Abstract
Objective
To evaluate the effectiveness of one rather than two hospital neonatal examinations in detection of abnormalities.
Design
Randomised controlled switchback trial.
Setting
Postnatal wards in a teaching hospital in north east Scotland.
Participants
All infants delivered at the hospital between March 1993 and February 1995.
Intervention
A policy of one neonatal screening examination compared with a policy of two.
Main outcome measures
Congenital conditions diagnosed in hospital; results of community health assessments at 8 weeks and 8 months; outpatient referrals; inpatient admissions; use of general practioner services; focused analysis of outcomes for suspected hip and heart abnormalities.
Results
4835 babies were allocated to receive one screening examination (one screen policy) and 4877 to receive two (two screen policy). More congenital conditions were suspected at discharge among babies examined twice (9.9 v 8.3 diagnoses per 100 babies; 95% confidence interval for difference 0.3 to 2.7). There was no overall significant difference between the groups in use of community, outpatient, or inpatient resources or in health care received. Although more babies who were examined twice attended orthopaedic outpatient clinics (340 (7%) v 289 (6%)), particularly for suspected congenital dislocation of the hip (176 (3.6/100 babies) v 137 (2.8/100 babies); difference −0.8; −1.5 to 0.1), there was no significant difference in the number of babies who required active management (12 (0.2%) v 15 (0.3%)).
Conclusions
Despite more suspected abnormalities, there was no evidence of net health gain from a policy of two hospital neonatal examinations. Adoption of a single examination policy would save resources both during the postnatal hospital stay and through fewer outpatient consultations.
Key messages
Neonatal screening in hospital after delivery can be safely carried out once rather than twice
Introduction of this policy would save hospital resources, both during the postnatal period and subsequently through fewer outpatient consultations
Later surveillance is an essential complement to hospital screening (whether performed once or twice) to detect abnormalities missed in hospital and conditions which develop after discharge
Introduction
Although there is wide acceptance that all newborn babies should be screened for abnormalities,1 there is no consensus on how this should be done. Biochemical screening for phenylketonuria and congenital hypothyroidism is effective, but clinical examination for defects in hips, vision, and hearing and other congenital abnormalities is less well founded on scientific evidence.2 Up to 12% of babies may have some detectable abnormality3 but not all will impact on health or require action.4
For babies born in hospital, clinical neonatal screening is usually carried out twice before discharge, once within 24 hours of birth and again a few days later. The rationale for the first examination is to detect abnormalities that may require early action. The second aims to detect those which may have been missed at the first screening and to detect others which may have become apparent later—such as cardiac defects as the fetal circulation adapts to extrauterine life.5
The need for a second examination, however, has been questioned.4,6,7 We therefore compared the policies for performing one rather than two hospital neonatal screening examinations as judged by their effectiveness in detecting congenital abnormalities and the consequent use of hospital and community resources.
Participants and methods
The trial was approved by the Grampian Health Board and University of Aberdeen joint ethics committee. All babies delivered at Aberdeen Maternity Hospital between March 1993 and February 1995 were eligible except those discharged home directly from the labour ward (domino births) and those transferred to the neonatal unit within 6 hours of birth. Eligible babies were randomised to one of two policies: one screen policy—one neonatal screening examination on day 3 or on the day before expected discharge if earlier; or two screen policy—one screening examination within 36 hours of birth and a second on the day of discharge or on the day before expected discharge if after day 3. The two screen policy was current practice in the hospital before the trial. Babies allocated to the one screen policy were examined at the latest on day 3 even if they stayed in for longer; babies allocated to the two screen policy and who stayed in hospital for more than 3 days had their second examination on a later day.
The trial adopted a controlled switchback design,8 which is in essence an extended crossover design. This allows the comparison of the screening policies to be unaffected by external changes over time. Babies were allocated to one or other policy depending on their postnatal ward and the calendar month. Each month half the wards in the hospital operated the one screen policy and the rest the two screen policy. Recruitment continued for 2 years. The initial month was allocated at random with crossover to the opposite policy on the first day of each subsequent month. The screening policy to which a ward was assigned could not therefore be influenced by patients or staff. Also, neither the mother nor doctor could choose which ward a baby would be in. Babies were examined by NHS staff, including an associate specialist (JAR), community medical officers, and paediatric senior house officers. Training in and execution of routine neonatal examinations remained unaltered throughout the trial period.
Babies were followed up for their first year of life. The main outcome measures were congenital conditions coded at discharge from hospital; results of the community health assessments at 8 weeks and 8 months; use of general practioner services in the first year of life (for a randomly selected 10% subsample only); outpatient referrals; and inpatient admissions that involved congenital conditions.
Hospital examinations
Conditions diagnosed in hospital were identified from the routinely collected and computerised Scottish Morbidity Records (SMR11 and SMR11(E)) by using the ICD-9 (international classification of diseases, ninth revision) codes which referred to congenital conditions likely to be detected by neonatal screening. They were then matched with hand extracted data that described the results of examinations.
Community resources
The results of the 8 week and 8 month assessments were linked by using the community health index number as a unique identifier. The general practitioners of a 10% subsample of babies, who were selected at random each month, were sent a questionnaire requesting details of all contacts during the baby’s first year of life. General practitioners were blind to the screening policy each baby had received.
Hospital resources
Babies referred to outpatient departments were tracked by using the computerised Scottish outpatient record (SMR0). Inpatient admissions in the first year of life were found by using the Scottish inpatient and day case record summary sheet (SMR1). This includes details of type of admission, specialty, and conditions diagnosed (with ICD-9 codes). These data collecting systems tracked babies admitted to hospitals throughout Scotland.
The hospital notes of the 10% of babies, whose general practitioners were sent questionnaires, were examined both to cross validate other methods of data collection and to provide more detailed information about hospital contacts. The data extractor was blinded to the screening policy which each baby had received.
Hip and cardiac anomalies
All babies suspected of having a hip anomaly were referred to a central orthopaedic clinic. Subsequent management of these babies during their first year of life was described with the clinic’s dedicated database and linked to hospital discharge findings with the unique hospital number. Similarly, all babies with cardiac problems were seen centrally (by PB). Data for babies confirmed as having a problem were linked to initial findings by using the hospital number.
Statistical analyses
As each baby could have more than one condition the results were calculated as the number of diagnoses per hundred babies. Analysis was by intention to treat. Comparisons of rates of abnormality and referral are presented as 95% confidence intervals for the difference with the normal approximation to the difference between two Poisson variables.9 We also calculated 95% confidence intervals for the difference between two proportions for all main comparisons.
We aimed to recruit between 9000 and 10 000 babies, on the basis of an anticipated rate of diagnoses before discharge of 12%. The study had a power of 80% to detect a relative reduction of 15% (to 10.2%, with 2P<0.05).
Results
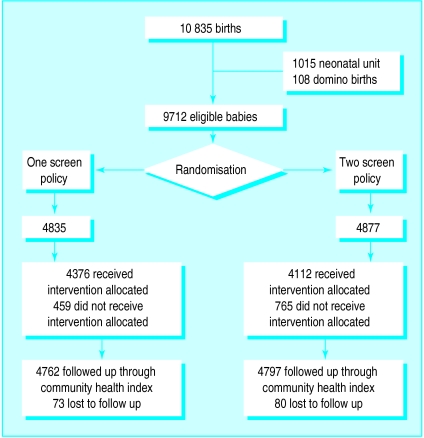
Of the 10 835 babies born alive during the 2 year recruitment period, 9712 (89.6%) were eligible for randomisation; 4835 were allocated to the one screen policy and 4877 to the two screen policy (figure). We could not link data on 1.6% of babies; the numbers lost to follow up because of death (n=7) or moving out of the area were similar in both groups. The groups’ baseline characteristics were similar in respect of sex, mode of delivery, birth order, and weight (table 1). Length of stay was the same in both groups (median (interquartile range) 4 (2 to 6) days).
Table 1.
Baseline characteristics of trial babies* according to whether they underwent one or two neonatal screening examinations before discharge from hospital. Values are numbers (percentages) of babies unless stated otherwise
| Characteristic | One screen policy (n=4835) | Two screen policy (n=4877) |
|---|---|---|
| Boys | 2424 (50.1) | 2462 (50.5) |
| Mode of delivery: | ||
| Spontaneous cephalic | 3441 (71.2) | 3474 (71.2) |
| Assisted vaginal | 680 (14.0) | 647 (13.3) |
| Breech | 43 (0.9) | 46 (0.9) |
| Caesarean section | 671 (13.9) | 710 (14.6) |
| Mother previously nulliparous | 2092 (43.3) | 2089 (42.8) |
| Mean (SD) birth weight (g) | 3460 (479) | 3457 (479) |
Data source: Scottish morbidity record 11.
In practice 459 (9.5%) of the one screen policy group were actually examined twice, and 766 (15.7%) of the two screen policy group were examined once. Reasons for failure to adhere to protocol included detection of serious physical abnormality within the first 24 hours, failure to change from one policy to the other at the start of a month, and babies going home early. Babies allocated to the one screen policy were less likely ever to be examined by a senior member of staff (56%) compared with 85% examined at least once by a senior doctor under the two screen policy (difference 28%; 95% confidence interval 26% to 30%). Of the two screen policy babies, 32% were examined on day 4 or later compared with 3% in the one screen policy group.
Significantly fewer conditions were diagnosed in hospital among one screen policy babies than two screen policy babies (8.3 v 9.9; difference 1.6; 0.3 to 2.7; table 2). This was largely because of fewer suspected musculoskeletal problems, especially suspected hip anomalies (2.8 v 3.6; difference 0.8; 0.1 to 0.5).
Table 2.
Congenital diagnoses in neonates at discharge from hospital after birth* according to whether they underwent one or two neonatal screening examinations
| Detail | One screen policy (No of diagnoses/100 babies; n=4835) | Two screen policy (No of diagnoses/100 babies; n=4877) | Difference in No of diagnoses/100 babies (95% CI) |
|---|---|---|---|
| No of diagnoses | 406 (8.3) | 482 (9.9) | 1.6 (0.3 to 2.7) |
| Musculoskeletal conditions: | |||
| Total No | 172 (3.6) | 236 (4.8) | 1.2 (0.4 to 1.9) |
| Hip anomalies | 137 (2.8) | 176 (3.6) | — |
| Musculoskeletal anomalies | 15 (0.3) | 28 (0.6) | — |
| Other deformities of limb | 20 (0.4) | 32 (0.7) | — |
| Cardiovascular disease | 59 (1.2) | 55 (1.1) | 0.1 (−0.3 to 0.5) |
| Abdominal | 4 (0.1) | 0 | — |
| Facial | 46 (1.0) | 53 (1.1) | −0.1 (−0.5 to 0.3) |
| Genitourinary | 75 (1.5) | 79 (1.6) | −0.1 (−0.6 to 0.4) |
| Other | 50 (1.0) | 59 (1.2) | −0.1 (−0.6 to 0.2) |
Data source: Scottish morbidity records 11 and 11(E).
Of the babies for whom we could link data, 37 (0.38%) failed to have an assessment at 8 weeks. There was no evidence of an excess of abnormal findings between the groups at either the 8 week or the 8 month community assessment (table 3). Nor were there more followed up in primary care or referred to secondary care from this community screening programme, both overall and among the 10% subsample.
Table 3.
Routine assessments at 8 weeks and 8 months of age and contacts with general practitioners in first year in babies according to whether they underwent one or two neonatal screening examinations before discharge from hospital. Values are numbers (percentages*) of babies
| Detail | One screen policy (n=4835) | Two screen policy (n=4877) | Difference in percentage (95% CI) |
|---|---|---|---|
| Assessment at 8 weeks†: | |||
| No of babies | 4741 | 4781 | |
| Abnormal | 498 (10.5) | 506 (10.6) | −0.1 (−1.3 to 1.2) |
| Follow up in primary care | 299 (6.3) | 272 (5.7) | 0.6 (−0.4 to 1.6) |
| Referral to secondary care | 138 (2.9) | 151 (3.2) | −0.2 (−0.9 to 0.4) |
| Assessment at 8 months†: | |||
| No of babies | 4649 | 4590 | |
| Abnormal | 984 (21.2) | 973 (21.2) | <−0.1 (−1.7 to 1.6) |
| Follow up in primary care | 794 (17.1) | 739 (16.1) | 1.0 (−0.5 to 2.5) |
| Referral to secondary care | 174 (3.7) | 199 (4.3) | −0.6 (−1.4 to 0.2) |
| Sample (10%) of trial babies‡ | 486 | 490 | — |
| Respondents | 431 (88.7) | 442 (90.2) | — |
| No of contacts with GP (No/100 babies) | 3738 (867) | 3697 (836) | — |
| Contacts resulting in secondary referral (No/100 babies) | 100 (23) | 110 (25) | — |
Percentages calculated with exclusion of categories with missing information.
Data source: community health index records.
Data source: general practitioner questionnaire for 10% subsample of babies.
Although the difference in the proportions of babies attending outpatient clinics in their first year of life was not significant (18.5% v 19.9%; difference −1.4%; 2.9% to 0.1%; table 4), the observed difference was largely explained by more attendances at the orthopaedic outpatient clinic (6.0% v 7.0%; difference −1%; −1.9% to −0.02%).
Table 4.
Outpatient and inpatient care in first year of life in babies according to whether they underwent one or two neonatal screening examinations before discharge from hospital after birth. Values are numbers (percentages) of babies
| Detail | One screen policy (n=4835) | Two screen policy (n=4877) | Difference in percentage (95% CI) |
|---|---|---|---|
| Outpatients* | |||
| Total No of referrals | 1091 | 1184 | — |
| No with at least one outpatient referral | 896 (18.5) | 973 (19.9) | −1.4 (−2.9 to 0.1) |
| Specialty: | |||
| Orthopaedic surgery | 289 (6.0) | 340 (7.0) | −1.0 (−1.9 to −0.02) |
| Ophthalmology | 126 (2.6) | 134 (2.7) | — |
| Medical paediatrics | 311 (6.4) | 316 (6.4) | — |
| Other | 365 (7.5) | 394 (8.1) | — |
| Inpatients† | |||
| Total No of admissions | 1034 | 1015 | — |
| No with at least one inpatient admission | 753 (15.6) | 718 (14.7) | 0.9 (−0.1 to 2.2) |
| First inpatient admission only: | |||
| Type of admission: | |||
| Planned admission | 183 (3.8) | 188 (3.9) | — |
| Emergency admission | 570 (11.9) | 530 (10.9) | — |
| Specialty: | |||
| Orthopaedic surgery | 13 (0.3) | 10 (0.2) | — |
| Ophthalmology | 15 (0.3) | 13 (0.3) | — |
| Medical paediatrics | 462 (9.6) | 428 (8.8) | — |
| Other | 263 (5.4) | 267 (5.5) | — |
Data source: Scottish morbidity record 0.
Data source: Scottish morbidity record 1.
In their first year of life 1471 (15%) babies were admitted as inpatients at least once; of these, 369 (3.8%) were admitted more than once (table 4). There were no apparent differences between the two groups in the proportion of admissions, the type (whether planned or emergency), or the specialty.
There were no clear differences between the groups in the number with a primary diagnosis of a congenital condition at their first admission (1.0 v 0.7 diagnoses per 100 babies; difference 0.3; −0.1 to 0.7) or any admission (1.6 v 1.4 diagnoses per 100 babies; difference 0.2; −0.3 to 0.7).
The larger number of hip anomalies suspected in hospital under the two screen policy (see table 2) was reflected in more babies being seen at the orthopaedic clinic (125 v 176; table 5). There was no difference, however, in the proportion who received active management (outpatient splinting or surgical reduction 0.3% v 0.2%; difference 0.1%; −0.1% to 0.3%; table 5). For babies who had been judged normal in hospital (on the basis of a negative Ortolani-Barlow manoeuvre10) there was no clear difference in the proportion subsequently referred nor in those who then required active management (0.2% v 0.1%; difference 0.1%; −0.1% to 0.2%; table 5). This applied also to those referred because of a family history (table 5). There was no difference in the proportion of babies confirmed to have a cardiac anomaly between the two groups irrespective of whether or not they were diagnosed in hospital (table 5).
Table 5.
Follow up of trial babies at orthopaedic and cardiac clinics according to whether they underwent one or two neonatal screening examinations before discharge from hospital after birth. Values are numbers (percentages) of babies
| Detail | One screen policy (n=4835) | Two screen policy (n=4877) | Difference in percentage (95% CI) |
|---|---|---|---|
| Orthopaedic clinic* | |||
| Suspected hip problem at birth: | |||
| Total No | 137 (2.8) | 176 (3.6) | −0.8 (−1.5 to −0.1) |
| No followed up at clinic | 125 (2.6) | 167 (3.4) | — |
| No who required treatment | 15 (0.3) | 12 (0.2) | |
| No who had reduction under general anaesthesia | 3 (0.1) | 1 (<0.1) | — |
| Hip problems not suspected at birth but babies later referred: | |||
| Total No | 71 (1.5) | 77 (1.6) | −0.1 (−0.6 to 0.4) |
| No who required treatment | 8 (0.2) | 5 (0.1) | — |
| No who had reduction under general anaesthesia | 5 (0.1) | 1 (<0.1) | — |
| Babies with no click at birth but referred because of family history of hip problem: | |||
| Total No | 46 (1.0) | 35 (0.7) | 0.2 (−0.1 to 0.6) |
| No who required treatment | 3 (<0.1) | 1 (<0.1) | — |
| No who had reduction under general anaesthesia | 0 | 0 | — |
| Cardiac clinic† | |||
| No with suspected heart problem at discharge from hospital after birth | 43 (0.9) | 49 (1.0) | −0.1 (−0.5 to 0.3) |
| Confirmed at heart clinic | 29 (0.6) | 35 (0.7) | −0.1 (−0.4 to 0.2) |
| No not suspected at discharge but later confirmed at heart clinic | 15 (0.3) | 13 (0.3) | <0.1 (−0.2 to 0.3) |
Data source: orthopaedic clinic database.
Data source: cardiac clinic records.
Discussion
Most (90%) babies born during the study period were included in the trial, representing all those for whom routine neonatal screening was appropriate. The lower than expected rate of diagnoses in the trial babies (10% rather than 12%) probably reflects the relatively higher risk among babies who were ineligible because they were admitted directly to the neonatal unit.
Methodological issues
The trial was a pragmatic comparison of two policies as they might be used in hospitals. It was therefore expected that some of those allocated one examination would actually have a second and that some allocated two examinations would have only one. As we based the analyses on “intention to treat,” however, we should have avoided the introduction of any bias. Possible bias introduced in assessing outcome was also minimised by using routinely collected data and blinding of providers of data to policy allocation.
We believe that the screening policies were similar to those implemented in other hospitals in the United Kingdom. Policies for type and grade of staff who carry out neonatal screening vary across the country. Our study reflects practice as carried out by experienced paediatricians or those in training rather than other specialties.
Congenital diagnoses
The trial has shown clearly that examining babies in hospital twice rather than once resulted in more congenital abnormalities being suspected at the time of discharge (see table 2). The excess may have resulted from more babies being examined by experienced staff, because a second examiner might detect something missed at first, or because of new conditions which developed over time. These extra “diagnoses,” however, did not lead to any detectable increase in interventions that might improve infant health nor did the infants from one group make extra use of emergency services, as might have been expected if important conditions had been missed in hospital. Thus there was no evidence that one examination was less effective than two in identifying babies who required medical attention.
Hip anomalies
The larger number of congenital abnormalities diagnosed at birth in the group examined twice was primarily attributable to an excess in suspected hip anomalies. This resulted in extra referrals to outpatient departments (2.6% v 3.4%, see table 5). These extra visits did not lead to more active management, and similar numbers underwent splinting or operation in both groups. A second examination did not there- fore seem to improve sensitivity but did reduce specificity, which led to apparently unnecessary intensive surveillance.
Indeed, the higher false positive rate associated with the two examination policy may be more likely to result in extra, iatrogenic damage (for example, avascular necrosis of the femoral head) than to prevent long term disability.11,12 If performance of neonatal screening only once did fail to detect significantly more genuine congenital displacement of the hip we would expect the “missed” babies to have appeared as extra referrals from the community. This was not the case, suggesting that the fluctuation in surgery rate was simply due to chance.
The poor detection rate for congenital displacement of the hip by neonatal screening corroborates a recent United Kingdom survey of hip screening (70% who required surgery were missed by routine screening). The overall surgery rate, of about 1 per 1000, was in keeping with the rate observed in that study.13
Heart anomalies
Most cases of serious cardiac anomaly first present with clinical symptoms14 and therefore the value of routine neonatal screening has been questioned.7 In this trial there was no clear evidence that a policy of more intensive screening led to a difference in the number of babies suspected of having serious cardiac problems at discharge.
Community screening
The trial confirmed the need for further surveillance after a baby leaves hospital. Of the 44 babies who received active management for congenital dislocation of the hip, 13 (30%) had negative findings on the Ortolani-Barlow manoeuvre in hospital and were later detected in the community. Of the 10 babies who required surgery, six (60%) were not detected in hospital.
In Scotland, later surveillance is provided by community screening at 8 weeks and 8 months, supplemented by opportunistic screening. As the numbers with later presenting problems were similar in the two groups there was no evidence that these cases were missed in hospital as a result of decreased neonatal screening but rather were identified as extra cases which were undetected equally in both groups. Therefore, any change in hospital policy must take into account the complementary nature of hospital and community screening.
Conclusion
Despite more suspected abnormalities among babies allocated a policy of two rather than one hospital neonatal examination the trial did not show any net health gain from this policy. A two screen policy does, however, carry additional resource implications for hospital services and extra anxiety for parents whose children are wrongly suspected of having abnormalities.
Figure.
Inclusion of babies in study
Acknowledgments
We thank the ward staff who made the study possible and the general practitioners who responded to the surveys. Ian Russell was an original grant holder and contributed to initial study design, Adrian Grant gave valuable comments on the analyses and draft reports, and James McLauchlan provided orthopaedic clinic data. The views expressed, however, are those of the authors.
Editorial by Hall
Footnotes
Funding: The Health Services Research Unit is funded by the Chief Scientist Office of the Scottish Office Department of Health, which also funded the study through a project grant.
Competing interests: None declared.
References
- 1.Maternity Services Advisory Committee to the Secretaries of State for Social Services and for Wales. Maternity care in action part III: care of the mother and baby (postnatal and neonatal care). London: HMSO; 1985. [Google Scholar]
- 2.Robinson R. Effective screening in child health. BMJ. 1998;316:1–2. doi: 10.1136/bmj.316.7124.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hall DMB. Health for all children. A programme for child health surveillance. Oxford: Oxford University Press; 1989. [Google Scholar]
- 4.Moss GD, Cartlidge PHT, Speidel BD, Chambers TL. Routine examination in the neonatal period. BMJ. 1991;302:878–879. doi: 10.1136/bmj.302.6781.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Manning DJ. One or two neonatal examinations? BMJ. 1991;302:1209. [PMC free article] [PubMed] [Google Scholar]
- 6.Hughes AP, Stoker AJ, Milligan DWA. One or two neonatal examinations? BMJ. 1991;302:1209. [PMC free article] [PubMed] [Google Scholar]
- 7.Cartlidge PHT. Routine discharge examination of babies: is it necessary? Arch Dis Child. 1992;67:1421–1422. doi: 10.1136/adc.67.12.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cochran WG, Cox GM. Experimental designs. New York: Wiley; 1957. [Google Scholar]
- 9.Daly LE, Bourke GJ, McGilvray J. Interpretation and uses of medical statistics. Oxford: Blackwell Scientific Publication; 1991. [Google Scholar]
- 10.Barlow TG. Early diagnosis and treatment of congenital dislocation of the hip. J Bone Joint Surg. 1962;44B:292–301. [Google Scholar]
- 11.Kalamchi A, MacEwen GD. Avascular necrosis following treatment of congenital dislocation of the hip. J Bone Joint Surg Am. 1980;62:876–888. [PubMed] [Google Scholar]
- 12.Bradley J, Wetherill M, Benson MK. Splintage for congenital dislocation of the hip. Is it safe and reliable? J Bone Joint Surg Br. 1987;69:257–263. doi: 10.1302/0301-620X.69B2.3818757. [DOI] [PubMed] [Google Scholar]
- 13.Godward S, Dezateux C. Surgery for congenital dislocation of the hip in the UK as a measure of outcome of screening. Lancet. 1998;351:1149–1152. doi: 10.1016/s0140-6736(97)10466-4. [DOI] [PubMed] [Google Scholar]
- 14.Abu-Harb M, Wyllie J, Hey E, Richmond S, Wren C. Presentation of obstructive left heart malformations in infancy. Arch Dis Child Fetal Neonatal Edition. 1994;71:179–183. doi: 10.1136/fn.71.3.f179. [DOI] [PMC free article] [PubMed] [Google Scholar]