Summary
One approach for the identification of therapeutic agents for Alzheimer's disease has focused on the research of α7 nAChR-selective agonists such as the partial agonists 3-(4-hydroxy,2-methoxybenzylidene)anabaseine (4OH-GTS-21) and, more recently, 2-[2-(4-bromophenyl)-2-oxoethyl]-1-methyl pyridinium (S 24795). An alternative approach for targeting α7 nAChR has been the development of positive modulators for this receptor. In this study we examined the interactions between full or partial agonists and positive modulators of α7 nAChRs in situ in brain tissue. Three positive modulators were used, 5-hydroxyindole (5-HI), 1-(5-chloro-2,4-dimethoxy-phenyl)-3-(5-methyl-isoxanol-3-yl)-urea (PNU-120596), and genistein. Whole-cell recordings were performed in stratum radiatum interneurons from rat brain slices. Hippocampal interneurons were stimulated by ACh, choline, S 24795, or 4OH-GTS-21, before and after bath perfusion with the positive modulators. 5-HI was not effective at potentiating 200 μM 4OH-GTS-21-evoked responses, however 5-HI induced a sustained potentiation of responses evoked by 30 μM 4OH-GTS-21. When 1 mM ACh and 200 μM 4OH-GTS-21 were applied alternately α7-mediated responses to both agonists were reduced, suggesting that high concentration of 4OH-GTS-21 produces residual inhibition or desensitization and that 5-HI is not effective at overcoming receptor desensitization. Similar results were obtained with α7 receptors expressed in Xenopus oocytes. Interestingly, responses evoked by S 24795 were potentiated by 5-HI but not by genistein. Additionally, PNU-120596 was able to potentiate α7-mediated responses, regardless of the nature of the agonist. We demonstrated that the potentiation of α7 nAChR response would depend on the nature and the effective concentration of the agonist involved and its particular interaction with the positive modulator.
Keywords: brain slices, patch-clamp, nAChRs, partial agonists, full agonists, hippocampus, positive allosteric modulators
Introduction
The α-bungarotoxin-sensitive, α7 nicotinic acetylcholine receptors (nAChRs) are highly expressed in hypothalamus, cortex, and hippocampus (DelToro et al., 1994; Seguela et al., 1993). Brain α7 nAChRs are homomeric receptors displaying very rapid desensitization to high concentrations of agonist, high levels of calcium permeability, and activation by choline (Castro and Albuquerque, 1995; Papke et al., 1996; Peng et al., 1994; Seguela et al., 1993).
The function and number of α7 receptors can be affected in several pathological conditions, such as schizophrenia, Alzheimer's disease (AD), and Parkinson's disease (Burghaus et al., 2003; Freedman et al., 1995; Guan et al., 1999; Guan et al., 2000). AD is characterized by loss of cholinergic projections; therefore drugs that act on or modulate nAChRs are likely to have great therapeutic potential. It has been shown that both nAChR agonists and partial agonists can enhance cognition in aged rats (Arendash et al., 1995; Socci et al., 1995). An alternative approach for targeting α7 receptors is the use of positive allosteric modulators (PAMs) that potentiate receptor function without causing direct activation.
Several positive modulators of α7 nAChRs have been documented, including ivermectin (Krause et al., 1998), 5-hydroxyindole (5-HI) (Grilli et al., 2006; Gurley et al., 2000; Mannaioni et al., 2003; Mok and Kew, 2006; Zwart et al., 2002), 1-(5-chloro-2,4-dimethoxy-phenyl)-3-(5-methyl-isoxazol-3-yl)-urea (PNU-120596) (Hurst et al., 2005), genistein (Charpantier et al., 2005; Cho et al., 2005), and compound 6 (Ng et al., 2007). Emergence of distinct PAMs has prompted a proposed classification into two types, based on their pharmacological profiles (Gronlien et al., 2007). Both types of PAMs increase the potency and efficacy of agonists. The effects of Type I, exemplified by 5-HI and genistein, are mostly observed in the agonist-evoked peak amplitude. Type II prolongs the agonist-evoked response in addition to increase peak amplitude. PNU-120596, the prototypical Type II PAM, has the apparent ability to reactivate receptors that are in a desensitized state (Hurst et al., 2005), while 5-HI potentiation was occluded by pretreatment with desensitizing concentrations of nicotine (Mok and Kew, 2006).
We performed whole-cell patch-clamp recordings in stratum radiatum interneurons from hippocampal rat brain slices, which robustly express functional α7 nAChRs (Alkondon et al., 1998; Frazier et al., 1998; Jones and Yakel, 1997), to examine the effect of 5-HI, PNU-120596, and genistein on the evoked responses by ACh and choline. Also to determine potential interactions that might occur in combinational therapies we investigated the effects of these modulators on the activity of two partial agonists proposed for clinical development, 3-(4-hydroxy,2-methoxybenzylidene)anabaseine (4OH-GTS-21) and 2-[2-(4-bromophenyl)-2-oxoethyl]-1-methyl pyridinium (S 24795). 4OH-GTS-21 is the active metabolite of GTS-21, one of the first α7-selective agonists to be identified (de Fiebre et al., 1995; Woodruff-Pak et al., 1994), while S 24795 is a second generation α7-selective partial agonist (López-Hernández et al, 2007). Through our studies we hope to provide new insights for the development of strategies to enhance nicotinic transmission in the brain and to better evaluate alternative approaches of using direct-acting agonists and/or positive modulators of receptor function.
Methods
Chemicals
4OH-GTS-21 was provided by Taiho Pharmaceuticals (Tokyo, Japan) and S 24795 by Servier (Paris, France). PNU-120596 was synthesized at the University of Florida and all other chemicals were purchased from Sigma-Aldrich (St. Louis, MO).
cDNA clones
The human α7 nAChR clone was obtained from Dr. Jon Lindstrom (University of Pennsylvania, Philadelphia PA). The RIC-3 clone was obtained from Dr. Millet Treinin (Hebrew University, Jerusalem Israel).
Preparation of RNA
Subsequent to linearization and purification of cloned cDNAs, RNA transcripts were prepared in vitro using the appropriate mMessage mMachine kit from Ambion Inc. (Austin TX).
Expression in Xenopus laevis oocytes
Mature (>9 cm) female Xenopus laevis African frogs (Nasco, Ft. Atkinson WI) were used as a source of oocytes. Before surgery the frogs were anesthetized by placing them in a 1.5 g/l solution of MS222 for 30 min. Oocytes were removed from an incision made in the abdomen.
Harvested oocytes were treated with 1.25 mg/ml collagenase (Worthington Biochemical Corporation, Freehold NJ) for two hours at room temperature in calcium-free Barth's solution (88 mM NaCl, 1 mM KCl, 2.38 mM NaHCO3, 0.82 mM MgSO4, 15 mM HEPES (pH 7.6), 12 mg/l tetracycline) in order to remove the follicular layer. Stage-5 oocytes were isolated and injected with 50 nl (5-20 ng) of α7 cRNA, usually in combination with human RIC-3 to accelerate and increase the level of nAChR expression (Halevi et al., 2003). Recordings were conducted 2-5 days post-injection.
Electrophysiology
Experiments were conducted using OpusXpress6000A (Molecular Devices, Union City, CA). OpusXpress is an integrated system that provides automated impalement and voltage clamp of up to eight oocytes in parallel. Both the voltage and current electrodes were filled with 3 M KCl. The oocytes were clamped at a holding potential of -60 mV.
Data were collected at 50 Hz and filtered at 20 Hz. The oocytes were bath-perfused with Ringer's solution. Agonist solutions were delivered from a 96-well plate using disposable tips. Flow rates were set at 2 ml/min. Drug applications usually alternated between ACh controls and test solutions of ACh or other experimental agonists at varying concentrations. PAMs were applied in the bath solution and then co-applied with agonist during the evoked responses.
Experimental protocols and data analysis
Responses of α7 receptors were calculated as net charge (Papke and Papke, 2002). Each oocyte received two initial control applications of ACh, then experimental drug applications, and follow-up control applications of ACh. The control ACh concentration were 60 μM, a concentration which is sufficient to evoke approximately 50% maximal net charge response (Papke and Papke, 2002). The initial ACh control responses from each cell were used to normalize the data for all subsequent responses and compensate for differences in the levels of channel expression among the oocytes. Mean values and standard errors (SEM) were calculated from the normalized responses of at least four oocytes for each experimental concentration, except where otherwise noted. For concentration-response relations, data were plotted using Kaleidagraph 3.0.2 (Abelbeck Software; Reading, PA), and curves were generated from the Hill equation:
Imax denotes the maximal response for a particular agonist/subunit combination, and n represents the Hill coefficient. Imax, n, and the EC50 were all unconstrained for the fitting procedures.
Brain slice preparation and Patch-clamp recording
All procedures involving animals were approved by the University of Florida Institutional Animal Care and Use Committee and were in accord with the NIH Guide for the Care and Use of Laboratory Animals. Male Sprague Dawley rats (p16-p30) were anesthetized with Halothane (Halocarbon Laboratories, River Edge NJ) and swiftly decapitated. Transverse (300 μm) whole brain slices were prepared using a vibratome (Pelco, Redding, CA) and a high Mg2+/low Ca2+ ice-cold artificial cerebral spinal fluid (ACSF) containing (in mM) 124 NaCl, 2.5 KCl, 1.2 NaH2PO4, 2.5 MgSO4, 10 D-glucose, 1 CaCl2, and 25.9 NaHCO3, saturated with 95% O2 - 5%CO2. Slices were incubated at 30°C for 30 minutes and then left at room temperature until they were transferred to a submersion chamber (Warner Instruments, Hamden, CT) for recording. During experiments, slices were perfused at a rate of 2 ml/min with normal ACSF containing (in mM) 126 NaCl, 3 KCl, 1.2 NaH2PO4, 1.5 MgSO4, 11 D-glucose, 2.4 CaCl2, 25.9 NaHCO3, and 0.004 atropine sulfate, saturated with 95% O2 - 5% CO2 at 30°C. Interneurons of the stratum radiatum were visualized with infrared differential interference contrast microscopy using a Nikon E600FN microscope. Patch-clamp recording pipettes were pulled from borosilicate glass (Sutter Instruments, Novato, CA) using a Flaming/Brown micropipette puller (P-97; Sutter Instruments, Novato, CA). Recording pipettes were filled with an internal solution of (in mM) 125 K-gluconate, 1 KCl, 0.1 CaCl2, 2 MgCl2, 1 EGTA, 2 MgATP, 0.3 Na3GTP, and 10 HEPES (pH 7.3 using KOH). The resistance of the recording pipette when filled with the internal solution was 3-5 MΩ. Cells were held at −70 mV, and a -10 mV/10ms test pulse was used to determine access resistance, input resistance, and whole-cell capacitance. Cells with access resistances > 60 MΩ or those requiring holding currents > 200 pA were not included in the final analyses. Signals were digitized using an Axon Digidata1322A and sampled at 20 kHz on a Dell computer using Clampex version 8 or 9. Data analysis was done with Clampfit version 8 or 9 (Axon Instruments, Union City, CA), Excel 2000 (Microsoft, Seattle, WA), GraphPad/Prism version 4.02 (Graphpad Software, San Diego, CA), and SigmaStat version 3.5 (Systat Software, Inc., San Jose, CA). Data are reported as mean ± SEM. Statistical analyses were done using two-tailed Student's t-test and one-way analysis of variance (ANOVA).
Drug application
Local somatic applications of ACh (1 mM pipette concentration), choline (2 mM pipette concentration), S 24595 (1 mM pipette concentration), and 4OH-GTS-21 (200 μM or 30 μM pipette concentration) were made using single- or double-barrel glass pipettes attached to a picospritzer (General Valve, Fairfield, NJ) with Teflon tubing (10-20 psi for 5-15 ms). ACh and either choline or S 24795 were alternately applied every 30 s, while ACh and 4OH-GTS-21 were alternately applied with an interstimulus interval of 1 min. Single-barrel pipettes were pulled from borosilicate glass with an outer diameter (o.d.) and inner diameter (i.d.) of 1.5 mm and 0.86 mm, respectively (Sutter Instrument, Novato, CA). Pipette opening size of the single barrel was typically 2-3 μm. Double-barrel pipettes were pulled from borosilicate theta glass with an o.d. of 1.5 mm; pipette opening size was around 3-4 μm. The application pipette was usually placed within 10-15 μm of the cell soma. For each cell, four baseline agonist-evoked responses were recorded followed by bath application of the positive modulator. 5-HI was bath-applied at a final concentration of 1 mM. PNU-120596 and genistein were bath-applied at a final concentration of 10 μM. Evoked responses were then recorded for 15-22 min. In some experiments 4OH-GTS-21 was bath-applied at a concentration of 1 μM.
When pipettes were loaded with 1 mM ACh, the average net charge of evoked responses did not differ significantly between single- and double-barrel experiments (data not shown). Experiments conducted to describe the error produced by alternating pressure applications using double-barrel pipettes showed an 85 ± 8% (n=5) correspondence in the peak amplitudes between the agonist applications from the two barrels (data not shown). In previous experiments we determined that pressure application from a pipette containing 1 mM ACh delivered an effective concentration of approximately 30 μM to the surface of the cell, thus a dilution factor of 3:100 would be expected for each agonist pressure-ejected in our system (Lopez-Hernandez et al., 2007). It should be noticed that this dilution factor was estimated using the pressure application method to deliver drugs with a double-barrel pipette to the soma of hippocampal neurons in brain slices and it may not be necessarily the same in other systems. This application method is suitable when applying small quantities of compounds to defined locations of in vitro brain slices, but leakage, dilution, and/or diffusion of agonist from the pipette tip can contribute to the difference of the agonist concentration at the application pipette and that experienced at the surface of the cell. However, this should not be the case with drug delivery using bath applications into the bulk solution.
Results
Potentiation of choline-evoked responses
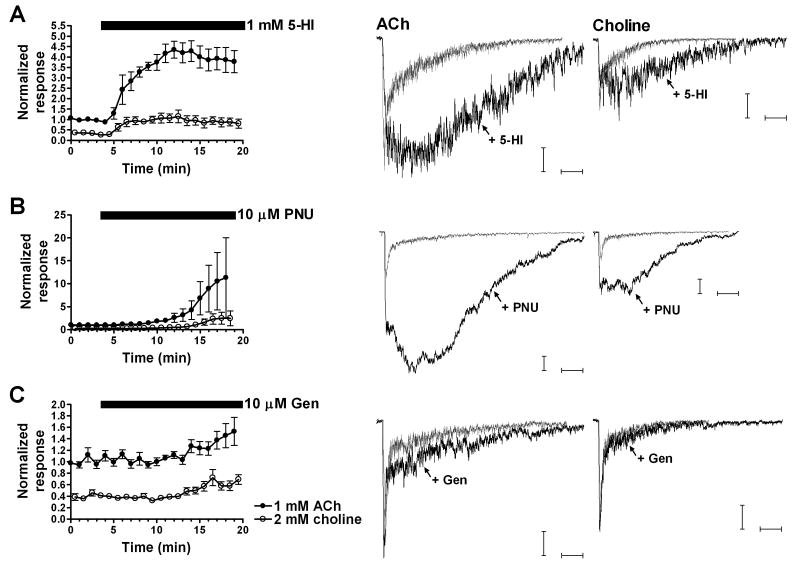
To evaluate the potentiation induced by each positive modulator, the percent change was estimated using the normalized area (relative to baseline ACh-evoked responses) for the last four evoked responses and the normalized response during baseline conditions (first four evoked responses in the absence of positive modulator). In the double-barrel experiments in which ACh and choline were in the barrels of the application pipette, all three positive modulators were able to significantly potentiate the responses evoked by either ACh or choline in rat hippocampus (Fig. 1). Following 5-HI application, the percent change relative to baseline was 191 ± 35 for choline-evoked responses and 285 ± 26 for ACh responses. For PNU-120596-induced potentiation, the percent change for choline was 1166 ± 416 while for ACh it was 832 ± 272. In the case of genistein (Fig. 1C) the percent change was 67 ± 14 for choline-evoked responses and 40 ± 9 for ACh responses.
Figure 1. Effects of 5-HI, PNU-120596, and genistein on ACh- and choline-evoked responses.
A-C. (Left panel) time courses for the 1 mM 5-HI-, 10 μM PNU-120596-, and 10 μM genistein-induced net charge potentiation of ACh- and choline-evoked responses. ACh and choline were applied alternately using double-barrel pressure application pipettes containing either 1 mM ACh or 2 mM choline with an interstimulus interval of 30 s. Four baseline evoked responses to each agonist were recorded followed by evoked responses in the presence of the positive modulator. Data were normalized to the average of the first four ACh-evoked responses prior to the bath application of the positive modulator. Black bars correspond to the application time courses of the PAMs (bars begin at the time when we switch to the perfusion buffer containing the positive modulator). Filled circles correspond to 1 mM ACh-evoked net charge responses, and open circles correspond to 2 mM choline. A-C. (Right panel) representative traces of the ACh- and choline-evoked responses and the potentiation of those responses by the positive modulators. Grey traces represent baseline evoked responses, and black traces correspond to the evoked responses in the presence of the positive modulator. Horizontal bars represent 250 ms. Vertical bars represent 100 pA for PNU-120596 and 50 pA for 5-HI and genistein. Data represent the averages of 7-11 interneurons.
We observed that the magnitude of genistein-induced potentiation of ACh-evoked responses varied across double-barrel experiments. Whereas it has been suggested that the effects of genistein could be mediated through tyrosine kinase inhibition (Charpantier et al., 2005; Cho et al., 2005), Grønlien et al. (Grønlien et al., 2007) provided some evidence supporting direct allosteric modulatory effects on α7 nAChRs. Although our data do not rule out that genistein can act directly as a PAM, the difference in magnitude of genistein-induced potentiation of ACh-evoked responses that we observed is arguably more consistent with an indirect interaction with α7 receptors. If genistein is not acting directly on α7 nAChRs, but is modulating the phosphorylation state of α7 receptors through the inhibition of tyrosine kinases (Cho et al., 2005), it is reasonable to assume that the magnitude of the genistein-induced potentiation of α7 nAChRs in hippocampal interneurons could be influenced by multiple factors in the brain slice preparation, especially under the conditions of whole-cell patch clamp and subsequent dialysis of the intracellular solution.
The reversible nature of the potentiation induced by 5-HI, PNU-120596, and genistein of ACh-evoked responses was not evaluated in the brain slice system. However, data obtained from oocytes expressing α7 nAChRs suggested that the effects of PNU-120596 reversed more slowly than the effects of 5-HI (Papke et al., submitted), with some potentiation by PNU-120596 still detectable after 30 min washout. Similar to PNU-120596, genistein effects were slowly reversible, presumably due to its ability to modulate the phosphorylation state of α7 receptors (data not shown).
Potentiation of partial agonist-evoked responses
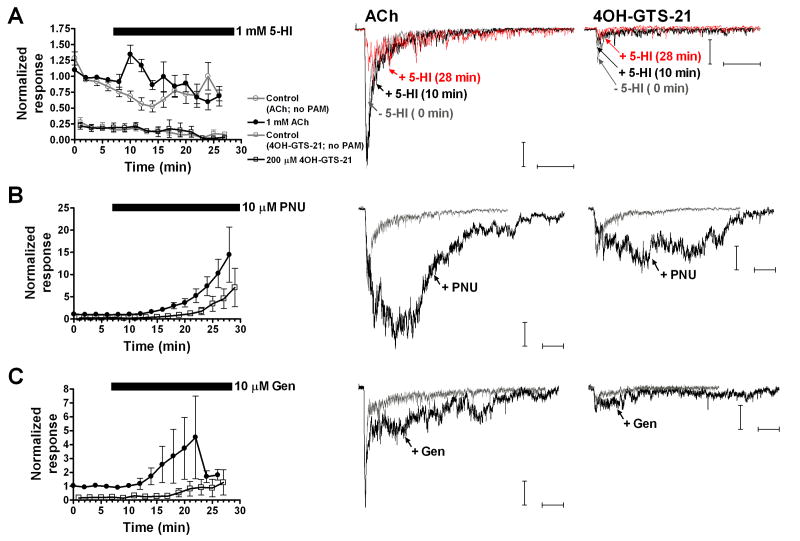
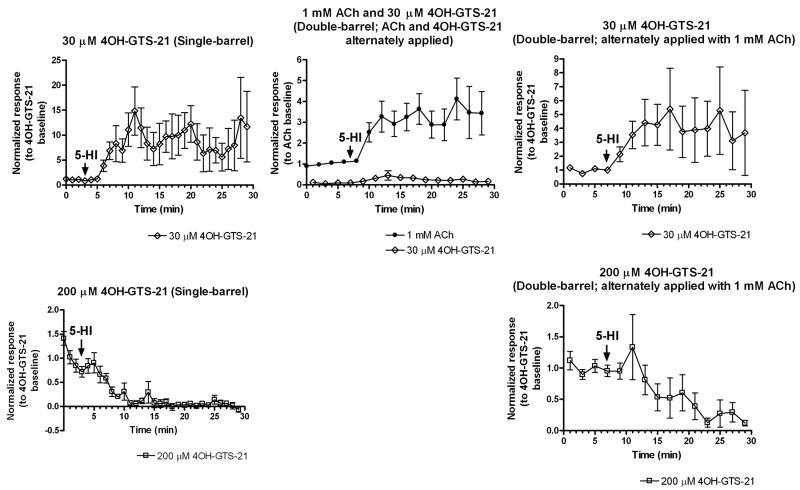
While all three positive allosteric modulators were able to increase (to varying degrees) responses to the high and low potency full agonists (ACh and choline), potentiation of evoked responses to alternated applications of ACh and 4OH-GTS-21 or S 24795 was qualitatively different (Fig. 2 and Fig. 5). PNU-120596 and genistein were able to significantly potentiate 200 μM 4OH-GTS-21-evoked net charge responses in rat hippocampal interneurons (Fig. 2B and 2C), but 200 μM 4OH-GTS-21-evoked responses were not potentiated by 1 mM 5-HI (Fig. 2A). Under control conditions (in the absence of positive modulators), a progressive rundown was observed in 1 mM ACh/200 μM 4OH-GTS-21 double-barrel experiments, for both ACh- and 4OH-GTS-21-evoked responses (Fig. 2A, gray symbols). This rundown was not observed on ACh-evoked responses when single-barrel pipettes were used for repeated applications of 1 mM ACh alone (Fig. 4A). It has previously been shown that 4OH-GTS-21 can cause use-dependent receptor blockade in α7 nAChRs (Papke et al., 2000; Uteshev et al., 2002; Uteshev et al., 2003). Thus the depression in both ACh- and 4OH-GTS-21-evoked responses in the double-barrel experiments were likely attributed to cross-desensitization or channel block induced by 200 μM 4OH-GTS-21. Bath application of 1 mM 5-HI did not significantly potentiate the responses by ACh or 4OH-GTS-21 under these conditions (Fig. 2A, black symbols). Thus, at the concentration used in this set of experiments, 4OH-GTS-21 might have noncompetitive antagonist properties on α7 limiting the potentiation effects of 1 mM 5-HI. To further evaluate if evoked responses by a lower concentration of 4OH-GTS-21 can be potentiated by 5-HI, we used single-barrel pipettes with either 200 or 30 μM 4OH-GTS-21 and compared the 5-HI-induced potentiation in these responses to that obtained in double-barrel experiments (Fig. 3). Responses evoked by 30 μM 4OH-GTS-21 exhibited a sustained potentiation induced by 1 mM 5-HI in both single- and double-barrel experiments (Fig. 3, upper panel). Moreover, 1 mM ACh evoked responses were effectively potentiated by 1 mM 5-HI when ACh was paired with 30 μM 4OH-GTS-21 (Fig. 3, middle upper panel). In experiments with acutely dissociated neurons, 4OH-GTS-21 at concentrations ≤ 30 μM did not induce the use-dependent inhibition of α7-mediated responses seen with higher concentrations (Uteshev et al., 2002). Similar results were obtained with mutant α4β2 receptors that responded to 4OH-GTS-21 as a super-agonist (4-5 folds more efficacious than ACh). At concentrations < 30 μM 4OH-GTS-21 only activation was observed, but at higher concentrations there were mixed agonist and antagonist effects (Horenstein et al., 2006). Thus the lack of 5-HI-induced potentiation of 200 μM 4OH-GTS-21-evoked responses is most likely due to the inability of this PAM to overcome the inhibitory after-effects of 4OH-GTS-21 applications, and is consistent with the finding of Mok and Kew in which 5-HI potentiation was occluded by pretreatment with desensitizing concentrations of nicotine (Mok and Kew, 2006).
Figure 2. Effects of 5-HI, PNU-120596, and genistein on ACh- and 4OH-GTS-21-evoked responses.
A-C. (Left panel) time courses for the induced potentiation of ACh- and 4OH-GTS-21-evoked net charge responses. ACh and 4OH-GTS-21 were applied alternately with an interstimulus interval of 1 min. Black bars correspond to the application time courses for the PAMs (bar begins at the time when we switch to the perfusion buffer containing the positive modulator). Filled black circles correspond to 1 mM ACh-evoked net charge responses, and open black squares correspond to 200 μM 4OH-GTS-21. Control experiments appear in A and consisted of alternative applications of 1 mM ACh (open gray circles) and 200 μM 4OH-GTS-21 (open gray squares) and no PAM was bath-applied for the duration of the recordings. ACh and 4OH-GTS-21 were applied alternately with an interstimulus interval of 1 min. A-C. (Right panel) representative traces of the ACh- and 4OH-GTS-21-evoked responses and the potentiation of those responses by 5-HI, PNU-120596, and genistein. Grey traces represent baseline evoked responses, and black traces correspond to the evoked responses in the presence of the positive modulator at the end of application. In the case of 5-HI, black traces correspond to the induced potentiation at time point 10 min, while red traces correspond to the induced potentiation at time point 28 min. Horizontal bars represent 250 ms and vertical bars represent 50 pA. Data represent the averages of 6-18 interneurons.
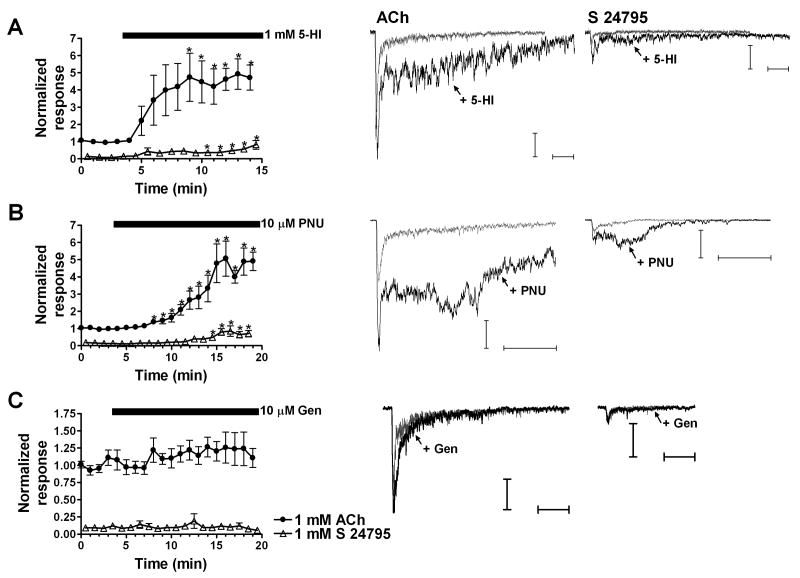
Figure 5. Effects of 5-HI, PNU-120596, and genistein on ACh- and S 24795-evoked responses.
A-C. (Left panel) time courses for the induced potentiation of ACh- and S 24795-evoked net charge responses. 1 mM ACh and 1 mM S 24795 were applied alternately with an interstimulus interval of 30 s. Black bars correspond to the application time courses of the PAMs (bars begin at the time when we switch to the perfusion buffer containing the positive modulator). Open triangles correspond to S 24795 data. A-C. (Right panel) representative traces of ACh- and S 24795-evoked responses and the potentiation of those responses by 5-HI, PNU-120596, and genistein. Horizontal bars represent 250 ms. Vertical bars represent 100 pA for PNU-120596 and 50 pA for 5-HI and genistein. Grey traces represent baseline evoked responses, and black traces correspond to the evoked responses in the presence of the positive modulator. Data represent the averages of 5-11 interneurons. Statistical analysis was included for the individual time points (relative to baseline) given the small S 24795-evoked responses.
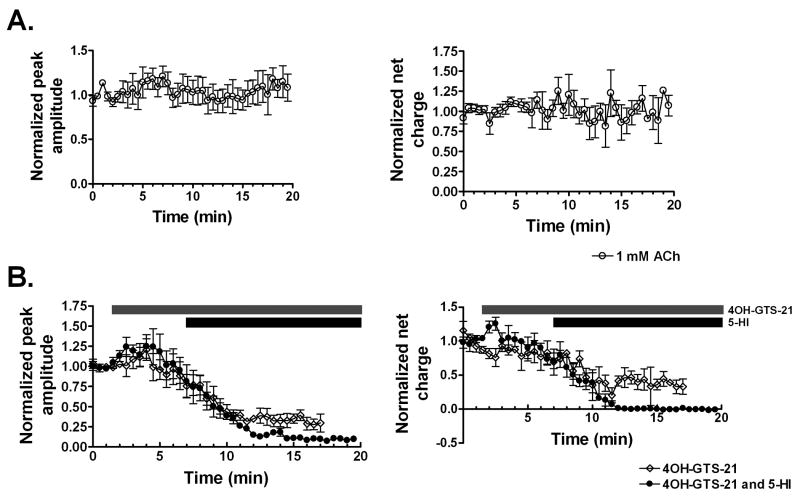
Figure 4. Effect of 4OH-GTS-21 bath application on ACh-evoked responses in the absence and presence of 5-HI modulation.
A. Consecutive 1 mM ACh applications at 30 s intervals showed basically no rundown both in terms of peak amplitude (left side) and net charge (right side). B. Four baseline ACh-evoked responses were recorded followed by evoked responses in the presence of 1 μM of 4OH-GTS-21 alone (open diamonds) or in the presence of 1 μM 4OH-GTS-21 and 1 mM 5-HI (filled circles). The changes in ACh-evoked responses are showed in terms of both peak amplitude (left side) and net charge (right side). The grey bar corresponds to the application time course of 1 μM 4OH-GTS-21 and the black bar corresponds to 1 mM 5-HI. Data were normalized to the average of the first four ACh-evoked responses prior to the bath application of 4OH-GTS-21. Data represent the averages of 4 interneurons for each condition.
Figure 3. 5-HI-induced potentiation of 4OH-GTS-21-evoked responses.
Upper panel, graphs represent the 1 mM 5-HI-induced potentiation of 30 μM 4OH-GTS-21-evoked responses in single-barrel (left side) or double-barrel (middle and right side) experiments. In double-barrel experiments 1 mM ACh and 30 μM 4OH-GTS-21 were applied alternately with an interstimulus interval of 1 min. For single-barrel experiments, data were normalized to the average of the first four 4OH-GTS-21-evoked responses prior to the bath application of 1 mM 5-HI (baseline). For double-barrel experiments, data were either normalized to 1 mM ACh baseline response (upper panel, middle) or to 4OH-GTS-21 baseline response (upper panel, right side). Data represent the averages of 7-8 interneurons. In the lower panel data from both single-barrel (left side) or double-barrel (right side) experiments with 200 μM 4OH-GTS-21 were also included. In both cases, data were normalized by the average of the first four 4OH-GTS-21-evoked responses prior to bath application of 1 mM 5-HI (n of 7-9 cells). Data normalized by ACh baseline response from double-barrel experiments with 1 mM ACh and 200 μM 4OH-GTS-21 alternately applied appeared on Fig. 2A. Arrows indicate the time at which 1 mM 5-HI application began. Filled black circles correspond to 1 mM ACh-evoked net charge responses, open black diamonds correspond to 30 μM 4OH-GTS-21, and open black squares correspond to 200 μM 4OH-GTS-21.
To determine whether the lack of 5-HI-induced potentiation of ACh responses was due to 4OH-GTS-21 cross-desensitization, 1 μM 4OH-GTS-21 was bath applied and the effects on the evoked responses by 1 mM ACh (delivered from a single-barrel application pipette at 30 s intervals) were measured in the absence and presence of 1 mM 5-HI (Fig. 4B). As seen in Figure 4, there was a reduction in terms of both peak amplitude and net charge following 1 μM 4OH-GTS-21 bath application regardless of the absence or presence of 5-HI. This decrease in evoked responses was not likely attributed by rundown since virtually no decrease in either peak or net charge responses was observed during consecutive applications of 1 mM ACh at 30 s intervals (Fig. 4A). In a similar manner, α7-mediated responses of dissociated neurons to 1 mM ACh were reduced by 3 min pretreatment with even lower concentrations of 4OH-GTS-21 (e.g. 200-400 nM) (Uteshev et al., 2003). Thus it is plausible that some 4OH-GTS-21 cross-desensitization interfered with the 5-HI-induced potentiation of ACh evoked responses in the double-barrel experiments in which 1 mM ACh was paired with 200 μM 4OH-GTS-21.
Regarding S 24795, both 5-HI and PNU-120596 significantly potentiated the net charge responses (Fig. 5A and 5B). Following 5-HI treatment, the estimated percent change value for 1 mM S 24795-evoked responses was 498 ± 117 and for ACh responses was 362 ± 39. The percent change values that resulted from PNU-120596 application were 391 ± 83 and 369 ± 43 for S 24795- and ACh-evoked responses, respectively. On the other hand, genistein induced no significant potentiation or inhibition of 1 mM S 24795-evoked responses (Fig. 5C and Fig. 7). In this paradigm, genistein-induced potentiation of ACh-evoked responses was similar to the experiments in which ACh and choline were in the double-barrel pipette (Fig. 7). The lack of potentiation of S 24795-evoked responses by genistein was unexpected, particularly because the other two positive modulators were able to potentiate the evoked responses by this partial agonist with an apparent lack of cross-over effects on ACh-evoked responses.
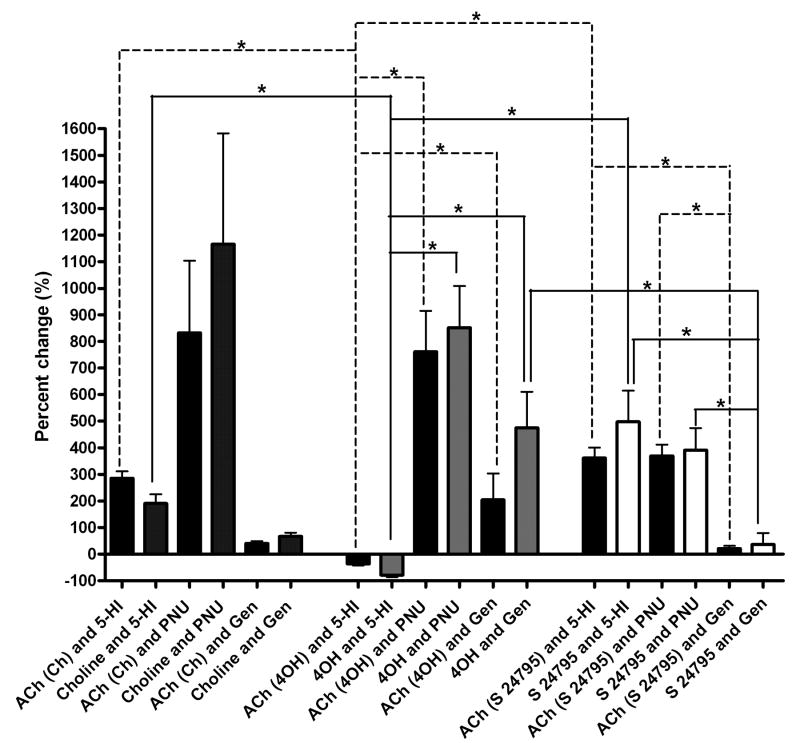
Figure 7. Comparison of the percent changes in the agonist-evoked responses induced by 5-HI, PNU-120596, and genistein.
Potentiation of the agonist-evoked net charge response values at the end of the positive modulator application, relative to initial baseline responses are represented by bar graphs. Experiments consisted of alternate applications from double-barrel pipettes of 1 mM ACh and either 2 mM choline, 200 μM 4OH-GTS-21, or 1 mM S 24795. For each positive modulator, the percent change was calculated using the normalized area for the last four evoked responses and the normalized response during baseline conditions (first four evoked responses in the absence of positive modulator). Each positive modulator was applied for 15-16 min in choline experiments, while the application period for the positive modulators in 4OH-GTS-21 experiments was 20-22 min. For S 24795, 5-HI, PNU-120596, and genistein were applied 11, 15, and 16 min, respectively. The relative potentiation of ACh-evoked net charge responses for each experiment is also included. Statistical analysis was done using Kruskal-Wallis one-way ANOVA with a Dunnett's multiple comparisons post-test; asterisks (*) indicate p value < 0.05. Results from the pairwise multiple comparisons for ACh are indicated with dashed lines, while for choline, 4OH-GTS-21, and S 24795 with solid lines. There was no significant statistical difference between the relative potentiation of choline-, 4OH-GTS-21-, or S 24795-evoked responses induced by the three positive modulators when compared to the induced potentiation of ACh-evoked responses.
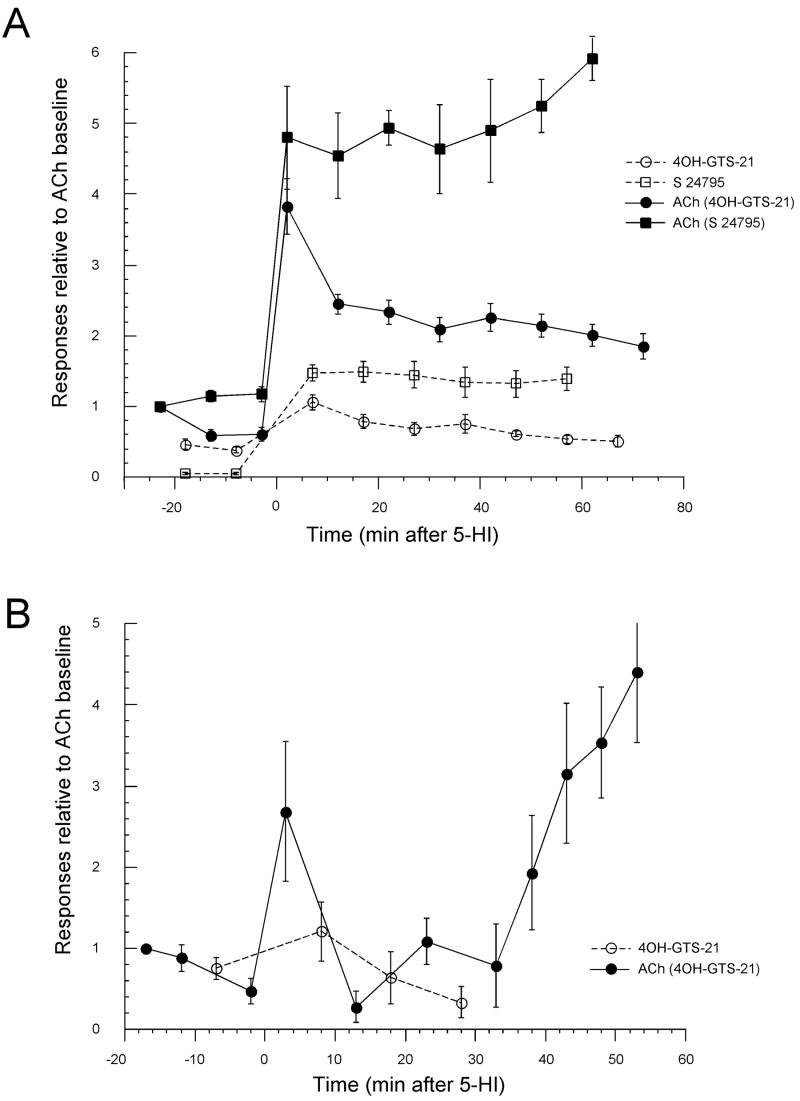
We conducted parallel experiments in Xenopus oocytes expressing α7 nAChRs to further confirm the effects of the Type 1 PAM 5-HI on alternating applications of 60 μM ACh and either 100 μM 4OH-GTS-21 or S 24795 (Fig. 6A). The results obtained from the oocyte expression system are consistent with our data from brain slices (Figs. 2A, 5A, and 6A). When 60 μM ACh and 100 μM S 24795 were alternately applied both evoked responses exhibited sustained potentiation induced by 1 mM 5-HI (Fig 6A), however the 5-HI-induced potentiation of responses evoked by alternated applications of 60 μM ACh and 100 μM 4OH-GTS-21 was transient; the magnitude of the potentiation decrease through time in both ACh- and 4OH-GTS-21-evoked responses. Also we evaluated in oocyte experiments the reversibility of 4OH-GTS-21 suppression of 5-HI-induced potentiation of ACh-evoked responses. When 60 μM ACh was applied alternately with 100 μM 4OH-GTS-21 the potentiation induced by 1 mM 5-HI was only transient but once the alternated 4OH-GTS-21 stimulations were stopped ACh-evoked responses were increasingly potentiated by 5-HI (Fig. 6B).
Figure 6. Effects of the Type I PAM 5-HI on ACh-, 4OH-GTS-21-, and S 24795-evoked currents of Xenopus oocytes expressing α7 nAChRs.
A. After initial control applications of 60 μM ACh, ACh was then applied alternately with applications of either 100 μM 4OH-GTS-21 or S 24795. The bath solution was then switched (at t = 0) to one containing 1 mM 5-HI. ACh was then co-applied with 5-HI and a rapid initial potentiation was observed. Subsequently, ACh applications alternated with applications of either 100 μM 4OH-GTS-21 or S 24795. Data from experiments where ACh and 4OH-GTS-21 were applied alternately are represented by filled circles for ACh (4OH-GTS-21) and open circles for 4OH-GTS-21. Data from experiments where ACh and S 24795 were applied alternately are represented by filled squares for ACh (S 24795) and open squares for S 24795. Responses were normalized to the first control ACh responses of each cell. The data represent the average responses of 5 cells ± SEM at each time point. B. To determine the reversibility of 4OH-GTS-21 suppression of 5-HI potentiation of ACh-evoked responses oocytes were stimulated with 60 μM ACh or 100 μM 4OH-GTS-21 prior to or subsequently to the addition of 1 mM 5-HI to the bath solution (t = 0). As in panel A, alternating applications of 4OH-GTS-21 prevented the 5-HI increase in ACh responses. This suppression of the potentiating effects of 5-HI was reversed when the cells were stimulated repeatedly with ACh without intervening 4OH-GTS-21 applications. Responses were normalized to the first control ACh responses of each cell. The data represent the average responses of 3 cells ± SEM at each time point.
The cross-desensitization effects of 200 μM 4OH-GTS-21 mostly affected the 1 mM 5-HI-induced potentiation and not the potentiation induced by either PNU-120596 or genistein (Fig. 7). Kruskal-Wallis one-way ANOVA on ranks with a Dunnett's multiple comparisons post-test showed that there was no statistically significant change in the PNU-129596- and genistein-induced potentiation of ACh-evoked responses, regardless if choline, 4OH-GTS-21, or S 24795 were present in the other side of the double-barrel application pipette. However, there were statistically significant differences (p < 0.05) between the 5-HI-induced potentiation of ACh-evoked responses in the double-barrel experiments depending on the drug paired with ACh. Specifically, there was a decrease in ACh-evoked responses when 1 mM ACh was paired with 200 μM 4OH-GTS-21 in the 5-HI experiments, but there was potentiation of ACh-evoked responses when 1 mM ACh was paired with either 2 mM choline or 1 mM S 24795.
Discussion
In this study we extend previous work on the evaluation of the effects of positive modulators on α7 nicotinic responses, but with emphasis on comparing and contrasting the positive modulation of full and partial agonist-evoked responses in rat hippocampus. The α7 nAChR is highly expressed in the hippocampus, an area in the brain involved in learning and memory processes. In the hippocampus, stratum radiatum interneurons of the CA1 area robustly express α7 nAChRs; α7-mediated responses can be readily detected with intracellular recording techniques (Frazier et al., 1998). More than 90% of all stratum radiatum neurons respond to ACh and are inhibited by MLA and α-bungarotoxin, suggesting that these are predominantly α7-mediated nicotinic responses (Frazier et al., 1998). Particularly, α7 nAChRs in stratum radiatum interneurons are thought to play critical roles in synaptic plasticity by mediating cellular mechanisms such as long-term potentiation and depression (LTP/LTD), which are mechanisms underlying learning and memory processes (Ji and Dani, 2000). Ji and Dani demonstrated that activation of nAChRs can inhibit or disinhibit pyramidal neurons depending on the connectivity of the hippocampal interneurons in which they are located, and suggested that by doing so nAChRs have the capacity to influence LTP/LTD (Ji and Dani, 2000). The α7 nAChRs were also involved in hippocampal γ oscillations, further suggesting that they can play important roles in cognitive functions (Song et al., 2005). Additionally, disruption of the normal function of nAChRs in hippocampus has been associated with AD (Guan et al., 2000). Since we aimed to evaluate the interactions between agonists and positive modulators of α7 nAChR function, we performed most of our experiments in rat hippocampal brain slices.
Our study, although carried out in fresh brain slices from rats, presents some similarities to Grønlien et al. findings (Grønlien et al., 2007) but also showed important differences in how these distinctly different potentiators will or will not modulate responses evoked by experimental partial agonists. Among the similarities, PNU-120596 effectively potentiated full and partial agonist-evoked responses and decreased current decay in both the oocyte heterologous expression system and in brain slices. Also PNU-120596 was ultimately more effective at potentiating α7 nAChR net charge responses than 5-HI and/or genistein. However, we demonstrated that the potentiation of α7 nAChR response would depend on the nature and the effective concentration of the agonist involved and its particular interaction with the positive modulator.
The case of 5-HI modulation of 4OH-GTS-21-evoked responses deserves special attention. We showed that in our ex vivo preparation there was no detectable 5-HI-induced potentiation of α7-mediated responses evoked by a high concentration of 4OH-GTS-21 (200 μM). It has been demonstrated that 200 μM 4OH-GTS-21 produced channel block of α7-mediated responses in tuberomammillary histamine neurons (Uteshev et al., 2002). However, in the same system, at lower concentrations (≤ 30 μM) the predominant effect of 4OH-GTS-21 was sustained channel activation. Higher concentrations of 4OH-GTS-21 stabilize the receptor in a desensitized state with a slower recovery rate than that obtained with lower concentrations (≤ 30 μM 4OH-GTS-21). As mentioned earlier, 5-HI was not effective in overcoming receptor blockade and/or desensitization (Mok and Kew, 2006; Zwart et al., 2002). Thus the lack of potentiation of 200 μM 4OH-GTS-21-evoked responses was mostly attributed to channel block and/or desensitization. This was further demonstrated when evoked responses by 30 μM 4OH-GTS-21, which produce no significant residual inhibition or desensitization, were effectively potentiated by 5-HI (Fig. 3). Moreover, our results in hippocampal brain slices are consistent with oocyte experiments performed recently by our laboratory, in which GTS-21 was found to produce a prolonged residual inhibition or desensitization that antagonized the potentiation induced by 5-HI (Papke et al., submitted). We also observed that in our 1 mM ACh/200 μM 4OH-GTS-21 double-barrel experiments the responses evoked by ACh were not potentiated by 1 mM 5-HI. Since applications of 1 mM ACh and 200 μM 4OH-GTS-21 were given alternately it is likely that the ACh-evoked responses were reduced due to the desensitization induced by 200 μM 4OH-GTS-21, and this, in turn, antagonized the potentiation effects of 5-HI in the evoked responses by both agonists. Similar inhibition of successive ACh responses when it was alternated with pressure application of a desensitizing concentration of another agonist (e.g. nicotine) was previously observed in hippocampal interneurons from rat brain slices (Frazier et al., 1998). Repetitive ACh-evoked responses can be stably recorded for a prolonged period of time in hippocampal brain slices with few rundown, as demonstrated by Figure 4. Thus, current rundown does not explain the lack of potentiation of ACh evoked responses by 5-HI in this set of double-barrel experiments. Moreover, 1 mM ACh-evoked responses were effectively potentiated in experiments in which ACh was paired with 2 mM choline, 30 μM 4OH-GTS-21, or 1 mM S 24795.
A lack of sustained potentiation of ACh-evoked responses by 1 mM 5-HI when 60 μM ACh was applied in alternation with 100 μM 4OH-GTS-21 was also observed in parallel experiments conducted in Xenopus oocytes (Fig. 6A). In contrast, when 60 μM ACh and 100 μM S 24795 were alternately applied to oocytes expressing α7 nAChRs, 1 mM 5-HI induced a sustained potentiation in both ACh and S 24795-evoked responses (Fig. 6A). Thereby, independent of the experimental setting, brain slices or Xenopus oocytes, high concentrations of 4OH-GTS-21 produces residual inhibition or desensitization in α7 nAChRs that cannot be overcome by treatment with 5-HI. Additionally, the suppression of 5-HI-induced potentiation of ACh-evoked responses was recovered with washout of 4OH-GTS-21 (Fig. 6B). Benzylidene anabaseines (e.g. GTS-21 and 4OH-GTS-21) have been reported to produce prolonged residual inhibitory or desensitizing effects in oocyte studies (Kem et al., 2004; Papke et al, 2004; Papke and Papke, 2002), which are not seen with S 24795 (Lopez-Hernandez et al., 2007). We hypothesize that it is the relatively slow reversibility of the desensitization produced by high concentration of 4OH-GTS-21 that limits the potentiating effects of 5-HI for both 4OH-GTS-21, and for ACh when applied in alternation with 200 μM 4OH-GTS-21. It seems unlikely that 4OH-GTS-21 is a low potency competitive antagonist of 5-HI at the allosteric modulatory site since 4OH-GTS-21 produced a decrease in ACh-evoked responses even in the absence of this PAM. Furthermore, the inhibitory effects observed in the presence of 5-HI were greater than those observed in the absence of 5-HI (Fig. 4B). Consistent with our data showing little or no potentiation of 200 μM 4OH-GTS-21 responses by 1 mM 5-HI, other recent findings in our laboratory have demonstrated that while 5-HI increases the frequency and amplitude of spontaneous synaptic events, this effect is not magnified when 5-HI and 4OH-GTS-21 are applied together (Thinschmidt et al., 2008).
We previously found that S 24795 is a partial agonist of α7 nAChRs and can modulate α7 receptor responsiveness to ACh in rat hippocampal interneurons (López-Hernández et al, 2007). Additionally, S 24795 has been found to be effective in alleviating impairments of working and declarative memory (Marighetto et al., 2008), and contextual memory (Beracochea et al., 2008) in aged mice. Here we demonstrated that S 24795-evoked net charge responses were effectively potentiated by both 5-HI and PNU-120596. Thus S 24795 might have an excellent therapeutic potential for approaches that target α7 nAChRs and involve a combination of a direct-acting agonist with a PAM, such as PNU-120596 or 5-HI, to enhance nicotinic receptor function. However, S 24795-evoked responses were not potentiated by genistein, in contrast to 200 μM 4OH-GTS-21-evoked responses which were significantly potentiated by this Type I PAM. This result can be explained in light of the findings by Jakubík and collaborators, which suggested that the direction of the allosteric interaction and its magnitude is going to depend on the receptor subtype and the nature of the agonist and allosteric modulator (Jakubík et al., 1997). Although both 4OH-GTS-21 and S 24795 are known to be α7-selective partial agonists, the interaction between the allosteric modulator and a particular agonist may be unique and depend on both the mechanism of the potentiator and the specific factors limiting the efficacy of the partial agonist. Perhaps the binding of S 24795 to α7 nAChR induces a conformational change different from the one produced by the binding of 4OH-GTS-21 that could be interfering with the allosteric modulation by genistein. However we have not provided any evidence in this study that supports the latter hypothesis; further experiments will be needed to address the lack of potentiation of S 24795-evoked responses by genistein.
Careful examination of the modulation of α7 nAChRs is critical for development of new strategies to enhance nicotinic transmission in the brain. The specific approach designed to target α7 nAChRs is going to depend on the pathological scenario. For example, it has been suggested that α7 nAChR function can be disrupted in AD (Guan et al., 2000). Thus, a PAM like PNU-120596 might have therapeutic potential, but it is interesting to note how effective this particular modulator was at enhancing the responses to choline at levels close to the physiological range. PNU-120596 induces a huge prolongation of the agonist-evoked response, and such potentiation of α7 receptor function in the presence of physiological concentrations of choline might be detrimental to cell survival given the high calcium permeability that α7 nAChRs exhibit. In fact, PNU-120596 was shown to induce cytotoxicity in the SH-SY5Y-α7 cell line (Ng et al., 2007). Thus it is critical to evaluate how a particular nicotinic full or partial agonist will interact with PNU-120596 in a co-therapy approach in order to select the pair that would not result in a larger prolongation of the evoked response.
Moreover, α7 expression throughout the body has to be considered when using a PAM. In the mammalian CNS α4, β2, and α7 are the most abundant nicotinic subunits, with α7 subunits most likely assembled into homopentamers and with well-documented functional significance ranging from the modulation of synaptic function (Dani, 2001) to whole animal behavior (Ren et al., 2007). In contrast to how extensively brain α7 nAChRs have been studied, their function in the peripheral nervous system (PNS) is less well understood. There is significant α7 subunit expression in the PNS (Brown and Fumagalli, 1977; Cuevas et al., 2000; Mandelzys et al., 1995; Sala et al., 2007; Tachikawa et al., 2001). However, the functional consequences of that expression are unclear. It has been demonstrated that ganglionic transmission in adult animals is, in general, insensitive to α7 antagonists (Brown and Fumagalli, 1977; Kristufek et al., 1999; Mandelzys et al., 1995; Tachikawa et al., 2001) and is mainly mediated by the activation of α3β4 nAChRs. However, contribution of α7 nAChRs in ganglionic transmission has not been excluded, and their presence in the autonomic nervous system might complicate subunit-selective targeting of brain nAChRs. Furthermore, it has been demonstrated that the nicotinic component of the anti-inflammatory pathway is mediated by α7-containing receptors (for review see Ulloa, 2005). Thus, inadvertent disruption of this pathway by therapies targeting α7 receptors in the CNS might produce undesired side effects. To our knowledge, the effects of PAMs on responses mediated by α7 nAChRs in the peripheral system have not been evaluated thus far but deserve to be addressed in the future in order to determine the full scope of potential side effects of combinational therapies and how these side effects can be counterbalanced.
In conclusion, the classification of PAMs into just two classes may be an oversimplification, and especially with the numerous drug development programs ongoing to identify additional modulators of α7 nAChRs (Dunlop et al., 2007), the simple classification proposed might be premature. Moreover, as suggested by the present study, specific interactions between particular activators and modulators may point to the need for broader classification of potential allosteric modulators. The development of pharmacological agents targeting α7 nAChRs remains a challenge, not only due to the difficulty in designing subtype-selective drugs, but also due to the complex interactions between full and/or partial agonists and positive modulators. The use of direct-acting agonists and/or PAMs as either monotherapies or co-therapies might provide alternative approaches to enhance nicotinic function, but their full potential will rely on careful evaluation of the positive modulation of α7-mediated responses to both natural and experimental agonists.
Acknowledgments
This work was supported by The McKnight Foundation, Institut De Recherches Internationales Servier, GM57481-05A2, T32-AG000196, and PO1 AG10485. We thank Dr. Edwin M. Meyer, Dr. Joseph Brown, Grace Ha, Clare Stokes, Lynda Cortés, Shehd Abdullah Abbas Al Rubaiy, and Sara Braley for their valuable input.
Footnotes
Disclosure; P. M. and C. T.-T. are employees of I.R.I.S which holds a patent on S 24795. RLP is a consultant to I.R.I.S. but has no financial interest in the commercial development of S 24795.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alkondon M, Pereira EF, Albuquerque EX. alpha-bungarotoxin- and methyllycaconitine-sensitive nicotinic receptors mediate fast synaptic transmission in interneurons of rat hippocampal slices. Brain Research. 1998;810:257–263. doi: 10.1016/s0006-8993(98)00880-4. [DOI] [PubMed] [Google Scholar]
- Arendash GW, Sengstock GJ, Sanberg PR, Kem WR. Improved learning and memory in aged rats with chronic administration of the nicotinic receptor agonist GTS-21. Brain Research. 1995;674:252–259. doi: 10.1016/0006-8993(94)01449-r. [DOI] [PubMed] [Google Scholar]
- Beracochea D, Boucard A, Trocme-Thibierge C, Morain P. Improvement of contextual memory by S 24795 in aged mice: comparison with memantine. Psychopharmacology. 2008;196:555–564. doi: 10.1007/s00213-007-0987-5. [DOI] [PubMed] [Google Scholar]
- Brown DA, Fumagalli L. Dissociation of α-bungarotoxin binding and receptor block in the rat superior cervical ganglion. Brain Research. 1977;129:165–168. [Google Scholar]
- Burghaus L, Schutz U, Krempel U, Lindstrom J, Schroder H. Loss of nicotinic acetylcholine receptor subunits alpha4 and alpha7 in the cerebral cortex of Parkinson patients. Parkinsonism and Related Disorders. 2003;9:243–246. doi: 10.1016/s1353-8020(03)00028-2. [DOI] [PubMed] [Google Scholar]
- Castro NG, Albuquerque EX. α-Bungarotoxin-sensitive hippocampal nicotinic receptor channel has a high calcium permeability. Biophysical Journal. 1995;68:516–524. doi: 10.1016/S0006-3495(95)80213-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charpantier E, Wiesner A, Huh KH, Ogier R, Hoda JC, Allaman G, Raggenbass M, Feuerbach D, Bertrand D, Fuhrer C. Alpha7 neuronal nicotinic acetylcholine receptors are negatively regulated by tyrosine phosphorylation and Src-family kinases. Journal of Neuroscience. 2005;25:9836–9849. doi: 10.1523/JNEUROSCI.3497-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho CH, Song W, Leitzell K, Teo E, Meleth AD, Quick MW, Lester RA. Rapid upregulation of alpha7 nicotinic acetylcholine receptors by tyrosine dephosphorylation. Journal of Neuroscience. 2005;25:3712–3723. doi: 10.1523/JNEUROSCI.5389-03.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuevas J, Roth AL, Berg DK. Two distinct classes of functional 7-containing nicotinic receptor on rat superior cervical ganglion neurons. Journal of Physiology. 2000;525(Pt 3):735–746. doi: 10.1111/j.1469-7793.2000.t01-1-00735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dani JA. Overview of nicotinic receptors and their roles in the central nervous system. Biological Psychiatry. 2001;49:166–174. doi: 10.1016/s0006-3223(00)01011-8. [DOI] [PubMed] [Google Scholar]
- de Fiebre CM, Meyer EM, Zoltewicz J, Henry JC, Muraskin S, Kem WR, Papke RL. Characterization of a family of anabaseine-derived compounds reveals that the 3-(4)-dimethylaminocinnamylidine derivative (DMAC) is a selective agonist at neuronal nicotinic α7/[125I]α-bungarotoxin receptor subtypes. Molecular Pharmacology. 1995;47:164–171. [PubMed] [Google Scholar]
- DelToro ED, Juiz JM, Peng X, Lindstrom J, Criado M. Immunocytochemical localization of the α7 subunit of the nicotinic acetylcholine receptor in the rat central nervous system. Journal of Comparative Neurology. 1994;349:325–342. doi: 10.1002/cne.903490302. [DOI] [PubMed] [Google Scholar]
- Dunlop J, Roncarati R, Jow B, Bothmann H, Lock T, Kowal D, Bowlby M, Terstappen GC. In vitro screening strategies for nicotinic receptor ligands. Biochemical Pharmacology. 2007;74:1172–1181. doi: 10.1016/j.bcp.2007.07.006. [DOI] [PubMed] [Google Scholar]
- Frazier CJ, Rollins YD, Breese CR, Leonard S, Freedman R, Dunwiddie TV. Acetylcholine activates an α-bungarotoxin-sensitive nicotinic current in rat hippocampal interneurons, but not pyramidal cells. Journal of Neuroscience. 1998;18:1187–1195. doi: 10.1523/JNEUROSCI.18-04-01187.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman R, Hall M, Adler LE, Leonard S. Evidence in postmortem brain tissue for decreased numbers of hippocampal nicotinic receptors in schizophrenia. Biological Psychiatry. 1995;38:22–33. doi: 10.1016/0006-3223(94)00252-X. [DOI] [PubMed] [Google Scholar]
- Grilli M, Raiteri L, Patti L, Parodi M, Robino F, Raiteri M, Marchi M. Modulation of the function of presynaptic alpha7 and non-alpha7 nicotinic receptors by the tryptophan metabolites, 5-hydroxyindole and kynurenate in mouse brain. British Journal of Pharmacology. 2006;149:724–732. doi: 10.1038/sj.bjp.0706914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grønlien JH, Haakerud M, Ween H, Thorin-Hagene K, Briggs CA, Gopalakrishnan M, Malysz J. Distinct profiles of {alpha}7 nAChR positive allosteric modulation revealed by structurally diverse chemotypes. Molecular Pharmacology. 2007 doi: 10.1124/mol.107.035410. [DOI] [PubMed] [Google Scholar]
- Guan ZZ, Zhang X, Blennow K, Nordberg A. Decreased protein level of nicotinic receptor alpha7 subunit in the frontal cortex from schizophrenic brain. Neuroreport. 1999;10:1779–1782. doi: 10.1097/00001756-199906030-00028. [DOI] [PubMed] [Google Scholar]
- Guan ZZ, Zhang X, Ravid R, Nordberg A. Decreased protein levels of nicotinic receptor subunits in the hippocampus and temporal cortex of patients with Alzheimer's disease. Journal of Neurochemistry. 2000;74:237–243. doi: 10.1046/j.1471-4159.2000.0740237.x. [DOI] [PubMed] [Google Scholar]
- Gurley D, Harris EW, Li C, Johnson EC, Lanthorn T. 5-Hydroxyindole potentiates the nicotinic acetylcholine receptor alpha7 subtype. Society Neuroscience. 2000 Abstract 716.15. [Google Scholar]
- Halevi S, Yassin L, Eshel M, Sala F, Sala S, Criado M, Treinin M. Conservation within the RIC-3 gene family. Effectors of mammalian nicotinic acetylcholine receptor expression. Journal of Biological Chemistry. 2003;278:34411–34417. doi: 10.1074/jbc.M300170200. [DOI] [PubMed] [Google Scholar]
- Horenstein NA, McCormack TJ, Stokes C, Ren K, Papke RL. Reversal of agonist selectivity by mutations of conserved amino acids in the binding site of nicotinic acetylcholine receptors. Journal of Biological Chemistry. 2006;282:5899–5909. doi: 10.1074/jbc.M609202200. [DOI] [PubMed] [Google Scholar]
- Hurst RS, Hajos M, Raggenbass M, Wall TM, Higdon NR, Lawson JA, Rutherford-Root KL, Berkenpas MB, Hoffmann WE, Piotrowski DW, Groppi VE, Allaman G, Ogier R, Bertrand S, Bertrand D, Arneric SP. A novel positive allosteric modulator of the alpha7 neuronal nicotinic acetylcholine receptor: in vitro and in vivo characterization. Journal of Neuroscience. 2005;25:4396–4405. doi: 10.1523/JNEUROSCI.5269-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakubík J, Bacakova L, El-Fakahany EE, Tucek S. Positive cooperativity of acetylcholine and other agonists with allosteric ligands on muscarinic acetylcholine receptors. Molecular Pharmacology. 1997;52:172–179. doi: 10.1124/mol.52.1.172. [DOI] [PubMed] [Google Scholar]
- Ji D, Dani JA. Inhibition and disinhibition of pyramidal neurons by activation of nicotinic receptors on hippocampal interneurons. Journal of Neurophysiology. 2000;83:2682–2690. doi: 10.1152/jn.2000.83.5.2682. [DOI] [PubMed] [Google Scholar]
- Jones S, Yakel J. Functional nicotinic ACh receptors on interneurones in the rat hippocampus. Journal of Physiology (Lond) 1997;504:603–610. doi: 10.1111/j.1469-7793.1997.603bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kem WR, Mahnir VM, Prokai L, Papke RL, Cao X, Le Francois S, Wildeboer K, Prokai-Tatrai K, Papke JKP, Soti F. Hydroxy metabolites of the Alzheimer's drug candidate DMXBA (GTS-21): their molecular properties, interactions with brain nicotinic receptors and brain penetration. Molecular Pharmacology. 2004;65:56–67. doi: 10.1124/mol.65.1.56. [DOI] [PubMed] [Google Scholar]
- Krause RM, Buisson B, Bertrand S, Corringer PJ, Galzi JL, Changeux JP, Bertrand D. Ivermectin: a positive allosteric effector of the alpha7 neuronal nicotinic acetylcholine receptor. Molecular Pharmacology. 1998;53:283–294. doi: 10.1124/mol.53.2.283. [DOI] [PubMed] [Google Scholar]
- Kristufek D, Stocker E, Boehm S, Huck S. Somatic and prejunctional nicotinic receptors in cultured rat sympathetic neurones show different agonist profiles. Journal of Physiology. 1999;516(Pt 3):739–756. doi: 10.1111/j.1469-7793.1999.0739u.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Hernández G, Placzek AN, Thinschmidt JS, Lestage P, Trocme-Thibierge C, Morain P, Papke RL. Partial agonist and neuromodulatory activity of S 24795 for alpha7 nAChR responses of hippocampal interneurons. Neuropharmacology. 2007;53:134–144. doi: 10.1016/j.neuropharm.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Mandelzys A, De Koninck P, Cooper E. Agonist and toxin sensitivities of ACh-evoked currents on neurons expressing multiple ACh receptor subunits. Journal of Neurophysiology. 1995;74:1212–1221. doi: 10.1152/jn.1995.74.3.1212. [DOI] [PubMed] [Google Scholar]
- Mannaioni G, Carpenedo R, Moroni F. 5-hydroxyindole causes convulsions and increases transmitter release in the CA1 region of the rat hippocampus. British Journal of Pharmacology. 2003;138:245–253. doi: 10.1038/sj.bjp.0705007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marighetto A, Valerio S, Desmedt A, Philippin JN, Trocmé-Thibierge C, Morain P. Comparative effects of the alpha7 nicotinic partial agonist, S 24795, and the cholinesterase inhibitor, donepezil, against aging-related deficits in declarative and working memory in mice. Psycopharmacology. 2008;197:499–508. doi: 10.1007/s00213-007-1063-x. [DOI] [PubMed] [Google Scholar]
- Mok MHS, Kew JN. Excitation of rat hippocampal interneurons via modulation of endogenous agonist activity at the alpha7 nicotinic ACh receptor. Journal of Physiology. 2006;574:699–710. doi: 10.1113/jphysiol.2006.104794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng HJ, Whittemore ER, Tran MB, Hogenkamp DJ, Broide RS, Johnstone TB, Zheng L, Stevens KE, Gee KW. Nootropic alpha7 nicotinic receptor allosteric modulator derived from GABAA receptor modulators. Procedings of the National Academy of Science U S A. 2007;104:8059–8064. doi: 10.1073/pnas.0701321104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papke RL, Bencherif M, Lippiello P. An evaluation of neuronal nicotinic acetylcholine receptor activation by quaternary nitrogen compounds indicates that choline is selective for the α7 subtype. Neuroscience Letters. 1996;213:201–204. doi: 10.1016/0304-3940(96)12889-5. [DOI] [PubMed] [Google Scholar]
- Papke RL, Meyer EM, Lavieri S, Bollampally S, Papke T, Horenstein B, Papke JKP. Effects at a distance in alpha7 nAChR selective agonists: Benzylidene substitutions regulate potency and efficacy. Journal of Neuropharmacology. 2004;46:1023–1038. doi: 10.1016/j.neuropharm.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Papke RL, Meyer E, Nutter T, Uteshev VV. Alpha7-selective agonists and modes of alpha7 receptor activation. European Journal of Pharmacology. 2000;393:179–195. doi: 10.1016/s0014-2999(00)00009-1. [DOI] [PubMed] [Google Scholar]
- Papke RL, Papke JK. Comparative pharmacology of rat and human alpha7 nAChR conducted with net charge analysis. British Journal of Pharmacology. 2002;137:49–61. doi: 10.1038/sj.bjp.0704833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng X, Katz M, Gerzanich V, Anand R, Lindstrom J. Human α7 acetylcholine receptor: cloning of the α7 subunit from the SH-SY5Y cell line and determination of pharmacological properties of native receptors and functional α7 homomers expressed in Xenopus oocytes. Molecular Pharmacology. 1994;45:546–554. [PubMed] [Google Scholar]
- Ren K, Thinshmidt J, Liu J, Ai L, Papke RL, King MA, Hughes JA, Meyer EM. alpha7 nicotinic receptor gene delivery into mouse hippocampal neurons leads to functional receptor expression, improved spatial memory-related performance, and tau hyperphosphorylation. Neuroscience. 2007;101:160–167. doi: 10.1016/j.neuroscience.2006.11.023. [DOI] [PubMed] [Google Scholar]
- Sala F, Nistri A, Criado M. Nicotinic Acetylcholine Receptors of Adrenal Chromaffin Cells. Acta Physiology (Oxford) 2007 doi: 10.1111/j.1748-1716.2007.01804.x. [DOI] [PubMed] [Google Scholar]
- Seguela P, Wadiche J, Dinely-Miller K, Dani JA, Patrick JW. Molecular cloning, functional properties and distribution of rat brain alpha 7: a nicotinic cation channel highly permeable to calcium. Journal of Neuroscience. 1993;13(2):596–604. doi: 10.1523/JNEUROSCI.13-02-00596.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Socci DJ, Sanberg PR, Arendash GW. Nicotine enhances Morris water maze performance of young and aged rats. Neurobiology of Aging. 1995;16:857–860. doi: 10.1016/0197-4580(95)00091-r. [DOI] [PubMed] [Google Scholar]
- Song C, Murray TA, Kimura R, Wakui M, Ellsworth K, Javedan SP, Marxer-Miller S, Lukas RJ, Wu J. Role of alpha7-nicotinic acetylcholine receptors in tetanic stimulation-induced gamma oscillations in rat hippocampal slices. Neuropharmacology. 2005;48:869–880. doi: 10.1016/j.neuropharm.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Tachikawa E, Mizuma K, Kudo K, Kashimoto T, Yamato S, Ohta S. Characterization of the functional subunit combination of nicotinic acetylcholine receptors in bovine adrenal chromaffin cells. Neuroscience Letters. 2001;312:161–164. doi: 10.1016/s0304-3940(01)02211-x. [DOI] [PubMed] [Google Scholar]
- Thinschmidt JS, Ren K, King MA, Meyer EM, Papke RL. Modulation of spontaneous hippocampal synaptic events with 5-hydroxyindole, 4OH-GTS-21, and rAAV- mediated alpha7 nicotinic receptor gene transfer. Brain Research. 2008;1203:51–60. doi: 10.1016/j.brainres.2008.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulloa L. The vagus nerve and the nicotinic anti-inflammatory pathway. National Review of Drug Discovery. 2005;4:673–684. doi: 10.1038/nrd1797. [DOI] [PubMed] [Google Scholar]
- Uteshev VV, Meyer EM, Papke RL. Activation and inhibition of native neuronal alpha-bungarotoxin-sensitive nicotinic ACh receptors. Brain Research. 2002;948:33–46. doi: 10.1016/s0006-8993(02)02946-3. [DOI] [PubMed] [Google Scholar]
- Uteshev VV, Meyer EM, Papke RL. Regulation of neuronal function by choline and 4OH-GTS-21 through alpha7 nicotinic receptors. Journal of Neurophysiology. 2003;89:33–46. doi: 10.1152/jn.00943.2002. [DOI] [PubMed] [Google Scholar]
- Woodruff-Pak DS, Li Y, Kem WR. A nicotinic agonist (GTS-21), eyeblink classical conditioning, and nicotinic receptor binding in rabbit brain. Brain Research. 1994;645:309–317. doi: 10.1016/0006-8993(94)91665-9. [DOI] [PubMed] [Google Scholar]
- Zwart R, De Filippi G, Broad LM, McPhie GI, Pearson KH, Baldwinson T, Sher E. 5-Hydroxyindole potentiates human alpha 7 nicotinic receptor-mediated responses and enhances acetylcholine-induced glutamate release in cerebellar slices. Neuropharmacology. 2002;43:374–384. doi: 10.1016/s0028-3908(02)00094-1. [DOI] [PubMed] [Google Scholar]