Abstract
Human epithelial mucin, the major glycoprotein component of mucus, plays a critical role in host innate defense response against invading microbes by facilitating the mucociliary clearance. Excess mucin production, however, overwhelms the mucociliary clearance, resulting in not only defective mucosal defense but also conductive hearing loss in the middle ear and mucus obstruction in the airway. Indeed, mucus overproduction is a hall-mark of otitis media (OM) and chronic obstructive pulmonary diseases (COPD). Thus, tight regulation of mucin production plays an important role in maintaining an appropriate balance between beneficial and detrimental outcomes. We previously reported that Streptococcus pneumoniae (S. pneumoniae) up-regulates MUC5AC mucin expression via a positive MAPK ERK1/2 and a negative JNK1/2 signaling pathway. However, the signaling components including the up-stream activators and the down-stream transcription factors involved in these two path-ways remain largely unknown. In the present study, we showed that positive regulation of MUC5AC mucin expression by ERK1/2 is dependent on Ras-Raf-1 signaling pathway, whereas the negative regulation of MUC5AC expression by JNK1/2 is dependent on MEKK3. Moreover, transcriptional factor AP-1 acts as a key regulator for both of the positive and negative regulation of MUC5AC mucin expression as evidenced by mutagenesis analysis of two AP-1 sites in the promoter region of human MUC5AC mucin gene. Ras-Raf1-ERK1/2-dependent AP-1 activation positively regulates MUC5AC mucin induction by S. pneumoniae, whereas MEKK3-JNK1/2-dependent AP-1 activation negatively regulates it. Therefore, our data unveiled a novel signaling mechanism underlying the tight regulation of MUC5AC mucin induction by S. pneumoniae and may lead to the development of new therapeutic strategy for reducing mucus overproduction in both OM and COPD.
Keywords: MUC5AC mucin, streptococcus pneumonia, ERK, JNK, AP-1, otitis media, COPD
Introduction
Mucosal epithelial cells in the respiratory tract act as the first line of host innate defense against inhaled microbes by producing a range of molecules for clearance. Respiratory defense against infection involves a diverse and complex system including mechanical barriers, which limit exposure of the respiratory tract to potential pathogenic organisms, the mucociliary apparatus and cough reflexes, which work to expel any microbes that may bypass the initial defenses [1–3]. Human epithelial mucins are high-molecular weight glycoproteins that constitute the major component of mucus secretions in the middle ear, trachea, digestive and reproductive tracts. Mucins play a critical role in host innate defense response against invading microbes by binding and trapping inhaled infectious particles, including bacteria and viruses, and facilitating the mucociliary clearance. Therefore, appropriate induction of mucin in infectious diseases represents an important host innate defensive response to microbes. However, excess mucin production, mainly resulting from up-regulation of mucin production, overwhelms the mucociliary clearance, resulting in defective mucosal defense and contributing to morbidity and mortality in related diseases [4, 5]. Indeed, mucus over-production is a hallmark of otitis media (OM) and chronic obstructive pulmonary disease (COPD), causing conductive hearing loss in the middle ear and mucus obstruction in the air-way, respectively [5–10]. Thus, tight regulation of mucin production plays an important role in maintaining an appropriate balance between beneficial and detrimental outcomes. However, the molecular mechanisms underlying tight regulation of mucus production in infectious diseases still remain largely unknown.
OM is the most common childhood infection and the leading cause of conductive hearing loss [11–13]. By 3 years of age, 80% of children have experienced at least one episode and more then 40% have suffered recurrent infections. It causes a substantial burden on community health programs. Three bacterial pathogens including Streptococcus pneumoniae (S. pneumoniae), nontypeable Haemophilus influenzae (NTHi), and Moraxella catarrhalis account for the majority of OM [14, 15]. Among them, S. pneumoniae is the most common bacterial pathogen causing OM, accounting for approximately 40% of the episodes [16, 17]. Although two pneumococcal vaccines have been recently developed using capsular polysaccharide antigens of 7 or 23 different capsule serotypes, recent clinical trials showed that these vaccines are unable to provide effective protection against OM [18, 19]. Moreover, inappropriate antibiotic treatment contributes to the worldwide emergence of antibiotic-resistant strains of S. pneumoniae. Therefore, development of alternative therapeutic strategies based on full understanding of the molecular pathogenesis of S. pneumoniae-induced OM is urgently needed. We previously reported that cytolytic toxin pneumolysin, a 53-kDa protein produced by all clinical isolates of S. pneumoniae, acts as a key virulence factor for respiratory infections induced by S. pneumoniae including acute lung injury (ALI), pneumonia, and OM [20–23]. Moreover, S. pneumoniae pneumolysin also plays an important role in inducing MUC5AC mucin in vitro and in vivo [20, 21]. S. pneumoniae up-regulates MUC5AC mucin expression via a positive ERK1/2 MAPK and a negative JNK1/2 MAPK signaling pathways [20, 21]. However, the signaling components including the up-stream activators and the down-stream transcription factors involved in these two pathways still remain largely unknown.
In the present study, we showed that positive regulation of MUC5AC mucin expression by ERK1/2 is dependent on Ras-Raf-1 signaling pathway, whereas the negative regulation of MUC5AC expression by JNK1/2 is dependent on MEKK3. Moreover, transcriptional factor AP-1 acts as a key regulator for both of the positive and negative regulation of MUC5AC mucin expression. Ras-Raf1-ERK1/2-dependent AP-1 activation positively regulates MUC5AC mucin induction by S. pneumoniae, whereas MEKK3-JNK1/2-dependent AP-1 activation negatively regulates it. Therefore, our studies may bring new insights into the tight regulation of MUC5AC mucin induction by S. pneumoniae and may lead to the development of new therapeutic strategy for reducing mucus overproduction in both OM and COPD.
Materials and Methods
Bacterial culture and pneumolysin
Clinical isolate S. pneumoniae strain D39 was used in this study. D39 isogenic pneumolysin-deficient mutant (PLN) was developed through insertion-duplication mutagenesis as described previously [24, 25]. Bacteria were grown on chocolate agar plate and in Todd-Hewitt broth supplemented with 0.5% yeast extract (THY) at 37°C in an atmosphere of 5% CO2. Water-jacketed incubator. Crude extracts of S. pneumoniae D39 and PLN were prepared as described previously [20–23], stored at -80°C, and used at the concentration of 5 μg/ml in all experiments, otherwise specifically indicated in the figure legends. His6 tag-fused pneumolysin was expressed and purified as described previously [20, 21].
Mammalian cell culture
All media described below were supplemented with 10% fetal bovine serum (Sigma-Aldrich) and Pen/Strep (100 units/ml penicillin and 0.1 mg/ml streptomycin; Gibco). Human middle ear epithelial HMEEC-1 cells were maintained in a 1:1 mixture of Bronchial Epithelial Basal Medium (BMEM; Clonetics) and Dulbecco's modified Eagle's medium (DMEM; Gibco) as described previously [23, 26–29]. Airway epithelial A549 cells were maintained in F-12K medium (Gibco). Human colon epithelial HM3 cells were maintained in DMEM H-21 (University of California Cell Culture Facility, San Francisco, CA) as described previously [23, 27–34].
RNA extraction and real-time quantitative RT-PCR analysis for MUC5AC mucin gene
The total RNA was isolated using TRIzol (Invitrogen) and synthesis of complementary DNA from total mRNA was performed with Multi-Scribe reverse transcriptase kit (TaqMan RT reagent, ABI) following manufacturer's instructions. Real-time quantitative PCR (Q-PCR) reactions were amplified and quantified using an ABI 7500 sequence detector and manufacturer's software (ABI). Primers and probes for human and mouse MUC5AC were described previously [35–37]. Relative quantity of MUC5AC mRNA was obtained using Comparative threshold cycle (CT) method and normalized using Predeveloped Taqman Assay Reagent (PDAR) human cyclophilin and mouse GAPDH for human MUC5AC and mouse Muc5ac, respectively.
Plasmids, Transfections and Luciferase assays
The expression plasmids ERK1DN (dominant-negative mutant), ERK2DN, Raf-1DN, RasDN, JNK1DN, JNK2DN, MEKK3DN were previously described [35–40]. The reporter constructs, 5'-flanking region of the human MUC5AC gene were also previously described [35–37]. The AP-1 mutants of MUC5AC-Luc constructs were generated by replacing either the AP-1 site with EcoRI site (p3.7MUC5AC-dAP1mt-Luc, p3.7MUC5AC-pAP1mt-Luc, pMUC5AC-dAP1mt-300TK-Luc, and pMUC5AC-pAP1mt-300TK-Luc). All transient transfections were carried out in triplicate using TransIT-LT1 reagent (Mirus, Medison, WI) following the manufacturer's instruction. Transfected cells were pretreated with or without chemical inhibitors including PD98059 (CalBiochem) and SP600125 (A.G. Scientific) for 1 hour followed by 5 hours incubation with S. pneumoniae lysates. Luciferase activity was normalized with respect to β-galactosidase activity using the Galacton-Plus substrate system (Tropix) following manufacturer's instruction. In all co-transfections with expression plasmids of signaling molecules, an empty vector was used as a control. Vehicle control was treated as a MOCK treatment in chemical inhibition experiments, and phosphate buffered saline (PBS) was used as a control for S. pneumoniae lysates treatment.
Antibodies and Western blot analysis
Antibodies against phospho-ATF-2, ATF-2, phospho-c-Jun, and c-Jun were purchased from Cell Signaling (Beverly, MA). Phosphorylation of ATF-2 and c-Jun and expression of total ATF-2 and c-Jun were detected by using Amersham ECL Plus Western Blotting Detection Reagents (GE Healthcare) following manufacturer's Instruction.
Animal Experiments
C57BL/6 mice were purchased from National Cancer Institute (NCI, NIH), and eight weeks-old male mice were used in this study. Intra-bulla inoculation of S. pneumoniae was conducted using transtympanic membrane inoculation method as described previously [20, 21, 23, 41]. The middle ears of anesthetized mice were inoculated with 10 μl of S. pneumoniae lysate (equivalent of 6.25 × 105 colony-forming units in saline), and 10 μl of saline was inoculated as a control. Low respiratory infection of S. pneumoniae was conducted using intratracheal inoculation method as described previously [22, 23, 27, 28, 31–33, 41]. Anesthetized mice were intratracheally inoculated with 50 μl of S. pneumoniae lysate (equivalent of 6.25 × 105 colony-forming units in saline), and 50 μl of saline was inoculated as a control. Bulla and lung tissues were dissected from mice 9 hours and 6 hours after inoculation, respectively, and then subjected to MUC5AC mRNA expression analysis by Q-PCR. All animal experiments were approved by the Institutional Animal Care and Use Committee at the University of Rochester.
Statistical Analysis
Data were analyzed using Student's t test, and a value of p<0.05 was considered significant.
Results and discussion
S. pneumoniae up-regulates of MUC5AC mucin expression both in epithelial cells in vitro and middle ear and lung tissues of mouse in vivo
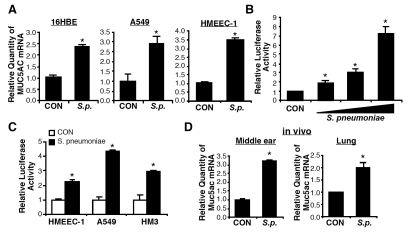
To identify the upstream activators and down-stream transcriptional factors for positive and negative regulation of MUC5AC mucin gene expression by S. pneumoniae, we first assessed the effect of S. pneumoniae on the expression of MUC5AC in human respiratory epithelial cells. As shown in Figure 1A, MUC5AC mRNA expression was up-regulated by S. pneumoniae D39 in airway epithelial A549, 16HBE, and HMEEC-1 cells. Up-regulation of MUC5AC was also induced by S. pneumoniae strains 6B and R6 [20]. Because transcriptional regulation plays an important role in regulating the gene expression at the mRNA steady-state level, we next sought to determine if S. pneumoniae induces MUC5AC mRNA by up-regulating its transcription using a MUC5AC promoter-driven luciferase reporter assay. As shown in Figure 1B, MUC5AC transcription was markedly up-regulated by S. pneumoniae in a dose-dependent manner in HM3 cells. In addition, MUC5AC induction was also observed in a variety of human epithelial cells transiently transfected with a full length MUC5AC promoter reporter construct (Figure 1C). To further determine if S. pneumoniae also up-regulates MUC5AC expression in the middle ear and lung tissues of mouse in vivo, we assessed the effect of S. pneumoniae on MUC5AC expression at the mRNA level in the mouse middle ear mucosa and lung tissue by inoculating S. pneumoniae into the middle ear of mouse via a trans-tympanic membrane and the lower respiratory tract via a intratracheal inoculation, respectively. As shown in Figure 1D, S. pneumoniae up-regulated MUC5AC at the mRNA level in both mouse middle ear and lung tissues. Taken together, our data indicate that S. pneumoniae up-regulates MUC5AC mucin gene expression in vitro and in vivo.
Figure 1.
S. pneumoniae up-regulates MUC5AC mucin expression both in epithelial cells in vitro and middle ear and lung tissues of mouse in vivo. A, Cells were treated with S. pneumoniae strain D39, and total RNA was extracted from S. pneumoniae- or control-treated cells 5 hours after treatment. mRNA expression levels of MUC5AC mucin gene were measured by Q-PCR analysis. B, HM3 cells stably transfected with full size MUC5AC-Luc constructs were treated with S. pneumoniae strain D39 at various concentrations, and MUC5AC transcription was measured 5 hours after treatment by performing luciferase analysis. C, Cells were transfected with p3.7MUC5AC-Luc and treated with S. pneumoniae strain D39. MUC5AC transcription was measured 5 hours after S. pneumoniae or control treatment. D, C57BL/6 mice were inoculated with S. pneumoniae lysate into the middle ear via transtympanic membrane or into the lung via intratracheal inoculation. Bulla were isolated from S. pneumoniae- or control-inoculated mice 9 hours after inoculation, and lung tissues were dissected 6 hours after inoculation. Total RNA was extracted from the bulla and lung tissues, and mRNA expression levels of MUC5AC mucin gene were measured by Q-PCR analysis. The values presented in A – D are Mean ± SD (n = 3). *, p<0.05 compared with CON.
Ras-Raf-1 signaling cascade and MEKK3 act as upstream activators for ERK1/2-mediated positive regulation and JNK1/2-mediated negative regulation of MUC5AC expression by S. pneumoniae, respectively
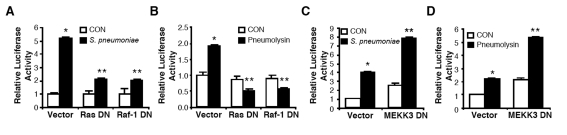
We previously reported that S. pneumoniae up-regulates MUC5AC mucin expression via a positive ERK1/2 MAPK and a negative JNK1/2 MAPK signaling pathways [20, 21]. However, the signaling components including up-stream activators of ERK1/2 and JNK1/2 MAPKs in-volved in S. pneumoniae-induced MUC5AC transcription are still unclear. Ras-Raf-1 path-way has been known as one of the major signaling cascades upstream of ERK1/2 MAPK pathway. [1, 42, 43]. To determine if this signaling pathway is involved in MUC5AC transcription, we next assessed the effect of blocking Ras-Raf-1 signaling on MUC5AC induction. As shown in Figure 2A & 2B, overexpression of a dominant-negative (DN) mutant of either Ras or Raf-1 inhibited MUC5AC induction by S. pneumoniae as well as pneumolysin, a key pathogenic factor causing S. pneumoniae-induced MUC5AC up-regulation. It has been recently shown that MEKK3 is an essential kinase transducing signals from the TLR4-MyD88-IRAK1-TRAF6 complex to JNK but not ERK [40]. Based on this recent report, we sought to determine if MEKK3 also acts as an up-stream activator of JNK1/2, thereby acting as a negative regulator for S. pneumoniae-induced MUC5AC expression in epithelial cells. To determine if MEKK3 is involved in MUC5AC transcription, we next assessed the effect of blocking MEKK3 signaling on MUC5AC induction. As shown in Figure 2C & D, expressing a DN mutant of MEKK3 (MEKK3 DN) enhanced MUC5AC induction by S. pneumoniae (Figure 2C) or pneumolysin (Figure 2D). Taken together, our data demonstrate that the Ras-Raf-1-ERK1/2 MAPK signaling pathway acts as a positive regulator for S. pneumoniae-induced MUC5AC transcription, whereas MEKK3-JNK1/2 MAPK signaling pathway acts as a negative regulator for it.
Figure 2.
Ras-Raf-1 signaling cascade and MEKK3 act as upstream activator for ERK1/2-mediated positive regulation and JNK1/2-mediated negative regulation of MUC5AC expression by S. pneumoniae, respectively. A & B, HM3 cells were transfected with p3.7MUC5AC-Luc with or without DN mutant of Ras (RasDN) or Raf-1 (Raf-1DN), and treated with S. pneumoniae strain D39 (A) or purified pneumolysin (B). MUC5AC transcription was measured 5 hours after S. pneumoniae or control treatment. C & D, HM3 cells were transfected with p3.7MUC5AC-Luc with or without DN mutant of MEKK3 (MEKK3 DN), and treated with S. pneumoniae strain D39 (C) or purified pneumolysin (D). MUC5AC transcription was measured 5 hours after S. pneumoniae or control treatment. The values presented in A – D are Mean ± SD (n = 3). *, p<0.05 compared with CON; **, p<0.05 compared with S. pneumoniae treatment in Vector transfected cells.
Two distinct AP-1 sites differentially regulate S. pneumoniae-induced MUC5AC mucin transcription
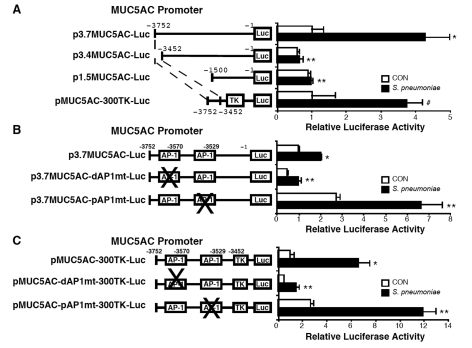
To define the downstream transcriptional factors mediating positive and negative regulation of MUC5AC mucin gene expression by S. pneumoniae, we next sought to define the S. pneumoniae-response elements in the promoter region of human MUC5AC gene. Analysis of luciferase activity from a panel of deletion mutants of MUC5AC promoter-luciferase reporter gene revealed a S. pneumoniae-response element between base pairs -3752/-3452
(Figure 3A). To determine whether this response element is sufficient to mediate MUC5AC induction, we made heterologus constructs in which MUC5AC promoter base pair -3752/-3452 was subcloned upstream of the thymidine kinase (TK) promoter (pMUC5AC-300TK-Luc). The result shown in Figure 3A indicates that the key response element in-deed resides in the base pair -3752/-3452 region. Further sequence analysis of this functional promoter region revealed that this region contains two AP-1 binding sites, one locates between base pairs -3576/-3570, the other locates between base pairs -3535/-3529. We next investigated if activation of these AP-1 sites is involved in S. pneumoniae-induced MUC5AC transcription by performing selective mutagenesis of the AP-1 binding sites. The two MUC5AC AP-1 mutant promoter constructs were obtained by replacing either the AP-1 site with EcoRI site. As shown in Figure 3B, the mutant construct containing a mutated AP-1 site located in the promoter region -3576/-3570bp (distal AP-1; p3.7MUC5AC-dAP1mt-Luc) markedly reduced the responsiveness of MUC5AC promoter construct to S. pneumoniae. In contrast, the mutant construct containing a mutated AP-1 site located in -3535/-3529bp (proximal AP-1; p3.7MUC5AC-pAP1mt-Luc) enhanced the responsiveness of MUC5AC promoter construct to S. pneumoniae. Similar results were also observed in cells treated with pneumolysin (data not shown). Consistent with these findings, mutation in distal AP-1 site of pMUC5AC-300TK-Luc (pMUC5AC-dAP1mt-300TK-Luc) resulted in markedly reduced responsiveness to S. pneumoniae, whereas mutation in proximal AP-1 site of pMUC5AC-300TK-Luc (pMUC5AC-pAP1mt-300TK-Luc) showed enhanced responsiveness to S. pneumoniae (Figure 3C). Indeed, S. pneumoniae greatly increased AP-1-driven luciferase activity in multiple epithelial cells (data not shown). Collectively, these results demonstrate that AP-1 activation is required for S. pneumoniae-induced MUC5AC transcription and two distinct AP-1 sites are differentially involved in mediating positive and negative regulations of MUC5AC transcription by S. pneumoniae.
Figure 3.
Two distinct AP-1 sites differentially regulate S. pneumoniae-induced MUC5AC mucin transcription. A, A549 cells were transfected with wild-type p3.7MUC5AC-Luc or truncated mutants of p3.7MUC5AC-Luc (p3.4MUC5AC-Luc, p1.5MUC5AC-Luc, or pMUC5AC-300TK-Luc), and treated with S. pneumoniae strain D39. MUC5AC transcription was measured 5 hours after S. pneumoniae or control treatment. B, HM3 cells were transfected with wild-type p3.7MUC5AC-Luc or mutants on either distal AP-1 site at -3576/-3570bp (p3.7MUC5AC-dAP1mt-Luc) or proximal AP-1 site at -3535/-3529bp (p3.7MUC5AC-pAP1mt-Luc), and treated with S. pneumoniae strain D39. MUC5AC transcription was measured 5 hours after S. pneumoniae or control treatment. C, HM3 cells were transfected with wild-type pMUC5AC-300TK-Luc or mutants on either distal AP-1 site at -3576/-3570bp (pMUC5AC-dAP1mt-300TK-Luc) or proximal AP-1 site at -3535/-3529bp (pMUC5AC-pAP1mt-300TK-Luc), and treated with S. pneumoniae strain D39. MUC5AC transcription was measured 5 hours after S. pneumoniae or control treatment. The values presented in A – C are Mean ± SD (n = 3). *, p<0.05 compared with CON in wild-type reporter gene transfected cells; **, p<0.05 compared with S. pneumoniae treatment in wild-type reporter gene transfected cells; #, p>0.05 compared with S. pneumoniae treatment in wild-type reporter gene transfected cells.
Two distinct AP-1 sites in MUC5AC promoter region mediate the ERK-dependent positive and the JNK-dependent negative regulation of MUC5AC induction, respectively
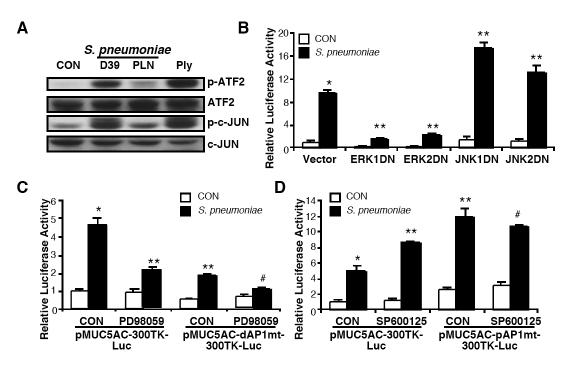
Although we identified that two distinct AP-1 sites in MUC5AC promoter region are differentially involved in positive and negative regulation of MUC5AC transcription by S. pneumoniae, it is still unclear how these two distinct AP-1 sites differentially regulate S. pneumoniae-induced MUC5AC induction. Interestingly, as assessed by Western blotting analysis, phosphorylation of ATF-2, one of the down-stream targets of ERK1/2, and phosphorylation of c-Jun, one of the downstream targets of JNK1/2, were observed in cells treated with either S. pneumoniae wild-type strain D39 or pneumolysin but not in cells treated with pneumolysin-deficient mutant strain PLN (Figure 4A). Given the facts that AP-1 can be activated by ERK and JNK pathways, and our data already indicated that ERK and JNK are differentially involved in MUC5AC induction by S. pneumoniae [1, 20, 21, 42, 44], we therefore investigated whether the two distinct AP-1 sites mediate the ERK1/2-dependent positive and the JNK1/2-dendent negative regulation of MUC5AC induction by S. pneumoniae, respectively. We first assessed the effect of blocking ERK and JNK signaling on MUC5AC induction by accessing the effects of DN mutants of ERK1/2 and JNK1/2 in MUC5AC induction by S. pneumoniae. As shown in Figure 4B, overexpression of DN mutant of either ERK1 or ERK2 inhibited MUC5AC induction by S. pneumoniae, whereas overexpression of DN mutant of either JNK1 or JNK2 enhanced it. Consistent with these findings, pretreatment with PD98059, a specific inhibitor for ERK1/2 MAPK, inhibited MUC5AC induction by S. pneumoniae, whereas pretreatment with SP600125, a specific inhibitor for JNK1/2 MAPK, enhanced MUC5AC induction by S. pneumoniae (data not shown). To further determine whether distal AP-1 site (-3576/-3570bp) mediates ERK1/2-mediated positive regulation of S. pneumoniae-induced MUC5AC transcription, we accessed the effect of PD98059 on S. pneumoniae-induced MUC5AC induction in cells transfected with either wild-type MUC5AC reporter gene or mutant containing mutation at distal AP-1 site (pMUC5AC-dAP1mt-300TK-Luc). As shown in Figure 4C, S. pneumoniae-induced MUC5AC induction was inhibited by pretreatment with PD98059 in cells transfected with wild-type MUC5AC reporter gene. However, PD98059 pretreatment did not show further inhibitory effect in cells transfected with pMUC5AC-dAP1mt-300TK-Luc. Next, to determine whether proximal AP-1 site (-3535/-3529bp) mediates JNK1/2-mediated negative regulation of S. pneumoniae-induced MUC5AC transcription, we accessed the effect of SP600125 on S. pneumoniae-induced MUC5AC induction in cells transfected with either wild-type MUC5AC reporter gene or mutant containing mutation at proximal AP-1 site (pMUC5AC-pAP1mt-300TK-Luc). As shown in Figure 4D, S. pneumoniae-induced MUC5AC induction was enhanced by pretreatment with SP600125 in cells transfected with wild-type MUC5AC reporter gene. However, SP600125 pretreatment did not show any further enhancing effect in cells transfected with pMUC5AC-pAP1mt-300TK-Luc. Collectively, these data indicate that two distinct AP-1 sites in MUC5AC promoter differentially mediate S. pneumoniae-induced MUC5AC transcription in epithelial cells. The distal AP-1 (-3576/-3570bp) mediates the positive regulation of S. pneumoniae-induced MUC5AC up-regulation via ERK1/2 MAPK signaling pathway, whereas the proximal AP-1 (-3535/-3529bp) mediates the negative regulation of MUC5AC induction via JNK1/2 MAPK signaling pathway.
Figure 4.
Two distinct AP-1 sites in MUC5AC promoter region mediate the ERK-dependent positive and the JNK-dependent negative regulation of MUC5AC induction, respectively. A, HM3 cells were treated with S. pneumoniae strain D39, pneumolysin-deficient strain PLN, or purified pneumolysin, and expression levels of total and phosphorylated ATF-2 and c-Jun were measured by western blotting analysis. B, HM3 cells were transfected with pMUC5AC-300TK-Luc with or without DN mutants of ERK1/2 or JNK1/2, and treated with S. pneumoniae strain D39. MUC5AC transcription was measured 5 hours after S. pneumoniae or control treatment. C, HM3 cells were transfected with pMUC5AC-300TK-Luc or pMUC5AC-dAP1mt-300TK-Luc, and treated with PD98059 for 1 hour followed by S. pneumoniae strain D39 treatment. MUC5AC transcription was measured 5 hours after S. pneumoniae or control treatment. D, HM3 cells were transfected with pMUC5AC-300TK-Luc or pMUC5AC-pAP1mt-300TK-Luc, and treated with SP600125 for 1 hour followed by S. pneumoniae strain D39 treatment. MUC5AC transcription was measured 5 hours after S. pneumoniae or control treatment. Data in A are representative result from three independent experiments. The values presented in B – D are Mean ± SD (n = 3). *, p<0.05 compared with CON in vector transfected cells (B) or wild-type reporter gene transfected cells (C & D); **, p<0.05 compared with S. pneumoniae treatment in vector transfected cells (B) or wild-type reporter gene transfected cells (C & D); #, p>0.05 compared with S. pneumoniae treatment in mutant reporter gene transfected cells (C & D).
Overproduction of mucin is hallmark of respiratory bacterial infectious disease [3, 4, 45, 46]. It causes mucus obstruction in the airway in COPD and conductive hearing loss in the middle ear in OM. To date, 20 mucin genes have been identified [45, 47]. Among these, at least MUC2, MUC5AC and MUC5B have been shown to play an important role in the pathogenesis of respiratory infectious disease [45, 47]. We recently reported that S. pneumoniae, the most common bacterial isolate in OM in children [16, 17], greatly up-regulates MUC5AC expression [20, 21]. However, the precise molecular mechanisms underlying S. pneumoniae-induced MUC5AC up-regulation still remains largely unknown. Given the fact that currently available vaccines for S. pneumoniae are unable to provide effective protection against OM [18, 19], and inappropriate antibiotic treatment of OM contributes significantly to the worldwide emergence of antibiotic-resistant strains of S. pneumoniae, there is an urgent need for developing novel therapeutic agents for the treatment of S. pneumoniae-induced OM based on full understanding of the molecular pathogenesis of OM, in particular S. pneumoniae-induced mucus overproduction. Previously, two MAPKs ERK1/2 and JNK1/2 have been identified as key signaling molecules mediating S. pneumoniae-induced MUC5AC expression, one as a positive regulator and the other as a negative regulator, respectively [20, 21]. However, the up-stream activators and down-stream transcriptional factors mediating these two MAPKs-mediated regulation of MUC5AC expression were unknown. In the present study (Fig. 5), we found that Ras-Raf-1 signaling cascade acts as an up-stream activator for ERK1/2 MAPK, thereby mediating the positive regulation of S. pneumoniae-induced MUC5AC transcription. In contrast, MEKK3 acts as an up-stream activator for JNK1/2 MAPK, thereby mediating the negative regulation of S. pneumoniae-induced MUC5AC transcription. Finally, two distinct AP-1 sites in the MUC5AC promoter region mediate the ERK-dependent positive and the JNK-dependent negative regulation of MUC5AC induction, respectively. Thus, the present study unveiled a novel signaling mechanism underlying the tight regulation of MUC5AC mucin induction by S. pneumoniae and may lead to the development of new therapeutic strategy for reducing mucus overproduction in both OM and COPD. Future studies will focus on developing novel therapeutic agents for treating these diseases.
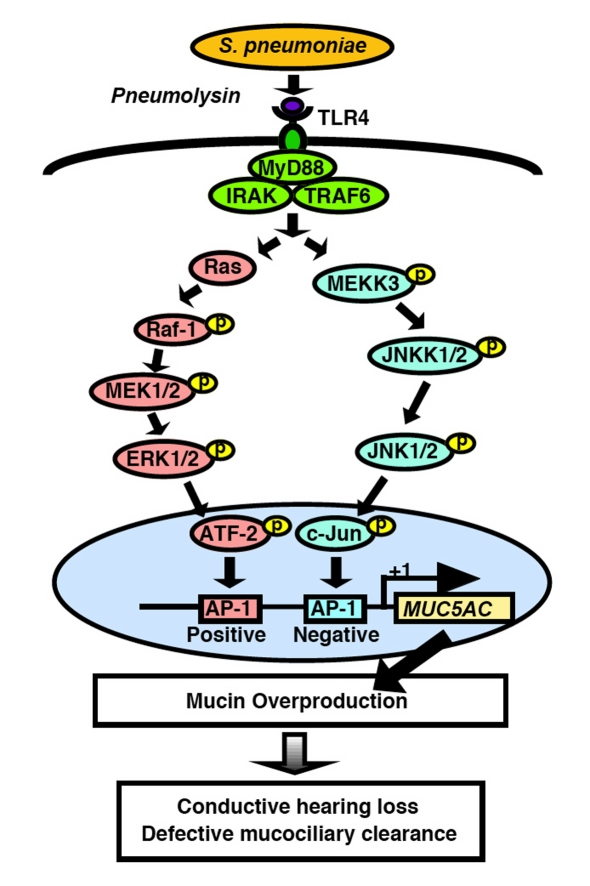
Figure 5.
Schematic diagram representing tight regulation of S. pneumoniae-induced MUC5AC mu-cin transcription via positive regulation by Ras-Raf-1-MEK1/2-ERK1/2-dependent AP-1 activation and negative regulation by MEKK3-JNKK1/ 2-JNK1/2-dependent AP-1 activation. Abbreviations: S. pneumoniae, Streptococcus pneumoniae; IRAK, interleukin-1-receptor-associated kinase; TRAF6, TNF receptor-associated factor 6; MEK, MAPK/ ERK kinase; ERK, extracellular signal regulated kinase; MEKK3, MAPK/ERK kinase kinase 3; JNKK, c-Jun N-terminal kinase kinase; JNK, c-Jun N-terminal kinase; ATF-2, activating transcription factor-2; AP-1, activating protein-1.
Acknowledgments
We thank Dr. Bing Su for providing MEKK3 DN plasmid. This work was supported by grants from National Institute of Health RO1DC004562 and RO1DC005843. We thank members of the Li laboratory for helpful discussion and suggestions.
References
- 1.Kopp E, Medzhitov R. Recognition of microbi-al infection by Toll-like receptors. Curr Opin Immunol. 2003;15:396–401. doi: 10.1016/s0952-7915(03)00080-3. [DOI] [PubMed] [Google Scholar]
- 2.Rose MC, Voynow JA. Respiratory tract mucin genes and mucin glycoproteins in health and disease. Physiol Rev. 2006;86:245–278. doi: 10.1152/physrev.00010.2005. [DOI] [PubMed] [Google Scholar]
- 3.Knowles MR, Boucher RC. Mucus clearance as a primary innate defense mechanism for mammalian airways. J Clin Invest. 2002;109:571–577. doi: 10.1172/JCI15217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rose MC, Nickola TJ, Voynow JA. Airway mucus obstruction: mucin glycoproteins, MUC gene regulation and goblet cell hyperplasia. Am J Respir Cell Mol Biol. 2001;25:533–537. doi: 10.1165/ajrcmb.25.5.f218. [DOI] [PubMed] [Google Scholar]
- 5.Majima Y, Hamaguchi Y, Hirata K, Takeuchi K, Morishita A, Sakakura Y. Hearing impairment in relation to viscoelasticity of middle ear effusions in children. Ann Otol Rhinol Laryngol. 1988;97:272–274. doi: 10.1177/000348948809700311. [DOI] [PubMed] [Google Scholar]
- 6.Reichman J, Healey WC. Learning disabilities and conductive hearing loss involving otitis media. J Learn Disabil. 1983;16:272–278. doi: 10.1177/002221948301600506. [DOI] [PubMed] [Google Scholar]
- 7.Foxwell AR, Kyd JM, Cripps AW. Nontypeable Haemophilus influenzae: pathogenesis and prevention. Microbiol Mol Biol Rev. 1998;62:294–308. doi: 10.1128/mmbr.62.2.294-308.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lim DJ, Chun YM, Lee HY, Moon SK, Chang KH, Li JD, Andalibi A. Cell biology of tubotympanum in relation to pathogenesis of otitis media - a review. Vaccine. 2000;19 Suppl 1:S17–25. doi: 10.1016/s0264-410x(00)00273-5. [DOI] [PubMed] [Google Scholar]
- 9.Chen YP, Tong HH, James, Demaria TF. Detection of mucin gene expression in normal rat middle ear mucosa by reverse transcriptase-polymerase chain reaction. Acta Otolaryngol. 2001;121:45–51. doi: 10.1080/000164801300006263. [DOI] [PubMed] [Google Scholar]
- 10.Rose AS, Prazma J, Randell SH, Baggett HC, Lane AP, Pillsbury HC. Nitric oxide mediates mucin secretion in endotoxin-induced otitis media with effusion. Otolaryngol Head Neck Surg. 1997;116:308–316. doi: 10.1016/S0194-59989770265-1. [DOI] [PubMed] [Google Scholar]
- 11.Gates GA. Cost-effectiveness considerations in otitis media treatment. Otolaryngol Head Neck Surg. 1996;114:525–530. doi: 10.1016/S0194-59989670243-7. [DOI] [PubMed] [Google Scholar]
- 12.Klein JO. The burden of otitis media. Vaccine. 2000;19(Suppl 1):S2–8. doi: 10.1016/s0264-410x(00)00271-1. [DOI] [PubMed] [Google Scholar]
- 13.Teele DW, Klein JO, Rosner B. Epidemiology of otitis media during the first seven years of life in children in greater Boston: a prospective, cohort study. J Infect Dis. 1989;160:83–94. doi: 10.1093/infdis/160.1.83. [DOI] [PubMed] [Google Scholar]
- 14.Murphy TF. Bacterial otitis media: pathogenetic considerations. Pediatr Infect Dis J. 2000;19:S9–15. doi: 10.1097/00006454-200005001-00003. discussion S15–16. [DOI] [PubMed] [Google Scholar]
- 15.Faden H. The microbiologic and immunologic basis for recurrent otitis media in children. Eur J Pediatr. 2001;160:407–413. doi: 10.1007/s004310100754. [DOI] [PubMed] [Google Scholar]
- 16.DeMaria TF, Bakaletz LO, Chonmaitree T, Heikkinen T, Hurst DS, Kawauchi H, Kurono Y, Patel JA, Sih TM, Stenfors LE, Suzuki M. Recent advances in otitis media. 6. Microbiology and immunology. Ann Otol Rhinol Laryngol Suppl. 2002;188:62–81. [PubMed] [Google Scholar]
- 17.Block SL. Causative pathogens, antibiotic resistance and therapeutic considerations in acute otitis media. Pediatr Infect Dis J. 1997;16:449–456. doi: 10.1097/00006454-199704000-00029. [DOI] [PubMed] [Google Scholar]
- 18.Straetemans M, Sanders EA, Veenhoven RH, Schilder AG, Damoiseaux RA, Zielhuis GA. Review of randomized controlled trials on pneumococcal vaccination for prevention of otitis media. Pediatr Infect Dis J. 2003;22:515–524. doi: 10.1097/01.inf.0000069763.08122.1c. [DOI] [PubMed] [Google Scholar]
- 19.Veenhoven R, Bogaert D, Uiterwaal C, Brouwer C, Kiezebrink H, Bruin J, E IJ, Hermans P, de Groot R, Zegers B, Kuis W, Rijkers G, Schilder A, Sanders E. Effect of conjugate pneumococcal vaccine followed by polysaccharide pneumococcal vaccine on recurrent acute otitis media: a randomised study. Lancet. 2003;361:2189–2195. doi: 10.1016/S0140-6736(03)13772-5. [DOI] [PubMed] [Google Scholar]
- 20.Ha U, Lim JH, Jono H, Koga T, Srivastava A, Malley R, Pages G, Pouyssegur J, Li JD. A novel role for IkappaB kinase (IKK) alpha and IKKbeta in ERK-dependent up-regulation of MUC5AC mucin transcription by Streptococcus pneumoniae. J Immunol. 2007;178:1736–1747. doi: 10.4049/jimmunol.178.3.1736. [DOI] [PubMed] [Google Scholar]
- 21.Ha UH, Lim JH, Kim HJ, Wu W, Jin S, Xu H, Li JD. MKP1 regulates the induction of MUC5AC mucin by Streptococcus pneumoniae pneumolysin by inhibiting the PAK4-JNK signaling pathway. J Biol Chem. 2008;283:30624–30631. doi: 10.1074/jbc.M802519200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lim JH, Stirling B, Derry J, Koga T, Jono H, Woo CH, Xu H, Bourne P, Ha UH, Ishinaga H, An-dalibi A, Feng XH, Zhu H, Huang Y, Zhang W, Weng X, Yan C, Yin Z, Briles DE, Davis RJ, Flavell RA, Li JD. Tumor suppressor CYLD regulates acute lung injury in lethal Streptococcus pneumoniae infections. Immunity. 2007;27:349–360. doi: 10.1016/j.immuni.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 23.Lim JH, Ha U, Sakai A, Woo CH, Kweon SM, Xu H, Li JD. Streptococcus pneumoniae syn-ergizes with nontypeable Haemophilus influenzae to induce inflammation via upregulating TLR2. BMC Immunol. 2008;9:40. doi: 10.1186/1471-2172-9-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Benton KA, Everson MP, Briles DE. A pneumolysin-negative mutant of Streptococcus pneumoniae causes chronic bacteremia rather than acute sepsis in mice. Infect Immun. 1995;63:448–455. doi: 10.1128/iai.63.2.448-455.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Benton KA, Paton JC, Briles DE. The hemolytic and complement-activating properties of pneumolysin do not contribute individually to virulence in a pneumococcal bacteremia model. Microb Pathog. 1997;23:201–209. doi: 10.1006/mpat.1997.0150. [DOI] [PubMed] [Google Scholar]
- 26.Chun YM, Moon SK, Lee HY, Webster P, Brackmann DE, Rhim JS, Lim DJ. Immortalization of normal adult human middle ear epithelial cells using a retrovirus containing the E6/E7 genes of human papillomavirus type 16. Ann Otol Rhinol Laryngol. 2002;111:507–517. doi: 10.1177/000348940211100606. [DOI] [PubMed] [Google Scholar]
- 27.Kweon SM, Wang B, Rixter D, Lim JH, Koga T, Ishinaga H, Chen LF, Jono H, Xu H, Li JD. Syn-ergistic activation of NF-kappaB by nontypeable H. influenzae and S. pneumoniae is me-diated by CK2, IKKbeta-IkappaBalpha, and p38 MAPK. Biochem Biophys Res Commun. 2006;351:368–375. doi: 10.1016/j.bbrc.2006.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jono H, Lim JH, Chen LF, Xu H, Trompouki E, Pan ZK, Mosialos G, Li JD. NF-kappaB is essential for induction of CYLD, the negative regulator of NF-kappaB: evidence for a novel inducible autoregulatory feedback pathway. J Biol Chem. 2004;279:36171–36174. doi: 10.1074/jbc.M406638200. [DOI] [PubMed] [Google Scholar]
- 29.Komatsu K, Jono H, Lim JH, Imasato A, Xu H, Kai H, Yan C, Li JD. Glucocorticoids inhibit nontypeable Haemophilus influenzae-induced MUC5AC mucin expression via MAPK phospha-tase-1-dependent inhibition of p38 MAPK. Biochem Biophys Res Commun. 2008;377:763–768. doi: 10.1016/j.bbrc.2008.10.091. [DOI] [PubMed] [Google Scholar]
- 30.Ishinaga H, Jono H, Lim JH, Komatsu K, Xu X, Lee J, Woo CH, Xu H, Feng XH, Chen LF, Yan C, Li JD. Synergistic induction of nuclear factor-kappaB by transforming growth factor-beta and tumour necrosis factor-alpha is mediated by protein kinase A-dependent RelA acetylation. Biochem J. 2009;417:583–591. doi: 10.1042/BJ20080781. [DOI] [PubMed] [Google Scholar]
- 31.Lim JH, Ha UH, Woo CH, Xu H, Li JD. CYLD is a crucial negative regulator of innate immune response in Escherichia coli pneumonia. Cell Microbiol. 2008;10:2247–2256. doi: 10.1111/j.1462-5822.2008.01204.x. [DOI] [PubMed] [Google Scholar]
- 32.Ishinaga H, Jono H, Lim JH, Kweon SM, Xu H, Ha UH, Koga T, Yan C, Feng XH, Chen LF, Li JD. TGF-beta induces p65 acetylation to enhance bacteria-induced NF-kappaB activation. Embo J. 2007;26:1150–1162. doi: 10.1038/sj.emboj.7601546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mikami F, Lim JH, Ishinaga H, Ha UH, Gu H, Koga T, Jono H, Kai H, Li JD. The transforming growth factor-beta-Smad3/4 signaling pathway acts as a positive regulator for TLR2 induction by bacteria via a dual mechanism involving functional cooperation with NF-kappaB and MAPK phosphatase 1-dependent negative cross-talk with p38 MAPK. J Biol Chem. 2006;281:22397–22408. doi: 10.1074/jbc.M602124200. [DOI] [PubMed] [Google Scholar]
- 34.Shuto T, Xu H, Wang B, Han J, Kai H, Gu XX, Murphy TF, Lim DJ, Li JD. Activation of NF-kappa B by nontypeable Hemophilus influenzae is mediated by toll-like receptor 2-TAK1-dependent NIK-IKK alpha /beta-I kappa B alpha and MKK3/6-p38 MAP kinase signaling pathways in epithelial cells. Proc Natl Acad Sci U S A. 2001;98:8774–8779. doi: 10.1073/pnas.151236098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang B, Lim DJ, Han J, Kim YS, Basbaum CB, Li JD. Novel cytoplasmic proteins of nonty-peable Haemophilus influenzae up-regulate human MUC5AC mucin transcription via a positive p38 mitogen-activated protein kinase pathway and a negative phosphoinositide 3-kinase-Akt pathway. J Biol Chem. 2002;277:949–957. doi: 10.1074/jbc.M107484200. [DOI] [PubMed] [Google Scholar]
- 36.Jono H, Xu H, Kai H, Lim DJ, Kim YS, Feng XH, Li JD. Transforming growth factor-beta-Smad signaling pathway negatively regulates nontypeable Haemophilus influenzae-induced MUC5AC mucin transcription via mitogen-activated protein kinase (MAPK) phosphatase-1-dependent inhibition of p38 MAPK. J Biol Chem. 2003;278:27811–27819. doi: 10.1074/jbc.M301773200. [DOI] [PubMed] [Google Scholar]
- 37.Chen R, Lim JH, Jono H, Gu XX, Kim YS, Bas-baum CB, Murphy TF, Li JD. Nontypeable Haemophilus influenzae lipoprotein P6 induces MUC5AC mucin transcription via TLR2-TAK1-dependent p38 MAPK-AP1 and IKKbeta-IkappaBalpha-NF-kappaB signaling pathways. Biochem Biophys Res Commun. 2004;324:1087–1094. doi: 10.1016/j.bbrc.2004.09.157. [DOI] [PubMed] [Google Scholar]
- 38.Jono H, Shuto T, Xu H, Kai H, Lim DJ, Gum JR, Jr, Kim YS, Yamaoka S, Feng XH, Li JD. Transforming growth factor-beta -Smad signaling pathway cooperates with NF-kappa B to mediate nontypeable Haemophilus influenzae-induced MUC2 mucin transcription. J Biol Chem. 2002;277:45547–45557. doi: 10.1074/jbc.M206883200. [DOI] [PubMed] [Google Scholar]
- 39.Shuto T, Imasato A, Jono H, Sakai A, Xu H, Watanabe T, Rixter DD, Kai H, Andalibi A, Linthicum F, Guan YL, Han J, Cato AC, Lim DJ, Akira S, Li JD. Glucocorticoids synergistically enhance nontypeable Haemophilus influenzae-induced Toll-like receptor 2 expression via a negative cross-talk with p38 MAP kinase. J Biol Chem. 2002;277:17263–17270. doi: 10.1074/jbc.M112190200. [DOI] [PubMed] [Google Scholar]
- 40.Huang Q, Yang J, Lin Y, Walker C, Cheng J, Liu ZG, Su B. Differential regulation of inter-leukin 1 receptor and Toll-like receptor signaling by MEKK3. Nat Immunol. 2004;5:98–103. doi: 10.1038/ni1014. [DOI] [PubMed] [Google Scholar]
- 41.Lim JH, Jono H, Koga T, Woo CH, Ishinaga H, Bourne P, Xu H, Ha UH, Li JD. Tumor suppressor CYLD acts as a negative regulator for non-typeable Haemophilus influenza-induced inflammation in the middle ear and lung of mice. PLoS ONE. 2007;2:e1032. doi: 10.1371/journal.pone.0001032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Raman M, Cobb MH. MAP kinase modules: many roads home. Curr Biol. 2003;13:R886–888. doi: 10.1016/j.cub.2003.10.053. [DOI] [PubMed] [Google Scholar]
- 43.Li JD, Feng W, Gallup M, Kim JH, Gum J, Kim Y, Basbaum C. Activation of NF-kappaB via a Src-dependent Ras-MAPK-pp90rsk pathway is required for Pseudomonas aeruginosa-induced mucin overproduction in epithelial cells. Proc Natl Acad Sci U S A. 1998;95:5718–5723. doi: 10.1073/pnas.95.10.5718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weston CR, Davis RJ. The JNK signal transduction pathway. Curr Opin Genet Dev. 2002;12:14–21. doi: 10.1016/s0959-437x(01)00258-1. [DOI] [PubMed] [Google Scholar]
- 45.Carrie S, Hutton DA, Birchall JP, Green GG, Pearson JP. Otitis media with effusion: components which contribute to the viscous properties. Acta Otolaryngol. 1992;112:504–511. doi: 10.3109/00016489209137432. [DOI] [PubMed] [Google Scholar]
- 46.Basbaum C, Lemjabbar H, Longphre M, Li D, Gensch E, McNamara N. Control of mucin transcription by diverse injury-induced signaling pathways. Am J Respir Crit Care Med. 1999;160:S44–48. doi: 10.1164/ajrccm.160.supplement_1.12. [DOI] [PubMed] [Google Scholar]
- 47.Kerschner JE. Mucin gene expression in human middle ear epithelium. Laryngoscope. 2007;117:1666–1676. doi: 10.1097/MLG.0b013e31806db531. [DOI] [PubMed] [Google Scholar]