Abstract
After pair-bonding, male prairie voles (Microtus ochrogaster) display aggression toward novel females but not toward their female partner. Here we show that this selective aggression in pair-bonded male prairie voles is associated with increased release of vasopressin (AVP) in the anterior hypothalamus (AH). Pharmacological activation of AVP-V1a receptors (V1aR) in the AH induced selective aggression in sexually naive males, whereas V1aR blockade diminished selective aggression in pair-bonded males. Pair-bonded males also showed an increased density in V1aR binding in the AH compared to their sexually naive counterparts and overexpression of V1aR in the AH, by viral vector-mediated gene transfer, facilitated aggression toward novel females. These data demonstrate that AH-AVP is both necessary and sufficient in the regulation of selective aggression associated with pair-bonding. In the second part of this study, we examined the effects of amphetamine (AMPH) exposure on female-directed aggression and revealed the potential role of AH-AVP underlying this behavior. Repeated AMPH administration in sexually naive male prairie voles enhanced V1aR expression in the AH and induced aggression toward a familiar or unfamiliar female. In addition, this AMPH-induced aggression was blocked by intra-AH administration of a V1aR antagonist. Together, our data reveal a socioneurobiological mechanism, highlighting a critical role of AH-AVP in the regulation of aggression induced by pair-bonding or drug experience in socially monogamous male prairie voles.
Keywords: behavioral pharmacology, drug abuse, microdialysis, neural plasticity, viral-vector gene transfer
Aggression is an agonistic behavior that plays an important role for survival and reproductive success. In both vertebrates (1) and invertebrates (2), aggression promotes fitness by acquiring territory, food, and mates, as well as by serving to protect offspring and avoid predation. In humans and nonhuman primates, aggressive behavior operates within social hierarchies to facilitate resource acquisition (3). Among the neurochemicals implicated in aggression (4, 5), arginine vasopressin (AVP), and its homolog vasotocin, have been found to regulate several forms of aggression in numerous species (6, 7) across diverse taxa (8, 9). Even in humans, central AVP correlates with aggressive behavior (10) and mediates anger (11). Therefore, the AVP system may have evolved to be primed by a wide variety of experiences to induce aggression, when appropriate, in social animals (12).
Drug use can override neurobiological programs to activate maladaptive forms of agonistic behavior, engaging inappropriate types of physical aggression (13), such as domestic violence (14) and intimate partner homicide (15). As a result, chronic drug abuse can cause permanent neural reorganization (16, 17), impairing the adaptive social brain (18), leading to the display of maladaptive social behavior (19). In rodent models such as hamsters, adolescent exposure to cocaine (20) or anabolic steroids (21, 22) induces agonistic behavior resembling male-male offensive aggression induced to establish territory through flank marking. Interestingly, these drug experiences are sufficient to reorganize AVP signaling at the level of the anterior hypothalamus (AH) (20–22), artificially activating a maladaptive form of physical aggression. However, the precise neurochemical properties regulating other adaptive forms of aggression, like mate guarding behavior, as well as their potential vulnerability to psychostimulants is still unknown.
Prairie voles (Microtus ochrogaster) are socially monogamous rodents that form mating-induced pair-bonds (23). Following 24 h of mating, male prairie voles display social preferences for their mates (partner preference) and intense aggression toward novel male and female conspecifics (selective aggression) (23). Although it has not been directly tested, selective aggression has been suggested to play an important role in mate guarding and thus in the maintenance of monogamous pair-bonds (24). Therefore, prairie voles represent a unique model system to investigate the neuromechansims underlying ethologically relevant aggression. Previous studies have shown that selective aggression is enduring (25, 26), associated with specific patterns of neuronal activation in the brain (26, 27), and can be blocked by central (intra-cerebroventricular; ICV) administration of an AVP-V1a receptor (V1aR) antagonist (28). Developmental exposure to AVP also facilitates aggression in adult prairie voles (29). However, previous work has focused on the neural mechanisms controlling male-male aggression, and therefore we know little about the neurobiology underlying aggression directed toward female conspecifics. Furthermore, the action site and dynamics of central AVP in the regulation of selective aggression and the impact that drugs of abuse may have on these neurobiological and behavioral systems are unknown.
In the present study, we first used in vivo brain microdialysis with ELISA to measure AVP release in the AH in pair-bonded males displaying selective aggression. Then, we pharmacologically manipulated AVP-V1aR in the AH to reveal its role in selective aggression. Next, we evaluated the neural plasticity of neuropeptide receptor systems associated with pair-bonding experience. As we found significant up-regulation of AVP-V1aR in the AH following pair-bonding, we performed site-specific viral vector-mediated gene transfer to enhance V1aR expression in the AH of sexually naive males and then examined their behavior. Because our data highlighted a critical role of anterior hypothalamic vasopressin (AH-AVP) in naturally occurring selective aggression toward novel females, we then examined if treatment with amphetamine (AMPH) could induce female-directed aggression, and whether this effect is mediated by AH-AVP in male prairie voles. We measured the effects of repeated AMPH exposure on aggression toward females as well as on the density of AVP-V1aR in the AH. We then pharmacologically blocked AVP-V1aR in the AH and examined its effects on AMPH-induced aggression. Together, our results reveal a neuroplastic change in AH-AVP signaling directly modulating female-directed aggression induced by either social or drug experiences.
Results
Intra-AH AVP Release During Selective Aggression.
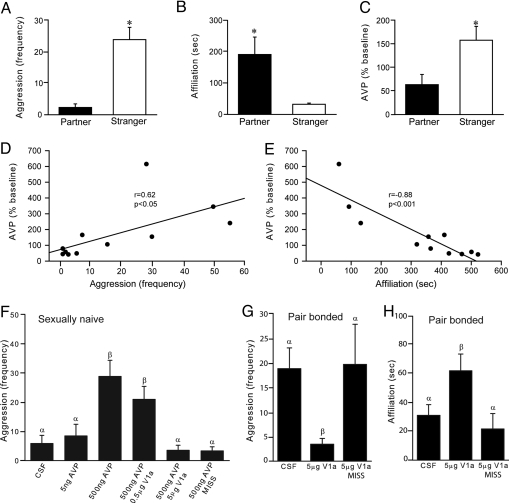
Based on the recent observation that selective aggression by male prairie voles was associated with neuronal activation in the AH, particularly in AVP-containing neurons (26), we first tested the hypothesis that selective aggression is associated with AH-AVP release. We performed in vivo brain microdialysis, with ELISA, on male prairie voles that were pair-bonded for 2 weeks. Subjects were implanted with a microdialysis probe aimed at the AH. After overnight recovery, their behavior toward a novel female or toward their female partner was examined by a 60-min resident-intruder test (RIT), during which microdialysis samples were continuously collected, and AVP contents were subsequently measured by ELISA. As found previously (25, 26), within the first 10 min of behavioral testing, pair-bonded males displayed significantly higher levels of aggression toward novel females (t = 5.79, P < 0.001) (Fig. 1A) but more side-by-side affiliation with their familiar partners (t = 2.57, P < 0.05) (Fig. 1B). ELISA analysis indicated that exposure to a novel female, compared to a familiar partner, increased AH-AVP release during the first 10 min of social interaction (t = 2.44, P < 0.05) (Fig. 1C). Correlation analyses, spanning the entire hour period, indicated that increased AH-AVP release was coupled positively with aggression (Fig. 1D) and negatively with affiliation (Fig. 1E).
Fig. 1.
AH-AVP is necessary and sufficient to regulate selective aggression. Male prairie voles that were pair-bonded for 2 weeks displayed robust aggression toward novel females (A) yet maintained a high level of affiliative side-by-side contact with their female partner (B) during a RIT. AVP release in the AH was significantly higher in males exposed to a novel female than in males re-exposed to their female partner (C). AH-AVP concentration was correlated positively with aggression (D) and negatively with affiliation (E). Sexually naive male prairie voles that received intra-AH infusions of a high (500 ng/side), but not a low (5 ng/side), dose of AVP displayed enhanced aggression toward a novel female relative to control males infused with CSF (F). Intra-AH administration of a high (5 μg/side), but not a low (0.5 μg/side), dose of the AVP-V1aR antagonist blocked AVP-induced aggression (F). Further, males with misplaced cannulae did not show AVP-induced aggression (F). For pair-bonded male prairie voles, intra-AH infusions of the AVP-V1aR antagonist blocked aggression (G) and induced affiliation (H) toward a novel female, relative to control males infused with CSF or males with misplaced cannulae. Bars indicate means ± standard error of the mean. Bars with different Greek letters differ significantly from each other. *, P < 0.05.
AH-V1aR Regulation of Selective Aggression.
As central (ICV) AVP regulates selective aggression (28) and the above data indicated increased AH-AVP release during aggression, we next tested the hypothesis that released AVP within the AH stimulates selective aggression. Bilateral cannulae, aimed at the AH, were stereotaxically implanted in the brain of sexually naive male prairie voles. After recovery, subjects received bilateral injections of cerebrospinal fluid (CSF; 200 nL/side) containing different doses of AVP or AVP with the V1aR antagonist, and then tested for aggression toward a novel female during a 10-min RIT. Intra-AH administration of AVP at a high (500 ng/side), but not a low (5 ng/side), dose induced aggression toward a novel female (F(5, 38) = 8.88, P < 0.01) (Fig. 1F). This AVP-induced aggression was mediated by V1aR, as it was blocked by concurrent administration of a 10-fold higher dose of the V1aR antagonist (Fig. 1F). This effect was also specific to the AH because males with misplaced cannulae did not show AVP-induced aggression (Fig. 1F).
To test if AVP-V1aR in the AH is necessary for naturally occurring selective aggression, males that were pair-bonded for 2 weeks were implanted with guide cannulae aimed at the AH received intra-AH injections of CSF or CSF containing the V1aR antagonist (5 μg/side) and then tested for aggression toward a novel female using a 10-min RIT. Compared to CSF controls, intra-AH infusions of the V1aR antagonist blocked aggression (F(2, 12) = 6.76, P < 0.01) (Fig. 1G) and facilitated social affiliation (F(2, 12) = 3.00, P < 0.05) (Fig. 1H) toward novel females, but such effects were not found in males with misplaced cannula. Therefore, AH-AVP is both necessary and sufficient for selective aggression in male prairie voles.
Increased AH-V1aR Binding Associated with Pair-Bonding.
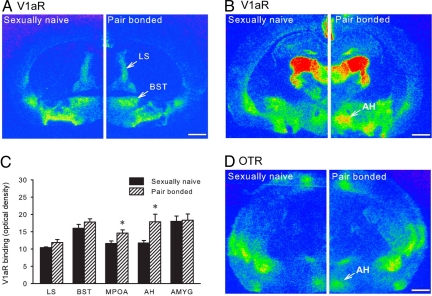
Environmental and endogenous factors can induce neuroplastic changes in the central nervous system, including neurons expressing AVP-V1aR (30). For example, social isolation increases the density of AH-V1aR to regulate offensive aggression in golden hamsters (31). Here we tested the hypothesis that the density of AH-V1aR changes with pair-bonding experience. Sexually naive males were compared with males that were pair-bonded for 2 weeks. Their brain sections were processed for AVP-V1aR autoradiographic binding. The two groups did not differ in the density of V1aR binding in the lateral septum (LS), bed nucleus of the stria terminalis (BST), or amygdala (AMYG) (Fig. 2 A and C). However, pair-bonded males showed higher densities of V1aR binding in the AH (t = 2.32, P < 0.05) (Fig. 2 B and C) and medial preoptic area (MPOA; t = 2.42, P < 0.05) (Fig. 2C). To examine if this effect was receptor-specific, an alternate set of brain sections was processed for oxytocin receptor (OTR) binding. OTR binding was visualized in the AH, but no group differences were found (Fig. 2D). Together, these data indicate that pair-bonding experience induces a neural plastic reorganization of AVP-V1aR in a region- and receptor-specific manner.
Fig. 2.
Neuropeptide receptor plasticity associated with pair-bonding experience. Sexually naive and pair-bonded male prairie voles were compared for AVP-V1aR binding. No group differences were observed in the densities of V1aR binding in the lateral septum (LS), bed nucleus of the stria terminalis (BST), and amygdala (AMYG) (A and C). However, pair-bonded males exhibited higher densities of V1aR binding in the anterior hypothalamus (AH) and medial preoptic area (MPOA) than sexually naive males (B and C). Oxytocin receptor (OTR) binding was also found in the AH, but no group differences were detected (D). Bars indicate means ± standard error of the mean. *, P < 0.05. (Scale bar, 1 mm.)
Enhanced AH-V1aR Expression Facilitates Female-Directed Aggression.
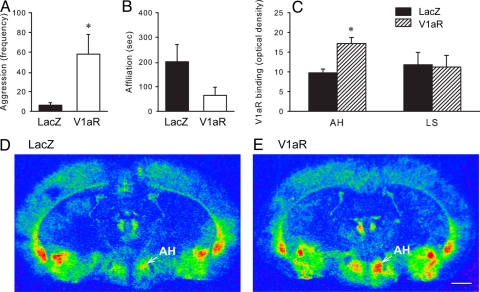
To determine whether the increase in V1aR density in the AH following pair-bonding is directly related to the emergence of aggression toward novel females, we used viral vector-mediated gene transfer to artificially elevate V1aR density in the AH of the vole brain. Sexually naive males were stereotaxically injected with an adeno-associated viral (AAV) vector, containing the prairie vole V1aR- or lacZ-gene (32–34), into the AH. Three weeks later, subjects were tested with a novel female in a 10-min RIT, and their brains were processed for AVP-V1aR binding. Males that received intra-AH infusions of the AAV-V1aR displayed higher levels of aggression toward a novel female compared to males that received infusions of the AAV-lacZ-gene (t = 2.45, P < 0.05) (Fig. 3A). The opposite trend was found in affiliative behavior, but the two groups did not differ significantly (Fig. 3B). AAV-V1aR males also showed an increased density of V1aR binding in the AH (t = 3.85, P < 0.01), but not LS, compared to AAV-lacZ controls (Fig. 3 C–E). These data suggest that enhanced expression of V1aR in the AH can facilitate female-directed aggression in male prairie voles.
Fig. 3.
V1aR gene transfer in the AH facilitates female-directed aggression. Sexually naive male prairie voles received intra-AH infusions of the AVP-V1aR viral vector (V1aR) or control LacZ viral vector (LacZ). Three weeks later, their behavioral interactions with a novel female were tested in a RIT. Males infused with the V1aR viral vector displayed increased aggression toward a novel female, relative to males infused with the LacZ viral vector (A). In addition, V1aR-males showed a significant increase in the density of V1aR binding in the anterior hypothalamus (AH), but not in the lateral septum (LS), than the LacZ-males (C–E). Bars indicate means ± standard error of the mean. *, P < 0.05. (Scale bar, 1 mm.)
AH-AVP Also Regulates AMPH-Induced Aggression.
It has been shown that exposure to drugs of abuse, such as cocaine, enhances male-male aggression by reorganizing the AH-AVP system in other social rodents (20). Therefore, we tested the hypothesis that AMPH, another commonly abused psychostimulant, would act in a similar fashion in affecting male-female aggression in prairie voles. We found that, compared to saline-treated controls, male prairie voles that received 3 days of repeated AMPH treatment displayed higher levels of aggression toward a novel female (t = 3.15, P < 0.01) (Fig. 4A), resembling the pattern of female-directed aggression that emerges after pair-bonding (25, 26). This AMPH treatment also induced an increase in the density of AVP-V1aR binding in the AH (t = 2.12, P < 0.05), but not MPOA, relative to saline controls (Fig. 4B). Furthermore, intra-AH infusions of CSF containing the V1aR antagonist, but not CSF alone, diminished AMPH-induced aggression toward novel females (t = 3.49, P < 0.01) (Fig. 4C). These data suggest that repeated exposure to AMPH can induce female-directed aggression and that this behavior is mediated by AH-AVP. Finally, to examine the selectivity of AMPH-induced aggression, males treated with saline or AMPH were tested for aggression toward an unfamiliar female or a familiar female (that cohabitated with the male across a wire mesh screen for 24 h without mating). Compared with saline-treated controls, AMPH-treated males displayed significantly higher levels of aggression toward either familiar or unfamiliar females (F(3, 24) = 12.18, P < 0.01) (Fig. 4D), indicating that AMPH exposure induces generalized aggression, rather than being selective to novel females.
Fig. 4.
AH-AVP underlies AMPH-induced aggression. Sexually naive male prairie voles received repeated AMPH (1 mg/kg) or saline (i.p.) for 3 days. Thereafter, their social behavior was tested with a novel female in a RIT. AMPH-treated males displayed significantly higher levels of aggression relative to their saline-treated counterparts (A) and had an increased density of AH-V1aR binding in the AH but not MPOA (B). Site-specific infusions of the AVP-V1aR antagonist into the AH of males that received 3-day repeated AMPH exposure significantly reduced their aggression toward novel females compared to males that received intra-AH infusions of CSF (C). Repeated AMPH exposure enhanced aggression toward both familiar and unfamiliar females (D). Bars indicate means ± standard error of the mean. Bars with different Greek letters differ significantly from each other. *, P < 0.05.
Discussion
Although male-male aggression has been studied in a variety of mammalian species, we know surprisingly little about male-female aggression and its underlying neuromechanisms. Here we show that pair-bonded male prairie voles display aggression toward novel conspecific females but not toward their familiar partners, and that this selective aggression is mediated by elevated AVP release and increased V1aR expression in the AH. This study demonstrates a direct role of AH-AVP in the regulation of selective aggression in male prairie voles. In addition, our data show that the same AH-AVP system also mediates generalized, female-directed aggression induced by AMPH. These data, together with previous research demonstrating AH-AVP involvement in a variety of aggressive behaviors in other species (35, 36), may highlight a unique point of convergence in the mammalian brain (37, 38). The AH-AVP system appears to be conserved and sensitive to multiple stimuli across diverse social animals and operates to control different forms of aggression; functioning to maintain a wide range of natural resources, from mates to offspring to territory.
Our approach by combining brain microdialysis with ELISA has generated intriguing data, demonstrating AH-AVP release during social interactions. These data corroborate previous work supporting the role of AH-AVP in territory-induced (39), drug-induced (20, 22), and early life stress-induced (35) aggression in other rodents, reported mostly from pharmacological studies. Further, an early study demonstrated the role of central (ICV) AVP on selective aggression in male prairie voles (28). Data from our current study provides evidence showing that the AH is a critical component of an AVP neural circuit in the regulation of selective aggression. Although it has not been examined in voles, it was speculated that the likely source of AVP in the AH may be from the nucleus circularis subpopulation of AVP neurons that are activated during offensive aggression in golden hamsters (40). The low doses of AVP used in our pharmacological manipulation likely reflect physiological effects on behavior and indicate the sensitivity of the AH to AVP-induced shifts in social behaviors ranging from intense physical aggression to social affiliation.
It has been shown that the social environment has a significant impact on signaling and structural components of AVP systems in the central nervous system. In marmoset monkeys, for example, prefrontal AVP-V1aR increases during fatherhood (30). In hamsters, several social and drug paradigms have been shown to directly alter the AH-AVP system to regulate offensive aggression (20–22, 39). In a previous study in male prairie voles, cohabitation with a female significantly increased the number of AVP mRNA labeled cells in the BST (41). Our current data showing a site- and neuropeptide-specific increase in AH-V1aR associated with pair-bonding or with AMPH experience also represent an interesting parallel to this literature.
In male prairie voles, pair-bonding experience appears to reorganize the AH-AVP system priming their brains to respond aggressively to novel females. This experience-dependent neuroplastic change may function to maintain monogamous bonds, as pair-bonded males aggressively reject potential mates. By overexpressing AVP-V1aR, site-specifically, in the AH in sexually naive males, we recreated the natural effects of AH-AVP receptor neuroplasticity observed from pair-bonding experience and revealed a direct function of AH-V1aR in facilitating aggression toward novel females. Similar viral vector-mediated increases in V1aR expression in the ventral pallidum in voles (32, 33) and lateral septum in mice (34) enhanced affiliation. Together, these data support the notion that region-specific AVP-V1aR expression regulates specific types of social behaviors (42), and multiple brain regions may serve as a circuit (23) in which AVP coordinates a range of adaptive social behaviors important for reproductive success.
Finally, AMPH has been shown to disrupt naturally occurring prosocial behaviors (43) and to induce intense aggression in several species including humans (44). Data from the present study show that AMPH experience in male prairie voles facilitated female-directed aggression. It should be noted that such AMPH-induced aggression is nonselective, and is directed equally toward unfamiliar or familiar females. Interestingly, this AMPH experience also induced a significant up-regulation of AH-V1aR expression, similarly, as observed in pair-bonded males. While it is not surprising that drugs of abuse act on the same neurobiological substrates mediating natural reward (45–48), our findings provide additional evidence to support the above notion and highlights the role of the neuropeptide AVP underlying naturally induced and drug-facilitated female-directed aggression in male prairie voles. These data also indicate the utility of the prairie vole model for evaluation of the effects of drug abuse on neural systems controlling adaptive forms of social behavior, such as mate guarding. Given that other neurochemicals, such as dopamine, have also been implicated in selective aggression associated with pair-bonding in prairie voles (25) and offensive aggression related to drug experience in other rodents (49), it will be important in future studies to examine neurochemical interactions in the regulation of agonistic behavior.
Materials and Methods
Subjects and Behavioral Testing.
Subjects were adult (90–150 days old) male prairie voles (M. ochrogaster) that were sexually naive or paired with a female for 2 weeks (pair-bonded). Subjects' behavioral interactions with an ovariectomized (OVX), estradiol-primed unfamiliar female, familiar female (24-h nonsexual cohabitation with partition), or female partner (2-weeks sexual and social cohabitation) were quantified during a 10-min resident-intruder test (RIT) (50) by trained independent observers blind to study conditions and treatment. The following behavioral patterns of the resident were subsequently quantified: the frequency of attacks, bites, chases, defensive/offensive upright postures, offensive sniffs, threats, and retaliatory attacks, and calculated as a composite score (aggression frequency) (26), as well as the duration of affiliative side-by-side physical contact (28). Procedures for stereotaxical cannulation, site-specific-infusions, and AMPH injections were described previously (25, 51). Bilateral guide cannulae were stereotaxically implanted and aimed into the AH (coordinates from bregma: posterior 0.55 mm, lateral ± 0.75 mm, ventral 5.9 mm). After a 4-day recovery, subjects were injected with CSF (200 nL/side) containing AVP, AVP-V1aR antagonist, or both, followed by RIT.
AH Microdialysis and AVP ELISA Analysis.
Microdialysis probe construction, cannulation, and dialysate collection were previously described (52). The dialysis membrane had a 1.0-mm active area and molecular weight cutoff of 18 kDa. Probes were perfused continuously at 1.0 μL/min with an isotonic solution using a glass Hamilton syringe connected to an automatic micropump (World Precision Instruments) (52). Dialysates were collected every 5 min, for five consecutive samples before RIT (baseline), and then for 1 h during RIT into vials containing 5 μL 0.1 N HCl, immediately frozen on dry ice, and stored at −80 °C. AVP concentration in dialysates was measured using a standard ELISA kit (Cayman Chemical). The AVP antiserum has high specificity and little cross reactivity with vasopressin intestinal peptide (<0.01%), oxytocin (<0.01%), Lys-vasopressin (<0.01%), Leu-enkephalin (<0.01%), Met-enkephalin (0.08%), or dynorphin A (1.6%). Samples were collapsed and analyzed as baseline and every 10 min during social interaction.
Receptor Autoradiographic Binding.
Receptor binding was performed, using previously established methods, with the [125I]-linear-AVP for V1aR (53) and [125I]-oxytocin for OTR binding (54). Binding density in selected brain areas was quantified bilaterally in three to four matched brain sections using National Institutes of Health (NIH)-IMAGE.
AH Viral Vector Injections.
The production and characteristics of the adeno-associated V1aR viral vector (NSE-V1aR) or control LacZ viral vector were previously described in detail (32–34). The NSE-V1aR and lacZ were dissolved in lactated Ringer's solution and injected (0.25 μL/side) bilaterally into the AH using an automatic micropump at a rate of 1 nL/s (32–34). Three weeks later, subjects were tested in a 10-min RIT, and their brain sections were then processed for AVP-V1aR autoradiographic binding.
Data Analysis.
The frequency and duration of behaviors during RIT were analyzed by a t-test or a one- or two-way analysis of variance (ANOVA) followed by a Student Newman-Keuls (SNK) posthoc test. Group differences in AVP content (percent change from baseline) in dialysates during the first 10 min of the RIT period were analyzed by a t-test. In addition, the mean AVP content (percent change from baseline) during the entire 60-min RIT was correlated with the corresponding aggression and affiliation averages. Group differences in the density of V1aR or OTR binding were analyzed by a t-test.
Experimental Procedures.
Experiment 1 used brain microdialysis with AVP ELISA analysis to measure AVP release in the AH during selective aggression. Males that were pair-bonded for 2 weeks were stereotaxically implanted with a microdialysis probe aimed at the AH, while the probe was infused continuously with an isotonic solution (52). After overnight recovery, baseline samples were collected before behavioral testing. Subjects were then randomly divided into two groups that were exposed to a novel female (n = 5) or re-exposed to their female partner (n = 6). Their behavioral interaction was videotaped for 60 min, and subsequently quantified for each 10-min interval throughout the entire 60-min RIT. Microdialysis samples were collected every 5 min continuously during the 60-min RIT. Following collection of the last sample, fast green dye (1%) was infused, and the subjects were overdosed with sodium pentobarbital (1 mg/10 g body weight). Their brains were removed, cut on a cryostat, and probe placement histologically verified. AVP contents in the microdialysate samples were measured using a standard ELISA kit.
Experiment 2 examined the role of AH-V1aR in selective aggression. Sexually naive male prairie voles were implanted with guide cannulae bilaterally aimed at the AH. After 4 days of recovery, they were randomly assigned into one of the following experimental groups that received injections of CSF (200 nL/side) (n = 8), or CSF containing 5 ng AVP (n = 7), 500 ng AVP (n = 7), 500 ng AVP with 0.5 μg V1a antagonist (n = 7), or 500 ng AVP with 5 μg V1a antagonist (n = 8). Injections were made using a Hamilton syringe connected to an automatic micropump. Thereafter, subjects were exposed to a novel female during a 10-min RIT, and their behavior was videotaped and subsequently quantified. Immediately after RIT, subjects were overdosed with sodium pentobarbital, decapitated, and their brains were sectioned for histological verification of cannula placement. Seven subjects that received injections of 500 ng AVP showed cannula placement outside the AH, and thus these animals formed an additional site-specific control group.
To examine the effects of AVP-V1aR blockade in the AH on pair-bonding-induced selective aggression, male prairie voles that had been paired with a female for 2 weeks were implanted with guide cannulae aimed at the AH. They were housed with their female partner continuously during the 4-day recovery period. Thereafter, the female partner was removed. Subjects were then divided into two groups receiving either intra-AH injections of CSF (n = 5) or AVP-V1aR antagonist (5 μg in 200 nL CSF/side) (n = 7), followed by a 10-min RIT toward a novel female. Cannula placement was verified histologically following RIT. Males injected with the AVP-V1aR antagonist that had cannula placed outside the AH (n = 3) were grouped and used as an additional control group.
Experiment 3 examined the effects of pair-bonding experience on AVP-V1aR binding. Sexually naive males (n = 6) were compared with males that were pair-bonded for 2 weeks (n = 8). Subjects were decapitated, brains harvested, frozen on dry ice, cut into coronal sections (14-μm thickness), and mounted onto Superfrost/plus slides (Fisher Scientific). Brain sections at 112-μm intervals were processed for AVP-V1aR binding using the [125I]-linear-AVP ligand (PerkinElmer). To examine receptor specificity, another set of brain sections was processed for OTR binding using the [125I]-oxytocin ligand (PerkinElmer).
Experiment 4 used viral vector-mediated V1aR gene transfer to examine if increased AVP-V1aR expression in the AH was directly related to selective aggression. Sexually naive male prairie voles were assigned into one of two experimental groups that received intra-AH injections of the adeno-associated viral (AAV) vector containing either the prairie vole V1aR (n = 8) or the control lacZ-gene (n = 7), as described in previous studies (32–34). Three weeks later, subjects were tested in a 10-min RIT, and their behavior toward a novel female was recorded and subsequently quantified. Immediately after RIT, subjects were decapitated, and their brain sections were processed for AVP-V1aR autoradiographic binding while injection placement was also verified.
Experiment 5 examined the effects of repeated AMPH exposure on aggression and on AVP-V1aR binding in male prairie voles. Because our recent data revealed that repeated AMPH treatment (1 mg/kg) for 3 consecutive days induced conditioned place preference (51), this treatment regimen was used. Sexually naive male prairie voles were divided into two groups: i.p. injected with AMPH (1 mg/kg, n = 8) or saline (n = 8) once daily for 3 consecutive days. On the fourth day, subject's social interaction with a novel female was tested in a 10-min RIT. Another set of male prairie voles also received 3 days of AMPH (n = 7) or saline (n = 5) treatment. These animals were killed on the fourth day, and their brain sections were processed for AVP-V1aR autoradiographic binding.
Experiment 6 examined if pharmacological blockade of V1aR in the AH diminished AMPH-induced aggression. Male prairie voles were stereotaxically implanted with guide cannulae aimed at the AH. After 4 days of recovery, they received 3 days of repeated AMPH injections. They were then randomly divided into two groups that received intra-AH injections of CSF (200 nL/side, n = 7) or CSF containing 5 μg V1a antagonist (n = 7), followed by a 10-min RIT toward a novel female. Cannula placement was verified histologically following RIT.
Experiment 7 tested the specificity of AMPH-induced aggression. Sexually naive male prairie voles were injected once daily with AMPH (1 mg/kg) (n = 16) or saline (n = 12) for 3 consecutive days followed by a 24-h nonsexual cohabitation with an OVX, estradiol-primed female. Subjects and the female were separated by a wire mesh screen to prevent mating but to allow for visual, tactile, and chemosensory exchange. As mating is essential for induction of selective aggression in male prairie voles (55), this paradigm allowed our subjects to establish familiarity with the female without developing selective aggression. Experimental males were then randomly divided into two groups: re-exposed to their familiar female (n = 6, saline; n = 8, AMPH) or exposed to a novel OVX and estradiol-primed female (n = 6, saline; n = 8, AMPH), and their social behavior was tested during a 10-min RIT.
Acknowledgments.
We thank K. Young, C. Lieberwirth, K. Lei, M. Martin, and A. Smith for critically reading this manuscript; H. Sandler, K. Mauk, M. Thairu, J. Kennett, and R. Shirley for their technical assistance; and E. Hammock and T. Curtis for their experimental design suggestions. This work was supported by National Institutes of Health Grants MHF31–79600 (to K.L.G.); MHR01–58616, DAR01–19627, and DAK02–23048 (to Z.W.), MH56897 and MH64692 (to L.J.Y.), and National Institutes of Health Program Training Grant T32 NS-07437.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Adams DB. Brain mechanisms of aggressive behavior: An updated review. Neurosci Biobehav Rev. 2006;30:304–318. doi: 10.1016/j.neubiorev.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 2.Kravitz EA, Huber R. Aggression in invertebrates. Curr Opin Neurobiol. 2003;13:736–743. doi: 10.1016/j.conb.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 3.Pedersen CA. Biological aspects of social bonding and the roots of human violence. Ann N Y Acad Sci. 2004;1036:106–127. doi: 10.1196/annals.1330.006. [DOI] [PubMed] [Google Scholar]
- 4.Siever LJ. Neurobiology of aggression and violence. Am J Psychiatry. 2008;165:429–442. doi: 10.1176/appi.ajp.2008.07111774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miczek KA, et al. Neurobiology of escalated aggression and violence. J Neurosci. 2007;27:11803–11806. doi: 10.1523/JNEUROSCI.3500-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caldwell HK, Lee HJ, Macbeth AH, Young WS., III Vasopressin: Behavioral roles of an “original” neuropeptide. Prog Neurobiol. 2008;84:1–24. doi: 10.1016/j.pneurobio.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Riters LV, Panksepp J. Effects of vasotocin on aggressive behavior in male Japanese quail. Ann N Y Acad Sci. 1997;807:478–480. doi: 10.1111/j.1749-6632.1997.tb51943.x. [DOI] [PubMed] [Google Scholar]
- 8.Goodson JL. Nonapeptides and the evolutionary patterning of sociality. Prog Brain Res. 2008;170:3–15. doi: 10.1016/S0079-6123(08)00401-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Backstrom T, Winberg S. Arginine-vasotocin influence on aggressive behavior and dominance in rainbow trout. Physiol Behav. 2009;96:470–475. doi: 10.1016/j.physbeh.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 10.Coccaro EF, et al. Cerebrospinal fluid vasopressin levels: Correlates with aggression and serotonin function in personality-disordered subjects. Arch Gen Psychiatry. 1998;55:708–714. doi: 10.1001/archpsyc.55.8.708. [DOI] [PubMed] [Google Scholar]
- 11.Thompson R, et al. The effects of vasopressin on human facial responses related to social communication. Psychoneuroendocrinology. 2004;29:35–48. doi: 10.1016/s0306-4530(02)00133-6. [DOI] [PubMed] [Google Scholar]
- 12.Donaldson ZR, Young LJ. Oxytocin, vasopressin, and the neurogenetics of sociality. Science. 2008;322:900–904. doi: 10.1126/science.1158668. [DOI] [PubMed] [Google Scholar]
- 13.Swartz MS, et al. Violence and severe mental illness: The effects of substance abuse and nonadherence to medication. Am J Psychiatry. 1998;155:226–231. doi: 10.1176/ajp.155.2.226. [DOI] [PubMed] [Google Scholar]
- 14.Moore TM, et al. Drug abuse and aggression between intimate partners: A meta-analytic review. Clin Psychol Rev. 2008;28:247–274. doi: 10.1016/j.cpr.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 15.Farooque RS, Stout RG, Ernst FA. Heterosexual intimate partner homicide: Review of ten years of clinical experience. J Forensic Sci. 2005;50:648–651. [PubMed] [Google Scholar]
- 16.Nestler EJ, Aghajanian GK. Molecular and cellular basis of addiction. Science. 1997;278:58–63. doi: 10.1126/science.278.5335.58. [DOI] [PubMed] [Google Scholar]
- 17.White FJ, Kalivas PW. Neuroadaptations involved in amphetamine and cocaine addiction. Drug Alcohol Depend. 1998;51:141–153. doi: 10.1016/s0376-8716(98)00072-6. [DOI] [PubMed] [Google Scholar]
- 18.Panksepp J, Knutson B, Burgdorf J. The role of brain emotional systems in addictions: A neuro-evolutionary perspective and new ‘self-report’ animal model. Addiction. 2002;97:459–469. doi: 10.1046/j.1360-0443.2002.00025.x. [DOI] [PubMed] [Google Scholar]
- 19.Wise RA. Brain reward circuitry: Insights from unsensed incentives. Neuron. 2002;36:229–240. doi: 10.1016/s0896-6273(02)00965-0. [DOI] [PubMed] [Google Scholar]
- 20.Jackson D, et al. Anterior hypothalamic vasopressin modulates the aggression-stimulating effects of adolescent cocaine exposure in Syrian hamsters. Neuroscience. 2005;133:635–646. doi: 10.1016/j.neuroscience.2005.02.047. [DOI] [PubMed] [Google Scholar]
- 21.Grimes JM, Ricci LA, Melloni RH., Jr Alterations in anterior hypothalamic vasopressin, but not serotonin, correlate with the temporal onset of aggressive behavior during adolescent anabolic-androgenic steroid exposure in hamsters (Mesocricetus auratus) Behav Neurosci. 2007;121:941–948. doi: 10.1037/0735-7044.121.5.941. [DOI] [PubMed] [Google Scholar]
- 22.Harrison RJ, et al. Chronic anabolic-androgenic steroid treatment during adolescence increases anterior hypothalamic vasopressin and aggression in intact hamsters. Psychoneuroendocrinology. 2000;25:317–338. doi: 10.1016/s0306-4530(99)00057-8. [DOI] [PubMed] [Google Scholar]
- 23.Young LJ, Wang Z. The neurobiology of pair bonding. Nat Neurosci. 2004;7:1048–1054. doi: 10.1038/nn1327. [DOI] [PubMed] [Google Scholar]
- 24.Carter CS, DeVries AC, Getz LL. Physiological substrates of mammalian monogamy: The prairie vole model. Neurosci Biobehav Rev. 1995;19:303–314. doi: 10.1016/0149-7634(94)00070-h. [DOI] [PubMed] [Google Scholar]
- 25.Aragona BJ, et al. Nucleus accumbens dopamine differentially mediates the formation and maintenance of monogamous pair bonds. Nat Neurosci. 2006;9:133–139. doi: 10.1038/nn1613. [DOI] [PubMed] [Google Scholar]
- 26.Gobrogge KL, Liu Y, Jia X, Wang Z. Anterior hypothalamic neural activation and neurochemical associations with aggression in pair-bonded male prairie voles. J Comp Neurol. 2007;502:1109–1122. doi: 10.1002/cne.21364. [DOI] [PubMed] [Google Scholar]
- 27.Wang Z, Hulihan TJ, Insel TR. Sexual and social experience is associated with different patterns of behavior and neural activation in male prairie voles. Brain Res. 1997;767:321–332. doi: 10.1016/s0006-8993(97)00617-3. [DOI] [PubMed] [Google Scholar]
- 28.Winslow JT, et al. A role for central vasopressin in pair bonding in monogamous prairie voles. Nature. 1993;365:545–548. doi: 10.1038/365545a0. [DOI] [PubMed] [Google Scholar]
- 29.Stribley JM, Carter CS. Developmental exposure to vasopressin increases aggression in adult prairie voles. Proc Natl Acad Sci USA. 1999;96:12601–12604. doi: 10.1073/pnas.96.22.12601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kozorovitskiy Y, Hughes M, Lee K, Gould E. Fatherhood affects dendritic spines and vasopressin V1a receptors in the primate prefrontal cortex. Nat Neurosci. 2006;9:1094–1095. doi: 10.1038/nn1753. [DOI] [PubMed] [Google Scholar]
- 31.Albers HE, et al. Role of V1a vasopressin receptors in the control of aggression in Syrian hamsters. Brain Res. 2006;1073–1074:425–430. doi: 10.1016/j.brainres.2005.12.081. [DOI] [PubMed] [Google Scholar]
- 32.Pitkow LJ, et al. Facilitation of affiliation and pair-bond formation by vasopressin receptor gene transfer into the ventral forebrain of a monogamous vole. J Neurosci. 2001;21:7392–7396. doi: 10.1523/JNEUROSCI.21-18-07392.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lim MM, et al. Enhanced partner preference in a promiscuous species by manipulating the expression of a single gene. Nature. 2004;429:754–757. doi: 10.1038/nature02539. [DOI] [PubMed] [Google Scholar]
- 34.Bielsky IF, et al. The V1a vasopressin receptor is necessary and sufficient for normal social recognition: A gene replacement study. Neuron. 2005;47:503–513. doi: 10.1016/j.neuron.2005.06.031. [DOI] [PubMed] [Google Scholar]
- 35.Veenema AH, et al. Effects of early life stress on adult male aggression and hypothalamic vasopressin and serotonin. Eur J Neurosci. 2006;24:1711–1720. doi: 10.1111/j.1460-9568.2006.05045.x. [DOI] [PubMed] [Google Scholar]
- 36.Ferris CF, et al. Vasopressin/serotonin interactions in the anterior hypothalamus control aggressive behavior in golden hamsters. J Neurosci. 1997;17:4331–4340. doi: 10.1523/JNEUROSCI.17-11-04331.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Choi GB, et al. Lhx6 delineates a pathway mediating innate reproductive behaviors from the amygdala to the hypothalamus. Neuron. 2005;46:647–660. doi: 10.1016/j.neuron.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 38.Motta SC, Goto M, Gouveia FV, Baldo MV, Canteras NS, Swanson LW. Dissecting the brain's fear system reveals the hypothalamus is critical for responding in subordinate conspecific intruders. Proc Natl Acad Sci USA. 2009;106:4870–4875. doi: 10.1073/pnas.0900939106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ferris CF, Axelson JF, Martin AM, Roberge LF. Vasopressin immunoreactivity in the anterior hypothalamus is altered during the establishment of dominant/subordinate relationships between hamsters. Neuroscience. 1989;29:675–683. doi: 10.1016/0306-4522(89)90140-1. [DOI] [PubMed] [Google Scholar]
- 40.Delville Y, De Vries GJ, Ferris CF. Neural connections of the anterior hypothalamus and agonistic behavior in golden hamsters. Brain Behav Evol. 2000;55:53–76. doi: 10.1159/000006642. [DOI] [PubMed] [Google Scholar]
- 41.Wang Z, Smith W, Major DE, De Vries GJ. Sex and species differences in the effects of cohabitation on vasopressin messenger RNA expression in the bed nucleus of the stria terminalis in prairie voles (Microtus ochrogaster) and meadow voles (Microtus pennsylvanicus) Brain Res. 1994;650:212–218. doi: 10.1016/0006-8993(94)91784-1. [DOI] [PubMed] [Google Scholar]
- 42.Insel TR, Wang ZX, Ferris CF. Patterns of brain vasopressin receptor distribution associated with social organization in microtine rodents. J Neurosci. 1994;14:5381–5392. doi: 10.1523/JNEUROSCI.14-09-05381.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miczek KA, Yoshimura H. Disruption of primate social behavior by d-amphetamine and cocaine: Differential antagonism by antipsychotics. Psychopharmacology (Berl) 1982;76:163–171. doi: 10.1007/BF00435272. [DOI] [PubMed] [Google Scholar]
- 44.Miczek KA, Tidey JW. Amphetamines: Aggressive and social behavior. NIDA Res Monogr. 1989;94:68–100. [PubMed] [Google Scholar]
- 45.Wheeler RA, et al. Behavioral and electrophysiological indices of negative affect predict cocaine self-administration. Neuron. 2008;57:774–785. doi: 10.1016/j.neuron.2008.01.024. [DOI] [PubMed] [Google Scholar]
- 46.Grigson PS, Hajnal A. Once is too much: Conditioned changes in accumbens dopamine following a single saccharin-morphine pairing. Behav Neurosci. 2007;121:1234–1242. doi: 10.1037/0735-7044.121.6.1234. [DOI] [PubMed] [Google Scholar]
- 47.Grigson PS, Twining RC. Cocaine-induced suppression of saccharin intake: A model of drug-induced devaluation of natural rewards. Behav Neurosci. 2002;116:321–333. [PubMed] [Google Scholar]
- 48.Nocjar C, Panksepp J. Chronic intermittent amphetamine pretreatment enhances future appetitive behavior for drug- and natural-reward: Interaction with environmental variables. Behav Brain Res. 2002;128:189–203. doi: 10.1016/s0166-4328(01)00321-7. [DOI] [PubMed] [Google Scholar]
- 49.Tidey JW, Miczek KA. Morphine withdrawal aggression: Modification with D1 and D2 receptor agonists. Psychopharmacology (Berl) 1992;108:177–184. doi: 10.1007/BF02245304. [DOI] [PubMed] [Google Scholar]
- 50.Miczek KA. A new test for aggression in rats without aversive stimulation: Differential effects of d-amphetamine and cocaine. Psychopharmacology (Berl) 1979;60:253–259. doi: 10.1007/BF00426664. [DOI] [PubMed] [Google Scholar]
- 51.Aragona BJ, Detwiler JM, Wang Z. Amphetamine reward in the monogamous prairie vole. Neurosci Lett. 2007;418:190–194. doi: 10.1016/j.neulet.2007.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Curtis JT, Stowe JR, Wang Z. Differential effects of intraspecific interactions on the striatal dopamine system in social and non-social voles. Neuroscience. 2003;118:1165–1173. doi: 10.1016/s0306-4522(03)00032-0. [DOI] [PubMed] [Google Scholar]
- 53.Wang Z, Young LJ, Liu Y, Insel TR. Species differences in vasopressin receptor binding are evident early in development: Comparative anatomic studies in prairie and montane voles. J Comp Neurol. 1997;378:535–546. [PubMed] [Google Scholar]
- 54.Wang Z, Young LJ. Ontogeny of oxytocin and vasopressin receptor binding in the lateral septum in prairie and montane voles. Brain Res Dev Brain Res. 1997;104:191–195. doi: 10.1016/s0165-3806(97)00138-7. [DOI] [PubMed] [Google Scholar]
- 55.Insel TR, Preston S, Winslow JT. Mating in the monogamous male: Behavioral consequences. Physiol Behav. 1995;57:615–627. doi: 10.1016/0031-9384(94)00362-9. [DOI] [PubMed] [Google Scholar]