Abstract
Approximately 35% of follicular thyroid carcinomas and a small fraction of follicular adenomas are associated with a t(2;3)(q13;p25) chromosomal translocation that fuses paired box gene 8 (PAX8) with the peroxisome proliferator-activated receptor-γ gene (PPARG), resulting in expression of a PAX8-PPARγ fusion protein, PPFP. The mechanism by which PPFP contributes to follicular thyroid neoplasia is poorly understood. Therefore, we have created mice with thyroid-specific expression of PPFP. At 1 yr of age, 25% of PPFP mice demonstrate mild thyroid hyperplasia. We bred these mice to mice with thyroid-specific single-allele deletion of the tumor suppressor Pten, denoted ThyPten+/−. In humans, PTEN deletion is associated with follicular adenomas and carcinomas, and in mice, deletion of one Pten allele causes mild thyroid hyperplasia. We found that PPFP synergizes with ThyPten+/− to cause marked thyroid hyperplasia, but carcinomas were not observed. AKT phosphorylation was increased as expected in the ThyPten+/− thyroids, and also was increased in the PPFP thyroids and in human PPFP follicular cancers. Staining for the cell cycle marker Ki-67 was increased in the PPFP, ThyPten+/−, and PPFP;ThyPten+/− thyroids compared with wild-type thyroids. Several genes with increased expression in PPFP cancers also were found to be increased in the thyroids of PPFP mice. This transgenic mouse model of thyroidal PPFP expression exhibits properties similar to those of PPFP thyroid cancers. However, the mice develop thyroid hyperplasia, not carcinoma, suggesting that additional events are required to cause follicular thyroid cancer.
PAX8-PPARγ fusion protein, which is expressed as a consequence of a t(2;3)(q13;p25) chromosomal translocation in follicular thyroid neoplasms, causes hyperplasia and increased AKT signaling in the thyroids of transgenic mice.
Well-differentiated thyroid cancer (papillary and follicular thyroid carcinoma) is the most common endocrine malignancy. Approximately 35% of follicular thyroid carcinomas, as well as a small fraction of benign follicular adenomas, are associated with a t(2,3)(q13;p25) chromosomal translocation that fuses paired box gene 8 (PAX8) with the peroxisome proliferator-activated receptor-γ (PPARγ) gene (PPARG) (1). The consequence of this translocation is that the PAX8 promoter drives expression of a PAX8-PPARγ fusion protein (PPFP) consisting of nearly the entire PAX8 protein fused to the entire PPARγ1 protein. The transcription factor PAX8 is a master regulator of thyroid development and function. PAX8 mutations in humans (2) and mice (3) result in failure of thyroid gland development. Furthermore, PAX8 induces the expression of many classic thyroid-specific genes such as those encoding thyroglobulin, thyroid peroxidase, and the sodium iodide symporter (4,5,6). The nuclear receptor PPARγ plays important roles in adipogenesis, the regulation of carbohydrate and lipid metabolism, and inflammation. In contrast to PAX8, PPARγ is expressed at very low levels in the normal thyroid and has no known function in that organ, although it has not been thoroughly studied.
When stably expressed in thyroid cell lines, PPFP increases the rate of cell proliferation, decreases apoptosis, and increases anchorage-independent colony growth in soft agar (7,8). PPFP was initially demonstrated to dominantly inhibit PPARγ induction of a luciferase reporter construct in transiently transfected U2OS osteosarcoma cells (1). This, combined with evidence that PPARγ has tumor suppressor properties in other cell types (9,10), led to the hypothesis that PPFP induces thyroid cancer by inhibiting the action of endogenous PPARγ. This hypothesis, however, seems in conflict with subsequent cDNA microarray data demonstrating increased expression of PPARγ-inducible genes in PPFP thyroid cancers, including aquaporin 7 (AQP7) and angiopoietin-like 4 (ANGPTL4) (11,12). Indeed, transient transfection studies in a thyroid cell line and primary thyrocyte cultures confirmed that PPFP has PPARγ-like activity on the AQP7 and ANGPTL4 promoters (11). Thus, the mechanisms by which PPFP contributes to the development and progression of follicular thyroid cancer remain unknown.
Cowden syndrome is an autosomal dominant disease characterized by the development of multiple benign and malignant neoplasms, including follicular thyroid adenomas and carcinomas. Cowden syndrome is caused by mutations in the tumor suppressor gene phosphatase and tensin homolog (PTEN) (13). Mice with thyroid-specific deletion of one Pten allele develop very mild thyroid hyperplasia, whereas mice lacking both Pten alleles develop more profound hyperplasia and adenomas (14). However, these mice do not develop follicular carcinomas.
Currently, there is no animal model of PPFP expression, although such a model is needed to obtain better insight into the role of PPFP in thyroid cancer. We report the development of transgenic mice that express PPFP in the thyroid gland. Because these mice demonstrate histologically normal thyroids or only mild hyperplasia, we bred them to mice with thyroid-specific expression of Cre recombinase and one floxed Pten allele. Our data indicate that PPFP expression and loss of one Pten allele synergize in the development of thyroid gland hyperplasia.
Materials and Methods
Generation of transgenic mice
The 2045-bp bovine thyroglobulin promoter (gift of Dr. James Fagin, Memorial Sloan Kettering Cancer Institute, New York, NY) was ligated to the SpeI and SalI sites of pRL-null (Promega, Madison, WI). PPFP with three Flag epitopes at the N terminus was ligated to the Nhe1 and Xba1 sites of the same plasmid. [PPFP contains the Pax8 splice variant that excludes exon 9 (1,7,11) using the nomenclature in which exon 2 is the first coding exon]. Both inserts were fully sequenced. In the resulting plasmid, the thyroglobulin promoter drives expression of a transcript that contains a small noncoding exon followed by an intron, an exon encoding PPFP, and an SV40 late polyadenylation signal. This fragment was excised by digestion with NdeI and ApaLI and was used to generate transgenic mice by the University of Michigan Transgenic Animal Core Facility. Purified DNA was microinjected into fertilized eggs obtained by mating FVB/N female mice with FVB/N male mice. Pronuclear microinjection was performed as described (15).
Mouse husbandry and mouse procedures
Mouse breeding and all procedures were approved by the Institutional Animal Care and Use Committee of the University of Michigan. PPFP mice were bred to FVB/N wild-type mice (Jackson Laboratory, Bar Harbor, ME). Tail DNA was analyzed by PCR using forward primer AACCTCTCGACTCACCAGACCTA (Pax8) and reverse primer ATCCCAAAGTTGGTGGGCCAGAAT (PPARγ), yielding a 191-bp band for PPFP mice. Transgenic mice in which the thyroid peroxidase promoter drives expression of Cre recombinase (TPO-Cre) on a nearly pure FVB/N background were obtained from Shioko Kimura, National Cancer Institute (Bethesda, MD) (16) and were bred to FVB/N mice. Genotyping was performed by PCR using primers AGGTGTAGAGAAGGCACTTAGC and CTAATCGCCATCTTCCAGCAGG, yielding a 412-bp band for TPO-Cre mice. FVB/N mice in which exon 5 of both Pten alleles is flanked by loxP sites (PtenL/L) were obtained from Sean Morrison, University of Michigan, and Hong Wu, University of California, Los Angeles (17), and were maintained by breeding PtenL/L mice to each other. Genotyping was performed by PCR as described (17). Mice of the genotype PtenL/+;TPO-Cre were obtained by breeding PtenL/L and TPO-Cre mice and for simplicity are denoted ThyPten+/− to indicate the excision of one Pten allele in thyrocytes. Excision was confirmed by PCR of thyroid gland DNA (data not shown). PPFP;ThyPten+/− mice were obtained by breeding PPFP;PtenL/+ mice with TPO-Cre mice.
Thyroid ultrasound examinations were performed on mice ranging from 6–12 months of age using a VisualSonics Vevo 770 high-resolution in vivo microimaging system (Toronto, Canada). The examinations were performed under isoflurane anesthesia with the neck fur having been removed using depilatory cream.
Immediately before being killed, mice were anesthetized with ketamine/xylazine, and blood was obtained for measurement of serum T4. For some mice, the thyroid glands were dissected en bloc with the trachea and were placed in formalin for paraffin embedding and subsequent histological analyses. For other mice, the thyroid glands were dissected free of the trachea and surrounding tissues, frozen in liquid nitrogen, stored at −70 C, and subsequently used for the isolation of RNA or protein.
Serum T4 assay
T4 concentrations were determined on 25-μl aliquots of sera using MP Biomedicals (Solon, OH) catalog no. 06-B254029 RIA kit.
Western blotting
Thyroid glands from seven mice age 10–12 months of each genotype were pooled. Total protein was isolated using M-Per reagent (Pierce, Rockford, IL) containing Halt protease and phosphatase inhibitor cocktail (Pierce). Protein concentrations were determined using Bio-Rad (Hercules, CA) reagent. Forty micrograms of protein per sample were loaded into each lane of a SDS-PAGE gel, electrophoresed, and transferred to a polyvinylidene difluoride membrane. Gels varied from 8–12% SDS depending on the size of the protein(s) being analyzed. The following primary antibodies from Cell Signaling Technology (Danvers, MA) were used at a 1:1000 dilution unless indicated otherwise: AKT catalog no. 9272, phosphorylated AKT (pAKT) (Ser-473) no. 9271, AKT1 no. 2938, AKT2 no. 3063, AKT3 no. 3788, PTEN no. 9188, pPTEN no. 9549 1:500, ribosomal protein S6 no. 2212, pS6 no. 2215, forkhead box O1 (FOXO1) no. 2880, and pFOXO1 no. 9461. Additional primary antibodies used include Flag M2 (Sigma Chemical Co., St. Louis, MO; no. F3165 1:2000), AQP7 (Abcam, Cambridge, MA; no. 32826 1:500), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Santa Cruz Biotechnology, Santa Cruz, CA; no. sc-32233 1:4000). CruzMarker compatible secondary antibodies from Santa Cruz Biotechnology were used at 1:100,000. Detection was with a Pierce Super Signal West Dura kit. Digital images were captured on a Bio-Rad Fluor-S Max Multi-Imager, and band intensities were quantified using Bio-Rad QuantityOne software.
Histology and immunohistochemistry
Paraffin-embedded thyroid glands were cut as 5-μm sections, deparaffinized, and subjected to antigen retrieval using Retrieve-All 1 (Covance, Madison,WI). Mouse thyroid immunohistochemistry was performed using a Vectastain Elite ABC kit (Vector Laboratories, Burlingame, CA) according to the vendor’s protocol. The following primary antibodies from Cell Signaling Technology were used: pAKT (Ser-473) (catalog no. 4060, 1:50), pS6 (no. 4857, 1:75), and FOXO1 (no. 2880, 1:30). Immunohistochemistry also was performed for Ki-67 (Dako, Carpinteria, CA: no. M7249, 1:25). The sections were counterstained lightly with hematoxylin. Hyperplasia was evaluated in standard hematoxylin- and eosin-stained sections and was considered the presence of increased numbers of follicular epithelial cells, typically manifesting as papillary projections of cells within the follicular lumen. A human tissue microarray containing four PPFP follicular thyroid cancers and normal thyroids was stained for pAKT (Ser-473) on the Dako Autostainer using Dako Envision+ and diaminobenzidine as the chromogen. Sections were labeled overnight with pAKT (Ser-473) antibody (Zymed, San Francisco, CA; no. 18-2484, 1:200), after microwave epitope retrieval in 1 mm EDTA (pH 8.0). Appropriate negative (no primary antibody) and positive (breast carcinoma) controls were stained in parallel.
RNA-level gene expression
Total RNA was prepared using an RNeasy mini kit (QIAGEN, Valencia, CA; no. 74104). RNA expression was quantified by real time RT-PCR. RT was performed using SuperScript III (Invitrogen, Carlsbad, CA) and random nonamer primers. Real-time PCR was performed using an Applied Biosystems (Foster City, CA) Step One Plus real-time PCR instrument and Power SYBR Green master mix. In general, the cDNA from 100 ng RNA was used in triplicate PCR, although for highly expressed genes, 10 ng was used. The cDNAs analyzed and their real-time PCR primers were acetyl-coenzyme A acyltransferase 1A (ACAA1), forward GGCAGTGGCCAACATTGC and reverse GGCCATGCCAATGTCATAAGA; cannabinoid receptor 1 (CNR1), forward CACGTTGAGCCTGGCCTAA and reverse TCTGCAAGGCCGTCTAAGATC; 24-dehydrocholesterol reductase (DHCR24), forward CCTGGAGGTGGACACCAAGA and reverse TCACCTGACCCATAGACACCAA; fructose bisphosphatase 1 (FBP1), forward GGAACCATTTTTGGCATTTACAG and reverse GGCTGCAGAGCATCCTTCTC; phosphoglycerate kinase 1 (PGK1), forward GGAAGCGGGTCGTGATGA and reverse TTTGGTTGTTTGTTATCTGGTTGTTC; PPARγ, forward AGGCGAGGGCGATCTTG and reverse CATGTCGTAGATGACAAATGGTGAT; PPFP, forward CGGACAGGGCAGCTATGC and reverse TCTCTGTGTCAACCATGGTCATT; and RAB15, forward CGCTCCTATCAGCATATCATGAA and reverse TGGACTCCTTCTGGAGCGTACT. The PCR protocol was 95 C for 10 min followed by 40 cycles of 95 C for 15 sec and 60 C for 1 min. The ΔΔCt method within the Step One software was used for calculations. Target gene expressions were normalized to that of PGK1. All amplicons were intron spanning except PPFP, for which control amplifications were performed on RNA that had not been reverse transcribed.
Results
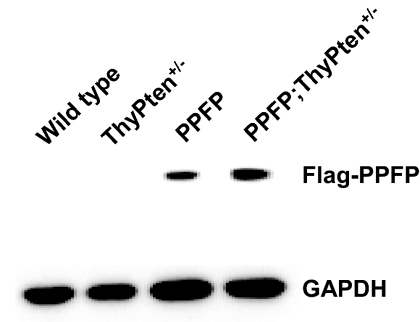
PPFP mice were born in the expected Mendelian ratios and appeared phenotypically normal. Expression of PPFP was validated by Western blot (Fig. 1) and real-time RT-PCR. PPFP protein expression appeared to be increased by 50% when combined with Pten deletion (Fig. 1, lane 4 vs. 3). However, at the RNA level (normalized to PPARγ), the difference was not significant; PPFP was expressed 1.4 ± 0.50-fold above PPARγ in PPFP mice (mean ± sd, five mice) and 1.6 ± 0.24-fold above PPARγ in PPFP;ThyPten+/− mice (P = 0.20, one-tailed t test). PPFP RNA is expressed 10- to 50-fold above PPARγ RNA in human thyroid cancer (11), but PPFP has not been quantified at the protein level in human neoplasms. Serum T4 levels were similar in all genotypes: control 1.91 ± 0.47 (n = 37), ThyPten+/− 1.88 ± 0.53 (n = 18), PPFP 1.99 ± 0.52 (n = 28), and PPFP;ThyPten+/− 2.06 ± 0.40 (n = 26) μg/dl (P = 0.57, ANOVA), indicating that PPFP does not cause significant disruption of thyroid function.
Figure 1.
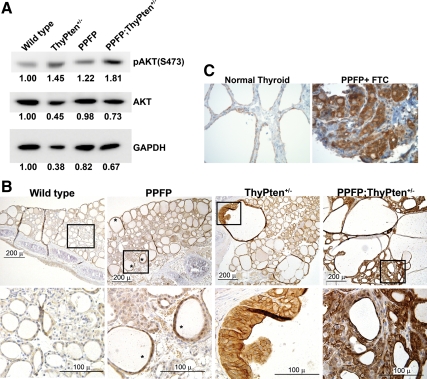
PPFP expression in transgenic mice. Protein was extracted and pooled from seven thyroid glands obtained from wild-type, PPFP, ThyPten+/−, or PPFP;ThyPten+/− mice. Forty micrograms of protein were loaded per lane of an SDS gel. PPFP expression was detected using an anti-Flag antibody. The same blot was reprobed with an anti-GAPDH antibody as a loading control.
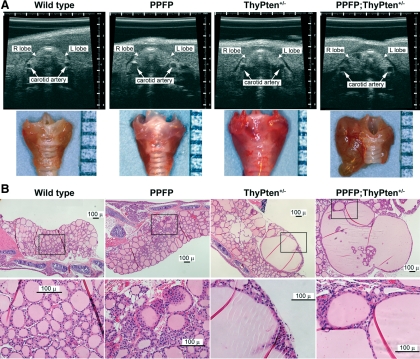
Thyroid anatomy was evaluated noninvasively by ultrasound. Because no gross abnormalities were apparent in PPFP mice, we bred those mice to TPO-Cre and PtenL/L mice and then studied mice with genotypes PPFP;ThyPten+/− and ThyPten+/− as well as the three control genotypes: PtenL/+, TPO-Cre, and wild type. At 10–12 months of age, only PPFP;ThyPten+/− mice demonstrated ultrasound abnormalities, and these abnormalities were confirmed upon gross dissection of the thyroid glands (Fig. 2A).
Figure 2.
Nodular hyperplastic changes due to expression of PPFP and loss of PTEN. A, Upper row, The thyroid glands of wild-type, PPFP, ThyPten+/−, and PPFP;ThyPten+/− mice were analyzed by ultrasound revealing that the PPFP;ThyPten+/− mouse had an enlarged right lobe with hypoechoic nodules; lower row, dissection of the thyroid glands en bloc with the trachea confirmed the PPFP;ThyPten+/− mouse had a grossly enlarged, nodular thyroid. A millimeter scale is shown at the right of each panel. B, Histological analysis of mouse thyroid glands by hematoxylin and eosin staining. The lower panel presents higher-magnification views of the boxed regions from the upper panel. Mild hyperplasia was apparent in about 25% of PPFP mice. Enlarged, hyperplastic follicles were apparent in about 50% of ThyPten+/− mice. All of the PPFP;ThyPten+/− mice showed extremely enlarged, hyperplastic follicles.
The thyroid glands also were examined histologically in mice 10–12 months of age (Fig. 2B). No abnormalities were observed in 21 control mice (wild-type, PtenL/+, or TPO-Cre). Most of the PPFP mice also were normal, but four of 15 demonstrated areas of mild hyperplasia. Abnormalities were slightly more common in ThyPten+/− mice, with five of 11 demonstrating small areas with hyperplasia and enlarged follicles. However, all 11 PPFP;ThyPten+/− mice showed markedly enlarged follicles and areas of hyperplasia. The abnormalities were independent of gender. No carcinomas were observed.
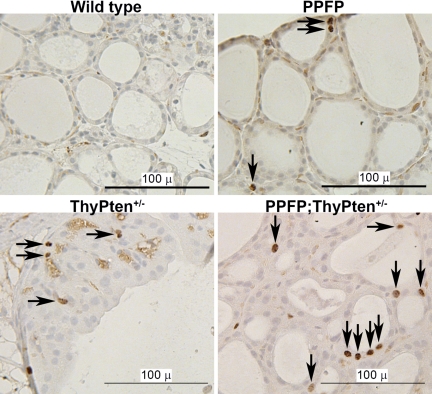
Immunohistochemical staining of the proliferation marker Ki-67 was performed (Fig. 3). Ki-67 expression was only rarely detected in control mice and was increased in PPFP, ThyPten+/−, and PPFP;ThyPten+/− mice.
Figure 3.
Proliferation analysis of mouse thyroid glands. Mouse thyroids were immunostained for Ki-67, which is expressed in the active phases of the cell cycle. Immunostaining was rare in wild-type mice and more prominent in the PPFP, ThyPten+/−, and PPFP;ThyPten+/− mice. Arrows point to positive nuclei. Sections were counterstained with hematoxylin.
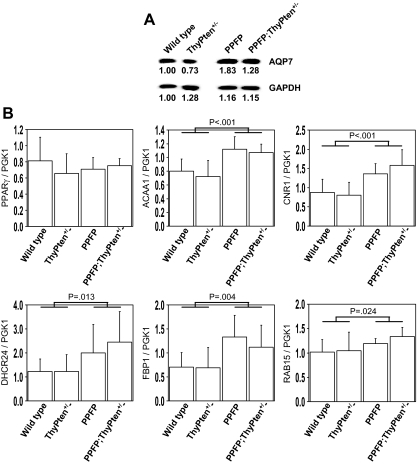
We analyzed the expression of several genes known to be induced in human PPFP thyroid cancers. We previously demonstrated that PPFP thyroid cancers have increased expression of AQP7 protein (11). Similarly, PPFP mice have increased expression of AQP7 protein (Fig. 4A). We used real-time RT-PCR to evaluate the expression of 12 genes that were shown to be induced at the RNA level in PPFP thyroid tumors by our group (11) and by Lacroix et al (12). For these studies, RNA was isolated from the thyroid glands of five mice of each of the following four groups: wild-type, ThyPten+/−, PPFP, and PPFP;ThyPten+/−. Given the limited amount of RNA available, we performed a screening assay in which we pooled the cDNAs generated from 20 ng RNA from each of the five mice of the same genotype and analyzed gene expression in that pool. Five of the 12 genes appeared to be induced in the genotypes PPFP and PPFP;ThyPten+/− (ACAA1, CNR1, DHCR24, FBP1, and RAB15). The expression of these five genes, along with PPARγ, PPFP, and PGK1 (as a neutral control) was then analyzed in the cDNA from each individual mouse using triplicate assays. The results confirmed that all five are induced in the thyroids of PPFP and PPFP;ThyPten+/− mice (Fig. 4B). Also, the results indicate that PPFP does not influence the expression of endogenous PPARγ. The seven genes that did not appear to be induced in the screening assay are ANGPTL4, ATP10B, ENO3, MYCL1, PMP22, SLC19A1, and TNFRSF21.
Figure 4.
Gene expression in PPFP mice. Thyroid glands were analyzed for the expression of genes previously shown to be induced in human PPFP thyroid cancer. A, Western blot analysis of AQP7 showing increased expression in PPFP mice. The blot was reprobed for GAPDH as a loading control. The numbers below each band indicate band intensities normalized to wild-type AQP7 (upper immunoblot) or wild-type GAPDH (lower immunoblot). Each lane contains 40 μg protein pooled from four mice. B, Real-time RT-PCR analysis of gene expression normalized to PGK1. The bars show the means ± sd for five mice. Statistical analyses were performed by one-tailed t test comparing the 10 mice expressing PPFP (PPFP and PPFP;ThyPten+/−) with the 10 mice without PPFP (wild-type and ThyPten+/−).
We next investigated the signaling pathways that might be activated in these mice. Because PTEN is a negative regulator of phosphatidylinositol 3-kinase (PI3K), loss of PTEN should increase PI3K activity and hence increase phosphorylation of AKT. This was confirmed in the ThyPten+/− mouse thyroids by Western blot (Fig. 5A). Surprisingly, we also found a small increase in AKT phosphorylation in the PPFP mice. If increased AKT phosphorylation occurs focally within the thyroid, this change could be difficult to detect when the entire thyroid is homogenized and assayed. Therefore, we also evaluated the phosphorylation status of AKT by immunohistochemistry (Fig. 5B). Indeed, scattered follicles in the PPFP mice showed increased staining. A similar but more pronounced increase was seen in the ThyPten+/− mice and the PPFP;ThyPten+/− mice. By probing a Western blot with isoform-specific antibodies, we found that AKT isoforms 1 and 2 were strongly expressed, and isoform 3 was only questionably detectable, in the thyroids from each of the four genotypes (data not shown).
Figure 5.
Analysis of AKT phosphorylation. A, Protein was extracted and pooled from seven thyroid glands obtained from wild-type, PPFP, ThyPten+/−, or PPFP;ThyPten+/− mice. Forty micrograms of protein were loaded per lane of an SDS gel. A Western blot analysis for phosphorylated AKT [pAKT; Ser-473 (S473)] shows increased expression in the ThyPten+/−, PPFP, and PPFP;ThyPten+/− mice. The same blot also was probed for total AKT and GAPDH. The numbers below each band indicate band intensities normalized to the wild-type mice. B, Immunohistochemistry of mouse thyroids for pAKT (Ser-473). *, Examples of follicles with increased immunostaining. The lower panel presents higher-magnification views of the boxed regions from the upper panel. Sections were counterstained with hematoxylin. C, Immunohistochemistry of a human PPFP follicular thyroid cancer and normal thyroid for pAKT (Ser-473). Images were photographed at ×400 magnification.
Given the observation of increased AKT phosphorylation in PPFP mice, AKT phosphorylation was evaluated in human PPFP-expressing thyroid cancers. There are no published studies addressing this issue, although increased PI3K/AKT signaling is a relatively common finding in thyroid cancer in general (18). Using a tissue microarray, we found increased pAKT immunostaining in four of four PPFP follicular thyroid cancers (Fig. 5C), confirming the relevance of our mouse data.
The increased phosphorylation of AKT in mice expressing PPFP prompted us to ask whether PTEN expression is decreased in these mice. However, by Western blot, no change was detected (data not shown). Because PTEN phosphorylation may negatively regulate its enzymatic activity (19), we also analyzed the expression of phosphorylated PTEN (Ser380/Thr382/383) by Western blot. However, phosphorylated PTEN also was unchanged in the PPFP mice (data not shown).
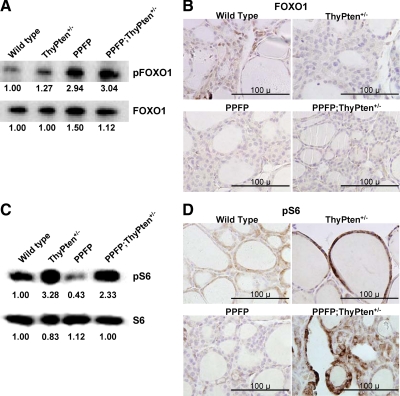
We also studied downstream targets of AKT signaling. Phosphorylation of FOXO1 was modestly increased in the ThyPten+/− mice and more markedly increased in the PPFP and the PPFP;ThyPten+/− mice, analyzed by Western blot (Fig. 6A). Because phosphorylation causes FOXO1 to exit the nucleus, these results predict that wild-type thyroids will have more nuclear FOXO1 than thyroids from the other genotypes. This was confirmed by immunohistochemistry (Fig. 6B). Ribosomal protein S6 phosphorylation was markedly increased in the ThyPten+/− mice and the PPFP;ThyPten+/− mice but was decreased in the PPFP mice, analyzed by Western blot (Fig. 6C) and confirmed by immunohistochemistry (Fig. 6D). Phosphorylation of glycogen synthase kinase-3β (GSK3β) was not increased in any of the genotypes (data not shown).
Figure 6.
Analysis of FOXO1 and S6 phosphorylation. A, Western blot analysis of pFOXO1 expression. Each lane contains 40 μg protein pooled from seven mice. The blot was reprobed for total FOXO1. The numbers below each band indicate band intensities normalized to the wild-type mice. B, Immunohistochemistry for total FOXO1. Patchy nuclear expression of FOXO1 (brown) is most prominent in the wild-type thyroid, consistent with a low level of FOXO1 phosphorylation. Sections were counterstained with hematoxylin. C, Western blot analysis of pS6 expression. Each lane contains 40 μg protein pooled from seven mice. The blot was reprobed for total S6. The numbers below each band indicate band intensities normalized to the wild-type mice. D, Immunohistochemistry for pS6. Sections were counterstained with hematoxylin. pS6, Phosphorylated S6; pFOXO1, phosphorylated FOXO1.
Discussion
Approximately 35% of follicular thyroid carcinomas contain a t(2,3)(q13;p25) chromosomal translocation that results in the expression of PPFP. Although this translocation originally was thought to be specific for follicular carcinomas, it has since been identified in a small fraction of adenomas (20). This suggests that PPFP expression may be an early event in follicular neoplasia and that additional alterations may be required to develop carcinoma. The mechanisms by which PPFP contributes to the development or progression of follicular cancer are not known. An understanding of the mechanisms could lead to improvements in the prevention, diagnosis, or therapy of this disease. For example, it is not known whether PPARγ ligands such as thiazolidinediones might be therapeutic or harmful.
The original hypothesis was that PPFP contributes to the development of thyroid cancer by functioning as a dominant-negative inhibitor of endogenous PPARγ, a putative tumor suppressor (1). This hypothesis fits with transient cotransfection data in U2OS osteosarcoma cells. It also fits with data showing decreased PPARγ expression in PPFP-negative thyroid cancers (21) as well as data showing that a PPARγ antagonist is growth promoting in Nthy-ori cells, an SV40 T-antigen immortalized human thyroid cell line (8). Furthermore, PPARγ agonists (thiazolidinediones) inhibit the growth of several thyroid cancer and melanoma cell lines, although this does not appear to correlate well with the expression level of PPARγ (22,23). [Some of these cell lines originally were thought to be of thyroid origin but a recent analysis indicates they probably are melanoma cell lines (24)]. In addition, loss of one Pparg allele exacerbates a mouse model of thyroid cancer caused by expression of a mutant thyroid hormone receptor (TR) (25). Unfortunately, thyroid cancer cell lines with the t(2,3)(q13;p25) translocation and PPFP expression have not been described.
PPFP follicular neoplasms have a unique gene expression profile when compared with PPFP-negative thyroid tumors (11,12). Surprisingly, two of the six most highly induced genes in PPFP thyroid cancer are AQP7 and ANGPTL4 (11), both of which are known to be induced by PPARγ. We have shown that PPFP induces the AQP7 and ANGPTL4 promoters in thyroid cells and that thiazolidinediones further stimulate this induction, confirming the PPARγ-like activity of PPFP (11). A pathway analysis of the PPFP cancer gene signature showed that the most enriched Gene Ontology terms were fatty acid metabolism and fatty acid β-oxidation. Because these pathways are induced by PPARs, this further indicates that PPFP can have PPAR-like activity in thyroid cancer. Thus, the mechanisms by which PPFP contributes to thyroid cancer, and its relationship to endogenous PPARγ, are not understood. It seems likely that the PPARγ portion of PPFP is important, because a very small fraction of follicular thyroid cancers is associated with a chromosomal translocation that creates a cAMP responsive element binding protein 3-like 2-PPARγ fusion protein (26). Although evidence is lacking for a specific role of the PAX8 portion of PPFP in thyroid carcinogenesis, PAX8 and its two closest relatives, PAX2 and PAX5, are associated with nonthyroid cancers, and chromosomal rearrangements involving PAX3 or PAX7 also are associated with cancer (27).
Herein we describe the first transgenic mouse model of PPFP expression in the thyroid. At 1 yr of age, most PPFP mice have histologically normal thyroid glands, although about 25% of these mice have areas of mild hyperplasia. Several explanations can be proposed for this mild phenotype. PPFP expression relative to PPARγ is lower in these mice than in human PPFP thyroid cancers. Additional mutations might need to accumulate, and 1 yr might not be sufficient time for this to occur with reasonable frequency in a mouse. The biology of PPFP might differ in mouse and human thyroids, or the thyroglobulin promoter used to drive PPFP expression might not have the appropriate functional properties. However, the thyroglobulin promoter was used to express the activated BRAF mutant V600E (28) and the ret proto-oncogene/papillary thyroid carcinoma fusion protein (29) to establish successful transgenic mouse models of papillary thyroid cancer, as well as activated NRAS (Q61K) to establish a model of mixed papillary/follicular cancer (30). Because TSH is a thyroid cancer growth factor, it is possible that a more exaggerated phenotype would be observed if the mice were rendered hypothyroid.
Many thyroid cancers of diverse histological types show evidence of increased AKT activation (18,31), although this has not been studied previously in PPFP thyroid cancers. We identified a mild increase in pAKT in PPFP mice and a strong increase in human PPFP cancers. Loss-of-function mutations in PTEN, a negative regulator of AKT, are associated with thyroid follicular adenomas and carcinomas in Cowden syndrome. Patients with Cowden syndrome inherit one nonfunctional PTEN allele (13). Because PTEN is a tumor suppressor, spontaneous mutations in the second allele over time lead to its inactivation and the growth of tumors in the thyroid and other organs. However, mice with a single-allele deletion of Pten develop only mild thyroid hyperplasia by 1 yr of age (14). Thus, we tested whether the expression of PPFP and the genetic loss of one Pten allele in the thyroid might synergize in the development of follicular thyroid cancer in mice. Although we did find synergy in the development of hyperplasia, we did not observe thyroid cancer in mice up to 1 yr of age.
We found that the PPFP thyroid glands exhibited increased phosphorylation of FOXO1, a downstream target of AKT. Because FOXO1 is inactivated by phosphorylation, AKT signaling blocks the FOXO-mediated transcription of genes involved in apoptosis and cell cycle arrest and may contribute to the hyperplasia seen in PPFP thyroids. In contrast, the phosphorylation of another target of AKT signaling, GSK3β, was not increased. These results are consistent with the observation that phosphorylation of AKT Ser-473 is necessary for its signaling to FOXO1 but not to GSK3β (32,33).
How might PPFP induce AKT phosphorylation? Mammalian target of rapamycin complex 2 (mTORC2) phosphorylates AKT Ser-473 (34); hence, our data suggest mTORC2 activity is increased. Interestingly, however, our data also suggest that the activity of mTORC1 is decreased, as judged by the phosphorylation status of S6. These findings could be explained if the primary effect of PPFP is to inhibit mTORC1 activity. Because a negative feedback loop from mTORC1 suppresses mTORC2 activity (35,36), decreased mTORC1 would lead to increased mTORC2 activity. Furthermore, a recent study has shown that mTORC2 activity is stimulated by the tuberous sclerosis complex TSC1-TSC2 (37), in contrast to the classic effect of TSC1-TSC2 to inhibit mTORC1. If PPFP stimulates the TSC, the result would be inhibition of mTORC1 and stimulation of mTORC2 activity. To address this hypothesis, we assessed by Western blotting the expression of TSC2, the catalytic component of the TSC. We also assessed TSC2 phosphorylation, which destabilizes the TSC (TSC2 phosphorylation itself is downstream of pAKT). Unfortunately, however, TSC2 expression was too low to reliably measure in our samples (data not shown).
Other pathways leading to increased AKT Ser-473 phosphorylation could be considered, although in general these would be predicted to increase both mTORC1 and mTORC2. Integrin-linked kinase 1 (ILK1) may have the potential to phosphorylate AKT Ser-473 (38), but we did not detect changes in ILK1 expression by Western blot (data not shown). Decreased PTEN would of course increase AKT phosphorylation. In fact, PPARβ/δ agonists have been reported to negatively regulate PTEN expression (39), and because PPAR isoforms share a common DNA response element, PPFP potentially could down-regulate PTEN. However, by Western blot, we were unable to detect a change in PTEN expression in PPFP thyroids. There also is evidence that PTEN activity may be negatively regulated by its own phosphorylation (19), but we did not detect increased phosphorylated PTEN (Ser380/Thr382/383) in the PPFP thyroids. It might be that multiple mechanisms contribute to the increased phosphorylation of AKT, each of which is too small to detect.
Mice with homozygous mutations in TRβ (TRβPV/PV) develop follicular thyroid cancer (40). However, TRβ mutations have not been described in human thyroid cancer. In addition, TRβPV/PV mice have extremely elevated circulating levels of TSH, which also is not a feature of human thyroid cancer. Nevertheless, the fact that the thyroid tumors in TRβPV/PV mice resemble human follicular thyroid cancer suggests that these diseases share common derangements of at least some signaling pathways. Thus, it is interesting to note that TRβPV binds directly to the p85α subunit of PI3K, resulting in PI3K activation via a nongenomic mechanism (41). Furthermore, deletion of a single Pten allele synergizes with TRβPV/PV to cause thyroid cancer of increased severity (42). In addition, other nuclear receptors such as the estrogen receptor are able to bind and activate PI3K by nongenomic mechanisms (43). Thus, even though immunohistochemistry demonstrates PPFP to be exclusively nuclear (1,11), it is possible that a small fraction is cytoplasmic and that this fraction activates PI3K by direct interaction, leading to increased AKT phosphorylation. Further studies will be required to address these possibilities and to achieve a more complete understanding of the mechanisms by which PPFP contributes to follicular thyroid cancer.
Acknowledgments
We thank Mark Deming, Supervisor, Pathology Imaging, for photography of the mouse thyroid glands. We acknowledge Galina Gavrilina and the Transgenic Animal Model Core of the University of Michigan Biomedical Research Core Facilities for preparation of the transgenic mice.
Footnotes
This work was supported by the Cell and Molecular Biology Core of the Michigan Diabetes Research and Training Center National Institutes of Health Grant P60 DK020572 and by the University of Michigan Comprehensive Cancer Center Support Grant P30 CA046592.
Disclosure Summary: The authors have nothing to disclose.
First Published Online September 24, 2009
Abbreviations: AQP7, Aquaporin 7; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; GSK3β, glycogen synthase kinase-3β; mTORC2, mammalian target of rapamycin complex 2; PAX8, paired box gene 8; PI3K, phosphatidylinositol 3-kinase; PPARγ, peroxisome proliferator-activated receptor-γ; PPFP, PAX8-PPARγ fusion protein; PTEN, phosphatase and tensin homolog; TR, thyroid hormone receptor; TSC, tuberous sclerosis complex.
References
- Kroll TG, Sarraf P, Pecciarini L, Chen CJ, Mueller E, Spiegelman BM, Fletcher JA 2000 PAX8-PPARγ1 fusion in oncogene human thyroid carcinoma. Science 289:1357–1360 [DOI] [PubMed] [Google Scholar]
- Macchia PE, Lapi P, Krude H, Pirro MT, Missero C, Chiovato L, Souabni A, Baserga M, Tassi V, Pinchera A, Fenzi G, Grüters A, Busslinger M, Di Lauro R 1998 PAX8 mutations associated with congenital hypothyroidism caused by thyroid dysgenesis. Nat Genet 19:83–86 [DOI] [PubMed] [Google Scholar]
- Mansouri A, Chowdhury K, Gruss P 1998 Follicular cells of the thyroid gland require Pax8 gene function. Nat Genet 19:87–90 [DOI] [PubMed] [Google Scholar]
- Esposito C, Miccadei S, Saiardi A, Civitareale D 1998 PAX 8 activates the enhancer of the human thyroperoxidase gene. Biochem J 331(Pt 1):37–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno M, Zannini M, Levy O, Carrasco N, di Lauro R 1999 The paired-domain transcription factor Pax8 binds to the upstream enhancer of the rat sodium/iodide symporter gene and participates in both thyroid-specific and cyclic-AMP-dependent transcription. Mol Cell Biol 19:2051–2060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabbro D, Pellizzari L, Mercuri F, Tell G, Damante G 1998 Pax-8 protein levels regulate thyroglobulin gene expression. J Mol Endocrinol 21:347–354 [DOI] [PubMed] [Google Scholar]
- Au AY, McBride C, Wilhelm Jr KG, Koenig RJ, Speller B, Cheung L, Messina M, Wentworth J, Tasevski V, Learoyd D, Robinson BG, Clifton-Bligh RJ 2006 PAX8-peroxisome proliferator-activated receptor γ (PPARγ) disrupts normal PAX8 or PPARγ transcriptional function and stimulates follicular thyroid cell growth. Endocrinology 147:367–376 [DOI] [PubMed] [Google Scholar]
- Gregory Powell J, Wang X, Allard BL, Sahin M, Wang XL, Hay ID, Hiddinga HJ, Deshpande SS, Kroll TG, Grebe SK, Eberhardt NL, McIver B 2004 The PAX8/PPARγ fusion oncoprotein transforms immortalized human thyrocytes through a mechanism probably involving wild-type PPARγ inhibition. Oncogene 23:3634–3641 [DOI] [PubMed] [Google Scholar]
- Demetri GD, Fletcher CD, Mueller E, Sarraf P, Naujoks R, Campbell N, Spiegelman BM, Singer S 1999 Induction of solid tumor differentiation by the peroxisome proliferator-activated receptor-γ ligand troglitazone in patients with liposarcoma. Proc Natl Acad Sci USA 96:3951–3956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarraf P, Mueller E, Smith WM, Wright HM, Kum JB, Aaltonen LA, de la Chapelle A, Spiegelman BM, Eng C 1999 Loss-of-function mutations in PPARγ associated with human colon cancer. Mol Cell 3:799–804 [DOI] [PubMed] [Google Scholar]
- Giordano TJ, Au AY, Kuick R, Thomas DG, Rhodes DR, Wilhelm Jr KG, Vinco M, Misek DE, Sanders D, Zhu Z, Ciampi R, Hanash S, Chinnaiyan A, Clifton-Bligh RJ, Robinson BG, Nikiforov YE, Koenig RJ 2006 Delineation, functional validation, and bioinformatic evaluation of gene expression in thyroid follicular carcinomas with the PAX8-PPARG translocation. Clin Cancer Res 12:1983–1993 [DOI] [PubMed] [Google Scholar]
- Lacroix L, Lazar V, Michiels S, Ripoche H, Dessen P, Talbot M, Caillou B, Levillain JP, Schlumberger M, Bidart JM 2005 Follicular thyroid tumors with the PAX8-PPARγ1 rearrangement display characteristic genetic alterations. Am J Pathol 167:223–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liaw D, Marsh DJ, Li J, Dahia PL, Wang SI, Zheng Z, Bose S, Call KM, Tsou HC, Peacocke M, Eng C, Parsons R 1997 Germline mutations of the PTEN gene in Cowden disease, an inherited breast and thyroid cancer syndrome. Nat Genet 16:64–67 [DOI] [PubMed] [Google Scholar]
- Yeager N, Klein-Szanto A, Kimura S, Di Cristofano A 2007 Pten loss in the mouse thyroid causes goiter and follicular adenomas: insights into thyroid function and Cowden disease pathogenesis. Cancer Res 67:959–966 [DOI] [PubMed] [Google Scholar]
- Nagy A, Gertsenstein M, Vintersten K, Behringer R 2003 Manipulating the mouse embryo: a laboratory manual. 3rd ed. New York: Cold Spring Harbor Press [Google Scholar]
- Kusakabe T, Kawaguchi A, Kawaguchi R, Feigenbaum L, Kimura S 2004 Thyrocyte-specific expression of Cre recombinase in transgenic mice. Genesis 39:212–216 [DOI] [PubMed] [Google Scholar]
- Lesche R, Groszer M, Gao J, Wang Y, Messing A, Sun H, Liu X, Wu H 2002 Cre/loxP-mediated inactivation of the murine Pten tumor suppressor gene. Genesis 32:148–149 [DOI] [PubMed] [Google Scholar]
- Hou P, Liu D, Shan Y, Hu S, Studeman K, Condouris S, Wang Y, Trink A, El-Naggar AK, Tallini G, Vasko V, Xing M 2007 Genetic alterations and their relationship in the phosphatidylinositol 3-kinase/Akt pathway in thyroid cancer. Clin Cancer Res 13:1161–1170 [DOI] [PubMed] [Google Scholar]
- Silva A, Yunes JA, Cardoso BA, Martins LR, Jotta PY, Abecasis M, Nowill AE, Leslie NR, Cardoso AA, Barata JT 2008 PTEN posttranslational inactivation and hyperactivation of the PI3K/Akt pathway sustain primary T cell leukemia viability. J Clin Invest 118:3762–3774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques AR, Espadinha C, Catarino AL, Moniz S, Pereira T, Sobrinho LG, Leite V 2002 Expression of PAX8-PPARγ1 rearrangements in both follicular thyroid carcinomas and adenomas. J Clin Endocrinol Metab 87:3947–3952 [DOI] [PubMed] [Google Scholar]
- Marques AR, Espadinha C, Frias MJ, Roque L, Catarino AL, Sobrinho LG, Leite V 2004 Underexpression of peroxisome proliferator-activated receptor (PPAR)γ in PAX8/PPARγ-negative thyroid tumours. Br J Cancer 91:732–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klopper JP, Hays WR, Sharma V, Baumbusch MA, Hershman JM, Haugen BR 2004 Retinoid X receptor-γ and peroxisome proliferator-activated receptor-γ expression predicts thyroid carcinoma cell response to retinoid and thiazolidinedione treatment. Mol Cancer Ther 3:1011–1020 [PubMed] [Google Scholar]
- Park JW, Zarnegar R, Kanauchi H, Wong MG, Hyun WC, Ginzinger DG, Lobo M, Cotter P, Duh QY, Clark OH 2005 Troglitazone, the peroxisome proliferator-activated receptor-γ agonist, induces antiproliferation and redifferentiation in human thyroid cancer cell lines. Thyroid 15:222–231 [DOI] [PubMed] [Google Scholar]
- Schweppe RE, Klopper JP, Korch C, Pugazhenthi U, Benezra M, Knauf JA, Fagin JA, Marlow LA, Copland JA, Smallridge RC, Haugen BR 2008 Deoxyribonucleic acid profiling analysis of 40 human thyroid cancer cell lines reveals cross-contamination resulting in cell line redundancy and misidentification. J Clin Endocrinol Metab 93:4331–4341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato Y, Ying H, Zhao L, Furuya F, Araki O, Willingham MC, Cheng SY 2006 PPARγ insufficiency promotes follicular thyroid carcinogenesis via activation of the nuclear factor-κB signaling pathway. Oncogene 25:2736–2747 [DOI] [PubMed] [Google Scholar]
- Lui WO, Zeng L, Rehrmann V, Deshpande S, Tretiakova M, Kaplan EL, Leibiger I, Leibiger B, Enberg U, Höög A, Larsson C, Kroll TG 2008 CREB3L2-PPARγ fusion mutation identifies a thyroid signaling pathway regulated by intramembrane proteolysis. Cancer Res 68:7156–7164 [DOI] [PubMed] [Google Scholar]
- Robson EJ, He SJ, Eccles MR 2006 A PANorama of PAX genes in cancer and development. Nat Rev Cancer 6:52–62 [DOI] [PubMed] [Google Scholar]
- Knauf JA, Ma X, Smith EP, Zhang L, Mitsutake N, Liao XH, Refetoff S, Nikiforov YE, Fagin JA 2005 Targeted expression of BRAFV600E in thyroid cells of transgenic mice results in papillary thyroid cancers that undergo dedifferentiation. Cancer Res 65:4238–4245 [DOI] [PubMed] [Google Scholar]
- Jhiang SM, Sagartz JE, Tong Q, Parker-Thornburg J, Capen CC, Cho JY, Xing S, Ledent C 1996 Targeted expression of the ret/PTC1 oncogene induces papillary thyroid carcinomas. Endocrinology 137:375–378 [DOI] [PubMed] [Google Scholar]
- Vitagliano D, Portella G, Troncone G, Francione A, Rossi C, Bruno A, Giorgini A, Coluzzi S, Nappi TC, Rothstein JL, Pasquinelli R, Chiappetta G, Terracciano D, Macchia V, Melillo RM, Fusco A, Santoro M 2006 Thyroid targeting of the N-ras(Gln61Lys) oncogene in transgenic mice results in follicular tumors that progress to poorly differentiated carcinomas. Oncogene 25:5467–5474 [DOI] [PubMed] [Google Scholar]
- Paes JE, Ringel MD 2008 Dysregulation of the phosphatidylinositol 3-kinase pathway in thyroid neoplasia. Endocrinol Metab Clin North Am 37:375–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guertin DA, Stevens DM, Thoreen CC, Burds AA, Kalaany NY, Moffat J, Brown M, Fitzgerald KJ, Sabatini DM 2006 Ablation in mice of the mTORC components raptor, rictor, or mLST8 reveals that mTORC2 is required for signaling to Akt-FOXO and PKCα, but not S6K1. Dev Cell 11:859–871 [DOI] [PubMed] [Google Scholar]
- Jacinto E, Facchinetti V, Liu D, Soto N, Wei S, Jung SY, Huang Q, Qin J, Su B 2006 SIN1/MIP1 maintains rictor-mTOR complex integrity and regulates Akt phosphorylation and substrate specificity. Cell 127:125–137 [DOI] [PubMed] [Google Scholar]
- Sarbassov DD, Guertin DA, Ali SM, Sabatini DM 2005 Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 307:1098–1101 [DOI] [PubMed] [Google Scholar]
- Harrington LS, Findlay GM, Gray A, Tolkacheva T, Wigfield S, Rebholz H, Barnett J, Leslie NR, Cheng S, Shepherd PR, Gout I, Downes CP, Lamb RF 2004 The TSC1-2 tumor suppressor controls insulin-PI3K signaling via regulation of IRS proteins. J Cell Biol 166:213–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning BD, Logsdon MN, Lipovsky AI, Abbott D, Kwiatkowski DJ, Cantley LC 2005 Feedback inhibition of Akt signaling limits the growth of tumors lacking Tsc2. Genes Dev 19:1773–1778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Dibble CC, Matsuzaki M, Manning BD 2008 The TSC1-TSC2 complex is required for proper activation of mTOR complex 2. Mol Cell Biol 28:4104–4115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troussard AA, Mawji NM, Ong C, Mui A, St-Arnaud R, Dedhar S 2003 Conditional knock-out of integrin-linked kinase demonstrates an essential role in protein kinase B/Akt activation. J Biol Chem 278:22374–22378 [DOI] [PubMed] [Google Scholar]
- Han S, Ritzenthaler JD, Zheng Y, Roman J 2008 PPARβ/δ agonist stimulates human lung carcinoma cell growth through inhibition of PTEN expression: the involvement of PI3K and NF-κB signals. Am J Physiol Lung Cell Mol Physiol 294:L1238–L1249 [DOI] [PubMed] [Google Scholar]
- Suzuki H, Willingham MC, Cheng SY 2002 Mice with a mutation in the thyroid hormone receptor β gene spontaneously develop thyroid carcinoma: a mouse model of thyroid carcinogenesis. Thyroid 12:963–969 [DOI] [PubMed] [Google Scholar]
- Furuya F, Hanover JA, Cheng SY 2006 Activation of phosphatidylinositol 3-kinase signaling by a mutant thyroid hormone β receptor. Proc Natl Acad Sci USA 103:1780–1785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guigon CJ, Zhao L, Willingham MC, Cheng SY 2009 PTEN deficiency accelerates tumour progression in a mouse model of thyroid cancer. Oncogene 28:509–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoncini T, Hafezi-Moghadam A, Brazil DP, Ley K, Chin WW, Liao JK 2000 Interaction of oestrogen receptor with the regulatory subunit of phosphatidylinositol-3-OH kinase. Nature 407:538–541 [DOI] [PMC free article] [PubMed] [Google Scholar]