Abstract
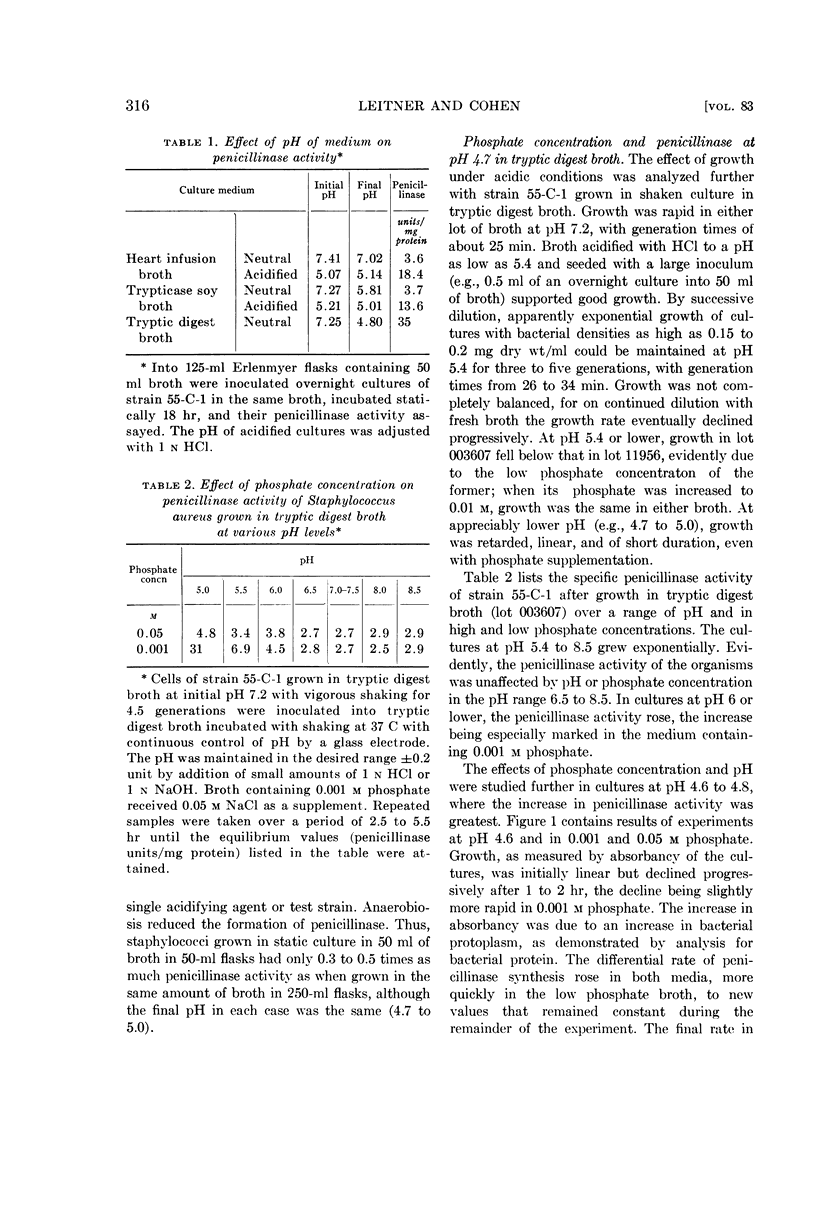
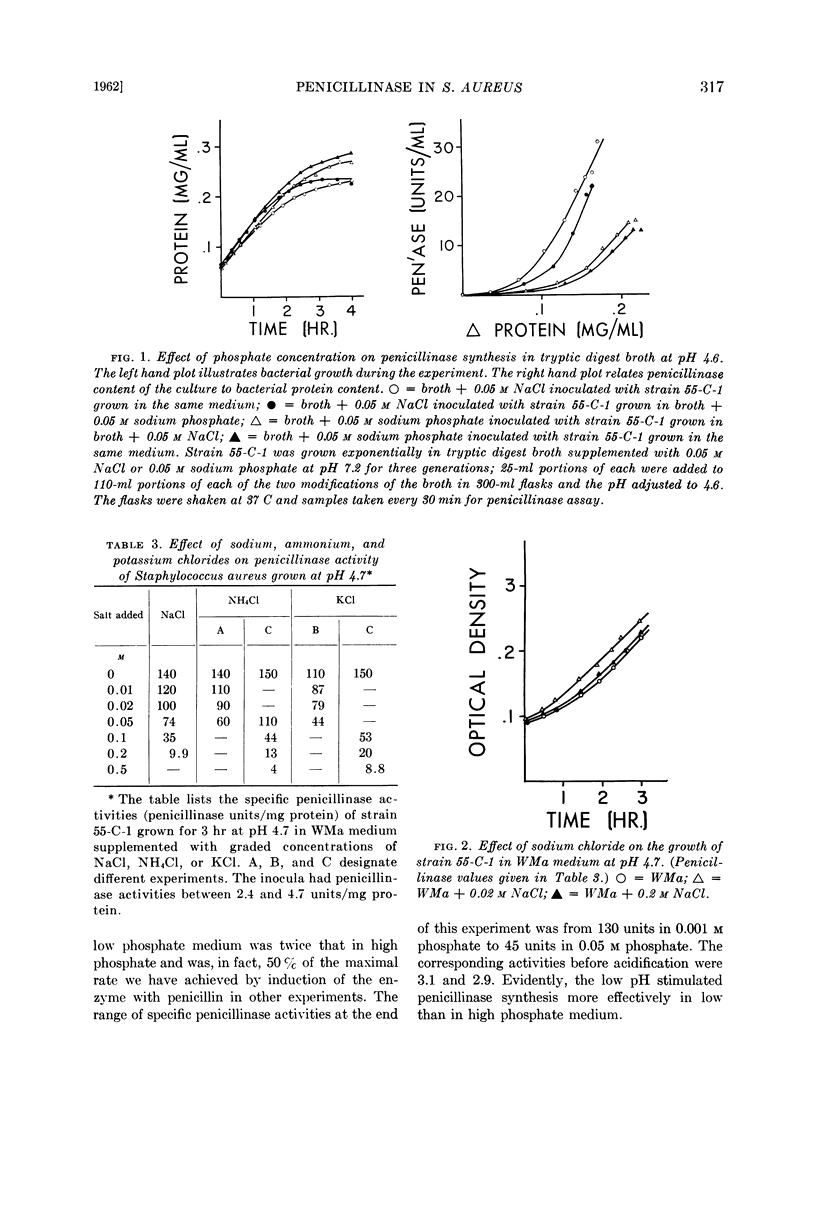
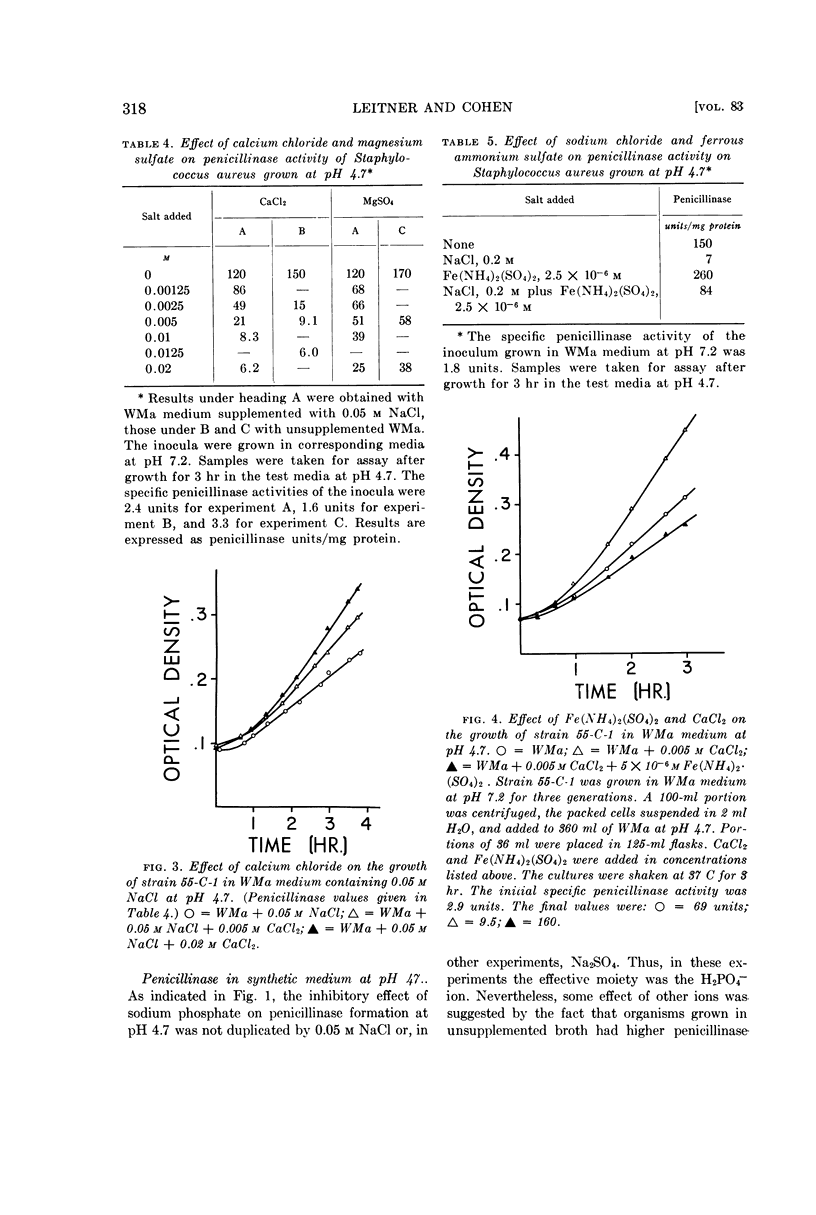
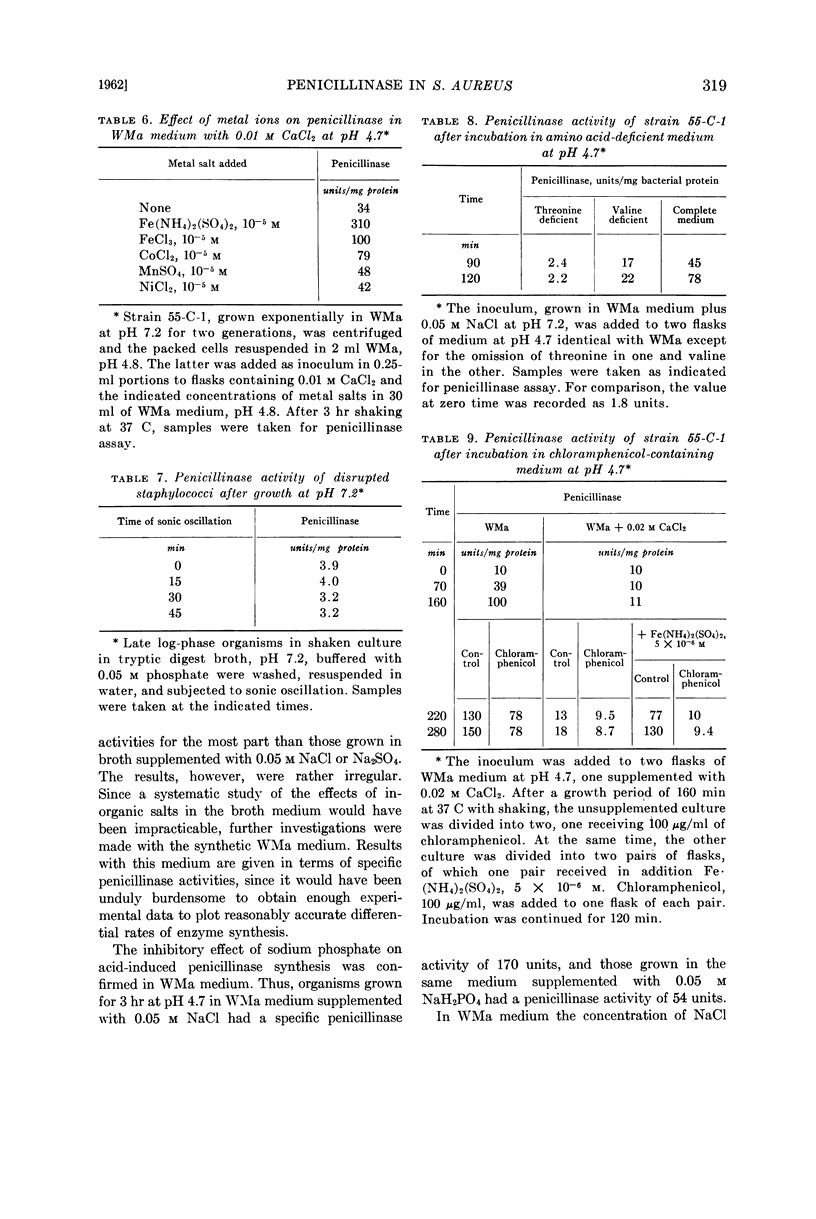
Leitner, Felix (Michael Reese Hospital, Chicago, Ill.) and Sidney Cohen. Effect of pH and inorganic salts on penicillinase formation in Staphylococcus aureus. J. Bacteriol. 83:314–323. 1962.—Staphylococcus aureus strain 55-C-1 exhibited a progressive increase in penicillinase activity as the pH of its growth medium dropped from 6.0 to 4.7. At pH 4.7, the increase in enzymic activity was inhibited markedly by Ca++, somewhat less by Mg++, and moderately by NaCl, KCl, and NH4Cl. Inhibition by NaH2PO4 was greater than by NaCl. Growth at the same pH, in small concentrations of Fe(NH4)2(SO4)2 (10−5 to 10−6m), elevated penicillinase activity strikingly, an effect shared to a lesser degree with FeCl3, CoCl2, and, very slightly, MnCl2. The available evidence indicated that the changes in enzymic activity were the result of changes in rate of formation of the enzyme.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- GERONIMUS L. H., COHEN S. Increased staphylococcal penicillinase activity accompanying penicillin treatment of experimentally infected mice. J Bacteriol. 1957 Oct;74(4):507–513. doi: 10.1128/jb.74.4.507-513.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GERONIMUS L. H., COHEN S. Induction of staphylococcal penicillinase. J Bacteriol. 1957 Jan;73(1):28–34. doi: 10.1128/jb.73.1.28-34.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale E. F. FACTORS INFLUENCING THE ENZYMIC ACTIVITIES OF BACTERIA. Bacteriol Rev. 1943 Sep;7(3):139–173. doi: 10.1128/br.7.3.139-173.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOROWITZ J., SAUKKONEN J. J., CHARGAFF E. Effects of fluoropyrimidines on the synthesis of bacterial proteins and nucleic acids. J Biol Chem. 1960 Nov;235:3266–3272. [PubMed] [Google Scholar]
- KJELDGAARD N. O., MAALOE O., SCHAECHTER M. The transition between different physiological states during balanced growth of Salmonella typhimurium. J Gen Microbiol. 1958 Dec;19(3):607–616. doi: 10.1099/00221287-19-3-607. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MITCHELL P. Transport of phosphate across the osmotic barrier of Micrococcus pyogenes; specificity and kinetics. J Gen Microbiol. 1954 Aug;11(1):73–82. doi: 10.1099/00221287-11-1-73. [DOI] [PubMed] [Google Scholar]
- MITCHELL P. Transport of phosphate across the surface of Micrococcus pyogenes; nature of the cell inorganic phosphate. J Gen Microbiol. 1953 Oct;9(2):273–287. doi: 10.1099/00221287-9-2-273. [DOI] [PubMed] [Google Scholar]
- McCARTHY B. J., HINSHELWOOD C. Variations in catalase activity during a bacterial growth cycle. Proc R Soc Lond B Biol Sci. 1959 Jan 27;150(938):13–23. doi: 10.1098/rspb.1959.0004. [DOI] [PubMed] [Google Scholar]
- POLLOCK M. R., TORRIANI A. M. Purification et caractéristiques physicochimiques de la pénicillinase de Bacillus cereus. C R Hebd Seances Acad Sci. 1953 Jul 20;237(3):276–278. [PubMed] [Google Scholar]
- WRIGHT E. S., MUNDY R. A. Defined medium for phenol coefficient tests with Salmonella typhosa and Staphylococcus aureus. J Bacteriol. 1960 Aug;80:279–280. doi: 10.1128/jb.80.2.279-280.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]


