Abstract
G protein-coupled receptors (GPCRs) are one of the most important classes of proteins in the genome, not only because of their tremendous molecular diversity but because they are the targets of nearly 50% of current pharmacotherapeutics. The majority of these drugs affect GPCR activity by binding to a similar molecular site as the endogenous cognate ligand for the receptor. These “orthosterically” targeted drugs currently dominate the existing pharmacopeia. Over the past two decades, novel opportunities for drug discovery have risen from a greater understanding of the complexity of GPCR signaling. A striking example of this is the appreciation that many GPCRs possess functional allosteric binding sites. Allosteric modulator ligands bind receptor domains topographically distinct from the orthosteric site, altering the biological activity of the orthosteric ligand by changing its binding affinity, functional efficacy, or both. This additional receptor signaling complexity can be embraced and exploited for the next generation of GPCR-targeted therapies. Despite the challenges associated with detecting and quantifying the myriad of possible allosteric effects on GPCR activity, allosteric ligands offer the prospect of engendering a facile stimulus-bias in orthosteric ligand signaling, paving the way for not only receptor-selective but also signaling pathway-selective therapies. Allosteric modulators possess specific advantages when considering the treatment of multifactorial syndromes, such as metabolic diseases or age-related cognitive impairment, because they may not greatly affect neurotransmitter or hormone release patterns, thus maintaining the integrity of complex signaling networks that underlie perception, memory patterns, or neuroendocrinological axes while introducing therapeutically beneficial signal bias.
Heptahelical GPCRs are ubiquitously expressed throughout eukaryotic organisms and can account for as much as 3 to 4% of the genome (Foord, 2002). By detecting ligands in the extracellular milieu, they transmit environmental information from outside a cell to the interior. At the same time, changes in the expression of GPCRs or receptor-associated regulatory proteins under varying physiological conditions can preprogram cells to respond in certain ways to external stimuli (Maudsley et al., 2004). GPCRs have evolved to allow cellular systems to sense their environment through selective recognition of almost every type of agent, e.g., photons, odorants, lipids, amino acids, carbohydrates, and complex polypeptides. Because of this unparalleled flexibility and their involvement in most of the physiological processes in an organism, it is not surprising that GPCRs have proven to be effective pharmacological drug targets. Appreciation of the functional complexity of GPCR biology in recent years has increased exponentially. It is now accepted that GPCRs interact with many proteins to exert their full range of activities (Brady and Limbird, 2002: Maudsley et al., 2004, 2007) and that, as with other transmembrane receptors (e.g., ligand-gated ion channels), they are subject to modulation by ligands that can act independently or cooperatively with the endogenous cognate ligands. This latter aspect of GPCR signaling, i.e., allosteric receptor modulation, will be the primary subject of this review as this capacity to synergize with the endogenous agents and their more subtle mode of activity makes allosteric modulators (AMs) prototypes for the next generation of therapeutics to treat complex disorders that affect multiple aspects of peripheral and central nervous tissue function (Christopoulos, 2002; Leach et al., 2007; Lewis et al., 2008; Conn et al., 2009).
Orthosteric and Allosteric Modulation of Heptahelical GPCRs
Modern classification of heptahelical GPCRs subdivides the superfamily into five major functional groups—glutamate receptors, rhodopsin-like receptors, adhesion family receptors, frizzled/taste receptors, and secretin-like receptors (Schiöth and Fredriksson, 2005)—that collectively mediate the cellular sensation of the most environmentally derived and endogenous somatic compounds. The rhodopsin-like class of GPCRs includes some of the most studied proteins in nature. Many of the GPCRs in this class have been subjected to extensive structure-function analysis by site-directed mutagenesis. Most rhodopsin-like GPCRs possess a distinctive orthosteric binding site (i.e., domain involved in docking of the endogenous ligand with the receptor), either deep within the helical bundle for small ligands (e.g., biogenic amines) or superficially across the extracellular loops and surface helical regions for larger ligands (e.g., small neuropeptides). This orthosteric binding site facilitates high-affinity ligand binding and allows transduction of the stimulus to the interior of the cell. It has been shown for most endogenous ligands that binding to this site initiates the majority of signaling activity associated with receptor-ligand engagement. Orthosteric ligand GPCR activation classically transmits a signal, mediated by conformational rearrangement, across the plasma membrane to the intracellular domains of the receptor. By contrast, AMs do not directly engage the orthosteric site. The binding of an AM may cause a conformational change in the receptor protein that is transmitted to the orthosteric site (and vice versa), in essence creating a “new” GPCR with its own set of binding and functional properties. In addition, AMs may engender collateral efficacy by biasing the stimulus, thus leading to signaling-pathway-selective allosteric modulation (either enhancement or blockade). In the context of pharmaceutical development, AMs are generally thought of as exogenous compounds, often small molecules that bind a region of the receptor that is distant from the native orthosteric site. However, it is important to consider that GPCRs interact with numerous intracellular proteins (e.g., heterotrimeric G proteins) that also affect receptor conformation. Thus, in the broad context, allosteric modulation of GPCR function can arise from the association of accessory proteins with the internal face of the receptor (Maudsley et al., 2005) or through AM interaction with intracellular binding sites (Espinoza-Fonseca and Trujillo-Ferrara, 2006). To understand the role of AMs in controlling GPCR responses to ligand-induced conformational changes, we shall first consider dynamic models of GPCR function.
Modeling Allosteric Modulation
In classical dynamic models of GPCR function, the receptor transmits the orthosteric ligand signal by functioning as a ligand-activated guanine nucleotide exchange factor for juxtamembrane heterotrimeric G proteins. G protein activation is initiated through ligand-driven changes in the tertiary structure of the transmembrane heptahelical receptor core (Ballesteros and Palczewski, 2001; Shapiro et al., 2002). These conformational changes are transmitted to the intracellular transmembrane loops and carboxyl terminus and alter the ability of the receptor to catalyze the rapid exchange of GDP for GTP on the heterotrimeric G protein α-subunit. The GTP-bound α-subunit then can stimulate its cognate downstream effectors, e.g., phospholipase C or adenylate cyclase, conveying information about the presence of the stimulus in the extracellular environment. In this basic conceptualization, the GPCR functions as a switch, existing in either an “off” or “on” state. Evidence that GPCR behavior is more complex originated with the finding that β-adrenergic receptors exhibit two affinity states for agonists, the relative proportions of which are modulated by the presence of guanine nucleotides (DeLean et al., 1980). The model advanced to explain these phenomena predicted that, in the presence of GDP, agonist binding promotes the formation of a long-lived ternary complex among agonist (H), GPCR (R), and heterotrimeric G protein (G) that exhibits high agonist binding affinity. In the absence of the G protein or when the presence of GTP allows for receptor-catalyzed G protein activation, the H-R-G complex is dissociated, and the receptor resides in a low-affinity (H-R) state. However, even this simplistic model accommodates a wide variety of orthosteric effects. Ligands can act as positive agonists (stimulating G protein turnover), inverse agonists (reducing constitutive G protein activation by the unliganded receptor), partial agonists (exhibiting lower intrinsic efficacy than a full agonist), or classical antagonists (binding the orthosteric site without G protein activation).
Along with the increasing complexity of orthosteric ligand-receptor interactions, the past decade has witnessed an increase in the number of potential therapeutic ligands that target GPCRs by binding to allosteric sites on the receptor. AMs may increase or decrease the ability of the orthosteric ligand to interact with the receptor and/or modulate its ability to stabilize the active conformation of the receptor. Although both modulatory processes may occur simultaneously, the most commonly observed AM effect is modulation of orthosteric ligand affinity. As with the ternary complex model for orthosteric ligand-receptor interactions, models have been generated for AM interactions (May et al., 2004). One model designed to quantify AM activity is described as the allosteric ternary complex model (ATCM; Fig. 1A). The ATCM is the simplest mass-action scheme applied to allosteric interactions, and its properties at equilibrium have been used to derive quantitative models for AM activity simulation (Stockton et al., 1983; Ehlert, 1988; Christopoulos and Kenakin, 2002). The ATCM can be used to quantify AM activity in terms of ligand affinity for the unoccupied receptor and its cooperativity factor (α). The cooperativity factor is a thermodynamic measure of the strength and direction of the allosteric change in affinity for one site when the other is occupied. Allosteric modulators can be broadly grouped as either positive AMs (PAMs, α > 1) or as negative AMs (NAMs, α < 1). For example, the binding of the orthosteric antagonist N-methylscopolamine (NMS) to the M2-muscarinic acetylcholine receptor (mAChR) is allosterically enhanced by alcuronium (Avlani et al., 2004) but is allosterically inhibited by gallamine, even though both AMs bind to a common allosteric site on the receptor (Lanzafame et al., 1997).
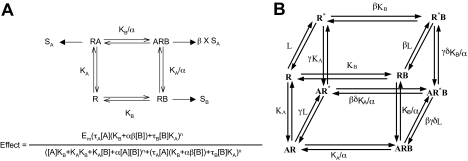
Fig. 1.
The basic and cubic allosteric ternary complex mechanisms. A, the ATCM, a concise framework for modeling the interaction of two ligands (e.g., an orthosteric agonist A and an allosteric modulator, B) on a receptor, in terms of their respective equilibrium dissociation constants (KA and KB), and a cooperativity factor, α, that denotes the magnitude and direction of the allosteric effect on ligand binding affinity. Stimulus is assumed to be imparted to the cell by the orthosteric agonist binding receptor (AR) and the modulator-orthosteric agonist binding species (ARB), and an additional proportionality factor, β, may be added to account for modulator-induced alterations in efficacy. B, the allosteric CTC model. This model allows the active state to interact with G protein and the inactive R* state. This model is formally identical with the allosteric two-state model of Hall (2000), which describes the interaction of an allosteric modulator and orthosteric ligand on a receptor that can adopt active and inactive conformations. The terms β, γ, and δ (for the CTC model) are ligand-related and describe the change in the receptor affinity, for the G protein, imparted by the ligand.
Beyond effects on orthosteric ligand affinity, AMs can produce changes in the intrinsic efficacy of the receptor-orthosteric ligand complex. This property is exemplified by a series of modulators of cannabinoid CB1 receptors. The allosteric modulator Org27569 enhances the binding of the orthosteric agonist CP55940 at mouse CB1 receptors but significantly reduces the efficacy of the orthosteric agonist WIN552122 for inhibition of electrically evoked contractions in a mouse vas deferens preparation and the efficacy of CP55940 at human CB1 receptors in a reporter-gene assay (Price et al., 2005). To accommodate these effects, an allosteric two-state model has been proposed that provides an additional cooperativity factor governing the transition of the receptor between a resting (R) and an activated (R*) state in the presence of an allosteric ligand, the allosteric cubic ternary complex (CTC) model (Fig. 1B).
Although most allosteric GPCR modulators are pharmacologically quiescent in the absence of an orthosteric ligand, it has been noted that some allosteric ligands, termed “ago-allosteric” modulators, act as agonists in their own right (Knudsen et al., 2006). Such “allosteric agonists” further expand the number of possible receptor-ligand interactions because they have the potential to modulate orthosteric ligand pharmacology in addition to perturbing cellular signaling in their own right. Two mAChR ligands suggested to act this way are the functionally selective partial agonists, McN-A-343 and AC-42. In addition to engendering partial agonist effects, they produce incomplete inhibition of the binding of the orthosteric antagonist NMS when present at saturating concentrations at rat M2 (McN-A-343) and human M1 (AC-42) mAChRs while retarding NMS dissociation (Langmead et al., 2006; Valant et al., 2008). It is noteworthy that it is also possible for a ligand to bind to an allosteric site without altering orthosteric regulation of receptor function, in effect acting as a “neutral” antagonist at the allosteric site.
Allosteric Receptor Modulation by GPCR Accessory Proteins
GPCRs are naturally allosteric proteins that interact with numerous other proteins that alter their ligand-binding affinity or signaling properties. In effect, heterotrimeric G proteins are AMs, in that they alter ligand affinity by contacting the receptor at a topographically distant site from the orthosteric binding site. Numerous other proteins interact with the intracellular face or transmembrane regions of GPCRs. GPCR-interacting proteins include kinases (e.g., G protein-coupled receptor kinases and protein kinase A) (Fraser et al., 2000), arrestins (Pfister et al., 1985), the 4.1 family of cytoskeletal proteins (e.g., ABP-280) (Li et al., 2000), amyloid precursor like protein 1 (APLP1) (Weber et al., 2006), receptor activity-modifying proteins/calcitonin gene-related peptide-receptor component protein (McLatchie et al., 1998; Evans et al., 2000), and PDZ-domain containing proteins (Hall et al., 1998). These interacting proteins influence GPCR signaling by regulating downstream effectors and by participating in scaffolding, endocytosis, trafficking, or recycling of the receptor (Engström et al., 2006; Tobin, 2008). For example, the interaction of the vasoactive intestinal polypeptide-pituitary adenylate cyclase-activating peptide receptor (VPAC1R) with the accessory protein receptor-activity-modifying protein 2 enhances Gq/11-mediated phosphoinositide accumulation while leaving receptor coupling to the Gs-coupled cAMP response essentially unaltered (Conner et al., 2005). Another example is the binding of neurochondrin to the C-terminal tail of the melanin-concentrating hormone receptor (MCHR1), which inhibits agonist stimulation of Gi/o and Gq/11 signaling but not agonist-induced internalization (Francke et al., 2006). In principal, sites of interaction with accessory proteins are potential points at which GPCRs can be allosterically manipulated, and any agent that targets one of these accessory proteins or their site of interaction with the receptor could be considered a functional AM.
A compelling example of such “indirect” allosteric modulation involves the regulation of GPCR heterodimers, wherein an orthosteric or allosteric ligand for one receptor modulates signaling of the dimer partner through conformational changes transmitted by contact between receptor transmembrane domains. It is now clear that most, if not all, GPCRs are able to form oligomers as either homodimers or heterodimers with other GPCRs. Dimerization affects many aspects of GPCR function, including subcellular trafficking and signaling. The first widely accepted demonstration involved the metabotropic GABAB receptor, which functions as an obligate heterodimer. The receptor is composed of two isoforms GABAB-1 and GABAB-2 that are nonfunctional when expressed individually but become functional when coexpressed, given that heterodimerization is required for post-translational trafficking to the plasma membrane (Jones et al., 1998; Kaupmann et al., 1998; Ng et al., 1999). Receptor heterodimerization also affects ligand binding. For example, positive cooperativity has been reported for the ligand binding of δ- and κ-opioid receptors when coexpressed (Jordan and Devi, 1999). Conversely, negative cooperativity in dopamine D2 receptor agonist binding in the presence of an adenosine A2 receptor agonist has been observed when the receptors were coexpressed (Franco et al., 2000). In practical terms, this means that the orthosteric ligand binding site of one receptor acts as an allosteric site for the heterodimer partner. In the context of μ-δ-opioid receptor dimers, antagonist occupancy of δ receptors enhances μ-opioid receptor agonist binding and signaling in vitro, and δ-opioid antagonists enhance morphine-induced analgesia in vivo (Gomes et al., 2004). Allosteric antagonism of GPCR heterodimers is also possible. In murine cardiomyocytes, antagonism of β-adrenergic receptors inhibits angiotensin AT1a receptor-mediated contractility and vice versa (Barki-Harrington et al., 2003). This phenomenon arises within β2-adrenergic/AT1a receptor heterodimers, wherein each receptor is uncoupled from its cognate G proteins when its heterodimer partner is bound to an orthosteric antagonist. Such a requirement for dual receptor occupancy may be a common phenomenon. For example, the interaction of M3AChR dimers with β-arrestin-1 and the subsequent activation of mitogen-activated protein kinase requires agonist binding to each receptor protomer (Novi et al., 2005).
Given the evidence that GPCRs form hetero-oligomers with unique ligand-binding and signaling properties, it is tempting to hypothesize that AMs might influence GPCR signaling in a multitude of ways, from changing ligand affinity/selectivity to altering downstream coupling preference or targeting other protomer receptors or accessory proteins to the complex. Allosteric regulation within di(oligo)mers implies that the pharmacological properties of a given receptor subtype can be influenced by the array of dimerization partners coexpressed in each particular cell type (George et al., 2000). Future work will be required to identify, at the molecular level, the conformational changes involved in these allosteric interactions, explain how agonists and antagonists exert positive and negative cooperative effects on dimer partners, and how these allosteric effects can be exploited to change the “texture” of these complex and diverse GPCR signaling states.
Allosteric Modulation of GPCR Functional Selectivity
Despite their utility in describing orthosteric signaling, it is now clear that two-state models cannot predict the full range of orthosteric ligand effects. Many ligands that behave as inverse agonists or classical “neutral” antagonists for one effector pathway have been found to exert opposing effects, e.g., agonist or partial agonist activity, for receptor coupling to an alternative G protein or G protein-independent pathway (Maudsley et al., 2000; Zhang and Neer, 2001; Maudsley et al., 2004). The phenomenon of ligand coupling a receptor to only a subset of its potential effectors is known by several different terms, including functional selectivity, agonist-directed trafficking of receptor stimulus, biased agonism, differential engagement, and stimulus trafficking (Berg et al., 1998; Bonhaus et al., 1998; Brink et al., 2000; MacKinnon et al., 2001; Kenakin, 2002, 2003).
The linkage between functional selectivity and receptor conformational state has been known for at least a decade. The phenomenon of reversal of potency, wherein a series of orthosteric agonists interacting with the same receptor exhibit different rank orders of potency when compared by using two or more readouts of receptor activation, implies that GPCRs can exist in more than one “active” conformation (Perez et al., 1996; Palanche et al., 2001; Galandrin and Bouvier, 2006). Evidence of the existence of distinct active receptor conformations has come from multiple sources using a wide array of receptor paradigms (Mundell et al., 1997, Maudsley et al., 1998, Kohout et al., 2004) and biophysical techniques (Ghanouni et al., 2001; Audet et al., 2008). The recent discovery of G protein-independent signals, e.g., transmitted by arrestin-bound signaling proteins (Luttrell et al., 1999), has led to even more dramatic examples of ligands that exhibit true reversal of efficacy. For example, the parathyroid hormone (PTH) analog, [d-Trp12, Tyr34]-PTH(7–34), acts as an inverse agonist for PTH1 receptor coupling to Gs-adenylyl cyclase and has no intrinsic efficacy for Gq/11-coupling but behaves as an agonist with respect to arrestin recruitment, receptor internalization, and arrestin-dependent extracellular signal-regulated kinase activation (Gesty-Palmer et al., 2006). Such behavior can only be modeled on the basis of multiple active receptor conformations and underscores the potential for pharmacologically biasing signal output.
AM binding may also affect receptor conformation to favor certain active states or change the interaction of the receptor with other juxtamembrane or transmembrane proteins, biasing the signal output generated by endogenous orthosteric ligands or even creating new “flavors” of receptor with unique functional properties (Maudsley et al., 2005). For example, in cortical astrocytes, an AM of the metabotropic glutamate receptor (mGluR) type 5, N-{4-chloro-2-[(1,3-dioxo-1,3-dihydro-2H-isoindol-2-yl) methyl] phenyl}-2-hydroxybenzamide (CPPHA), potentiates calcium mobilization by the orthosteric agonist 3,3′-difluorobenzaldazine but decreases the maximal extracellular signal-regulated kinase activation stimulated by the same orthosteric agonist (Zhang et al., 2005). Signaling pathway-selective effects could also enable AMs to generate “collateral” efficacy, offering the potential for “selecting” desired pharmacological effects and excluding nondesired effects by targeting allosteric sites. Thus, the binding of the PAM peptide ASLW to the CXCR4 chemokine receptor induces a stronger chemotactic immune cell response than the orthosteric ligand, CXCL12, but does not promote receptor internalization like CXCL12 (Sachpatzidis et al., 2003).
Designing Allosteric Modulators
Unlike orthosteric agents, the discovery of AMs has primarily been through the use of functional assays. Because many allosteric recognition sites lie outside of the classical orthosteric regions, the recent elucidation of higher resolution molecular GPCR structures and refined structural models (Rasmussen et al., 2007) is likely to aid the design of the next generation of AMs. Using such high-resolution crystal structures, the commonalities of AM binding pockets may become more apparent. However, there are specific considerations that are pertinent to the discovery process for AMs, e.g., the correct attribution of insurmountable drug effects to orthosteric or allosteric effects using well defined experimental criteria (Kenakin et al., 2006). An improved molecular dynamic understanding of the true range of functional activities of GPCRs via the use of techniques such as bioluminescence resonance energy transfer (Angers et al., 2000), optical dynamic mass redistribution (Kebig et al., 2009), and GPCR cellular impedance assays (Peters and Scott, 2009) will undoubtedly also prove valuable for future AM design. In recent years, significant progress in the rational design of AM agents has been made, especially for muscarinic acetylcholine, metabotropic glutamate, adenosinergic, and GABAergic receptors. Past attempts at therapeutic targeting of muscarinic acetylcholine receptors with orthosteric agents have largely failed due to poor receptor subtype selectivity. Recently, multiple AMs displaying excellent subtype selectivity for M1 muscarinic receptors have been developed, as well as two agents for the M4 subtype (Lazareno et al., 2004; Shirey et al., 2008). Perhaps paving the way for future AM design, the latter M4-selective agent, VU100010, was designed from a pre-existing compound (LY2033298) from Eli Lilly and Company (Indianapolis, IN) using a medicinal chemistry-chemoinformatic hybrid approach.
The ability to predict structure-activity relationships in AMs is in its infancy, although recent work with metabotropic glutamate receptors has begun to provide insight. Recently developed AMs for mGluR1 (Knoflach et al., 2001; Zheng et al., 2005; Wu et al., 2007), mGluR2 (Johnson et al., 2005), mGluR4 (Stachowicz et al., 2004), and mGluR5 (Lindsley et al., 2004; Roppe et al., 2004; Kinney et al., 2005; Porter et al., 2005; Zhao et al., 2007) have shown in vitro or clinical efficacy for chronic pain, anxiety, Parkinson's disease, and schizophrenia. Several of these series, most notably the PAMs (−)-PHCCC (mGluR4) (Stachowicz et al., 2004) and CPPHA (mGluR5) (Zhao et al., 2007) demonstrate a surprisingly sensitive structure-activity relationship, because even a slight modification of the parent compound almost invariably results in inactive derivatives. Whether such features are characteristic of PAMs compared with NAMs, in general, remains to be seen, but these distinctions may be indicative of a qualitative difference in the pharmacological nature of the dynamic relationship of PAM interaction with GPCRs compared with NAMs. From this finding, one could posit that there may be greater molecular diversity for NAMs and therefore a greater number of potential NAM binding sites on GPCRs.
The sheer molecular diversity of AMs adds an additional layer of complexity to compound design and screening programs. Unlike orthosteric ligands, which are structural mimetics of endogenous compounds that evolved to confer fidelity in receptor activation, AMs have much greater potential to produce off-target effects via receptor or nonreceptor interactions. There are many examples of allosteric modulating agents that possess other significant pharmacological functions. Although its therapeutic effect results from blockade of epithelial sodium channels, the diuretic amiloride also exerts an allosteric effect at α2A/2B adrenergic receptors (Leppik and Birdsall, 2000). The cyclooxygenase inhibitor salicylic acid not only acts via modulation of the prostanoid system, it also allosterically modulates the endothelin A receptor (Talbodec et al., 2000). The endogenous soporific lipid, oleamide, is both an endogenous cannabinoid CB1 receptor agonist and a potent allosteric modulator of multiple serotonin receptor subtypes (Thomas et al., 1997). The greater potential for cross reactivity inherent in small molecules needs to be considered both when screening AMs for activity and when attempting to ascribe physiologic effects on complex systems to a specific receptor target.
Allosteric Modulators in the Clinic
The two AMs that have been approved to date for clinical use illustrate the diverse effects attainable through allosteric modulation of GPCR function. The first to enter the market was Cinacalcet, a PAM of the calcium sensing receptor (CaSR) approved for treatment of secondary hyperparathyroidism and parathyroid carcinoma (Goodman et al., 2002; Nemeth et al., 2004; Szmuilowicz and Utiger, 2006). In chronic kidney disease, loss of renal 1α hydroxylation of [25-OH]-vitamin D2 impairs intestinal calcium absorption, leading to chronic hypocalcemia. This reduces tonic inhibition of PTH secretion by the CaSR in the parathyroid glands, leading to excessive PTH secretion. Elevated levels of PTH, the hallmark of secondary hyperparathyroidism, are associated with altered metabolism of calcium and phosphorus, bone pain, fractures, and an increased risk for cardiovascular death. Cinacalcet increases CaSR affinity for calcium, and effectively suppresses PTH secretion despite hypocalcemia. Conversely, Maraviroc (UK-427, 857) is a noncompetitive allosteric antagonist of the chemokine receptor, CCR5, that was recently approved as salvage therapy in advanced HIV disease (Wood and Armour, 2005). CCR5 acts as the cell surface coreceptor for the HIV virus. Maraviroc binding to CCR5 alters receptor conformation so as to block HIV binding, reducing HIV infectivity and producing a marked fall in systemic viral load (Rosario et al., 2008).
Allosteric Modulators in the Treatment of Complex Disorders
Over 90% of nonsensory GPCRs are expressed in the brain, where they regulate myriad neuronal and endocrine functions. Exploiting GPCR pharmacology to develop treatments for age-related conditions such as dementia will require a more advanced understanding of the complex changes in neuroendocrine interactions that occur with the aging process (Martin et al., 2008). Because of the immense complexity and interconnectedness of central nervous system signaling networks it is likely that therapeutic agents will need to produce highly specific and perhaps subtle changes. It is in these areas that the pleiotropic nature of AM actions offer potential advantages over orthosteric ligands (Fig. 2).
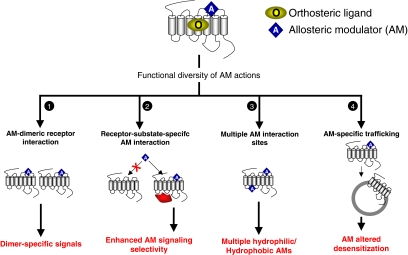
Fig. 2.
Multiplicity of activities of GPCR-targeted allosteric modulating agents. There are multiple functional mechanisms thought to underlie the pleiotropy of AM. Compared with conventional orthosteric ligands, AMs may be more sensitive to subtle alterations in GPCR function induced by receptor dimerization (1) or interaction with intracellular scaffolding proteins (2). This sensitivity may influence the signal-conditioning effects of the AM. The identification of multiple extracellular or intracellular functional binding sites for AMs on GPCRs (3) lends the capacity to target site-specific compounds based on their ability to partition into the plasma membrane. Not only can AMs modify direct stimulatory signaling processes induced by the orthosteric ligand but they may also control the directionality of tachyphylactic mechanisms as well such as homologous desensitization (4).
One significant barrier to orthosteric drug design is the difficulty of achieving selective drug targeting to well conserved and singular orthosteric binding regions. Although GPCRs respond to the widest range of compounds of any ‘receptive’ biological system, most are clustered into groups of closely related receptors that share a common endogenous ligand. The muscarinic M1–M5 receptors, metabotropic mGlu1–mGlu8 receptors, and the approximately 13 members of the serotonin 5-HT receptor family exemplify this property. While selectivity between families of receptors that bind structurally distinct ligands is usually achievable (e.g., a compound that interacts with mGluRs but not 5-HT receptors), it is often difficult to obtain subtype selectivity among members of an individual family using orthosteric ligands. A high degree of conservation in the amino acid sequences coding for the orthosteric binding site across all subtypes within a receptor family often precludes discovery of highly selective compounds. Despite much effort by medicinal chemists, a number of traditional orthosteric agonists of the muscarinic acetylcholine receptor evaluated in clinical trials for the treatment of Alzheimer's disease, including milameline, sabcomeline, cevimeline, and talsaclidine, have all shown therapeutic efficacy but ultimately failed because of poor subtype selectivity and associated side effects. In contrast, AMs can exhibit exquisite selectivity between closely related receptors (Lazareno et al., 1998; Ellis and Seidenberg, 2000). One reason for this may be that allosteric sites are placed under less evolutionary pressure with respect to conservation of function and thus display wider protein sequence divergence across receptor subtypes relative to orthosteric sites (Bridges and Lindsley, 2008). For example thiochrome binds to an allosteric site on all five subtypes of mAChR, but selectively enhances the affinity of acetylcholine only at the M4AChR because the cooperativity between the orthosteric and the allosteric sites is neutral (α = 1) at all other subtypes (Lazareno et al., 2004). Likewise, agents such as the M1/M4AChR allosteric agonist xanomeline and the M1AChR allosteric agonists AC-42, N-desmethylclozapine, and 1-(1′-2-methylbenzyl)-1,4′-bipiperidin-4-yl)-1H-benzo[d]imidazol-2(3H)-one have displayed unprecedented subtype selectivity (Spalding et al., 2002; Mirza et al., 2003; Sur et al., 2003; Jones et al., 2008).
In addition to these novel M1-selective allosteric agonists, exciting progress has been made in the discovery of novel PAMs of the M1AChR. For instance, VU0090157, VU0029767, and BQCA were recently identified in a high-throughput functional screening program (Marlo et al., 2009). None of these M1AChR PAMs had agonist activity or competed for binding at the orthosteric acetylcholine binding site. However, each induced parallel leftward shifts in acetylcholine affinity, indicating that they enhance M1AChR activity by increasing affinity for the orthosteric ligand. Furthermore, VU0090157 produced equivalent potentiation of M1AChR activation of phospholipase C and phospholipase D activity, whereas VU0029767 enhanced phospholipase C, but not phospholipase D, signaling. This illustrates another potential advantage of AMs, the ability to bias receptor output by differentially regulating receptor coupling to downstream pathways. As with VU0090157 and VU0029767, BQCA has no activity at M2, M3, or M4AChRs. In a contextual fear-conditioning model of episodic-like memory, BQCA fully reverses the cognitive impairment induced by scopolamine through a possible cognition-enhancing effect (Marion et al., 2008; Marlo et al., 2009). These findings suggest that it will be possible to develop highly selective M1AChR PAMs with potential benefit in cognitive disorders. Other subtype-selective AMs have shown promise in modifying cortical brain function. LY2033298 is a highly selective PAM of the M4AChR that has shown significant efficacy in preclinical models predictive of antipsychotic drug behavior (Chan et al., 2008). In addition, the mGluR5 potentiator 3,3′-difluorobenzaldazine is the first highly selective allosteric activator of mGluRs. Its discovery stimulated further efforts leading to the identification of other series of mGluR5 potentiators that have now been shown to have antipsychotic-like activity in animal models (Maj et al., 2003; Marino et al., 2003; Kinney et al., 2005).
Allosteric modulators, with little inherent intrinsic activity that act by enhancing or attenuating the response elicited by the endogenous transmissive compound, offer several potential advantages over conventional agonists and antagonists in treating complex conditions and syndromes. First, AM effects are often saturable and therefore less likely to elicit adverse effects from overdose. Second, their effects are often exerted primarily in the presence of the native orthosteric ligand. Thus, AM activity is tied to the temporal pattern of synaptic signaling or endocrine hormone release, such that they only amplify or reduce the receptor signal when the hormone or neurotransmitter is released. Hence, an AM will preserve the physiological receptor signaling hierarchy in complex neuroendocrine axes and neurotransmissive events, while at the same time, boosting the efficiency of the endogenous neurotransmitter/hormone. Furthermore, a lack of chronic receptor activation may cause less receptor desensitization or internalization over time, overcoming the problem of diminishing therapeutic efficacy that is seen with many chronically administered orthosteric agonists. This could prove especially important in the case of neurodegenerative diseases such as Alzheimer's disease where decreased levels of acetylcholine in the forebrain impair cognition (Hasselmo and Giocomo, 2006; Fisher, 2008) or in complex endocrine disorders such as diabetes (Martin et al., 2008, 2009). Third, AMs can bias signal output in favor of only part of the receptor response profile. This results from conformational constraints placed on the receptor that limit its ability to engage effector/accessory proteins, e.g., GRKs or arrestins, as well as G proteins. This property is well known for orthosteric ligands, such as morphine, which promote G protein coupling without causing arrestin-dependent desensitization. The physiological responsiveness to psychostimulants and morphine suggests the involvement of these other GPCR regulators in neurological/pathological states, such as addiction, Parkinson's disease, aging, mood disorders, and schizophrenia (Jacoby et al., 2006; Eglen et al., 2007). With high receptor subtype-specificity and functional selectivity, AMs may be better able to dissociate therapeutic and side effects, e.g., the hypotensive and sedative properties of α2A-adrenoceptors (Kukkonen, 2004).
Finally, AMs may be able to “reprogram” cellular responses to GPCR activation. During aging, it is highly likely that both the direct receptor system and its regulatory mechanisms become disrupted and modified by accumulated changes in the transcriptome and functional proteome. Restoring normal function may be difficult using simple orthosteric pharmacology, but AMs that selectively modify receptor interactions with their effectors or regulators may restore balance or even establish new functional receptor systems with unique signaling capability.
Conclusions
In recent years, the concept of allosteric modulation of GPCRs has matured and now represents an increasingly viable approach to drug discovery and development. This is evident in the fact that AMs have been reported and designed for many types of rhodopsin-like GPCRs, and several are currently in clinical trials, with two drugs already in clinical use. While still a relatively new concept in GPCR pharmacology, AMs have the potential to provide a remarkable precision in the targeting of drugs to closely related GPCR subtypes and engendering stimulus-bias in orthosteric ligand signaling, opening up new avenues for not only receptor-selective but also signaling-pathway-selective therapies. Many aspects of allosteric receptor pharmacology lend themselves to the treatment of pathological states that affect large complicated biological systems that possess multiple levels of activity of the same hormone or neurotransmitter, e.g., neurotransmissive memory patterns or multi-organ endocrine axes feedback loops.
Acknowledgments
We apologize to the multitude of investigators whose important contributions to this field were not recognized due to space constraints.
This research was supported in part by the Intramural Research Program of the National Institutes of Health National Institute on Aging; the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases [Grant R01-DK055524] (L.M.L.); and the Research Service of the Ralph H. Johnson Veterans Affairs Medical Center (L.M.L.).
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.109.156380
- GPCR
- G protein-coupled receptor
- AM
- allosteric modulator
- PAM
- positive allosteric modulator
- NAM
- negative allosteric modulator
- ATCM
- allosteric ternary complex model
- NMS
- N-methylscopolamine
- AchR
- acetylcholine receptor
- mAChR
- muscarinic acetylcholine receptor
- mGluR
- metabotropic glutamate receptor
- CTC
- cubic ternary complex
- CB1
- cannabinoid 1
- PTH
- parathyroid hormone
- CPPHA
- N-{4-chloro-2-[(1,3-dioxo-1,3-dihydro-2H-isoindol-2-yl) methyl] phenyl}-2-hydroxybenzamide
- WIN55212
- (R)-(+)-[2,3-dihydro-5-methyl-3-(4-morpholinylmethyl) pyrrolo-[1,2,3-d,e]-1,4-benzoxazin-6-yl]-1-naphthalenyl-methanone
- McN-A-343
- 4-(m-chlorophenyl-carbamoyloxy)-2-butynyltrimethylammonium chloride
- AC-42
- 4-N-butyl-1-[4-(2-methylphenyl)-4-oxo-1-butyl]-piperidine hydrogen chloride
- Org27569
- 5-chloro-3-ethyl-N-[2-[4-(1-piperidinyl)phenyl]ethyl-1H-indole-2-carboxamide
- VU0090157
- cyclopentyl 1,6-dimethyl-4-(6-nitrobenzo[d][1,3]-dioxol-5-yl)-2-oxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate
- VU0029767
- (E)-2-(4-ethoxyphenylamino)-N′-((2-hydroxynaphthalen-1-yl) methylene) acetohydrazide
- BQCA
- benzyl quinolone carboxylic acid
- VU10010
- 3-amino-N-(4-chlorobenzyl)-4,6-dimethylthieno[2,3-b] pyridine-2-carboxamide 2,2,2-trifluoroacetate
- CP55940
- (1R,3R,4R)-3-[2-hydroxy-4-(1,1-dimethylheptyl)phenyl]-4-(3-hydroxypropyl)cyclohexan-1-ol
- LY2033298
- 3-amino-5-chloro-N-cyclopropyl-6-methoxy-4-methyl-thieno[2,3-b]pyridine-2-carboxamide
- ABP-280
- 280-kDa actin-binding protein.
References
- Angers et al., 2000.Angers S, Salahpour A, Joly E, Hilairet S, Chelsky D, Dennis M, Bouvier M. (2000) Detection of beta2-adrenergic receptor dimerization in living cells using bioluminescence resonance energy transfer (BRET). Proc Natl Acad Sci U S A 28:3684–3689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audet et al., 2008.Audet N, Galés C, Archer-Lahlou E, Vallières M, Schiller PW, Bouvier M, Pineyro G. (2008) Bioluminescence resonance energy transfer assays reveal ligand-specific conformational changes within preformed signaling complexes containing delta-opioid receptors and heterotrimeric G proteins. J Biol Chem 283:15078–15088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avlani et al., 2004.Avlani V, May LT, Sexton PM, Christopoulos (2004), Application of a kinetic model to the apparently complex behavior of negative and positive allosteric modulators of muscarinic acetylcholine receptors. J Pharmacol Exp Ther 308:1062–1072 [DOI] [PubMed] [Google Scholar]
- Ballesteros and Palczewski, 2001.Ballesteros J, Palczewski K. (2001) G protein-coupled receptor drug discovery: implications from the crystal structure of rhodopsin. Curr Opin Drug Discov Devel 4:561–574 [PMC free article] [PubMed] [Google Scholar]
- Barki-Harrington et al., 2003.Barki-Harrington L, Luttrell LM, Rockman HA. (2003) Dual inhibition of β-adrenergic and angiotensin II receptors a single antagonist: a functional role for receptor dimerization in vivo. Circulation 108:1611–1618 [DOI] [PubMed] [Google Scholar]
- Berg et al., 1998.Berg KA, Maayani S, Goldfarb J, Scaramellini C, Leff P, Clarke WP. (1998) Effector pathway-dependent relative efficacy at serotonin type 2A and 2C receptors: evidence for agonist-directed trafficking of receptor stimulus. Mol Pharmacol 54:94–104 [PubMed] [Google Scholar]
- Bonhaus et al., 1998.Bonhaus DW, Chang LK, Kwan J, Martin GR. (1998) Dual activation and inhibition of adenylyl cyclase by cannabinoid receptor agonists: evidence for agonist-specific trafficking of intracellular responses. J Pharmacol Exp Ther 287:884–888 [PubMed] [Google Scholar]
- Brady and Limbird, 2002.Brady AE, Limbird LE. (2002) G protein-coupled receptor interacting proteins: emerging roles in localization and signal transduction. Cell Signal 14:297–309 [DOI] [PubMed] [Google Scholar]
- Bridges and Lindsley, 2008.Bridges TM, Lindsley CW. (2008) G-protein-coupled receptors: from classical modes of modulation to allosteric mechanisms. ACS Chem Biol 3:530–541 [DOI] [PubMed] [Google Scholar]
- Brink et al., 2000.Brink CB, Wade SM, Neubig RR. (2000) Agonist-directed trafficking of porcine alpha(2A)-adrenergic receptor signaling in Chinese hamster ovary cells: l-isoproterenol selectively activates G(s). J Pharmacol Exp Ther 294:539–547 [PubMed] [Google Scholar]
- Chan et al., 2008.Chan WY, McKinzie DL, Bose S, Mitchell SN, Witkin JM, Thompson RC, Christopoulos A, Lazareno S, Birdsall NJ, Bymaster FP, et al. (2008) Allosteric modulation of the muscarinic M4 receptor as an approach to treating schizophrenia. Proc Natl Acad Sci U S A 105:10978–10983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christopoulos, 2002.Christopoulos A. (2002) Allosteric binding sites on cell-surface receptors: novel targets for drug discovery. Nat Rev Drug Discov 1:198–210 [DOI] [PubMed] [Google Scholar]
- Christopoulos and Kenakin, 2002.Christopoulos A, Kenakin T. (2002) G protein-coupled receptor allosterism and complexing. Pharmacol Rev 54:323–374 [DOI] [PubMed] [Google Scholar]
- Conn et al., 2009.Conn PJ, Christopoulos A, Lindsley CW. (2009) Allosteric modulators of GPCRs: a novel approach for the treatment of CNS disorders. Nat Rev Drug Discov 8:41–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conner et al., 2005.Conner AC, Hay DL, Simms J, Howitt SG, Schindler M, Smith DM, Wheatley M, Poyner DR. (2005) A key role for transmembrane prolines in calcitonin receptor-like receptor agonist binding and signalling: implications for family B G-protein-coupled receptors. Mol Pharmacol 67:20–31 [PubMed] [Google Scholar]
- De Lean et al., 1980.De Lean A, Stadel JM, Lefkowitz RJ. (1980) A ternary complex model explains the agonist-specific binding properties of the adenylate cyclase-coupled β-adrenergic receptor. J Biol Chem 255:7108–7117 [PubMed] [Google Scholar]
- Ehlert, 1988.Ehlert FJ. (1988) Estimation of the affinities of allosteric ligands using radioligand binding and pharmacological null methods. Mol Pharmacol 33:187–194 [PubMed] [Google Scholar]
- Eglen et al., 2007.Eglen RM, Bosse R, Reisine T. (2007) Emerging concepts of guanine nucleotide-binding protein-coupled receptor (GPCR) function and implications for high throughput screening. Assay Drug Dev Technol 5:425–451 [DOI] [PubMed] [Google Scholar]
- Ellis and Seidenberg, 2000.Ellis J, Seidenberg M. (2000) Interactions of alcuronium, TMB-8, and other allosteric ligands with muscarinic acetylcholine receptors: studies with chimeric receptors. Mol Pharmacol 58:1451–1460 [DOI] [PubMed] [Google Scholar]
- Engström et al., 2006.Engström M, Savola JM, Wurster S. (2006) Differential efficacies of somatostatin receptor agonists for G-protein activation and desensitization of somatostatin receptor subtype 4-mediated responses. J Pharmacol Exp Ther 316:1262–1268 [DOI] [PubMed] [Google Scholar]
- Espinoza-Fonseca and Trujillo-Ferrara, 2006.Espinoza-Fonseca LM, Trujillo-Ferrara JG. (2006) The existence of a second allosteric site on the M1 muscarinic acetylcholine receptor and its implications for drug design. Bioorg Med Chem Lett 16:1217–1220 [DOI] [PubMed] [Google Scholar]
- Evans et al., 2000.Evans BN, Rosenblatt MI, Mnayer LO, Oliver KR, Dickerson IM. (2000) CGRP-RCP, a novel protein required for signal transduction at calcitonin gene-related peptide and adrenomedullin receptors. J Biol Chem 275:31438–31443 [DOI] [PubMed] [Google Scholar]
- Fisher, 2008.Fisher A. (2008) M1 muscarinic agonists target major hallmarks of Alzheimer's disease—the pivotal role of brain M1 receptors. Neurodegener Dis 5:237–240 [DOI] [PubMed] [Google Scholar]
- Foord, 2002.Foord SM. (2002) Receptor classification: post genome. Curr Opin Pharmacol 2:561–566 [DOI] [PubMed] [Google Scholar]
- Francke et al., 2006.Francke F, Ward RJ, Jenkins L, Kellett E, Richter D, Milligan G, Bächner D. (2006) Interaction of neurochondrin with the melanin-concentrating hormone receptor 1 interferes with G protein-coupled signal transduction but not agonist-mediated internalization. J Biol Chem 281:32496–32507 [DOI] [PubMed] [Google Scholar]
- Franco et al., 2000.Franco R, Ferré S, Agnati L, Torvinen M, Ginés S, Hillion J, Casadó V, Lledó P, Zoli M, Lluis C, et al. (2000) Evidence for adenosine/dopamine receptor interactions: indications for heteromerization. Neuropsychopharmacology 23:S50–S59 [DOI] [PubMed] [Google Scholar]
- Fraser et al., 2000.Fraser ID, Cong M, Kim J, Rollins EN, Daaka Y, Lefkowitz RJ, Scott JD. (2000) Assembly of an A kinase-anchoring protein-β2-adrenergic receptor complex facilitates receptor phosphorylation and signaling. Curr Biol 10:409–412 [DOI] [PubMed] [Google Scholar]
- Galandrin and Bouvier, 2006.Galandrin S, Bouvier M. (2006) Distinct signaling profiles of beta1 and beta2 adrenergic receptor ligands toward adenylyl cyclase and mitogen-activated protein kinase reveals the pluridimensionality of efficacy. Mol Pharmacol 70:1575–1584 [DOI] [PubMed] [Google Scholar]
- George et al., 2000.George SR, Fan T, Xie Z, Tse R, Tam V, Varghese G, O'Dowd BF. (2000) Oligomerization of mu- and delta-opioid receptors. Generation of novel functional properties. J Biol Chem 275:26128–26135 [DOI] [PubMed] [Google Scholar]
- Gesty-Palmer et al., 2006.Gesty-Palmer D, Chen M, Reiter E, Ahn S, Nelson CD, Wang S, Eckhardt AE, Cowan CL, Spurney RF, Luttrell LM, et al. (2006) Distinct beta-arrestin- and G protein-dependent pathways for parathyroid hormone receptor-stimulated ERK1/2 activation. J Biol Chem 281:10856–10864 [DOI] [PubMed] [Google Scholar]
- Ghanouni et al., 2001.Ghanouni P, Gryczynski Z, Steenhuis JJ, Lee TW, Farrens DL, Lakowicz JR, Kobilka BK. (2001) Functionally different agonists induce distinct conformations in the G protein coupling domain of the β2 adrenergic receptor. J Biol Chem 276:24433–24436 [DOI] [PubMed] [Google Scholar]
- Gomes et al., 2004.Gomes I, Gupta A, Filipovska J, Szeto HH, Pintar JE, Devi LA. (2004) A role for heterodimerization of μ and δ opiate receptors in enhancing morphine analgesia. Proc Natl Acad Sci U S A 101:5135–5139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman et al., 2002.Goodman WG, Hladik GA, Turner SA, Blaisdell PW, Goodkin DA, Liu W, Barri YM, Cohen RM, Coburn JW. (2002) The calcimimetic agent AMG073 lowers plasma parathyroid hormone levels in hemodialysis patients with secondary hyperparathyroidism. J Am Soc Nephrol 13:1017–1024 [DOI] [PubMed] [Google Scholar]
- Hall, 2000.Hall DA. (2000) Modeling the functional effects of allosteric modulators at pharmacological receptors: an extension of the two-state model of receptor activation. Mol Pharmacol 58:1412–1423 [DOI] [PubMed] [Google Scholar]
- Hall et al., 1998.Hall RA, Premont RT, Chow CW, Blitzer JT, Pitcher JA, Claing A, Stoffel RH, Barak LS, Shenolikar S, Weinman EJ, et al. (1998) The beta2-adrenergic receptor interacts with the Na+/H+-exchanger regulatory factor to control Na+/H+ exchange. Nature 392:626–630 [DOI] [PubMed] [Google Scholar]
- Hasselmo and Giocomo, 2006.Hasselmo ME, Giocomo LM. (2006) Cholinergic modulation of cortical function. J Mol Neurosci 30:133–135 [DOI] [PubMed] [Google Scholar]
- Jacoby et al., 2006.Jacoby E, Bouhelal R, Gerspacher M, Seuwen K. (2006) The 7 TM G-protein-coupled receptor target family. Chem Med Chem 1:761–782 [DOI] [PubMed] [Google Scholar]
- Johnson et al., 2005.Johnson MP, Barda D, Britton TC, Emkey R, Hornback WJ, Jagdmann GE, McKinzie DL, Nisenbaum ES, Tizzano JP, Schoepp DD. (2005) Metabotropic glutamate 2 receptor potentiators: receptor modulation, frequency-dependent synaptic activity, and efficacy in preclinical anxiety and psychosis model(s). Psychopharmacology 179:271–283 [DOI] [PubMed] [Google Scholar]
- Jones et al., 2008.Jones CK, Brady AE, Davis AA, Xiang Z, Bubser M, Tantawy MN, Kane AS, Bridges TM, Kennedy JP, Bradley SR, et al. (2008) Novel selective allosteric activator of the M1 muscarinic acetylcholine receptor regulates amyloid processing and produces antipsychotic-like activity in rats. J Neurosci 28:10422–10433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones et al., 1998.Jones KA, Borowsky B, Tamm JA, Craig DA, Durkin MM, Dai M, Yao WJ, Johnson M, Gunwaldsen C, Huang LY, et al. (1998) GABA(B) receptors function as a heteromeric assembly of the subunits GABA(B)R1 and GABA(B)R2. Nature 396:674–679 [DOI] [PubMed] [Google Scholar]
- Jordan and Devi, 1999.Jordan BA, Devi LA. (1999) G-protein-coupled receptor heterodimerization modulates receptor function. Nature 399:697–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaupmann et al., 1998.Kaupmann K, Malitschek B, Schuler V, Heid J, Froestl W, Beck P, Mosbacher J, Bischoff S, Kulik A, Shigemoto R, et al. (1998) GABAB receptor subtypes assemble into functional heteromeric complexes. Nature 396:683–687 [DOI] [PubMed] [Google Scholar]
- Kebig et al., 2009.Kebig A, Kostenis E, Mohr K, Mohr-Andrä M. (2009) An optical dynamic mass redistribution assay reveals biased signaling of dualsteric GPCR activators. J Recept Signal Transduct Res 29:140–145 [DOI] [PubMed] [Google Scholar]
- Kenakin, 2002.Kenakin T. (2002) Efficacy at G-protein-coupled receptors. Nat Rev Drug Discov 1:103–110 [DOI] [PubMed] [Google Scholar]
- Kenakin, 2003.Kenakin T. (2003) Predicting therapeutic value in the lead optimization phase of drug discovery. Nat Rev Drug Discov 2:429–438 [DOI] [PubMed] [Google Scholar]
- Kenakin et al., 2006.Kenakin T, Jenkinson S, Watson C. (2006) Determining the potency and molecular mechanism of action of insurmountable antagonists. J Pharmacol Exp Ther 319:710–723 [DOI] [PubMed] [Google Scholar]
- Kinney et al., 2005.Kinney GG, O'Brien JA, Lemaire W, Burno M, Bickel DJ, Clements MK, Chen TB, Wisnoski DD, Lindsley CW, Tiller PR, Smith S, Jacobson MA, Sur C, Duggan ME, Pettibone DJ, Conn PJ, Williams DL., Jr (2005). A novel selective positive allosteric modulator of metabotropic glutamate receptor subtype 5 has in vivo activity and antipsychotic-like effects in rat behavioral models. J Pharmacol Exp Ther 313:199–206 [DOI] [PubMed] [Google Scholar]
- Knoflach et al., 2001.Knoflach F, Mutel V, Jolidon S, Kew JN, Malherbe P, Vieira E, Wichmann J, Kemp JA. (2001) Positive allosteric modulators of metabotropic glutamate 1 receptor: characterization, mechanism of action, and binding site. Proc Natl Acad Sci U S A 98:13402–13407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen et al., 2006.Knudsen LB, Kiel D, Teng M, Behrens C, Bhumralkar D, Kodra JT, Holst JJ, Jeppesen CB, Johnson MD, de Jong JC, et al. (2006) Small molecule agonists for the glucagon-like peptide 1 receptor. Proc Natl Acad Sci U S A 104:937–942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohout et al., 2004.Kohout TA, Nicholas SL, Perry SJ, Reinhart G, Junger S, Struthers RS. (2004) Differential desensitization, receptor phosphorylation, beta-arrestin recruitment, and ERK1/2 activation by the two endogenous ligands for the CC chemokine receptor. J Biol Chem 279:23214–23222 [DOI] [PubMed] [Google Scholar]
- Kukkonen, 2004.Kukkonen JP. (2004) Regulation of receptor-coupling to (multiple) G proteins. A challenge for basic research and drug discovery. Receptors Channels 10:167–183 [DOI] [PubMed] [Google Scholar]
- Langmead et al., 2006.Langmead CJ, Fry VA, Forbes IT, Branch CL, Christopoulos A, Wood MD, Herdon HJ. (2006) Probing the molecular mechanism of interaction between 4-n-butyl-1-[4-(2-methylphenyl)-4-oxo-1-butyl]-piperidine (AC-42) and the muscarinic M(1) receptor: direct pharmacological evidence that AC-42 is an allosteric agonist. Mol Pharmacol 69:236–246 [DOI] [PubMed] [Google Scholar]
- Lanzafame et al., 1997.Lanzafame A, Christopoulos A, Mitchelson F. (1997) Three allosteric modulators act at a common site, distinct from that of competitive antagonists, at muscarinic acetylcholine M2 receptors. J Pharmacol Exp Ther 282:278–285 [PubMed] [Google Scholar]
- Lazareno et al., 1998.Lazareno S, Popham A, Birdsall NJ. (1998) Muscarinic interactions of bisindolylmaleimide analogues. Eur J Pharmacol 360:281–284 [DOI] [PubMed] [Google Scholar]
- Lazareno et al., 2004.Lazareno S, Dolezal V, Popham A, Birdsall NJ. (2004) Thiochrome enhances acetylcholine affinity at muscarinic M4 receptors: receptor subtype selectivity via cooperativity rather than affinity. Mol Pharmacol 65:257–266 [DOI] [PubMed] [Google Scholar]
- Leach et al., 2007.Leach K, Sexton PM, Christopoulos A. (2007) Allosteric GPCR modulators: taking advantage of permissive receptor pharmacology. Trends Pharmacol Sci 28:382–389 [DOI] [PubMed] [Google Scholar]
- Leppik and Birdsall, 2000.Leppik RA, Birdsall NJ. (2000) Agonist binding and function at the human α2A-adrenoceptor: allosteric modulation by amilorides. Mol Pharmacol 58:1091–1099 [DOI] [PubMed] [Google Scholar]
- Lewis et al., 2008.Lewis JA, Lebois EP, Lindsley CW. (2008) Allosteric modulation of kinases and GPCRs: design principles and structural diversity. Curr Opin Chem Biol 12:269–280 [DOI] [PubMed] [Google Scholar]
- Li et al., 2000.Li M, Bermak JC, Wang ZW, Zhou QY. (2000) Modulation of dopamine D2 receptor signaling by actin-binding protein ABP-280. Mol Pharmacol 57:446–452 [DOI] [PubMed] [Google Scholar]
- Lindsley et al., 2004.Lindsley CW, Wisnoski DD, Leister WH, O'Brien JA, Lemaire W, Williams DL, Jr, Burno M, Sur C, Kinney GG, Pettibone DJ, et al. (2004) Discovery of positive allosteric modulators for the metabotropic glutamate receptor subtype 5 from a series of N-(1,3-diphenyl-1H-pyrazol-5-yl)benzamides that potentiate receptor function in vivo. J Med Chem 47:5825–5828 [DOI] [PubMed] [Google Scholar]
- Luttrell et al., 1999.Luttrell LM, Ferguson SS, Daaka Y, Miller WE, Maudsley S, Della Rocca GJ, Lin F, Kawakatsu H, Owada K, Luttrell DK, et al. (1999) Beta-arrestin-dependent formation of beta2 adrenergic receptor-Src protein kinase complexes. Science 283:655–661 [DOI] [PubMed] [Google Scholar]
- MacKinnon et al., 2001.MacKinnon AC, Waters C, Jodrell D, Haslett C, Sethi T. (2001) Bombesin and substance P analogues differentially regulate G protein coupling to the bombesin receptor: direct evidence for biased agonism. J Biol Chem 276:28083–28091 [DOI] [PubMed] [Google Scholar]
- Maj et al., 2003.Maj M, Bruno V, Dragic Z, Yamamoto R, Battaglia G, Inderbitzin W, Stoehr N, Stein T, Gasparini F, Vranesic I, et al. (2003) (−)-PHCCC, a positive allosteric modulator of mGluR4: characterization, mechanism of action, and neuroprotection. Neuropharmacology 45:895–906 [DOI] [PubMed] [Google Scholar]
- Marino et al., 2003.Marino MJ, Williams DL, Jr, O'Brien JA, Valenti O, McDonald TP, Clements MK, Wang R, DiLella AG, Hess JF, Kinney GG, et al. (2003) Allosteric modulation of group III metabotropic glutamate receptor 4: a potential approach to Parkinson's disease treatment. Proc Natl Acad Sci U S A 100:13668–13673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marion et al., 2008.Marion W, Guangping X, Michelle P, Susan G, Scott D, John JR, Andrew D, Christopher PR, Guy RS, William JR. (2008) In vivo pharmacodynamic effects of BQCA, a novel selective allosteric M1 receptor modulator. Alzheimers Dement 4 (Suppl 1):T770 [Google Scholar]
- Marlo et al., 2009.Marlo JE, Niswender CM, Days EL, Bridges TM, Xiang Y, Rodriguez AL, Shirey JK, Brady AE, Nalywajko T, Luo Q, et al. (2009) Discovery and characterization of novel allosteric potentiators of M1 muscarinic receptors reveals multiple modes of activity. Mol Pharmacol 75:577–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin et al., 2008.Martin B, Golden E, Keselman A, Stone M, Mattson MP, Egan JM, Maudsley S. (2008) Therapeutic perspectives for the treatment of Huntington's disease: treating the whole body. Histol Histopathol 23:237–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin et al., 2009.Martin B, Golden E, Carlson OD, Pistell P, Zhou J, Kim W, Frank BP, Thomas S, Chadwick WA, Greig NH, et al. (2009) Exendin-4 improves glycemic control, ameliorates brain and pancreatic pathologies, and extends survival in a mouse model of Huntington's disease. Diabetes 58:318–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maudsley et al., 1998.Maudsley S, Gent JP, Findlay JB, Donnelly D. (1998) The relationship between the agonist-induced activation and desensitization of the human tachykinin NK2 receptor expressed in Xenopus oocytes. Br J Pharmacol 124:675–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maudsley et al., 2000.Maudsley S, Pierce KL, Zamah AM, Miller WE, Ahn S, Daaka Y, Lefkowitz RJ, Luttrell LM. (2000) The beta(2)-adrenergic receptor mediates extracellular signal-regulated kinase activation via assembly of a multi-receptor complex with the epidermal growth factor receptor. J Biol Chem 275:9572–9580 [DOI] [PubMed] [Google Scholar]
- Maudsley et al., 2004.Maudsley S, Davidson L, Pawson AJ, Chan R, López de Maturana R, Millar RP. (2004) Gonadotropin-releasing hormone (GnRH) antagonists promote proapoptotic signaling in peripheral reproductive tumor cells by activating a Galphai-coupling state of the type I GnRH receptor. Cancer Res 64:7533–7544 [DOI] [PubMed] [Google Scholar]
- Maudsley et al., 2005.Maudsley S, Martin B, Luttrell LM. (2005) The origins of diversity and specificity in G protein coupled receptor signaling. J Pharmacol Exp Ther 314:485–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maudsley et al., 2007.Maudsley S, Martin B, Luttrell LM. (2007) G protein-coupled receptor signaling complexity in neuronal tissue: implications for novel therapeutics. Curr Alzheimer Res 4:3–19 [DOI] [PubMed] [Google Scholar]
- May et al., 2004.May LT, Avlani VA, Sexton PM, Christopoulos A. (2004) Allosteric modulation of G protein-coupled receptors. Curr Pharm Des 10:2003–2013 [DOI] [PubMed] [Google Scholar]
- McLatchie et al., 1998.McLatchie LM, Fraser NJ, Main MJ, Wise A, Brown J, Thompson N, Solari R, Lee MG, Foord SM. (1998) RAMPs regulate the transport and ligand specificity of the calcitonin-receptor-like receptor. Nature 393:333–339 [DOI] [PubMed] [Google Scholar]
- Mirza et al., 2003.Mirza NR, Peters D, Sparks RG. (2003) Xanomeline and the antipsychotic potential of muscarinic receptor subtype selective agonists. CNS Drug Rev 9:159–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundell et al., 1997.Mundell SJ, Benovic JL, Kelly E. (1997) A dominant negative mutant of the G protein-coupled receptor kinase 2 selectively attenuates adenosine A2 receptor desensitization. Mol Pharmacol 51:991–998 [DOI] [PubMed] [Google Scholar]
- Nemeth et al., 2004.Nemeth EF, Heaton WH, Miller M, Fox J, Balandrin MF, Van Wagenen BC, Colloton M, Karbon W, Scherrer J, Shatzen E, et al. (2004) Pharmacodynamics of the type II calcimimetic compound cinacalcet HCl. J Pharmacol Exp Ther 308:627–635 [DOI] [PubMed] [Google Scholar]
- Ng et al., 1999.Ng GY, Clark J, Coulombe N, Ethier N, Hebert TE, Sullivan R, Kargman S, Chateauneuf A, Tsukamoto N, McDonald T, et al. (1999) Identification of a GABAB receptor subunit, gb2, required for functional GABAB receptor activity. J Biol Chem 274:7607–7610 [DOI] [PubMed] [Google Scholar]
- Novi et al., 2005.Novi F, Stanasila L, Giorgi F, Corsini GU, Cotecchia S, Maggio R. (2005) Paired activation of two components within muscarinic M3 receptor dimers is required for recruitment of beta-arrestin-1 to the plasma membrane. J Biol Chem 280:19768–19776 [DOI] [PubMed] [Google Scholar]
- Palanche et al., 2001.Palanche T, Ilien B, Zoffmann S, Reck MP, Bucher B, Edelstein SJ, Galzi JL. (2001) The neurokinin A receptor activates calcium and cAMP responses through distinct conformational states. J Biol Chem 276:34853–34861 [DOI] [PubMed] [Google Scholar]
- Perez et al., 1996.Perez DM, Hwa J, Gaivin R, Mathur M, Brown F, Graham RM. (1996) Constitutive activation of a single effector pathway: evidence for multiple activation states of a G protein-coupled receptor. Mol Pharmacol 49:112–122 [PubMed] [Google Scholar]
- Peters and Scott, 2009.Peters MF, Scott CW. (2009) Evaluating cellular impedance assays for detection of GPCR pleiotropic signaling and functional selectivity. J Biomol Screen 14:246–255 [DOI] [PubMed] [Google Scholar]
- Pfister et al., 1985.Pfister C, Chabre M, Plouet J, Tuyen VV, De Kozak Y, Faure JP, Kühn H. (1985) Retinal S antigen identified as the 48K protein regulating light-dependent phosphodiesterase in rods. Science 228:891–893 [DOI] [PubMed] [Google Scholar]
- Porter et al., 2005.Porter RH, Jaeschke G, Spooren W, Ballard TM, Büttelmann B, Kolczewski S, Peters JU, Prinssen E, Wichmann J, Vieira E, et al. (2005) Fenobam: a clinically validated nonbenzodiazepine anxiolytic is a potent, selective, and noncompetitive mGlu5 receptor antagonist with inverse agonist activity. J Pharmacol Exp Ther 315:711–721 [DOI] [PubMed] [Google Scholar]
- Price et al., 2005.Price MR, Baillie GL, Thomas A, Stevenson LA, Easson M, Goodwin R, McLean A, McIntosh L, Goodwin G, Walker G, et al. (2005) Allosteric modulation of the cannabinoid CB1 receptor. Mol Pharmacol 68:1484–1495 [DOI] [PubMed] [Google Scholar]
- Rasmussen et al., 2007.Rasmussen SG, Choi HJ, Rosenbaum DM, Kobilka TS, Thian FS, Edwards PC, Burghammer M, Ratnala VR, Sanishvili R, Fischetti RF, et al. (2007) Crystal structure of the human beta2 adrenergic G-protein-coupled receptor. Nature 450:383–387 [DOI] [PubMed] [Google Scholar]
- Roppe et al., 2004.Roppe JR, Wang B, Huang D, Tehrani L, Kamenecka T, Schweiger EJ, Anderson JJ, Brodkin J, Jiang X, Cramer M, et al. (2004) 5-[2-Methyl-1,3-thiazol-4-yl)ethynyl]-2,3′-bipyridine: a highly potent, orally active metabotropic glutamate subtype 5 (mGlu5) receptor antagonist with anxiolytic activity. Bioorg Med Chem Lett 14:3993–3996 [DOI] [PubMed] [Google Scholar]
- Rosario et al., 2008.Rosario MC, Jacqmin P, Dorr P, James I, Jenkins TM, Abel S, van der Ryst E. (2008) Population pharmacokinetic/pharmacodynamic analysis of CCR5 receptor occupancy by maraviroc in healthy subjects and HIV-positive patients. Br J Clin Pharmacol 65:86–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachpatzidis et al., 2003.Sachpatzidis A, Benton BK, Manfredi JP, Wang H, Hamilton A, Dohlman HG, Lolis E. (2003) Identification of allosteric peptide agonists of CXCR4. J Biol Chem 278:896–907 [DOI] [PubMed] [Google Scholar]
- Schiöth and Fredriksson, 2005.Schiöth HB, Fredriksson R. (2005) The GRAFS classification system of G-protein coupled receptors in comparative perspective. Gen Comp Endocrinol 142:94–101 [DOI] [PubMed] [Google Scholar]
- Shapiro et al., 2002.Shapiro DA, Kristiansen K, Weiner DM, Kroeze WK, Roth BL. (2002) Evidence for a model of agonist-induced activation of 5-hydroxytryptamine 2A serotonin receptors that involves the disruption of a strong ionic interaction between helices 3 and 6. J Biol Chem 277:11441–11449 [DOI] [PubMed] [Google Scholar]
- Shirey et al., 2008.Shirey JK, Xiang Z, Orton D, Brady AE, Johnson KA, Williams R, Ayala JE, Rodriguez AL, Wess J, Weaver D, et al. (2008) An allosteric potentiator of M4 mAChR modulates hippocampal synaptic transmission. Nat Chem Biol 4:42–50 [DOI] [PubMed] [Google Scholar]
- Spalding et al., 2002.Spalding TA, Trotter C, Skjaerbaek N, Messier TL, Currier EA, Burstein ES, Li D, Hacksell U, Brann MR. (2002) Discovery of an ectopic activation site on the M(1) muscarinic receptor. Mol Pharmacol 61:1297–1302 [DOI] [PubMed] [Google Scholar]
- Stachowicz et al., 2004.Stachowicz K, Kłak K, Kłodzińska A, Chojnacka-Wojcik E, Pilc A. (2004) Anxiolytic-like effects of PHCCC, an allosteric modulator of mGlu4 receptors, in rats. Eur J Pharmacol 498:153–156 [DOI] [PubMed] [Google Scholar]
- Stockton et al., 1983.Stockton JM, Birdsall NJ, Burgen AS, Hulme EC. (1983) Modification of the binding properties of muscarinic receptors by gallamine. Mol Pharmacol 23:551–557 [PubMed] [Google Scholar]
- Sur et al., 2003.Sur C, Mallorga PJ, Wittmann M, Jacobson MA, Pascarella D, Williams JB, Brandish PE, Pettibone DJ, Scolnick EM, Conn PJ. (2003) N-Desmethylclozapine, an allosteric agonist at muscarinic 1 receptor, potentiates N-methyl-d-aspartate receptor activity. Proc Natl Acad Sci U S A 100:13674–13679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szmuilowicz and Utiger, 2006.Szmuilowicz ED, Utiger RD. (2006) A case of parathyroid carcinoma with hypercalcemia responsive to cinacalcet therapy. Nat Clin Pract Endocrinol Metab 2:291–296 [DOI] [PubMed] [Google Scholar]
- Talbodec et al., 2000.Talbodec A, Berkane N, Blandin V, Breittmayer JP, Ferrari E, Frelin C, Vigne P. (2000) Aspirin and sodium salicylate inhibit endothelin ETA receptors by an allosteric type of mechanism. Mol Pharmacol 57:797–804 [DOI] [PubMed] [Google Scholar]
- Thomas et al., 1997.Thomas EA, Carson MJ, Neal MJ, Sutcliffe JG. (1997) Unique allosteric regulation of 5-hydroxytryptamine receptor-mediated signal transduction by oleamide. Proc Natl Acad Sci U S A 94:14115–14119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin, 2008.Tobin AB. (2008) G-protein-coupled receptor phosphorylation: where, when and by whom. Br J Pharmacol 153:S167–S176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valant et al., 2008.Valant C, Gregory KJ, Hall NE, Scammells PJ, Lew MJ, Sexton PM, Christopoulos A. (2008) A novel mechanism of G protein-coupled receptor functional selectivity. Muscarinic partial agonist McN-A-343 as a bitopic orthosteric/allosteric ligand. J Biol Chem 283:29312–29321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber et al., 2006.Weber B, Schaper C, Scholz J, Bein B, Rodde C, H Tonner P. (2006) Interaction of the amyloid precursor-like protein 1 with the alpha2A-adrenergic receptor increases agonist-mediated inhibition of adenylate cyclase. Cell Signal 18:1748–1157 [DOI] [PubMed] [Google Scholar]
- Wood and Armour, 2005.Wood A, Armour D. (2005) The discovery of the CCR5 receptor antagonist, UK-427,857, a new agent for the treatment of HIV infection and AIDS. Prog Med Chem 43:239–271 [DOI] [PubMed] [Google Scholar]
- Wu et al., 2007.Wu WL, Burnett DA, Domalski M, Greenlee WJ, Li C, Bertorelli R, Fredduzzi S, Lozza G, Veltri A, Reggiani A. (2007) Discovery of orally efficacious tetracyclic metabotropic glutamate receptor 1 (mGluR1) antagonists for the treatment of chronic pain. J Med Chem 50:5550–5553 [DOI] [PubMed] [Google Scholar]
- Zhang and Neer, 2001.Zhang W, Neer EJ. (2001) Reassembly of phospholipase C-beta2 from separated domains: analysis of basal and G protein-stimulated activities. J Biol Chem 276:2503–2508 [DOI] [PubMed] [Google Scholar]
- Zhang et al., 2005.Zhang Y, Rodriguez AL, Conn PJ. (2005) Allosteric potentiators of metabotropic glutamate receptor subtype 5 have differential effects on different signaling pathways in cortical astrocytes. J Pharmacol Exp Ther 315:1212–1219 [DOI] [PubMed] [Google Scholar]
- Zhao et al., 2007.Zhao Z, Wisnoski DD, O'Brien JA, Lemaire W, Williams DL, Jr, Jacobson MA, Wittman M, Ha SN, Schaffhauser H, Sur C, et al. (2007) Challenges in the development of mGluR5 positive allosteric modulators: the discovery of CPPHA. Bioorg Med Chem Lett 17:1386–1391 [DOI] [PubMed] [Google Scholar]
- Zheng et al., 2005.Zheng GZ, Bhatia P, Daanen J, Kolasa T, Patel M, Latshaw S, El Kouhen OF, Chang R, Uchic ME, Miller L, et al. (2005) Structure-activity relationship of triazafluorenone derivatives as potent and selective mGluR1 antagonists. J Med Chem 48:7374–7388 [DOI] [PubMed] [Google Scholar]