Abstract
Rationale: Obesity is a heritable trait that contributes to hypertension and subsequent cardiorenal disease risk; thus, the investigation of genetic variation that predisposes individuals to obesity is an important goal. Circulating peptide YY (PYY) is known for its appetite and energy expenditure-regulating properties; linkage and association studies have suggested that PYY genetic variation contributes to susceptibility for obesity, rendering PYY an attractive candidate for study of disease risk.
Design: To explore whether common genetic variation at the human PYY locus influences plasma PYY or metabolic traits, we systematically resequenced the gene for polymorphism discovery and then genotyped common single-nucleotide polymorphisms across the locus in an extensively phenotyped twin sample to determine associations. Finally, we experimentally validated the marker-on-trait associations using PYY 3′-untranslated region (UTR)/reporter and promoter/reporter analyses in neuroendocrine cells.
Results: Four common genetic variants were discovered across the locus, and three were typed in phenotyped twins. Plasma PYY was highly heritable (P < 0.0001), and genetic pleiotropy was noted between plasma PYY and body mass index (BMI) (P = 0.03). A PYY haplotype extending from the proximal promoter (A-23G, rs2070592) to the 3′-UTR (C+1134A, rs162431) predicted not only plasma PYY (P = 0.009) but also other metabolic syndrome traits. Functional studies with transfected luciferase reporters confirmed regulatory roles in altering gene expression for both 3′-UTR C+1134A (P < 0.001) and promoter A-23G (P = 0.0016).
Conclusions: Functional genetic variation at the PYY locus influences multiple heritable metabolic syndrome traits, likely conferring susceptibility to obesity and subsequent cardiorenal disease.
Genetic variation in peptide YY (PYY) contributes to susceptibility for obesity, as illustrated by identification of polymorphisms that confer functional changes in gene expression.
Obesity is an expanding global health problem whose prevalence is increasing in the United States (from ∼56% in 1988–1994 to ∼65% in 1999–2002) (1). Obesity is a well-established and sometimes modifiable risk factor for the metabolic syndrome and its downstream clinical consequences such as type 2 diabetes and cardiorenal disease. Prevention of serious downstream health consequences is of great importance, especially for individuals in whom diet and exercise alone do not achieve weight reduction targets.
Peptide-YY (PYY), a 36-amino acid polypeptide secreted from L cells of the gastrointestinal tract, is known for its appetite-regulating (2,3) and energy-expenditure properties (4). Upon proteolytic cleavage by dipeptidyl peptidase IV, PYY exists in the circulation in two molecular forms, PYY1-36 and PYY3-36. PYY1-36 acts on all four functional neuropeptide Y receptors (Y1, Y2, Y4, and Y5), whereas PYY3-36 is a specific Y2 receptor agonist (5). When administered iv, PYY3-36 suppresses appetite in rodents and humans (2). In a recent human study, subjects receiving PYY3-36 developed satiety, whereas subjects receiving PYY1-36 displayed no change in appetite (6). On the other hand, PYY produces hyperphagia when injected into the rodent central nervous system, either cerebral ventricles, paraventricular nucleus, or hippocampus (7). Perhaps reflecting the appetite regulation properties of PYY, obese human subjects have decreased fasting levels of circulating PYY (8). These intriguingly opposing data on different feeding behaviors as a function of injection site and peptide form not only confirm the importance of PYY in energy regulation and weight development but also emphasize our incomplete understanding of PYY actions on energy intake and expenditure.
The human PYY gene (NM_004160), on chromosome 17q21.1, is composed of four exons and three introns that span about 1.2 kb. Exons 2–4 give rise to the prepro-PYY protein open reading frame. Linkage and association studies indicate that the PYY gene contributes to susceptibility for obesity (9), abdominal fat mass (10), and type 2 diabetes (11). Better understanding of the mechanism whereby PYY genetic variation alters circulating PYY or metabolic syndrome traits might uncover novel pathways suggesting therapeutic interventions into obesity.
To explore whether common genetic variants at the human PYY locus influence PYY biosynthesis or additional metabolic syndrome traits, we applied a systematic approach beginning with genomic resequencing of PYY to identify both common and rare single-nucleotide polymorphisms (SNPs), thus defining allelic and haplotypic frequencies in four ethnic groups. We then genotyped a set of potentially functional polymorphisms spanning PYY in a carefully phenotyped twin sample to determine trait heritability and associations. Finally, we used experimental tools to explore mechanisms by which the associated variants might influence gene expression, using transfected PYY promoter and 3′-untranslated region (UTR) luciferase reporter analyses in neuroendocrine cells.
Subjects and Methods
Human subjects
Polymorphism discovery panel
A series of 80 unrelated individuals of four biogeographic ancestries (24 European/Caucasian, 25 sub-Saharan African, 15 Hispanic, and 16 East Asian) were selected from urban southern California (San Diego, CA) volunteers for systematic resequencing of the PYY gene. Ethnicity was established by self-identification. Subject characteristics are defined in previous work (12).
Phenotyped twin pairs
Twin pairs were recruited by a population-based twin registry in southern California (13) and by newspaper advertisement. These twins are of European, African-American, and Asian ancestry to improve the power of allelic association studies and generalizability of the finding. Ethnicity was established by self-identification, as well as the ethnicity for both parents and all four grandparents. Self-reported zygosity was confirmed by extensive SNP typing. The twin cohort consists of 198 Caucasian pairs [123 monozygotic (MZ) and 64 dizygotic (DZ); 92 males and 304 females], 15 African-American pairs (seven MZ and eight DZ; 11 males and 19 females), and 25 Hispanic pairs (16 MZ and nine DZ; 17 males and 33 females). Clinical characteristics of subjects are presented in Supplemental Table 1 (published as supplemental data on The Endocrine Society’s Journals Online web site at http://jcem.endojournals.org). http://hyper.ahajournals.org/cgi/content/full/49/5/1015-R19-081679. Body mass index (BMI) was defined as weight (in kilograms) divided by square of the height (in meters). All subjects gave informed, written consent; the protocol was approved by the University of California at San Diego Human Research Protection Program.
Molecular genetics: systematic polymorphism discovery and genotyping
Genomic DNA extracted from EDTA-anticoagulated blood was used for resequencing and genotyping. Resequencing was done using source clone L25648.1 on an ABI 3100 automated sequencer (Applied Biosystems, Foster City, CA). Genotyping was performed using MALDI-TOF mass spectrometry. Please refer to the supplemental data for detailed procedural protocol.
Genetic background class assignment
Generalized analysis of molecular variance (GAMOVA) was performed (14) to determine the utility of self-identified ethnicity vs. genetic background class. Genetic background classes were inferred and assigned to study subjects using an Analysis of Population Structure [implemented in BAPS v. 5.1 (15)]. Please refer to supplemental data for detailed method.
Statistical analyses
Haplotypes were estimated using the HAP algorithm (16). Haplotype or SNP associations on traits were estimated using Generalized Estimating Equation (GEE) modeling in SAS (SAS Institute Inc., Cary, NC) to account for intrapair correlations. Goodness of fit to Hardy-Weinberg expected proportions was examined by χ2 test. Pair-wise linkage disequilibrium (LD) between markers was estimated with the D′ method (17) using the Haploview software (18) in Caucasian twin families. Heritability (h2), the fraction of phenotypic variance attributable to additive genetic variance (h2 = VG/VP), as well as shared environmental effects (environmental covariance, rE) and pleiotropy (shared genetic determination or genetic covariance, rG) were analyzed using variance components in SOLAR [multipoint quantitative-trait linkage analysis in general pedigrees (19)]. Quantitative traits were log10-transformed for association analysis, if the distribution initially deviated from normality. Kendall’s rank test was used to determine partial correlation coefficients between circulating PYY and metabolic syndrome traits, adjusting for effects of age and sex. Generalized linear models were used to determine the percentage of variance of BMI that can be explained by the 3′-UTR polymorphism, accounting for covariates used in the association analyses. Associations were analyzed using QTDT 2.6.0 (20) for inferential statistics and GEE modeling in SAS for descriptive genotypic means and se values. Association tests accounted for effects of covariates (age, sex, and genetic admixture class assignment).
Human plasma PYY assay
Human peptide-YY (total) was measured using Linco HRP-TMB ELISA kit (catalog no. EZHPYYT66K; Linco Research Inc., Minneapolis, MN). Assay details can be found in the supplemental data.
Bioinformatics: inter-species sequence homology and RNA motif prediction
Primate and nonprimate sequence alignment was performed for both PYY promoter A-23G and 3′-UTR C1134A variants using Clustal-W (21). Detection of possible regulatory mRNA motifs and elements was performed using regulatory RNA motifs and elements finder software (22). Differences in mRNA folding free energy and/or stem/loop structures were probed using the Genebee server (23).
Functional consequences of PYY genetic variation in cella: PYY promoter/luciferase and 3′-UTR/luciferase reporter plasmid transfections
PYY promoter and 3′-UTR luciferase reporters were constructed using promoterless pG3-Basic and pGL3-Promoter (Promega, Madison, WI), respectively. Transcription of the 3′-UTR luciferase reported is under the control of the SV40 early promoter (Supplemental Fig. 3). Single-nucleotide variants for both promoter A-23G and 3′-UTR C1134A were created by site-directed mutagenesis (QuikChange; Stratagene, La Jolla, CA). We transfected the promoterless empty vector (pGL3-Basic) in promoter experiments and the SV40 minimal promoter alone (pGL3-Promoter) in 3′-UTR experiments to monitor changes over baseline levels for both PYY constructs. Please refer to the supplemental data for detailed protocol.
Results
PYY genomics: systematic human polymorphism discovery
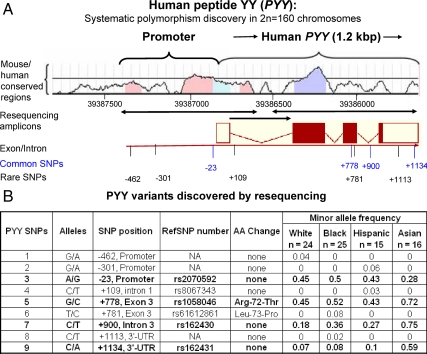
In n = 80 unrelated individuals (n = 160 chromosomes), all four exons and adjacent promoter and intronic regions were resequenced to determine the presence of previously unidentified polymorphisms; the three amplicons are illustrated in Fig. 1A. As shown in Fig. 1B, a total of nine SNPs were detected, of which four were common (minor allele frequency >0.05), with previously assigned RefSNP numbers. Three SNPs were located in the first approximately 750 bp of proximal promoter, two in the coding region (exon 3), two in introns, and two in the 3′-UTR.
Figure 1.
A, Resequencing strategy for variants based on human PYY genomic source clone L25648.1 (AceView PYY.cApr07). Sequences conserved between mouse and human PYY were visualized with VISTA (51). Locations of common (blue) and rare (black) SNPs are indicated, numbered (±) with respect to the cap site. In the four exons, the coding region (ORF) is indicated by solid/filled rectangles, whereas the 5′- and 3′-UTRs are open rectangles. Arrows on the amplicons indicate the direction of resequencing. B, PYY variants discovered by resequencing. Summary of nine SNPs discovered is shown. Four SNPs have minor allele frequency greater than 5% (in bold), meeting a criterion for common SNPs (no. 3, 5, 7, and 9).
Of the four common (MAF>0.05) SNPs, one is located in the very proximal promoter immediately adjacent to the TATA box (A-23G), one in exon-3 (G+778C, nonsynonymous Arg72Thr), one in intron 3 (C+888T), and one in the 3′-UTR (C+1134A). Frequencies of each of the nine discovered polymorphisms are presented in Fig. 1B.
Twin pair descriptive statistics and metabolic syndrome trait heritabilities (h2)
Twins constitute a compelling natural experiment to establish evidence of trait heritability as well as genetic association. The study population for association analysis consisted of 198 Caucasian twin pairs, 15 African-American twin pairs, and 25 Hispanic twin pairs. Their ages ranged from 15 to 82 yr, with a mean (±sem) value of 39.7 ± 0.7. Clinical characteristics of the twins are shown in online supplemental Table S1. Metabolic syndrome traits, including plasma PYY and BMI, displayed substantial heritability, with h2 values for such traits ranging from about 35 ± 7 to about 82 ± 2% (online supplemental Table S1). Perhaps as a result of their relatively young mean ages, only a modest number had clinical hypertension (e.g. systolic blood pressure >140 kg/m2), at 23%. Circulating PYY in twins correlated with BMI (P = 0.05), plasma C-reactive protein (P = 0.004), and plasma insulin (P = 0.001) (online supplemental Table S2).
Genomic background classification
Bayesian analysis of population structure identified eight population groups as the optimal genomic partition of the total sample, with a probability of near 1.0 and log10 (marginal likelihood) of −28042.67. GAMOVA suggested that self-identified ethnicity accounted for only 4.8% (P < 0.0001) of cumulative percent variability explained in the IBS gene matrix, whereas the eight genomic ancestry groups identified by Bayesian analysis of population structure explained a substantially higher fraction of variance at 49.9% (P < 0.0001) of cumulative percent variability explained, indicating that genomic ancestry classification serves as a more powerful variable for covariate adjustment. Therefore, in the following SNP- and haplotype-phenotype association analyses, we applied the assigned genomic ancestry class in place of the self-identified race category during covariate adjustments. Genomic ancestry group did not predict plasma PYY concentration (P = 0.41).
PYY LD pattern: haplotypes and clinical associations
Patterns of LD across three common SNPs at the PYY locus are illustrated in online supplemental Fig. S1A (plotted for a single ethnicity, Caucasian). Pair-wise LD was significant (D′ > 0.98) across the entire gene region, from the 5′ promoter to 3′-UTR. To assess the effect of this local genomic region on a proximal trait (circulating PYY concentration) as well as other metabolic syndrome traits, PYY haplotypes were inferred using the HAP algorithm (12) in all twins. Six different haplotypes were observed in n = 476 twins (2n = 908 chromosomes), of which three were common (frequency >2%) and accounted for more than 99% of the chromosomes studied. Haplotype-1 (A-23→G+778→C+1134) is the most common haplotype, accounting for 69.2% of chromosomes, haplotype-2 (−23G→+778C→C+ 1134) accounts for 26.1%, whereas haplotype-3 (−23G→+778C→+1134A) occurs at a frequency of 4.0% (online supplemental Fig. S1B).
Each of the three common haplotypes was examined for effects on plasma PYY and other metabolic syndrome traits in twins. Whereas no significant associations were found for haplotype-1 or -2, haplotype-3 (−23G→+778C→+1134A) displayed copy number-dependent effects on total cholesterol (173.2 ± 2.5 vs. 193.2 ± 5.3 mg/dl, P = 0.009), high-density lipoprotein (HDL) cholesterol (48 ± 1 vs. 53.4 ± 2.7 mg/dl, P = 0.023), and plasma PYY (128.2 ± 7.7 vs. 97.0 ± 8.3 pg/ml, P = 0.009) (Table 1).
Table 1.
PYY common haplotype 3 (-23G → +778C → +1134A) and PYY 3′-UTR variant C+1134A: Trait associations in phenotyped twins
| Phenotypes |
PYY haplotype-3
|
P-value | |||
|---|---|---|---|---|---|
| Zero copies (n = 348)
|
One or two (n = 41)
|
||||
| Mean | se | Mean | se | ||
| Hemodynamic | |||||
| SBP, mmHg | 133.4 | 1.2 | 133.9 | 2.4 | 0.681 |
| DBP, mmHg | 72.8 | 0.9 | 72.5 | 2.2 | 0.810 |
| Heart rate, bpm | 68.8 | 0.8 | 69.1 | 1.9 | 0.840 |
| Metabolic Traits | |||||
| BMI, kg/m2 | 25.2 | 0.4 | 26.4 | 1.0 | 0.220 |
| Total cholesterol, mg/dl | 172.7 | 2.6 | 192.9 | 6.2 | 0.005 |
| HDL, mg/dl | 47.7 | 1.0 | 53.6 | 2.7 | 0.033 |
| LDL, mg/dl | 102.6 | 2.3 | 113.9 | 6.2 | 0.118 |
| Apo A1, mg/dl | 130.6 | 1.7 | 140.6 | 4.2 | 0.057 |
| Apo B-100, mg/dl | 74.8 | 1.6 | 81.9 | 4.4 | 0.060 |
| CRP, ng/ml | 2127.7 | 194.8 | 2215.0 | 558.5 | 0.481 |
| PYY, pg/mL | 128.6 | 7.9 | 97.1 | 7.8 | 0.002 |
| Leptin, ng/ml | 9.3 | 0.4 | 12.4 | 1.9 | 0.110 |
| Insulin, μUnit/ml | 13.1 | 0.7 | 17.2 | 3.7 | 0.600 |
| Phenotypes |
PYY 3′-UTR variant C+1134A
|
P-value | |||
|---|---|---|---|---|---|
| CC (n = 348)
|
CA+AA (n = 41)
|
||||
| Mean | se | Mean | se | ||
| Hemodynamic | |||||
| SBP, mmHg | 133.3 | 1.2 | 133.7 | 2.4 | 0.7 |
| DBP, mmHg | 72.5 | 0.9 | 72.9 | 2.1 | 0.8 |
| Heart rate, bpm | 68.9 | 0.8 | 69.4 | 1.8 | 0.94 |
| Metabolic Traits | |||||
| BMI, kg/m2 | 25.1 | 0.3 | 26.7 | 1.0 | 0.05 |
| Total cholesterol, mg/dl | 173.2 | 2.5 | 193.3 | 6.3 | 0.01 |
| HDL, mg/dl | 48.0 | 1.0 | 53.4 | 2.7 | 0.08 |
| LDL, mg/dl | 105.4 | 2.2 | 115.2 | 6.0 | 0.22 |
| Apo A1, mg/dl | 130.9 | 1.7 | 138.5 | 4.3 | 0.47 |
| Apo B-100, mg/dl | 74.9 | 1.6 | 84.2 | 4.4 | 0.03 |
| CRP, ng/ml | 2153.0 | 201.0 | 2596.0 | 718.0 | 0.61 |
| PYY, pg/mL | 128.2 | 7.7 | 97.0 | 8.3 | 0.02 |
| Leptin, ng/ml | 9.3 | 0.4 | 13.4 | 1.8 | 0.01 |
| Insulin, μUnit/ml | 13.1 | 0.7 | 17.8 | 3.7 | 0.51 |
Haplotype descriptive and inferential statistics were performed by Generalized Estimating Equations (GEE). PYY 3′-UTR variant inferential statistics were tested using Quantitative Transmission Disequilibrium Test (QTDT) (51) for total evidence of association. Descriptive statistics were calculated using the GEE model. All associations have been adjusted for the effects of age, sex, and genetic admixture classes. Bold, P-value < 0.05; Italicized, 0.05 < P-value < 0.10. Apo A1, Apolipoprotein A1; Apo B-100, apolipoprotein B-100; CRP, C-reactive protein; DBP, diastolic blood pressure; LDL, low-density lipoprotein; SBP, systolic blood pressure.
Individual PYY marker-on-trait associations in twins
Although the three common polymorphisms examined are in significant LD with each other (D′ ≥ 0.98, online supplemental Figure S1A), 3′-UTR C+1134A was the only individual SNP showing significant association with metabolic traits, including BMI, total cholesterol, apolipoprotein (Apo) B, circulating PYY, and leptin levels (Table 1). Given the relatively low minor (A) allele frequency (∼7%) of C+1134A, heterozygotes and minor allele (A/A) homozygotes were combined to test for a minor allele carrier effect (adjusted for age, sex, and admixture class as potential confounders). There were significant associations between the minor allele (+1134A) and increased BMI (26.7 ± 1.0 vs. 25.1 ± 0.3 kg/m2, P = 0.047), higher total cholesterol (193.3 ± 6.3 vs. 173.2 ± 2.5 mg/dl, P = 0.006), higher ApoB (84.2 ± 4.4 vs. 74.9 ± 1.6 mg/dl, P = 0.032), decreased PYY (97.0 ± 8.3 vs. 128.2 ± 7.7 pg/ml, P = 0.021), and increased leptin (13.4 ± 1.8 vs. 9.3 ± 0.4 ng/ml, P = 0.014). PYY variant C+1134A explained about 2.3% of BMI variance, with an allelic effect size on BMI of about 0.674 kg/m2.
Individual associations for plasma PYY were not found for either exon 3 G+778C (Arg72Thr) or promoter A-23G polymorphisms.
Pleiotropy (shared heritability) and shared environmental determinants of PYY and BMI
In addition to positive associations found in the univariate analyses, the +1134A allele also seemed to exert pleiotropic effects on BMI and circulating PYY (Fig. 2). Carriers of the minor allele (i.e. C/A or A/A diploid genotypes) had increased PYY (P = 0.021) as well as decreased BMI (P = 0.047). h2 values for serum PYY and BMI were estimated in twins. Both PYY and BMI were significantly heritable at 51% for PYY (h2: 0.51 ± 0.057; P < 0.0001) and 82% for BMI (h2: 0.82 ± 0.024, P < 0.0001). Evidence of shared genetic determination (pleiotropy) was found for PYY and BMI (RhoG = −0.18 ± 0.085, P = 0.03; the minus sign indicates that the two traits correlate inversely), whereas there was no evidence of shared environmental determination for the two traits (RhoE = 0.028 ± 0.08, P = 0.72) (Fig. 2). Multivariate ANOVA was carried out to test whether the PYY 3′-UTR variant (C+1134A) coordinately influences both PYY and BMI as codependent variables in the same model. The significant result (P = 0.0007) confirmed heritability and further documented shared genetic determination (or pleiotropy) for C+1134A on metabolic syndrome traits (Fig. 2).
Figure 2.
Plasma PYY and BMI: heritability and shared genetic determination (pleiotropy). Genotypic mean and se values are shown for PYY and BMI, adjusting for age, sex, and genetic admixture. MANOVA, Multivariate ANOVA.
PYY polymorphism bioinformatic analyses
PYY proximal promoter variant A-23G
Mammalian (primate and nonprimate) interspecies homology in the PYY proximal promoter variant A-23G revealed substantial conservation around the TATA box across the mammals, which is extended for one base by the A allele (i.e. 5′-ATATAAA-3′). The human minor allele G appears to be the ancestral allele (in all other primates) (online supplemental Fig. S2).
PYY 3′-UTR variant C+1134A
Mammalian (primate and nonprimate) interspecies homology (Fig. 1A, top panel) indicates that the PYY 3′-UTR C+1134A region is highly conserved, especially in nonrodents, with the likely human ancestral allele being wild-type allele C (online supplemental Fig. S4A).
Functional consequences of PYY polymorphisms probed in cella by transfection and expression of luciferase reporters
In light of the observed associations of PYY genetic variation with plasma PYY and other metabolic traits (Table 2) as well as bioinformatic analyses suggesting functional potential of these two gene regions, we developed in cella gene expression (transfected luciferase reporter) assays for promoter A-23G and 3′-UTR C+1134A polymorphisms, using neurendocrine (rat PC12 chromaffin) cells.
We assayed variant-specific gene expression in PC12 chromaffin cells (Fig. 3) with PYY promoter/luciferase reporters for promoter A-23G and 3′-UTR/luciferase reporters for C+1134A.
Figure 3.
Functional studies of trait-associated variants at the human PYY locus. Left panel, Transfected promoter studies. PYY promoter variants were ligated into the promoterless vector pGL3-basic, just upstream of the luciferase ORF. The transfected empty vector (pGL3-basic) yielded an expression value of about 0.7 × 106 luciferase/β-galactosidase units. Right panel, Transfected 3′-UTR studies. PYY 3′-UTR variants were ligated into the XbaI site just downstream of the luciferase ORF in vector pGL3-promoter, in which expression is driven by the Simian virus 40 early promoter. In each case, plasmids were transfected into PC12 chromaffin cells, which were harvested for luciferase reporter activity at 24 h. The transfected empty vector (pGL3-promoter) showed an expression value of about 4.4 × 105 luciferase/β-galactosidase units. Results were corrected for transfection efficiency by cotransfection of a β-galactosidase cDNA whose expression was driven by the cytomegalovirus promoter. Error bars, ±sem in four replicate transfections.
PYY promoter A-23G
The variant (-23G) promoter allele demonstrated about 1.2-fold greater (P = 0.0016) luciferase reporter activity than the wild-type (A-23) allele (Fig. 3, left panel).
PYY 3′-UTR C+1134A
PYY luciferase reporter/3′-UTR variant assay showed the wild-type variant (allele C) to be significantly less in reporter activity comparing to the mutant variant (allele A) (P < 0.001), suggesting correlation between the variant allele and greater transcript abundance (Fig. 3, right panel).
Discussion
Overview
This study demonstrates four new findings about human PYY genetic variation: 1) the PYY gene harbors at least nine SNPs, of which four are common with minor allele frequency greater than 5%; 2) metabolic syndrome traits in healthy twins are highly heritable, and plasma PYY shares significant genetic determination (pleiotropy) with BMI; 3) a haplotype spanning PYY as well as a SNP located in the 3′-UTR of PYY associates with not only circulating (plasma) PYY but also multiple metabolic syndrome traits including BMI, HDL, and total cholesterol; 4) in cella luciferase reporter expression and bioinformatic analyses demonstrate functional roles for both a PYY promoter and the 3′-UTR SNPs in regulating gene expression, thereby supporting the human marker-on-trait associations and revealing functional mechanisms whereby PYY genetic variation contributes to obesity and related metabolic syndrome risk traits.
Context
Previously published studies demonstrated associations between polymorphism at the human PYY locus and metabolic syndrome traits but with variable results. Hung et al. (24) discovered two rare PYY variants (Leu73Pro and IVS2+32delG) in 101 English subjects with early-onset obesity. Ahituv et al. (25) observed association of genetic variation at PYY with obesity in subjects with BMI extreme values in the population; findings included association of common variant Arg72Thr as well as segregation of rare variant Gln62Pro with body mass. Torekov et al. (11) found association of the PYY Arg72 allele with type 2 diabetes, elevated BMI, and glucose intolerance in the Danish population; however, neither Santoro et al. (26) nor Lavebratt et al. (27) found association for Arg72Thr with BMI or blood pressure. Ma et al. (28) found that obesity was predicted by two PYY genetic variants in Pima Indian men. Siddiq et al. (29) found association of an intronic PYY variant with childhood obesity but not in adults in a European sample. However, the mechanism whereby PYY genetic variation influences metabolic syndrome traits has remained elusive.
In the Genomewide Investigation of ANThropometric measures consortion (GIANT) consortium metaanalysis of 15 genome-wide association cohorts (30), a BMI association signal was not found in the PYY region on chromosome 17q21; however, even though GIANT identified eight loci contributing to BMI, the cumulative effect of such variants accounted for only about 0.84% of BMI variance. The finding of about 82% heritability for BMI (online supplemental Table S1) indicated that the majority of genetic influence on BMI remains to be defined and may be referable to less common genetic variation (31) such as that at PYY 3′-UTR C+1134A, whose minor allele frequency is only about 7%.
The somewhat inconsistent PYY marker-on-trait associations in the literature may reflect not only genetic heterogeneity or population stratification but also hit-or-miss candidate SNP selection bias. By contrast, to assess whether PYY genetic variation affects susceptibility to metabolic syndrome traits, we applied a systematic, three-stage approach by first systemically resequencing the human PYY gene across its established domains (Fig. 1A) (32), followed by both haplotype and individual SNP marker-on-trait association analysis of PYY common SNPs in a twin study and then bioinformatic predictions and in cella expression assays to validate the functional significance of the associated PYY variants.
Altered regulation of gene expression is consistent with PYY marker-on-trait associations: plausible role of 3′-UTR microRNA and promoter motifs
Upon resequencing the human PYY gene, nine SNPs were discovered, of which four are common in the population, with minor allele frequency greater than 5%. Association analysis in twins revealed significant associations of a PYY 3-SNP haplotype (−23G→+778C→+1134A) with circulating PYY, total cholesterol, and HDL levels, suggesting PYY as a susceptibility gene for these traits. Individual SNP analysis reinforced the haplotype-based test and revealed significant association of 3′-UTR variant C+1134A and multiple traits: PYY, BMI, total cholesterol, ApoA, and leptin.
The individual SNP maker-on-trait association results lead us to bioinformatic and in cella studies to probe a functional role for 3′-UTR SNP C+1134A. In most vertebrates, 3′-UTRs are substantially longer than their 5′ counterparts, suggesting importance for posttranscriptional regulation. Additionally, the average length of 3′-UTR sequences has increased during evolution, indicating likely contributions to organismal complexity (33). Indeed, sequence alignment showed the local region of the 3′-UTR polymorphism to be well conserved among primates, with the major allele (C) likely to be ancestral (online supplemental Fig. S4A).
3′-UTR regulatory motif prediction found that 3′-UTR allele C+1134 spanned a partial sequence match for a human microRNA hsa-miR-663 binding motif (online supplemental Fig. S4B). Animal microRNAs are typically complementary to sites in the 3′-UTR regions of mature mRNAs, at which annealing of the microRNA to the mRNA can lead to inhibition of protein translation or (in the case of a perfect sequence match) to cleavage of the mRNA (34). Therefore, the predicted binding of hsa-miR-663 to the wild-type allele C+1134 is suggestive of decreased PYY translational activity. To further validate the functional role of the 3′-UTR allele, a transfected luciferase reporter gene/3′-UTR plasmid expression assay was carried out. A reporter plasmid containing the 3′-UTR minor allele +1134A showed significantly increased gene expression compared with that containing the major allele C+1134. Although the spectrum of expression of the newly recognized human microRNA hsa-miR-663 is not yet well defined, these experimental luciferase results support the viewpoint that in vivo PYY haplotype and SNP marker-on-trait associations may be attributable to functional changes at the 3′-UTR variant, a domain predicted to alter the binding motif for microRNA hsa-miR-663.
To further assess functional roles of PYY SNPs in gene regulation, we also performed the gene expression assay on the promoter SNP A-23G, which extends the TATA box by one nucleotide when the wild-type (A) allele is present (online supplemental Fig. S2). Promoter allele −23G cotransmits with 3′-UTR allele+1134A on haplotype 3, which displays a significant association with decreased plasma PYY, increased BMI, and other metabolic syndrome traits (Table 1). Directionally consistent with the in cella result of the 3′-UTR study, a promoter/reporter plasmid carrying promoter variant −23G demonstrated increased expression when compared with the wild-type A-23 allele (Fig. 3). A decline in promoter strength with elongation of the TATA box underlies another common trait, Gilbert syndrome, in which extension of the UGT1A1 TATA box from A(TA)6TAA to A(TA)7TAA reduced expression of a luciferase reporter (35).
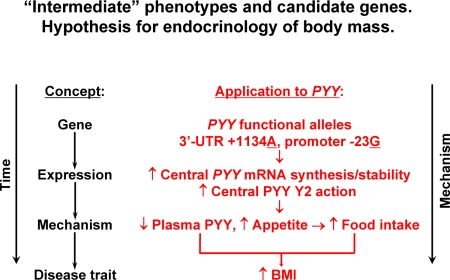
Endocrinology of human body mass: hypothesis
The opposing associations of variant +1134A, with increased luciferase activity in cella (Fig. 3) but decreased circulating PYY in vivo (Table 1), can be reconciled by a consideration of the very different biological actions on appetite exerted by central vs. peripheral PYY.
Circulating PYY derives largely from the gut (ileum and large intestine); however, PYY is present also in the brain (36), with PYY-containing neurons found in the hypothalamus and hindbrain regions (37) as well as sympathetic neurons (38). It has been suggested that circulating PYY exerts a stimulatory action on appetite through Y1 (NPY1R) receptors and an inhibitory action through Y2 (NPY2R) receptors (39). Although recent studies focused largely on the anorexigenic effect of PYY administered peripherally (40), centrally infused PYY was originally discovered as a potent orexigenic agent, producing hyperphagia in rodents when injected into cerebral ventricles, paraventricular nucleus, or hippocampus (41,42). Chronic central administration of PYY augmented feeding and drinking, without development of tolerance, suggesting PYY may play a role in the pathogenesis of bulimic syndromes (43). Rahardjo et al. (44) also noted a reciprocal relationship between central and peripheral PYY actions in experimental animals with diet-induced obesity: increased brain stem PYY and NPY2R binding density coupled to decreased peripheral/plasma PYY concentration.
Our in cella expression results (Fig. 3) indicate that the alleles associated with decreased plasma PYY, 3′-UTR +1134A and promoter −34G (Table 1), each gave rise to elevated reporter expression (Fig. 3). In the context of the observed reciprocal actions of central and peripheral PYY (44), we therefore propose a parsimonious scheme integrating our in vivo and in cella results (Fig. 4), in which the pertinent action of PYY genetic variation on gene expression might occur in the brain. In subjects with haplotype-3 (promoter −23G→exon 3+778C→3′-UTR+1134A; Table 1) elevated PYY expression would trigger increased appetite, thereby elevating BMI and suppressing circulating/peripheral PYY.
Figure 4.
Hypothetical schema integrating the clinical and experimental observations in this study.
It has been suggested that initial postmeal release of PYY is activated through neural mechanisms until ingested nutrients reach the small intestine and the colon, locally triggering additional PYY release into the bloodstream (45), with subsequent inhibition of gastric motility (46). Whereas nonobese human volunteers showed a progressive rise in plasma PYY during meals of increasing caloric content, obese subjects displayed a lower endogenous PYY response after a meal (47). Perhaps in obese individuals, the postmeal PYY release process is impaired, resulting in an insufficient satiety signal given the calorie intake, with consequent overeating.
In addition to central mechanisms of appetite regulation, PYY has also been shown to affect cognitive processes. Increased plasma PYY correlated positively with indices of subclinical disordered eating (or drive for thinness) in a sample of women including exercise-induced amenorrhea (48).
Observed correlations between high BMI with low PYY (online supplemental Table S2), coupled with the satiety-inducing property of iv administered exogenous PYY, provide convincing support for a PYY regulatory role in control of body mass, yet it remains unclear whether endogenous basal plasma PYY concentration simply serves as a obesity risk biomarker or is instead causally involved in the development of obesity. Our observation of pleiotropy (shared genetic determination, or genetic covariance) between plasma PYY and BMI (Fig. 2), coupled with evidence that the PYY 3′-UTR variant coordinately influences both traits (Fig. 2) suggests that endogenous PYY is an important determinant of BMI. Thus, we conclude that diminished plasma PYY is likely not only a biomarker for obesity but also contributes to the pathogenesis of this phenotype as well as related metabolic syndrome traits.
Advantages and limitations of this study
The twin study design enabled us to probe the role of pleiotropy (genetic covariance) in determination of multiple metabolic syndrome traits, such as plasma PYY and BMI. Furthermore, systematic resequencing allowed us to discover and characterize all common genetic variation across the PYY locus as well as establish patterns of LD. Scoring multiple variants in the phenotyped individuals enabled both haplotype and single SNP associations to traits. Finally, luciferase reporter plasmids for two PYY variants in crucial functional domains (3′-UTR and promoter) established the functional consequences in cella of the discovered genetic variation.
One limitation of our study is that the human PYY assay detected PYY1-36 and PYY3-36 with equal affinity; therefore, we were unable to determine separately the influence of PYY variation on the two different processed forms of the peptide. However, previous studies have shown that the ratio of plasma PYY1-36 to PYY3-36 was similar in normal-weight and obese subjects (47) as well as in fetal intestinal tissues as early as 9.5 wk of age (49). Additionally, although our functional data provide support for regulatory actions of the 3′-UTR and promoter PYY variants on PYY gene expression, we have not yet directly probed effects of specific factors, such as hsa-miR-663, on mRNA translation or stability.
We studied functional consequences both for the promoter (A-23G) and the 3′-UTR (C+1134A) variants because the very high degree of LD across the locus (D′ ≥ 0.98; online supplemental Fig. S1A) would suggest that the functional variant might be anywhere within the LD block conferring the haplotype-3 on-trait associations (Table 1). We did not study intronic variant C888T because it did not disrupt splice donor/acceptor recognition motifs and was not in an area of sequence conservation (Fig. 1A) nor did we pursue exon 3 G+778C (Arg72Thr) because it lays within the propeptide (or flanking peptide) of the PYY open reading frame (ORF), rather than in the functional PYY1-36 product peptide. However, Arg72Thr is located only five to six amino acids downstream from the Lys66Arg67 dibasic cleavage site of pro-PYY and may thus alter the efficiency of posttranslational proteolytic liberation of PYY from its flanking peptide (50). Torekov et al. (11) found modest association with obesity/overweight for Arg72 carriers but not other obesity-related traits, including BMI. Our sample consists of healthy, normal-weight-range American twins, whereas Torekov et al. studied a Danish population-based sample; inconsistencies in association within the same locus between the Torekov and our sample may thus result from the differences in genetic background. However, both studies provide evidence to support human PYY as a susceptibility gene for human body mass development.
Conclusions and perspectives
We conducted a comprehensive study of variants across the PYY gene, probing associations with plasma PYY and metabolic syndrome traits and then tested mechanisms underlying the associations. In addition to demonstrating the heritable nature of the metabolic syndrome traits, we present data on PYY genetic variation as a significant contributor to BMI and several lipid components and uncovered a potential central mechanism underlying the marker-on-trait associations. Our study is the first to observe associations between 3′-UTR and promoter region polymorphisms of PYY with plasma PYY expression and metabolic syndrome traits. The association of specific 3′-UTR and promoter variants with PYY and BMI, coupled with alterations of luciferase reporter expression in cella, led us to propose the hypothesis that PYY genetic variation may influence risk traits via changes in the central nervous system actions of PYY on appetite. Future studies on the precise biological mechanisms whereby genetically programmed changes in PYY expression occur may yield new insights into the pathogenesis of obesity and its associated traits, perhaps allowing more effective intervention into the increasingly widespread epidemic of obesity.
Supplementary Material
Footnotes
This work was supported by National Kidney Foundation (fellowship to P.B.S.), National Institutes of Health, and Department of Veterans Affairs.
Disclosure Summary: The authors have nothing to disclose.
First Published Online October 9, 2009
Abbreviations: Apo, Apolipoprotein; BMI, body mass index; DZ, dizygotic; h2, heritability; HDL, high-density lipoprotein; LD, linkage disequilibrium; MZ, monozygotic; ORF, open reading frame; PYY, peptide YY; SNP, single-nucleotide polymorphism; UTR, untranslated region.
References
- Hedley AA, Ogden CL, Johnson CL, Carroll MD, Curtin LR, Flegal KM 2004 Prevalence of overweight and obesity among US children, adolescents, and adults, 1999–2002. JAMA 291:2847–2850 [DOI] [PubMed] [Google Scholar]
- Batterham RL, Cowley MA, Small CJ, Herzog H, Cohen MA, Dakin CL, Wren AM, Brynes AE, Low MJ, Ghatei MA, Cone RD, Bloom SR 2002 Gut hormone PYY(3–36) physiologically inhibits food intake. Nature 418:650–654 [DOI] [PubMed] [Google Scholar]
- Batterham RL, Bloom SR 2003 The gut hormone peptide YY regulates appetite. Ann N Y Acad Sci 994:162–168 [DOI] [PubMed] [Google Scholar]
- Doucet E, Laviolette M, Imbeault P, Strychar I, Rabasa-Lhoret R, Prud'homme D 2008 Total peptide YY is a correlate of postprandial energy expenditure but not of appetite or energy intake in healthy women. Metabolism 57:1458–1464 [DOI] [PubMed] [Google Scholar]
- Vincent RP, le Roux CW 2008 The satiety hormone peptide YY as a regulator of appetite. J Clin Pathol 61:548–552 [DOI] [PubMed] [Google Scholar]
- Sloth B, Davidsen L, Holst JJ, Flint A, Astrup A 2007 Effect of subcutaneous injections of PYY1-36 and PYY3-36 on appetite, ad libitum energy intake, and plasma free fatty acid concentration in obese males. Am J Physiol Endocrinol Metab 293:E604–E609 [DOI] [PubMed] [Google Scholar]
- Hagan MM 2002 Peptide YY: a key mediator of orexigenic behavior. Peptides 23:377–382 [DOI] [PubMed] [Google Scholar]
- Batterham RL, Cohen MA, Ellis SM, Le Roux CW, Withers DJ, Frost GS, Ghatei MA, Bloom SR 2003 Inhibition of food intake in obese subjects by peptide YY3-36. N Engl J Med 349:941–948 [DOI] [PubMed] [Google Scholar]
- Cai G, Cole SA, Butte NF, Voruganti VS, Comuzzie AG 2008 Genome-wide scan revealed genetic loci for energy metabolism in Hispanic children and adolescents. Int J Obes (Lond) 32:579–585 [DOI] [PubMed] [Google Scholar]
- Perusse L, Rice T, Chagnon YC, Despres JP, Lemieux S, Roy S, Lacaille M, Ho-Kim MA, Chagnon M, Province MA, Rao DC, Bouchard C 2001 A genome-wide scan for abdominal fat assessed by computed tomography in the Quebec Family Study. Diabetes 50:614–621 [DOI] [PubMed] [Google Scholar]
- Torekov SS, Larsen LH, Glumer C, Borch-Johnsen K, Jorgensen T, Holst JJ, Madsen OD, Hansen T, Pedersen O 2005 Evidence of an association between the Arg72 allele of the peptide YY and increased risk of type 2 diabetes. Diabetes 54:2261–2265 [DOI] [PubMed] [Google Scholar]
- O'Connor DT, Kailasam MT, Kennedy BP, Ziegler MG, Yanaihara N, Parmer RJ 2002 Early decline in the catecholamine release-inhibitory peptide catestatin in humans at genetic risk of hypertension. J Hypertens 20:1335–1345 [DOI] [PubMed] [Google Scholar]
- Cockburn M, Hamilton A, Zadnick J, Cozen W, Mack TM 2002 The occurrence of chronic disease and other conditions in a large population-based cohort of native Californian twins. Twin Res 5:460–467 [DOI] [PubMed] [Google Scholar]
- Nievergelt CM, Schork NJ 2005 Admixture mapping as a gene discovery approach for complex human traits and diseases. Curr Hypertens Rep 7:31–37 [DOI] [PubMed] [Google Scholar]
- Corander J, Marttinen P 2006 Bayesian identification of admixture events using multilocus molecular markers. Mol Ecol 15:2833–2843 [DOI] [PubMed] [Google Scholar]
- Halperin E, Eskin E 2004 Haplotype reconstruction from genotype data using Imperfect Phylogeny. Bioinformatics 20:1842–1849 [DOI] [PubMed] [Google Scholar]
- Lewontin RC The Interaction of Selection and Linkage. Ii. Optimum Models. Genetics 50:757–782, 1964 Oct [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett JC, Fry B, Maller J, Daly MJ 2005 Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics 21:263–265 [DOI] [PubMed] [Google Scholar]
- Almasy L, Blangero J 1998 Multipoint quantitative-trait linkage analysis in general pedigrees. Am J Hum Genet 62:1198–1211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abecasis GR, Cardon LR, Cookson WO 2000 A general test of association for quantitative traits in nuclear families. Am J Hum Genet 66:279–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JD, Gibson TJ, Higgins DG 2002 Multiple sequence alignment using ClustalW and ClustalX. Curr Protoc Bioinformatics Chapter 2:Unit 2 3 [DOI] [PubMed] [Google Scholar]
- Huang HY, Chien CH, Jen KH, Huang HD 2006 RegRNA: an integrated web server for identifying regulatory RNA motifs and elements. Nucleic Acids Res 34:W429–W434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodskii LI, Ivanov VV, Kalaidzidis Ia L, Leontovich AM, Nikolaev VK, Feranchuk SI, Drachev VA 1995 [GeneBee-NET: An Internet based server for biopolymer structure analysis]. Biokhimiia 60:1221–1230 [PubMed] [Google Scholar]
- Hung CC, Pirie F, Luan J, Lank E, Motala A, Yeo GS, Keogh JM, Wareham NJ, O'Rahilly S, Farooqi IS 2004 Studies of the peptide YY and neuropeptide Y2 receptor genes in relation to human obesity and obesity-related traits. Diabetes 53:2461–2466 [DOI] [PubMed] [Google Scholar]
- Ahituv N, Kavaslar N, Schackwitz W, Ustaszewska A, Collier JM, Hebert S, Doelle H, Dent R, Pennacchio LA, McPherson R 2006 A PYY Q62P variant linked to human obesity. Hum Mol Genet 15:387–391 [DOI] [PubMed] [Google Scholar]
- Santoro N, Del Giudice EM, Grandone A, Marzuillo P, Cozzolino D, Di Salvo G, Pacileo G, Calabro R, Perrone L 2008 Y2 receptor gene variants reduce the risk of hypertension in obese children and adolescents. J Hypertens 26:1590–1594 [DOI] [PubMed] [Google Scholar]
- Lavebratt C, Alpman A, Persson B, Arner P, Hoffstedt J 2006 Common neuropeptide Y2 receptor gene variant is protective against obesity among Swedish men. Int J Obes (Lond) 30:453–459 [DOI] [PubMed] [Google Scholar]
- Ma L, Tataranni PA, Hanson RL, Infante AM, Kobes S, Bogardus C, Baier LJ 2005 Variations in peptide YY and Y2 receptor genes are associated with severe obesity in Pima Indian men. Diabetes 54:1598–1602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiq A, Gueorguiev M, Samson C, Hercberg S, Heude B, Levy-Marchal C, Jouret B, Weill J, Meyre D, Walley A, Froguel P 2007 Single nucleotide polymorphisms in the neuropeptide Y2 receptor (NPY2R) gene and association with severe obesity in French white subjects. Diabetologia 50:574–584 [DOI] [PubMed] [Google Scholar]
- Willer CJ, Speliotes EK, Loos RJ, Li S, Lindgren CM, Heid IM, Berndt SI, Elliott AL, Jackson AU, Lamina C, Lettre G, Lim N, Lyon HN, McCarroll SA, Papadakis K, Qi L, Randall JC, Roccasecca RM, Sanna S, Scheet P, Weedon MN, Wheeler E, Zhao JH, Jacobs LC, Prokopenko I,et al. 2009 Six new loci associated with body mass index highlight a neuronal influence on body weight regulation. Nat Genet 41:25–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodmer W, Bonilla C 2008 Common and rare variants in multifactorial susceptibility to common diseases. Nat Genet 40:695–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hort Y, Baker E, Sutherland GR, Shine J, Herzog H 1995 Gene duplication of the human peptide YY gene (PYY) generated the pancreatic polypeptide gene (PPY) on chromosome 17q21.1. Genomics 26:77–83 [DOI] [PubMed] [Google Scholar]
- Mazumder B, Seshadri V, Fox PL 2003 Translational control by the 3’-UTR: the ends specify the means. Trends Biochem Sci 28:91–98 [DOI] [PubMed] [Google Scholar]
- Standart N, Jackson RJ 2007 MicroRNAs repress translation of m7Gppp-capped target mRNAs in vitro by inhibiting initiation and promoting deadenylation. Genes Dev 21:1975–1982 [DOI] [PubMed] [Google Scholar]
- Bosma PJ, Chowdhury JR, Bakker C, Gantla S, de Boer A, Oostra BA, Lindhout D, Tytgat GN, Jansen PL, Oude Elferink RP, et al. 1995 The genetic basis of the reduced expression of bilirubin UDP-glucuronosyltransferase 1 in Gilbert’s syndrome. N Engl J Med 333:1171–1175 [DOI] [PubMed] [Google Scholar]
- Ekman R, Wahlestedt C, Bottcher G, Sundler F, Hakanson R, Panula P 1986 Peptide YY-like immunoreactivity in the central nervous system of the rat. Regul Pept 16:157–168 [DOI] [PubMed] [Google Scholar]
- Lundberg JM, Terenius L, Hokfelt T, Tatemoto K 1984 Comparative immunohistochemical and biochemical analysis of pancreatic polypeptide-like peptides with special reference to presence of neuropeptide Y in central and peripheral neurons. J Neurosci 4:2376–2386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Happola O, Wahlestedt C, Ekman R, Soinila S, Panula P, Hakanson R 1990 Peptide YY-like immunoreactivity in sympatheticneurons of the rat. Neuroscience 39:225–230 [DOI] [PubMed] [Google Scholar]
- Yang H 2002 Central and peripheral regulation of gastric acid secretion by peptide YY. Peptides 23:349–358 [DOI] [PubMed] [Google Scholar]
- Grudell AB, Camilleri M 2007 The role of peptide YY in integrative gut physiology and potential role in obesity. Curr Opin Endocrinol Diabetes Obes 14:52–57 [DOI] [PubMed] [Google Scholar]
- Clark JT, Sahu A, Kalra PS, Balasubramaniam A, Kalra SP 1987 Neuropeptide Y (NPY)-induced feeding behavior in female rats: comparison with human NPY ([Met17]NPY), NPY analog ([norLeu4]NPY) and peptide YY. Regul Pept 17:31–39 [DOI] [PubMed] [Google Scholar]
- Stanley BG, Daniel DR, Chin AS, Leibowitz SF 1985 Paraventricular nucleus injections of peptide YY and neuropeptide Y preferentially enhance carbohydrate ingestion. Peptides 6:1205–1211 [DOI] [PubMed] [Google Scholar]
- Morley JE, Levine AS, Grace M, Kneip J 1985 Peptide YY (PYY), a potent orexigenic agent. Brain Res 341:200–203 [DOI] [PubMed] [Google Scholar]
- Rahardjo GL, Huang XF, Tan YY, Deng C 2007 Decreased plasma peptide YY accompanied by elevated peptide YY and Y2 receptor binding densities in the medulla oblongata of diet-induced obese mice. Endocrinology 148:4704–4710 [DOI] [PubMed] [Google Scholar]
- Korner J, Leibel RL 2003 To eat or not to eat - how the gut talks to the brain. N Engl J Med 349:926–928 [DOI] [PubMed] [Google Scholar]
- Pappas TN, Debas HT, Goto Y, Taylor IL 1985 Peptide YY inhibits meal-stimulated pancreatic and gastric secretion. Am J Physiol 248:G118–123 [DOI] [PubMed] [Google Scholar]
- le Roux CW, Batterham RL, Aylwin SJ, Patterson M, Borg CM, Wynne KJ, Kent A, Vincent RP, Gardiner J, Ghatei MA, Bloom SR 2006 Attenuated peptide YY release in obese subjects is associated with reduced satiety. Endocrinology 147:3–8 [DOI] [PubMed] [Google Scholar]
- Scheid JL, Williams NI, West SL, VanHeest JL, De Souza MJ 2009 Elevated PYY is associated with energy deficiency and indices of subclinical disordered eating in exercising women with hypothalamic amenorrhea. Appetite 52:184–192 [DOI] [PubMed] [Google Scholar]
- Xiao Q, Han X, Arany E, Hill D, Challis JR, McDonald TJ 1998 Human placenta and fetal membranes contain peptide YY1–36 and peptide YY3–36. J Endocrinol 156:485–492 [DOI] [PubMed] [Google Scholar]
- Brakch N, Rholam M, Simonetti M, Cohen P 2000 Favourable side-chain orientation of cleavage site dibasic residues of prohormone in proteolytic processing by prohormone convertase 1/3. Eur J Biochem 267:1626–1633 [DOI] [PubMed] [Google Scholar]
- Mayor C, Brudno M, Schwartz JR, Poliakov A, Rubin EM, Frazer KA, Pachter LS, Dubchak I 2000 VISTA: visualizing global DNA sequence alignments of arbitrary length. Bioinformatics 16: 1046–1047 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.