Abstract
Pendrin is expressed in the apical regions of type B and non-A, non-B intercalated cells, where it mediates Cl− absorption and HCO3− secretion through apical Cl−/HCO3− exchange. Since pendrin is a robust I− transporter, we asked whether pendrin is upregulated with dietary I− restriction and whether it modulates I− balance. Thus I− balance was determined in pendrin null and in wild-type mice. Pendrin abundance was evaluated with immunoblots, immunohistochemistry, and immunogold cytochemistry with morphometric analysis. While pendrin abundance was unchanged when dietary I− intake was varied over the physiological range, I− balance differed in pendrin null and in wild-type mice. Serum I− was lower, while I− excretion was higher in pendrin null relative to wild-type mice, consistent with a role of pendrin in renal I− absorption. Increased H2O intake enhanced differences between wild-type and pendrin null mice in I− balance, suggesting that H2O intake modulates pendrin abundance. Raising water intake from ∼4 to ∼11 ml/day increased the ratio of B cell apical plasma membrane to cytoplasm pendrin label by 75%, although circulating renin, aldosterone, and serum osmolality were unchanged. Further studies asked whether H2O intake modulates pendrin through the action of AVP. We observed that H2O intake modulated pendrin abundance even when circulating vasopressin levels were clamped. We conclude that H2O intake modulates pendrin abundance, although not likely through a direct, type 2 vasopressin receptor-dependent mechanism. As water intake rises, pendrin becomes increasingly critical in the maintenance of Cl− and I− balance.
Keywords: chloride, apical anion exchange, vasopressin, intercalated cells, vasopressin escape
how the kidney regulates I− excretion is poorly understood. In humans, I− elimination occurs primarily through the kidney, being excreted in urine as inorganic I− (38). I− is eliminated to a lesser extent in stool as organic I− (38). In kidney, I− is filtered at the glomerulus and absorbed along the tubular epithelium through both passive and active mechanisms (38). Renal I− clearance follows changes in glomerular filtration rate (GFR), although I− clearance is lower than GFR due to I− absorption along the nephron (38). However, the molecular mechanisms responsible for renal tubular I− absorption are poorly characterized.
Pendrin is a robust I− transporter that is expressed in the apical regions of type B and non-A, non-B intercalated cells found within the distal convoluted tubule (DCT), the connecting tubule (CNT), the initial collecting tubule (iCT), and the cortical collecting duct (CCD) (13, 21, 34). In heterologous expression systems, pendrin mediates I−, Cl−, and HCO3− transport as Cl−/HCO3−, Cl−/Cl−, Cl−/I−, and I−/HCO3− exchange (22, 23, 25, 40) in an electroneutral, 1:1 exchange relationship (24). In rabbit CCD, electroneutral apical Cl−/HCO3− exchange is observed in type B intercalated cells, which occurs, at least partly, through the action of pendrin (21, 27, 35).
Pendrin is upregulated with dietary Cl− intake restriction, which increases Cl− uptake in the CCD (20, 30, 32), thereby helping maintain NaCl balance (32, 35). Whether pendrin abundance is regulated by dietary intake of other halides, such as I−, and whether pendrin contributes to the maintenance of I− homeostasis are unexplored. Thus the purpose of the present study was to determine whether pendrin is upregulated with dietary I− restriction and to determine whether and how pendrin participates in the renal regulation of I− excretion.
METHODS
Animals
Slc26a4 (−/−) mice (9) were bred in parallel with coisogenic wild-type mice (129S6/SvEv Tac, Taconic Farms, Germantown, NY). Ration-fed, age- and sex-matched male and female Slc26a4 (−/−) and Slc26a4 (+/+) mice were studied. Table 1 shows the composition and I− content of these diets as determined by our analysis (2, 3). Each diet was prepared as a gel (31), thus enabling H2O and food intake to be predetermined.
Table 1.
Diet composition for mouse studies
| Series | Name | Daily H2O Intake, ml | Daily NaCl Intake, meq | Daily I− Intake, μg | Daily Dietary Intake |
|---|---|---|---|---|---|
| 1 | Effect of I− intake on pendrin abundance | ||||
| Low-iodide content | 9.0 | 0.78 | 0.9 | I−-deficient, synthetic diet: 3.2 g/day, AIN-93M, cat. no. 520000, Zeigler Bros., Gardners, PA, 89 mg/day gelatin, 0.32 g/day sucrose | |
| High-iodide content | 9.0 | 0.78 | 13.0 | ||
| 2 | Effect of H2O intake on pendrin abundance | ||||
| High water content | 9 or 13 | 0.78 | 12 | Grain-based diet: 3.2 g/day, Zeigler Bros., 53881300, 99 mg/day agar | |
| Low water content | 4 | 0.78 | 12 | ||
| 3 | Effect of vasopressin on pendrin abundance | ||||
| Vehicle | 6 | 0.87 | 6.9 | Grain-based diet: 3.5 g/day Zeigler Bros., 53881300, 379 mg/day gelatin | |
| dDAVP | 6 | 0.87 | 6.9 | ||
| 4 | Effect of H2O intake on pendrin abundance when circulating vasopressin levels are clamped | ||||
| dDAVP+high water content | 11 | 0.87 | 6.9 | Grain-based diet: 3.5 g/day, Zeigler Bros., 53881300, 379 mg/day gelatin | |
| dDAVP+low water content | 5 | 0.87 | 6.9 | ||
dDAVP, [deamino-Cys1, d-Arg8]-vasopressin acetate salt hydrate.
Treatment Conditions
Effect of I− intake on pendrin abundance (series 1).
Mice received a synthetic, I−-restricted diet (0.9 μg I−/day, Table 1, series 1) that provided an I− intake slightly above the minimum daily requirement for a mouse (28) or the same diet supplemented with potassium iodide (KI), giving an I− intake (13 μg I−/day), which is slightly above that of standard rodent diets.1 After 2 wk of treatment, mice were killed, and pendrin abundance was examined.
Effect of H2O intake on pendrin abundance (series 2).
For 7 days, mice ate a balanced, grain-based, NaCl-replete diet giving mice a high (9 or 13 ml/day) or low water intake (4 ml/day).
Effect of vasopressin on pendrin abundance (series 3).
For 7 days, mice received [deamino-Cys1, d-Arg8]-vasopressin acetate salt hydrate (dDAVP; 0.25 ng/h; V1005, Sigma) or vehicle by minipump (Alzet model 1002, Alzet, Palo Alto, CA) and consumed a grain-based diet with 6 ml H2O daily.
Effect of H2O intake on pendrin abundance when circulating vasopressin levels were clamped (series 4).
For 10 days, mice received dDAVP (0.25 ng/h) by minipump and consumed a grain-based diet with 5 ml H2O/day. A second group received dDAVP over the 10-day treatment period and consumed the same diet as the group above (5 ml H2O/day) for the first 3 days and then received 11 ml/day of H2O for the remaining 7 days. The nutrient intake was otherwise the same in each group.
In some experiments, mice were placed in metabolic cages 48 h before death. Urine was then collected under oil at 4°C for the 24 h before death. Mice were then killed under anesthesia with 1–2% isoflurane in 100% O2 at 1 liter/min.
The Institutional Animal Care and Use Committee at Emory University approved all animal treatment protocols.
Measurement of Blood Pressure, Serum and Urine Chemistries, Thyroid-Stimulating Hormone, Aldosterone, Renin, and Arterial Blood Gases
Blood was collected for serum chemistries through the abdominal aorta under isoflurane anesthesia. Serum chemistries were measured by IDEXX Laboratories (West Sacramento, CA) (31). Urine Cl− was measured with a Chloride Analyzer 926S (Nelson Jameson, Marshfield, WI). Arterial and urinary pH and Pco2 were measured using an ABL5 (Radiometer America, Westlake, OH) (31) or a microelectrode (MI 410, Microelectrodes, Londonderry, NH). Serum and urine creatinine were measured by HPLC (8). Plasma renin concentration was measured using methods described previously (17). Serum osmolality was measured with a vapor pressure osmometer (Wescor, Logan, UT). Serum total and inorganic iodine, urine inorganic iodine, and iodine content of the diets were measured using methods published previously (2, 3). Serum inorganic iodine was calculated as the difference between total and hormonal (or organic) iodine. Serum thyroid-stimulating hormone (TSH) was measured by radioimmunoassay (19). Serum aldosterone was measured by RIA using a kit (Coat-A-Count, Diagnostic Products, Los Angeles, CA.).
Antibodies
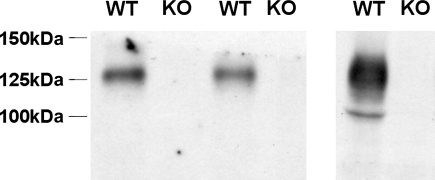
The rabbit anti-rat aquaporin-2 (AQP2) antibody (4) was a generous gift of Dr. Mark Knepper. The primary rabbit anti-Slc26a4 antibody employed in immunohistochemistry and immunogold cytochemistry recognizes amino acids 766–780 of the human Slc26a4 sequence, the gene encoding pendrin. Polyclonal antibodies that target this amino acid sequence have been characterized previously in studies of mouse kidney (21). The antibody employed in immunoblots recognizes the terminal 29 amino acids of the rat pendrin protein sequence (16) and was a generous gift of Dr. Peter Aronson. We asked whether this anti-pendrin antibody (16) specifically detects pendrin protein by immunoblotting. This antibody detects a protein of the expected size in kidney lysates from wild-type, but not from pendrin null mice (20) (Fig. 1). Thus this antibody specifically detects pendrin protein by immunoblotting and was employed in all experiments below that quantified pendrin abundance by Western blot analysis.
Fig. 1.
Pendrin abundance in the kidney can be detected by immunoblot. Pendrin band density was compared in kidney lysates from 3 wild-type and 3 pendrin null mice run in 2 separate gels. As shown, the antibody raised against the terminal 29 amino acids of the rat pendrin sequence (16) detects a protein in lysates from wild-type mice, but not from pendrin null mice, that migrates at ∼130 kDa, the expected mobility of pendrin (20). Therefore, this antibody is specific for pendrin protein.
Immunoblotting
Semiquantitative immunoblotting of kidney lysates from Slc26a4 (−/−) and Slc26a4 (+/+) mice were performed as reported previously (36). Tissue was homogenized in dissecting buffer (0.3 M sucrose, 25 mM imidazole, 1 mM EDTA, pH 7.2, containing 8.5 μM leupeptin, 1 mM phenylmethylsulfonyl fluoride) and then dissolved in Laemmli buffer and resolved by SDS-PAGE. Equal protein loading was confirmed by Coomassie blue staining of gels run in parallel (29). Protein was electrophoretically transferred onto nitrocellulose membranes and probed with the antibody that recognizes the terminal 29 amino acids of the rat pendrin sequence (16). Immunolabeling was detected with a horseradish peroxidase-conjugated goat anti-rabbit secondary antibody (Upstate Biotechnology, Lake Placid, NY) using an enhanced chemiluminescence system (Amersham Biosciences, Little Chalfont, UK). Band density was quantified using Quantity One Image software (Bio-Rad, Hercules, CA) and compared between groups.
Immunohistochemistry
In situ fixation of mouse kidneys was performed as described previously (31). For paraffin embedding, tissues were dehydrated in a graded series of ethyl alcohol followed by xylene and then embedded in paraffin. The sections were deparaffinized and rehydrated. Endogenous peroxidase was blocked with 0.5% H2O2 in absolute methanol for 30 min at room temperature. To reveal antigens, sections were incubated in 1 mM Tris solution (pH 9.0) supplemented with 0.5 mM EGTA and heated in a microwave oven for 10 min. Nonspecific binding of IgG was prevented by blocking in PBS supplemented with 1% BSA, 0.05% saponin, and 0.2% gelatin. Sections were incubated overnight at 4°C with primary antibodies diluted in PBS supplemented with 0.1% BSA and 0.3% Triton X-100. After sections were rinsed with PBS supplemented with 0.1% BSA, 0.05% saponin, and 0.2% gelatin, labeling was visualized with a horseradish peroxidase-conjugated secondary antibody (1:200, DAKO), followed by incubation with 3,3′-diaminobenzidine (brown stain). Sections were washed with distilled water, dehydrated with graded ethanol and xylene, mounted in Eukitt, and examined by light microscopy.
Immunogold Cytochemistry
Kidneys were prepared for electron microscopy as described previously (34). For electron microscopy, pendrin immunoreactivity was localized in ultrathin sections using immunogold cytochemistry (34). The CCD, CNT, and iCT were identified as described previously (34). Type A, type B, and non-A, non-B intercalated cell subtypes were identified using morphological characteristics established in studies of rat and mouse under basal conditions (34).
Morphometrical Analysis
Apical plasma membrane boundary length, cytoplasmic area, and gold label along the apical plasma membrane and over the cytoplasm, including cytoplasmic vesicles, were quantified in type B and non-A, non-B intercalated cells from each treatment group as described previously (34). In each animal, at least five cells of each intercalated cell subtype were selected at random and photographed at a primary magnification of ×5,000 and examined at a final magnification of about ×18,200.
Statistical Analysis
Comparisons were made between two groups using an unpaired Student's t-test. A P < 0.05 indicates statistical significance. Data are displayed as means ± SE.
RESULTS
I− Intake Does Not Change Pendrin Expression
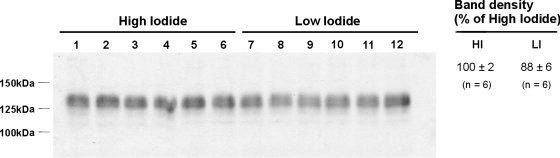
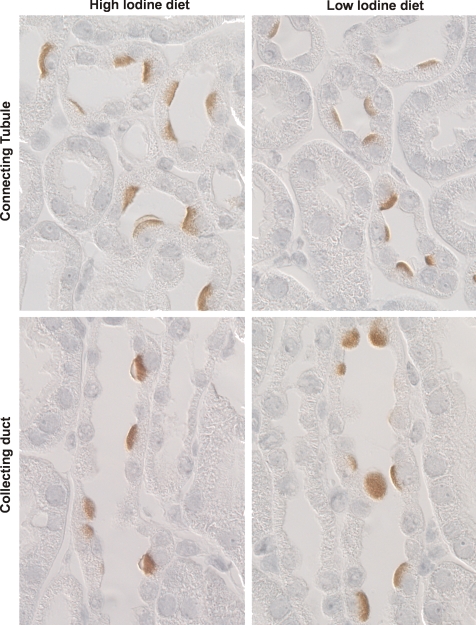
Because Cl− intake modulates pendrin expression (20, 30, 32), we asked if renal total pendrin abundance is regulated by intake of other halides. Thus the effect of I− intake on pendrin abundance and subcellular distribution was explored (Table 1, series 1). Figure 2 shows that pendrin abundance was similar in kidney lysates from wild-type mice given either the low (0.9 μg I−/day)- or the high-I− (13 μg I−/day) synthetic diet for 2 wk. Moreover, no marked difference in the subcellular distribution of pendrin immunolabel was detected when I− consumption was varied (Fig. 3).2 We conclude that I− intake, unlike Cl− intake, has little affect on pendrin abundance or subcellular distribution.
Fig. 2.
Effect of I− intake on pendrin abundance. Wild-type mice were given a synthetic diet that provided either 0.9 (LI) or 13 μg I−/day (HI) for 14 days and then killed (series 1). Pendrin abundance was quantified by immunoblotting kidney lysates from mice in each group. Band density was normalized to the mean band density of renal lysates from mice given the high-I− diet. As shown, no difference in pendrin abundance was detected between groups.
Fig. 3.
Effect of I− intake on pendrin immunolabel. Wild-type mice were given a synthetic, I−-restricted or an I−-replete diet for 14 days (series 1). Pendrin labeling in cortical sections from mice in each group is shown. No distinct difference in label intensity or subcellular distribution was noted between these 2 groups.
Pendrin Null Mice Have Lower Serum I− and Greater I− Clearance than Wild-Type Mice Particularly When Water Intake is High
To determine whether pendrin modulates I− balance, serum total and inorganic I− concentrations were measured in pendrin null and wild-type mice following 2 wk of the I−-restricted diet employed above (0.9 μg I−/day, series 1). As shown (Table 2), serum I− was lower in pendrin null than in wild-type mice. Genetic disruption of Slc26a4 does not reduce serum I− concentration from hypothyroidism since TSH is similar in pendrin null and wild-type mice (Table 2).
Table 2.
Effect of I− restriction on serum I− in wild-type and pendrin null mice
| Water Intake, ml/day | Total I−, μg/ml | Inorganic I−, μg/ml | Thyroid-Stimulating Hormone, mU/l | |
|---|---|---|---|---|
| Wild-type | 9 | 7.0±0.3 (n = 4) | 3.1±0.3 (n = 4) | 22±2 (n = 5) |
| Pendrin null | 9 | 5.9±0.4 (n = 3) | 1.5±0.1*(n = 3) | 21±6 (n = 5) |
Values are means ± SE. Mice were given the synthetic, low I− diet (0.9 mg I−/day, series 1).
P < 0.05.
Further experiments explored whether pendrin null mice have a lower serum I− concentration from greater renal I− excretion. Thus serum I− and fractional excretion of I− (FEI−)3 were compared in wild-type and pendrin null mice after animals received 7 days of a standard rodent diet prepared as a gel, which gave 4 ml H2O/day (series 2, Table 1), which is the typical daily ad libitum H2O consumption for a mouse. As shown (Table 3), serum I− was lower in pendrin null relative to wild-type mice, although no difference in FEI− was detected.
Table 3.
Urinary iodide and chloride balance in wild-type and pendrin null mice when H2O consumption is varied
| Wild-Type | Pendrin Null | Wild-Type | Pendrin Null | |
|---|---|---|---|---|
| H2O intake, ml/day | 4 | 4 | 13 | 13 |
| Urine volume, ml/day | 0.96±0.02 (n = 5) | 1.00±0.03(n = 5) | 8.5±0.2 (n = 5) | 9.0±0.2 (n = 5) |
| Serum total I−, μg/ml | 0.184±0.005 (n = 5) | 0.123±0.006*(n = 5) | 0.250±0.008 (n = 5) | 0.118±0.011*(n = 5) |
| Serum inorganic I−, μg/ml | 0.137±0.004 (n = 5) | 0.079±0.007*(n = 5) | 0.202±0.008 (n = 5) | 0.076±0.009*(n = 5) |
| I− excretion, μg/day | 14.4±1.83 (n = 5) | 14.8±1.55 (n = 5) | ||
| Inorganic I− clearance, μl/min | 49±5 (n = 5) | 136±6*(n = 5) | ||
| Fractional excretion of I−, % ([U/P I−]/[U/P creatinine] × 100) | 23.6±4.7 (n = 3) | 37.4±6.2 (n = 3) | 69.2±15.6 (n = 3) | 192.8±29.1 (n = 3)* |
| Serum Cl−, meq/l | 115±1 (n = 5) | 115±1 (n = 5) | ||
| Cl− clearance, μl/min | 2.76±0.10 (n = 5) | 3.46±0.08*(n = 5) |
Values are means ± SE. To determine serum creatinine and I− concentrations that were used in the calculation of the fractional excretion of I−, I− and creatinine were measured in the same serum samples that were pooled from 2 mice. For each “n”, I− and creatinine concentrations were measured separately in urine samples from each of these 2 mice and averaged. The mean of these 2 concentrations were used in the calculations. Twenty-four-hour solute excretion was not quantified in mice given the lower water intake since it was not possible to achieve full solute recovery. Mice were given the grain-based diet(series 2).
P < 0.05.
Because water intake changes the driving force for the passive absorption of solutes such as I−, further studies compared serum I− concentration in pendrin null and wild-type mice after 7 days of an identical diet, but when water intake was increased from 4 to 13 ml/day (series 2). As shown (Table 3), wild-type and mutant mice had a greater difference in I− balance when H2O intake increased. Whereas FEI− was similar in pendrin null and wild-type mice when consuming 4 ml H2O/day, FEI− was 2.8-fold higher in the mutant mice when water consumption increased to 13 ml/day. Clearance of both I− and Cl− were greater in pendrin null than in wild-type mice (Table 3). However, mutant and wild-type mice had greater differences in I− clearance than in Cl− clearance. We conclude that pendrin expression modulates the renal excretion of I−, particularly when water intake is high.
Pendrin Abundance is Regulated by Water Intake
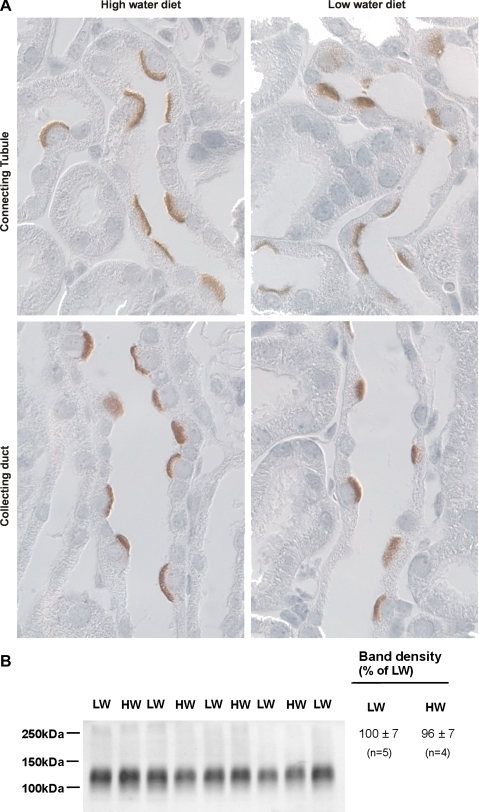
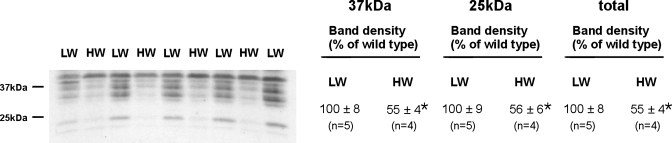
Since wild-type and pendrin null mice had greater differences in serum I− concentration as water intake rose, we asked whether water intake modulates pendrin abundance. By light microscopy (Fig. 4A), pendrin immunolabel appeared more intense and more discrete in the apical regions of cells in cortical sections from mice that consumed more H2O. Thus pendrin abundance and subcellular distribution were quantified in kidneys from wild-type mice after consuming 4 or 9 ml H2O per day for 7 days (series 2). As shown (Table 4), the ratio of apical plasma membrane to cytoplasm pendrin immunolabel in B cells was ∼75% higher in mice with the higher H2O intake, although apical plasma membrane pendrin immunolabel was unchanged in non-A, non-B cells. However, water intake did not affect total pendrin protein abundance when quantified either by immunoblotting or by immunogold cytochemistry with morphometrical analysis (Table 4, Fig. 4B). We conclude that the subcellular distribution of pendrin changes as water intake is varied. With increased H2O consumption, the subcellular localization of pendrin in type B intercalated cells shifts from the cytoplasm to the apical plasma membrane.
Fig. 4.
Effect of water intake on pendrin immunolabel. A: wild-type mice were given a balanced diet prepared as a gel and either 4 (LW) or 9 ml H2O/day (HW; series 2). After 7 days of either diet, pendrin labeling was examined in cortical sections taken from kidneys in each group. As shown, label was more distinct and discrete in the apical regions of cells in kidneys from mice given the higher water intake. B: mice were treated as described in A. Pendrin band density was quantified by immunoblotting of kidney lysates taken from mice in each group. Values were normalized to the band density obtained from kidney lysates taken from mice given the low-water diet. As shown, pendrin band density was similar in kidneys from each group.
Table 4.
Subcellular distribution of pendrin labeling in type B and non-A, non-B intercalated cells when H2O intake is varied
| Type B | Non-A, Non-B | |||
|---|---|---|---|---|
| Water intake, ml/day | 4 | 9 | 4 | 9 |
| No. of animals studied | 3 | 4 | 3 | 4 |
| Apical plasma membrane boundary length, mm × 10−2 | 0.831±0.209 | 1.14±0.104 | 2.18±0.32 | 2.48±0.40 |
| Apical plasma membrane label density, gold particles/mm apical membrane | 422±125 | 411±40 | 835±70 | 1,329±162 |
| Apical plasma membrane Gold, gold particles/cell | 3.54±1.15 | 4.66±0.582 | 18.4±6.2 | 34.9±8.6 |
| Subcellular label distribution ratio, apical plasma membrane gold/cytoplasmic gold × 10−2 | 3.83±0.76 | 6.66±0.667* | 0.632±0.02 | 0.853±0.16 |
| Cytoplasmic area, mm2 × 10−5 | 4.44±0.555 | 4.01±0.151 | 3.62±0.32 | 4.14±0.49 |
| Cytoplasmic gold, gold particles/cell | 86.1±15.7 | 70.4±5.89 | 28.9±4.6 | 39.0±3.6 |
| Total gold, gold particles/cell | 89.6±16.9 | 75.0±6.21 | 47.3±8.1 | 73.8±12.1 |
Values are means ± SE. Mice were given the grain-based diet(series 2).
P < 0.05.
Since pendrin is regulated in vivo by the renin-angiotensin-aldosterone system (18, 31, 39) and possibly by changes in arterial pH (1, 11, 33), arterial pH and circulating concentrations of aldosterone or renin were measured when dietary H2O intake was varied (Table 5). As shown, increased H2O intake did not alter arterial pH, serum osmolality, serum aldosterone, or plasma renin concentration and thus cannot explain the increase in pendrin abundance observed as water intake increased. However, urinary pH was higher in mice consuming more H2O, consistent with increased pendrin-mediated HCO3− secretion.
Table 5.
Effect of water intake on serum aldosterone, plasma renin concentration, and arterial and urinary pH in wild-type mice
| Daily H2O Intake, ml | Serum Aldosterone Concentration, nM | Plasma Renin Concentration μg ANG I·ml sample−1·h−1 | Serum Osmolality, mosmol/kgH2O | Arterial pH | Arterial Pco2 | Arterial HCO3−, (calculated) mM | Urine pH | |
|---|---|---|---|---|---|---|---|---|
| High water intake | 9 | 1.57±0.36 (n = 3) | 0.40±0.02 (n = 3) | 306±(n = 4) | 7.37±0.01(n = 8) | 42±1 | 23±1 | 6.44±0.09 (n = 9) |
| Low water intake | 4 | 1.59±0.35 (n = 3) | 0.43±0.01 (n = 3) | 305±2 (n = 5) | 7.37±0.01 (n = 10) | 42±1 | 23±1 | 6.11±0.05*(n = 10) |
Values are means ± SE. Mice were given the grain-based diet(series 2).
P < 0.05.
Water Intake Increases Pendrin Expression Independently of Vasopressin
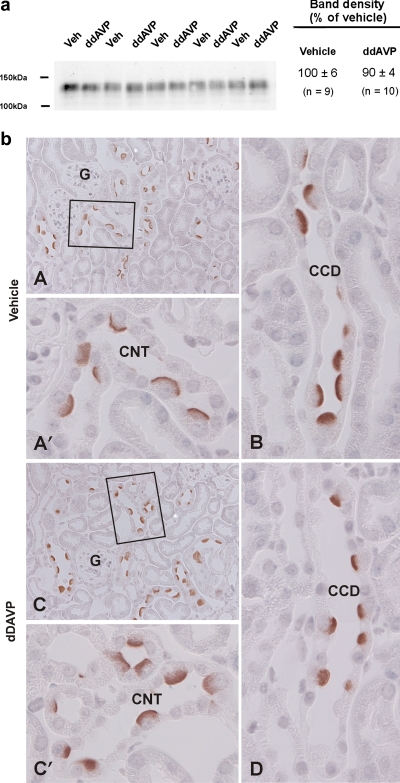
Since circulating vasopressin levels rise as water intake falls and because vasopressin modulates salt and water transport in the collecting duct, we asked whether water intake modulates pendrin abundance through the action of vasopressin. Since AQP2 abundance is highly regulated by changes in circulating vasopressin concentration, AQP2 abundance was quantified in kidney lysates from mice consuming 4 vs. 13 ml H2O/day (series 2). With reduced H2O intake, renal AQP 2 abundance increased, consistent with the expected increase in circulating vasopressin (Fig. 5). Thus we hypothesized that lowering water intake reduces pendrin abundance because vasopressin release increases. To explore the effect of vasopressin on pendrin abundance, mice received 7 days of dDAVP or vehicle (series 3), while consuming 6 ml H2O each day. As shown, dDAVP did not change pendrin protein abundance (Fig. 6). However, pendrin immunolabel appeared slightly more diffuse in kidneys from mice given dDAVP, consistent with reduced apical plasma membrane abundance. Thus we cannot exclude the possibility that water intake modulates pendrin abundance, at least in part, through the action of vasopressin.
Fig. 5.
Effect of water intake on aquaporin-2 (AQP2) abundance. Wild-type mice were given a balanced diet prepared as a gel and either 4 (LW) or 13 ml H2O (HW) each day (series 2). After 7 days of either diet, AQP2 band density was examined in kidney lysates taken from mice in each group. As shown, AQP2 band density was higher in kidneys from mice with the lower H2O intake.
Fig. 6.
Effect of a V2R agonist (dDAVP) on pendrin abundance and subcellular distribution. Mice were given 7 days of dDAVP or vehicle, while consuming 6 ml H2O daily (series 3). Pendrin abundance was quantified by immunoblotting of kidney lysates from mice receiving dDAVP or vehicle (a). One representative gel is shown. Values were normalized to the band density of kidney lysates from vehicle-treated mice run on the same gel. As shown, total protein abundance by immunoblot was similar in both groups. b: Pendrin immunolabel in cortical sections from mice given vehicle (A) or dDAVP (C; series 3). Connecting tubules (CNTs) from each of these micrographs are shown at higher magnification (A′ and C′). Pendrin immunolabel in cortical collecting ducts (CCDs) from mice given dDAVP or vehicle are shown in B and D. Pendrin label appeared more diffuse in cortical sections from mice given dDAVP than those given vehicle, consistent with increased apical plasma membrane pendrin abundance.
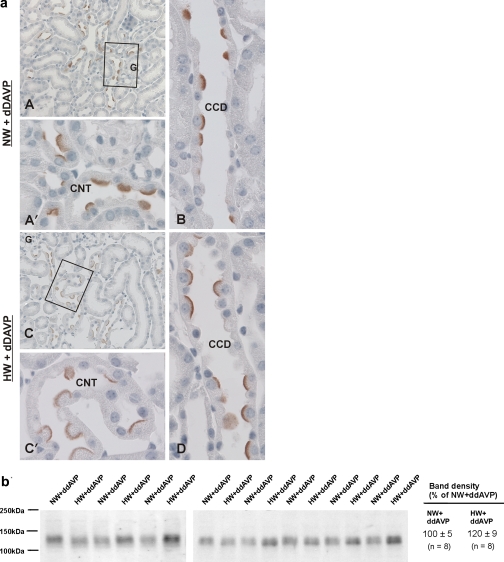
Vasopressin might modulate pendrin abundance through a direct, type 2 vasopressin receptor (V2R)-mediated mechanism, or through an indirect effect, such as through changes in luminal flow rate. Therefore, further experiments explored whether H2O intake modulates pendrin total protein abundance and subcellular distribution when circulating vasopressin concentration was clamped. Thus mice received dDAVP by minipump and either 5 or 11 ml H2O daily for 7 days (series 4). As shown (Fig. 7), in dDAVP-treated mice, pendrin immunolabel was much more intense and discrete in the region of the apical plasma membrane when animals consumed 11 ml H2O/day relative to those consuming 5 ml H2O/day, despite similar circulating levels of vasopressin. However, pendrin total protein abundance was similar in kidney lysates from vasopressin-treated mice consuming either 5 or 11 ml H2O/day (Fig. 7). Thus H2O intake modulates pendrin's subcellular distribution independent of circulating vasopressin levels.
Fig. 7.
Effect of water intake on pendrin abundance and subcellular distribution when circulating vasopressin levels are clamped. Series 4: Mice received dDAVP and 5 ml H2O/day (NW+dDAVP) or dDAVP and 11 ml H2O/day (HW+dDAVP). Pendrin labeling is shown in cortical sections from mice in each group (a). Pendrin immunolabel is shown in cortical sections from mice given 5 (A) or 11 ml H2O/day (C; series 3). CNTs from each of these micrographs are shown at higher magnification (A′ and C′). Pendrin immunolabel in CCDs from dDAVP-treated mice with the lower or higher water intake are shown in B and D. Pendrin labeling appeared more distinct and discrete in the region of the apical plasma membrane in sections from mice that consumed more H2O. b: Pendrin abundance in kidney lysates from mice in each of these treatment groups. As shown, H2O intake did not increase pendrin total protein abundance when quantified by immunoblotting (P = 0.072).
Further experiments determined whether pendrin attenuates the fall in serum Cl− concentration following vasopressin treatment when H2O intake is high. As shown (Table 6), serum Cl− is lower in pendrin null than in wild-type mice in this treatment model, although differences in Cl− clearance were not detected. Therefore, pendrin is critical in maintaining serum Cl− and I− concentration, particularly when H2O intake is high.
Table 6.
Serum Na+ and Cl− concentration and Cl− clearance in wild-type and pendrin null mice receiving dDAVP with a high H2O intake (vasopressin escape)
| Na+, meq | Cl−, meq | Cl− Excretion, meq/24 h | Cl− Clearance, μl/min | |
|---|---|---|---|---|
| Wild-type | 126±2 (n = 4) | 104±3 (n = 8) | 0.700±0.08 (n = 8) | 4.62±0.50 (n = 8) |
| Pendrin null | 118±4 (n = 3) | 88±3*(n = 5) | 0.680±0.09 (n = 5) | 5.46±1.03 (n = 5) |
Values are means ± SE. Mice were given dDAVP by minipump and consumed the grain-based diet and 5 ml H2O/day for 3 days, 11 ml H2O/day for the next 7 days, and then killed (series 4, vasopressin escape).
P < 0.05.
DISCUSSION
The present study demonstrates that water intake regulates pendrin abundance in the kidney. Water intake does not alter pendrin abundance through changes in arterial pH, serum osmolality, or through changes in circulating TSH, aldosterone, or renin concentration. However, since pendrin abundance might be reduced with administration of the V2R agonist dDAVP, water intake might modulate pendrin abundance and pendrin-mediated I− uptake through the release of vasopressin.
The body tightly controls serum NaCl concentration largely through the regulated release of vasopressin. The antidiuretic action of vasopressin is mediated by V2R, which is expressed along the collecting duct and in the CNT (10, 15). However, since the V2R is not expressed within intercalated cells (10) and because water intake regulates pendrin abundance even when circulating vasopressin concentration is clamped4, it is unlikely that vasopressin alters pendrin abundance through a direct, V2R-mediated signaling event. Instead, dDAVP most likely regulates pendrin abundance through an indirect effect, such as through changes in luminal flow rate, luminal solute concentration, or through changes in epithelial properties, such as solvent drag. Whether flow rate increases pendrin-mediated transport in vitro remains to be determined, however.
How the kidney helps maintain serum Na+ and Cl− concentration when circulating vasopressin levels are inappropriately high has been studied extensively in rats given the V2R agonist dDAVP and a high dietary water intake (vasopressin escape). Although hyponatremia is observed during vasopressin escape, the kidney mitigates the fall in serum Na+ by reducing the abundance of the vasopressin-sensitive H2O channel AQP2, which reduces water absorption (6), and by increasing the abundance and activity of Na+ transporters such as the epithelial Na+ channel (ENaC) and the thiazide-sensitive NaCl cotransporter (NCC) (5), which enhances Na+ absorption. In addition, the present study shows that the kidney helps maintain serum Cl− concentration during vasopressin escape by upregulating pendrin. The increase in pendrin abundance observed during vasopressin escape likely contributes to the hypertension observed in this treatment model (26). In the absence of pendrin, the kidney has an impaired ability to mitigate the fall in serum Cl− expected when circulating vasopressin levels are inappropriately high.
During vasopressin escape, ENaC activity rises through increased abundance of the α-ENaC subunit and through the enhanced posttranslational processing of γ-ENaC, which involves protein cleavage and glycosylation. The increased γ-ENaC processing observed during vasopressin escape occurs in the renal cortex, where pendrin is highly abundant, but does not occur in the medulla, where pendrin abundance is low (26). Since pendrin impacts ENaC subunit abundance and γ-ENaC posttranslational processing (14), the increase in pendrin abundance observed during vasopressin escape contributes to the enhanced γ-NaC cleavage present in this treatment model.
Since increased water intake upregulates pendrin abundance, increased pendrin-mediated I− uptake is expected. With greater water intake, pendrin-mediated I− uptake should increase in wild-type mice. In the absence of pendrin, I− balance is more negative, particularly when water consumption is high, consistent with a role of pendrin in renal tubular I− absorption.
Pendrin is upregulated not only with increased H2O consumption but also by dietary Cl− restriction (20, 30, 32, 35), which further impacts I− balance. As early as 1911, it was recognized that renal I− absorption increases dramatically following dietary Cl− restriction (37). The increment in renal I− absorption observed during NaCl restriction occurs in part from increased pendrin abundance. Moreover, if I− and Cl− compete for a common extracellular pendrin binding site, Cl− restriction should augment pendrin-mediated I− uptake by increasing the occupancy of this binding site with I−. This possibility remains to be tested, however.
Renal I− absorption occurs through both active and passive mechanisms (12). Passive solute absorption depends on luminal solute concentration, which increases as water is absorbed (37). With the increase in water absorption that follows reduced H2O intake, luminal solute concentration rises, which increases the driving force for solute absorption. Thus reduced H2O consumption increases luminal I− concentration, which reduces I− excretion by increasing the driving force for renal I− absorption. Conversely, increased H2O consumption reduces the driving force for renal I− absorption, which should increase I− excretion. Since the pendrin-dependent component of renal I− absorption increases with greater H2O consumption, it is unlikely that pendrin modulates renal tubular I− absorption through a passive transport mechanism.
While Cl− intake modulates pendrin abundance and subcellular distribution, we did not observe a change in pendrin expression when I− intake was limited to 0.9 μg/day for 14 days. However, we cannot exclude the possibility that further dietary I− restriction increases transporter abundance.
In conclusion, pendrin is critical to the maintenance of serum I− and Cl−, particularly when H2O intake is high. Increased water intake raises pendrin abundance, which most probably increases pendrin-mediated I− absorption. However, water intake does not likely increase pendrin abundance through a direct, vasopressin V2R-mediated signaling event.
GRANTS
This study was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-PO1-061521, Project 2 (to S. M. Wall). Thyroid-stimulating hormone assays were performed by the laboratory of Dr. Samuel Refetoff, which is supported by R37DK-15070.
ACKNOWLEDGMENTS
We thank Drs. Ken Spring, Peter Kopp, and Dominique Eladari for helpful suggestions.
Footnotes
1The I− content of the standard rodent diet employed in the Emory University vivarium is LabDiet 5001 (Purina Mills, St. Louis, MO), which gives a mouse ∼3 μg I−/day. The diet preparation used in most of our previous studies (no. 538813, Ziegler Brothers) gives a mouse ∼10 μg I−/day.
2Because I− intake might have a biphasic effect on pendrin abundance, pendrin expression was examined over different ranges of I− intake. No difference in pendrin abundance was noted when I− intake was varied (not shown).
3Mice kidneys secrete a substantial amount of creatinine (7). Therefore the urine I−/creatinine ratio should overestimate the amount of I− absorbed.
4During vasopressin escape, serum aldosterone increases (26), which contributes to the increase in pendrin abundance observed in this treatment model.
REFERENCES
- 1. Adler L, Efrati E, Zelikovic I. Molecular mechanisms of epithelial cell-specific expression and regulation of the human anion exchanger (pendrin) gene. Am J Physiol Cell Physiol 294: C1261–C1276, 2008 [DOI] [PubMed] [Google Scholar]
- 2. Benotti J, Benotti N. Protein-bound iodine, total iodine and butanol-extractable iodine by partial automation. Clin Chem 9: 408–416, 1963 [PubMed] [Google Scholar]
- 3. Benotti J, Benotti N, Pino S, Gardyna H. Determination of total iodine in urine, stool, diets and tissue. Clin Chem 11: 932–936, 1965 [PubMed] [Google Scholar]
- 4. DiGiovanni SR, Nielsen S, Christensen EI, Knepper MA. Regulation of collecting duct water channel expression by vasopressin in Brattleboro rat. Proc Natl Acad Sci USA 91: 8984–8988, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ecelbarger CA, Knepper MA, Verbalis JG. Increased abundance of distal sodium transporters in rat kidney during vasopressin escape. J Am Soc Nephrol 12: 207–217, 2001 [DOI] [PubMed] [Google Scholar]
- 6. Ecelbarger CA, Nielsen S, Olson BR, Murase T, Baker EA, Knepper MA, Verbalis JG. Role of renal aquaporins in escape from vasopressin-induced antidiuresis in rat. J Clin Invest 99: 1852–1863, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Eisner CB, Faulhaber-Walter R, Wang Y, Levine M, Briggs J, Schnermann J. Gender differences of creatinine excretion in mice (Abstract). FASEB J . 23, 804.18 2009 [Google Scholar]
- 8. Erdely A, Freshour G, Smith C, Engels K, Olson JL, Baylis C. Protection against puromycin aminonucleoside-induced chronic renal disease in the Wistar-Furth rat. Am J Physiol Renal Physiol 287: F81–F89, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Everett LA, Belyantseva IA, Noben-Trauth K, Cantos R, Chen A, Thakkar SI, Hoogstraten-Miller SL, Kachar B, Wu DK, Green ED. Targeted disruption of mouse Pds provides insight about the inner-ear defects encountered in Pendred syndrome. Hum Mol Genet 10: 153–161, 2001 [DOI] [PubMed] [Google Scholar]
- 10. Fenton RA, Brond L, Nielsen S, Praetorius J. Cellular and subcellular distribution of the type-2 vasopressin receptor. Am J Physiol Renal Physiol 293: F748–F760, 2007 [DOI] [PubMed] [Google Scholar]
- 11. Frische S, Kwon TH, Frøkiær J, Madsen KM, Nielsen S. Regulated expression of pendrin in rat kidney in response to chronic NH4Cl or NaHCO3 loading. Am J Physiol Renal Physiol 284: F584–F593, 2003 [DOI] [PubMed] [Google Scholar]
- 12. Halmi NS, King LT, Widner RR, Hass AC, Stuelke RG. Renal excretion of radioiodide in rats. Am J Physiol 193: 379–385, 1958 [DOI] [PubMed] [Google Scholar]
- 13. Kim YH, Kwon TH, Frische S, Kim J, Tisher CC, Madsen KM, Nielsen S. Immunocytochemical localization of pendrin in intercalated cell subtypes in rat and mouse kidney. Am J Physiol Renal Physiol 283: F744–F754, 2002 [DOI] [PubMed] [Google Scholar]
- 14. Kim YH, Pech V, Spencer KB, Beierwaltes WH, Everett LA, Green ED, Shin WK, Verlander JW, Sutliff RL, Wall SM. Reduced ENaC expression contributes to the lower blood pressure observed in pendrin null mice. Am J Physiol Renal Physiol 293: F1314–F1324, 2007 [DOI] [PubMed] [Google Scholar]
- 15. Kishore BK, Mandon B, Oza NB, DiGiovanni SR, Coleman RA, Ostrowski NL, Wade JB, Knepper MA. Rat vasopressin V2 receptor. J Clin Invest 97: 2763–2771, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Knauf F, Yang CL, Thomson RB, Mentone SA, Giebisch G, Aronson PS. Identification of a chloride-formate exchanger expressed on the brush border membrane of renal proximal tubule cells. Proc Natl Acad Sci USA 98: 9425–9430, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lum C, Shesely EG, Potter DL, Beierwaltes WH. Cardiovascular and renal phenotype in mice with one or two renin genes. Hypertension 43: 79–86, 2004 [DOI] [PubMed] [Google Scholar]
- 18. Pech V, Kim YH, Weinstein AM, Everett LA, Pham TD, Wall SM. Angiotensin II increases chloride absorption in the cortical collecting duct in mice through a pendrin-dependent mechanism. Am J Physiol Renal Physiol 292: F914–F920, 2007 [DOI] [PubMed] [Google Scholar]
- 19. Pohlenz J, Maqueem A, Cua K, Weiss RE, Van Sande J, Refetoff S. Improved radioimmunoassay for measurement of mouse thyrotropin in serum: strain differences in thyrotropin concentration and thyrotroph sensitivity to thyroid hormone. Thyroid 9: 1265–1271, 1999 [DOI] [PubMed] [Google Scholar]
- 20. Quentin F, Chambrey R, Trinh-Trang-Tan MM, Fysekidis M, Cambillau M, Paillard M, Aronson PS, Eladari D. The Cl−/HCO3− exchanger pendrin in the rat kidney is regulated in response to chronic alterations in chloride balance. Am J Physiol Renal Physiol 287: F1179–F1188, 2004 [DOI] [PubMed] [Google Scholar]
- 21. Royaux IE, Wall SM, Karniski LP, Everett LA, Suzuki K, Knepper MA, Green ED. Pendrin, encoded by the pendred syndrome gene, resides in the apical region of renal intercalated cells and mediates bicarbonate secretion. Proc Natl Acad Sci USA 98: 4221–4226, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Scott DA, Karniski LP. Human pendrin expressed in Xenopus laevis oocytes mediates chloride/formate exchange. Am J Physiol Cell Physiol 278: C207–C211, 2000 [DOI] [PubMed] [Google Scholar]
- 23. Scott DA, Wang R, Kreman TM, Sheffield VC, Karniski LP. The Pendred syndrome gene encodes a chloride-iodide transport protein. Nat Genet 21: 440–443, 1999 [DOI] [PubMed] [Google Scholar]
- 24. Shcheynikov N, Yang D, Wang Y, Zeng W, Karniski LP, So I, Wall SM, Muallem S. Slc26a4 functions as an electroneutral Cl−/I−/HCO3− exchanger: role of Slc26a4 and Slc26a6 in I- and HCO3− secretion and in regulation of CFTR in the parotid duct. J Physiol 586: 3814–3824, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Soleimani M, Greeley T, Petrovic S, Wang Z, Amlal H, Kopp P, Burnham CE. Pendrin: an apical Cl−/OH−/HCO3− exchanger in the kidney cortex. Am J Physiol Renal Physiol 280: F356–F364, 2001 [DOI] [PubMed] [Google Scholar]
- 26. Song J, Hu X, Khan O, Tian Y, Verbalis JG, Ecelbarger CA. Increased blood pressure, aldosterone activity, and regional differences in renal ENaC protein during vasopressin escape. Am J Physiol Renal Physiol 287: F1076–F1083, 2004 [DOI] [PubMed] [Google Scholar]
- 27. Star RA, Burg MB, Knepper MA. Bicarbonate secretion and chloride absorption by rabbit cortical collecting ducts: role of chloride/bicarbonate exchange. J Clin Invest 76: 1123–1130, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Subcommittee on Laboratory Animal Nutrition Nutrient requirements of the mouse. In: Nutrient Requirements of Laboratory Animals, edited by Benevenga NJ. Washington, DC: National Academy Press, 1995, p. 80–97 [Google Scholar]
- 29. Terris J, Ecelbarger CA, Nielsen S, Knepper MA. Long-term regulation of four renal aquaporins in rats. Am J Physiol Renal Fluid Electrolyte Physiol 271: F414–F422, 1996 [DOI] [PubMed] [Google Scholar]
- 30. Vallet M, Picard N, Loffing-Cueni D, Fysekidis M, Bloch-Faure M, Deschenes G, Breton S, Meneton P, Loffing J, Aronson PS, Chambrey R, Eladari D. Pendrin regulation in mouse kidney primarily is chloride-dependent. J Am Soc Nephrol 17: 2153–2163, 2006 [DOI] [PubMed] [Google Scholar]
- 31. Verlander JW, Hassell KA, Royaux IE, Glapion DM, Wang ME, Everett LA, Green ED, Wall SM. Deoxycorticosterone upregulates Pds (Slc26a4) in mouse kidney: role of pendrin in mineralocorticoid-induced hypertension. Hypertension 42: 356–362, 2003 [DOI] [PubMed] [Google Scholar]
- 32. Verlander JW, Kim YH, Shin WK, Pham TD, Hassell KA, Beierwaltes WH, Green ED, Everett LA, Matthews SW, Wall SM. Dietary Cl− restriction upregulates pendrin expression within the apical plasma membrane of type B intercalated cells. Am J Physiol Renal Physiol 291: F833–F839, 2006 [DOI] [PubMed] [Google Scholar]
- 33. Wagner CA, Finberg KE, Stehberger PA, Lifton RP, Giebisch GH, Aronson PS, Geibel JP. Regulation of the expression of the Cl−/anion exchanger pendrin in mouse kidney by acid-base status. Kidney Int 62: 2109–2117, 2002 [DOI] [PubMed] [Google Scholar]
- 34. Wall SM, Hassell KA, Royaux IE, Green ED, Chang JY, Shipley GL, Verlander JW. Localization of pendrin in mouse kidney. Am J Physiol Renal Physiol 284: F229–F241, 2003 [DOI] [PubMed] [Google Scholar]
- 35. Wall SM, Kim YH, Stanley L, Glapion DM, Everett LA, Green ED, Verlander JW. NaCl restriction upregulates renal Slc26a4 through subcellular redistribution: role in Cl− conservation. Hypertension 44: 1–6, 2004 [DOI] [PubMed] [Google Scholar]
- 36. Wall SM, Knepper MA, Hassell KA, Fischer MP, Shodeinde A, Shin WK, Pham TD, Meyer JW, Lorenz JN, Beierwaltes WH, Dietz JR, Shull GE, Kim YH. Hypotension in NKCC1 null mice: role of the kidneys. Am J Physiol Renal Physiol 290: F409–F416, 2006 [DOI] [PubMed] [Google Scholar]
- 37. Walser M, Rahill WJ. Renal tubular reabsorption of iodide as compared with chloride. J Clin Invest 44: 1371–1381, 1965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wayne EJ, Koutras DA, Alexander WD. Excretion of iodine. In: Clinical Aspects of Iodine Metabolism. Philadelphia, PA: Davis, 1964, p. 73–82 [Google Scholar]
- 39. Weiner ID, New AR, Milton AE, Tisher CC. Regulation of luminal alkaliniation and acidification in the cortical collecting duct by angiotensin II. Am J Physiol Renal Fluid Electrolyte Physiol 269: F730–F738, 1995 [DOI] [PubMed] [Google Scholar]
- 40. Yoshida T, Hisatome I, Taniguchi S, Sasaki N, Yamamoto Y, Miake J, Fukui H, Shimizu H, Okamura T, Okura T, Igawa O, Shigemasa C, Green ED, Kohn LD, Suzuki K. Mechanism of iodide/chloride exchange by pendrin. Endocrinology 145: 4301–4308, 2004 [DOI] [PubMed] [Google Scholar]