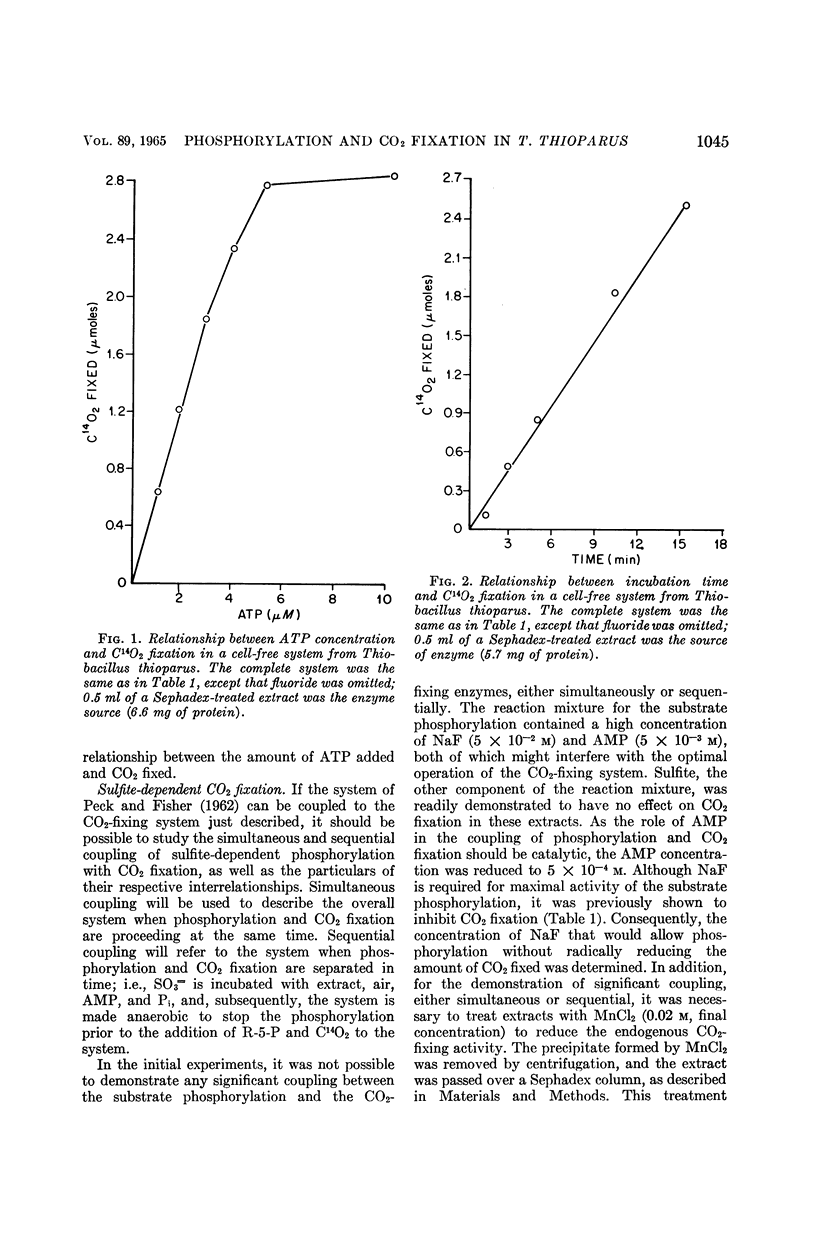
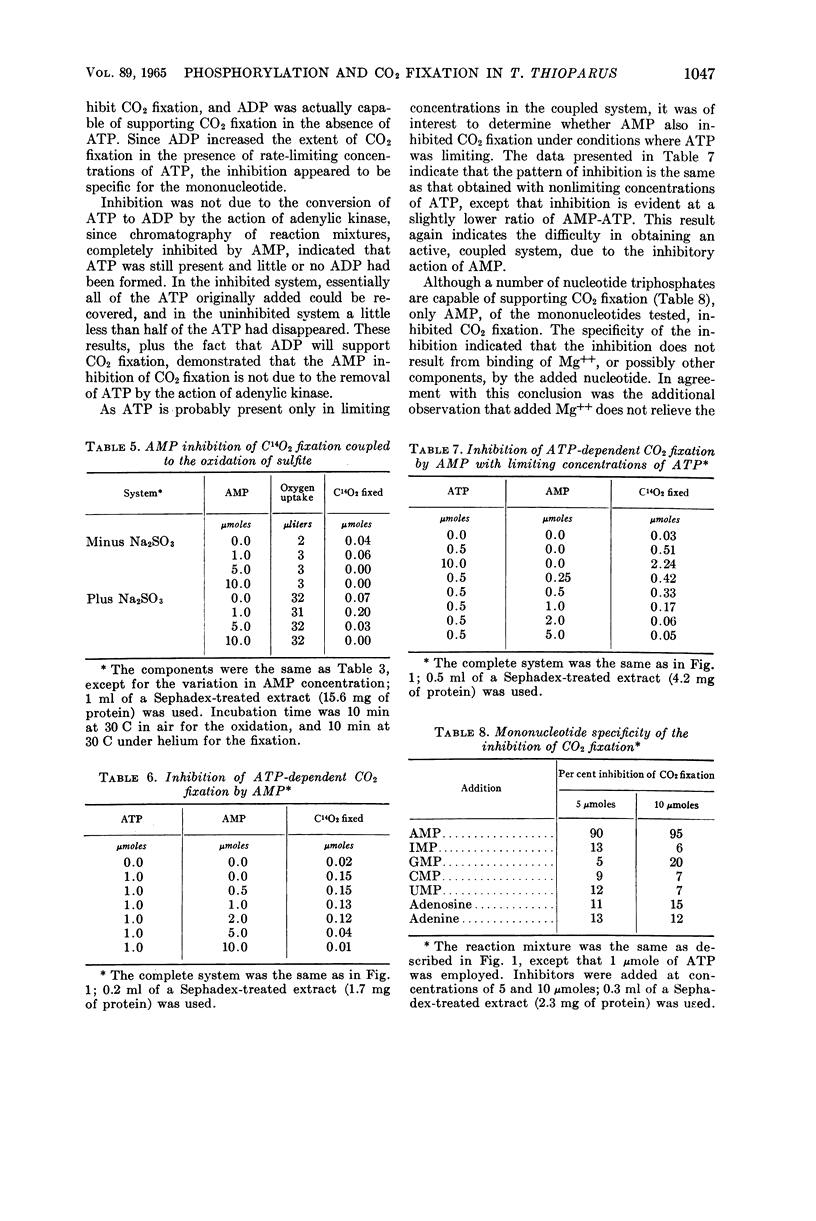
Abstract
Johnson, Emmett J. (University of Mississippi Medical Center, Jackson), and Harry D. Peck, Jr. Coupling of phosphorylation and carbon dioxide fixation in extracts of Thiobacillus thioparus. J. Bacteriol. 89:1041–1050. 1965.—A cell-free system from Thiobacillus thioparus which fixes large quantities of C14O2 in the presence of ribose-5-phosphate, adenosine triphosphate (ATP), and Mg++ has been described. The specific activity (0.041 μmole of ribulose-1,5-diphosphate min−1 mg−1 protein) of the CO2-fixing system approaches that of green plants, and is further evidence for the importance of the role of carboxydismutase in the thiobacilli. In addition to ATP, adenosine diphosphate (ADP) and other nucleoside triphosphates served with varying degrees of effectiveness for the fixation of C14O2. The ATP requirement for CO2 fixation was partially replaced under aerobic conditions by a combination of SO3=, PO4≡, and adenosine monophosphate (AMP). Phosphorylation and CO2 fixation were separated in time by first incubating SO3= and AMP aerobically, and then anaerobically introducing C14O3= and ribose-5-phosphate into the reaction mixture. During the first incubation, P32O4≡ was esterified into nucleotides, mainly ADP, and in the second incubation C14O2 was fixed, with the concomitant utilization of almost equal amounts of the esterified phosphate. These data provide the first in vitro evidence for the mechanism of the coupling of CO2 fixation and phosphorylation in T. thioparus. The fixation of C14O2 was shown to be almost completely inhibited by AMP. This inhibition was not due to the conversion of ATP to ADP by adenylic kinase, or to the binding of magnesium by the nucleotide. The inhibition was specific for AMP, since other mononucleotides, adenosine, and adenine did not inhibit. The AMP regulation of CO2 fixation may represent a basic control mechanism in autotrophic metabolism.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AUBERT J. P., MONCEL C., MILHAUD G., MILLET J. Existence, isolement et propriétés physicochimiques d'un cytochrome C de Thiobacillus denitrificans. C R Hebd Seances Acad Sci. 1958 Mar 10;246(10):1616–1619. [PubMed] [Google Scholar]
- COOK T. M., UMBREIT W. W. The occurrence of cytochrome and coenzyme Q in Thiobacillus thiooxidans. Biochemistry. 1963 Jan-Feb;2:194–196. doi: 10.1021/bi00901a036. [DOI] [PubMed] [Google Scholar]
- HANES C. S., ISHERWOOD F. A. Separation of the phosphoric esters on the filter paper chromatogram. Nature. 1949 Dec 31;164(4183):1107-12, illust. doi: 10.1038/1641107a0. [DOI] [PubMed] [Google Scholar]
- HURWITZ J., WEISSBACH A., HORECKER B. L., SMYRNIOTIS P. Z. Spinach phosphoribulokinase. J Biol Chem. 1956 Feb;218(2):769–783. [PubMed] [Google Scholar]
- KELLY D. P., SYRETT P. J. INHIBITION OF FORMATION OF ADENOSINE TRIPHOSPHATE IN THIOBACILLUS THIOPARUS BY 2:4-DINITROPHENOL. Nature. 1964 May 9;202:597–598. doi: 10.1038/202597a0. [DOI] [PubMed] [Google Scholar]
- MILHAUD G., AUBERT J. P., MILLET J. Métabolisme du carbone dans la chimioautotrophie; cycle d'assimilation de l'anhydride carbonique. C R Hebd Seances Acad Sci. 1956 Jul 2;243(1):102–105. [PubMed] [Google Scholar]
- PECK H. D., Jr Evidence for the reversibility of the reaction catalyzed by adenosine 5'-phosphosulfate reductase. Biochim Biophys Acta. 1961 May 27;49:621–624. doi: 10.1016/0006-3002(61)90273-6. [DOI] [PubMed] [Google Scholar]
- PECK H. D., Jr, FISHER E., Jr The oxidation of thiosulfate and phosphorylation in extracts of Thiobacillus thioparus. J Biol Chem. 1962 Jan;237:190–197. [PubMed] [Google Scholar]
- PECK H. D., Jr Symposium on metabolism of inorganic compounds. V. Comparative metabolism of inorganic sulfur compounds in microorganisms. Bacteriol Rev. 1962 Mar;26:67–94. doi: 10.1128/br.26.1.67-94.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBBINS P. W., LIPMANN F. Separation of the two enzymatic phases in active sulfate synthesis. J Biol Chem. 1958 Sep;233(3):681–685. [PubMed] [Google Scholar]
- SANTER M., BOYER J., SANTER U. Thiobacillus novellus. I. Growth on organic and inorganic media. J Bacteriol. 1959 Aug;78:197–202. doi: 10.1128/jb.78.2.197-202.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANTER M., VISHNIAC W. CO2 incorporation by extracts of Thiobacillus thioparus. Biochim Biophys Acta. 1955 Sep;18(1):157–158. doi: 10.1016/0006-3002(55)90033-0. [DOI] [PubMed] [Google Scholar]
- SUZUKI I., WERKMAN C. H. Chemoautotrophic carbon dioxide fixation by extracts of Thiobacillus thiooxidans. II. Formation of phosphoglyceric acid. Arch Biochem Biophys. 1958 Sep;77(1):112–123. doi: 10.1016/0003-9861(58)90047-x. [DOI] [PubMed] [Google Scholar]
- TRUDINGER P. A. Fixation of carbon dioxide by extracts of the strict autotroph Thiobacillus denitrificans. Biochem J. 1956 Oct;64(2):274–286. doi: 10.1042/bj0640274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TRUDINGER P. A. Thiosulphate oxidation and cytochromes in Thiobacillus X. 1. Fractionation of bacterial extracts and properties of cytochromes. Biochem J. 1961 Apr;78:673–680. doi: 10.1042/bj0780673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VISHNIAC W., TRUDINGER P. A. Symposium on autotrophy. V. Carbon dioxide fixation and substrate oxidation in the chemosynthetic sulfur and hydrogen bacteria. Bacteriol Rev. 1962 Jun;26:168–175. [PMC free article] [PubMed] [Google Scholar]


