Abstract
The extracellular matrix (ECM), once thought to be a static structural component of tissues, is now known to play a complex and dynamic role in a variety of cellular functions in a number of diverse tissues. A significant body of literature attests to the ability of the ECM to communicate both spatial and temporal information to adherent cells, thereby directing cell behavior via interactions between the ECM and cell-surface receptors. Moreover, volumes of experimental data show that a great deal of communication travels in the opposite direction, from the cell to the ECM, allowing for regulation of the cues transmitted by the ECM. As such, the ECM, with respect to its components and their organization, is not a fixed reflection of the state the local microenvironment in which a cell finds itself at a particular time, but rather is able to respond to and effect changes in its local microenvironment. As an example of the developmental consequences of ECM interactions, this review gives an overview of the ‘give and take’ relationship between the ECM and the cells of the developing skeletal elements, in particular, the chondrocyte.
Keywords: Extracellular matrix, Endochondral ossification, Chondrocyte, Osteoblast
1. Introduction—the extracellular matrix and development
The extracellular matrix (ECM) is an essential player in functions such as cell survival, migration, proliferation, and differentiation (Lukashev and Werb, 1998). ECM regulation of cell behavior during development is a vast and somewhat cumbersome topic and has been observed in a number of developmental and repair processes including cardiac remodeling (Goldsmith and Borg, 2002), vascular morphogenesis (Brooke et al., 2003), angiogenesis (Li et al., 2003) and skeletal development (Aszódi et al., 2000). The ECM provides cues to the cells it contacts by a variety of means. The ECM marks locations and sets boundaries within developing tissue elements. Certain ECM components signal directly to the cells they contact, or bind morphogens and/or growth factors, sequestering them from cells, activating them, and/or concentrating them in local microenvironments. The importance of cell shape changes in the differentiation and maturation processes of a number of cell types has also begun to emerge, and the ECM appears to play a prominent role in this effect. The cells that interface with the ECM and receive ECM cues are themselves able to regulate ECM signaling. This regulation may occur by differential expression of ECM components and/or proteases, and post-translational modification or organization of ECM components.
In the interest of effectiveness and clarity, this review will focus on the interplay between the ECM and the developing chondrocyte, a major cell type of developing skeletal tissue, in directing and regulating skeletal development. Skeletal development by the process of endochondral ossification requires the regulation and coordination of multiple distinct cell types and numerous distinct ECM microenvironments within this tissue (Fig. 1; Erlebacher et al., 1995). It has been more than 30 years since the influence of the ECM on the process of skeletal development was first recognized, when it was shown that the implantation of demineralized bone matrix is sufficient to reconstitute endochondral ossification (Urist, 1965; Reddi and Anderson, 1976). More recent genetic studies attest to the dependence of normal physiological bone development on the ECM (Ramirez, 1996; Quondamatteo et al., 2002) and, in particular, on ECM remodeling through the activities of nearby cells (Ortega et al., 2003).
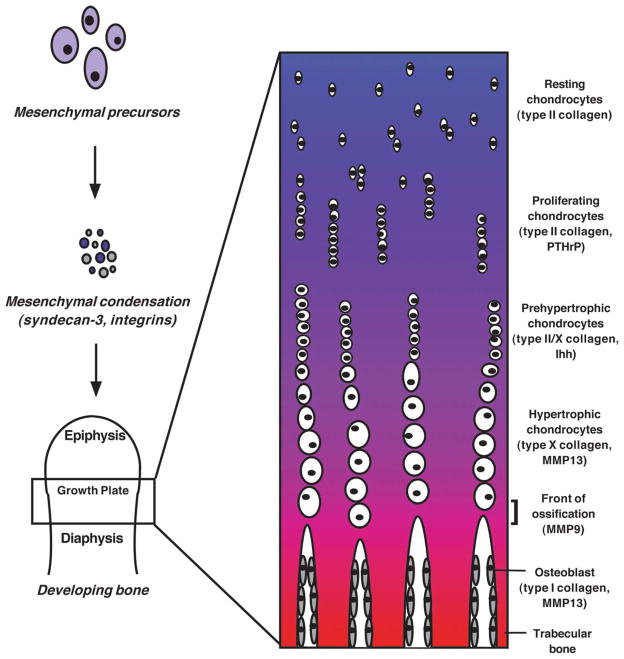
Fig. 1. Chondrocyte development and endochondral ossification.
Upon receiving the appropriate cues, mesenchymal precursors in the developing embryo congregate to form mesenchymal condensations, in which they express syndecan-3 as well as a number of integrins on their respective cell surfaces. These condensations then take on the shape of the skeletal element for which they will serve as a template, and the constituent mesenchymal precursors begin to differentiate into osteoblasts and chondrocytes. As bone development progresses, the maturing chondrocytes become organized according to their developmental stage and establish the epiphyseal growth plate (closeup). Within the growth plate, each distinct population of chondrocytes expresses a specific repertoire of ECM components and signaling molecules. At the bottom of the growth plate, the hypertrophic chondrocytes apoptose and their matrix calcifies; this region is invaded by osteoblasts and other cells, and new bone is formed.
2. The life of a chondrocyte
The chondrocyte is one of a number of well-defined cell types in developing bone. Chondrocytes are the constituent cells of cartilage, and through their function in endochondral ossification—the process by which most vertebrate bones are formed—play a central role in determining the rate of bone growth (Hunziker, 1994). Endochondral ossification involves the replacement of an avascular cartilaginous template with a highly vascularized, mineralized tissue, and initiates during embryogenesis with the condensation of mesenchymal precursors at the future sites of skeletal elements (Poole, 1991). These mesenchymal condensations take on the shape of the skeletal elements for which they will serve as templates, and the mesenchymal precursors in these dense cell masses then differentiate along an osteogenic pathway (ultimately producing osteoblasts, bone-depositing cells) or a chondrogenic pathway (ultimately producing chondrocytes, cartilage cells). Chondrogenic precursors follow a differentiation pathway mediated by a variety of signaling molecules including Indian hedgehog (Ihh), bone morphogenetic proteins (BMPs) and parathyroid hormone (PTH)-related peptide (PTHrP) (Jüppner, 2000; Katagiri and Takahashi, 2002). This differentiation process gradually proceeds outward from the midline (the diaphysis) of the bone anlage toward the opposite ends (the epiphyses), and the chondrocytes become organized according to their differentiation state. This establishes the epiphyseal growth plate. The differentiating chondrocytes within the growth plate are organized along a continuum beginning at the region most proximal to the epiphysis, where the resting and actively proliferating chondrocytes are located, and continuing through to the region most proximal to the diaphysis, where hypertrophic and apoptosing chondrocytes are located (Fig. 1). At each stage along this differentiation continuum, the chondrocytes express and secrete a distinct repertoire of collagens, proteoglycans and other ECM molecules (Karsenty and Wagner, 2002). In addition, hypertrophic chondrocytes secrete matrix vesicles containing enzymes that actively degrade and mineralize their surrounding matrix (Wuthier et al., 1985; Kirsch et al., 2000); these cells undergo apoptosis as this area is invaded by blood vessels, osteogenic cells and mesenchymal precursors. The residual calcified cartilage matrix is then used as a scaffold for the deposition of mineralized bone matrix, resulting in the production of new trabecular bone (Horton, 1993; Wagner and Karsenty, 2001; Ortega et al., 2003).
This review will focus on the ‘give and take’ relationship between the ECM and the developing chondrocyte: the effect of changes in the ECM on the development of the chondrocyte, and the effect of changes that the chondrocyte undergoes during its differentiation on the development of the ECM.
3. Initiation of skeletal development—the impact of a changing ECM on the chondrocyte
The composition and organization of the ECM can serve as a landmark for an adherent cell: it can tell the cell ‘where’ it is and ‘when’ it is. As the developing chondrocyte moves among microenvironments within the developing bone, each with different ECM landmarks, it modulates its differentiation state and behavior accordingly, at each step tweaking its gene expression in response to what it sees and touches.
3.1. Formation of the mesenchymal condensation—the ECM as a landmark
As cells are able to receive cues and signals from the ECMs to which they adhere, and because these ECMs may differ in composition among local microenvironments (topics which will be discussed in more detail later), local ECMs are able to mark regions and create boundaries within tissues. To initiate the process of endochondral ossification, dispersed mesenchymal precursor cells must migrate to the location of a future skeletal element and form a dense cell mass, the pre-chondrogenic condensation (Hall and Miyake, 1992). It is in these condensations that the precursors begin to produce and deposit ECM (Knudson and Toole, 1985).
The ECM appears to play an important role at two distinct stages of condensation: initiation of the condensation and boundary setting. The expression of the ECM molecule fibronectin is upregulated at the onset of condensation formation and chondrogenesis (Kulyk et al., 1989). Fibronectin mediates the formation of mesenchymal condensations by binding the cell-surface receptor N-CAM, which is transiently expressed on the condensing mesenchymal precursors (Widelitz et al., 1993; reviewed in Hall and Miyake, 2000). Although a precise mechanism for the initiation of condensation is unknown, it is likely that fibronectin marks locations where condensations will form. In developing chick limbs, the ECM molecule tenascin-C, a large glycoprotein known to associate with a variety of matrix components and interact with cells via cell-surface syndecans and integrins (Salmivirta et al., 1991; Prieto et al., 1993; Sriramarao et al., 1993), is a marker of the outer boundaries of these mesenchymal condensations (Koyama et al., 1995, 1996). Tenascin-C is found at high levels in the ECM at the outer rim of mesenchymal condensations in early chick limb development (Mackie et al., 1987; Gould et al., 1992), where adherent mesenchymal precursors highly express the cognate receptor, syndecan-3 (Salmivirta et al., 1991; Koyama et al., 1995, 1996). Curiously, animals deficient for tenascin-C show no apparent skeletal phenotype (Saga et al., 1992; Forsberg et al., 1996), although no skeleton-specific study has been performed. The possibility remains that other ECM components or intrinsic programming of cells within the condensation may compensate in the absence of tenascin-C. Nonetheless, it appears that components of local ECMs may provide a developmental ‘zipcode’ such that participating cells can safely find the way to their places of business.
The ability of ECM molecules to mark the locations and bounds of these mesenchymal condensations is just one example of the evidence that the ECM (and in some cases, associated molecules) is able to signal directly to cells in the developing skeleton, thereby influencing their behavior. Decelluarlized, demineralized matrix from bone, when implanted subcutaneously in rats, is sufficient to reconstitute the process of endochondral ossification at these locations (Urist, 1965; Reddi and Anderson, 1976). Of particular interest, this demineralized implanted matrix allows for recruitment of the appropriate mesenchymal precursor cells and their differentiation into functional chondrocytes and osteoblasts, as well as recruitment of endothelial and hematopoietic cells at the site of implantation. These results indicate that matrix components (and their tightly associated factors, such as BMPs) are sufficient to not only mark the location of new bone formation, but also to recruit the appropriate circulating cell types of varying origin.
Alterations in the ECM in a particular local microenvironment are often a hallmark of disorder and disease. A large body of in vitro evidence suggests a requirement for minimum cell number within condensations for the differentiation of mesenchymal precursors along the chondrogenic and osteogenic pathways (reviewed in Hall and Miyake, 1992). Indeed, growing mesenchymal precursors in low-density micromasses, to mimic a low-density condensation, causes these cells to assume a fibroblastic phenotype and abolishes chondrogenesis (Hattori and Ide, 1984; Cottrill et al., 1987; Hurle et al., 1989). Given that ECM components may be required to mark the location of future skeletal elements and recruit a threshold amount of precursors to these locations, one could then imagine a situation where the absence of the requisite ECM components would lead to decreased recruitment of precursors to the condensations, causing a subsequent deficiency in chondrocyte and osteoblast differentiation. According to in vitro studies, the ability of mesenchymal precursors to initiate chondrogenic differentiation is dependent upon cell configuration within the mesenchymal condensation, which varies by the density of the condensation (Archer et al., 1985). In addition, inhibitory factors that block chondrogenesis in the developing condensation act by altering the expression of matrix components, thereby altering the ECM of the condensation and preventing cartilage development (Solursh, 1984). Thus, it is apparent that the fidelity of the ECM to this point in the program of skeletal development is necessary for the proper formation and function of pre-chondrogenic condensations—it is required for precursor cells to reach the proper place at the proper time, and differentiate accordingly.
3.2. Life in the growth plate—influences exerted by the ECM
Following formation of the pre-chondrogenic condensations, the constituent mesenchymal precursors initiate a program of either osteogenic or chondrogenic differentiation. Those that differentiate along the chondrogenic lineage, when appropriately stimulated, progress through the distinct maturational stages previously mentioned. These maturing chondrocyte populations become organized into the epiphyseal growth plates, where they are arranged by differentiation stage into distinct zones with distinct ECMs and distinct collections of signaling molecules, including growth factors and morphogens (Fig. 1; Erlebacher et al., 1995). As we will see, the developing chondrocytes rely heavily on the integrity of their surrounding ECM as they progress through these developmental stages.
While components of a particular ECM microenvironment may be able to signal directly to adherent cells, as appears to be the case during the formation of mesenchymal condensations, certain ECM proteins are also able to bind, sequester and/or activate endogenous signaling molecules such as growth factors and morphogens, and thereby modulate the effects of these molecules on surrounding cells, adding another layer of complexity to ECM signaling.
Many soluble signaling factors are able to influence the developing chondrocyte, among them Ihh and members of the BMP family. Ihh is secreted by pre-hypertrophic chondrocytes and signals through its cognate receptor Patched (Ptc) to stimulate production of PTHrP in the resting/proliferating chondrocyte zone (Vortkamp et al., 1996; Lanske et al., 1996; Chung et al., 1998; St-Jacques et al., 1999). PTHrP, in turn, promotes chondrocyte proliferation while retarding chondrocyte differentiation (Kobayashi et al., 2002). Although direct evidence has not yet been reported, ECM components in the cartilage microenvironment are thought to mediate the exposure of developing chondrocytes to Ihh, thereby mediating its effects on these cells and allowing for proper tissue organization and development.
The BMPs were initially discovered due to their ability to induce ectopic endochondral bone formation (Urist, 1965), and certain members of this family are able to induce the expression of chondrocyte-specific genes in chondroblast cell lines (Chen et al., 1991; Duprez et al., 1996). BMP-2, -4 and -7 have distinct stage-specific expression patterns in the developing mouse, although the precise molecular and cellular mechanisms by which these molecules regulate endochondral ossification are unknown (reviewed in Hogan, 1996; Enomoto-Iwamoto et al., 1998). Recently, a potential interaction has been suggested between fibrillin-2, a structural component of cartilage extracellular microfibrils, and BMP-7 in the process of limb patterning (Arteaga-Solis et al., 2001). Mice heterozygous for null alleles of the genes encoding each of these proteins show the combined phenotype of the individual nullizygous animals, suggesting a link between the presence of fibrillin-2 and the ability of BMP-7 to signal within the developing limb. Fibrillin-2 is expressed differentially throughout the developing limb, and by binding BMP-7, may help to establish a subsequent gradient of this molecule throughout the tissue. Alternatively, interaction with fibrillin-2 may be required for BMP-7 activity, such that differential amounts of fibrillin-2 in the developing limb would cause differential amounts of active BMP-7 within the tissue, allowing for the various cell types to receive different cues based on their location within the tissue.
The importance of this function of the ECM in skeletal development becomes especially clear when disease conditions are considered. A number of disorders are connected with loss of function mutations specifically in genes encoding the fibrillins, including Marfan syndrome (fibrillin-1), characterized by skeletal and limb symptoms including arachnodactyly and spine curvature, and congenital contractural arachnodactyly (CCA, fibrillin-2), characterized by skeletal and limb symptoms including scoliosis, multiple joint contractures, and limited extension of fingers and toes (Ramirez, 1996; Quondamatteo et al., 2002). In addition, a recent study has linked autosomal dominant Weill–Marchesani syndrome, a skeletal dysplasia which causes symptoms including short stature and brachydactyly, with a deletion of the latent transforming growth factor-β binding protein (LTBP) motif of fibrillin-1 (Faivre et al., 2003). Although the specific mechanism of each condition is unclear, it is possible that the causative mutations render the bone ECMs in affected individuals unable to properly establish and/or regulate the necessary gradients of important signaling molecules.
In addition, new evidence suggests that post-translational modification of the cartilage ECM is required for chondrocyte maturation. The protein cross-linking enzyme transglutaminase-2 (TG2) is required for chondrocyte hypertrophy, suggesting that developing chondrocytes respond to ECM complexes with higher-order organization (Johnson et al., 2003).
3.3. Chondrocyte organization—the further impact of a developing ECM on the developing chondrocyte
Studies performed in null animals provide the majority of the evidence for the impact of the developing ECM on the differentiating chondrocyte. Of particular interest are mice deficient in production of type II collagen, the ECM component characteristically expressed and secreted by resting and proliferating chondrocytes. In humans, a number of mutations in the gene encoding the α1 chain of type II collagen (COL2A1) result in hypochondroplasia, a condition characterized by a lack of chondrocytes in the developing bone (Vissing et al., 1989; Horton et al., 1992; Bogaert et al., 1992), in conjunction with growth plate disorganization and a reduced ECM density (Freisinger et al., 1994). COL2A1-null mice display severe chondrocyte disorganization and a lack of epiphyseal growth plates and mineralized bone matrix in their developing long bones, despite normal development processes in their craniofacial and axial skeletons (Li et al., 1995). More recent studies on these animals show that vertebral chondrocytes in these animals retain the ability not only to express cartilage-specific matrix components, but also to fully mature to hypertrophy (Aszódi et al., 1998). These results suggest that type II collagen is required for organization of differentiating chondrocytes within the developing endochondral bones, and that this organization, while required for proper development of these bones, is not required for the differentiation and function of the developing chondrocytes.
A similar situation exists for perlecan (also called HSPG2), a major heparan sulfate proteoglycan of the cartilage (SundarRaj et al., 1995), which is able to interact with other ECM molecules and bind local growth factors (Aviezer et al., 1994; Arikawa-Hirasawa et al., 1999). Perlecan is a component of virtually all basement membranes (Iozzo et al., 1994; Handler et al., 1997) and is thought to guard the integrity of the ECM—particularly that of the developing cartilage—through its interactions with other ECM components (Costell et al., 1999). As in the case of type II collagen, mice deficient for perlecan show severe disorganization of the cartilage in the developing bone and a defect in endochondral ossification, but their chondrocytes retain the ability to differentiate normally (Arikawa-Hirasawa et al., 1999; Costell et al., 1999). Perlecan deficiency has been implicated in the human condition Schwartz–Jampel syndrome, a disorder also characterized by chondrodysplasia (Nicole et al., 2000). These results suggest that perlecan, like type II collagen, is required for organization of the cartilage in the developing endochondral bone, but that this organization is not required for chondrocyte differentiation. Overall, this collection of literature points to a vital organizational role for the ECM in order to promote normal endochondral bone development.
These examples attest to a potentially dramatic impact of the ECM on the developing chondrocyte. However, this communication is not by any means unidirectional. As we will see, the chondrocyte also exerts significant influence on the development of the ECM.
4. Chondrogenic development—the impact of a changing chondrocyte on the ECM
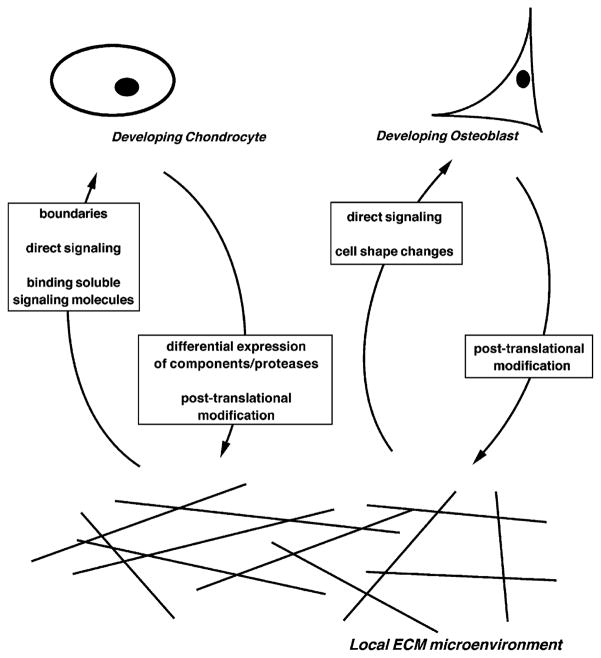
Just as the ECM may exert both direct and indirect influences on adherent cells and consequently modulate their behavior, so can these cells exert influences back on the ECM. This may be accomplished in a variety of fashions, including differential expression of particular ECM components and/or proteases by cells in a local microenvironment (Fig. 2). As a result, the ECM in a particular microenvironment paints a vivid picture of the cell types present, as well as their developmental status.
Fig. 2. Give and take between the ECM and mesenchymal cells of the developing bone.
The mesenchymal cells of the developing bone, in particular the developing chondrocyte and osteoblast, experience a bidirectional ‘give and take’ relationship with their respective ECMs. The ECM is able to influence the development of these cell types by marking locations and boundaries, signaling directly to the cells, binding soluble signaling molecules, and/or mediating cell shape changes. The cells, in turn, are able to influence the development of their respective ECMs by differentially expressing ECM components and proteases that cleave ECM components and/or post-translationally modifying/organizing ECM components.
4.1. Differential expression of ECM components
Mesenchymal precursors that differentiate along the chondrogenic pathway initiate a specific genetic program in which they express a number of characteristic ECM components including type II collagen, type IX collagen, and type XI collagen (Hall, 1988; Wagner and Karsenty, 2001). These cells progress through maturation to hypertrophy, when they express yet another cadre of ECM components including type X collagen (Wagner and Karsenty, 2001). When the cartilaginous epiphyseal growth plates have been established, the chondrocytes are organized within this zone according to their differentiation stage, and the zone in which each distinct chondrocyte population is located is bounded by its distinct ECM (Fig. 1).
Signals received by cells in a particular ECM micro-environment may be modulated by the differential production of particular ECM components. Targeted deletion studies of a variety of ECM components in cartilage and bone as well as other tissues attest to the essential roles of individual components in developmental processes (Hynes, 1996). The progression from mesenchymal precursors through cartilage to bone in the processes of bone development and bone repair requires a number of different ECMs, made possible by the differential expression of several ECM components by local cells, in particular the fibrillar collagens (reviewed in Sandberg et al., 1993). According to experiments involving in vivo reconstitution of endochondral ossification, a shift in collagen expression may be observed throughout the process of chondrocyte development, from mesenchymal precursors that primarily express type III collagen, through chondrocytes that primarily express type II and then type X collagen (Reddi et al., 1977). More recent studies using this model have shown that the expression patterns of other ECM components including fibronectin, aggrecan and other proteoglycans, and the integrins that allow chondrocytes to bind these molecules, are modulated during the process of endochondral ossification (Yu et al., 1991; Alini et al., 1992). Mutations in type I and type II collagens that cause even minor structural alterations impact the ability of these molecules to participate in matrix assembly and result in bone and cartilage disorders (Byers, 1990). Thus, the differential expression of matrix components by chondrocytes, in particular specific collagens, is a vital fixture in their differentiation program, and is required for proper cartilage and subsequent bone development.
4.2. Differential expression of proteases
Just as ECM signaling can be modulated by the differential expression of ECM components, so can it be regulated by the differential expression of proteases that cleave ECM components. Such cleavage may expose cryptic epitopes, allowing for activation of the cleaved ECM substrates, or conversely, deactivate the ECM substrates by this cleavage process. Cleavage of ECM components may be mediated by a number of proteases, including members of the matrix metalloproteinase (MMP; Sternlicht and Werb, 2001), ADAM-TS (Carpizo and Iruela-Arispe, 2000; Caterson et al., 2000; Tang, 2001) and cathepsin families (McGrath, 1999), which may be differentially expressed and are selective in their substrates (Oksjoki et al., 2001). Localized remodeling of the ECM by proteases is required during developmental processes including angiogenesis (Ingber and Folkman, 1989) and skeletal development (Vu et al., 1998).
The MMPs comprise a specialized family of zinc-dependent extracellular proteinases. MMPs are able to cleave a variety of substrates, including ECM proteins, extracellular non-matrix proteins, and cell-surface proteins. Collectively, the MMPs are able to cleave virtually all ECM components, and by virtue of their wide variety of substrates, play a role in a number of developmental processes (Sternlicht and Werb, 2001). In particular, MMP9 (gelatinase-B), MMP13 (collagenase-3) and MMP14 (MT1-MMP) are expressed by cells in the developing bone, and are important mediators of endochondral ossification (Vu et al., 1998; Pendas et al., 1997; Enomoto et al., 2000; Holmbeck et al., 1999). From the perspective of the developing chondrocyte, these proteases are essential for local ECM remodeling and subsequent normal chondrocyte maturation. Indeed, the absence of MMP9, MMP13 or both results in an enlargement of the hypertrophic zone of the epiphyseal growth plate and a subsequent delay in the ossification process (Vu et al., 1998; Ortega et al., 2003). Further characterization of these phenotypes in the authors’ laboratory have shown that the ECM in the single or double-null mice is altered and as such, chondrocytes within the local microenvironments of null bones may be exposed to molecules and epitopes not generally seen by cells within the local microenvironments of wild-type bones (D. Behonick, unpublished observations). Such results suggest that the ability of the cells in this microenvironment, particularly the chondrocytes, to express proteases such as MMPs, is required for proper ECM remodeling and the support of normal skeletal development. Similarly, the closely related ADAM-TS family of metalloproteases, which includes the enzymes that cleave cartilage aggrecan and procollagen peptidases (Caterson et al., 2000; Tang, 2001), also appear to play a role in remodeling the proteoglycan portion of the skeleton.
Following the terminal differentiation of the chondrocyte to the hypertrophic state, this cell secretes large amounts of a specialized matrix high in type X collagen, as well as enzymes that mineralize and partially degrade this matrix, and then dies a quiet, apoptotic death. The microenvironment is then invaded by a variety of cells that the chondrocyte never sees. These cell types establish the front of ossification, the junction between the hypertrophic zone of the cartilaginous growth plate and the newly formed trabecular bone, and in their own ways, are influenced by and exert influence on the development of the ECM.
5. A second ‘give and take’ relationship: the ECM and the osteoblast
A mesenchymal cousin to the chondrocyte, the osteoblast also has a ‘give and take’ relationship with the ECM in the developing skeleton. The primary responsibility of the osteoblast is the deposition of matrix that will mineralize to give newly formed bone; however, the ECM is able to exert its own influence in turn, and shape the fate of the osteoblast.
5.1. The effect of the ECM on the osteoblast
The osteoblast is an especially appropriate example of a cell that receives direct signals from the ECM. The primary function of the osteoblast is the deposition of a type I collagen matrix at the front of ossification in the developing endochondral long bone. Lack of an organized type I collagen ECM blocks the expression of osteoblast-specific genes required for development of the mature osteoblast phenotype (Franceschi and Iyer, 1992), and more recent studies have demonstrated that the ability of osteoblasts to properly produce and secrete a highly organized collagen I matrix is required for differentiation of osteogenic precursors into mature, fully-functional osteoblasts (reviewed in Franceschi, 1999). The mechanism for this cellular responsiveness to the ECM requires binding of osteoblasts to the type I collagen matrix they secrete via β1 integrins (Xiao et al., 1998; Zimmerman et al., 2000) and transduction of this signal intracellularly by the MAP kinase (MAPK) pathway (Takeuchi et al., 1997; Xiao et al., 2002). Phosphorylation of the osteoblast-specific transcription factor core binding factor 1 (Cbfa1, also called Runx2) by the MAPK pathway is thought to result in upregulation of osteoblast-specific genes at the promoter level (Xiao et al., 1998; reviewed in Franceschi and Xiao, 2003). More recently, Ziros et al. (2002) has shown that osteoblasts respond to mechanical signals including mechanical loading and stretching by upregulating a variety of osteoblast-specific genes through the activation of Cbfa1. These mechanical signals are transduced extracellularly by the ECM, and are most likely transduced intracellularly via the MAPK pathway.
As is true for the developing chondrocyte, communication between the developing bone ECM and the developing osteoblast is hardly unidirectional. The developing osteoblast exerts influence on the ECM of the developing bone by way of its unique function in bone development.
5.2. The effect of the osteoblast on the ECM
As we have seen, cells in the bone microenvironment are able to remodel the ECMs they see and touch by a variety of means—these include post-translational modification of the ECM by cells in a local microenvironment. A vital modification step in the process of skeletal development is the mineralization of the bone matrix by osteoblasts. Osteoblastic cells provide for this modification by depositing a number of macromolecules, including osteonectin (also called SPARC), a phosphorylated glycoprotein (Holland et al., 1987), and bone sialoprotein (BSP), a glycosylated matrix protein (zur Nieden et al., 2003). Based on in vitro experiments, osteonectin and BSP play a large role in bone matrix mineralization. While osteonectin binds hydroxyapatite and calcium ions, the main mineral components of bone (Termine and Robey, 1996), BSP is a known nucleator of mineralization (Bianco et al., 1991). Although a BSP-null mouse has yet to be made, animals deficient for osteonectin display a decrease in bone mass and bone ECM, particularly type I collagen, in conjunction with reduced numbers of functional osteoblasts and osteoclasts (Delany et al., 2000). By providing for this ECM modification (mineralization), then the osteoblast is able to modulate the composition of its surrounding matrix and as a result, may impact the responses of other local cell types to this matrix.
6. Conclusions and future directions
The requirement for appropriate ECM microenvironments exists in a wide range of developing tissues. It is likely that many of the burgeoning cell types within these developing tissues experience a ‘give and take’ with their respective ECMs similar to that which exists between the developing chondrocyte (and osteoblast) and its developing ECM. It is evident that as the tissue develops, so develops the ECM, providing a vivid reflection of the events occurring at a particular time and place. In this age of recombinant DNA technology and fully sequenced genomes, many tools exist to aid in the further investigation of this field. It is likely that the use of transgenic animals, for example the variety of MMP-null animals, will bring great insight into the precise mechanisms by which the ECM regulates cell behavior in a variety of tissues.
Acknowledgments
The authors give great thanks to Dr. Dominique Stickens and Jennifer Lilla for stimulating discussions and helpful suggestions for this manuscript. This work was supported by grants from the NIH (AR46238 and AG23218).
References
- Alini M, Matsui Y, Dodge GR, Poole AR. The extracellular matrix of cartilage in the growth plate before and during calcification: changes in composition and degradation of type II collagen. Calcif Tissue Int. 1992;50:327–335. doi: 10.1007/BF00301630. [DOI] [PubMed] [Google Scholar]
- Archer CW, Rooney P, Cottrill CP. Cartilage morphogenesis in vitro. J Embryol Exp Morphol. 1985;90:33–48. [PubMed] [Google Scholar]
- Arikawa-Hirasawa E, Watanabe H, Takami H, Hassell JR, Yamada Y. Perlecan is essential for cartilage and cephalic development. Nat Genet. 1999;23:354–358. doi: 10.1038/15537. [DOI] [PubMed] [Google Scholar]
- Arteaga-Solis E, Gayraud B, Lee SY, Shum L, Sakai L, Ramirez F. Regulation of limb patterning by extracellular microfibrils. J Cell Biol. 2001;154:275–281. doi: 10.1083/jcb.200105046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aszódi A, Chan D, Hunziker E, Bateman JF, Fässler R. Collagen II is essential for the removal of the notochord and the formation of intervertebral discs. J Cell Biol. 1998;143:1399–1412. doi: 10.1083/jcb.143.5.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aszódi A, Bateman JF, Gustafsson E, Boot-Handford R, Fässler R. Mammalian skeletogenesis and extracellular matrix: what can we learn from knockout mice? Cell Struct Funct. 2000;25:73–84. doi: 10.1247/csf.25.73. [DOI] [PubMed] [Google Scholar]
- Aviezer D, Hecht D, Safran M, Eisinger M, David G, Yayon A. Perlecan, basal lamina proteoglycan, promotes basic fibroblast growth factor-receptor binding, mitogenesis, and angiogenesis. Cell. 1994;79:1005–1013. doi: 10.1016/0092-8674(94)90031-0. [DOI] [PubMed] [Google Scholar]
- Bianco P, Fisher LW, Young MF, Termine JD, Robey PG. Expression of bone sialoprotein (BSP) in developing human tissues. Calcif Tissue Int. 1991;49:421–426. doi: 10.1007/BF02555854. [DOI] [PubMed] [Google Scholar]
- Bogaert R, Tiller GE, Weis MA, Gruber HE, Rimoin DL, Cohn DH, Eyre DR. An amino acid substitution (Gly853 → Glu) in the collagen alpha 1 (II) chain produces hypochondrogenesis. J Biol Chem. 1992;267:22522–22526. [PubMed] [Google Scholar]
- Brooke BS, Karnik SK, Li DY. Extracellular matrix in vascular morphogenesis and disease: structure versus signal. Trends Cell Biol. 2003;13:51–56. doi: 10.1016/s0962-8924(02)00007-7. [DOI] [PubMed] [Google Scholar]
- Byers PH. Brittle bones-fragile molecules: disorders of collagen gene structure and expression. Trends Genet. 1990;6:293–300. doi: 10.1016/0168-9525(90)90235-x. [DOI] [PubMed] [Google Scholar]
- Carpizo D, Iruela-Arispe ML. Endogenous regulators of angiogenesis-emphasis on proteins with thrombospondin—type I motifs. Cancer Metastasis Rev. 2000;19:159–165. doi: 10.1023/a:1026570331022. [DOI] [PubMed] [Google Scholar]
- Caterson B, Flannery CR, Hughes CE, Little CB. Mechanisms involved in cartilage proteoglycan catabolism. Matrix Biol. 2000;19:333–344. doi: 10.1016/s0945-053x(00)00078-0. [DOI] [PubMed] [Google Scholar]
- Chen P, Carrington JL, Hammonds RG, Reddi AH. Stimulation of chondrogenesis in limb bud mesoderm cells by recombinant human bone morphogenetic protein 2B (BMP-2B) and modulation by transforming growth factor beta 1 and beta 2. Exp Cell Res. 1991;195:509–515. doi: 10.1016/0014-4827(91)90403-h. [DOI] [PubMed] [Google Scholar]
- Chung U, Lanske B, Lee K, Li E, Kronenberg H. The parathyroid hormone/parathyroid hormone-related peptide receptor coordinates endochondral bone development by directly controlling chondrocyte differentiation. Proc Natl Acad Sci USA. 1998;95:13030–13035. doi: 10.1073/pnas.95.22.13030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costell M, Gustafsson E, Aszódi A, Morgelin M, Bloch W, Hunziker E, Addicks K, Timpl R, Fässler R. Perlecan maintains the integrity of cartilage and some basement membranes. J Cell Biol. 1999;147:1109–1122. doi: 10.1083/jcb.147.5.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottrill CP, Archer CW, Wolport L. Cell sorting and chondrogenic aggregate formation in micromass culture. Dev Biol. 1987;122:503–515. doi: 10.1016/0012-1606(87)90314-9. [DOI] [PubMed] [Google Scholar]
- Delany AM, Amling M, Priemel M, Howe C, Baron R, Canalis E. Osteopenia and decreased bone formation in osteonectin-deficient mice. J Clin Invest. 2000;105:915–923. doi: 10.1172/JCI7039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duprez DM, Coltey M, Amthor H, Brickell PM, Tickle C. Bone morphogenetic protein-2 (BMP-2) inhibits muscle development and promotes cartilage formation in chick limb bud cultures. Dev Biol. 1996;174:448–452. doi: 10.1006/dbio.1996.0087. [DOI] [PubMed] [Google Scholar]
- Enomoto H, Enomoto-Iwamoto M, Iwamoto M, Nomura S, Himeno M, Kitamura Y, Kishimoto T, Komori T. Cbfa1 is a positive regulatory factor in chondrocyte maturation. J Biol Chem. 2000;275:8695–8702. doi: 10.1074/jbc.275.12.8695. [DOI] [PubMed] [Google Scholar]
- Enomoto-Iwamoto M, Iwamoto M, Mukudai Y, Kawakami Y, Nohno T, Higuchi Y, Takemoto S, Ohuchi H, Noji S, Kurisu K. Bone morphogenetic protein signaling is required for maintenance of differentiated phenotype, control of proliferation, and hypertrophy in chondrocytes. J Cell Biol. 1998;140:409–418. doi: 10.1083/jcb.140.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlebacher A, Filvaroff EH, Gitelman SE, Derynck R. Toward a molecular understanding of skeletal development. Cell. 1995;80:371–378. doi: 10.1016/0092-8674(95)90487-5. [DOI] [PubMed] [Google Scholar]
- Faivre L, Gorlin RJ, Wirtz MK, Godfrey M, Dagoneau N, Samples JR, Le Merrer M, Collod-Beroud G, Boileau C, Munnich A, Cormier-Daire V. In frame fibrillin-1 gene deletion in autosomal dominant Weill–Marchesani syndrome. J Med Genet. 2003;40:34–36. doi: 10.1136/jmg.40.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsberg E, Hirsch E, Frohlich L, Meyer M, Ekblom P, Aszódi A, Werner S, Fässler R. Skin wounds and severed nerves heal normally in mice lacking tenascin-C. Proc Natl Acad Sci USA. 1996;93:6594–6599. doi: 10.1073/pnas.93.13.6594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschi RT, Iyer BS. Relationship between collagen synthesis and expression of the osteoblast phenotype in MC3T3-E1 cells. J Bone Miner Res. 1992;7:235–246. doi: 10.1002/jbmr.5650070216. [DOI] [PubMed] [Google Scholar]
- Franceschi RT. Developmental control of osteoblast-specific gene expression: role of specific transcription factors and the extracellular matrix environment. Crit Rev Oral Biol Med. 1999;10:40–57. doi: 10.1177/10454411990100010201. [DOI] [PubMed] [Google Scholar]
- Franceschi RT, Xiao G. Regulation of the osteoblast-specific transcription factor Runx2: responsiveness to multiple signal transduction pathways. J Cell Biochem. 2003;88:446–454. doi: 10.1002/jcb.10369. [DOI] [PubMed] [Google Scholar]
- Freisinger P, Ala-Kokko L, LeGuellec D, Franc S, Bouvier R, Ritvaniemi P, Prockop DJ, Bonaventure J. Mutation in the COL2A1 gene in a patient with hypchondrogenesis. J Biol Chem. 1994;269:13663–13669. [PubMed] [Google Scholar]
- Goldsmith EC, Borg TK. The dynamic interaction of the extracellular matrix in cardiac remodeling. J Card Fail. 2002;8:S314–S318. doi: 10.1054/jcaf.2002.129258. [DOI] [PubMed] [Google Scholar]
- Gould SE, Upholt WB, Kosher RA. Syndecan 3: a member of the syndecan family of membrane-intercalated proteoglycans that is expressed in high amounts at the onset of chicken limb cartilage differentiation. Proc Natl Acad Sci USA. 1992;89:3271–3275. doi: 10.1073/pnas.89.8.3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall BK. The Embryonic development of bone. Am Sci. 1988;76:174–181. [Google Scholar]
- Hall BK, Miyake T. The membranous skeleton: the role of cell condensations in vertebrate skeletogenesis. Anat Embryol. 1992;186:107–124. doi: 10.1007/BF00174948. [DOI] [PubMed] [Google Scholar]
- Hall BK, Miyake T. All for one and one for all: condensations and the initiation of skeletal development. Bioessays. 2000;22:138–147. doi: 10.1002/(SICI)1521-1878(200002)22:2<138::AID-BIES5>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Handler M, Yurchenco PD, Iozzo RV. Developmental expression of perlecan during murine embryogenesis. Dev Dyn. 1997;210:130–145. doi: 10.1002/(SICI)1097-0177(199710)210:2<130::AID-AJA6>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Hattori T, Ide H. Limb bud chondrogenesis in cell culture, with particular reference to serum concentration in the culture medium. Exp Cell Res. 1984;150:338–346. doi: 10.1016/0014-4827(84)90577-9. [DOI] [PubMed] [Google Scholar]
- Hogan BLM. Bone morphogenetic proteins: multifunctional regulators of vertebrate development. Genes Dev. 1996;10:1580–1594. doi: 10.1101/gad.10.13.1580. [DOI] [PubMed] [Google Scholar]
- Holland PW, Harper SJ, McVey JH, Hogan BL. In vivo expression of mRNA for the Ca++-binding protein SPARC (osteonectin) revealed by in situ hybridization. J Cell Biol. 1987;105:473–482. doi: 10.1083/jcb.105.1.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmbeck K, Bianco P, Caterina J, Yamada S, Kromer M, Kuznetsov SA, Mankani M, Robey PG, Poole AR, Pidoux I, Ward JM, Birkedal-Hansen H. MT1-MMP-deficient mice develop dwarfism, osteopenia, arthritis, and connective tissue disease due to inadequate collagen turnover. Cell. 1999;99:81–92. doi: 10.1016/s0092-8674(00)80064-1. [DOI] [PubMed] [Google Scholar]
- Horton WA, Machado MA, Ellard J, Campbell D, Bartley J, Ramirez F, Vitale E, Lee B. Characterization of a type II collagen gene (COL2A1) mutation identified in cultured chondrocytes from human hypochondrogenesis. Proc Natl Acad Sci USA. 1992;89:4583–4587. doi: 10.1073/pnas.89.10.4583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton WA. Morphology of connective tissue: cartilage. In: Royce RN, Steinmann B, editors. Connective Tissue and its Heritable Disorders. Molecular Genetic, and Medical Aspects. Wiley-Liss; New York: 1993. pp. 73–84. [Google Scholar]
- Hunziker EB. Mechanism of longitudinal bone growth and its regulation by growth plate chondrocytes. Microsc Res Tech. 1994;28:505–519. doi: 10.1002/jemt.1070280606. [DOI] [PubMed] [Google Scholar]
- Hurle JM, Ganan Y, Macias D. Experimental analysis of the in vivo chondrogenic potential of the interdigital mesenchyme of the chick leg bud subjected to local ectodermal removal. Dev Biol. 1989;132:368–374. doi: 10.1016/0012-1606(89)90233-9. [DOI] [PubMed] [Google Scholar]
- Hynes RO. Targeted mutations in cell adhesion genes: what have we learned from them? Dev Biol. 1996;180:402–412. doi: 10.1006/dbio.1996.0314. [DOI] [PubMed] [Google Scholar]
- Ingber DE, Folkman J. How does extracellular matrix control capillary morphogenesis? Cell. 1989;58:803–805. doi: 10.1016/0092-8674(89)90928-8. [DOI] [PubMed] [Google Scholar]
- Iozzo RV, Cohen IR, Grassel S, Murdoch AD. The biology of perlecan: the multifaceted heparan sulphate proteoglycan of basement membranes and pericellular matrices. Biochem J. 1994;302:625–639. doi: 10.1042/bj3020625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KA, Van Etten D, Nanda N, Graham RM, Terkeltaub RA. Distinct transgultaminaseII/TG2-independent and TG2-dependent pathways mediate articular chondrocyte hypertrophy. J Biol Chem. 2003;278:18824–18832. doi: 10.1074/jbc.M301055200. [DOI] [PubMed] [Google Scholar]
- Jüppner H. Role of parathyroid hormone-related peptide and Indian hedgehog in skeletal development. Pediatr Nephrol. 2000;14:606–611. doi: 10.1007/s004670000343. [DOI] [PubMed] [Google Scholar]
- Karsenty G, Wagner EF. Reaching a genetic and molecular understanding of skeletal development. Cell. 2002;2:389–406. doi: 10.1016/s1534-5807(02)00157-0. [DOI] [PubMed] [Google Scholar]
- Katagiri T, Takahashi N. Regulatory mechanisms of osteoblast and osteoclast differentiation. Oral Dis. 2002;8:147–159. doi: 10.1034/j.1601-0825.2002.01829.x. [DOI] [PubMed] [Google Scholar]
- Kirsch T, Harrison G, Worch KP, Golub EE. Regulatory roles of zinc in matrix vesicle-mediated mineralization of growth plate cartilage. J Bone Miner Res. 2000;15:261–270. doi: 10.1359/jbmr.2000.15.2.261. [DOI] [PubMed] [Google Scholar]
- Knudson CB, Toole BP. Changes in the pericellular matrix during differentiation of limb bud mesoderm. Dev Biol. 1985;112:308–318. doi: 10.1016/0012-1606(85)90401-4. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Chung U, Schipani E, Starbuck M, Karsenty G, Katagiri T, Goad DL, Lanske B, Kronenberg HM. PTHrP and Indian hedgehog control differentiation of growth plate chondrocytes at multiple steps. Development. 2002;129:2977–2986. doi: 10.1242/dev.129.12.2977. [DOI] [PubMed] [Google Scholar]
- Koyama E, Leatherman JL, Shimazu A, Nah HD, Pacifici M. Syndecan-3, Tenascin-C, and the development of cartilaginous skeletal elements and joints in chick limbs. Dev Dyn. 1995;203:152–162. doi: 10.1002/aja.1002030204. [DOI] [PubMed] [Google Scholar]
- Koyama E, Shimazu A, Leatherman JL, Golden EB, Nah HD, Pacifici M. Expression of Syndecan-3 and Tenascin-C: possible involvement in periosteum development. J Orthop Res. 1996;14:403–412. doi: 10.1002/jor.1100140310. [DOI] [PubMed] [Google Scholar]
- Kulyk WM, Upholt WB, Kosher RA. Fibronectin gene expression during cartilage differentiation. Development. 1989;106:449–455. doi: 10.1242/dev.106.3.449. [DOI] [PubMed] [Google Scholar]
- Lanske B, Karaplis AC, Lee K, Luz A, Vortkamp A, Pirro A, Karperien M, Defize LH, Ho C, Mulligan RC, Abou-Samra AB, Juppner H, Segre GV, Kronenberg HM. PTH/PTHrP receptor in early development and Indian hedgehog-related bone growth. Science. 1996;273:663–666. doi: 10.1126/science.273.5275.663. [DOI] [PubMed] [Google Scholar]
- Li J, Zhang YP, Kirsner RS. Angiogenesis in wound repair: angiogenic growth factors and the extracellular matrix. Microsc Res Tech. 2003;60:107–114. doi: 10.1002/jemt.10249. [DOI] [PubMed] [Google Scholar]
- Li SW, Prockop DJ, Helminen H, Fässler R, Lapvetelainen T, Kiraly K, et al. Transgenic mice with targeted inactivation of the Col2 alpha 1 gene for collagen II develop a skeleton with membranous and periosteal bone but no endochondral bone. Genes Dev. 1995;9:2821–2830. doi: 10.1101/gad.9.22.2821. [DOI] [PubMed] [Google Scholar]
- Lukashev ME, Werb Z. ECM signaling: orchestrating cell behavior and misbehavior. Trends Cell Biol. 1998;8:437–441. doi: 10.1016/s0962-8924(98)01362-2. [DOI] [PubMed] [Google Scholar]
- Mackie EJ, Thesleff I, Chiquet-Ehrismann R. Tenascin is associated with chondrogenic and osteogenic differentiation in vivo and promotes chondrogenesis in vitro. J Cell Biol. 1987;105:2569–2579. doi: 10.1083/jcb.105.6.2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath ME. The lysosomal cysteine proteases. Ann Rev Biophys Biomol Struct. 1999;28:181–204. doi: 10.1146/annurev.biophys.28.1.181. [DOI] [PubMed] [Google Scholar]
- Nicole S, Davoine CS, Topaloglu H, Cattolico L, Barral D, Beighton P, Hamida CB, Hammouda H, Cruaud C, White PS, Samson D, Urtizberea JA, Lehmann-Horn F, Weissenbach J, Hentati F, Fontaine B. Perlecan, the major proteoglycan of basement membranes, is altered in patients with Schwartz–Jampel syndrome (chondrodystrophic myotonia) Nat Genet. 2000;26:480–483. doi: 10.1038/82638. [DOI] [PubMed] [Google Scholar]
- zur Nieden NI, Kempka G, Ahr HJ. In vitro differentiation of embryonic stem cells into mineralized osteoblasts. Differentiation. 2003;71:18–27. doi: 10.1046/j.1432-0436.2003.700602.x. [DOI] [PubMed] [Google Scholar]
- Oksjoki S, Soderstrom M, Vuorio E, Anttila L. Differential expression of cathepsins B, H, K, L and S in the mouse ovary. Mol Hum Reprod. 2001;7:27–34. doi: 10.1093/molehr/7.1.27. [DOI] [PubMed] [Google Scholar]
- Ortega N, Behonick D, Stickens D, Werb Z. How proteases regulate bone morphogenesis. Ann NY Acad Sci. 2003;995:109–116. doi: 10.1111/j.1749-6632.2003.tb03214.x. [DOI] [PubMed] [Google Scholar]
- Pendas AM, Balbin M, Llano E, Jimenez MG, Lopez-Otin C. Structural analysis and promoter characterization of the human collagenase-3 gene (MMP13) Genomics. 1997;40:222–233. doi: 10.1006/geno.1996.4554. [DOI] [PubMed] [Google Scholar]
- Poole AR. The growth plate: cellular physiology, cartilage assembly and mineralization. In: Hall BK, Newman SA, editors. Cartilage: Molecular Aspects. CRC Press; Boca Raton: 1991. pp. 179–211. [Google Scholar]
- Prieto AL, Edelman GM, Crossin KL. Multiple integrins mediate cell attachment to cytotactin/tenascin. Proc Natl Acad Sci USA. 1993;90:10154–10158. doi: 10.1073/pnas.90.21.10154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quondamatteo F, Reinhardt DP, Charbonneau NL, Pophal G, Sakai LY, Herken R. Fibrillin-1 and fibrillin-2 in human embryonic and early fetal development. Matrix Biol. 2002;21:637–646. doi: 10.1016/s0945-053x(02)00100-2. [DOI] [PubMed] [Google Scholar]
- Ramirez F. Fibrillin mutations in Marfan syndrome and related phenotypes. Curr Opin Genet Dev. 1996;6:309–315. doi: 10.1016/s0959-437x(96)80007-4. [DOI] [PubMed] [Google Scholar]
- Reddi AH, Anderson WA. Collagenous bone matrix-induced endochondral ossification and hemopoiesis. J Cell Biol. 1976;69:557–571. doi: 10.1083/jcb.69.3.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddi AH, Gay R, Gay S, Miller EJ. Transitions in collagen types during matrix-induced cartilage, bone and bone marrow formation. Proc Natl Acad Sci USA. 1977;74:5589–5592. doi: 10.1073/pnas.74.12.5589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saga Y, Yagi T, Ikawa Y, Sakakura T, Aizawa S. Mice develop normally without tenascin. Genes Dev. 1992;6:1821–1831. doi: 10.1101/gad.6.10.1821. [DOI] [PubMed] [Google Scholar]
- Salmivirta M, Elenius K, Vainio S, Hofer U, Chiquet-Ehrismann R, Thesleff I, Jalkanen M. Syndecan from embryonic tooth mesenchyme binds tenascin. J Biol Chem. 1991;266:7733–7739. [PubMed] [Google Scholar]
- Sandberg MM, Aro HT, Vuorio EI. Gene expression during bone repair. Clin Orthop. 1993;289:292–312. [PubMed] [Google Scholar]
- Solursh M. Ectoderm as a determinant of early tissue pattern in the limb bud. Cell Differ. 1984;15:17–24. doi: 10.1016/0045-6039(84)90025-3. [DOI] [PubMed] [Google Scholar]
- Sriramarao P, Mendler M, Bourdon MA. Endothelial cell attachment and spreading on human tenascin is mediated by alpha 2 beta 1 and alpha v beta 3 integrins. J Cell Sci. 1993;105:1001–1012. doi: 10.1242/jcs.105.4.1001. [DOI] [PubMed] [Google Scholar]
- Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol. 2001;17:463–516. doi: 10.1146/annurev.cellbio.17.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St-Jacques B, Hammerschmidt M, McMahon AP. Indian hedgehog signaling regulates proliferation and differentiation of chondrocytes and is essential for bone formation. Genes Dev. 1999;13:2072–2086. doi: 10.1101/gad.13.16.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SundarRaj N, Fite D, Ledbetter S, Chakravarti S, Hassell JR. Perlecan is a component of cartilage matrix and promotes chondrocyte attachment. J Cell Sci. 1995;108:2663–2672. doi: 10.1242/jcs.108.7.2663. [DOI] [PubMed] [Google Scholar]
- Takeuchi Y, Suzawa M, Kikuchi T, Nishida E, Fujita T, Matsumoto T. Differentiation and transforming growth factor-beta receptor down-regulation by collagen-alpha2 beta1 integrin interaction is mediated by focal adhesion kinase and its downstream signals in murine osteoblastic cells. J Biol Chem. 1997;272:29309–29316. doi: 10.1074/jbc.272.46.29309. [DOI] [PubMed] [Google Scholar]
- Tang BL. ADAMTS: a novel family of extracellular matrix proteases. Int J Biochem Cell Biol. 2001;33:33–44. doi: 10.1016/s1357-2725(00)00061-3. [DOI] [PubMed] [Google Scholar]
- Termine JD, Robey PG. Bone matrix proteins and the mineralization process. In: Favus MJ, editor. Primer on the metabolic bone diseases and disorders of mineral metabolism. Lippincott-Raven; Philadelphia: 1996. pp. 24–28. [Google Scholar]
- Urist MR. Bone: formation by autoinduction. Science. 1965;150:893–899. doi: 10.1126/science.150.3698.893. [DOI] [PubMed] [Google Scholar]
- Vissing H, D’Alessio M, Lee B, Ramirez F, Godfrey M, Hollister DW. Glycine to serine substitution in the triple helical domain of pro-alpha 1 (II) collagen results in a lethal perinatal form of short-limbed dwarfism. J Biol Chem. 1989;264:18265–18267. [PubMed] [Google Scholar]
- Vortkamp A, Lee K, Lanske B, Segre GV, Kronenberg HM, Tabin C. Regulation of rate of cartilage differentiation by Indian hedgehog and PTH-related protein. Science. 1996;273:613–622. doi: 10.1126/science.273.5275.613. [DOI] [PubMed] [Google Scholar]
- Vu TH, Shipley JM, Bergers G, Berger JE, Helms JA, Hanahan D, Shapiro SD, Senior RM, Werb Z. MMP-9/Gelatinase B is a key regulator of growth plate angiogenesis and apoptosis of hypertrophic chondrocytes. Cell. 1998;93:411–422. doi: 10.1016/s0092-8674(00)81169-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner EF, Karsenty G. Genetic control of skeletal development. Curr Opin Gen Dev. 2001;11:527–532. doi: 10.1016/s0959-437x(00)00228-8. [DOI] [PubMed] [Google Scholar]
- Widelitz RB, Jiang TX, Murray BA, Chuong CM. Adhesion molecules in skeletogenesis: II. Neural cell adhesion molecules mediate precartilaginous mesenchymal condensations and enhance chondrogenesis. J Cell Physiol. 1993;156:399–411. doi: 10.1002/jcp.1041560224. [DOI] [PubMed] [Google Scholar]
- Wuthier RE, Chin JE, Hale JE, Register TC, Hale LV, Ishikawa Y. Isolation and characterization of calcium-accumulating matrix vesicles from chondrocytes of chicken epiphyseal growth plate cartilage in primary culture. J Biol Chem. 1985;260:15972–15979. [PubMed] [Google Scholar]
- Xiao G, Wang D, Benson MD, Karsenty G, Franceschi RT. Role of the alpha2-integrin in osteoblast-specific gene expression and activation of the Osf2 transcription factor. J Biol Chem. 1998;273:32988–32994. doi: 10.1074/jbc.273.49.32988. [DOI] [PubMed] [Google Scholar]
- Xiao G, Gopalakrishnan R, Di J, Reith E, Benson MD, Franceschi RT. Bone morphogenic proteins, extracellular matrix, and mitogen-activated protein kinase signaling pathways are required for osteoblast-specific gene expression and differentiation in MC3T3-E1 cells. J Bone Miner Res. 2002;17:101–110. doi: 10.1359/jbmr.2002.17.1.101. [DOI] [PubMed] [Google Scholar]
- Yu YM, Becvar R, Yamada Y, Reddi AH. Changes in the gene expression of collagens, fibronectin, integrin and proteoglycans during matrix-induced bone morphogenesis. Biochem Biophys Res Commun. 1991;177:427–432. doi: 10.1016/0006-291x(91)92001-z. [DOI] [PubMed] [Google Scholar]
- Zimmerman D, Jin F, Leboy P, Hardy S, Damsky C. Impaired bone formation in transgenic mice resulting from altered integrin function in osteoblasts. Dev Biol. 2000;220:2–15. doi: 10.1006/dbio.2000.9633. [DOI] [PubMed] [Google Scholar]
- Ziros PG, Rojas Gil AP, Georgakopoulos T, Haeos I, Kletsas D, Basdra EK, Papavassiliou AG. The bone-specific transcriptional regulator Cbfa1 is a target of mechanical signals in osteoblastic cells. J Biol Chem. 2002;277:23934–23941. doi: 10.1074/jbc.M109881200. [DOI] [PubMed] [Google Scholar]