Abstract
Objective
We recently reported that 60% of newly diagnosed CF children who had pancreatic insufficiency (PI) responded to treatment initiation and achieved catch-up weight gain to a level comparable to their birth weight Z-score within 2 years of diagnosis (“responders”), while the remaining 40% failed to do so (“non-responders”). The present study examined the impact of this early weight recovery on subsequent growth pattern and pulmonary status at age 6 years.
Patients and Methods
Sixty-three children with CF who had PI but no meconium ileus, and were enrolled in the Wisconsin CF Neonatal Screening Project, were studied. “Responders” were defined by a recovery of weight Z-score comparable to that at birth within 2 years of diagnosis. During ages 2–6, growth was evaluated with the combination of height and body mass index. Pulmonary status was evaluated by symptoms, spirometry, quantitative chest radiography and respiratory microbiology.
Results
The majority (71%) of the responders maintained their early weight recovery through age 6 years while only 32% of the non-responders achieved substantial growth improvement during age 2 to 6 years. Proportionately fewer responders reported cough symptoms (10% daytime cough, p =0.02; 22% nighttime cough, p=0.05) compared to non-responders (41% daytime cough, 45% nighttime cough) at age 6. Percent predicted FEV1 (%FEV1) at age 6 was 11% higher in responders (99.5 ± 13.9%) compared to non-responders (88.3 ± 18.5%), p = 0.015. Responders had significantly better Brasfield (20.1 ± 1.4, p = 0.01) and Wisconsin chest radiographic scores (8.3 ± 3.3, p = 0.04) compared to non-responders (Brasfield 18.9 ± 1.8, Wisconsin 12.3 ± 8.3). Respiratory microbiology was not significantly different. Multiple regression analyses indicated that the positive association between responder and %FEV1 at age 6 years remained statistically significant after controlling for infections with Pseudomonas aeruginosa and Staphlococcus aureus and chest radiographic scores. Growth patterns during 2–6 years of age were not associated with pulmonary measures at age 6.
Conclusion
CF patients with PI who achieved early growth recovery within 2 years of diagnosis had fewer cough symptoms, higher lung function and better chest radiography scores at 6 years of age.
Keywords: Cystic Fibrosis, Growth, Malnutrition, Height, Weight, Body mass index, Pulmonary function, Chest radiograph, Newborn screening
Cystic fibrosis (CF) is a life-threatening, genetic disorder that is generally characterized by intestinal malabsorption, impaired growth and lung disease. Malnutrition is prevalent (1-5), as indicated by the observation that nearly half of newly diagnosed CF children have a height or weight below the 5th percentile (3), and is associated with poor clinical outcomes (1,6-10). Therefore, optimizing nutritional status is critical for patients with CF. However, it is unclear why some CF patients respond to treatment initiation and succeed in recovering from malnutrition and growth faltering/failure experienced prior to diagnosis, while others fail to do so. In our recent study (11), we reported that approximately 60% of CF children who had pancreatic insufficiency (PI) but no meconium ileus (MI) responded to treatment initiation and achieved catch-up weight gain to a level comparable to their birth weight Z-score within 2 years of CF diagnosis (“responders”), while the remaining 40% did not achieve this (“non-responders”). Diagnosis through newborn screening, less severe malnutrition and pulmonary disease at the time of diagnosis, sustained high energy intake at >120% of estimated energy requirement (12), and sustained normal plasma linoleic acid level were found to be significant determinants of this early treatment response (11).
In the present study, we hypothesize that the early weight recovery experienced by the responders leads to better pulmonary status at age 6 years, when reliable pulmonary function data can be obtained in the majority of CF children (13). Specifically, we examined whether: 1) responders maintained their early weight recovery beyond 2 years post-diagnosis up to 6 years of age, 2) non-responders achieved substantial growth improvement during age 2 to 6 years, 3) high energy intake contributed to growth maintenance in responders and growth improvement in non-responders during age 2 to 6 years, and 4) responders experienced better pulmonary status compared to non-responders at 6 years of age. Data from CF children enrolled in the Wisconsin CF Neonatal Screening Project (14, 15) were utilized to address these questions.
SUBJECTS AND METHODS
Study population
The study population eligible for the present study consists of 80 CF children who have PI but not MI and are enrolled in the Wisconsin CF Neonatal Screening Project, which is a prospective longitudinal investigation initiated in 1985 to assess the benefits and risks of newborn screening for CF (14, 15). The design and purpose of the Wisconsin CF Neonatal Screening Project has been described in detail elsewhere (14, 15). Briefly, for half of the randomly assigned newborns, early diagnosis of CF was established through neonatal screening, while the diagnosis of CF in newborns randomized to the control arm was generally established by traditional methods (i.e., through signs and symptoms of CF). The study protocol was approved by the human subjects committee at the University of Wisconsin and the Research and Publications Committee/Human Rights Board at Children’s Hospital of Wisconsin.
Of the 80 eligible patients, 9 were excluded from our first study of treatment responsiveness (11) for various reasons stated in detail in that report (e.g., false negative screening result, lack of birth weight data, or low birth weight). Another 8 patients were lost to follow up before 6 years of age; the remaining 63 patients were included in the present study. No significant differences on baseline characteristics (i.e., gender, responder status, age of diagnosis, height and weight z-scores at diagnosis) were found between the 63 patients included in the present study and the entire 80 eligible patients.
Measurements of nutritional status
Growth
Birth weight was obtained from medical records or newborn screening records. Anthropometric measurements were obtained according to a standardized protocol and performed by trained research nurses and dietitians. Recumbent length (before age 2 years), standing height (after age 2 years) and weight were measured at diagnosis, every 6 weeks for the first year of life, and every 3 months thereafter (14, 16). Recumbent length was measured with the use of a calibrated wooden board, and standing height was measured with a stadiometer, to the nearest 0.5 cm. Weight was measured in unclothed infants < 2 y on a springless infant scale, and older children were weighed without shoes and outer clothing on a springless adult scale, both to the nearest 0.1 kg. Age- and sex-specific Z-scores for weight (WTz), length/height (HTz) and body mass index (BMIz) were computed by using the 2000 CDC growth charts available from http://www.cdc.gov/growthcharts (17).
Energy intake
Three-day food records were distributed to the family at diagnosis and every 6 months thereafter to assess dietary intake, as described in detail elsewhere (11, 14, 18, 19). Computerized nutrient analysis programs, Nutritionist III, IV and V (N-Squared Computing, Silverton, OR) were used to calculate daily energy intake (11, 14, 19, 20). Energy intake was expressed as a percentage of the estimated energy requirement (EER) based on the Dietary Reference Intakes released in 2002 (12) and used an ‘active’ physical activity factor (21).
Essential fatty acid status
Essential fatty acid status was assessed at diagnosis and every 6 months thereafter (16, 22). Plasma fatty acid composition was measured in total lipid extracts by using the gas-chromatography methods described by Farrell et al. (23, 24).
Definition of responders within 2 years of diagnosis
As described in detail previously (11), we evaluated early treatment response based on recovery from malnutrition and growth faltering, which is a common presentation in CF children with newly diagnosed, untreated CF (2, 3, 25, 26). CF children were referred to as responders if their catch-up weight gain within 2 years of diagnosis resulted in achieving a WTz similar to their WTz at birth. On the other hand, those who did not gain sufficient weight to achieve a WTz comparable to their birth WTz within 2 years of diagnosis were referred to as non-responders. The rationale of using birth WTz to define adequate weight gain is based on the observations that birth weights of CF children tend to be similar (25, 26) or only slightly lower (18, 26) compared to those of healthy children, but a large percentage of CF children fall below the 5th percentile by the time of diagnosis (3, 25, 26). In addition, physical growth of infants and young children varies greatly due to intrauterine, genetic and nutritional influences (27). Therefore, recovery of weight to a level comparable to one’s birth WTz represents a more individualized indicator of treatment responsiveness than using a common reference level (such as weight > 5th or 10th percentile) for all CF children.
Classification of growth patterns beyond 2 years of diagnosis up to age 6 years
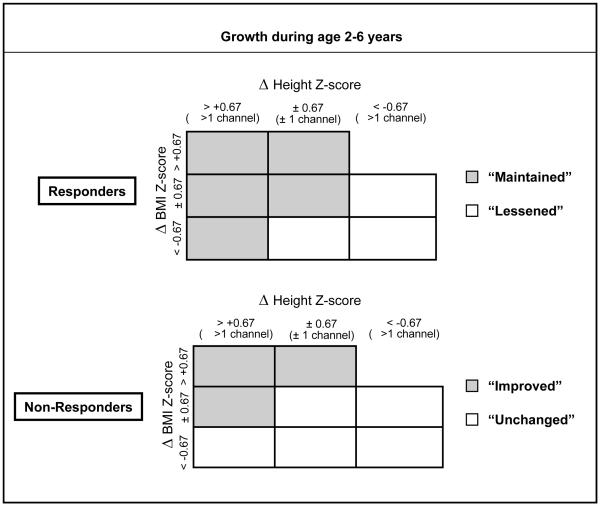
Growth beyond 2 years of diagnosis up to age 6 years was evaluated based on the combination of changes in HTz and BMIz. WTz was not used in combination with HTz to define growth pattern between ages 2–6 because WTz is influenced by both age and height and thus highly correlated with HTz. For example, a child may have a low WTz simply because of being short for age, and not due to a low weight for height. On the other hand, BMIz is an independent indicator of weight for height proportion, thus providing a complimentary measure to HTz. Specifically, the cumulative changes in HTz and BMIz from ages 2 to 6 (i.e., ΔHTz and ΔBMIz) were estimated by using linear regression technique for each individual CF child (i.e., slope multiplied by the 4-year interval). Thereafter, ΔHTz and ΔBMIz were evaluated relative to a one-channel difference (equivalent to approximately 0.67 Z-score) on the respective CDC growth charts (Figure 1). For responders, those whose ΔHTz and/or ΔBMIz increased or remained stable were classified as “Maintained” (Figure 1, gray boxes) because their responder status was maintained over the period of age 2–6. On the other hand, responders whose ΔHTz and/or ΔBMIz declined were classified as “Lessened” (Figure 1, clear boxes). For non-responders, those whose ΔHTz and/or ΔBMIz increased were classified as “Improved” (Figure 1, gray boxes), while those whose ΔHTz and ΔBMIz remained stable or declined were classified as “Unchanged” (Figure 1, clear boxes) because their non-responder status did not change over the age period of 2 to 6.
Figure 1.
Schematic of growth pattern definition from diagnosis to age six years. Recovery of birth weight z-score is represented by responders, who achieved catch-up weight gain to a level comparable to their birth weight Z-score within 2 years of CF diagnosis. Non-responders are those who did not gain sufficient weight to achieve a weight z-score comparable to their birth weight z-score within 2 years of diagnosis.
Pulmonary and clinical outcome measures
Measures of pulmonary status included parent-reported respiratory symptoms (cough and wheezing) (28), lung function by spirometry (13, 29), lung image by quantitative chest radiology (30-32), and pulmonary infection by respiratory microbiology (33, 34).
Respiratory symptoms (cough and wheezing) were reported by the parents/caregivers at each protocol visit (at diagnosis, every 6 weeks during the first year of life, and every 3 months thereafter). The frequency of cough was recorded as 0 = none, 1 = rare/occasional, 2 = mild dry cough, 3 = mild productive cough, and 4 = frequent/severe. Wheezing experiences were recorded as 0 = clear, 1 = mild, 2 = moderate, and 3 = severe. We considered cough to be present if the score was two or above and wheezing to be present if the score was one or above, as reported previously (28).
Pulmonary function tests (PFT) were obtained every 6 months beginning when the children reached age 4 (to allow for a training period between age 4–6 years), as described in detail previously (13). The quality of data was ensured using the Pediatric Alternate Spirometry System (PASS) we developed and validated previously (13, 29). Only data accepted by at least two of the three raters were considered valid (13, 29). Fifty-five (87%) had at least one valid PFT measurements between 4–8 years of age; the valid PFT measurement that was closest to age 6 years (mean age at PFT: 6.2 ± 0.9 years, range 4.5 to 8.7 years) for each patient was retained for analysis. The remaining 8 patients had PFT measurements between age 4–8 years but none of the measurements were accepted by the PASS system. Percent predicted forced vital capacity (%FVC), forced expiratory volume in one second (%FEV1), and FEV1 to FVC ratio were calculated by using equations from Wang et al (35).
Chest radiographic (CXR) scores were used to evaluate hyperinflation (“air trapping”) and structural abnormalities of the lungs such as bronchiectasis (30, 31). Chest radiographs were obtained every 6 months from diagnosis to 4 years of age and annually thereafter (30, 31). The original films obtained at diagnosis, age 2 years, age 4 years and every year thereafter were scored using the Wisconsin Chest Radiograph Scoring System (13, 30, 31) and the Brasfield system (32). Two raters, who were unaware of patient identities and demonstrated previously to score the images similarly with standardized methods (30, 31), assigned subscores according to disease categories; the total score was computer generated (30, 31). To evaluate pulmonary infections, cultures of respiratory secretions were obtained by vigorous oropharyngeal swabbing during forced coughing at the time of diagnosis, every 6 months thereafter, and at all sick visits as described elsewhere (33). These secretions were cultured to detect the presence of microbial pathogens. The two respiratory pathogens that are most likely to infect young children with CF, Staphylococcus aureus (SA) and Pseudomonas aeruginosa (PA), were analyzed in the present study. PA infection was examined in multiple ways: 1) the traditional approach of “never positive” vs. “ever positive” (33) at age 2 and age 6 years, and 2) the relatively new approach (36-38) of “never”, “transient” (1–2 positives), “intermittent” (3–4 positives but no more than 2 consecutive positives) and “persistent” (5–10 positives and no more than 2 consecutive negatives) between age 2 to 6 years.
In addition, Shwachman-Kulczki (39) scores were obtained to indicate the overall clinical severity of CF at each protocol visit. The Shwachman-Kulczki scoring system includes four inter-related components: general activity, physical examination, nutrition and chest radiographic findings with a scoring range of 5–25 points for each component and a total score of 20–100 points (39). The four components of the score were determined by the managing physician, who was unaware of patient status with regard to responder or non-responder.
Statistical Analysis
Analyses focused on comparing clinical characteristics between responders and non-responders, and among the four growth patterns during age 2 to 6, as illustrated in Figure 1. SAS (version 8.02, SAS Institute, Inc, Cary NC, 2001) and R (http://www.r-project.org) (40) were used for data processing and statistical analyses. One-way analysis of variance was used to compare means when the data appeared normally distributed, and the Wilcoxon rank sum test was used to compare means when the data appeared skewed. Non-parametric analysis of variance was used to compare medians. Chi-square (when sample size was larger than 5 in all subgroups), Fisher’s exact (when sample size was smaller than 5 in a given subgroup) and Mantel-Haenszel chi-square tests were used to compare proportions. Multiple regression analysis was used to evaluate the association between responder status and %FEV1 while adjusting for relevant covariates. When the overall p-value from type III analysis indicated statistical significance, multiple comparisons were performed to identify differences between subgroups of interest. All analyses on pulmonary function at age 6 utilized 55 CF children who had valid %FEV1 data.
RESULTS
Comparison of responders and non-responders at 2 years of age
At two years of age, responders had significantly better growth (HTz, WTz and BMIz) and Shwachman-Kulczcki scores (physical exam subscore, growth subscore and total score) compared to non-responders (Table 1). Parent-reported cough symptoms, Brasfield and Wisconsin CXR scores, as well as SA and PA infections at age 2 years did not differ significantly between responders and non-responders.
Table 1.
Characteristics of CF in responders and non-responders at two years of age
| Responder | Non-Responder | P-value | |
|---|---|---|---|
| N (%) | 41 (65%) | 22 (35%) | |
| Age of diagnosis (mo) | |||
| mean ± SD | 4.0 ± 6.2 | 2.6 ± 3.2 | 0.39 |
| Median | 1.8 | 1.7 | 0.44 |
| No. (%) diagnosed by newborn screening | 28 (68%) | 14 (64%) | 0.71 |
| Growth indices | |||
| Weight-for-age Z-score (WTz) | 0.28 ± 0.93 | −1.23 ± 0.81 | <0.0001 |
| Height-for-age Z-score (HTz) | 0.06 ± 0.89 | −1.03 ± 0.79 | <0.0001 |
| BMI Z-score (BMIz) | 0.43 ± 0.92 | −0.38 ± 0.90 | 0.001 |
| Shwachman-Kulczycki scores* | |||
| Activity | 24.8 ± 0.9 | 24.5 ± 1.2 | 0.27 |
| Physical Exam | 24.2 ± 1.7 | 23.1 ± 2.8 | 0.05 |
| Growth | 24.1 ± 2.1 | 21.1 ± 3.9 | 0.0003 |
| Chest radiographic score | 22.7 ± 1.9 | 22.0 ± 2.1 | 0.24 |
| Total score | 95.7 ± 3.8 | 90.7 ± 7.3 | 0.001 |
| Parent-reported cough and wheezing | |||
| Presence of daytime coughing, N (%) | 4 (10%) | 6 (27%) | 0.25 |
| Presence of nighttime coughing, N (%) | 7 (17%) | 8 (36%) | 0.09 |
| Presence of wheezing, N (%) | 2 (5%) | 2 (9%) | 0.61 |
| Brasfield and Wisconsin CXR scores† | |||
| Brasfield score | 21.1 ± 1.5 | 20.5 ± 2.2 | 0.16 |
| Wisconsin score | 5.9 ± 3.5 | 8.0 ± 5.8 | 0.08 |
| Respiratory Infection‡ | |||
| Positive SA, N (%) | 7 (18%) | 6 (27%) | 0.37 |
| Positive PA, N (%) | 6 (15%) | 6 (27%) | 0.24 |
1 responder did not have Shwachman-Kulczycki score at age 2
7 responders and 1 non-responder did not have Brasfield and Wisconsin CXR scores at age 2
1 responder did not have respiratory infection data at age 2
Growth patterns of responders and non-responders during 2– 6 years of age
As shown in Table 2, the majority of responders (71%) experienced improved or stable growth. On the other hand, less than one-third of the non-responders experience substantial growth improvement over the subsequent 4 years.
Table 2.
Growth status, energy intake, and plasma linoleic acid concentration in responders and non-responders*
| Responder | Non-Responder | P-value | |||
|---|---|---|---|---|---|
| Maintained | Lessened | Improved | Unchanged | ||
| N (%) | 29 (71%) | 12 (29%) | 7 (32%) | 15 (68%) | |
| Growth indices | |||||
| Weight-for-age Z-score (HTz) at age 6 | 0.40 ± 0.67a | −0.27 ± 0.53b | −0.70 ± 0.60bc | −0.86 ± 0.68c | <0.0001 |
| Height-for-age Z-score (HTz) at age 6 | 0.26 ± 0.96a | −0.34 ± 0.81ab | −1.22 ± 0.61c | −0.90 ± 0.82bc | <0.0001 |
| BMI Z-score (BMIz) at age 6 | 0.53 ± 0.51a | 0.01 ± 0.8bc | 0.26 ± 0.67ab | −0.27 ± 0.61c | 0.001 |
| Average energy intake (%EER†) over age 2–6‡ | 124 ± 26bc | 108 ± 22c | 148 ± 17a | 136 ± 29ab | 0.009 |
| Linoleic acid (% total plasma fatty acids) over age 2–6 | 27.4 ± 3.1 | 26.7 ± 5.0 | 27.6 ± 4.4 | 27.0 ± 3.4 | 0.9 |
Values with different superscripts within the same row are significantly different
EER: estimated energy requirement (EER), based on the Dietary Reference Intakes (DRI) released in 2002 (12)
Three responders and four non-responders did not have dietary data
At 6 years of age, responders whose growth was maintained during age 2 to 6 (Table 2) had the best HTz and BMIz; both were above the 50th percentile (i.e., z-scores greater than zero). Responders whose growth lessened during age 2 to 6 had significantly lower BMIz but not HTz compared to responders whose growth maintained during age 2 to 6 (Table 2). Nevertheless, HTz, WTz, and BMIz scores of responders whose growth lessened during age 2 to 6 remained somewhat better compared to non-responders whose growth was unchanged during age 2 to 6 (Table 2). Non-responders whose growth improved during age 2 to 6 had the lowest HTz, but BMIz was better than non-responders whose growth remained unchanged during age 2 to 6 (Table 2).
Further analysis was performed to examine whether energy intake and plasma linoleic acid concentrations during age 2–6 years differed between responders and non-responders. On average, non-responders consumed more calories (141 ± 25 %EER) than responders (119 ± 26 %EER) during age 2–6 years, p = 0.005. Energy intake did not differ significantly in responders whose growth maintained compared to those whose growth lessened, nor in non-responders whose growth improved compared to those whose growth was unchanged (Table 2). Average plasma linoleic acid concentrations did not differ significantly among the four growth categories (Table 2).
Pulmonary status of responders and non-responders at 6 years of age
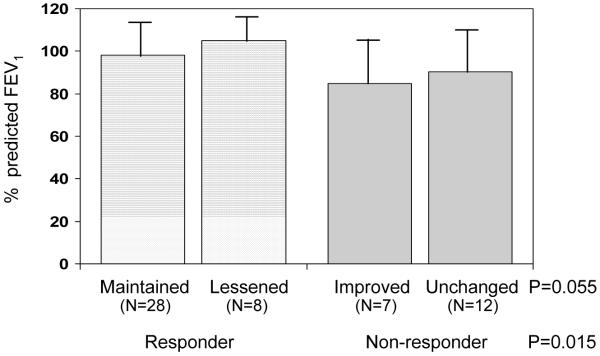
As shown in Figure 2, responders had significantly higher %FEV1 (99.5 ± 13.9%) compared to non-responders (88.3 ± 18.5%), p = 0.015. Percent predicted FVC (responders: 103.1 ± 11.2%, non-responders: 97.6 ± 16.1%) and percent predicted FEV1/FVC ratio (responders: 96.4 ± 10.2%, non-responders: 93.5 ± 14.5 %) did not differ significantly in responders compared to non-responders. No significant differences were found in %FEV1 (Figure 2), %FVC or percent predicted FEV1/FVC ratio at 6 years of age between responders whose growth were maintained or lessened, and between non-responders whose growth were improved or unchanged, during 2–6 years of age.
Figure 2.
Pulmonary function as indicated by percent predicted FEV1 according to responder status and growth category.
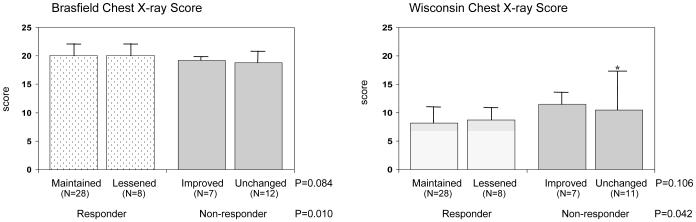
Consistent with pulmonary function data, chest radiographic scores were better in responders than in non-responders. As shown in Figure 3, responders had higher Brasfield CXR score (20.1 ± 1.4, normal score is 25, p = 0.010) and lower Wisconsin CXR score (8.3 ± 3.3, normal score is 0, p = 0.042) compared to non-responders (Brasfield CXR = 18.9 ± 1.8; Wisconsin CXR = 12.3 ± 8.3) at age 6 years. The average CXR scores during age 2 to 6 were also significantly better in responders (Brasfield CXR = 20.7 ± 1.1; Wisconsin CXR = 6.7 ± 2.1) than in non-responders (Brasfield CXR = 19.8 ± 2.0; Wisconsin CXR = 9.2 ± 5.5), p = 0.030 and p = 0.009 for Brasfield and Wisconsin scores, respectively. No significant differences were found in either CXR score at 6 years of age between responders whose growth maintained or lessened, and between non-responders whose growth improved or was unchanged, during 2–6 years of age (Figure 3).
Figure 3.
Chest radiographic scores according to responder status and growth category. The first panel shows mean Brasfield score (32) within each group. A better Brasfield result is indicated by a higher score. The second panel shows mean scores using the Wisconsin scoring method (30, 31). Lower values from the Wisconsin method indicate fewer chest x-ray abnormalities. *One extreme value was excluded in the calculation of mean and SD.
With regard to pulmonary infections, no significant differences were found in the rates of ever-positive PA and SA at age 6 between responders (PA: 37%, SA: 29%) and non-responders (PA: 27%, SA: 41%). Further analysis showed that the percentage of patients with never (responders: 37%, non-responders: 32%), transient (responders: 39%, non-responders: 36%), intermittent (responders: 7%, non-responders: 14%) and persistent (responders: 17%, non-responders: 18%) PA colonization during age 2–6 years also did not differ significantly between responders and non-responders. Lastly, no significant differences were found in the rate of ever-positive PA and SA at 6 years of age between responders whose growth maintained or lessened, and between non-responders whose growth improved or unchanged, during 2–6 years of age.
In addition to the objective measures of lung function and chest radiographic scores, proportionately fewer responders reported to experience less daytime cough (10%, p = 0.02) and nighttime cough (22%, p = 0.05) at age 6 years compared to non-responders (41% daytime cough and 45% nighttime cough). Total Shwachman-Kulczki score at age 6 was significantly higher in responders (94 ± 5) than non-responders (89 ± 6), p = 0.002.
Factors associated with %FEV1 at age 6 years
Regression analyses were performed to identify significant predictors of pulmonary function at age 6. Univariate analysis demonstrated that responder status (i.e., achieving a catch-up weight gain within 2 years of diagnosis) is the single strongest predictor of %FEV1 at age 6 (Table 3). Of the three variables indicative of PA status, PA at age 6 had a stronger association with %FEV1 at age 6 than PA at age 2 or PA during age 2 to 6. On the other hand, SA at age 2 had a stronger association with %FEV1 at age 6 than SA at age 6. Regarding chest radiographic scores, mean CXR score during age 2 to 6 had a stronger association with %FEV1 at age 6 than CXR score at age 2 or at age 6. Brasfield and Wisconsin CXR scores had similar strength of associations with %FEV1 at age 6.
Table 3.
Regression analyses on factors associated with % predicted FEV1 at age 6 years
| regression coefficient | P-value | |
|---|---|---|
| Univariate regression with %FEV1 as the response variable | ||
| Responder (vs. non-responder) | +11.2 | 0.015 |
| Growth during age 2 to 6 (vs. “maintained”) | +6.9 (“lessened”) | 0.280 |
| −7.6 (“unchanged”) | 0.167 | |
| −13.3 (“improved”) | 0.050 | |
| SA positive at age 2 (vs. negative) | −7.9 | 0.141 |
| SA positive at age 6 (vs. negative) | +1.2 | 0.798 |
| PA positive at age 2 (vs. negative) | −0.7 | 0.898 |
| PA positive at age 6 (vs. negative) | −4.3 | 0.362 |
| PA positive during age 2 to 6 (vs. never) | +0.1 (transient) | 0.981 |
| −8.4 (intermittent) | 0.319 | |
| −2.2 (persistent) | 0.741 | |
| Brasfield CXR at age 2 | +1.7 | 0.170 |
| Wisconsin CXR at age 2 | −0.4 | 0.438 |
| Brasfield CXR at age 6 | +1.2 | 0.367 |
| Wisconsin CXR at age 6 | −0.5 | 0.256 |
| Mean Brasfield CXR during age 2 to 6 | +2.1 | 0.135 |
| Mean Wisconsin CXR during age 2 to 6 | −0.7 | 0.193 |
| Multiple regression with %FEV1 as the response variable | ||
| Model 1: adjusting for PA and SA | ||
| Responder (vs. non-responder) | +10.9 | 0.019 |
| SA positive at age 2 (vs. negative) | −5.7 | 0.272 |
| PA positive at age 6 (vs. negative) | −5.4 | 0.235 |
| Model 2: adjusting for CXR | ||
| Responder (vs. non-responder) | +10.0 | 0.041 |
| Mean Brasfield CXR during age 2 to 6 | +1.0 | 0.475 |
| Model 3: adjusting for both PA and CXR | ||
| Responder (vs. non-responder) | +9.9 | 0.046 |
| SA positive at age 2 (vs. negative) | −5.7 | 0.281 |
| PA positive at age 6 (vs. negative) | −5.1 | 0.268 |
| Mean Brasfield CXR during age 2 to 6 | +0.8 | 0.582 |
| Logistic regression with %FEV1 < 5th percentile (i.e., < 82.5%) as the response variable | ||
| Model 4: adjusting for both PA and CXR | ||
| Responder (vs. non-responder) | 0.09 (0.02, 0.44)* | 0.003 |
| SA positive at age 6 (vs. negative) | 1.63 (0.32, 8.24)* | 0.557 |
| PA positive at age 6 (vs. negative) | 1.42 (0.29, 6.84)* | 0.664 |
| Mean Brasfield CXR during age 2 to 6 | 1.05 (0.89, 1.23)* | 0.851 |
odds ratio and 95% confidence interval
Based on observations from the univariate analyses, SA at age 2, PA at age 6 and mean Brasfield CXR score were included as covariates in multiple regression analyses to examine their influences on the association between responder status and %FEV1 at age 6. All multivariate models in Table 3 showed that responder status remained a significant and the strongest predictor of %FEV1 at age 6. This association did not change when other PA, SA or CXR variables were adjusted for in the multivariate models.
DISCUSSION
This study utilized a prospective cohort to investigate the impact of early recovery of birth weight z-score within 2 years of diagnosis of CF, on pulmonary status at age 6. Our results demonstrate that, in CF children who have PI but no MI, early attainment of birth weight status following CF diagnosis is associated with significantly better pulmonary outcomes at age 6 years, which are consistently reflected in the symptom (cough scores), function (%FEV1) and structural appearance (chest radiographic scores) of the lungs. The difference in pulmonary function, i.e., ~10% in %FEV1 between responders and non-responders, is large enough to be clinically significant, especially in view of the average annual decline of %FEV1 typically estimated at 2–3% (5). In addition, compared to non-responders, these early responders required lower caloric intake to maintain adequate growth and achieve better pulmonary function, which may be associated with lower work of breathing, i.e., less energy expenditure for respiration. The third novel finding from the present study is that this early weight recovery has a stronger effect on pulmonary status at age 6 years than subsequent maintenance or improvement of growth experienced during age 2 to 6 years.
Results from the present and our previous (11) report advance our knowledge in understanding the patterns of growth experienced by CF children who have PI but no MI during the first 6 years of life. Specifically, we showed that 60% of these children responded to treatment initiation and achieved catch-up weight gain to a level similar to their birth weight z-score within 2 years of diagnosis (11); the majority (~70%) of these responders maintained this growth recovery through age 6 years, but the remaining ~30% exhibited a decline in growth during age 2 to 6 years. Conversely, 40% of CF children who had PI but not MI did not achieve early weight recovery (11) and only few of these non-responders (~30%) experienced substantial growth improvement during age 2 to 6 years. Our data suggest that high caloric intake contributed to growth maintenance within responders (average intake of >120% of estimated requirement) and growth improvement within non-responders (average intake of >140% of estimated requirement) during ages 2–6 years (Table 2).
The temporal relationships between energy intake and growth patterns observed in our studies demonstrate the complex interactions between treatment and response that change over time for a chronic disease such as CF. First, we observed that sustained high-energy intake following diagnosis contributed to initial treatment response (11). However, average caloric intake during age 2 to 6 was significantly lower in responders than in non-responders (Table 2). The latter observation should not be interpreted as a negative association between energy intake and growth during age 2 to 6. Instead, it is an example of reverse causality in that poor growth in non-responders leads to the recommendation of and adherence to a higher caloric intake during this period. However, it may also be possible that non-responders’ dietary intakes were over-reported since the CF care team was likely to be closely monitoring food intake to ensure adherence to high calorie intake.
The complex interactions between treatment and response also make the search for factors determining treatment responsiveness difficult. One major challenge is to define what constitutes treatment and response. In our studies, we developed a novel approach that utilizes individualized growth parameters to define nutritional response, i.e., catch-up weight gain with birth weight as the reference point to define initial treatment response and changes in height and BMI patterns within one growth channel as the reference point to define subsequent response. However, we are not able to quantify treatment received by individual patients. Although therapeutic protocols are well defined for our study population, the amount of treatment received is likely to be continuously influenced, and adjusted, by individual’s response. An example to support this argument is the observation by Konstan et al (9) that CF children with the best %FEV1 at age 3 had the most rapid decline of %FEV1 during the subsequent 3 years, which the authors attributed to less comprehensive therapy. Delineating the impact of therapeutic variation on treatment response will require the development of new research methods.
In the present study, neither PA nor SA infection explained the difference in pulmonary outcomes between responders and non-responders. One possible explanation is that, in this young population, the prevalence of PA and SA infection was relatively low, with most infections transient. A longer evaluation period is likely needed to assess the impact of respiratory infections on pulmonary function in responders and non-responders.
Although we showed that CF children diagnosed by newborn screening were more likely to be responders compared to those diagnosed by conventional methods (11), and responders had better pulmonary status at age 6 compared to non-responders, our previous results did not reveal significantly better pulmonary function in screened patients compared to those diagnosed by traditional methods (29). Several possibilities may contribute to these findings. First, the benefit of newborn screening on pulmonary status at age 6 years may be dependent on early nutritional response. This is supported by our observations that screened responders have better %FEV1 and chest radiographic scores than screened non-responders (data not presented). Second, only 77% (55 out of 71) of the study population was included in pulmonary analyses at age 6 due to lost to follow up or invalid %FEV1 data. Reduction in sample size as well as the somewhat imbalanced sample attrition between screened responders (75% retention) and control non-responders (92% retention), although not statistically significant (p = 0.25), may have affected comparisons between screened and conventional diagnosis groups.
In conclusion, results from this study provide clear evidence that in CF children with PI but without MI, response to treatment initiation as indicated by achieving an early weight status recovery within 2 years of diagnosis is associated with better pulmonary status at age 6 years. In addition, these early responders require lower energy intake to maintain adequate growth compared to that required for promoting further growth improvement in non-responders. The indication that the benefit of newborn screening on pulmonary status at age 6 years may be dependent on early nutritional response emphasizes the need for comprehensive therapy following screening. Further research is needed to determine whether the nutritional and pulmonary benefits experienced by these early responders persist throughout childhood.
ACKNOWLEDGEMENT
The following faculty members have participated in this project: Jeff Douglas, PhD, Norman Fost, MD, MPH, Christopher Green, MD, Ronald Gregg, PhD, Michael Kosorok, PhD, Ronald Laessig, PhD, HuiChuan Lai, PhD, Mari Palta, PhD, Michael Rock, MD, Margie Rosenberg, PhD, Audrey Tluezek, PhD, L.J. Wei, PhD, Susan West, PhD, and Benjamin Wilfond, MD, University of Wisconsin Medical School, Madison; W. Theodore Bruns, MD, William Gershan, MD, Elaine Mischler, MD, Mark Splaingard, MD, and Lee Rusakow, Medical College of Wisconsin, Milwaukee. The investigation has been coordinated and managed superbly on a day-to-day basis at both sites by Anita Laxova. In addition, the Group includes outstanding teams of biostatisticians (Rebecca Koscik, Sharon Shen, Lan Zeng, Zhanhai Li), nurses (Karen Moucha, Miriam Block, Holly Colby, Lynn Feenan, Mary Ellen Freeman, Catherine McCarthy, Darci Pfeil), nutritionists (Lisa Davis, Mary Marcus, Tami Miller), and superb leaders of the Wisconsin State Laboratory of Hygiene’s Newborn Screening Program (David Hassamer, Gary Hoffman).
Supported by National Institutes of Health Grants 1R01DK072126, 2R01 DK34108, 1ULRR025011 and M01RR00058.
Abbreviations
- CF
cystic fibrosis
- EER
estimated energy requirement
- HTz
height z-score
- MI
meconium ileus
- PI
pancreatic insufficiency
- WTz
weight z-score
- BMIz
body mass index z-score
- PFT
pulmonary function test
- PASS
pediatric alternate spirometry system
- FVC
forced vital capacity
- FEV1
forced expiratory volume in one second
- CXR
chest x-ray
- PA
Pseudomonas aeruginosa
- SA
Staphylococcus aureus
Footnotes
None of the authors have conflict of interest.
A complete list of the researchers who have participated in this project is provided in the acknowledgement.
REFERENCES
- 1.Kraemer R, Rudeberg A, Hadorn B, Rossie E. Relative underweight in cystic fibrosis and its prognostic value. Acta Paediatr Scand. 1978;67:33–7. doi: 10.1111/j.1651-2227.1978.tb16273.x. [DOI] [PubMed] [Google Scholar]
- 2.Giglio L, Candusso M, D’Orazio C, Mastella G, Faraguna D. Failure to thrive: the earliest feature of cystic fibrosis in infants diagnosed by neonatal screening. Acta Paediatrica. 1997;86:1162–5. doi: 10.1111/j.1651-2227.1997.tb14836.x. [DOI] [PubMed] [Google Scholar]
- 3.Lai HC, Kosorok MR, Sondel SA, Chen ST, FitzSimmons SC, Green CG, Shen G, Walker S, Farrell PM. Growth status in children with cystic fibrosis based on the National Cystic Fibrosis Patient Registry data: evaluation of various criteria used to identify malnutrition. J Pediatr. 1998;132:478–85. doi: 10.1016/s0022-3476(98)70024-1. [DOI] [PubMed] [Google Scholar]
- 4.McNaughton SA, Stormont DA, Shepherd RW, Francis PW, Dean B. Growth failure in cystic fibrosis. J Paediatr Child Health. 1999;35:86–92. doi: 10.1046/j.1440-1754.1999.00329.x. [DOI] [PubMed] [Google Scholar]
- 5.Cystic Fibrosis Foundation . Cystic Fibrosis Foundation Patient Registry Annual Data Report 2002. Cystic Fibrosis Foundation; Bethesda, MD: 2003. [Google Scholar]
- 6.Shwachman H, Redmond A, Khaw KT. Studies in cystic fibrosis. Report of 130 patients diagnosed under 3 months of age over a 20-year period. Pediatrics. 1970;46:335–43. [PubMed] [Google Scholar]
- 7.Zemel BS, Jawad AF, FitzSimmons S, Stallings VA. Longitudinal relationship among growth, nutritional status, and pulmonary function in children with cystic fibrosis: analysis of the Cystic Fibrosis Foundation National CF Patient Registry. J Pediatr. 2000;137:374–80. doi: 10.1067/mpd.2000.107891. [DOI] [PubMed] [Google Scholar]
- 8.Beker LT, Russek-Cohen E, Fink RJ. Stature as a prognostic factor in cystic fibrosis survival. J Am Dietetic Assoc. 2001;101:438–42. doi: 10.1016/S0002-8223(01)00113-4. [DOI] [PubMed] [Google Scholar]
- 9.Konstan MW, Butler SM, Wohl ME, Stoddard M, Matousek R, Wagener JS, Johnson CA, Morgan WJ. Growth and nutritional indexes in early life predict pulmonary function in cystic fibrosis. J Pediatr. 2003;142:624–30. doi: 10.1067/mpd.2003.152. [DOI] [PubMed] [Google Scholar]
- 10.Milla CE. Association of nutritional status and pulmonary function in children with cystic fibrosis. Curr Opin Pulm Med. 2004;10:505–9. doi: 10.1097/01.mcp.0000138995.08494.69. [DOI] [PubMed] [Google Scholar]
- 11.Shoff SM, Ahn H, Davis L, Lai HJ, the Wisconsin CF Neonatal Screening Group Temporal associations among energy intake, plasma linoleic acid, and growth improvement in response to treatment initiation after diagnosis of cystic fibrosis. Pediatr. 2006;117:391–400. doi: 10.1542/peds.2004-2832. [DOI] [PubMed] [Google Scholar]
- 12.National Academy of Sciences . Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids (Macronutrients) National Academies Press; Washington DC: 2002. [DOI] [PubMed] [Google Scholar]
- 13.Farrell PM, Li Z, Kosorok MR, Laxova A, Green CG, Collins J, Lai HC, Rock MJ, Splaingard ML. Longitudinal evaluation of bronchopulmonary disease in children with cystic fibrosis. Pediatr Pulmonol. 2003;36:230–240. doi: 10.1002/ppul.10336. [DOI] [PubMed] [Google Scholar]
- 14.Farrell PM, Kosorok MR, Rock MJ, Laxova A, Zeng L, Lai HC, et al. Early diagnosis of cystic fibrosis through neonatal screening prevents severe malnutrition and improves long-term growth. Pediatrics. 2001;107:1–13. doi: 10.1542/peds.107.1.1. [DOI] [PubMed] [Google Scholar]
- 15.Fost N, Farrell PM. A prospective randomized trial of early diagnosis and treatment of cystic fibrosis: a unique ethical dilemma. Clin Res. 1989;37:495–500. [PubMed] [Google Scholar]
- 16.Farrell PM, Kosorok MR, Laxova A, Shen G, Koscik RE, Bruns WT, Splaingard M, Mischler EH. Nutritional benefits of neonatal screening for cystic fibrosis. New Engl J Med. 1997;337:963–9. doi: 10.1056/NEJM199710023371403. [DOI] [PubMed] [Google Scholar]
- 17.Kuczmarski RJ, Ogden CL, Guo SS, et al. 2000 CDC Growth Charts for the United States: Methods and Development. Vital & Health Statistics - Series 11: Data From the National Health Survey. 2000:iii–x. 1–190. [PubMed] [Google Scholar]
- 18.Marcus MS, Sondel SA, Farrell PM, Laxova A, Carey PM, Langhough R, Mischler EH. Nutritional status of infants with cystic fibrosis associated with early diagnosis and intervention. Am J Clin Nutr. 1991;54:578–85. doi: 10.1093/ajcn/54.3.578. [DOI] [PubMed] [Google Scholar]
- 19.Lai HC, Kosorok MR, Laxova A, Davis LA, FitzSimmon SC, Farrell PM. Nutritional status of patients with cystic fibrosis with meconium ileus: a comparison with patients without meconium ileus and diagnosed early through neonatal screening. Pediatr. 2000;105:53–61. doi: 10.1542/peds.105.1.53. [DOI] [PubMed] [Google Scholar]
- 20.Farrell PM, Lai HC, Li Z, Kosorok MR, Laxova A, Green CG, Collins J, et al. Evidence on improved outcomes with early diagnosis of cystic fibrosis through neonatal screening: Enough is enough! J Pediatr. 2005;147:S30–S36. doi: 10.1016/j.jpeds.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 21.Trabulsi J, Ittenbach RF, Schall JI, Olsen IE, Yudkoff M, Daikhin Y, Zemel BS, Stallings VA. Evaluation of formulas for calculating total energy requirements of preadolescent children with cystic fibrosis. Am J Clin Nutr. 2007;85:144–151. doi: 10.1093/ajcn/85.1.144. [DOI] [PubMed] [Google Scholar]
- 22.Mischler EH, Parrell SW, Farrell PM, Raynor WJ, Lemen RJ. Correction of linoleic acid deficiency in cystic fibrosis. Pediatr Res. 1986;20:36–41. doi: 10.1203/00006450-198601000-00010. [DOI] [PubMed] [Google Scholar]
- 23.Farrell PM, Mischler EH, Engle MJ, Brown DJ, Lau SM. Fatty acid abnormalities in cystic fibrosis. Pediatr Res. 1985;19:104–9. doi: 10.1203/00006450-198501000-00028. [DOI] [PubMed] [Google Scholar]
- 24.van Egmond AW, Kosorok MR, Koscik R, Laxova A, Farrell PM. Effect of linoleic acid intake on growth of infants with cystic fibrosis. Am J Clin Nutr. 1996;63:746–52. doi: 10.1093/ajcn/63.5.746. [DOI] [PubMed] [Google Scholar]
- 25.Pederzini F, D’Orazio C, Tamiazzo G, Faraguna D, Giglio L, Mastella G. Growth evaluation at one year of life in infants with cystic fibrosis diagnosed by neonatal screening. Pediatr Pulmonol. 1991;7(suppl):64–8. doi: 10.1002/ppul.1950110713. [DOI] [PubMed] [Google Scholar]
- 26.Bronstein MN, Sokol RJ, Abman SH, Chatfield BA, Hammond KB, Hambidge KM, et al. Pancreatic insufficiency, growth, and nutrition in infants identified by newborn screening as having cystic fibrosis. J Pediatr. 1992;120:533–40. doi: 10.1016/s0022-3476(05)82478-3. [DOI] [PubMed] [Google Scholar]
- 27.Greco L, Santamaria F, Salvatore D, de Ritis G. Growth dynamics in cystic fibrosis. Acta Paediatr. 1993;82:254–60. doi: 10.1111/j.1651-2227.1993.tb12654.x. [DOI] [PubMed] [Google Scholar]
- 28.Lai HC, Kosorok MR, Laxova A, Makholm LM, Farrell PM. Delayed diagnosis in females with cystic fibrosis in the United States. Am J Epidemiol. 2002;156:165–173. doi: 10.1093/aje/kwf014. [DOI] [PubMed] [Google Scholar]
- 29.Farrell PM, Li Z, Kosorok MR, Laxova A, Green CG, Collins J, Lai HC, Rock MJ, Splaingard ML. Bronchopulmonary disease in children with cystic fibrosis after early or delayed diagnosis. Am J Respir Crit Care Med. 2003;168:1100–1108. doi: 10.1164/rccm.200303-434OC. [DOI] [PubMed] [Google Scholar]
- 30.Weatherly MR, Palmer CGS, Peters ME, Green CG, Fryback D, Langhough R, Farrell PM. Wisconsin cystic fibrosis chest radiograph scoring system. Pediatrics. 1993;91:488–495. [PubMed] [Google Scholar]
- 31.Koscik RE, Kosorok MR, Farrell PM, Collins J, Peters ME, Laxova A, Green CG, Zeng L, Rusakow LS, Hardie RC, Campbell PW, Gurney JW. Wisconsin cystic fibrosis chest radiograph scoring system: validation and standardization for application to longitudinal studies. Pediatr Pulmonol. 2000;29:457–467. doi: 10.1002/(sici)1099-0496(200006)29:6<457::aid-ppul8>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 32.Brasfield D, Hicks G, Song S, Tiller RE. The chest roentgenogram in cystic fibrosis: a new scoring system. Pediatrics. 1979;63:24–29. [PubMed] [Google Scholar]
- 33.Farrell PM, Shen G, Splaingard M, Colby CE, Laxova A, Kosorok MR, et al. Acquisition of Pseudomonas aeruginosa in children with cystic fibrosis. Pediatrics. 1997;100(5):E2. doi: 10.1542/peds.100.5.e2. [DOI] [PubMed] [Google Scholar]
- 34.West SEH, Zeng L, Lee BL, Kosorok MR, Laxova A, Rock MJ, Splaingard MJ, Farrell PM. Respiratory infections with Pseudomonas aeruginosa in children with cystic fibrosis: early detection by serology and assessment of risk factors. JAMA. 2002;287:2958–2967. doi: 10.1001/jama.287.22.2958. [DOI] [PubMed] [Google Scholar]
- 35.Wang X, Dockery DW, Wypij D, Fay ME, Ferris BG. Pulmonary function between 6 and 18 years of age. Pediatr Pulmonol. 1993;15:75–88. doi: 10.1002/ppul.1950150204. [DOI] [PubMed] [Google Scholar]
- 36.Lee TW, Brownlee KG, Denton M, Littlewood JM, Conway SP. Reduction in prevalence of chronic Pseudomonas aeruginosa infection at a regional pediatric cystic fibrosis center. Pediatric Pulmonology. 2004;37(2):104–10. doi: 10.1002/ppul.10401. [DOI] [PubMed] [Google Scholar]
- 37.Johansen HK, Norregaard L, Gotzsche PC, Pressler T, Koch C, Hoiby N. Antibody response to Pseudomonas aeruginosa in cystic fibrosis patients: a marker of therapeutic success?--A 30-year cohort study of survival in Danish CF patients after onset of chronic P. aeruginosa lung infection. Pediatric Pulmonology. 2004;37(5):427–32. doi: 10.1002/ppul.10457. [DOI] [PubMed] [Google Scholar]
- 38.Deretic V, Schurr MJ, Yu H. Pseudomonas aeruginosa, mucoidy and the chronic infection phenotype in cystic fibrosis. Trends in Microbiology. 1995;3(9):351–6. doi: 10.1016/s0966-842x(00)88974-x. [DOI] [PubMed] [Google Scholar]
- 39.Shwachman H, Kulczycki L. Long term study of one hundred five patients with cystic fibrosis. Am J Dis Chil. 1958;96:6–15. doi: 10.1001/archpedi.1958.02060060008002. [DOI] [PubMed] [Google Scholar]
- 40.Ihaka R, Gentleman R. R: a language for data analysis and graphics. J Computat Graphic Stat. 1996;5:299–314. [Google Scholar]