Abstract
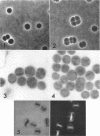
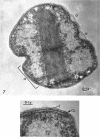
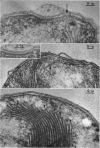
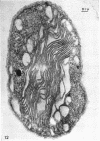
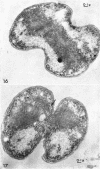
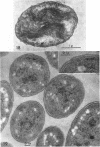
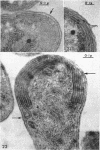
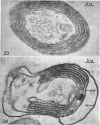
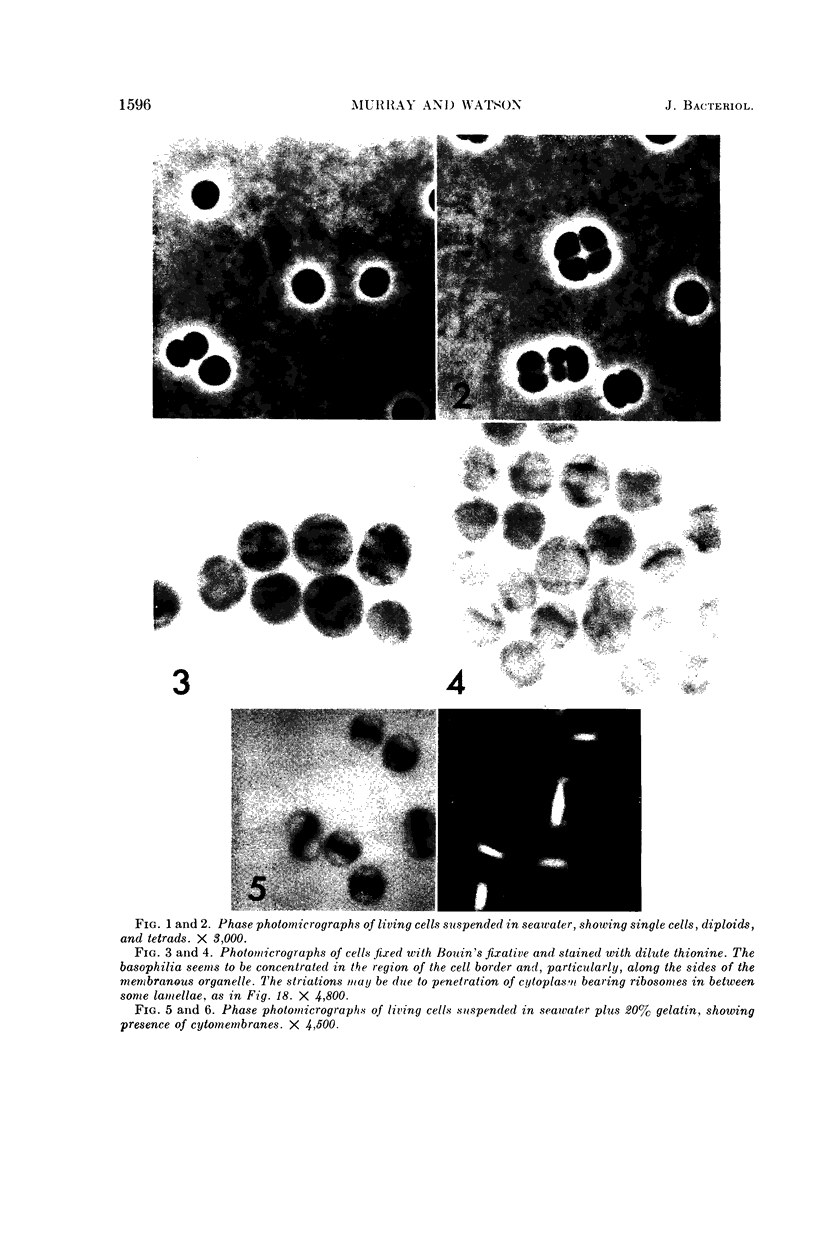
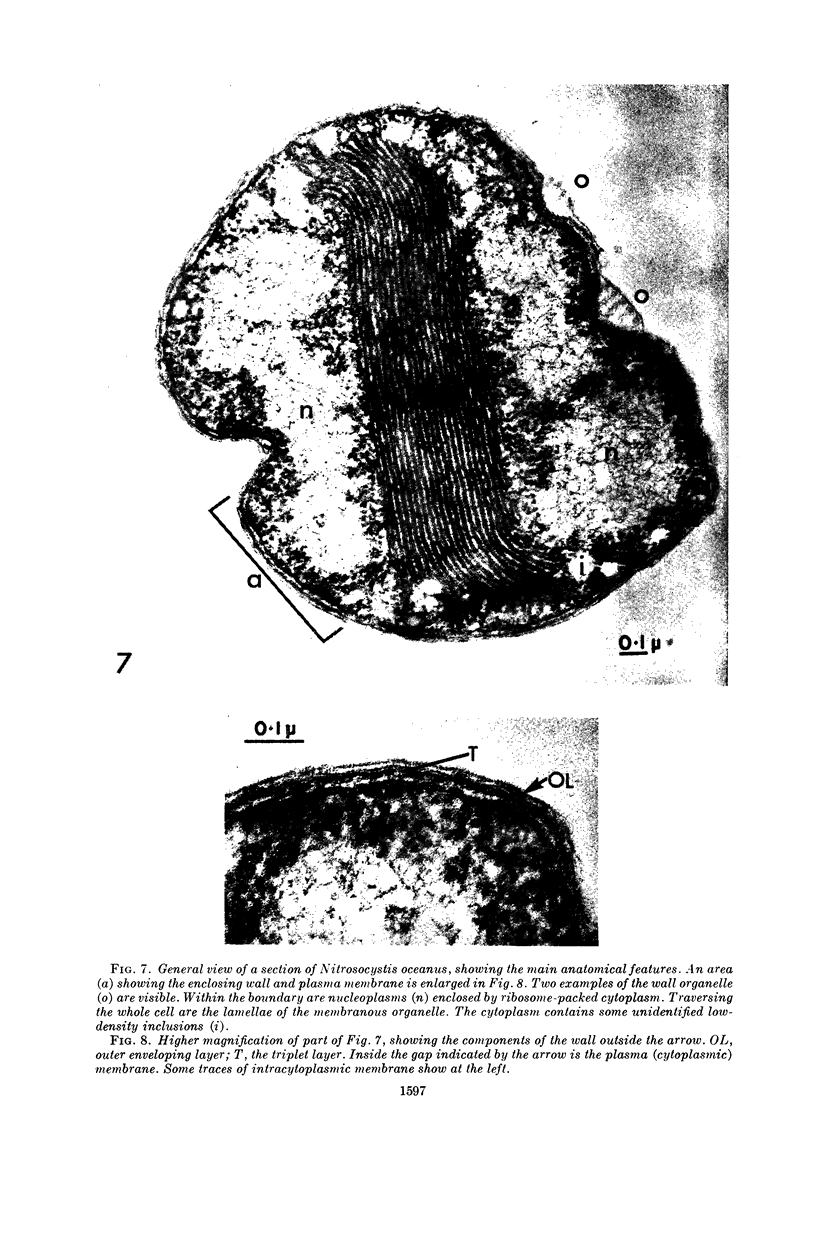
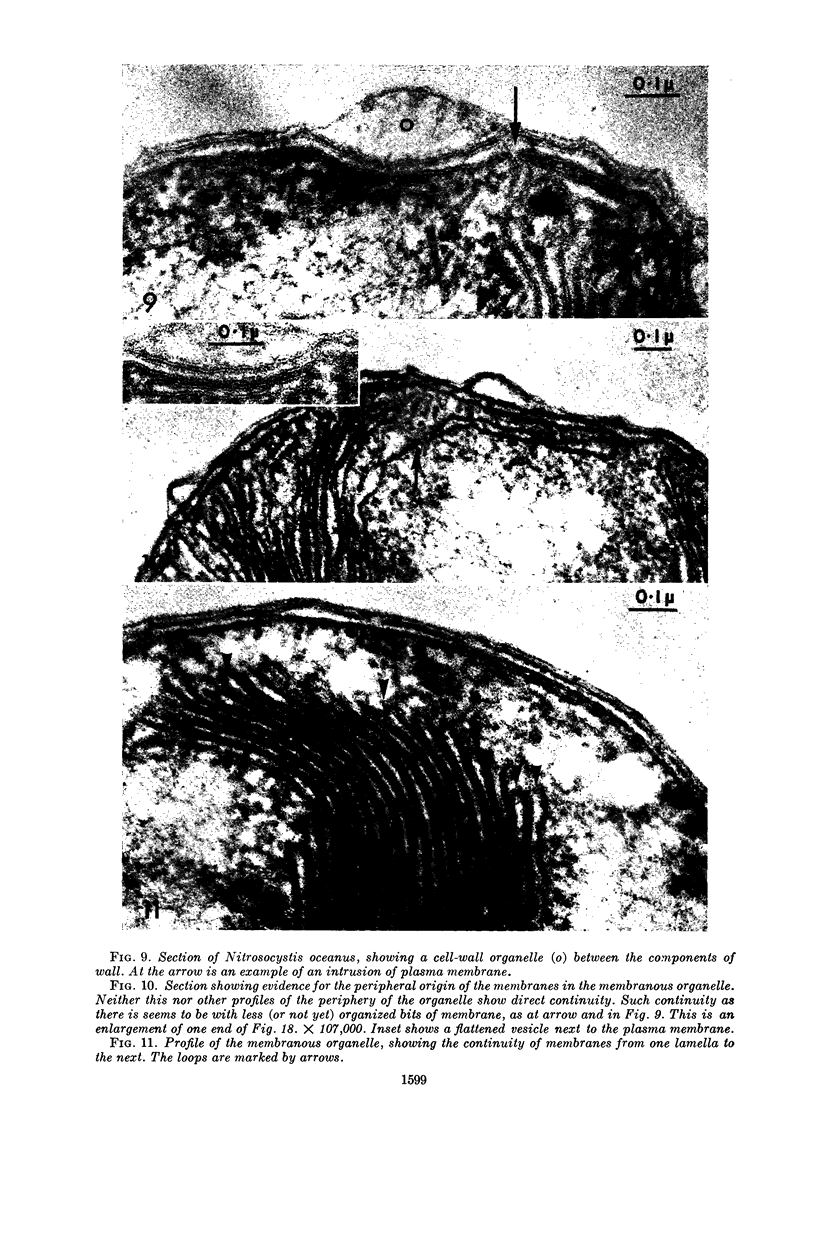
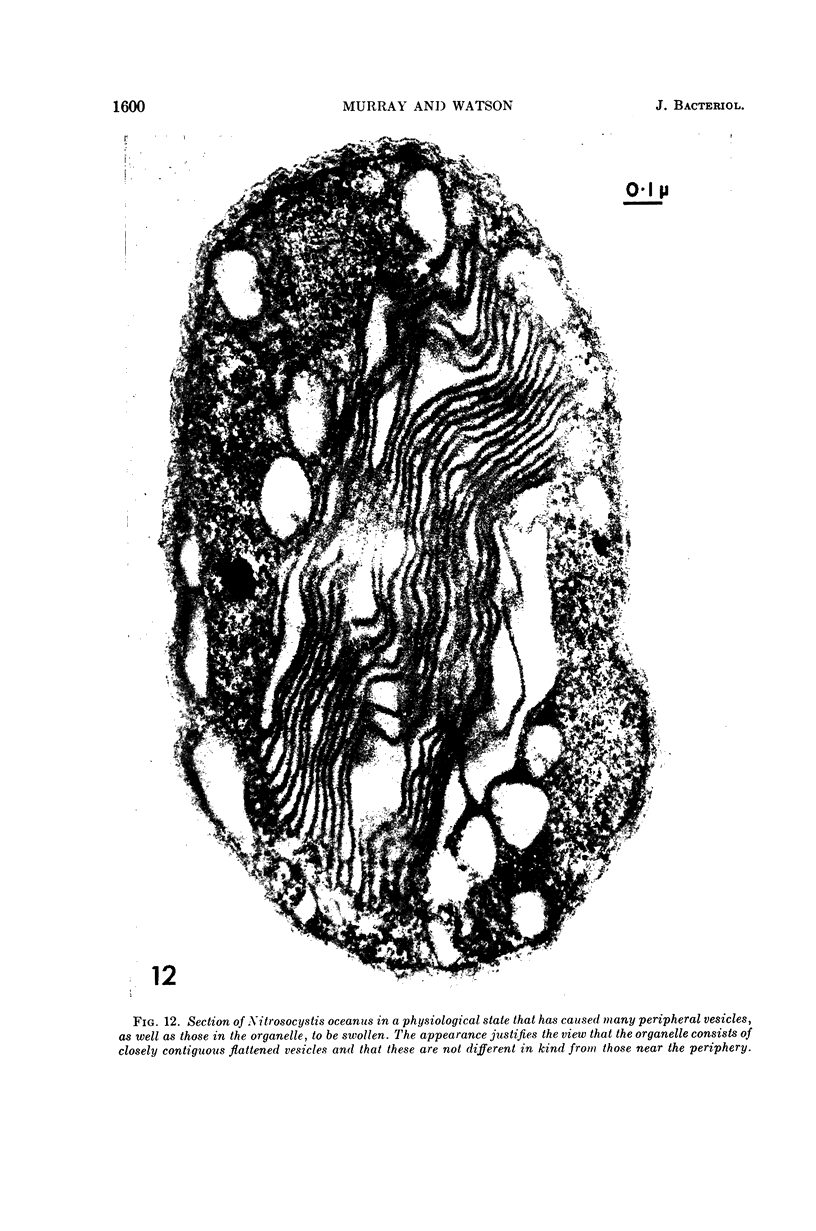
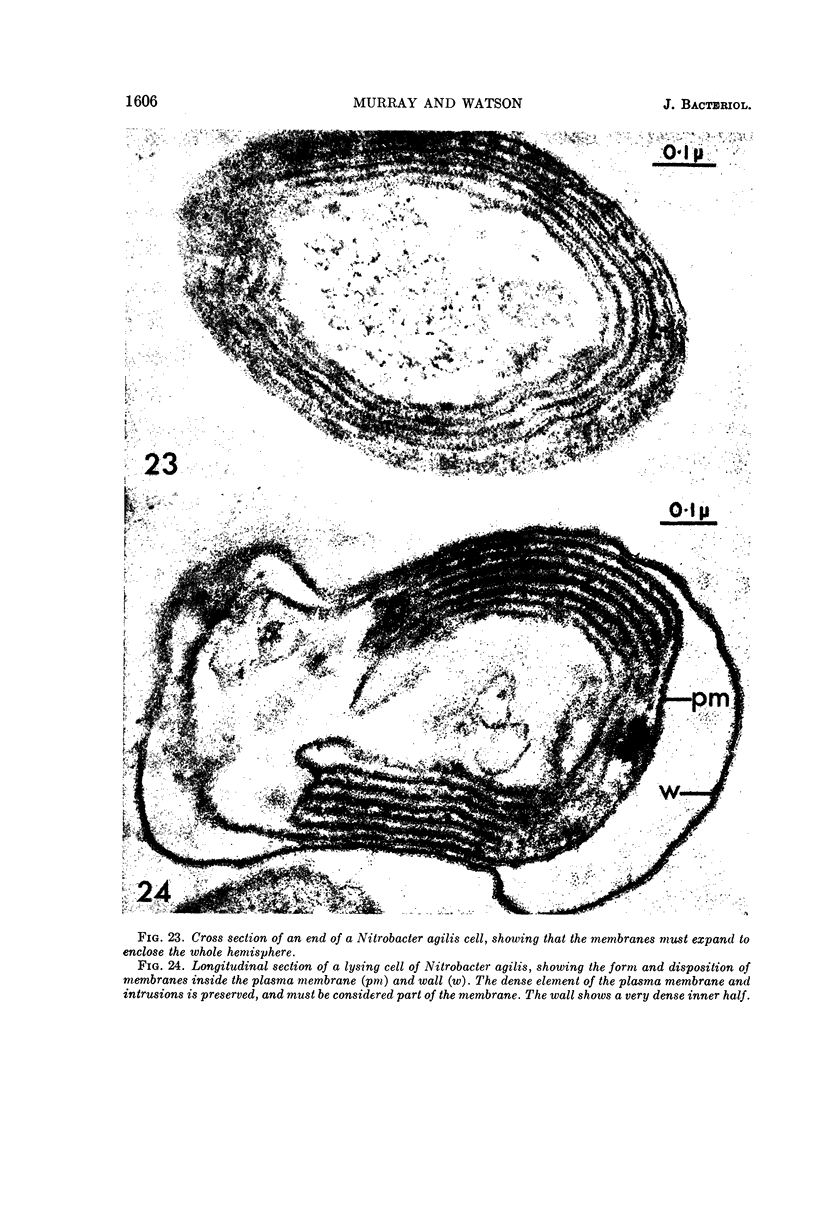
Murray, R. G. E. (University of Western Ontario, London, Ont., Canada), and S. W. Watson. Structure of Nitrosocystis oceanus and comparison with Nitrosomonas and Nitrobacter. J. Bacteriol. 89:1594–1609. 1965.—Nitrosocystis oceanus has distinctive features: the cell wall (overall thickness, 250 A) has an inner triplet structure and a dense enveloping layer; between these lie the “cell-wall organelles” (two or more per cell; plaques about 0.5 μ in diameter and 0.1 μ thick) of unknown function and genesis. The plasma membrane (ca. 80 A) shows rare intrusions that form irregular peripheral vesicles, which appear to form the component lamellae of the “membranous organelle” and probably detach from the periphery. The membranous organelles consist of about 20 vesicles so flattened that the lumen is only 100 A thick. The outer surfaces are in contact and form a triplet structure with an accentuated center line; these lamellae almost traverse the cell, displace the cytoplasm and the nucleoplasms, and form the prominent, seemingly permanent, feature of the cell. Division is constrictive without trace of a septum, and the act of division divides the membranous organelle. No mesosomes appear to be formed. Nitrosomonas europaea shows no sign of a cell-wall organelle or of the outer enveloping layer of wall. The cytoplasm contains intrusive paired lamellae, which might or might not remain connected to the periphery, and they do not fuse or form regular associations. These are thought to be the equivalent of the vesicles in Nitrosocystis but remaining almost parallel and close to the plasma membrane. Nitrobacter agilis has a unique plasma membrane with a (50 A) dense layer applied to the inside of the usual unit membrane. All of the components are represented in the intrusions, which are arranged over and shape the poles of the cells, with close and regular spacing. Each nitrifier was distinctive; in common they have membrane systems which, it is considered, must relate to the specialized mechanisms for acquiring energy adopted by these organisms.
Full text
PDF


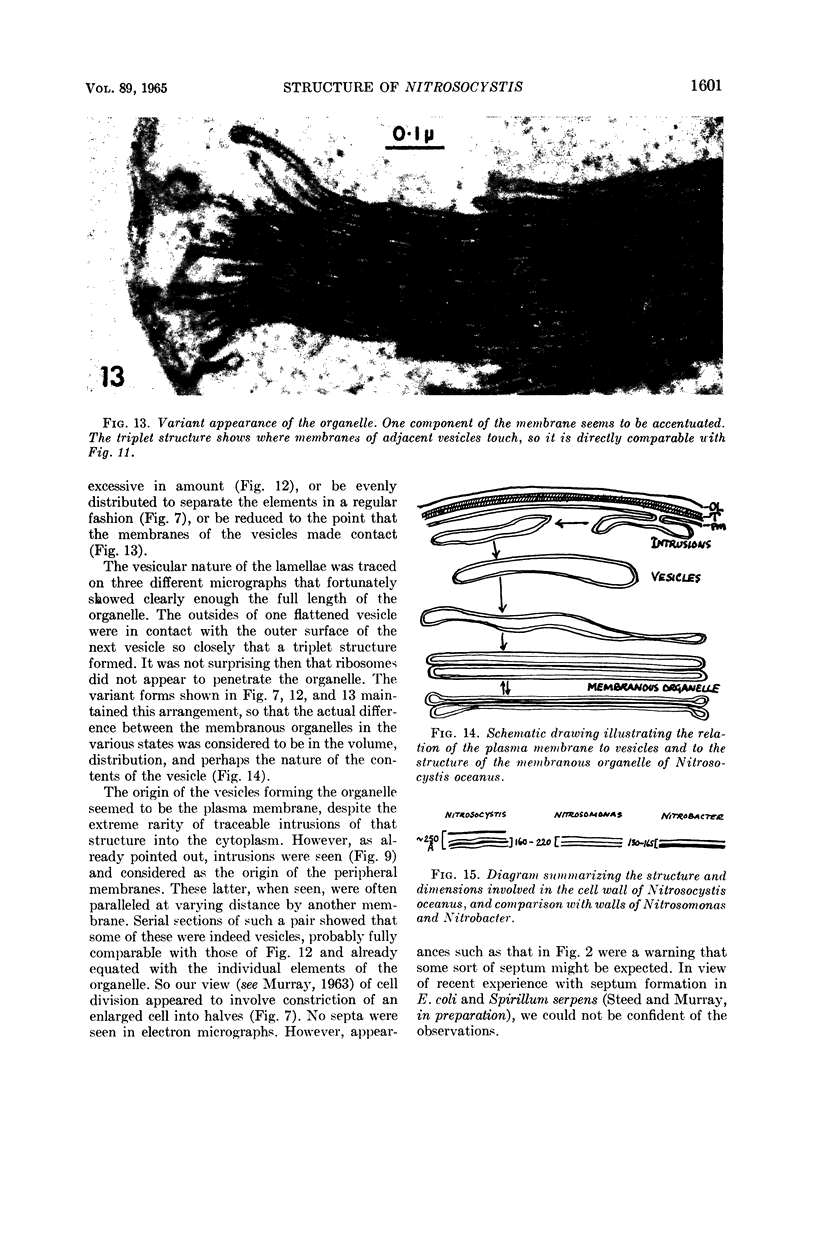

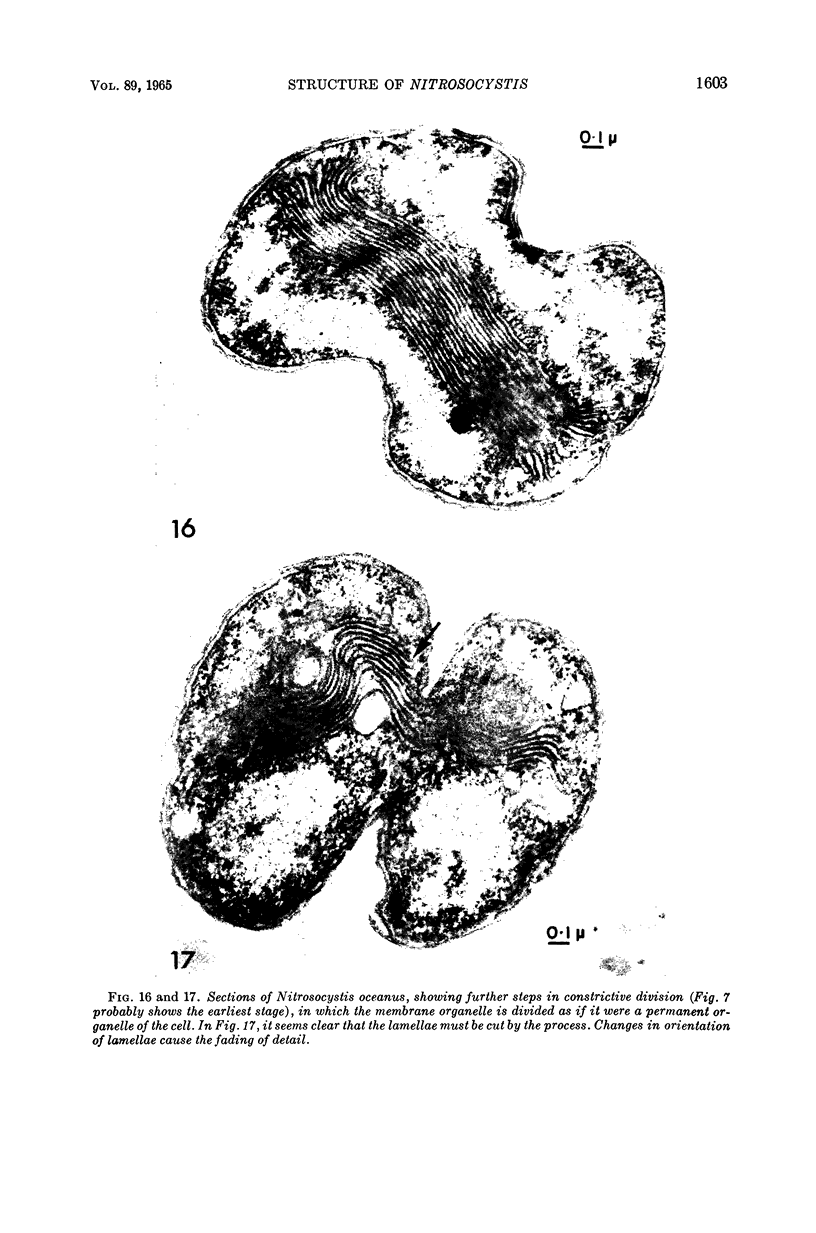
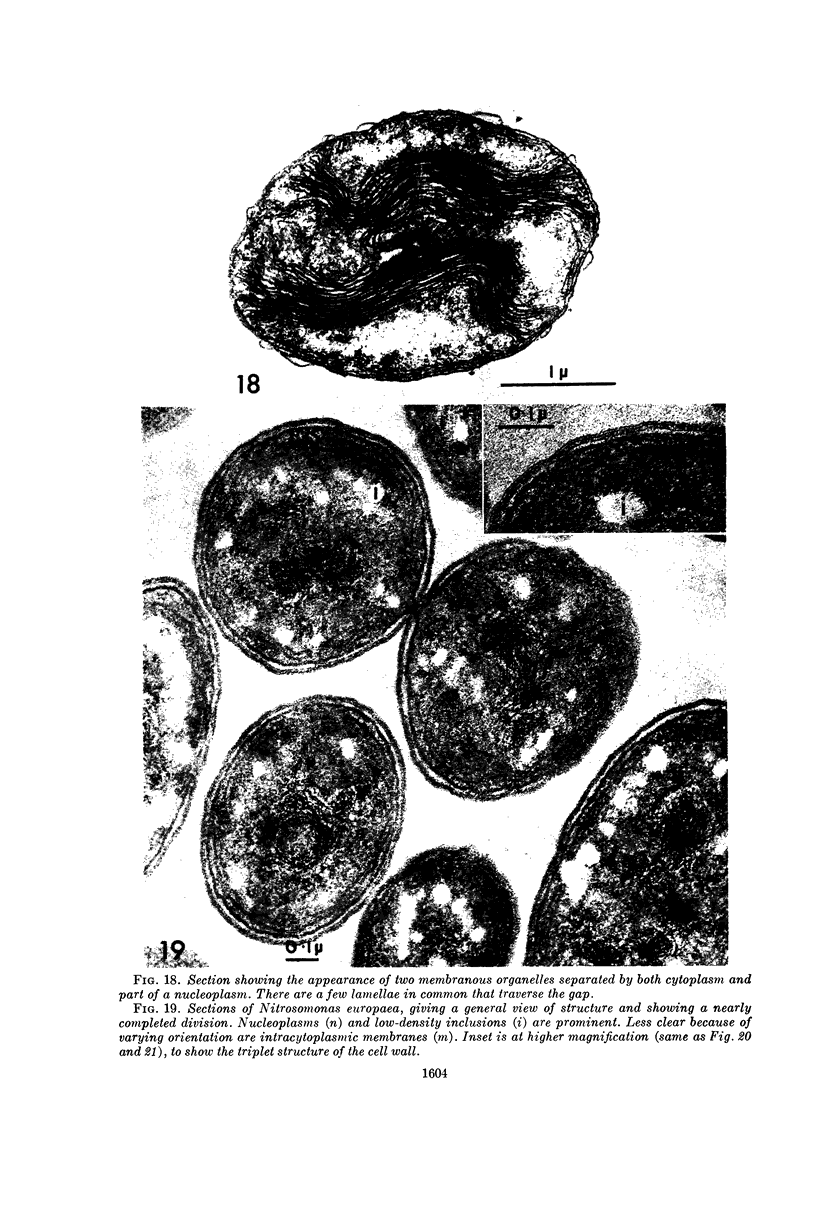
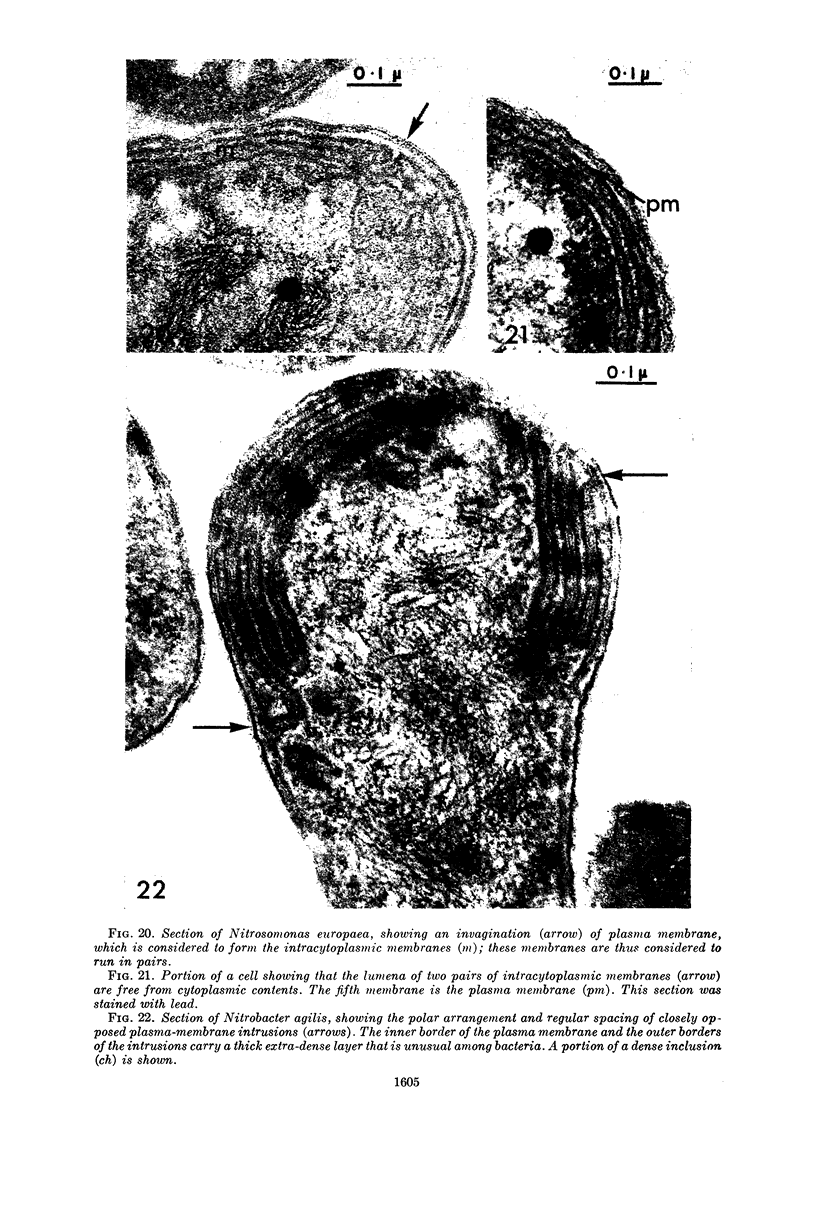



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BEER M. Disposition of membranes in Alcaligenes faecalis. J Bacteriol. 1960 Nov;80:659–664. doi: 10.1128/jb.80.5.659-664.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOATMAN E. S., DOUGLAS H. C. Fine structure of the photosynthetic bacterium Rhodomicrobium vannielii. J Biophys Biochem Cytol. 1961 Nov;11:469–483. doi: 10.1083/jcb.11.2.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROWN A. D., TURNER H. P. MEMBRANE STABILITY AND SALT TOLERANCE IN GRAM-NEGATIVE BACTERIA. Nature. 1963 Jul 20;199:301–302. doi: 10.1038/199301a0. [DOI] [PubMed] [Google Scholar]
- CEDERGREN B., HOLME T. On the glycogen in Escherichia coli B; electron microscopy of ultrathin sections of cells. J Ultrastruct Res. 1959 Oct;3:70–73. doi: 10.1016/s0022-5320(59)80016-2. [DOI] [PubMed] [Google Scholar]
- CLAUS G. W., ROTH L. E. FINE STRUCTURE OF THE GRAM-NEGATIVE BACTERIUM ACETOBACTER SUBOXYDANS. J Cell Biol. 1964 Feb;20:217–233. doi: 10.1083/jcb.20.2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COSTERTON J. W., MURRAY R. G., ROBINOW C. F. Observations on the motility and the structure of Vitreoscilla. Can J Microbiol. 1961 Jun;7:329–339. doi: 10.1139/m61-040. [DOI] [PubMed] [Google Scholar]
- FITZ-JAMES P. C. Participation of the cytoplasmic membrane in the growth and spore fromation of bacilli. J Biophys Biochem Cytol. 1960 Oct;8:507–528. doi: 10.1083/jcb.8.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIESBRECHT P., DREWS G. [Electron microscope studies on the development of "chromatophores" by Rhodospirillum molischianum Giesberger]. Arch Mikrobiol. 1962;43:152–161. [PubMed] [Google Scholar]
- KELLENBERGER E., RYTER A., SECHAUD J. Electron microscope study of DNA-containing plasms. II. Vegetative and mature phage DNA as compared with normal bacterial nucleoids in different physiological states. J Biophys Biochem Cytol. 1958 Nov 25;4(6):671–678. doi: 10.1083/jcb.4.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEES H. Symposium on autotrophy. IV. Some thoughts on the energetics of chemosynthesis. Bacteriol Rev. 1962 Jun;26:165–167. [PMC free article] [PubMed] [Google Scholar]
- MASON D. J., POWELSON D. M. Nuclear division as observed in live bacteria by a new technique. J Bacteriol. 1956 Apr;71(4):474–479. doi: 10.1128/jb.71.4.474-479.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBINOW C. F. On the plasma membrane of some bacteria and fungi. Circulation. 1962 Nov;26:1092–1104. doi: 10.1161/01.cir.26.5.1092. [DOI] [PubMed] [Google Scholar]
- RYTER A., KELLENBERGER E., BIRCHANDERSEN A., MAALOE O. Etude au microscope électronique de plasmas contenant de l'acide désoxyribonucliéique. I. Les nucléoides des bactéries en croissance active. Z Naturforsch B. 1958 Sep;13B(9):597–605. [PubMed] [Google Scholar]
- STANIER R. Y., VAN NIEL C. B. The concept of a bacterium. Arch Mikrobiol. 1962;42:17–35. doi: 10.1007/BF00425185. [DOI] [PubMed] [Google Scholar]