Abstract
Prostate cancer (PCa), like most human cancers, features dysregulated CD44 expression. Expression of CD44 standard (CD44s), present in benign epithelium, is lost in PCa while pro-invasive splice variant isoform CD44v7-10 is overexpressed. The role of CD44 in silibinin's anti-growth effects was uncertain. To assess silibinin's effects on CD44 promoter activity, PC-3M PCa cells were transfected with luciferase-CD44 promoter construct 24 h prior to 25–200 μM silibinin treatment for 48 h. Also, cells' expression of CD44 RNA (by qRT-PCR) and protein (Western blot analysis) was studied. Silibinin was further tested preoperatively on a pilot cohort of 6 men with PCa compared with 7 matched placebo-treated men, with immunostaining for CD44v7-10 in their prostates. In PC-3M cells, silibinin dose-dependently inhibited CD44 promoter activity up to 87%, caused a 90% inhibition of total CD44 and 70% decrease in CD44v7-10 RNA, and at the protein level, decreased total CD44 at 100–200 μM dose and decreased CD44v7-10 after 3 days. Silibinin decreased adhesion to hyaluronan and fibronectin. Silibinin at 100–200 μM inhibited Egr-1, a regulator of CD44 promoter activity. Men treated with silibinin did not differ in tissue CD44v7-10 expression. In conclusion, CD44 inhibition is one mechanism by which silibinin reduces PCa tumorigenicity.
Keywords: Silibinin, prostatic neoplasms; CD44; plasmid; alternate splicing; adhesion
Introduction
About 30% of prostate cancer (PCa) cases undergo transition from quiescent to aggressive, and this transition relies on altered expression of adhesion glycoproteins such as CD44 that allow tumor cells to detach, interact with proteins that digest stromal matrix, migrate through matrix and intravasate into lymphovascular channels. CD44 enables cell-cell and cell-matrix adhesion, primarily to hyaluronan but also to other ligands, and links the cell membrane to the actin cytoskeleton, modulating motility. CD44 is a transmembrane molecule encoded by an alternately spliced gene. It is expressed as ubiquitous, standard (CD44s) isoform but inclusion of one or more of 10 variant (v) exons produces tissue-specific (CD44v) isoforms. Moreover, a unique variant isoform of CD44 is overexpressed in PCa and facilitates PCa cell growth and invasion [1–6].
Silibinin is a nutritional supplement that has gained attention as intervention to prevent or treat cancer. Silibinin is a polyphenolic flavonolignan isolated from milk thistle and artichoke that arrests PCa proliferation in vitro [7] with cell cycle arrest in G1 [8], potentiates a chemotherapeutic drugs [9], and in vivo increases apoptosis and inhibits angiogenesis [10]. It inhibits cancer invasion by inactivating the PI3K-Akt and MAPK signaling pathways [11] as well as decreasing production of urokinase-plasminogen activator (uPA) and matrix metalloprotease-2 [12]. The early growth response-1 (Egr-1) transcription factor binds to and activates CD44 promoter [13]. Here we investigate these effects of silibinin on CD44 total and variant, adhesion, and Egr-1 expression.
Materials and Methods
Cell lines and treatment
PC-3M cells, a metastasis-derived variant of PC-3, were obtained from Dr. Girish Shah, U. of Louisiana—Monroe. LNCaP and DU145 cells were from ATCC (Manassas, VA). Cells were grown in RPMI 1640 (Invitrogen) with 10% fetal calf serum and antibiotics. Cells were grown in 5% CO2 incubator at 37°C. For each experiment, cells in a flask were trypsinized, washed with sterile PBS to remove trypsin, resuspended in basal medium, and counted after dilution with Trypan blue dye using the grid method.
Cells were treated with a dose of 25 up to 200 μM silibinin; based on the fact that 100 μM most effectively inhibited invasion, MMP-2, and uPA [11,12]. Vehicle for both agents was DMSO, which was applied to control cells.
CD44 promoter luciferase assays
Using the PXP2 plasmid, 1150 bases of CD44 sequence including the start site, and beginning 964 bases upstream to the start site, was cloned in between Xho I and Hind III sites. The efficacy of the promoter construct had been validated based on its inhibition by HOXC6 [14]. Luciferase activity was measured 48 h after transfection using the firefly luciferase assay (Gold Biotechnologies, St. Louis, MO). The cells were harvested in 20 mM K2HPO4 pH 7.8 with 5 mM MgCl2 and 0.5% Triton X-100 buffer for 15 min on ice, and the mixture was centrifuged for 10 min at 4°C. 50 μl of lysate were added to 350 μl of luciferase assay buffer as per the manufacturer. The relative luciferase units (RLU) were normalized to protein concentration as determined by Bradford assay.
Real time TaqMan RNA analysis
Total RNA was prepared from cell pellets using Trizol (Invitrogen, Carlsbad, CA) as described by the manufacturer. RNA was further purified by isopropanol precipita-tion, resuspended in RNAse-free water, and its concentration measured. Complementary DNA (cDNA) was synthesized from 4 μg total RNA in 20 μl reaction mixture as we did previously [15]. At least triplicate samples were run using a primer set that brackets the entire variant region with a probe for CD44v9 [15], primers for CD44 total with a probe that binds a standard exon, and 18S ribosomal RNA. Quantitative PCR reactions were optimized to 4 μg cDNA (0.16 μg with 18S) plus the manufacturer's master mix and primer/probe sets (Applied Biosystems, Foster City, CA) in a volume of 20 μl. The amplification protocol was as follows: hold 50°C 2 min, 95°C 10 min, then 40 cycles of (95°C for 0:15 and 60°C for 1:00) using the ABI 7500 cycler (Perkin-Elmer, Waltham, MA). Primer/probe sets for CD44v were: forward, AACGCTTCAGCCTACTGCAAA; reverse, TCTTCCAAGCCTTCATGTGATG; probe, GATTTGGACAGGACAGGACCTCTTTCAATG. For CD44 total we used forward, CAACTCCAT CTGTGCAGCAAA; reverse, GTAACCTCCTGAAG TGCTGCTC; probe, CATATTGCTTCAATGCTTCAG CTCCACCTG. Primer and probe sets for 18S were proprietary to the manufacturer.
Western blot analysis
Cultured cells were directly lysed in their wells using RIPA buffer (Upstate Biologicals, Lake Placid, NY) plus the protease inhibitor mini tablets (Roche, Indianapolis, IN). Protein concentration of the cell lysate was estimated by Bradford method. SDS-PAGE was performed on 25 μg sample/lane according to Laemmli method using the NuPAGE system (Invitrogen, Carlsbad, CA). 10 μl of Kaleido-scope Precision Plus Protein Standards (Bio-Rad) was run in at least one lane. After electrophoresis for 2 hr, the protein was transferred to nitrocellulose. Primary antibodies were used. Polyclonal anti-Egr-1 C-terminus (Santa Cruz) was used at 1:200. To assess the overexpressed CD44v7-10 the membrane was reacted with neat supernatant from the hybridoma cell line HB-258 (ATCC). CD44 total (standard + variant) was assessed using monoclonal antibody 156-3C11 (Cell Signaling Technology, Danvers, MA) at 1:1000. Anti-β-actin-HRP antibody (Sigma, St. Louis) was used at a dilution of 1:10,000. Membranes were washed 3 x 15 min in TBS with 0.1% Tween 20% and 1:2000 dilution of goat anti-mouse IgG antibody labeled with HRP (Bio-Rad, Hercules, CA) was added in TBS + 5% skim milk for 1 hr. Reactivity was detected using the SuperSignal West Pico Substrate chemiluminescent system (Pierce Biotechnology, Rockford, IL). Each experimental run was conducted at least twice.
Cell adhesion assays
Cellular adhesion assays as described [15], were carried out using trypsinized confluent untreated, PC-3M cells after 48 hour 100 μM silibinin treatment or vehicle only. Each test condition was set in 8 wells. 96-well black-edged, clear flat bottom Costar plates (Cole-Parmer, Vernon Hills, IL) were coated with 20 μg/ml fibronectin (Becton Dickinson, Bedford, MA) or 2 mg/ml hyaluronan (Sigma, St. Louis, MO) [15] at 37°C overnight. To measure baseline nonspecific binding, other wells were coated with 1 mg/ml BSA. 1x106 cells suspended in 1 mL PBS were incubated with the dye BCECF-AM (Dojindo, Tokyo) for 15 minutes at 37°C [16]. After two washes of the cells with PBS, cells were added to plates at a density of 3x104 per well and incubated at 37°C for 90 min. Fluorescence intensities at 530 nm were measured using a Bio-Tek FL-600 fluorescent plate reader. Nonadherent cells were removed with successive PBS washes, reading fluorescence intensities with PBS in the wells after each. Adhesion was calculated [15] as % cells bound=(100) fluorescence intensity after second wash / fluorescence intensity of total cells plated. The assay was repeated twice.
Immunostaining for CD44v9 in prostate cancer patients
Under written informed consent, six men planning to undergo radical prostatectomy received 13 g silibinin-phytosome daily in 3 divided doses for a 2–4 week duration, and 7 men were untreated controls. Plasma levels of silibinin peaked at 25–150 μM with a half-life ranging from 2–5 hours [17]. The primary antibody was neat supernatant from the hybridoma cell line HB-258 (ATCC) secreting mouse anti-human antibody to CD44v9. The secondary antibody was goat anti-mouse IgG antibody labeled with HRP (Bio-Rad, Hercules, CA).
Statistical analysis
CD44 promoter activity data were analyzed by ANOVA and post t-test, comparing activity at various doses. TaqMan data were analyzed by the 2(-ΔΔCT) method [18] to determine fold change in gene expression (untreated cells=1.00). The ΔCT was taken as the difference between the CD44v or CD44 total and the 18S ribosomal RNA CTs. The ΔΔCT was obtained using the mean ΔCT of untreated cells as calibrator. Each TaqMan result was compared to 1.00 using 2-tailed paired t-test. Statistical significance was set at p <0.05. Adhesion assay data are expressed as mean ±SD. The significance of differences among group means was tested by 2-tailed paired t-test.
Results
Silibinin suppresses CD44 promoter activity, RNA and protein
Transfection of our CD44 promoter construct into PC-3M prostate cancer cells for 24 h was followed by silibinin treatment for 48 h to assess the effects of these agents. Silibinin significantly dose-dependently inhibited promoter activity, up to 86.7% (p<0.001) at 200 μM (Figure 1). Doses from 25 μM to 200 μM differed significantly from no treatment; and a dose of 200 μM caused significantly less promoter activity than all lower doses. Mean RNA levels after all doses of silibinin treatment, normalized to untreated cells, were more than 90% lower for total CD44. CD44v7-10 was noted to be increased at 25 μM dose but decreased at higher doses (Figure 2). At the protein level, silibinin's effect at the protein level was minimal after 1 day, with western blot analysis (Figure 3) demonstrating inhibition of total CD44 and CD44 variant mainly at the highest, 200 μM dose. We then performed a time course experiment, however, and noted that the earlier CD44v7-10 stimulation turned to a strong inhibition at 3 days, consistent with silibinin's inhibition of CD44v7-10 RNA.
Figure 1.
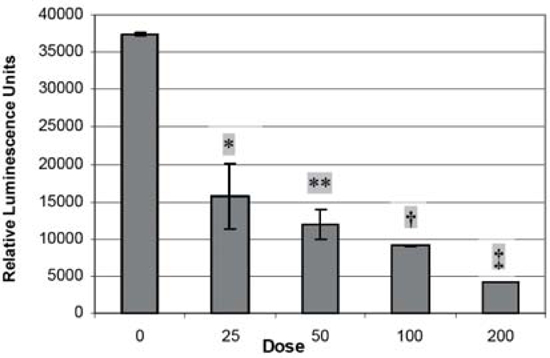
CD44 promoter assays. Transfected PC-3M cells overexpress PXP2 plasmid containing cloned in CD44 promoter + luciferase. Cells were incubated with silibinin for 48 hr after promoter transfection. There is a dose-dependent inhibition of promoter activity. ANOVA is significant at p<0.0001; post-tests using t-test show *p=.002 versus dose 0; **p=.0001 versus dose 0; .19 versus 25; †p=.001 versus dose 0; .06 versus 25; .07 versus 50; ‡p=.002 versus dose 0, .01 versus 25; .005 versus 50; .0001 versus 100.
Figure 2.
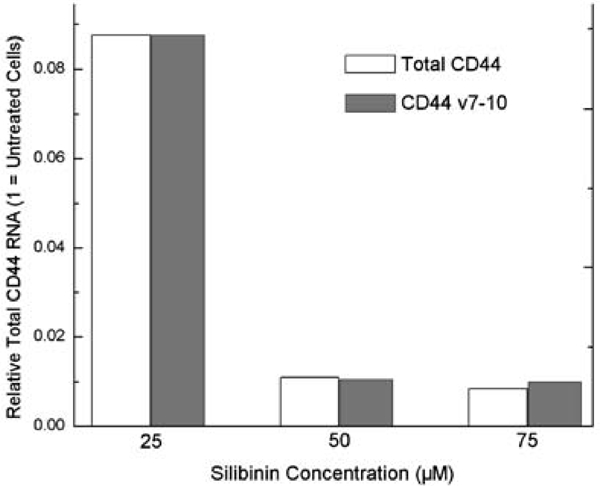
qRT-PCR demonstrates mean CD44 RNA levels after 25–100 μM silibinin treatment, normalized to untreated cells. Total CD44 was 91–99% inhibited. CD44v7-10 was 2.4x control value after 25 μM dose but inhibited at higher doses.
Figure 3.
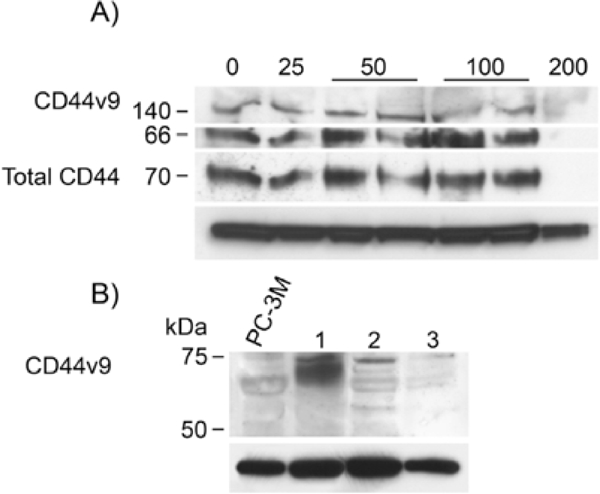
A) Western blot analysis of 25–200 μM silibinin-treated PC-3M cells at 24 h, using monoclonal anti-CD44v9. Predominant band is 66kDa, characteristic for CD44 variant cleavage product [3] although there is some full-length protein at 140 kDa. Monoclonal anti-total CD44, predominantly CD44s, is also detected. At 24 hr, total CD44 and CD44v9 are decreased only at 200 μM dose. Below: β-actin is shown as a normalizer. B) Extending the silibinin exposure of cells to 3 days caused a most marked inhibitory effect on CD44v9. Below: β-actin is shown as a normalizer.
Adhesion assays
Compared to untreated cells, PC-3M cells after 100 μM silibinin had a 66% reduction in rate of adhesion to hyaluronan and 47% reduction in adhesion to fibronectin (Figure 4).
Figure 4.
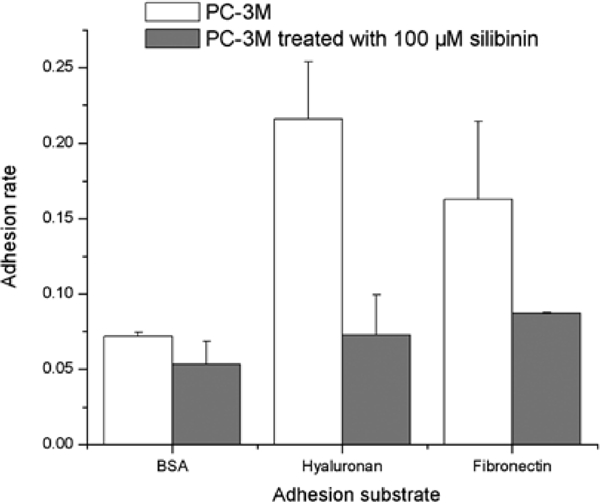
Adhesion assay with PC-3M cells after 100 μM silibinin treatment. Adhesion to both hyaluronan and fibronectin is diminished to baseline level, near that of BSA-coated wells, after silibinin treatment.
Clinical treatment with silibinin
Prostate tissues from 6 men with prostate cancer treated with silibinin and 7 treated with placebo were immunostained for the functionally important CD44 v7-10 using anti-CD44v9, directed against the largest portion of the included protein product CD44v7-10. Immunoreactivity was scored on a scale of 0–3+. The mean reactivity in treated and untreated men respectively were 1.0 and 1.3 for cancer, 2.9 and 2.6 for benign basal cells, 0.7 and 0.8 for benign secretory cells, and 0.8 and 0.7 for atrophic glands (p=NS). No significant differences were noted.
Candidate mediators of silibinin effect
Silibinin dose of 100 or 200 μM inhibited Egr-1 expression (Figure 5). Bands were observed at 55 kD consistent with the theoretical monomeric form of Egr-1, with dimeric form bands near 110 kD [19]. 100 μM concentration equals the plasma level attained in patients [17]. As a negative control, slow growing, androgen-sensitive LNCaP cells were negative, while more aggressive DU145 cells expressed Egr-1. Immunoblots against EGFR and phospho-EGFR disclosed some silibinin effect (data not shown).
Figure 5.
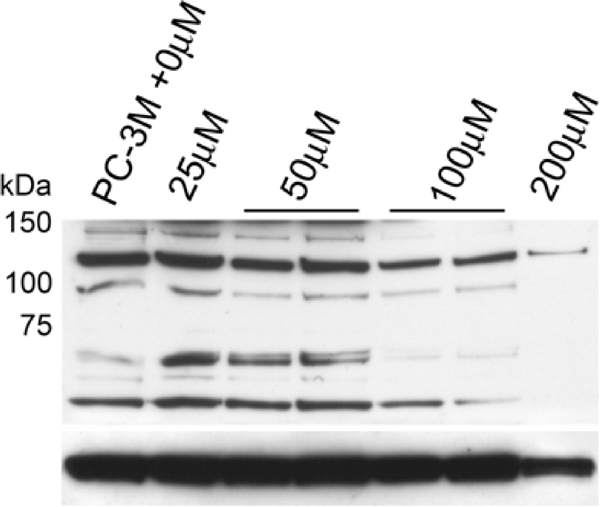
Western blot analysis of 25–200 μM silibinin-treated PC-3M cells using polyclonal anti-Egr-1. LNCaP and DU145 whole cell lysates are used as negative controls. Monomeric 55-kD and dimeric 110-kD cytoplasmic Egr-1 bands are seen, as well as a 75-kD band that had been described as specific to nuclear Egr-1 [19]. Below: β-actin is shown as a normalizer.
Discussion
We discovered that the nutrient silibinin reduces CD44 promoter activity, resulting in decreased total CD44 RNA. Inhibition of CD44v7-10 was less marked; however, at the protein level, this inhibition was strongly evident after 3 days of treatment. Our interest in CD44v7-10 variant isoform expression in PCa extends back several years. By isolating RNA from clinical PCa specimens, we found CD44v7-10 to be a unique PCa signature, consistently overexpressed in androgen-independent cells such as PC-3, primary PCa, and PCa metastatic to other tissues [1–3]. Interference against CD44v7-10 reduced invasion index by 69% compared to untreated control cells [3]. Moreover, PCa loses the splicing ability to produce the standard isoform expressed in benign prostate that constitutes most of total CD44 [3–6] along with moderate levels of CD44v3-10 variant [6]. In PC-3 prostate tumor, CD44s transfection reduced invasion in vitro [20] and its tumorigenicity in mouse xenografts [21]. Silibinin's ability to reduce pro-invasive CD44v7-10 undoubtedly contributes to its effects.
CD44's adhesive affinities depend on its ability to cluster and multimerize, which is altered by a change in CD44s to CD44 variant ratio. Thus we tested PCa cell adhesion to hyaluronan and fibronectin, two ligands that are most functionally important for CD44 in PCa cells. Binding to these two ligands was most strongly altered in PCa cells compared to benign BPH-1 cells [20], which bound hyaluronan strongly and fibronectin minimally. The androgen-sensitive LNCaP cells bound both ligands equally, while PC-3 and their derivatives bound most strongly to fibronectin, probably representing an integrin-independent mechanism of binding to fibronectin. Moreover, re-expression of CD44s significantly increased adhesion to hyaluronan and decreased adhesion to fibronectin. Here, we observed a loss of total CD44 (which is largely CD44s) after silibinin treatment, consistent with our decreased hyaluronan binding, and lower CD44v7-10 which would mediate less fibronectin binding. Because of the decreased hyaluronan binding, silibinin does not strictly cause reversion to the benign phenotype but is consistent with the decreased migration [7], invasion [11,12] and possibly decreased in vivo growth [8] of cancer cells after silibinin treatment. The only prior study of silibinin's effects on PCa cell adhesion were done with type I collagen, showing inhibited adhension of PC-3 cells to that ligand [7].
By immunostaining, prostatectomy tissue from silibinin-treated men did not differ in CD44v9 expression from that of untreated men. This lack of effect has two good explanations. First, the treatment was only for 2–4 weeks, minimizing the opportunity for the agent to alter CD44 expression in the tumor. Second, samples from this cohort of men are being tested to determine the tissue absorption into the prostate. Despite the high serum levels achieved, we have not confirmed reliable tissue penetration.
Silibinin's suppression of CD44 promoter activity which diminishes total CD44 RNA and protein is probably indirect. Factors that regulate CD44 expression by binding its promoter include Egr-1 [13,22], epidermal growth factor (EGF) [23], activating protein-1 (AP-1) [24], and H-ras [25]. Early growth response (EGR) genes encode zinc finger DNA-binding nuclear transcription factors, control cell proliferation, and show divergent expression in various human tumors. In PCa, Egr-1 binds to the androgen receptor [26] with important physiologic implications. One of us [23] demonstrated increased Egr-1, but not Egr-2 or EGR-α expression, in malignant prostate tissue as compared with weak expression in benign tissue. By in situ hybridization, the expression of Egr-1 was highest in tumors of Gleason score 8–10 than in those of lower Gleason score. Here we showed for the first time that the therapeutically relevant 100 μM dose of silibinin suppresses the high level of Egr-1 in prostate cancer (PCa) cells, suggesting that Egr-1 modulates, at least in part, the effects of silibinin on CD44.
Our findings are not meant to imply that Egr-1 is the sole mediator of silibinin's effects on CD44. Silibinin was reported to act on the MAP kinase pathway [11]. We have shown that MEK and p38 components of the MAP kinase pathway are functionally important for PCa cell expression of CD44 as well as possibly in its splicing [27]. Silibinin also inhibits the PI3K-Akt signaling pathway [11]. Further, it is likely that Egr-1, by binding to GC-rich motifs of other genes, exerts pro-growth effects relevant to PCa that are not dependent on CD44. These include activation of ICAM-1/CD54 [13], which is relevant to PCa growth [28], repression of Fas/CD95 gene [13], required for apoptosis, and repression of transforming growth factor-β Type II receptor, shutting off a growth-inhibitory pathway [29].
In conclusion, silibinin exerts functional effects on CD44 through the CD44 promoter, leading to reduced CD44 total and CD44v7-10 expression. These changes are accompanied by altered adhesion to known extracellular matrix substrates of CD44.
Acknowledgments
This work was supported by Department of Defense Prostate Cancer Research Program, Grant PC060671 to K.A.I. We thank Dr. Steve K. Nordeen for advice.
References
- 1.Iczkowski KA, Pantazis CG, Collins J. The loss of expression of CD44 standard and variant isoforms is related to prostatic carcinoma development and tumor progression. J Urol Pathol. 1997;6:119–129. [Google Scholar]
- 2.Iczkowski KA, Bai S, Pantazis CG. Prostate cancer overexpresses CD44 variants 7–9 at the messenger RNA and protein level. Anticancer Res. 2003;23:3129–40. [PubMed] [Google Scholar]
- 3.Omara-Opyene AL, Qiu J., Shah GV, Iczkowski KA. Prostate cancer invasion is influenced more by expression of a CD44 isoform including variant 9 than by Muc18. Lab Invest. 2004;84:894–907. doi: 10.1038/labinvest.3700112. [DOI] [PubMed] [Google Scholar]
- 4.Vis AN, van Rhijn BW, Noordzij MA, Schroder FH., van der Kwast TH. Value of tissue markers p27(kip1), MIB-1, and CD44s for the pre-operative prediction of tumour features in screen-detected prostate cancer. J Pathol. 2002;197:148–54. doi: 10.1002/path.1084. [DOI] [PubMed] [Google Scholar]
- 5.Vis AN, Noordzij MA, Fitoz K, Wildhagen MF, Schroder FH, van der Kwast TH. Prognostic value of cell cycle proteins p27(kip1) and MIB-1, and the cell adhesion protein CD44s in surgically treated patients with prostate cancer. J Urol. 2000;164:2156–61. [PubMed] [Google Scholar]
- 6.Harrison GM, Davies G, Martin TA, Mason MD, Jiang WG. The influence of CD44v3-v10 on adhesion, invasion and MMP-14 expression in prostate cancer cells. Oncol Rep. 2006;15:199–206. [PubMed] [Google Scholar]
- 7.Mokhtari MJ, Motamed N, Shokrgozar MA. Evaluation of silibinin on the viability, migration and adhesion of the human prostate adenocarcinoma (PC-3) cell line. Cell Biol Int. 2008;32:888–92. doi: 10.1016/j.cellbi.2008.03.019. [DOI] [PubMed] [Google Scholar]
- 8.Xi X, Agarwal R. Silibinin decreases prostate-specific antigen with cell growth inhibition via G1 arrest, leading to differentiation of prostate carcinoma cells: implications for prostate cancer intervention. Proc Natl Acad Sci USA. 1999;22(96):7490–5. doi: 10.1073/pnas.96.13.7490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flaig TW, Su LJ, Harrison G, Agarwal R, Glodé LM. Silibinin synergizes with mitoxantrone to inhibit cell growth and induce apoptosis in human prostate cancer cells. Int J Cancer. 2007;120:2028–33. doi: 10.1002/ijc.22465. [DOI] [PubMed] [Google Scholar]
- 10.Raina K, Rajamanickam S, Singh RP, Deep G, Chittezhath M, Agarwal R. Stage-specific inhibitory effects and associated mechanisms of silibinin on tumor progression and metastasis in transgenic adenocarcinoma of the mouse prostate model. Cancer Res. 2008;68:6822–30. doi: 10.1158/0008-5472.CAN-08-1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen PN, Hsieh YS, Chiou HL, Chu SC. Silibinin inhibits cell invasion through inactivation of both PI3K-Akt and MAPK signaling pathways. Chem Biol Interact. 2005;20(156):141–50. doi: 10.1016/j.cbi.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 12.Chu SC, Chiou HL, Chen PN, Yang SF, Hsieh YS. Silibinin inhibits the invasion of human lung cancer cells via decreased productions of urokinas-plasminogen activator and matrix metalloproteinase-2. Mol Carcinog. 2004;40:143–9. doi: 10.1002/mc.20018. [DOI] [PubMed] [Google Scholar]
- 13.Abdel-Latif MM, Windle HJ, Fitzgerald KA, Ang YS, Eidhin DN, Li-Weber M, Sabra K, Kelleher D. Helicobacter pylori activates the early growth response 1 protein in gastric epithelial cells. Infect Immun. 2004;72:3549–60. doi: 10.1128/IAI.72.6.3549-3560.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang K, Handorean AM, Iczkowski KA. MicroRNA 373 and 520c are downregulated in prostate cancer, suppress CD44 translation, and modulate prostate cancer, suppress CD44 translation, and modulate prostate cancer cell invasion. Int J Clin Exp Pathol 2008, in Press. Prostate. 1997;31:77–83. [PMC free article] [PubMed] [Google Scholar]
- 15.Iczkowski KA, Omara-Opyene AL, Kulkarni TR, Pansara M, Shah GV. Paracrine calcitonin in prostate cancer is linked to CD44 variant expression and invasion. Anticancer Res. 2005;25:2075–83. [PubMed] [Google Scholar]
- 16.Harada N, Mizoi T, Kinouchi M, Hoshi S, Shiiba K, Sasaki I, Matsuno S. Introduction of antisense CD44s cDNA downregulates expression of overall CD44 isoforms and inhibits tumor growth and metastasis in highly metastatic colon carcinoma cells. Int J Cancer. 2001;91:67–75. doi: 10.1002/1097-0215(20010101)91:1<67::aid-ijc1011>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 17.Flaig TW, Gustafson DL, Su LJ, Zirrolli JA, Crighton F, Harrison GS, Pierson AS, Agarwal R, Glodé LM. A phase I and pharmacokinetic study of silybin-phytosome in prostate cancer patients. Invest New Drugs. 2007;25:139–46. doi: 10.1007/s10637-006-9019-2. [DOI] [PubMed] [Google Scholar]
- 18.Livak KL, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-ΔΔCT) method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 19.Vollmann H, Wolfel S, Ohneseit P, Stransky E, Vonthein R, Wick W, Meyermann R, Simon P. Differential expression of Egr1 and activation of microglia following irradiation in the rat brain. Strahlenther Onkol. 2001;183:248–55. doi: 10.1007/s00066-007-1664-7. [DOI] [PubMed] [Google Scholar]
- 20.Iczkowski KA, Omara-Opyene AL, Shah GV. The predominant CD44 splice variant in prostate cancer binds fibronectin, and calcitonin stimulates its expression. Anticancer Res. 2006;26:2863–72. [PubMed] [Google Scholar]
- 21.Miyake H, Hara I, Okamoto I, Gohji K, Yamanaka K, Arakawa S, Saya H, Kamidono S. Interaction between CD44 and hyaluronic acid regulates human prostate cancer development. J Urol. 1998;160:1562–6. [PubMed] [Google Scholar]
- 22.Fitzgerald KA, O'Neill LA. Characterization of CD44 induction by IL-1: a critical role for Egr-1. J Immunol. 1999;162:4920–7. [PubMed] [Google Scholar]
- 23.Eid MA, Kumar V, Iczkowski KA, Bostwick DG, Tindall DJ. Expression of early growth response genes in human prostate cancer. Cancer Res. 1998;58:2461–8. [PubMed] [Google Scholar]
- 24.Foster LC, Wiesel P, Huggins GS, Panares R, Chin MT, Pellacani A, Perrella MA. Role of activating protein-1 and high mobility group-I(Y) protein in the induction of CD44 gene expression by interleukin-1beta in vascular smoooth muscle cells. FASEB J. 2000;14:368–78. doi: 10.1096/fasebj.14.2.368. [DOI] [PubMed] [Google Scholar]
- 25.Orian-Rousseau V, Morrison H, Matzke A, Kastilan T, Pace G, Herrlich P, Ponta H. Hepatocyte growth factor-induced Ras activation requires ERM proteins linked to both CD44v6 and F-actin. Mol Biol Cell. 2007;18:76–83. doi: 10.1091/mbc.E06-08-0674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang SZ, Abdulkadir SA. Early growth response gene 1 modulates androgen receptor signaling in prostate carcinoma cells. J Biol Chem. 2003;278:39906–39911. doi: 10.1074/jbc.M307250200. [DOI] [PubMed] [Google Scholar]
- 27.Robbins EW, Travanty EA, Yang K, Iczkowski KA. MAP kinase pathways and calcitonin influence CD44 alternate isoform expression in prostate cancer cells. BioMed Central Cancer. 2008;8:260. doi: 10.1186/1471-2407-8-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brooks KJ, Coleman EJ, Vitetta ES. The antitumor activity of an anti-CD54 antibody in SCID mice xenografted with human breast, prostate, non-small cell lung, and pancreatic tumor cell lines. Int J Cancer. 2008;123:2438–45. doi: 10.1002/ijc.23793. [DOI] [PubMed] [Google Scholar]
- 29.Du B, Fu C, Kent KC, Bush H, Jr, Schulick AH, Kreiger K, Collins T, McCaffrey TA. Elevated Egr-1 in human atherosclerotic cells transcriptionally represses the transforming growth factor-beta type II receptor. J Biol Chem. 2000;275:39039–47. doi: 10.1074/jbc.M005159200. [DOI] [PubMed] [Google Scholar]


