Abstract
Accumulating evidence indicates that alterations in the IGF axis contribute to the development of chemo- and radio-resistant, advanced-stage cancers. Additionally, they contribute to hormonal insensitivity in adenocarcinomas such as those derived from prostate and breast. The ligands, IGF-I and IGF-II, along with their receptors, IGF-IR and IGF-IIR, have been implicated in a wide range of disease. Activation and subsequent signal transduction through the receptors is attenuated, and/or potentiated, by the interactions of IGF axis ligands, IGF-I/II, with the high affinity IGF-binding proteins 1 to 6 (IGFBP1-6). New evidence indicates that the IGFBPs, irrespective of ligand interactions, correlate with the development and metastatic behavior of several cancers. Increased expression of insulin-like growth factor binding protein 2 (IGFBP-2) is found in advanced cancers of the ovary, breast, stomach, adrenal gland, bladder, CNS, and prostate. Further, IGFBP-2 seemingly has ligand-independent effects that participate in the development and dissemination of advanced cancer cells. As such, IGFBP-2 can assist in the development of the lethal phenotype for some cancers. While several reports have shown an important role for IGFBP-2 in the development of androgen insensitivity and the proliferation of AI PCa cells in vivo, these studies have not tested a role for IGFBP-2 in the metastatic spread of AI PCa cells. Additionally, the mechanism of IGFBP-2 action in these events has not been elucidated. The redundancy and abundance of the IGFBPs have precluded a clear understanding of the means by which IGFBP-2 signals. Components of these signaling pathways, particularly IGFBP-2, are being evaluated currently in clinical trials.
Keywords: Insulin-like growth factor, IGF; prostate cancer, PCa; androgen insensitivity, AI; androgen sensitive, AS; neoplasm, bone, metastasis
Introduction
It has been recognized for some time that steroid hormones contribute, either directly or by a poorly defined contributory manner, to the initiation, promotion, and progression of tumors to an advanced, more aggressive, and malignant state [1, 2]. It was realized that certain tumors are hormone-sensitive. This was exploited for the design of the first effective clinical treatments for advanced breast and prostate cancers (PCa). [3–9]. The presence of steroid hormone receptors is the most widely used prognostic marker for breast cancer responsiveness to anti-estrogen therapy. Indeed, more than 60 years ago Charles Huggins proposed and espoused hormone ablation as an effective therapy for men with advanced PCa. Prevailingly for patients, this extended lifespan and reduced metastasis-associated pain [10, 11]. Huggins' approach evolved into some version of hormone blockade or ablation, now the mainstay clinical treatment for advanced, metastatic PCa [12]. Unfortunately, chemical/surgical castration or combined androgen blockade eventually results in the development of hormonally insensitive, very aggressive PCa. Thus, a search continues for new molecular targets of which can be exploited for slowing progression to an advanced, androgen insensitive (AI) state.
The members of growth hormone (GH)-insulin-like growth factor (IGF) axis possess pleotropic actions in cell proliferation [13, 14], growth [15–18], survival [17–23], angiogenesis [18, 23, 24], chemoresistance [25] and signaling cross-talk to both steroid hormone receptors and other growth factor receptor signaling cascades [3, 13, 19, 24, 26–30]. In the proper context, these molecules are central to disregulated processes in the development and progression of neoplasias from a wide array of tissues. The IGF axis consists of two ligands (IGF-I and IGF-II), two receptors (IGF1R and IGF2R), as well as a family of closely related IGF binding proteins (IGFBP 1–6) of which are distinct from the CCN proteins [31]. Altered expression in IGF axis members is generally considered associated with the development of several cancers including prostate [13, 32]. Elevated serum levels of IGF-1 have been associated with increased risk for developing breast cancer in women [28, 33–35], colorectal cancer [36–39], and prostate cancers [36, 40–45], as well as enhancing the likelihood of developing additional primary tumors from head and neck cancer [46, 47]. In breast cancer patients, decreased serum ratios of free/total IGF-II correlated with primary tumor size [48], further implicating the more stable, complexed form of the ligand as the critical compound. The ligands, IGF-I and -II show similar evidence of altered expression in both prostate cancer tissues and cell lines [49]. Generally speaking, the level of IGF ligands appears to increase in carcinoma cell lines with aggressiveness [50, 51]. In normal tissue, the expression pattern of these ligands is predominately stromal, but in cancer their stromal expression is enhanced, combined with some epithelial expression [13, 49]. In the isogenic cell lines of the LNCaP human PCa progression model, IGF-II secretion increases from LNCaP to C4-2 and remains stable in the bone-adapted cell line, C4-2B4 [50]. However, IGF-I expression is not detected in LNCaP to C4-2 while C4-2B4 cells express significantly detectable IGF-I [51]. Furthermore, in both the LNCaP and LAPC-9 xenograft models, succession from AS to AI is accompanied by a dramatic increase in IGF-I and IGF-IR, as well as alterations in additional IGF-axis members [52]. Elevated IGF-II levels increase aggressiveness of MCF-7 cells both in vitro and in vivo [48, 53–56]. Synonymously, reduced IGF-1 and II levels inhibit PC-3 prostate cancer cell growth [57–59].
Based upon the co-expression of IGFBPs, IGFs, and steroid hormone receptors [19, 20, 35, 54, 60, 61] the role of the IGF type I receptor (IGF1R) in cancer development and progression is tissue dependent as well as contextually dependent. In part this is because the receptor has a dual role of controlling cell proliferation or cell differentiation. The IGF1R is at least somewhat produced in almost all expression studies for human benign and malignant tissues, and is infrequently lost.
Insulin-Like Growth Factor Binding Protein-2 (IGFBP-2) has garnered interest as a candidate target/molecule for PCa development and progression [62]. In addition to the possible targeting of IGFBP-2 to prevent the progression to AI PCa, IGFBP-2 may have utility in clinical screening for the detection of both advanced PCa and AI PCa. Herein we briefly summarize the evidence for ligand-independent effects of IGFBPs with an emphasis on the role of IGFBP-2 in cancer progression. Based on the literature and personal experience, we describe the ability of IGFBP-2 (1) to promote the survival of PCa cells in a castrate environment, (2) to facilitate progression to AI, and (3) to possibly enhance the aggressive behavior of AI PCa cells. By this, IGFBP-2 maybe an important driving force in the development of metastatic, hormonally refractive, bone-colonizing PCa.
IGFBPs
Six well characterized IGFBPs (1–6) are known, ranging in molecular weights from 24 kDa (IGFBP-4) to 45 kDa (IGFBP-3). All six IGFBPs have affinities for IGF-I and IGF-II in the same order of magnitude as the ligands have for IGFIR [63–69]. The IGFBPs are best understood to regulate IGF bioavailability and to maintain the half-life of circulating IGF-I/II in all tissues, including that of the prostate [70]. Based on physiological context (i.e. cell type, growth factor milieu present, etc), individual IGFBPs may act to either attenuate or potentiate IGF signaling. Thus, the action of IGFBPs is described classically as “IGF-dependent”. Via poorly understood mechanisms, IGFBPs clearly extend the serum half-life of IGFs and their resulting presentation to cognate receptors [63, 71, 72]. This can result in pleiotropic but tissue specific signaling cascades and effects observed for the IGFs [63, 71, 72]. In contrast to their involvement in signal promotion, some evidence indicates that IGFBPs participate in the scavenging of free, bioactive IGFs thereby dampening the signaling responses [65, 73, 74].
Ligand-dependent Actions of IGFBPs and Cancer
Although its expression is not detected in PCa tissue [75], serum IGFBP-1 levels are significantly greater in patients with PCa (mean = 23.7ng/mL) as compared to healthy individuals of related age (mean = 14.4ng/mL) [76]. Interestingly, however, IGFBP-1 has been shown to modulate the bioavailability of IGF-I and decreases LNCaP cell growth in vitro [16]. Because IGFBP-1 is nutritionally regulated [77], the physical condition of the patients from which the sera was derived in the prior study may be the cause for this discrepancy. Here, patients in a fasting, or nutritionally poor, condition would allow for misinterpretation. Its potential inhibitory effects displayed in Ngo et al. proffer a therapeutic role for IGFBP-1, but there is no evidence for its expression in the prostate or prostate cancer [75]. Additional evidence for a disease-inhibitory role of IGFBP-1 extends beyond PCa. The risk of colorectal cancer is inversely correlated with increased IGFBP-1 levels [78].
As discussed above, the expression of IGFBP-2 increases during prostate disease progression from benign prostatic hyperplasia (BPH) to metastatic prostate cancer [79]. This trend continues with increased IGFBP-2 expression in AI PCa [80]. IGFBP-2 promotes glioma cell invasion [14] and increased IGFBP-2 levels are found in the sera of patients with aggressive ovarian cancer [81]. Elevated IGFBP-2 levels positively correlate with the presence of cancer antigen 125 (CA 125), a putative cancer biomarker [81]. In this regard, IGFBP-2 holds promise as a potential biomarker for the screening of such cancers. By contrast, IGFBP-2 also may have anti-tumorigenic properties. Elevated IGFBP-2 levels were inversely correlated with the risk of colorectal cancer [78] as well as primate mammary epithelial cell proliferation and survival [82, 83].
IGFBP-3 expression decreases during the progression from BPH to metastatic PCa [79]. IGF/IGFBP-3 ratios indicate a man's relative risk for developing prostate cancer [36, 41], while plasma concentrations of IGFBP-3 hold promise as a predictor of advanced stage disease [84]. Since the risk for non-small cell lung cancer (NSCLC) is correlated to a particular polymorphic variation in the IGFBP-3 gene [85], genetic polymorphisms may explain its role in the development of cancer. In contrast to PCa, increased serum levels of IGFBP-3 portend a higher risk of developing breast cancer (BCa) in premenopausal women [34, 82, 86–88]; were positively correlated with BCa tumor size and increased in ER/PR-negative patients [88]; and finally, were reported in patients diagnosed with colorectal cancer [89]. This is controversial for colorectal malignancies however, as other groups report an inverse correlation between IGFBP-3 levels and disease risk [32]. It has been suggested that the discrepancies between these data resulted from differential techniques in assaying for intact and/or proteolytically cleaved IGFBP-3 [78]. The discrepancy may also be the result of the actual IGFBP-3 levels in patients at risk versus those with colon cancer.
IGFBP-4 has been detected in endothelial cells [90], spinal cord [91], bone mesenchyme [92–95], and human fibroblasts [96–98], but seems to be absent in the normal protate [99–101]. Tennant et al. detected IGFBP-4 in prostate epithelial cell primary cultures and observed a decrease in expression in the more aggressively tumorigenic derivative cell lines [102]. However, other groups reported LNCaP cells display no IGFBP-4 protein and low mRNA expression [103, 104]. Prostatic stromal cell primary cultures have shown some expression of IGFBP-4 [105]. IGFBP-4 expression is seen in a variety of cancers including neuroblastoma [106, 107], osteosarcoma [95, 108], and that of the breast [109], lung [110], and prostate [100–102, 104, 111, 112]. In vitro studies indicate an IGF-inhibitory action for IGFBP-4 [65, 77, 113–115]. In an IGF-dependent manner, IGFBP-4 was also shown to inhibit the invasion and proliferation of colon cancer cells [116], osteosacroma growth [117], and delayed the onset of prostate tumor formation [15]. These data strongly implicate IGFBP-4 as a negative modulator for PCa growth.
IGFBP-5 is expressed in various cell types including human fibroblasts [118], ovarian granulose cells [50], and articular chondrocytes [119]. Further, expression of IGFBP-5 has been found in the rat ventral prostate, as well as various PCa model systems including LNCaP cells [120], PC3 cells [120], and CWR22 human prostate xenografts [49]. IGFBP-5 has been found to inhibit the actions of IGF-I in osteosarcoma [121] and smooth muscle cells [122]. However it appears to potentiate the effects of IGF-I in prostatic disease, consistently [118, 123]. It mimics IGFBP-2 expression as it increases with the progression of prostatic disease from BPH to metastatic PCa [49, 102, 111, 112, 123]. Through IGF-I, IGFBP-5 accelerates the progression of prostate cancer cells to AI [124]. Interestingly, the expression of IGFBP-5 appears to be stromally derived and not a direct result of PCa expression [102]. Therefore, the generation of a reactive stroma or microenvironment rich in IGFBP-5 may be a prerequisite for rapid PCa establishment and growth.
Seemingly, IGFBP-6 possesses an initial anti-proliferative role that is altered during disease progression to become an opposite stimulatory role. Although mechanistically unclear, IGFBP-6 can stimulate osteosarcoma cell growth and survival [125], while it hinders IGF-2 signaling in healthy tissue [107, 126], likely reducing cell growth. The aggressive nature of P69 cell lines is inversely correlated with IGFBP-6 expression [127]. P69, PC-3, ALVA-31, and DU145 prostate cancer cell lines all make IGFBP-6 [103, 127, 128] while LNCaP cells do not [103, 104]. IGFBP-6 is present in ovarian tissue [64], fibroblasts [126], primary cultures of prostate epithelial cells [127], and osteoblasts [129, 130]. Cancers including osteosarcoma [125], neuroblastoma [106, 107], and that of the breast [109] and prostate [104, 111, 127, 128, 131] produce IGFBP-6.
The molecular context in which the cancer lays includes the dynamic interplay between the tumor cells and the neighboring microenvironment. Perhaps the functions of IGFBP-2, along with the actions of other IGF-axis members, depend more on this context than previously thought.
Ligand-independent actions of IGFBPs and Cancers
IGFBPs also act in manners that have no obligatory requirement for ligand or, at least, signal transduction through the IGFIR. Accordingly, the ability of IGFBPs to act via these non-classical pathways is referred to as IGF-, IGFIR-, or ligand-independent. Autocrine production of IGF axis members in cancer cells complicates the analysis of IGFBP ligand-independent effects. Nonetheless, evidence supports the ability of IGFBPs to act through non-IGFIR mediated pathways that alter cell extracellular matrix (ECM) production and cell surface interactions [132–134], nuclear localization [135], apoptosis [136], growth [136], and the modulation of transcriptional activity [137].
Frommer et al. showed that exogenously added recombinant IGFBP-2 induced apoptosis in the IGFIR-null human BCa cell line (Hs578T). Microarray and RT-PCR data generated by the same group indicates that IGFBP-2 induces the expression of genes that regulate cellular proliferation, adhesion, and apoptosis [138]. Butt et al. [63] demonstrated that IGFBP-3 induced growth inhibition and apoptosis of human breast cancer cells even when blocking signal transduction through the IGF-IR. Additional data suggests the presence of IGFBP-3 specific membrane receptors on the surfaces of some cell types, including human breast cancer epithelial cells [139], human airway smooth muscle cells (ASMs) [136], and prostate cancer epithelial cells [140]. Interestingly, the TGFβ type V receptor can seemingly interact with both TGF-β1 and IGFBP-3 [141–143]. In the absence of IGFs, IGFBP-4 inhibits anchorage-independent colony formation of colon cancer cells [116]. Furthermore, data to supports that bone formation is stimulated by IGFBP-5 in an IGF-independent fashion [144].
Ligand-independent actions of IGFBPs and PCa
Normally, post-translational modifications of IGFBPs alter their interaction with ligand, however, this may also change the ligand independent functions of the IGFBPs. Such modifications include phosphorylation (IGFBP-1, -3, and –5), N-glycosylation (IGFBP-3, and –4), and proteolysis (IGFBP1–6). Of these modifications, IGFBP proteolysis may be the most crucial as it acts to (1) decrease the magnitude of intact IGFBP present [145], (2) decrease the affinity of a given IGFBP for IGF-I and/or IGF-II (thus increasing the concentration of free, bioactive IGFs) [77], and/or (3) liberate otherwise cryptic domains, resulting in additional IGF-independent effects not observed in intact IGFBPs [146, 147]. Thus, the ability of IGFBPs to undergo regulated proteolysis has important implications regarding their ability to act in both an IGF-dependent and IGF-independent manner. Moreover, the inability to regulate levels of free, bioactive IGFs via IGFBP proteolysis, or the inability to produce bioactive IGFBP fragments, may have important consequences in regard to disease progression.
As an example and introduction to this topic we use IGFBP-3, which has been studied extensively in this context. Independent of ligand, IGFBP-3 has been shown to induce apoptosis in PC-3 cells in a [148]. In support this ligand independent action, ligand binding mutants still demonstrated IGFBP-3-induced apoptosis [59]. As previously eluded to, IGFBP-3 maybe signaling through the TGFβ type V receptor [140], and possibly via other specific, yet unidentified cell surface proteins [136, 139, 140]. Rajah et al. showed that the pro-apoptotic effects of TGF-β1 in PCa required IGFBP-3 stimulation, and that IGFBP-3 still induced apoptosis in IGF-receptor null mouse fibroblasts [140]. Other IGF-independent growth effects of IGFBP-3 occur via the RXR nuclear receptor and what maybe a IGFBP-3-specific cell surface receptor [63]. Cathepsins [120], matrix metalloproteinases [149], and PSA [150] are all proteases of which cleave IGFBP-3 at various sites. The proteolysis results in the production of lower molecular weight fragments that have a decreased affinity for ligand [151]. In this way, proteolysis may lead to the increased availability of IGFs to interact with their receptors.
IGFBP-2 and progression to AI PCa
It remains to be determined whether increased IGFBP-2 expression drives progression to AI-PCa or is an adaptive response to an androgen depleted environment. A comparison of the androgen sensitive CWR22 and androgen insensitive CWR22R xenograft tissues provided the early evidence for a role of IGFBP-2 in progression to AI-PCa. In these tumor xenografts IGFBP-2 was over-expressed greater than two fold in the CWR22R tumors [152]. Here, patient samples expressed strong IGFBP-2 staining in 100% of hormone refractory PCa versus 36% of primary PCa and in 0% of benign prostatic hyperplasia specimens. Subsequent studies validated these findings [62, 80, 153–155], thereby supporting that increased IGFBP-2 expression is associated with progression to AI. Tissue microarray data from repeated biopsies of intermittent androgen ablation trials indicated that IGFBP-2 protein levels are increased with time and number of cycles following androgen ablation, especially in samples derived from patients converting to AI PCa [80]. Furthermore, IGFBP-2 mRNA levels were increased in both castrated rats [99] and in rats undergoing chemical castration using 10 mg/kg bicalutamide [52]. In these studies the highest levels of IGFBP-2 mRNA were coincident with peak levels of apoptosis, suggesting that increased IGFBP-2 expression is important for the expansion of AI clones in a castrate environment. This assertion is supported by a report indicating that IGFBP-2 stimulates the proliferation of AI PCa cells [154]. Also, tumors derived from LNCaP cells selected to overexpress IGFBP-2 displayed enhanced rebound following castration as well as increased tumor size and PSA levels post-castration [80]. Treatment of mice bearing LNCaP tumors with IGFBP-2 anti-sense oligonucleotides (ASOs) resulted in decreased PSA levels and tumor volumes in the castrate environment. This lends support to the idea that the tumor cells have adapted or acquired a dependence upon the expression of IGFBP-2. Thus, it appears that IGFBP-2, in addition to promoting progression to AI, may be an excellent molecular target for preventing this deadly process. However, the actual molecular mechanism by which IGFBP-2 acts in the genesis of AI-PCa is unclear. Elucidation of this adaptive or promotional pathway may provide other significant targets for therapeutic development.
A role for IGFBP-2 in the metastatic phenotype of AI PCa?
Classically, IGFBP-2 functions to modulate of IGF-I and IGF-II induced cell signaling. Evidence indicates that increased levels of IGFBP-2 are correlated both with the presence of advanced disease and may promote several steps of the metastatic process (see table). For example, invasive breast cancer was shown to express higher amounts of IGFBP-2 compared to carcinomas in situ [156] and IGFBP-2 facilitates the migration of breast cancer cells in response to IGF signaling [157]. Similarly, IGFBP-2 over expression is positively associated with advanced gastric [29] and ovarian cancers [158]. In vitro, the invasive capability of glioblastoma [159] and ovarian cancer cells [158] is increased significantly with over expression of IGFBP-2.
Table 1.
Evidence Relating IGFBP-2 to Advanced Cancer
| In vitro | In vivo | |
|---|---|---|
| Adrenal Cancer | no reports | Increased in the serum of patients [165] |
| Increased expression in malignant vs. benign human tumors [166] | ||
| Bladder Cancer | Increased invasion of bladder cancer cells [167] | Increased expression in invasive disease [168] |
| Breast Cancer | Denotes anti-estrogen resistant BCa cell lines [169] | Increased expression in invasive disease [156] |
| CNS cancers | Increased migration of SHEP cells [14] | Correlated with decreased survival [171] |
| Increases glioma cell invasion [170] | Increased with tumor grade [172] | |
| Assoc. with expression of Invasion genes [159] | Increased with progression [173] | |
| Gastric Cancer | Expressed in multiple cancer cell lines [174] | Increased in peritoneal metastasis [176] |
| Associated with increased proliferation [175] | Associated with advanced disease [137] | |
| Ovarian Cancer | Increased invasion [175] | Increased in serum of ovarian cancer patients [177] |
| Increased in ovarian tissue during progression [178] | ||
| Increased in, and associated with increased risk of advanced disease [175] [179] | ||
| Prostate Cancer | Increased in metastatic, AI PCa cell lines [51] | Serum levels have inverse correlation to advanced disease [180] |
| Loss of proteolytic degradation in AI PCa [145] | Increased expression in AI PCa [75] | |
| Decreased time to attain AI [80] |
Finally, IGFBP-2 negatively modulates cell adhesion [133], perhaps facilitating the entry of metastatic cells into a distal site. In contrast to these reports, there are examples of IGFBP-2 negatively regulating the malignant behavior of breast cancer cells [132, 157]. This supports the pleiotropic and tissue-specific functions of IGFBPs as described previously [64, 72, 77, 160]. These data notwithstanding, the molecular determinants associated with the metastatic process, which clearly vary by cellular context, are only starting to be elucidated. To date, however, the majority of accumulated data strongly implies that increased production and/or accumulation of IGFBP-2 is associated with the presence of more clinically aggressive cancers and increased metastatic phenotype in vitro.
Data published recently from our laboratory suggests that IGFBP-2 is proteolyzed by AS PCa cells in the presence of androgen. Therefore, a consequence of castration may be to increase the levels of intact IGFBP-2 [145]. Furthermore, the ability of PCa cells to proteolyze IGFBP-2 in the presence of androgen is lost during progression to AI [145]. The post-castration loss of IGFBP-2 proteolysis would result in increased levels of IGFBP-2, thus providing a local microenvironment suitable for PCa adaptation to high levels of IGFBP-2. This would lead to the aforementioned increased proliferation of PCa cells in an androgen depleted environment [154], and facilitate the subsequent clonal expansion and development of AI PCa [80]. Our work and that of others [51] has shown that AI PCa cells isolated from the bone microenvironment produce higher levels of IGFBP-2. This all argues strongly that IGFBP-2 plays a major role in the development of both AI PCa as well as metastasis to bone.
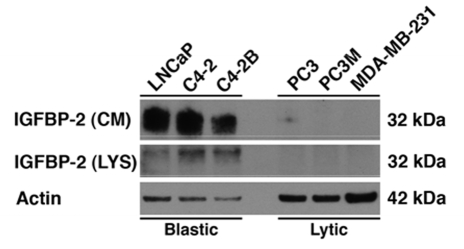
Yet still unclear are the mechanisms involved by which PCa cells promote the formation of osteosclerotic lesions in bone. IGFBP-2 may be correlated with the extent of how osteoblastic or osteolytic these lesions become. In vitro data from our lab suggests that IGFBP-2 is present in both the cell lysate and culture media of osteoblastic prostate cancer cell lines while absent from that of osteolytic cancer cell lines (Figure 1). The presence of IGFBP-2 possibly possesses a causal relationship for the difference in bone turnover among different forms of advanced disease.
Figure 1.
Western blot analysis for IGFBP-2 in both the whole cell lysate (WCL) and conditioned media (CM) of osteoblastic and osteolytic cancer cell lines. Actin served as a loading control for WCL only. Cell lines were cultured in 5% FBS and serum starved for 48hrs before lysis. IGFBP-2 is present in the osteoblastic/sclerotic prostate cancer cell lines (LNCaP, C4-2, and C4-2B4) while absent in the osteolytic prostate cancer cell lines (PC3 and PC3M) and breast cancer cell line (MDA-MB-231).
Any role that IGFBP-2 has in facilitating dissemination of metastatic cancer cells almost certainly arises from the combination of ligand dependent and independent actions. It is well known that increased IGF signaling can stimulate the aggressive behavior of a variety of cancer cells, including neuroblastoma [161] and PCa [162, 163], as well as numerous others. Indeed, prostate cancer cells of the LNCaP progression series express progressively more IGF-II [164] and begin to express IGF-I following bone adaptation [51]. Further, we have shown that expression of IGF ligands increases the survival of AI-PCa cells in an androgen receptor independent manner [19]. So, in the cases where increased IGFBP-2 production/activity facilitates IGF signaling, it is conceptually straightforward to conceive how this would contribute to metastasis. On the other hand, the mechanism by which IGFBP-2 acts independently of IGF signaling to facilitate metastasis is not well understood, but may be rooted in the presence of a putative heparin-binding domain [14], as well as an RGD sequence in the C-terminus of IGFBP-2. These two domains were critical for the ability of IGFBP-2 to interact with ECM components [14] and cell surface integrins [132, 133]. Therefore, these two domains may allow IGFBP-2 to act as a molecular “bridge” between the cell surface and the ECM. Increased expression/accumulation of IGFBP-2 may result in the increased ability of a metastatic cell to interact with the local environment, thus facilitating the aggressive behavior of metastatic cells.
Future studies on the role of IGFBP-2 in PCa metastases
Currently, studies are needed to determine (1) if increased levels of IGFBP-2 promote the selection of or adaptation of AI PCa cells, (2) if IGFBP-2 selectively promotes the growth of AI-PCa cells, (3) if IGFBP-2 operates in an IGF-dependent and/or IGF-independent manner to facilitate the metastasis of AI PCa cells, (4) the cell signaling pathways activated by IGFBP-2 binding, (5) if the action of IGFBP-2 in the context of androgen is altered in a castrate environment, (6) the molecular basis for IGFBP-2 induced migration, metastasis, and invasion, and (7) better therapeutic targets for AI PCa and PCa metastasis.
Acknowledgments
This work was supported by NSF IGERT 0221651 and NIH INBRE P20 RR016472 program. The authors wish to thank Drs. Mary C. Farach-Carson and Carlton R. Cooper for their critical reading and suggestions during preparation of the manuscript.
References
- 1.Frasor J, Danes JM, Komm B, Chang KCN, Lyttle CR, Katzenellenbogen BS. Profiling of Estrogen Up- and Down-Regulated Gene Expression in Human Breast Cancer Cells: Insights into Gene Networks and Pathways Underlying Estrogenic Control of Proliferation and Cell Phenotype. Endocrinology. 2003;144:4562–4574. doi: 10.1210/en.2003-0567. [DOI] [PubMed] [Google Scholar]
- 2.Evangelou A, Letarte M, Marks A, Brown TJ. Androgen Modulation of Adhesion and Antiadhesion Molecules in PC-3 Prostate Cancer Cells Expressing Androgen Receptor. Endocrinology. 2002;143:3897–3904. doi: 10.1210/en.2002-220156. [DOI] [PubMed] [Google Scholar]
- 3.Stoll BA. Endocrine therapy in cancer. Practitioner. 1979;222:211–217. [PubMed] [Google Scholar]
- 4.Huggins CB. Propositions in Hormonal Treatment of Advanced Cancers. Jama. 1965;192:1141–1145. doi: 10.1001/jama.1965.03080260029008. [DOI] [PubMed] [Google Scholar]
- 5.Huggins CB. Two principles in endocrine therapy of cancers: hormone-deprival and hormone-interference. Proc Rudolf Virchow Med Soc City N Y. 1964;23:151–164. [PubMed] [Google Scholar]
- 6.Huggins CB. The Hormone-Dependent Cancers. Bull N Y Acad Med. 1963;39:752–757. [PMC free article] [PubMed] [Google Scholar]
- 7.Huggins CB. On hormone-dependent cancers. J R Coll Surg Edinb. 1959;4:191–198. [PubMed] [Google Scholar]
- 8.Huggins C, Hodges CV. Studies on prostatic cancer. I. The effect of castration, of estrogen and androgen injection on serum phosphatases in metastatic carcinoma of the prostate. CA Cancer J Clin. 1972;22:232–240. doi: 10.3322/canjclin.22.4.232. [DOI] [PubMed] [Google Scholar]
- 9.Huggins C. Endocrine methods of treatment of cancer of the breast. J Natl Cancer Inst. 1954;15:1–25. [PubMed] [Google Scholar]
- 10.Huggins C, Hodges CV. Studies on prostate cancer: I. The effect of castration , of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. Cancer Research. 1941;1:293–297. [Google Scholar]
- 11.Huggins C, Stevens RE, Hodges CV. Studies on prostate cancer: II. The effects of castration on advanced carcinoma of the prostate gland. Archives of Surgery. 1941;43:209–223. [Google Scholar]
- 12.Denmeade SR, Isaacs JT. A history of prostate cancer treatment. Nat Rev Cancer. 2002;2:389–396. doi: 10.1038/nrc801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Samani AA, Yakar S, LeRoith D, Brodt P. The role of the IGF system in cancer growth and metastasis: overview and recent insights. Endocr Rev. 2007;28:20–47. doi: 10.1210/er.2006-0001. [DOI] [PubMed] [Google Scholar]
- 14.Russo VC, Schutt BS, Andaloro E, Ymer SI, Hoeflich A, Ranke MB, Bach LA, Werther GA. Insulin-Like Growth Factor Binding Protein-2 Binding to Extracellular Matrix Plays a Critical Role in Neuroblastoma Cell Proliferation, Migration, and Invasion. Endocrinology. 2005;146:4445–4455. doi: 10.1210/en.2005-0467. [DOI] [PubMed] [Google Scholar]
- 15.Damon SE, Maddison L, Ware JL, Plymate SR. Overexpression of an inhibitory insulin-like growth factor binding protein (IGFBP), IGFBP-4, delays onset of prostate tumor formation. Endocrinology. 1998;139:3456–3464. doi: 10.1210/endo.139.8.6150. [DOI] [PubMed] [Google Scholar]
- 16.Ngo TH, Barnard RJ, Leung PS, Cohen P, Aronson WJ. Insulin-like growth factor I (IGF-I) and IGF binding protein-1 modulate prostate cancer cell growth and apoptosis: possible mediators for the effects of diet and exercise on cancer cell survival. Endocrinology. 2003;144:2319–2324. doi: 10.1210/en.2003-221028. [DOI] [PubMed] [Google Scholar]
- 17.Urbanska K, Trojanek J, Del Valle L, Eldeen MB, Hofmann F, Garcia-Echeverria C, Khalili K, Reiss K. Inhibition of IGF-I receptor in anchorage-independence attenuates GSK-3beta constitutive phosphorylation and compromises growth and survival of medulloblastoma cell lines. Oncogene. 2007;26:2308–2317. doi: 10.1038/sj.onc.1210018. [DOI] [PubMed] [Google Scholar]
- 18.Menu E, Jernberg-Wiklund H, De Raeve H, De Leenheer E, Coulton L, Gallagher O, Van Valckenborgh E, Larsson O, Axelson M, Nilsson K, Van Camp B, Croucher P, Vanderkerken K. Targeting the IGF-1R using picropodophyllin in the therapeutical 5T2MM mouse model of multiple myeloma: beneficial effects on tumor growth, angiogenesis, bone disease and survival. Int J Cancer. 2007;121:1857–1861. doi: 10.1002/ijc.22845. [DOI] [PubMed] [Google Scholar]
- 19.Krueckl SL, Sikes RA, Edlund NM, Bell RH, Hurtado-Coll A, Fazli L, Gleave ME, Cox ME. Increased insulin-like growth factor I receptor expression and signaling are components of androgen-independent progression in a lineage-derived prostate cancer progression model. Cancer Res. 2004;64:8620–8629. doi: 10.1158/0008-5472.CAN-04-2446. [DOI] [PubMed] [Google Scholar]
- 20.Guvakova MA, Surmacz E. Overexpressed IGF-I receptors reduce estrogen growth requirements, enhance survival, and promote E-cadherin-mediated cell-cell adhesion in human breast cancer cells. Exp Cell Res. 1997;231:149–162. doi: 10.1006/excr.1996.3457. [DOI] [PubMed] [Google Scholar]
- 21.Van Den Berg CL. Igf-I influence on breast cancer cell survival. Breast Dis. 2003;17:49–59. doi: 10.3233/bd-2003-17106. [DOI] [PubMed] [Google Scholar]
- 22.Linnerth NM, Baldwin M, Campbell C, Brown M, McGowan H, Moorehead RA. IGF-II induces CREB phosphorylation and cell survival in human lung cancer cells. Oncogene. 2005;24:7310–7319. doi: 10.1038/sj.onc.1208882. [DOI] [PubMed] [Google Scholar]
- 23.Kurmasheva RT, Houghton PJ. IGF-I mediated survival pathways in normal and malignant cells. Biochim Biophys Acta. 2006;1766:1–22. doi: 10.1016/j.bbcan.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 24.Strammiello R, Benini S, Manara MC, Perdichizzi S, Serra M, Spisni E, Picci P, Scotlandi K. Impact of IGF-I/IGF-IR circuit on the angiogenetic properties of Ewing's sarcoma cells. Horm Metab Res. 2003;35:675–684. doi: 10.1055/s-2004-814149. [DOI] [PubMed] [Google Scholar]
- 25.Gooch JL, Van Den Berg CL, Yee D. Insulin-like growth factor (IGF)-I rescues breast cancer cells from chemotherapy-induced cell death–proliferative and anti-apoptotic effects. Breast Cancer Res Treat. 1999;56:1–10. doi: 10.1023/a:1006208721167. [DOI] [PubMed] [Google Scholar]
- 26.Wang Y, Kreisberg JI, Ghosh PM. Cross-talk between the androgen receptor and the phosphatidylinositol 3-kinase/Akt pathway in prostate cancer. Curr Cancer Drug Targets. 2007;7:591–604. doi: 10.2174/156800907781662248. [DOI] [PubMed] [Google Scholar]
- 27.Allegra JC, Lippman ME, Thompson EB, Simon R, Barlock A, Green L, Huff KK, Do HM, Aitken SC, Warren R. Relationship between the progesterone, androgen, and glucocorticoid receptor and response rate to endocrine therapy in metastatic breast cancer. Cancer Res. 1979;39:1973–1979. [PubMed] [Google Scholar]
- 28.Haffner MC, Petridou B, Peyrat JP, Revillion F, Muller-Holzner E, Daxenbichler G, Marth C, Doppler W. Favorable prognostic value of SOCS2 and IGF-I in breast cancer. BMC Cancer. 2007;7:136. doi: 10.1186/1471-2407-7-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mochizuki T, Sakai K, Iwashita M. Effects of insulin-like growth factor (IGF) binding protein-3 (IGFBP-3) on endometrial cancer (HHUA) cell apoptosis and EGF stimulated cell proliferation in vitro. Growth Horm IGF Res. 2006;16:202–210. doi: 10.1016/j.ghir.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 30.Liu W, Bloom DA, Cance WG, Kurenova EV, Golubovskaya VM, Hochwald SN. Fak and Igf-Ir Interact to Provide Survival Signals in Human Pancreatic Adenocarcinoma Cells. Carcinogenesis. 2008 doi: 10.1093/carcin/bgn026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grotendorst GR, Lau LF, Perbal B. CCN proteins are distinct from and should not be considered members of the insulin-like growth factor-binding protein superfamily. Endocrinology. 2000;141:2254–2256. doi: 10.1210/endo.141.6.7485. [DOI] [PubMed] [Google Scholar]
- 32.Jenkins PJ, Bustin SA. Evidence for a link between IGF-I and cancer. Eur J Endocrinol. 2004;151:S17–22. doi: 10.1530/eje.0.151s017. [DOI] [PubMed] [Google Scholar]
- 33.Schairer C, Hill D, Sturgeon SR, Fears T, Pollak M, Mies C, Ziegler RG, Hoover RN, Sherman ME. Serum concentrations of IGF-I, IGFBP-3 and c-peptide and risk of hyperplasia and cancer of the breast in postmenopausal women. Int J Cancer. 2004;108:773–779. doi: 10.1002/ijc.11624. [DOI] [PubMed] [Google Scholar]
- 34.Rinaldi S, Peeters PH, Berrino F, Dossus L, Biessy C, Olsen A, Tjonneland A, Overvad K, Clavel-Chapelon F, Boutron-Ruault MC, Tehard B, Nagel G, Linseisen J, Boeing H, Lahmann PH, Trichopoulou A, Trichopoulos D, Koliva M, Palli D, Panico S, Tumino R, Sacerdote C, van Gils CH, van Noord P, Grobbee DE, Bueno-de-Mesquita HB, Gonzalez CA, Agudo A, Chirlaque MD, Barricarte A, Larranaga N, Quiros JR, Bingham S, Khaw KT, Key T, Allen NE, Lukanova A, Slimani N, Saracci R, Riboli E, Kaaks R. IGF-I, IGFBP-3 and breast cancer risk in women: The European Prospective Investigation into Cancer and Nutrition (EPIC) Endocr Relat Cancer. 2006;13:593–605. doi: 10.1677/erc.1.01150. [DOI] [PubMed] [Google Scholar]
- 35.McCarty MF. Androgenic progestins amplify the breast cancer risk associated with hormone replacement therapy by boosting IGF-I activity. Med Hypotheses. 2001;56:213–216. doi: 10.1054/mehy.2000.1152. [DOI] [PubMed] [Google Scholar]
- 36.Weiss JM, Huang WY, Rinaldi S, Fears TR, Chatterjee N, Chia D, Crawford ED, Kaaks R, Hayes RB. IGF-1 and IGFBP-3: Risk of prostate cancer among men in the Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial. Int J Cancer. 2007;121:2267–2273. doi: 10.1002/ijc.22921. [DOI] [PubMed] [Google Scholar]
- 37.Ma J, Stampfer M, Pollak M. RESPONSE: more about: prospective study of colorectal cancer risk in men and plasma levels of insulin-like growth factor (IGF)-I and IGF- binding protein-3. J Natl Cancer Inst. 2000;92:1949. doi: 10.1093/jnci/92.23.1949. [DOI] [PubMed] [Google Scholar]
- 38.Kaaks R, Toniolo P, Akhmedkhanov A, Lukanova A, Biessy C, Dechaud H, Rinaldi S, Zeleniuch-Jacquotte A, Shore RE, Riboli E. Serum C-peptide, insulin-like growth factor (IGF)-I, IGF-binding proteins, and colorectal cancer risk in women. J Natl Cancer Inst. 2000;92:1592–1600. doi: 10.1093/jnci/92.19.1592. [DOI] [PubMed] [Google Scholar]
- 39.Giovannucci E, Pollak M, Platz EA, Willett WC, Stampfer MJ, Majeed N, Colditz GA, Speizer FE, Hankinson SE. Insulin-like growth factor I (IGF-I), IGF-binding protein-3 and the risk of colorectal adenoma and cancer in the Nurses' Health Study. Growth Horm IGF Res. 2000;10 Suppl A:S30–31. doi: 10.1016/s1096-6374(00)90014-5. [DOI] [PubMed] [Google Scholar]
- 40.Yu H, Nicar MR, Shi R, Berkel HJ, Nam R, Trachtenberg J, Diamandis EP. Levels of insulin-like growth factor I (IGF-I) and IGF binding proteins 2 and 3 in serial postoperative serum samples and risk of prostate cancer recurrence. Urology. 2001;57:471–475. doi: 10.1016/s0090-4295(00)01003-7. [DOI] [PubMed] [Google Scholar]
- 41.Winter DL, Hanlon AL, Raysor SL, Watkins-Bruner D, Pinover WH, Hanks GE, Tricoli JV. Plasma levels of IGF-1, IGF-2, and IGFBP-3 in white and African-American men at increased risk of prostate cancer. Urology. 2001;58:614–618. doi: 10.1016/s0090-4295(01)01273-0. [DOI] [PubMed] [Google Scholar]
- 42.Stattin P, Bylund A, Rinaldi S, Biessy C, Dechaud H, Stenman UH, Egevad L, Riboli E, Hallmans G, Kaaks R. Plasma insulin-like growth factor-I, insulin-like growth factor-binding proteins, and prostate cancer risk: a prospective study. J Natl Cancer Inst. 2000;92:1910–1917. doi: 10.1093/jnci/92.23.1910. [DOI] [PubMed] [Google Scholar]
- 43.Li L, Yu H, Schumacher F, Casey G, Witte JS. Relation of serum insulin-like growth factor-I (IGF-I) and IGF binding protein-3 to risk of prostate cancer (United States) Cancer Causes Control. 2003;14:721–726. doi: 10.1023/a:1026383824791. [DOI] [PubMed] [Google Scholar]
- 44.Nam RK, Trachtenberg J, Jewett MA, Toi A, Evans A, Emami M, Narod SA, Pollak M. Serum insulin-like growth factor-I levels and prostatic intraepithelial neoplasia: a clue to the relationship between IGF-I physiology and prostate cancer risk. Cancer Epidemiol Biomarkers Prev. 2005;14:1270–1273. doi: 10.1158/1055-9965.EPI-04-0430. [DOI] [PubMed] [Google Scholar]
- 45.Allen NE, Key TJ, Appleby PN, Travis RC, Roddam AW, Rinaldi S, Egevad L, Rohrmann S, Linseisen J, Pischon T, Boeing H, Johnsen NF, Tjonneland A, Gronbaek H, Overvad K, Kiemeney L, Bueno-de-Mesquita HB, Bingham S, Khaw KT, Tumino R, Berrino F, Mattiello A, Sacerdote C, Palli D, Quiros JR, Ardanaz E, Navarro C, Larranaga N, Gonzalez C, Sanchez MJ, Trichopoulou A, Travezea C, Trichopoulos D, Jenab M, Ferrari P, Riboli E, Kaaks R. Serum insulin-like growth factor (IGF)-I and IGF-binding protein-3 concentrations and prostate cancer risk: results from the European Prospective Investigation into Cancer and Nutrition. Cancer Epidemiol Biomarkers Prev. 2007;16:1121–1127. doi: 10.1158/1055-9965.EPI-06-1062. [DOI] [PubMed] [Google Scholar]
- 46.Slomiany MG, Black LA, Kibbey MM, Day TA, Rosenzweig SA. IGF-1 induced vascular endothelial growth factor secretion in head and neck squamous cell carcinoma. Biochem Biophys Res Commun. 2006;342:851–858. doi: 10.1016/j.bbrc.2006.02.043. [DOI] [PubMed] [Google Scholar]
- 47.Wu X, Zhao H, Do K-A, Johnson MM, Dong Q, Hong WK, Spitz MR. Serum Levels of Insulin Growth Factor (IGF-I) and IGF-Binding Protein Predict Risk of Second Primary Tumors in Patients with Head and Neck Cancer. Clin Cancer Res. 2004;10:3988–3995. doi: 10.1158/1078-0432.CCR-03-0762. [DOI] [PubMed] [Google Scholar]
- 48.Singer CF, Mogg M, Koestler W, Pacher M, Marton E, Kubista E, Schreiber M. Insulin-Like Growth Factor (IGF)-I and IGF-II Serum Concentrations in Patients with Benign and Malignant Breast Lesions: Free IGF-II Is Correlated with Breast Cancer Size. Clin Cancer Res. 2004;10:4003–4009. doi: 10.1158/1078-0432.CCR-03-0093. [DOI] [PubMed] [Google Scholar]
- 49.Gregory CW, Kim D, Ye P, D'Ercole AJ, Pretlow TG, Mohler JL, French FS. Androgen receptor up-regulates insulin-like growth factor binding protein-5 (IGFBP-5) expression in a human prostate cancer xenograft. Endocrinology. 1999;140:2372–2381. doi: 10.1210/endo.140.5.6702. [DOI] [PubMed] [Google Scholar]
- 50.Liu XJ, Malkowski M, Guo Y, Erickson GF, Shimasaki S, Ling N. Development of specific antibodies to rat insulin-like growth factor-binding proteins (IGFBP-2 to -6): analysis of IGFBP production by rat granulosa cells. Endocrinology. 1993;132:1176–1183. doi: 10.1210/endo.132.3.7679972. [DOI] [PubMed] [Google Scholar]
- 51.Rubin J, Chung LW, Fan X, Zhu L, Murphy TC, Nanes MS, Rosen CJ. Prostate carcinoma cells that have resided in bone have an upregulated IGF-I axis. Prostate. 2004;58:41–49. doi: 10.1002/pros.10299. [DOI] [PubMed] [Google Scholar]
- 52.Nickerson T, Chang F, Lorimer D, Smeekens SP, Sawyers CL, Pollak M. In vivo progression of LAPC-9 and LNCaP prostate cancer models to androgen independence is associated with increased expression of insulin-like growth factor I (IGF-I) and IGF-I receptor (IGF-IR) Cancer Res. 2001;61:6276–6280. [PubMed] [Google Scholar]
- 53.Daly RJ, Harris WH, Wang DY, Darbre PD. Autocrine production of insulin-like growth factor II using an inducible expression system results in reduced estrogen sensitivity of MCF-7 human breast cancer cells. Cell Growth Differ. 1991;2:457–464. [PubMed] [Google Scholar]
- 54.Brunner N, Moser C, Clarke R, Cullen K. IGF-I and IGF-II expression in human breast cancer xenografts: relationship to hormone independence. Breast Cancer Res Treat. 1992;22:39–45. doi: 10.1007/BF01833332. [DOI] [PubMed] [Google Scholar]
- 55.Noble A, Towne C, Chopin L, Leavesley D, Upton Z. Insulin-like growth factor-II bound to vitronectin enhances MCF-7 breast cancer cell migration. Endocrinology. 2003;144:2417–2424. doi: 10.1210/en.2002-221138. [DOI] [PubMed] [Google Scholar]
- 56.Lee AV, Darbre P, King RJ. Processing of insulin-like growth factor-II (IGF-II) by human breast cancer cells. Mol Cell Endocrinol. 1994;99:211–220. doi: 10.1016/0303-7207(94)90010-8. [DOI] [PubMed] [Google Scholar]
- 57.Csernus VJ, Schally AV, Kiaris H, Armatis P. Inhibition of growth, production of insulin-like growth factor-II (IGF-II), and expression of IGF-II mRNA of human cancer cell lines by antagonistic analogs of growth hormone-releasing hormone in vitro. Proc Natl Acad Sci U S A. 1999;96:3098–3103. doi: 10.1073/pnas.96.6.3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Plonowski A, Schally AV, Letsch M, Krupa M, Hebert F, Busto R, Groot K, Varga JL. Inhibition of proliferation of PC-3 human prostate cancer by antagonists of growth hormone-releasing hormone: lack of correlation with the levels of serum IGF-I and expression of tumoral IGF-II and vascular endothelial growth factor. Prostate. 2002;52:173–182. doi: 10.1002/pros.10105. [DOI] [PubMed] [Google Scholar]
- 59.Hong J, Zhang G, Dong F, Rechler MM. Insulin-like Growth Factor (IGF)-binding Protein-3 Mutants That Do Not Bind IGF-I or IGF-II Stimulate Apoptosis in Human Prostate Cancer Cells. J. Biol. Chem. 2002;277:10489–10497. doi: 10.1074/jbc.M109604200. [DOI] [PubMed] [Google Scholar]
- 60.Kahan Z, Gardi J, Nyari T, Foldesi I, Hajnal-Papp R, Ormandi K, Lazar G, Thurzo L, Schally AV. Elevated levels of circulating insulin-like growth factor-I, IGF-binding globulin-3 and testosterone predict hormone-dependent breast cancer in postmenopausal women: a case-control study. Int J Oncol. 2006;29:193–200. [PubMed] [Google Scholar]
- 61.Mazzoccoli G, Giuliani A, Bianco G, De Cata A, Balzanelli M, Carella AM, La Viola M, Tarquini R. Decreased serum levels of insulin-like growth factor (IGF)-I in patients with lung cancer: temporal relationship with growth hormone (GH) levels. Anticancer Res. 1999;19:1397–1399. [PubMed] [Google Scholar]
- 62.Kanety H, Madjar Y, Dagan Y, Levi J, Papa MZ, Pariente C, Goldwasser B, Karasik A. Serum insulin-like growth factor-binding protein-2 (IGFBP-2) is increased and IGFBP-3 is decreased in patients with prostate cancer: correlation with serum prostate-specific antigen. J Clin Endocrinol Metab. 1993;77:229–233. doi: 10.1210/jcem.77.1.7686915. [DOI] [PubMed] [Google Scholar]
- 63.Butt AJ, Fraley KA, Firth SM, Baxter RC. IGF-Binding Protein-3-Induced Growth Inhibition and Apoptosis Do Not Require Cell Surface Binding and Nuclear Translocation in Human Breast Cancer Cells. Endocrinology. 2002;143:2693–2699. doi: 10.1210/endo.143.7.8876. [DOI] [PubMed] [Google Scholar]
- 64.Gockerman A, Prevette T, Jones JI, Clemmons DR. Insulin-like growth factor (IGF)-binding proteins inhibit the smooth muscle cell migration responses to IGF-I and IGF-II. Endocrinology. 1995;136:4168–4173. doi: 10.1210/endo.136.10.7545099. [DOI] [PubMed] [Google Scholar]
- 65.Clemmons DR, Busby WH, Arai T, Nam TJ, Clarke JB, Jones JI, Ankrapp DK. Role of insulin-like growth factor binding proteins in the control of IGF actions. Prog Growth Factor Res. 1995;6:357–366. doi: 10.1016/0955-2235(95)00013-5. [DOI] [PubMed] [Google Scholar]
- 66.Mohan S, Baylink DJ. IGF-binding proteins are multifunctional and act via IGF-dependent and -independent mechanisms. J Endocrinol. 2002;175:19–31. doi: 10.1677/joe.0.1750019. [DOI] [PubMed] [Google Scholar]
- 67.Monzavi R, Cohen P. IGFs and IGFBPs: role in health and disease. Best Pract Res Clin Endocrinol Metab. 2002;16:433–447. doi: 10.1053/beem.2002.0212. [DOI] [PubMed] [Google Scholar]
- 68.Baxter RC. Insulin-like growth factor (IGF)-binding proteins: interactions with IGFs and intrinsic bioactivities. Am J Physiol Endocrinol Metab. 2000;278:E967–976. doi: 10.1152/ajpendo.2000.278.6.E967. [DOI] [PubMed] [Google Scholar]
- 69.Bach LA, Rechler MM. Measurement of insulin-like growth factor (IGF)-II binding to purified IGF binding proteins 1–6: comparison of charcoal adsorption and high performance size exclusion chromatography. Biochim Biophys Acta. 1996;1313:79–88. doi: 10.1016/0167-4889(96)00053-5. [DOI] [PubMed] [Google Scholar]
- 70.Stewart CE, Bates PC, Calder TA, Woodall SM, Pell JM. Potentiation of insulin-like growth factor-I (IGF-I) activity by an antibody: supportive evidence for enhancement of IGF-I bioavailability in vivo by IGF binding proteins. Endocrinology. 1993;133:1462–1465. doi: 10.1210/endo.133.3.7689959. [DOI] [PubMed] [Google Scholar]
- 71.Yakar S, Leroith D, Brodt P. The role of the growth hormone/insulin-like growth factor axis in tumor growth and progression: Lessons from animal models. Cytokine Growth Factor Rev. 2005;16:407–420. doi: 10.1016/j.cytogfr.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 72.Long L, Nip J, Brodt P. Paracrine growth stimulation by hepatocyte-derived insulin-like growth factor-1: a regulatory mechanism for carcinoma cells metastatic to the liver. Cancer Res. 1994;54:3732–3737. [PubMed] [Google Scholar]
- 73.Wolf E, Hoeflich A, Lahm H. What is the function of IGF-II in postnatal life? Answers from transgenic mouse models. Growth Horm IGF Res. 1998;8:185–193. doi: 10.1016/s1096-6374(98)80110-x. [DOI] [PubMed] [Google Scholar]
- 74.Bermont L, Fauconnet S, Lamielle F, Adessi GL. Cell-associated insulin-like growth factor-binding proteins inhibit insulin-like growth factor-I-induced endometrial cancer cell proliferation. Cell Mol Biol (Noisy-le-grand) 2000;46:1173–1182. [PubMed] [Google Scholar]
- 75.Mita K, Nakahara M, Usui T. Expression of the insulin-like growth factor system and cancer progression in hormone-treated prostate cancer patients. Int J Urol. 2000;7:321–329. doi: 10.1046/j.1442-2042.2000.00200.x. [DOI] [PubMed] [Google Scholar]
- 76.Signorello LB, Brismar K, Bergstrom R, Andersson SO, Wolk A, Trichopoulos D, Adami HO. Insulin-like growth factor-binding protein-1 and prostate cancer. J Natl Cancer Inst. 1999;91:1965–1967. doi: 10.1093/jnci/91.22.1965. [DOI] [PubMed] [Google Scholar]
- 77.Collett-Solberg PF, Cohen P. The role of the insulin-like growth factor binding proteins and the IGFBP proteases in modulating IGF action. Endocrinol Metab Clin North Am. 1996;25:591–614. doi: 10.1016/s0889-8529(05)70342-x. [DOI] [PubMed] [Google Scholar]
- 78.Sandhu MS, Dunger DB, Giovannucci EL. Insulin, Insulin-Like Growth Factor-I (IGF-I), IGF Binding Proteins, Their Biologic Interactions, and Colorectal Cancer. J. Natl. Cancer Inst. 2002;94:972–980. doi: 10.1093/jnci/94.13.972. [DOI] [PubMed] [Google Scholar]
- 79.Miyake H, Pollak M, Gleave ME. Castration-induced Up-Regulation of Insulin-like Growth Factor Binding Protein-5 Potentiates Insulin-like Growth Factor-I Activity and Accelerates Progression to Androgen Independence in Prostate Cancer Models. Cancer Res. 2000;60:3058–3064. [PubMed] [Google Scholar]
- 80.Kiyama S, Morrison K, Zellweger T, Akbari M, Cox M, Yu D, Miyake H, Gleave ME. Castration-induced increases in insulin-like growth factor-binding protein 2 promotes proliferation of androgen-independent human prostate LNCaP tumors. Cancer Res. 2003;63:3575–3584. [PubMed] [Google Scholar]
- 81.Flyvbjerg A, Mogensen O, Mogensen B, Nielsen OS. Elevated Serum Insulin-Like Growth Factor-Binding Protein 2 (IGFBP-2) and Decreased IGFBP-3 in Epithelial Ovarian Cancer: Correlation with Cancer Antigen 125 and Tumor-Associated Trypsin Inhibitor. J Clin Endocrinol Metab. 1997;82:2308–2313. doi: 10.1210/jcem.82.7.4085. [DOI] [PubMed] [Google Scholar]
- 82.Krajcik RA, Borofsky ND, Massardo S, Orentreich N. Insulin-like Growth Factor I (IGF-I), IGF-binding Proteins, and Breast Cancer. Cancer Epidemiol Biomarkers Prev. 2002;11:1566–1573. [PubMed] [Google Scholar]
- 83.Zhou J, Anderson K, Bievre M, Ng S, Bondy CA. Primate mammary gland insulin-like growth factor system: cellular localization and regulation by sex steroids. J Investig Med. 2001;49:47–55. doi: 10.2310/6650.2001.34090. [DOI] [PubMed] [Google Scholar]
- 84.Chan JM, Stampfer MJ, Ma J, Gann P, Gaziano JM, Pollak M, Giovannucci E. Insulin-Like Growth Factor-I (IGF-I) and IGF Binding Protein-3 as Predictors of Advanced-Stage Prostate Cancer. J. Natl. Cancer Inst. 2002;94:1099–1106. doi: 10.1093/jnci/94.14.1099. [DOI] [PubMed] [Google Scholar]
- 85.Rudd MF, Webb EL, Matakidou A, Sellick GS, Williams RD, Bridle H, Eisen T, Houlston RS. Variants in the GH-IGF axis confer susceptibility to lung cancer. Genome Res. 2006;16:693–701. doi: 10.1101/gr.5120106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Muti P, Quattrin T, Grant BJ, Krogh V, Micheli A, Schunemann HJ, Ram M, Freudenheim JL, Sieri S, Trevisan M, Berrino F. Fasting glucose is a risk factor for breast cancer: a prospective study. Cancer Epidemiol Biomarkers Prev. 2002;11:1361–1368. [PubMed] [Google Scholar]
- 87.Yu H, Jin F, Shu XO, Li BD, Dai Q, Cheng JR, Berkel HJ, Zheng W. Insulin-like growth factors and breast cancer risk in Chinese women. Cancer Epidemiol Biomarkers Prev. 2002;11:705–712. [PubMed] [Google Scholar]
- 88.Vadgama JV, Wu Y, Datta G, Khan H, Chillar R. Plasma insulin-like growth factor-I and serum IGF-binding protein 3 can be associated with the progression of breast cancer, and predict the risk of recurrence and the probability of survival in African-American and Hispanic women. Oncology. 1999;57:330–340. doi: 10.1159/000012052. [DOI] [PubMed] [Google Scholar]
- 89.el Atiq F, Garrouste F, Remacle-Bonnet M, Sastre B, Pommier G. Alterations in serum levels of insulin-like growth factors and insulin-like growth-factor-binding proteins in patients with colorectal cancer. Int J Cancer. 1994;57:491–497. doi: 10.1002/ijc.2910570409. [DOI] [PubMed] [Google Scholar]
- 90.Dahlfors G, Arnqvist HJ. Vascular endothelial growth factor and transforming growth factor-beta1 regulate the expression of insulin-like growth factor-binding protein-3, -4, and -5 in large vessel endothelial cells. Endocrinology. 2000;141:2062–2067. doi: 10.1210/endo.141.6.7481. [DOI] [PubMed] [Google Scholar]
- 91.Arnold PM, Ma JY, Citron BA, Zoubine MN, Festoff BW. Selective developmental regulation of gene expression for insulin-like growth factor-binding proteins in mouse spinal cord. Spine. 2000;25:1765–1770. doi: 10.1097/00007632-200007150-00005. [DOI] [PubMed] [Google Scholar]
- 92.Okazaki R, Riggs BL, Conover CA. Glucocorticoid regulation of insulin-like growth factor-binding protein expression in normal human osteoblast-like cells. Endocrinology. 1994;134:126–132. doi: 10.1210/endo.134.1.7506203. [DOI] [PubMed] [Google Scholar]
- 93.Wang D, Nagpal ML, Shimasaki S, Ling N, Lin T. Interleukin-1 induces insulin-like growth factor binding protein-3 gene expression and protein production by Leydig cells. Endocrinology. 1995;136:4049–4055. doi: 10.1210/endo.136.9.7544275. [DOI] [PubMed] [Google Scholar]
- 94.van Kleffens M, Groffen C, Rosato RR, van den Eijnde SM, van Neck JW, Lindenbergh-Kortleve DJ, Zwarthoff EC, Drop SL. mRNA expression patterns of the IGF system during mouse limb bud development, determined by whole mount in situ hybridization. Mol Cell Endocrinol. 1998;138:151–161. doi: 10.1016/s0303-7207(98)00007-0. [DOI] [PubMed] [Google Scholar]
- 95.Zazzi H, Nikoshkov A, Hall K, Luthman H. Structure and transcription regulation of the human insulin-like growth factor binding protein 4 gene (IGFBP4) Genomics. 1998;49:401–410. doi: 10.1006/geno.1998.5283. [DOI] [PubMed] [Google Scholar]
- 96.Camacho-Hubner C, Busby WH, Jr., McCusker RH, Wright G., Clemmons DR. Identification of the forms of insulin-like growth factor-binding proteins produced by human fibroblasts and the mechanisms that regulate their secretion. J Biol Chem. 1992;267:11949–11956. [PubMed] [Google Scholar]
- 97.Conover CA, Clarkson JT, Bale LK. Effect of glucocorticoid on insulin-like growth factor (IGF) regulation of IGF-binding protein expression in fibroblasts. Endocrinology. 1995;136:1403–1410. doi: 10.1210/endo.136.4.7534698. [DOI] [PubMed] [Google Scholar]
- 98.Yoshizawa A, Clemmons DR. Testosterone and insulin-like growth factor (IGF) I interact in controlling IGF-binding protein production in androgen-responsive foreskin fibroblasts. J Clin Endocrinol Metab. 2000;85:1627–1633. doi: 10.1210/jcem.85.4.6517. [DOI] [PubMed] [Google Scholar]
- 99.Nickerson T, Pollak M, Huynh H. Castration-induced apoptosis in the rat ventral prostate is associated with increased expression of genes encoding insulin-like growth factor binding proteins 2,3,4 and 5. Endocrinology. 1998;139:807–810. doi: 10.1210/endo.139.2.5912. [DOI] [PubMed] [Google Scholar]
- 100.Bruyninx M, Ammar H, Reiter E, Cornet A, Closset J. Genes upregulated during castration-induced rat prostatic apoptosis: cloning and characterization of new cDNAs. BJU Int. 2000;85:1134–1142. doi: 10.1046/j.1464-410x.2000.00654.x. [DOI] [PubMed] [Google Scholar]
- 101.Barni T, Vannelli BG, Sadri R, Pupilli C, Ghiandi P, Rizzo M, Selli C, Serio M, Fiorelli G. Insulin-like growth factor-I (IGF-I) and its binding protein IGFBP-4 in human prostatic hyperplastic tissue: gene expression and its cellular localization. J Clin Endocrinol Metab. 1994;78:778–783. doi: 10.1210/jcem.78.3.7510307. [DOI] [PubMed] [Google Scholar]
- 102.Tennant MK, Thrasher JB, Twomey PA, Birnbaum RS, Plymate SR. Insulin-like growth factor-binding proteins (IGFBP)-4, -5, and -6 in the benign and malignant human prostate: IGFBP-5 messenger ribonucleic acid localization differs from IGFBP-5 protein localization. J Clin Endocrinol Metab. 1996;81:3783–3792. doi: 10.1210/jcem.81.10.8855838. [DOI] [PubMed] [Google Scholar]
- 103.Kimura G, Kasuya J, Giannini S, Honda Y, Mohan S, Kawachi M, Akimoto M, Fujita-Yamaguchi Y. Insulin-like growth factor (IGF) system components in human prostatic cancer cell-lines: LNCaP, DU145, and PC-3 cells. Int J Urol. 1996;3:39–46. doi: 10.1111/j.1442-2042.1996.tb00628.x. [DOI] [PubMed] [Google Scholar]
- 104.Goossens K, Esquenet M, Swinnen JV, Manin M, Rombauts W, Verhoeven G. Androgens decrease and retinoids increase the expression of insulin-like growth factor-binding protein-3 in LNcaP prostatic adenocarcinoma cells. Mol Cell Endocrinol. 1999;155:9–18. doi: 10.1016/s0303-7207(99)00122-7. [DOI] [PubMed] [Google Scholar]
- 105.Cohen P, Peehl DM, Baker B, Liu F, Hintz RL, Rosenfeld RG. Insulin-like growth factor axis abnormalities in prostatic stromal cells from patients with benign prostatic hyperplasia. J Clin Endocrinol Metab. 1994;79:1410–1415. doi: 10.1210/jcem.79.5.7525636. [DOI] [PubMed] [Google Scholar]
- 106.Chambery D, de Galle B, Babajko S. Retinoic acid stimulates IGF binding protein (IGFBP)-6 and depresses IGFBP-2 and IGFBP-4 in SK-N-SH human neuroblastoma cells. J Endocrinol. 1998;159:227–232. doi: 10.1677/joe.0.1590227. [DOI] [PubMed] [Google Scholar]
- 107.Babajko S, Binoux M. Modulation by retinoic acid of insulin-like growth factor (IGF) and IGF binding protein expression in human SK-N-SH neuroblastoma cells. Eur J Endocrinol. 1996;134:474–480. doi: 10.1530/eje.0.1340474. [DOI] [PubMed] [Google Scholar]
- 108.Durham SK, Riggs BL, Harris SA, Conover CA. Alterations in insulin-like growth factor (IGF)-dependent IGF-binding protein-4 proteolysis in transformed osteoblastic cells. Endocrinology. 1995;136:1374–1380. doi: 10.1210/endo.136.4.7534697. [DOI] [PubMed] [Google Scholar]
- 109.Sheikh MS, Shao ZM, Hussain A, Clemmons DR, Chen JC, Roberts CT, Jr., LeRoith D., Fontana JA. Regulation of insulin-like growth factor-binding-protein-1, 2, 3, 4, 5, and 6: synthesis, secretion, and gene expression in estrogen receptor-negative human breast carcinoma cells. J Cell Physiol. 1993;155:556–567. doi: 10.1002/jcp.1041550314. [DOI] [PubMed] [Google Scholar]
- 110.Noll K, Wegmann BR, Havemann K, Jaques G. Insulin-like growth factors stimulate the release of insulin-like growth factor-binding protein-3 (IGFBP-3) and degradation of IGFBP-4 in nonsmall cell lung cancer cell lines. J Clin Endocrinol Metab. 1996;81:2653–2662. doi: 10.1210/jcem.81.7.8675593. [DOI] [PubMed] [Google Scholar]
- 111.Figueroa JA, De Raad S, Tadlock L, Speights VO, Rinehart JJ. Differential expression of insulin-like growth factor binding proteins in high versus low Gleason score prostate cancer. J Urol. 1998;159:1379–1383. [PubMed] [Google Scholar]
- 112.Thomas LN, Wright AS, Lazier CB, Cohen P, Rittmaster RS. Prostatic involution in men taking finasteride is associated with elevated levels of insulin-like growth factor-binding proteins (IGFBPs)-2, -4, and -5. Prostate. 2000;42:203–210. doi: 10.1002/(sici)1097-0045(20000215)42:3<203::aid-pros6>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 113.Mohan S, Nakao Y, Honda Y, Landale E, Leser U, Dony C, Lang K, Baylink DJ. Studies on the mechanisms by which insulin-like growth factor (IGF) binding protein-4 (IGFBP-4) and IGFBP-5 modulate IGF actions in bone cells. J Biol Chem. 1995;270:20424–20431. doi: 10.1074/jbc.270.35.20424. [DOI] [PubMed] [Google Scholar]
- 114.Singh P, Dai B, Dhruva B, Widen SG. Episomal expression of sense and antisense insulin-like growth factor (IGF)-binding protein-4 complementary DNA alters the mitogenic response of a human colon cancer cell line (HT-29) by mechanisms that are independent of and dependent upon IGF-I. Cancer Res. 1994;54:6563–6570. [PubMed] [Google Scholar]
- 115.Duan C, Clemmons DR. Differential expression and biological effects of insulin-like growth factor-binding protein-4 and -5 in vascular smooth muscle cells. J Biol Chem. 1998;273:16836–16842. doi: 10.1074/jbc.273.27.16836. [DOI] [PubMed] [Google Scholar]
- 116.Diehl D, Hoeflich A, Wolf E, Lahm H. Insulin-like Growth Factor (IGF)-binding Protein-4 Inhibits Colony Formation of Colorectal Cancer Cells by IGF-independent Mechanisms. Cancer Res. 2004;64:1600–1603. doi: 10.1158/0008-5472.can-03-2844. [DOI] [PubMed] [Google Scholar]
- 117.Hayden JM, Strong DD, Baylink DJ, Powell DR, Sampath TK, Mohan S. Osteogenic protein-1 stimulates production of insulin-like growth factor binding protein-3 nuclear transcripts in human osteosarcoma cells. Endocrinology. 1997;138:4240–4247. doi: 10.1210/endo.138.10.5457. [DOI] [PubMed] [Google Scholar]
- 118.Jones JI, Gockerman A, Busby WH, Jr., Camacho-Hubner C., Clemmons DR. Extracellular matrix contains insulin-like growth factor binding protein-5: potentiation of the effects of IGF-I. J Cell Biol. 1993;121:679–687. doi: 10.1083/jcb.121.3.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sunic D, McNeil JD, Rayner TE, Andress DL, Belford DA. Regulation of insulin-like growth factor-binding protein-5 by insulin-like growth factor I and interleukin-1alpha in ovine articular chondrocytes. Endocrinology. 1998;139:2356–2362. doi: 10.1210/endo.139.5.5983. [DOI] [PubMed] [Google Scholar]
- 120.Conover CA, Perry JE, Tindall DJ. Endogenous cathepsin D-mediated hydrolysis of insulin-like growth factor-binding proteins in cultured human prostatic carcinoma cells. J Clin Endocrinol Metab. 1995;80:987–993. doi: 10.1210/jcem.80.3.7533776. [DOI] [PubMed] [Google Scholar]
- 121.Imai Y, Busby WH, Jr., Smith CE, Clarke JB, Garmong AJ, Horwitz GD, Rees C., Clemmons DR. Protease-resistant form of insulin-like growth factor-binding protein 5 is an inhibitor of insulin-like growth factor-I actions on porcine smooth muscle cells in culture. J Clin Invest. 1997;100:2596–2605. doi: 10.1172/JCI119803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kiefer MC, Schmid C, Waldvogel M, Schlapfer I, Futo E, Masiarz FR, Green K, Barr PJ, Zapf J. Characterization of recombinant human insulin-like growth factor binding proteins 4, 5, and 6 produced in yeast. J Biol Chem. 1992;267:12692–12699. [PubMed] [Google Scholar]
- 123.Miyake H, Nelson C, Rennie PS, Gleave ME. Overexpression of insulin-like growth factor binding protein-5 helps accelerate progression to androgen-independence in the human prostate LNCaP tumor model through activation of phosphatidylinositol 3′-kinase pathway. Endocrinology. 2000;141:2257–2265. doi: 10.1210/endo.141.6.7520. [DOI] [PubMed] [Google Scholar]
- 124.Gleave ME, Miyake H. Castration-induced upregulation of insulin-like growth factor binding protein-5 potentiates IGF-1 and accelerates androgen-independent progression in prostate cancer. Prostate Cancer Prostatic Dis. 2000;3:S16. doi: 10.1038/sj.pcan.4500440. [DOI] [PubMed] [Google Scholar]
- 125.Schmid C, Keller C, Gosteli-Peter M, Zapf J. Mitogenic and antiapoptotic effects of insulin-like growth factor binding protein-6 in the human osteoblastic osteosarcoma cell line Saos-2/B-10. Biochem Biophys Res Commun. 1999;263:786–789. doi: 10.1006/bbrc.1999.1451. [DOI] [PubMed] [Google Scholar]
- 126.Claussen M, Buergisser D, Schuller AG, Matzner U, Braulke T. Regulation of insulin-like growth factor (IGF)-binding protein-6 and mannose 6-phosphate/IGF-II receptor expression in IGF-IL-overexpressing NIH 3T3 cells. Mol Endocrinol. 1995;9:902–912. doi: 10.1210/mend.9.7.7476972. [DOI] [PubMed] [Google Scholar]
- 127.Tennant MK, Thrasher JB, Twomey PA, Drivdahl RH, Birnbaum RS, Plymate SR. Protein and messenger ribonucleic acid (mRNA) for the type 1 insulin-like growth factor (IGF) receptor is decreased and IGF-II mRNA is increased in human prostate carcinoma compared to benign prostate epithelium. J Clin Endocrinol Metab. 1996;81:3774–3782. doi: 10.1210/jcem.81.10.8855837. [DOI] [PubMed] [Google Scholar]
- 128.Drivdahl RH, Loop SM, Andress DL, Ostenson RC. IGF-binding proteins in human prostate tumor cells: expression and regulation by 1,25-dihydroxyvitamin D3. Prostate. 1995;26:72–79. doi: 10.1002/pros.2990260203. [DOI] [PubMed] [Google Scholar]
- 129.Gabbitas B, Canalis E. Growth factor regulation of insulin-like growth factor binding protein-6 expression in osteoblasts. J Cell Biochem. 1997;66:77–86. doi: 10.1002/(sici)1097-4644(19970701)66:1<77::aid-jcb9>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 130.Yeh LC, Adamo ML, Olson MS, Lee JC. Osteogenic protein-1 and insulin-like growth factor I synergistically stimulate rat osteoblastic cell differentiation and proliferation. Endocrinology. 1997;138:4181–4190. doi: 10.1210/endo.138.10.5465. [DOI] [PubMed] [Google Scholar]
- 131.Angelloz-Nicoud P, Binoux M. Autocrine regulation of cell proliferation by the insulin-like growth factor (IGF) and IGF binding protein-3 protease system in a human prostate carcinoma cell line (PC-3) Endocrinology. 1995;136:5485–5492. doi: 10.1210/endo.136.12.7588299. [DOI] [PubMed] [Google Scholar]
- 132.Pereira JJ, Meyer T, Docherty SE, Reid HH, Marshall J, Thompson EW, Rossjohn J, Price JT. Bimolecular interaction of insulin-like growth factor (IGF) binding protein-2 with alphavbeta3 negatively modulates IGF-I-mediated migration and tumor growth. Cancer Res. 2004;64:977–984. doi: 10.1158/0008-5472.can-03-3056. [DOI] [PubMed] [Google Scholar]
- 133.Schutt BS, Langkamp M, Rauschnabel U, Ranke MB, Elmlinger MW. Integrin-mediated action of insulin-like growth factor binding protein-2 in tumor cells. J Mol Endocrinol. 2004;32:859–868. doi: 10.1677/jme.0.0320859. [DOI] [PubMed] [Google Scholar]
- 134.Charlton A, Blair V, Shaw D, Parry S, Guilford P, Martin IG. Hereditary diffuse gastric cancer: predominance of multiple foci of signet ring cell carcinoma in distal stomach and transitional zone. Gut. 2004;53:814–820. doi: 10.1136/gut.2002.010447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Santer FR, Bacher N, Moser B, Morandell D, Ressler S, Firth SM, Spoden GA, Sergi C, Baxter RC, Jansen-Durr P, Zwerschke W. Nuclear insulin-like growth factor binding protein-3 induces apoptosis and is targeted to ubiquitin/proteasome-dependent proteolysis. Cancer Res. 2006;66:3024–3033. doi: 10.1158/0008-5472.CAN-05-2013. [DOI] [PubMed] [Google Scholar]
- 136.Cohen P, Rajah R, Rosenbloom J, Herrick DJ. IGFBP-3 mediates TGF-beta1-induced cell growth in human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2000;278:L545–551. doi: 10.1152/ajplung.2000.278.3.L545. [DOI] [PubMed] [Google Scholar]
- 137.Shi LH, Zhu XQ, Zhao GH, Xia YB, Zhang YS. Expression of insulin-like growth factor binding protein-2 in gastric carcinoma and its relationship with cell proliferation. World J Gastroenterol. 2006;12:6285–6289. doi: 10.3748/wjg.v12.i39.6285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Frommer KW, Reichenmiller K, Schutt BS, Hoeflich A, Ranke MB, Dodt G, Elmlinger MW. IGF-independent effects of IGFBP-2 on the human breast cancer cell line Hs578T. J Mol Endocrinol. 2006;37:13–23. doi: 10.1677/jme.1.01955. [DOI] [PubMed] [Google Scholar]
- 139.Oh Y, Muller HL, Pham H, Rosenfeld RG. Demonstration of receptors for insulin-like growth factor binding protein-3 on Hs578T human breast cancer cells. J Biol Chem. 1993;268:26045–26048. [PubMed] [Google Scholar]
- 140.Rajah R, Nunn SE, Herrick DJ, Grunstein MM, Cohen P. Leukotriene D4 induces MMP-1, which functions as an IGFBP protease in human airway smooth muscle cells. Am J Physiol. 1996;271:L1014–1022. doi: 10.1152/ajplung.1996.271.6.L1014. [DOI] [PubMed] [Google Scholar]
- 141.O'Grady P, Huang SS, Huang JS. Expression of a new type high molecular weight receptor (type V receptor) of transforming growth factor beta in normal and transformed cells. Biochem Biophys Res Commun. 1991;179:378–385. doi: 10.1016/0006-291x(91)91381-l. [DOI] [PubMed] [Google Scholar]
- 142.Leal SM, Liu Q, Huang SS, Huang JS. The type V transforming growth factor beta receptor is the putative insulin-like growth factor-binding protein 3 receptor. J Biol Chem. 1997;272:20572–20576. doi: 10.1074/jbc.272.33.20572. [DOI] [PubMed] [Google Scholar]
- 143.O'Grady P, Liu Q, Huang SS, Huang JS. Transforming growth factor beta (TGF-beta) type V receptor has a TGF-beta-stimulated serine/threonine-specific autophosphorylation activity. J Biol Chem. 1992;267:21033–21037. [PubMed] [Google Scholar]
- 144.Miyakoshi N, Richman C, Kasukawa Y, Linkhart TA, Baylink DJ, Mohan S. Evidence that IGF-binding protein-5 functions as a growth factor. J Clin Invest. 2001;107:73–81. doi: 10.1172/JCI10459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Degraff DJ, Malik M, Chen Q, Miyako K, Rejto L, Aguiar AA, Bancroft DR, Cohen P, Sikes RA. Hormonal regulation of IGFBP-2 proteolysis is attenuated with progression to androgen insensitivity in the LNCaP progression model. J Cell Physiol. 2007;213:261–268. doi: 10.1002/jcp.21123. [DOI] [PubMed] [Google Scholar]
- 146.Salahifar H, Baxter RC, Martin JL. Insulin-like growth factor binding protein (IGFBP)-3 protease activity secreted by MCF-7 breast cancer cells: inhibition by IGFs does not require IGF-IGFBP interaction. Endocrinology. 1997;138:1683–1690. doi: 10.1210/endo.138.4.5064. [DOI] [PubMed] [Google Scholar]
- 147.Andress DL, Birnbaum RS. Human osteoblast-derived insulin-like growth factor (IGF) binding protein-5 stimulates osteoblast mitogenesis and potentiates IGF action. J Biol Chem. 1992;267:22467–22472. [PubMed] [Google Scholar]
- 148.Bhattacharyya N, Pechhold K, Shahjee H, Zappala G, Elbi C, Raaka B, Wiench M, Hong J, Rechler MM. Nonsecreted insulin-like growth factor binding protein-3 (IGFBP-3) can induce apoptosis in human prostate cancer cells by IGF-independent mechanisms without being concentrated in the nucleus. J Biol Chem. 2006;281:24588–24601. doi: 10.1074/jbc.M509463200. [DOI] [PubMed] [Google Scholar]
- 149.Manes S, Llorente M, Lacalle RA, Gomez-Mouton C, Kremer L, Mira E, Martinez AC. The matrix metalloproteinase-9 regulates the insulin-like growth factor-triggered autocrine response in DU-145 carcinoma cells. J Biol Chem. 1999;274:6935–6945. doi: 10.1074/jbc.274.11.6935. [DOI] [PubMed] [Google Scholar]
- 150.Cohen P, Graves HC, Peehl DM, Kamarei M, Giudice LC, Rosenfeld RG. Prostate-specific antigen (PSA) is an insulin-like growth factor binding protein-3 protease found in seminal plasma. J Clin Endocrinol Metab. 1992;75:1046–1053. doi: 10.1210/jcem.75.4.1383255. [DOI] [PubMed] [Google Scholar]
- 151.Lassarre C, Duron F, Binoux M. Use of the ligand immunofunctional assay for human insulin-like growth factor ((IGF) binding protein-3 (IGFBP-3) to analyze IGFBP-3 proteolysis and igf-i bioavailability in healthy adults, GH-deficient and acromegalic patients, and diabetics. J Clin Endocrinol Metab. 2001;86:1942–1952. doi: 10.1210/jcem.86.5.7467. [DOI] [PubMed] [Google Scholar]
- 152.Bubendorf L, Kolmer M, Kononen J, Koivisto P, Mousses S, Chen Y, Mahlamaki E, Schraml P, Moch H, Willi N, Elkahloun AG, Pretlow TG, Gasser TC, Mihatsch MJ, Sauter G, Kallioniemi OP. Hormone therapy failure in human prostate cancer: analysis by complementary DNA and tissue microarrays. J Natl Cancer Inst. 1999;91:1758–1764. doi: 10.1093/jnci/91.20.1758. [DOI] [PubMed] [Google Scholar]
- 153.Inman BA, Harel F, Audet JF, Meyer F, Douville P, Fradet Y, Lacombe L. Insulin-like growth factor binding protein 2: an androgen-dependent predictor of prostate cancer survival. Eur Urol. 2005;47:695–702. doi: 10.1016/j.eururo.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 154.Moore MG, Wetterau LA, Francis MJ, Peehl DM, Cohen P. Novel stimulatory role for insulin-like growth factor binding protein-2 in prostate cancer cells. Int J Cancer. 2003;105:14–19. doi: 10.1002/ijc.11015. [DOI] [PubMed] [Google Scholar]
- 155.Cohen P, Peehl DM, Stamey TA, Wilson KF, Clemmons DR, Rosenfeld RG. Elevated levels of insulin-like growth factor-binding protein-2 in the serum of prostate cancer patients. J Clin Endocrinol Metab. 1993;76:1031–1035. doi: 10.1210/jcem.76.4.7682560. [DOI] [PubMed] [Google Scholar]
- 156.Busund LT, Richardsen E, Busund R, Ukkonen T, Bjornsen T, Busch C, Stalsberg H. Significant expression of IGFBP2 in breast cancer compared with benign lesions. J Clin Pathol. 2005;58:361–366. doi: 10.1136/jcp.2004.020834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kricker JA, Towne CL, Firth SM, Herington AC, Upton Z. Structural and functional evidence for the interaction of insulin-like growth factors (IGFs) and IGF binding proteins with vitronectin. Endocrinology. 2003;144:2807–2815. doi: 10.1210/en.2002-221086. [DOI] [PubMed] [Google Scholar]
- 158.Hwang PH, Kim SY, Lee JC, Kim SJ, Yi HK, Lee DY. PTEN/MMAC1 enhances the growth inhibition by anticancer drugs with downregulation of IGF-II expression in gastric cancer cells. Exp Mol Med. 2005;37:391–398. doi: 10.1038/emm.2005.49. [DOI] [PubMed] [Google Scholar]
- 159.Wang H, Wang H, Shen W, Huang H, Hu L, Ramdas L, Zhou YH, Liao WS, Fuller GN, Zhang W. Insulin-like growth factor binding protein 2 enhances glioblastoma invasion by activating invasion-enhancing genes. Cancer Res. 2003;63:4315–4321. [PubMed] [Google Scholar]
- 160.Ricort JM. Insulin-like growth factor binding protein (IGFBP) signalling. Growth Horm IGF Res. 2004;14:277–286. doi: 10.1016/j.ghir.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 161.van Golen CM, Schwab TS, Kim B, Soules ME, Su Oh S, Fung K, van Golen KL, Feldman EL. Insulin-like growth factor-I receptor expression regulates neuroblastoma metastasis to bone. Cancer Res. 2006;66:6570–6578. doi: 10.1158/0008-5472.CAN-05-1448. [DOI] [PubMed] [Google Scholar]
- 162.Kuang Z, Yao S, Keizer DW, Wang CC, Bach LA, Forbes BE, Wallace JC, Norton RS. Structure, dynamics and heparin binding of the C-terminal domain of insulin-like growth factor-binding protein-2 (IGFBP-2) J Mol Biol. 2006;364:690–704. doi: 10.1016/j.jmb.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 163.Marelli MM, Moretti RM, Procacci P, Motta M, Limonta P. Insulin-like growth factor-I promotes migration in human androgen-independent prostate cancer cells via the alphavbeta3 integrin and PI3-K/Akt signaling. Int J Oncol. 2006;28:723–730. [PubMed] [Google Scholar]
- 164.Guo N, Ye JJ, Liang SJ, Mineo R, Li SL, Giannini S, Plymate SR, Sikes RA, Fujita-Yamaguchi Y. The role of insulin-like growth factor-II in cancer growth and progression evidenced by the use of ribozymes and prostate cancer progression models. Growth Horm IGF Res. 2003;13:44–53. doi: 10.1016/s1096-6374(02)00121-1. [DOI] [PubMed] [Google Scholar]
- 165.Boulle N, Baudin E, Gicquel C, Logie A, Bertherat J, Penfornis A, Bertagna X, Luton JP, Schlumberger M, Le Bouc Y. Evaluation of plasma insulin-like growth factor binding protein-2 as a marker for adrenocortical tumors. Eur J Endocrinol. 2001;144:29–36. doi: 10.1530/eje.0.1440029. [DOI] [PubMed] [Google Scholar]
- 166.Boulle N, Logie A, Gicquel C, Perin L, Le Bouc Y. Increased levels of insulin-like growth factor II (IGF-II) and IGF-binding protein-2 are associated with malignancy in sporadic adrenocortical tumors. J Clin Endocrinol Metab. 1998;83:1713–1720. doi: 10.1210/jcem.83.5.4816. [DOI] [PubMed] [Google Scholar]
- 167.Miyake H, Hara I, Yamanaka K, Muramaki M, Gleave M, Eto H. Introduction of insulin-like growth factor binding protein-2 gene into human bladder cancer cells enhances their metastatic potential. Oncol Rep. 2005;13:341–345. [PubMed] [Google Scholar]
- 168.Miyake H, Hara I, Yamanaka K, Muramaki M, Eto H. Prognostic significance of insulin-like growth factor (IGF) binding protein-2 to IGF-binding protein-3 ratio in patients undergoing radical cystectomy for invasive transitional cell carcinoma of the bladder. BJU Int. 2005;95:987–991. doi: 10.1111/j.1464-410X.2005.05453.x. [DOI] [PubMed] [Google Scholar]
- 169.Juncker-Jensen A, Lykkesfeldt AE, Worm J, Ralfkiaer U, Espelund U, Jepsen JS. Insulin-like growth factor binding protein 2 is a marker for antiestrogen resistant human breast cancer cell lines but is not a major growth regulator. Growth Horm IGF Res. 2006;16:224–239. doi: 10.1016/j.ghir.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 170.Song SW, Fuller GN, Khan A, Kong S, Shen W, Taylor E, Ramdas L, Lang FF, Zhang W. IIp45, an insulin-like growth factor binding protein 2 (IGFBP-2) binding protein, antagonizes IGFBP-2 stimulation of glioma cell invasion. Proc Natl Acad Sci U S A. 2003;100:13970–13975. doi: 10.1073/pnas.2332186100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.McDonald KL, O'Sullivan MG, Parkinson JF, Shaw JM, Payne CA, Brewer JM, Young L, Reader DJ, Wheeler HT, Cook RJ, Biggs MT, Little NS, Teo C, Stone G, Robinson BG. IQGAP1 and IGFBP2: valuable biomarkers for determining prognosis in glioma patients. J Neuropathol Exp Neurol. 2007;66:405–417. doi: 10.1097/nen.0b013e31804567d7. [DOI] [PubMed] [Google Scholar]
- 172.Elmlinger MW, Deininger MH, Schuett BS, Meyermann R, Duffner F, Grote EH, Ranke MB. In vivo expression of insulin-like growth factor-binding protein-2 in human gliomas increases with the tumor grade. Endocrinology. 2001;142:1652–1658. doi: 10.1210/endo.142.4.8084. [DOI] [PubMed] [Google Scholar]
- 173.Fuller GN, Rhee CH, Hess KR, Caskey LS, Wang R, Bruner JM, Yung WK, Zhang W. Reactivation of insulin-like growth factor binding protein 2 expression in glioblastoma multiforme: a revelation by parallel gene expression profiling. Cancer Res. 1999;59:4228–4232. [PubMed] [Google Scholar]
- 174.Yip RG, Wolfe MM. GIP biology and fat metabolism. Life Sci. 2000;66:91–103. doi: 10.1016/s0024-3205(99)00314-8. [DOI] [PubMed] [Google Scholar]
- 175.Lee EJ, Mircean C, Shmulevich I, Wang H, Liu J, Niemisto A, Kavanagh JJ, Lee JH, Zhang W. Insulin-like growth factor binding protein 2 promotes ovarian cancer cell invasion. Mol Cancer. 2005;4:7. doi: 10.1186/1476-4598-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.He T, Wu XT, Yang ZM, Da MX, Zhang MM, Qian K. [IGFBP-2 mRNA expression in gastric cancer tissues and its roles in proliferation, differentiation and peritoneal metastasis of gastric cancer] Sichuan Da Xue Xue Bao Yi Xue Ban. 2005;36:187–189. [PubMed] [Google Scholar]
- 177.Lancaster JM, Sayer RA, Blanchette C, Calingaert B, Konidari I, Gray J, Schildkraut J, Schomberg DW, Marks JR, Berchuck A. High expression of insulin-like growth factor binding protein-2 messenger RNA in epithelial ovarian cancers produces elevated preoperative serum levels. Int J Gynecol Cancer. 2006;16:1529–1535. doi: 10.1111/j.1525-1438.2006.00623.x. [DOI] [PubMed] [Google Scholar]
- 178.Wang H, Rosen DG, Wang H, Fuller GN, Zhang W, Liu J. Insulin-like growth factor-binding protein 2 and 5 are differentially regulated in ovarian cancer of different histologic types. Mod Pathol. 2006;19:1149–1156. doi: 10.1038/modpathol.3800637. [DOI] [PubMed] [Google Scholar]
- 179.Lukanova A, Lundin E, Micheli A, Akhmedkhanov A, Rinaldi S, Muti P, Lenner P, Biessy C, Krogh V, Riboli E, Hallmans G, Berrino F, Zeleniuch-Jacquotte A, Toniolo P, Kaaks R. Risk of ovarian cancer in relation to prediagnostic levels of C-peptide, insulin-like growth factor binding proteins-1 and -2 (USA, Sweden, Italy) Cancer Causes Control. 2003;14:285–292. doi: 10.1023/a:1023688603547. [DOI] [PubMed] [Google Scholar]
- 180.Shariat SF, Lamb DJ, Kattan MW, Nguyen C, Kim J, Beck J, Wheeler TM, Slawin KM. Association of preoperative plasma levels of insulin-like growth factor I and insulin-like growth factor binding proteins-2 and -3 with prostate cancer invasion, progression, and metastasis. J Clin Oncol. 2002;20:833–841. doi: 10.1200/JCO.2002.20.3.833. [DOI] [PubMed] [Google Scholar]