNT/A inhibits transmission across a subpopulation of neuromuscular junctions. This dichotomy is also revealed at later time after BoNT/A administration when muscle performance was normal but CMAP amplitude had not yet fully recovered. In agreement with these in vivo measurements, we observed that capsaicin partially protected neurally evoked twitch tension from BoNT/A-induced weakness. Conversely, electrophysiological evaluation of superficial motor end-plates demonstrated that capsaicin prevented BoNT/A-induced reduction or failure of ACh release in response to 1, 20, 50, and 70 Hz of nerve stimulation. Similarly, capsaicin pretreatment preserved exocytosis-induced FM 1-43 uptake into Neuro 2a cells. Henkel et al. (1996) previously demonstrated that BoNT/A reduces FM 1-43 uptake into nerve endings of the mouse diaphragm muscle. Thus, capsaicin has an in vivo and in vitro action on cholinergic neurons which protects their exocytotic mechanisms from BoNT/A inhibition.
A possible prophylactic action of capsaicin is reduction of BoNT/A binding and/or uptake into cholinergic nerves (Kalandakanond and Coffield, 2001). To test for such an action, we examined uptake of fluorescently labeled BoNT/A into motor nerve terminals and Neuro 2a cells. For both cell types, brief preincubation in capsaicin reduced BoNT/A uptake. The data do not allow us to distinguish capsaicin-induced reduction of extracellular binding or endocytosis of BoNT/A. However, capsaicin failed to protect against the inhibitory effect of BoNT/A on FM 1-43 uptake into Neuro 2a cells that were incubated in Ca2+-free solution. In addition, the TRPV1 antagonist capsazepine inhibited capsaicin's protective effect. Thus, our working hypothesis is that capsaicin inactivates endocytotic events critical to BoNT/A uptake. It remains to be determined whether other endovanilloids (Starowicz et al., 2007) similarly protect the neuromuscular junction against BoNT/A.
The inhibition of BoNT/A uptake into motor nerve endings by capsaicin was not complete. This may explain the failure of in vivo pretreatment with capsaicin to completely protect CMAP amplitude and twitch tension from BoNT/A. Furthermore, capsaicin produced a window of protection that lasted for 12 h; BoNT/A administered at later times produced muscle inactivity that was indistinguishable from mice treated by BoNT/A alone. Thus, capsaicin concentration may have decreased from the extracellular synaptic space or the molecular events activated by TRPV1 terminated after 12 h.
Although this study suggests that capsaicin has a prophylactic action, recovery of CMAP amplitude after BoNT/A indicates that capsaicin may also have a therapeutic effect. That is, CMAP amplitude recovered more rapidly when capsaicin injection followed BoNT/A. However, understanding the mechanism of this enhanced recovery requires further study.
Effects of high capsaicin concentrations on the fluidity and elasticity of a large number of membrane proteins (Lundbaek et al., 2005) are not antagonized by capsazepine. Furthermore, both capsaicin and capsazepine promote inactivation of voltage-dependent Na+ channels by altering lipid bilayer elasticity. However, our data suggest that nonselective action is not responsible for the protective action of capsaicin against BoNT/A. That is, the protective effect of capsaicin was observed only in the presence of extracellular Ca2+ and antagonized by the TRPV1 antagonist capsazepine. In addition, we have observed an anti-BoNT/A effect of capsaicin at concentrations as low as 300 nM (Supplemental Fig. 1). These data strongly suggest that capsaicin exerts its protective effects via TRPV1 activation.
In summary, the presence of TRPV1 channels on motor nerve endings indicates the need to study their influence on the function and pathology of the neuromuscular junction. At the same time, the finding that activation of TRPV1 by capsaicin protects against BoNT/A suggests a novel mechanism for preventing and treating the action of this potent neurotoxin.
Supplementary Material
Acknowledgments
We thank Drs. Young Jin Son and Latha Potluri of Drexel University for assisting with the use of the upright confocal microscope at Drexel University to produce some of the images, and Dr. David Lagunoff and Peter Carroll of University of Medicine and Dentistry of New Jersey confocal core facility for assistance with use of the LSM-410 and LSM-510 confocal microscopes.
This work was supported by The Defense Threat Reduction Agency [Grant HDTRA1-07-C-0031 CBT BAA]; the National Institutes of Health [Grant 2R01-NS045979]; the Kirby Foundation; and the Toohey Foundation.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.109.156901
 The online version of this article (available at http://jpet.aspetjournals.org) contains supplemental material.
The online version of this article (available at http://jpet.aspetjournals.org) contains supplemental material.
- BoNT
- botulinum neurotoxin
- αBnTX
- alpha bungarotoxin
- BoNT/A
- botulinum neurotoxin A
- CAP
- capsaicin
- CPZ
- capsazepine
- CMAP
- compound muscle action potential
- EDL
- Extensor digitorum longus
- EPP
- end-plate potentials
- EPC
- end-plate currents
- MNE
- motor nerve ending
- TRPV1
- transient receptor potential protein 1 of vanilloid subfamily
- TS
- triangularis sterni
- TSR
- toe spread reflex
- ACh
- acetylcholine
- HC
- heavy chain
- LC
- light chain
- PBS
- phosphate-buffered saline
- YFP
- yellow fluorescence protein.
References
- Arnon et al., 2001.Arnon SS, Schechter R, Inglesby TV, Henderson DA, Bartlett JG, Ascher MS, Eitzen E, Fine AD, Hauer J, Layton M, et al. (2001) Botulinum toxin as a biological weapon: medical and public health management. J Am Med Assoc 285:1059–1070 [DOI] [PubMed] [Google Scholar]
- Benedetto, 1999.Benedetto AV. (1999) The cosmetic uses of botulinum toxin type A. Int J Dermatol 38:641–655 [DOI] [PubMed] [Google Scholar]
- Bilici et al., 2007.Bilici A, Karcaaltincaba M, Ilica AT, Bukte Y, Senol A. (2007) Treatment of hypertension from renal artery entrapment by percutaneous CT-guided botulinum toxin injection into diaphragmatic crus as alternative to surgery and stenting. AJR Am J Roentgenol 189:W143–W145 [DOI] [PubMed] [Google Scholar]
- Blasi et al., 1993.Blasi J, Chapman ER, Link E, Binz T, Yamasaki S, De Camilli P, Südhof TC, Niemann H, Jahn R. (1993) Botulinum neurotoxin A selectively cleaves the synaptic protein SNAP-25. Nature 365:160–163 [DOI] [PubMed] [Google Scholar]
- Brunger et al., 2007.Brunger AT, Breidenbach MA, Jin R, Fischer A, Santos JS, Montal M. (2007) Botulinum neurotoxin heavy chain belt as an intramolecular chaperone for the light chain. PLoS Pathog 3:1191–1194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong et al., 2006.Dong M, Yeh F, Tepp WH, Dean C, Johnson EA, Janz R, Chapman ER. (2006) SV2 is the protein receptor for botulinum neurotoxin A. Science 312:592–596 [DOI] [PubMed] [Google Scholar]
- Eubanks et al., 2007.Eubanks LM, Hixon MS, Jin W, Hong S, Clancy CM, Tepp WH, Baldwin MR, Malizio CJ, Goodnough MC, Barbieri JT, et al. (2007) An in vitro and in vivo disconnect uncovered through high-throughput identification of botulinum neurotoxin A antagonists. Proc Natl Acad Sci U S A 104:2602–2607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henkel et al., 1996.Henkel AW, Lübke J, Betz WJ. (1996) FM1-43 dye ultrastructural localization in and release from frog motor nerve terminals. Proc Natl Acad Sci U S A 93:1918–1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalandakanond and Coffield, 2001.Kalandakanond S, Coffield JA. (2001) Cleavage of SNAP-25 by botulinum toxin type A requires receptor-mediated endocytosis, pH-dependent translocation, and zinc. J Pharmacol Exp Ther 296:980–986 [PubMed] [Google Scholar]
- Lakshmi and Joshi, 2005.Lakshmi S, Joshi PG. (2005) Co-activation of P2Y2 receptor and TRPV channel by ATP: implications for ATP induced pain. Cell Mol Neurobiol 25:819–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundbaek et al., 2005.Lundbaek JA, Birn P, Tape SE, Toombes GE, Søgaard R, Koeppe RE, 2nd, Gruner SM, Hansen AJ, Andersen OS. (2005) Capsaicin regulates voltage-dependent sodium channels by altering lipid bilayer elasticity. Mol Pharmacol 68:680–689 [DOI] [PubMed] [Google Scholar]
- Mantyh et al., 1996.Mantyh CR, Pappas TN, Lapp JA, Washington MK, Neville LM, Ghilardi JR, Rogers SD, Mantyh PW, Vigna SR. (1996) Substance P activation of enteric neurons in response to intraluminal Clostridium difficile toxin A in the rat ileum. Gastroenterology 111:1272–1280 [DOI] [PubMed] [Google Scholar]
- McArdle et al., 1981.McArdle JJ, Angaut-Petit D, Mallart A, Bournaud R, Faille L, Brigant JL. (1981) Advantages of the triangularis sterni muscle of the mouse for investigations of synaptic phenomena. J Neurosci Methods 4:109–115 [DOI] [PubMed] [Google Scholar]
- Meng et al., 2007.Meng J, Wang J, Lawrence G, Dolly JO. (2007) Synaptobrevin I mediates exocytosis of CGRP from sensory neurons and inhibition by botulinum toxins reflects their anti-nociceptive potential. J Cell Sci 120:2864–2874 [DOI] [PubMed] [Google Scholar]
- Montal et al., 1992.Montal MS, Blewitt R, Tomich JM, Montal M. (1992) Identification of an ion channel-forming motif in the primary structure of tetanus and botulinum neurotoxins. FEBS Lett 313:12–18 [DOI] [PubMed] [Google Scholar]
- Montell, 2005.Montell C. (2005) The TRP superfamily of cation channels. Sci STKE 2005 [DOI] [PubMed] [Google Scholar]
- Oguma et al., 1995.Oguma K, Fujinaga Y, Inoue K. (1995) Structure and function of Clostridium botulinum toxins. Microbiol Immunol 39:161–168 [DOI] [PubMed] [Google Scholar]
- Schnider et al., 1994.Schnider P, Schmied M, Berger T, Auff E. (1994) [Therapeutic use of botulinum A toxin in neurology]. Wien Klin Wochenschr 106:335–344 [PubMed] [Google Scholar]
- Schulte-Mattler, 2008.Schulte-Mattler WJ. (2008) Use of botulinum toxin A in adult neurological disorders: efficacy, tolerability and safety. CNS Drugs 22:725–738 [DOI] [PubMed] [Google Scholar]
- Scott, 2004.Scott AB. (2004) Development of botulinum toxin therapy. Dermatol Clin 22:131–133 [DOI] [PubMed] [Google Scholar]
- Simpson, 1981.Simpson LL. (1981) The origin, structure, and pharmacological activity of botulinum toxin. Pharmacol Rev 33:155–188 [PubMed] [Google Scholar]
- Souayah et al., 2006.Souayah N, Karim H, Kamin SS, McArdle J, Marcus S. (2006) Severe botulism after focal injection of botulinum toxin. Neurology 67:1855–1856 [DOI] [PubMed] [Google Scholar]
- Starowicz et al., 2007.Starowicz K, Maione S, Cristino L, Palazzo E, Marabese I, Rossi F, de Novellis V, Di Marzo V. (2007) Tonic endovanilloid facilitation of glutamate release in brainstem descending antinociceptive pathways. J Neurosci 27:13739–13749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuboi et al., 2002.Tsuboi M, Furukawa Y, Kurogouchi F, Nakajima K, Hirose M, Chiba S. (2002) Botulinum neurotoxin A blocks cholinergic ganglionic neurotransmission in the dog heart. Jpn J Pharmacol 89:249–254 [DOI] [PubMed] [Google Scholar]
- Van Beek et al., 2007.Van Beek AL, Lim PK, Gear AJ, Pritzker MR. (2007) Management of vasospastic disorders with botulinum toxin A. Plast Reconstr Surg 119:217–226 [DOI] [PubMed] [Google Scholar]
- Vyklicky et al., 2008.Vyklicky L, Novakova-Tousova K, Benedikt J, Samad A, Touska F, Vlachova V. (2008) Calcium-dependent desensitization of vanilloid receptor TRPV1: a mechanism possibly involved in analgesia induced by topical application of capsaicin. Physiol Res 57 (Suppl 3):S59–S68 [DOI] [PubMed] [Google Scholar]
- Yowler et al., 2002.Yowler BC, Kensinger RD, Schengrund CL. (2002) Botulinum neurotoxin A activity is dependent upon the presence of specific gangliosides in neuroblastoma cells expressing synaptotagmin I. J Biol Chem 277:32815–32819 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.