Abstract
Angiogenesis is required for wound healing and repair, but dysregulated angiogenesis is involved in gastrointestinal inflammation. Bacillus polyfermenticus (B.P.) is a probiotic bacterium clinically used for a variety of intestinal disorders in East Asia. Here we investigated the effect of B.P. on angiogenesis of human intestinal microvascular endothelial cells (HIMECs) and wound healing in intestinal mucosa. Exposure of HIMECs to the conditioned medium of B.P. cultures (B.P. CM) increased cell migration, permeability, and tube formation. Production of the proangiogenic cytokine IL-8 was increased by B.P. CM, and neutralizing antibodies against IL-8 or IL-8 receptor CXCR2 reduced tube formation as well as actin stress fiber formation. B.P. CM also increased NF-κB activation, and inhibitors of NF-κB suppressed B.P. CM-induced tube formation and IL-8 production. Furthermore, B.P. facilitated recovery of mice from colitis as shown by increased body weight and reduced rectal bleeding and histological severity. B.P. also increased angiogenesis and mouse IL-8 production in the mucosal layer. Collectively, these results show that B.P. increases angiogenesis of HIMECs in a NF-κB/IL-8/CXCR2-dependent manner. Moreover, B.P. promotes angiogenesis in the mucosa during recovery of mice from colitis, suggesting that this probiotic may be clinically used to facilitate intestinal wound healing.
Keywords: inflammatory bowel diseases, wound healing, CXCR
use of probiotic therapy has progressively increased for prevention and treatment of gastrointestinal disorders (16, 49, 55). Probiotics are defined as live nonpathogenic microorganisms that benefit the host when provided in adequate amounts (24). Probiotics relieve symptoms of irritable bowel syndrome and inflammatory bowel disease (IBD), enhance immunity against pathogenic bacterial or viral infection, and ameliorate antibiotic-associated diarrhea (24, 30). However, the molecular mechanisms by which probiotics mediate beneficial effects remain unclear.
The probiotic Bacillus polyfermenticus (B.P.), first found in the air by Dr. Terakado in 1933, is sold commercially in Japan and Korea to treat a variety of intestinal disorders (40). B.P. is relatively resistant to digestive enzymes, gastric acid, and bile salts because of its endospore-forming feature that contributes to its longer presence in the gastrointestinal tract (40). B.P. also produces the antimicrobial agent bacteriocin (40). Oral administration of B.P. to humans stimulates IgG production and modulates the number of CD4+, CD8+, or natural killer cells (35). Anticancer effect of this bacterium was also reported in studies using a dimethylhydrazine-induced colon cancer model in rats (41, 47). However, the effect of B.P. on intestinal angiogenesis has not been investigated yet.
The angiogenic program consists of a deliberately orchestrated series of cellular events by which new vessels arise from preexisting ones. Dysregulated angiogenesis underlies major human diseases such as cancer, diabetic retinopathy, and IBD including Crohn's disease (CD) (14) and ulcerative colitis (UC) (10, 12, 17, 27). Moreover, angiogenesis is necessary for wound healing to occur, which requires delineated cellular responses to regenerate damaged tissues (45). Interleukin-8 (IL-8/CXCL-8), a CXC chemokine, is known as an angiogenic and permeability factor in nonimmune cells including endothelial cells (29, 54, 60). IL-8 exerts its biological activity via binding to two receptors, CXCR1 and CXCR2 (1). IL-8 is also implicated in tumor angiogenesis of gastrointestinal carcinomas (19, 37). In human intestinal microvascular endothelial cells (HIMECs), IL-8 increases tube formation and migration through its CXCR2 receptor (28).
In the present study, we investigated the effects of B.P. on intestinal angiogenesis during experimental colitis in vivo and in HIMECs in vitro. Our results show that B.P. enhances several angiogenic responses, including tube formation, cell migration, and permeability, and these responses are mediated through NF-κB and IL-8 signaling pathways. Moreover, B.P. accelerates the recovery of mice from colitis and increases angiogenesis in the mucosal layer. Together, these results suggest that B.P. exerts its probiotic effect on intestinal wound healing through increasing angiogenesis.
MATERIALS AND METHODS
Reagents.
Antibodies against p-p105 and p-p65, NF-κB subunits, were purchased from Cell Signaling Technology (Danvers, MA). Antibodies against β-actin (Sigma, St. Louis, MO), CXCR2 (BD Pharmingen, San Diego, CA), and IL-8 (R&D, Minneapolis, MN) were purchased. Anti-CD31 antibody and its isotype control rat IgG were from BD Pharmingen. Biotinylated anti-rat antibody was purchased from Vector Laboratories (Burlingame, CA). Other IgGs were from Santa Cruz Biotechnology (Santa Cruz, CA). Human recombinant IL-8 was purchased from R&D Systems. NF-κB inhibitors, SN50, SN50M, and celastrol were purchased from Calbiochem (La Jolla, CA). SB 225002 was purchased from Tocris Bioscience (Ellisville, MI).
Cell cultures.
HIMECs were isolated as previously described (6). Briefly, HIMECs were obtained from normal areas of the intestine of patients admitted for bowel resection. HIMECs were isolated by enzymatic digestion and subsequently cultured in MCDB131 medium (Sigma) supplemented with 20% fetal bovine serum (BioWhittaker, Walkersville, MD), antibiotics (BioWhittaker), heparin (Sigma), and endothelial cell growth factor (Roche Applied Science, Indianapolis, IN). Cultures of HIMECs were maintained at 37°C in 5% CO2. HIMECs were used between passages 7 and 12.
Human colonic epithelial cells (NCM460) were cultivated in M3D medium (Incell, San Antonio, TX) supplemented with 10% (vol/vol) heat-inactivated fetal bovine serum, 1% l-glutamine, and 10 units/ml penicillin, and 100 μg/ml streptomycin at 37°C in air supplemented with 5% CO2 as previously described (53). The Institutional Biosafety Committee approved all the procedures involving human cell lines.
Preparation of B.P. CM and B.S. CM.
Freeze-dried B.P. (4 × 109 CFU/g) was provided by Binex (Busan, South Korea). Frozen Bacillus subtilis (B.S.) stock was purchased from ATCC (Manassas, VA). For the conditioned medium of B.P. (B.P. CM) and B.S. (B.S. CM), we followed the previously described protocol by Grabig et al. (22). Briefly, B.P. (2 × 109 CFU) and B.S. (2.3 × 109 CFU) were incubated for 16 h at 37°C (B.P.) or 30°C (B.S.) in 100 ml Luria-Bertani broth. Cultures were then collected by centrifugation (1,000 g for 15 min), and pellets were washed twice in phosphate-buffered saline and then resuspended in M3D medium containing 10% fetal calf serum without antibiotics. After 2 h incubation at 37°C in 5% CO2, culture medium was collected and filtered through a 0.22 μm-pore-size filter. B.P CM or B.S. CM was then mixed with complete M3D medium (1:2 ratio) before treatments.
Tube formation assay.
HIMECs (4 × 104 cells/well) were plated on 400 μl of Matrigel (BD Biosciences, San Jose, CA)-coated 24-well plate and supplemented with either complete MCDB131 medium alone or a combination of B.P. CM or B.S. CM and complete MCDB131 medium. After 6 h, images were visualized with Axio Observer D1 inverted microscope (Carl Zeiss), captured with an AxioCam digital camera, and processed with Adobe photoshop as previously described (31). Total tube length was measured with Image J.
Quantitative cell migration assay using Boyden chambers.
Cell migration was quantified by use of a quantitative cell migration assay kit (Millipore, Billerica, MA) following the manufacturer's instructions. Briefly, the Boyden chamber assay kit consists of a hollow plastic chamber sealed at one end with a porous membrane. Cells were seeded in this hollow chamber with complete M3D medium. The hollow chamber resided in another chamber filled with B.P. CM. Cells were allowed to migrate overnight through the pores to the other side of the membrane. The inner tube was then removed and carefully washed, and any nonmigratory cells inside of the membrane were carefully scraped away. Cells migrated on the opposite side of the membrane were stained, extracted, and quantified on a spectrophotometer (Spectra Max M5, Molecular Devices, Sunnyvale, CA).
Permeability assay.
The permeability assay was conducted as previously described (48). Briefly, HIMECs (1 × 105 cells/well) in 100 μl of culture medium were plated on Matrigel-coated Transwell inserts of 3-μm pore size. After 30 min, 100 μl of culture medium alone was added to the insert and 1 ml of culture medium was added to the lower chamber. After 24 h, the medium from the insert was removed and an additional 1 × 105 cells/well in 100 μl of culture medium was plated. Twenty-four hours after the second plating, B.P. CM or VEGF (100 ng/ml) was added to the lower chamber along with 10 μg of 3-kDa FITC-dextran. For all assays, 10-μl aliquots were removed at the indicated time points from the upper chamber, and fluorescence intensity was quantified by use of a fluorimeter (Fluoroskan ascent, Thermo Fisher Scientific, Fair Lawn, NJ) with excitation at 485 nm and emission at 535 nm.
Quantitative real-time PCR.
Total RNA from mouse colon tissues was isolated via RNeasy Plus Mini Kit (Qiagen) and equal amount of RNA (2 μg) was transcribed into cDNA, by use of High Capacity Reverse Transcription Kit (Applied Biosystems). Subsequently, quantitative real-time PCR was performed on Applied Biosystems 7500 Fast Real-Time PCR System with TaqMan Universal Master Mix, using the standard conditions from Applied Biosystems. Annealing/extension temperature was 60°C (1 min). The primer pairs and FAM dye-labeled TaqMan MGB (minor groove binding) probes or GAPDH gene for the internal control were purchased from Applied Biosystems. The level of expression was calculated on the basis of the PCR cycle number at which the exponential growth in fluorescence from the probe passes a certain threshold value (CT). Relative gene expression was determined by the difference in the CT values of the target genes after normalization to RNA input level, using CT value of GAPDH. Relative quantification was represented by standard 2−ΔCT calculations. ΔCT = (CT-target gene − CT-GAPDH) (44). Each reaction was performed in triplicate.
ELISA of human IL-8 or mouse IL-8 (KC).
For human IL-8, HIMECs were plated on Matrigel and then supplemented with B.P. CM, B.S. CM, or celastrol for 6 h. The culture supernatant was collected and the concentration of human IL-8 was determined by ELISA (Biosource). For mouse IL-8, the whole colon was dissected, homogenized, and lysed in RIPA. After centrifugation, supernatant was collected and used for mouse IL-8 [keratinocyte-derived cytokine (KC)] ELISA (Invitrogen). Experiments were carried out in triplicate and results are shown as mean pg/ml (21).
Rhodamine-phalloidin staining.
F-actin polymerization was assessed in HIMECs cultured on fibronectin-coated glass chamber slides (LabTek, Thermo Fisher Scientific). Cells were stimulated with anti-CXCR2 antibody (5 μg/ml) or its isotype control IgG 15 min prior to B.P. CM stimulation (15 min). The cells then washed with PBS, fixed with 10% formalin for 15 min, permeabilized with Triton X-100 (0.1%) for 5 min, and stained with rhodamine-phalloidin (Molecular Probes, Eugene, OR) for 15 min. After the washing, slides were mounted with 50% glycerol and examined with an Axio Imager Z1 microscope (Carl Zeiss) and captured with an AxioCam MRm digital camera and processed with Adobe Photoshop.
Immunoblotting analysis.
Equal amounts of protein from cell lysates were subjected to SDS-PAGE analysis, and immunoblotting using the appropriate antibodies was performed as we previously described (51, 52).
DSS-induced colitis and histological analysis.
CD-1 mice (8-wk-old males) were from Charles River Laboratory. The Institutional Animal Care and Use Committee of University of California-Los Angeles approved all animal procedures. To induce colitis, mice were fed with dextran sulfate sodium (DSS, 4%) (MP Biomedicals, Irvine, CA) dissolved in regular tap water for 7 days followed by 1% DSS for 4 additional days, as previously described (51, 62). For oral administration, B.P. or B.S. was dissolved in sterilized water (108 CFU/ml) and orally administered everyday in 100 μl total volume by gastric gavage when mice were fed with 1% DSS. Control mice were treated with the same volume of sterilized water. For the SB225002 inhibitor experiment, mice were injected daily with 50 μl SB225002 [0.3 mg/kg ip in saline (Sigma) supplemented with 0.3% Ethanol (Sigma)] when B.P. or B.S. was administered. Mice were weighed for body weight changes and monitored for rectal bleeding everyday. For histological evaluation, the entire mouse colon was excised, fixed, paraffin embedded, and stained with hematoxylin and eosin. The histological severity of colitis was graded on a scale of 0–4, as described by our laboratory (51).
Immunohistochemistry.
For CD31 staining, segments of the transverse colon were embedded in optimal cutting temperature compound and frozen immediately. Five-micrometer sections were cut and then processed for peroxidase immunohistochemistry using CD31 antibody (1:100 dilution) as previously described (50). Hematoxylin solution (Vector Laboratories) was used for counterstaining. Total vessel length was measured via Image J.
Statistical analysis.
Results are represented as means ± SD. Paired and two-tailed Student's t-tests or one-way ANOVA followed by the multiple-comparison Newman-Keuls post hoc test were used to assess differences between groups. A P value of <0.05 was considered statistically significant.
RESULTS
Probiotic B.P. increases angiogenesis of HIMECs.
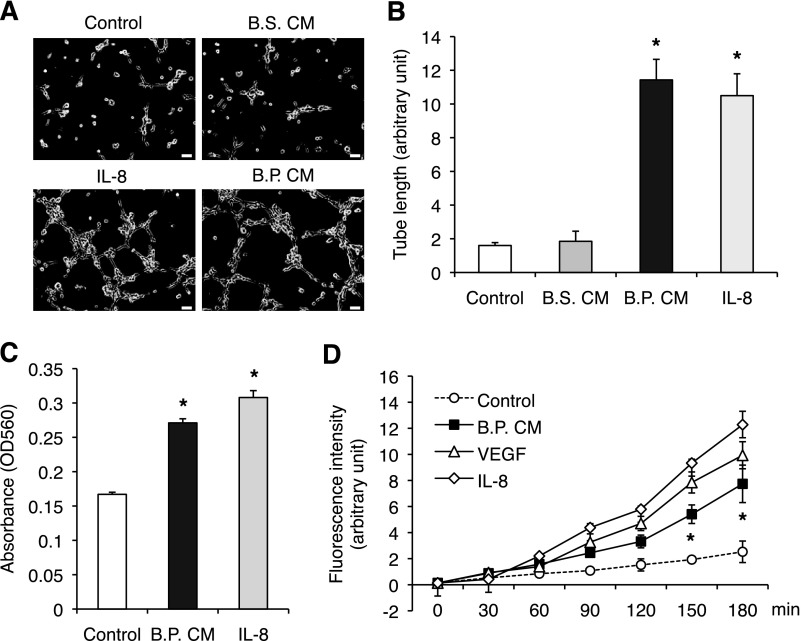
Angiogenesis is required for wound healing to support epithelial cell proliferation by supplying oxygen and nutrients on site. In the intestine, mucosal wound healing is a key process to maintain homeostasis. We assume that intestinal wound healing also requires angiogenesis. To this end, we investigated whether B.P. induces angiogenesis in vitro by measuring tube formation, migration, permeability, and proliferation of HIMECs. The B.P. CM enhanced tube formation of HIMECs when cultured on Matrigel for 6 h (Fig. 1, A and B). However, the B.S. CM (has similar characteristics as B.P.) did not increase tube formation, suggesting that this effect is specific to B.P. (Fig. 1, A and B). Additionally, the effect of B.P. CM on tube formation was similar to that of 5 ng/ml proangiogenic factor IL-8 (Fig. 1, A and B). Transmigration of endothelial cells through a Boyden chamber was also increased in B.P. CM-treated cells and again, the level of increased migration was similar to that induced by IL-8 (Fig. 1C). Although endothelial cell proliferation is observed in an angiogenic process, it is not always required. We found that B.P. CM increased endothelial cell proliferation by only 9% after 24 h of stimulation (Supplemental Fig. S1A). Since tubes formed within 6 h, it does not seem that proliferation of endothelial cells contributed to tube formation in this setting.
Fig. 1.
The conditioned medium (CM) of Bacillus polyferminticus (B.P.; B.P. CM) increases angiogenesis of human intestinal microvascular endothelial cells (HIMECs). A and B: HIMECs were plated on a Matrigel, and then medium containing either B.P. CM or Bacillus subtilis (B.S.) CM or IL-8 was added. After 6 h in culture, 3 randomly selected fields were photographed (A) and tube lengths were measured (B). Error bars represent SD of triplicate samples. B.P. CM increased angiogenesis of HIMECs, whereas B.S. CM did not. Similar results were observed in 3 independent experiments; *P < 0.05 vs. B.S. CM. Bar, 50 μm. C: the number of cells transmigrated through the pores of Boyden chamber was increased in the presence of B.P. CM or IL-8 (5 ng/ml). Error bars represent SD of triplicate samples. Similar results were observed in 3 independent experiments; *P < 0.01 vs. control. D: HIMECs were plated on a Matrigel-coated Transwell chamber and treated with B.P. CM or 100 ng/ml VEGF (a positive control) or 5 ng/ml IL-8 (a positive control) along with FITC-dextran for the indicated times. IL-8, VEGF, and B.P. CM increased permeability as measured by increased fluorescence intensity. Error bars represent SD of triplicate samples. Similar results were observed in 3 independent experiments; *P < 0.05 vs. control.
Increased permeability indicates loosened cell-cell contact and migration of endothelial cells, and therefore it is a prerequisite for angiogenesis. To test the ability of B.P. to alter permeability, confluent monolayers of HIMECs were generated on a Matrigel-coated Transwell chamber and treated with B.P. CM, 100 ng/ml VEGF, or 5 ng/ml IL-8. VEGF and IL-8 were used as positive controls. We found that B.P. CM stimulated endothelial permeability, and this permeability was similar to that induced by VEGF or IL-8, albeit to a lesser degree (Fig. 1D). These results indicate that B.P. CM increases angiogenesis of HIMECs by increasing endothelial cell migration and permeability.
B.P. CM-induced angiogenesis is mediated by IL-8/CXCR2.
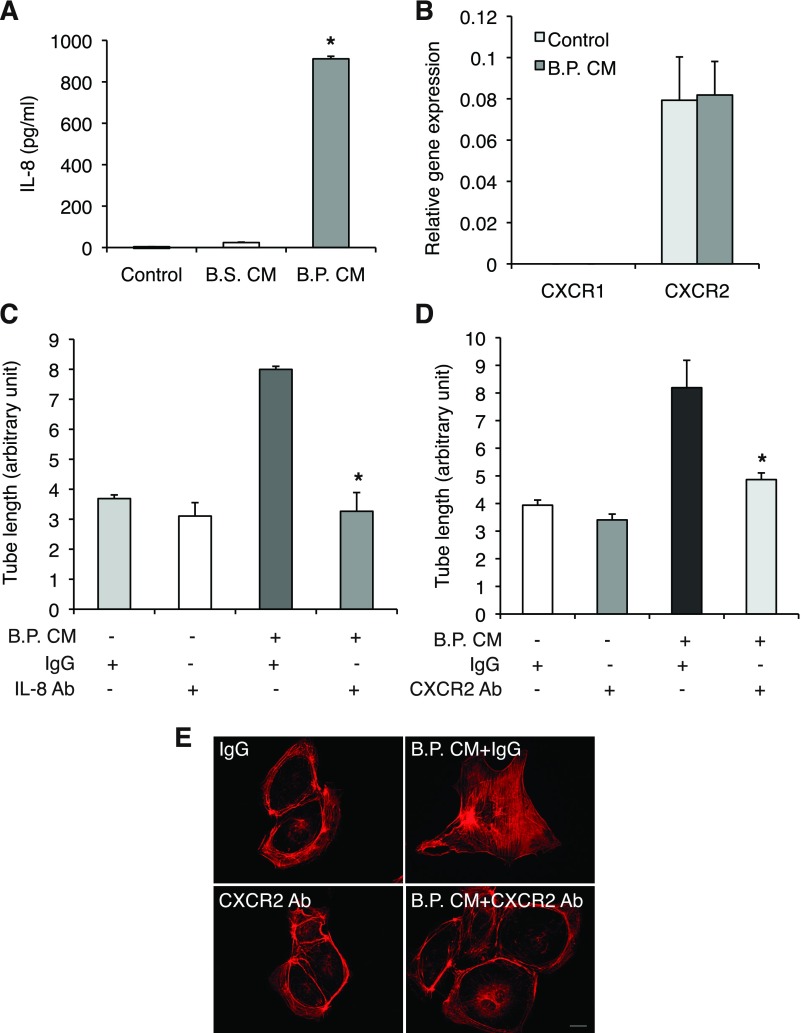
The chemokine IL-8 increases endothelial permeability during angiogenesis (5). IL-8 also induced angiogenesis in HIMECs through CXCR2 (28). Thus we investigated whether IL-8 is a mediator of B.P. CM-induced angiogenesis. B.P. CM, not B.S. CM, dramatically increased IL-8 production in HIMECs (Fig. 2A). In accordance with the previous report, HIMECs express CXCR2 and the stimulation with B.P. CM did not alter the receptor expression (Fig. 2B). Moreover, B.P. CM-induced tube formation was significantly inhibited in the presence of anti-IL-8 neutralizing antibody (0.5 μg/ml) or anti-CXCR2 neutralizing antibody (5 μg/ml) (Fig. 2, C and D).
Fig. 2.
B.P. CM enhances angiogenesis of HIMECs through activation of the IL-8/CXCR2 pathway. A: the result of ELISA for IL-8 in HIMEC supernatant showed that B.P. CM, not B.S. CM, increased IL-8 production. Error bars represent SD of triplicate samples. Similar results were observed in 3 independent experiments; *P < 0.01 vs. B.S. CM. B: quantitative PCR of CXCR1 and CXCR2 in HIMECs indicates that only CXCR2 is expressed in HIMECs. Moreover, B.P. CM did not alter the expression profile. Error bars represent SD of triplicate samples. Similar results were observed in 3 independent experiments. NCM460 cDNA was used as a calibrator and GAPDH was included as the internal control of PCR. C and D: tube assays of HIMECs were performed on Matrigel with B.P. CM along with IL-8 (0.5 μg/ml)- or CXCR2 (5 μg/ml)-neutralizing antibodies or their isotype control goat IgG or mouse IgG, respectively. B.P. CM-induced angiogenesis was reduced in the presence of IL-8- or CXCR2-neutralizing antibodies, suggesting that angiogenic effect of B.P. CM is IL-8/CXCR2 dependent. Error bars represent SD of triplicate samples. Similar results were observed in 3 independent experiments; *P < 0.05 vs. IgG isotype control. E: stress fiber polymerization was assessed in HIMECs stimulated with B.P. CM along with CXCR2 neutralizing antibody (5 μg/ml) or IgG control. F-actin was detected by staining rhodamine-phalloidin. B.P. CM strongly increased stress fiber assembly, but neutralizing CXCR2 blocked it. Bar, 20 μm.
Furthermore, endothelial cell migration requires actin reorganization, and the previous study indicated that IL-8 induces stress fibers in HIMECs (20, 28). Indeed, stress fiber formation was enhanced when stimulated with B.P. CM and attenuated when the cells were preincubated with anti-CXCR2 antibody (5 μg/ml) (Fig. 2E). These results suggest that B.P. CM induces IL-8 production in HIMECs and as a result autocrine activation of CXCR2 increases angiogenic features including tube formation and F-actin polymerization.
In addition, a recent study suggests that IL-8-induced endothelial cell permeability increased transactivation of VEGFR2 in a VEGF-dependent manner (48). Moreover, inhibition of VEGFR abolishes RhoA activation and gap formation by IL-8 suggesting that VEGFR2 activation is required for IL-8-induced permeability (48). Since the above data indicated that B.P. CM increased permeability of HIMECs and induced IL-8, we tested whether B.P. CM can activate VEGFR2. However, B.P. CM did not affect phosphorylation of VEGFR2 (Supplemental Fig. S1B). Although B.P. CM produces IL-8, the effect of IL-8 on VEGFR2 activation on other endothelial cells may not be applicable to HIMECs. Moreover, since B.P. CM did not increase VEGF production in HIMECs (data not shown), it is unlikely that B.P. CM will transactivate VEGFR2.
IL-8 production by B.P. CM is NF-κB dependent.
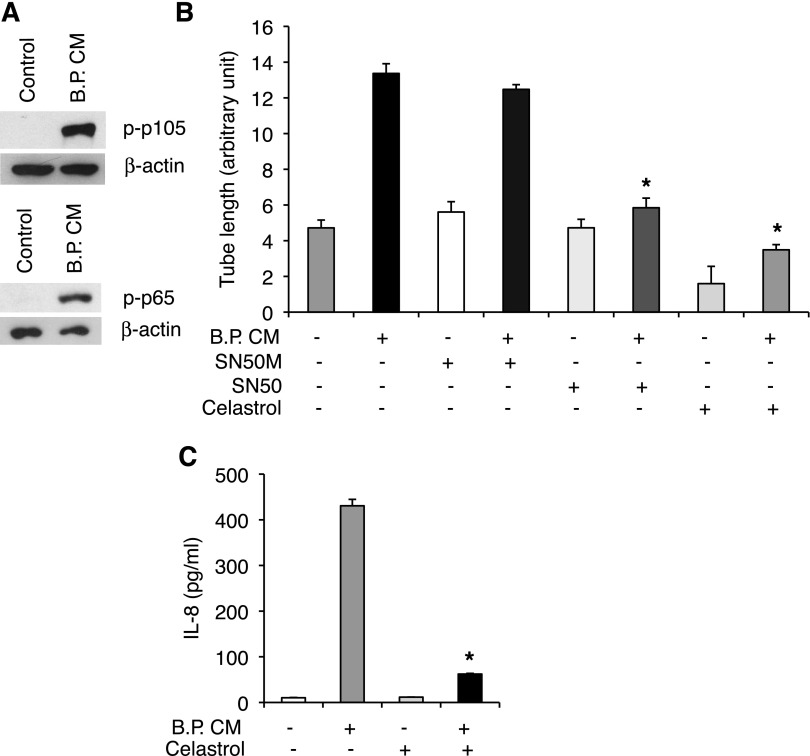
Production of many cytokines including IL-8 is regulated by an inducible cytoplasmic transcription factor NF-κB (59). In immune cells, NF-κB is involved in inflammatory responses (34). However, in nonimmune cells including epithelial and endothelial cells, NF-κB is also involved in cell survival, proliferation, and wound healing (34, 43). Thus we tested whether B.P. CM activates NF-κB pathway to increase IL-8 production in HIMECs. We found that B.P. CM induced phosphorylation of two NF-κB subunits, p105 and p65 (Fig. 3A). Moreover, pharmacological inhibitors of NF-κB, SN50 (20 μM), and celastrol (2.2 μM) suppressed B.P. CM-induced tube formation, whereas a negative control for SN50 (SN50M, 20 μM) did not affect tube length (Fig. 3B). Furthermore, increased IL-8 production by B.P. CM was inhibited in the presence of NF-κB inhibitor celastrol (Fig. 3C). These results indicate that IL-8 production by B.P. CM is, at least in part, through NF-κB.
Fig. 3.
IL-8 production and angiogenesis by B.P. CM are NF-κB dependent. A: B.P. CM increased phosphorylation of NF-κB as tested by Western blot analysis. B: HIMECs were subjected to a tube assay and stimulated with B.P. CM along with NF-κB inhibitor SN50 (20 μM) and its negative control peptide SN50M (20 μM) or another NF-κB inhibitor, celastrol (2.2 μM), for 6 h. Inhibitors of NF-κB blocked B.P. CM-induced angiogenesis. Error bars represent SD of triplicate samples. Similar results were observed in 3 independent experiments; *P < 0.05 vs. B.P. CM. C: the result of ELISA for IL-8 showed that B.P. CM-increased IL-8 production was decreased in the presence of the NF-κB inhibitor celastrol. Error bars represent SD of triplicate samples. Similar results were observed in 3 independent experiments; *P < 0.001 vs. B.P. CM.
B.P. facilitates mucosal wound healing.
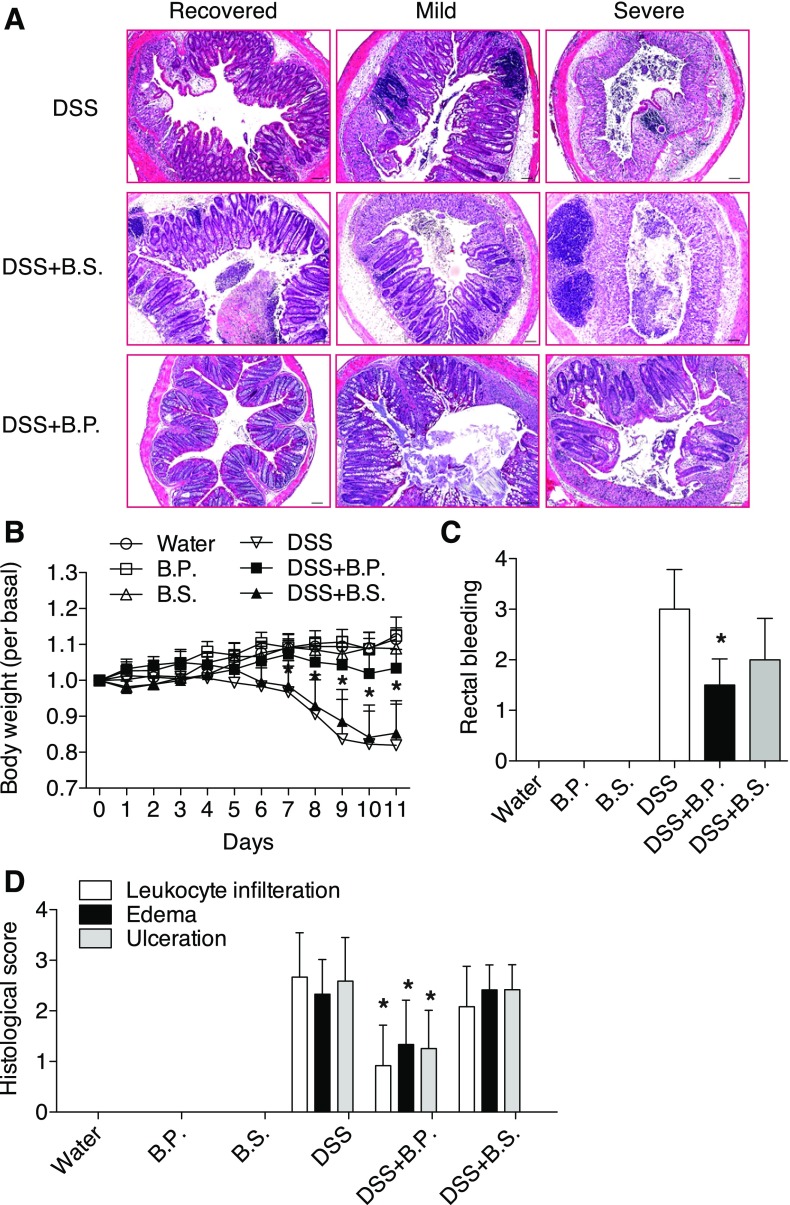
We next determined the angiogenic effect of B.P. in vivo using a mouse model of colitis. We hypothesized that increased angiogenesis by B.P. may accelerate mucosal wound healing and recovery from colitis. To test this, mice were first given 4% DSS (colitis-inducing agent) for a week, which was then followed by 1% DSS for 4 additional days with or without gastric gavage of B.P. or B.S. Continuous treatment of 4% DSS aggravated inflammation as indicated by declining body weight (Supplemental Fig. S2A). During the low dose (1%) of DSS treatment, however, we expected mice would recover gradually and the inflamed tissues would start healing in intestinal mucosa, and therefore this procedure would allow us to investigate a role of B.P. on wound healing. For histological evaluations, hematoxylin and eosin-stained colonic tissues from DSS alone or DSS with B.P. treated or DSS with B.S.-treated mice were examined. Among 16 mice per group, tissues can be divided into three subgroups based on histological severity of colitis: recovered, mild, and severe, as shown in Fig. 4A. These figures indicated presence of mucosal healing from inflammation, and notably B.P.-treated mice showed accelerated mucosal healing compared with B.S.-treated or nontreated mice in DSS-induced colitic tissues (Fig. 4A). Mice treated with B.S. or B.P. alone in the non-DSS control group showed intact mucosal layers (Supplemental Fig. S2B). Increased body weight of B.P.-treated mice compared with B.S.-treated mice also indicated that B.P. facilitated recovery of mice from colitis (Fig. 4B). Rectal bleeding was significantly reduced in B.P.-treated mice compared with B.S.-treated mice when monitored at day 11 (Fig. 4C). Histological scores of inflammation (leukocyte infiltration, edema, and ulceration) showed better recovery in B.P.-treated mice than B.S.-treated mice (Fig. 4D).
Fig. 4.
B.P. facilitates mucosal wound healing. Mice were supplied with 4% dextran sulfate sodium (DSS) in drinking water for 7 days followed by 1% DSS for 4 days. One group of mice was supplied with B.P. and the other group of mice was supplied with B.S. via oral gavage. A: tissues were collected, fixed, and stained for hematoxylin and eosin. Tissues from the mice fed with B.P. showed facilitated wound healing. Bar, 100 μm; n = 16 per group. B: mice fed with B.P. showed increased body weight compared with B.S.-treated mice. Data are shown as means ± SD; n = 4–7 per group; *P < 0.05 vs. DSS+B.S. C: rectal bleeding was reduced in B.P.-treated mice compared with B.S.-treated mice. Data are shown as means ± SD; n = 7–15 per group; *P < 0.01 vs. DSS+B.S. D: histological scoring for several parameters of colonic inflammation including neutrophil infiltration, edema, and ulceration were graded on a scale of 0–4: 0, normal; 4, severe. Mice fed with B.P. showed reduced histological scores compared with B.S.-treated mice. Data are shown as means ± SD; n = 6 per group; *P < 0.05 vs. DSS+B.S.
B.P. increases angiogenesis during wound healing.
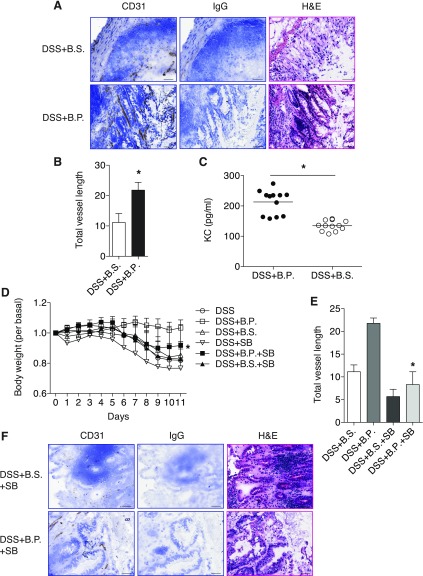
Since B.P. facilitated wound healing, we next tested whether increased angiogenesis plays a role in wound healing. To test this, we chose frozen tissue sections from B.S.- or B.P.-treated groups and performed immunohistochemistry with a CD31 antibody to detect blood vessels. We found that increased angiogenesis was evident in tissues from B.P.-administered mice compared with B.S.-administered mice (Fig. 5A). Total vessel length was also increased in B.P. group compared with B.S. group (Fig. 5B). Moreover, since our in vitro results indicate that IL-8 was involved in B.P.-induced angiogenesis, we next tested whether IL-8 was increased by B.P.-induced wound healing and angiogenesis in vivo. Production of KC (mouse IL-8) was increased in B.P.-treated mice tissues than B.S.-treated mice, and this suggested B.P.-dependent angiogenesis in vivo was also, at least in part, dependent on KC (Fig. 5C).
Fig. 5.
B.P. increases angiogenesis during wound healing. Mice were supplied with 4% DSS in drinking water for 7 days followed by 1% DSS for 4 days. One group of mice was supplied with B.P. and the other group of mice was supplied with B.S. via oral gavage. Tissues were collected and embedded for frozen sections. A: immunohistochemistry for CD31 was performed in both B.P.-treated and B.S.-treated groups by using sections. Tissues from the mice with B.P. treatment showed increased CD31 staining compared with B.S.-treated mice, suggesting increased angiogenesis. Rat IgG2a control was used instead of antibody as a negative control. Bar, 50 μm. B: total vessel length was measured by use of the Image J program. The B.P.-treated group showed increased total vessel length compared with B.S.-treated mice. Data are shown as means ± SD; n = 5 per group; *P < 0.01 vs. DSS+B.S. C: the result of ELISA for KC indicated that mice fed with B.P. showed increased KC production compared with B.S.-treated mice. n = 12; *P < 0.01 vs. DSS+B.S. D: mice treated with B.P. plus SB showed increased body weight compared with B.P. alone-treated mice. Data are shown as means ± SD; n = 4–7 per group; *P < 0.01 vs. DSS+B.P. E: total vessel length was measured with the Image J program. B.P. plus SB-treated group showed reduced total vessel length compared with B.P. alone-treated mice. Data are shown as means ± SD; n = 3–5 per group; *P < 0.001 vs. DSS+B.P. F: immunohistochemistry for CD31 was performed. Tissues from the mice with B.P. plus SB treatment showed reduced CD31 staining compared with B.P. alone-treated mice (A). Rat IgG2a control was used instead of the CD31 antibody as a negative control. Bar, 50 μm.
Given the observation that increased angiogenesis by B.P. in wound healing is related to induced IL-8 production, we further tested whether the pharmacological inhibitor of CXCR2 could block B.P.-increased angiogenic response and wound healing in colitis. To test this, mice were first given 4% DSS for a week, followed by 1% DSS for 4 additional days with gastric gavage of B.P. or B.S. and injection of SB225002 (SB, 0.3 mg/kg ip) once per day. B.P.-facilitated recovery of mice from colitis was impaired in mice injected with SB as indicated by reduced body weight of B.P. plus SB-treated mice compared with B.P. alone-treated mice (Fig. 5D). The overall vessel density and total vessel length in the tissues from B.P. plus SB-treated mice were reduced compared with B.P. alone-treated mice as shown by CD31 staining (Fig. 5, A, B, E, and F). These results imply that B.P.-induced angiogenesis is through the activation of CXCR2. In B.S.-treated or nontreated control mice, SB treatment did not significantly alter body weight (Fig. 5D), although the mice showed slightly increased diarrhea and bleeding (data not shown). The total vessel length was slightly reduced in B.S. plus SB- or SB alone-treated mice compared with B.S. alone-treated or nontreated mice, respectively (Fig. 5, E and F). Taken together, these results suggest that B.P. facilitates wound healing by a mechanism involving increased angiogenesis through IL-8.
DISCUSSION
In this study we report two major findings. First, the observation that the probiotic bacterium B.P. regulates angiogenesis of intestinal microvascular endothelial cells. Second, that B.P. modulates endothelial IL-8 production by a NF-κB-dependent pathway. These findings suggest a previously unappreciated role of the probiotic B.P. in angiogenesis of intestinal endothelial cells, which is essential for wound healing process.
Angiogenesis in both inflammation and wound healing.
Angiogenesis is an intrinsic component to chronic inflammatory process by promoting recruitment of inflammatory cells, producing cytokines, matrix-degrading enzymes, chemokines, and supplying nutrients (27, 32, 45). Very recently, pathological angiogenesis has been implicated in IBD including CD and UC (14). A notable feature in IBD tissues is the presence of aberrant vasculature and increased vessel density in the inflamed mucosa (58, 61). Additionally, IBD vessels showed impaired NO-dependent vasodilation, suggesting their contribution to reduced perfusion, poor wound healing, and persistent inflammation (26). Mucosal extracts from IBD patients induced migration of HIMECs in an IL-8-dependent manner and increased angiogenic responses in a cornea pocket assay and a chorioallantoic membrane assay (12). Moreover, clinical studies have shown elevated local concentrations of vascular endothelial growth factor (23) and increases in serum levels of basic fibroblast growth factor (b-FGF) and transforming growth factor (TGF)-β in patients with active UC or CD, suggesting increased angiogenic responses (8, 33, 38). In IL-10-deficient colitis model, an antiangiogenic compound decreased angiogenesis and alleviated clinical severity and histological inflammation (13). Therefore, increased angiogenesis is attributed to worsening experimental IBD and angiogenesis blockade (i.e., decreased angiogenesis) appears to be beneficial to treat IBD.
In this report, however, we show that angiogenesis is beneficial to heal experimental IBD by focusing on the role of angiogenesis in wound healing (instead of inflammation). Angiogenesis is indispensable for all the processes of wound healing that require cellular events to facilitate regeneration and recovery of damaged tissues (45). These responses consist of inflammation, angiogenesis, epithelialization, and collagen remodeling (36). Among them, angiogenesis is important because oxygen supply is a prerequisite for epithelial cells and fibroblasts to act on the repair process (7). In a low-oxygen environment like the wound area, macrophages and platelets produce angiogenic factors that attract endothelial cells to form new capillaries (7). Studies indicate that disrupted angiogenesis results in impaired wound healing. For instance, eNOS-deficient mice exerted impaired wound healing due to reduced angiogenesis (42). Moreover, angiopoietin-1 promoted wound healing in diabetic mice by increasing angiogenesis (11). Our findings also suggest that the probiotic bacterium when applied at the healing phase of experimental IBD increased angiogenesis and thus enhanced wound healing and facilitated recovery of mice from colitis. Taken together, angiogenesis is essential for both inflammation and wound healing, and therefore it is important to apply pro- or antiangiogenic reagents in a timely manner. Additionally, in terms of the therapeutic application, proangiogenic therapy should be applied when there is a requirement for wound healing. In contrast, antiangiogenic therapy should be applied when there is active inflammation.
IL-8 and CXCR-2 in inflammation, wound healing, and angiogenesis.
The role of IL-8 and its receptors, CXCR1 and CXCR2, in IBD has been demonstrated in several studies. In chronic DSS-induced experimental colitis, CXCR2−/− mice showed limited signs of tissue damages and reduced colitic symptoms (9). Additionally, neutralizing CXCR2 using anti-CXCR2 serum relieved clinical symptoms in an acute DSS colitis model and blocking CXCR2 with its selective antagonist SB225002 ameliorated acute 2,4,6-trinitrobenzene sulfonic acid (TNBS)-induced colitis (4, 15). However, contrasting roles for CXCR2 in experimental colitis were also reported. Blocking CXCR2 by its neutralizing antibody reduced leukocyte influx during the early phase of TNBS-induced acute colitis but failed to reduce leukocyte accumulation during the late phase of colitis (2). Similarly, CXCR2 exerted contrasting effects on the recruitment of inflammatory cells during the early and late phase of fungus-induced allergic inflammation (56). Furthermore, a recent study showed that mice deficient with the CXCR2 ligand KC showed increased susceptibility to DSS-induce colitis, suggesting a protective role of KC in colitis (57). These studies suggest that IL-8 and CXCR2 can play contrasting roles in IBD.
Our study extends a role of IL-8 and CXCR2 in the wound healing from colitis by increasing angiogenesis. Previous studies demonstrated that CXC chemokines, which contain a Glu-Leu-Arg (ELR) motif adjacent to their first cysteine amino acid in the NH2 terminus, are potent angiogenic promoters (3). Moreover, as described above, angiogenesis is a critical component of wound healing, and therefore it is logical that IL-8-induced angiogenesis could facilitate wound healing. In an incisional skin wound-healing model, IL-8 was strongly expressed and its expression was correlated with an increased number of vessels (18). Another study showed the chicken CXC chemokine 9E3/CEF4, highly homologous to human IL-8, was highly upregulated within wounded areas with neovascularization (46). The present study also demonstrates that increased IL-8 production by B.P. can facilitate wound healing and increase angiogenesis in DSS-injured intestinal mucosal layers. Thus the data from other groups, coupled with our own, clearly indicate that the characteristics of IL-8 as an angiogenic promoter seem to constitute its protective role in mucosal healing.
What is the mechanism by which B.P. induces angiogenesis?
There is only a handful of reports suggesting that probiotics induce angiogenesis. A probiotic strain Lactobacillus rhamnosus GG increased the expression of proangiogenic VEGF and therefore enhanced wound healing from gastric ulcers in rats (39). Additionally, supernatants from cultures of two strains of Lactobacillus acidophilus induced proliferation of blood vessels when injected into rodent ears subcutaneously (25). However, the molecular mechanism by which probiotics modulate angiogenesis has not yet been studied.
Microbial products often trigger activation of NF-κB and its dependent signaling cascades including cytokine productions. Those products involve lipopolysaccharide, flagellin, double stranded RNA among others and activate Toll-like receptors (TLRs). Our recent data indicate that B.P. exerts its activity at least in part through TLR2 and TLR4, suggesting a possibility that B.P. produces microbial products that are able to activate TLRs (unpublished data). Furthermore, activation of NF-κB often leads to production of cytokines including IL-8, an inducer of inflammation and angiogenesis. Intestinal endothelial cells express most of TLRs and IL-8 receptors (CXCR1 and 2). Therefore, it is possible that functional molecule(s), possibly TLR ligands, produced from B.P. activate endothelial TLRs and its downstream NF-κB. Sequentially, activated NF-κB increases transcription of IL-8, which in turn enhances angiogenesis of endothelial cells via its receptor as an autocrine loop.
In summary, our study shows that B.P. increases angiogenesis in HIMECs in vitro and mucosal tissues in vivo. B.P. exerts its angiogenic effect through activation of NF-κB/IL-8/CXCR2 pathway. Future studies will evaluate the therapeutic effect of B.P. for revascularization in intestinal wound healing after chronic inflammation.
GRANTS
This work was supported by a research grant from Binex (S. H. Rhee), by a Young Clinical Scientist Award from Flight Attendant Medical Research Institute (E. Im and S. H. Rhee), and by National Institute of Diabetes and Digestive and Kidney Diseases Grants 1KO1 DK083336 (E. Im), RO1 DK50894 and RO1 DK69854 (C. Fiocchi), PO1 DK33506 and RO1 DK072471 (C. Pothoulakis), and 1KO1 DK079015 (S. H. Rhee).
Supplementary Material
ACKNOWLEDGMENTS
We thank Elise Ma and Jinyoung Choi for technical assistance.
REFERENCES
- 1. Ahuja SK, Ozcelik T, Milatovitch A, Francke U, Murphy PM. Molecular evolution of the human interleukin-8 receptor gene cluster. Nat Genet 2: 31–36, 1992. [DOI] [PubMed] [Google Scholar]
- 2. Ajuebor MN, Zagorski J, Kunkel SL, Strieter RM, Hogaboam CM. Contrasting roles for CXCR2 during experimental colitis. Exp Mol Pathol 76: 1–8, 2004. [DOI] [PubMed] [Google Scholar]
- 3. Belperio JA, Keane MP, Arenberg DA, Addison CL, Ehlert JE, Burdick MD, Strieter RM. CXC chemokines in angiogenesis. J Leukoc Biol 68: 1–8, 2000. [PubMed] [Google Scholar]
- 4. Bento AF, Leite DF, Claudino RF, Hara DB, Leal PC, Calixto JB. The selective nonpeptide CXCR2 antagonist SB225002 ameliorates acute experimental colitis in mice. J Leukoc Biol 84: 1213–1221, 2008. [DOI] [PubMed] [Google Scholar]
- 5. Biffl WL, Moore EE, Moore FA, Carl VS, Franciose RJ, Banerjee A. Interleukin-8 increases endothelial permeability independent of neutrophils. J Trauma 39: 98–102; discussion 102–103, 1995. [DOI] [PubMed] [Google Scholar]
- 6. Binion DG, West GA, Ina K, Ziats NP, Emancipator SN, Fiocchi C. Enhanced leukocyte binding by intestinal microvascular endothelial cells in inflammatory bowel disease. Gastroenterology 112: 1895–1907, 1997. [DOI] [PubMed] [Google Scholar]
- 7. Bishop A. Role of oxygen in wound healing. J Wound Care 17: 399–402, 2008. [DOI] [PubMed] [Google Scholar]
- 8. Bousvaros A, Zurakowski D, Fishman SJ, Keough K, Law T, Sun C, Leichtner AM. Serum basic fibroblast growth factor in pediatric Crohn's disease. Implications for wound healing. Dig Dis Sci 42: 378–386, 1997. [DOI] [PubMed] [Google Scholar]
- 9. Buanne P, Di Carlo E, Caputi L, Brandolini L, Mosca M, Cattani F, Pellegrini L, Biordi L, Coletti G, Sorrentino C, Fedele G, Colotta F, Melillo G, Bertini R. Crucial pathophysiological role of CXCR2 in experimental ulcerative colitis in mice. J Leukoc Biol 82: 1239–1246, 2007. [DOI] [PubMed] [Google Scholar]
- 10. Carmeliet P. Angiogenesis in life, disease and medicine. Nature 438: 932–936, 2005. [DOI] [PubMed] [Google Scholar]
- 11. Cho CH, Sung HK, Kim KT, Cheon HG, Oh GT, Hong HJ, Yoo OJ, Koh GY. COMP-angiopoietin-1 promotes wound healing through enhanced angiogenesis, lymphangiogenesis, and blood flow in a diabetic mouse model. Proc Natl Acad Sci USA 103: 4946–4951, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Danese S, Sans M, de la Motte C, Graziani C, West G, Phillips MH, Pola R, Rutella S, Willis J, Gasbarrini A, Fiocchi C. Angiogenesis as a novel component of inflammatory bowel disease pathogenesis. Gastroenterology 130: 2060–2073, 2006. [DOI] [PubMed] [Google Scholar]
- 13. Danese S, Sans M, Spencer DM, Beck I, Doñate F, Plunkett ML, de la Motte C, Redline R, Shaw DE, Levine AD, Mazar AP, Fiocchi C. Angiogenesis blockade as a new therapeutic approach to experimental colitis. Gut 56: 855–862, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dhanabal M, Wu F, Alvarez E, McQueeney KD, Jeffers M, MacDougall J, Boldog FL, Hackett C, Shenoy S, Khramtsov N, Weiner J, Lichenstein HS, LaRochelle WJ. Recombinant semaphorin 6A-1 ectodomain inhibits in vivo growth factor and tumor cell line-induced angiogenesis. Cancer Biol Ther 4: 659–668, 2005. [DOI] [PubMed] [Google Scholar]
- 15. Farooq SM, Stillie R, Svensson M, Svanborg C, Strieter RM, Stadnyk AW. Therapeutic effect of blocking CXCR2 on neutrophil recruitment and dextran sodium sulfate-induced colitis. J Pharmacol Exp Ther 329: 123–129, 2009. [DOI] [PubMed] [Google Scholar]
- 16. Fedorak RN, Madsen KL. Probiotics and the management of inflammatory bowel disease. Inflamm Bowel Dis 10: 286–299, 2004. [DOI] [PubMed] [Google Scholar]
- 17. Gariano RF, Gardner TW. Retinal angiogenesis in development and disease. Nature 438: 960–966, 2005. [DOI] [PubMed] [Google Scholar]
- 18. Gillitzer R, Goebeler M. Chemokines in cutaneous wound healing. J Leukoc Biol 69: 513–521, 2001. [PubMed] [Google Scholar]
- 19. Giorgini S, Trisciuoglio D, Gabellini C, Desideri M, Castellini L, Colarossi C, Zangemeister-Wittke U, Zupi G, Del Bufalo D. Modulation of bcl-xL in tumor cells regulates angiogenesis through CXCL8 expression. Mol Cancer Res 5: 761–771, 2007. [DOI] [PubMed] [Google Scholar]
- 20. Gong C, Stoletov KV, Terman BI. VEGF treatment induces signaling pathways that regulate both actin polymerization and depolymerization. Angiogenesis 7: 313–321, 2004. [DOI] [PubMed] [Google Scholar]
- 21. Goumans MJ, Valdimarsdottir G, Itoh S, Rosendahl A, Sideras P, ten Dijke P. Balancing the activation state of the endothelium via two distinct TGF-beta type I receptors. EMBO J 21: 1743–1753, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Grabig A, Paclik D, Guzy C, Dankof A, Baumgart DC, Erckenbrecht J, Raupach B, Sonnenborn U, Eckert J, Schumann RR, Wiedenmann B, Dignass AU, Sturm A. Escherichia coli strain Nissle 1917 ameliorates experimental colitis via toll-like receptor 2- and toll-like receptor 4-dependent pathways. Infect Immun 74: 4075–4082, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gragoudas ES, Adamis AP, Cunningham ET, Jr, Feinsod M, Guyer DR, VEGF Inhibition Study in Ocular Neovascularization Clinical Trial Group Pegaptanib for neovascular age-related macular degeneration. N Engl J Med 351: 2805–2816, 2004. [DOI] [PubMed] [Google Scholar]
- 24. Guarner F, Malagelada JR. Gut flora in health and disease. Lancet 361: 512–519, 2003. [DOI] [PubMed] [Google Scholar]
- 25. Halper J, Leshin LS, Lewis SJ, Li WI. Wound healing and angiogenic properties of supernatants from Lactobacillus cultures. Exp Biol Med (Maywood) 228: 1329–1337, 2003. [DOI] [PubMed] [Google Scholar]
- 26. Hatoum OA, Binion DG, Otterson MF, Gutterman DD. Acquired microvascular dysfunction in inflammatory bowel disease: loss of nitric oxide-mediated vasodilation. Gastroenterology 125: 58–69, 2003. [DOI] [PubMed] [Google Scholar]
- 27. Hatoum OA, Heidemann J, Binion DG. The intestinal microvasculature as a therapeutic target in inflammatory bowel disease. Ann NY Acad Sci 1072: 78–97, 2006. [DOI] [PubMed] [Google Scholar]
- 28. Heidemann J, Ogawa H, Dwinell MB, Rafiee P, Maaser C, Gockel HR, Otterson MF, Ota DM, Lugering N, Domschke W, Binion DG. Angiogenic effects of interleukin 8 (CXCL8) in human intestinal microvascular endothelial cells are mediated by CXCR2. J Biol Chem 278: 8508–8515, 2003. [DOI] [PubMed] [Google Scholar]
- 29. Hu DE, Hori Y, Fan TP. Interleukin-8 stimulates angiogenesis in rats. Inflammation 17: 135–143, 1993. [DOI] [PubMed] [Google Scholar]
- 30. Huebner ES, Surawicz CM. Probiotics in the prevention and treatment of gastrointestinal infections. Gastroenterol Clin North Am 35: 355–365, 2006. [DOI] [PubMed] [Google Scholar]
- 31. Im E, Venkatakrishnan A, Kazlauskas A. Cathepsin B regulates the intrinsic angiogenic threshold of endothelial cells. Mol Biol Cell 16: 3488–3500, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jackson JR, Seed MP, Kircher CH, Willoughby DA, Winkler JD. The codependence of angiogenesis and chronic inflammation. FASEB J 11: 457–465, 1997. [PubMed] [Google Scholar]
- 33. Kanazawa S, Tsunoda T, Onuma E, Majima T, Kagiyama M, Kikuchi K. VEGF, basic-FGF, and TGF-beta in Crohn's disease and ulcerative colitis: a novel mechanism of chronic intestinal inflammation. Am J Gastroenterol 96: 822–828, 2001. [DOI] [PubMed] [Google Scholar]
- 34. Karin M. Nuclear factor-kappaB in cancer development and progression. Nature 441: 431–436, 2006. [DOI] [PubMed] [Google Scholar]
- 35. Kim HS, Park H, Cho IY, Paik HD, Park E. Dietary supplementation of probiotic Bacillus polyfermenticus, Bispan strain, modulates natural killer cell and T cell subset populations and immunoglobulin G levels in human subjects. J Med Food 9: 321–327, 2006. [DOI] [PubMed] [Google Scholar]
- 36. Kirsner RS, Eaglstein WH. The wound healing process. Dermatol Clin 11: 629–640, 1993. [PubMed] [Google Scholar]
- 37. Kitadai Y, Haruma K, Sumii K, Yamamoto S, Ue T, Yokozaki H, Yasui W, Ohmoto Y, Kajiyama G, Fidler IJ, Tahara E. Expression of interleukin-8 correlates with vascularity in human gastric carcinomas. Am J Pathol 152: 93–100, 1998. [PMC free article] [PubMed] [Google Scholar]
- 38. Konno S, Iizuka M, Yukawa M, Sasaki K, Sato A, Horie Y, Nanjo H, Fukushima T, Watanabe S. Altered expression of angiogenic factors in the VEGF-Ets-1 cascades in inflammatory bowel disease. J Gastroenterol 39: 931–939, 2004. [DOI] [PubMed] [Google Scholar]
- 39. Lam EK, Yu L, Wong HP, Wu WK, Shin VY, Tai EK, So WH, Woo PC, Cho CH. Probiotic Lactobacillus rhamnosus GG enhances gastric ulcer healing in rats. Eur J Pharmacol 565: 171–179, 2007. [DOI] [PubMed] [Google Scholar]
- 40. Lee KH, Jun KD, Kim WS, Paik HD. Partial characterization of polyfermenticin SCD, a newly identified bacteriocin of Bacillus polyfermenticus. Lett Appl Microbiol 32: 146–151, 2001. [DOI] [PubMed] [Google Scholar]
- 41. Lee NK, Park JS, Park E, Paik HD. Adherence and anticarcinogenic effects of Bacillus polyfermenticus SCD in the large intestine. Lett Appl Microbiol 44: 274–278, 2007. [DOI] [PubMed] [Google Scholar]
- 42. Lee PC, Salyapongse AN, Bragdon GA, Shears LL, 2nd, Watkins SC, Edington HD, Billiar TR. Impaired wound healing and angiogenesis in eNOS-deficient mice. Am J Physiol Heart Circ Physiol 277: H1600–H1608, 1999. [DOI] [PubMed] [Google Scholar]
- 43. Liu ZG, Hsu H, Goeddel DV, Karin M. Dissection of TNF receptor 1 effector functions: JNK activation is not linked to apoptosis while NF-kappaB activation prevents cell death. Cell 87: 565–576, 1996. [DOI] [PubMed] [Google Scholar]
- 44. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25: 402–408, 2001. [DOI] [PubMed] [Google Scholar]
- 45. Majno G. Chronic inflammation: links with angiogenesis and wound healing. Am J Pathol 153: 1035–1039, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Martins-Green M, Bissell MJ. Localization of 9E3/CEF-4 in avian tissues: expression is absent in Rous sarcoma virus-induced tumors but is stimulated by injury. J Cell Biol 110: 581–595, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Park E, Jeon GI, Park JS, Paik HD. A probiotic strain of Bacillus polyfermenticus reduces DMH induced precancerous lesions in F344 male rat. Biol Pharm Bull 30: 569–574, 2007. [DOI] [PubMed] [Google Scholar]
- 48. Petreaca ML, Yao M, Liu Y, Defea K, Martins-Green M. Transactivation of vascular endothelial growth factor receptor-2 by interleukin-8 (IL-8/CXCL8) is required for IL-8/CXCL8-induced endothelial permeability. Mol Biol Cell 18: 5014–5023, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Reid G, Jass J, Sebulsky MT, McCormick JK. Potential uses of probiotics in clinical practice. Clin Microbiol Rev 16: 658–672, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rhee SH, Im E, Pothoulakis C. Toll-like receptor 5 engagement modulates tumor development and growth in a mouse xenograft model of human colon cancer. Gastroenterology 135: 518–528, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Rhee SH, Im E, Riegler M, Kokkotou E, O'Brien M, Pothoulakis C. Pathophysiological role of Toll-like receptor 5 engagement by bacterial flagellin in colonic inflammation. Proc Natl Acad Sci USA 102: 13610–13615, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Rhee SH, Jones BW, Toshchakov V, Vogel SN, Fenton MJ. Toll-like receptors 2 and 4 activate STAT1 serine phosphorylation by distinct mechanisms in macrophages. J Biol Chem 278: 22506–22512, 2003. [DOI] [PubMed] [Google Scholar]
- 53. Rhee SH, Keates AC, Moyer MP, Pothoulakis C. MEK is a key modulator for TLR5-induced interleukin-8 and MIP3alpha gene expression in non-transformed human colonic epithelial cells. J Biol Chem 279: 25179–25188, 2004. [DOI] [PubMed] [Google Scholar]
- 54. Salcedo R, Resau JH, Halverson D, Hudson EA, Dambach M, Powell D, Wasserman K, Oppenheim JJ. Differential expression and responsiveness of chemokine receptors (CXCR1–3) by human microvascular endothelial cells and umbilical vein endothelial cells. FASEB J 14: 2055–2064, 2000. [DOI] [PubMed] [Google Scholar]
- 55. Sartor RB. Probiotic therapy of intestinal inflammation and infections. Curr Opin Gastroenterol 21: 44–50, 2005. [PubMed] [Google Scholar]
- 56. Schuh JM, Blease K, Hogaboam CM. CXCR2 is necessary for the development and persistence of chronic fungal asthma in mice. J Immunol 168: 1447–1456, 2002. [DOI] [PubMed] [Google Scholar]
- 57. Shea-Donohue T, Thomas K, Cody MJ, Aiping Z, DeTolla LJ, Kopydlowski KM, Fukata M, Lira SA, Vogel SN. Mice deficient in the CXCR2 ligand, CXCL1 (KC/GRO-alpha), exhibit increased susceptibility to dextran sodium sulfate (DSS)-induced colitis. Innate Immun 14: 117–124, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Spalinger J, Patriquin H, Miron MC, Marx G, Herzog D, Dubois J, Dubinsky M, Seidman EG. Doppler US in patients with crohn disease: vessel density in the diseased bowel reflects disease activity. Radiology 217: 787–791, 2000. [DOI] [PubMed] [Google Scholar]
- 59. Stegmaier JC, Kirchhoff C, Bogner V, Matz M, Kanz KG, Mutschler W, Biberthaler P. Dynamics of neutrophilic NF-kB translocation in relation to IL-8 mRNA expression after major trauma. Inflamm Res 57: 547–554, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Strieter RM, Polverini PJ, Kunkel SL, Arenberg DA, Burdick MD, Kasper J, Dzuiba J, Van Damme J, Walz A, Marriott D, Chan SY, Roczniak S, and Shanafelt AB. The functional role of the ELR motif in CXC chemokine-mediated angiogenesis. J Biol Chem 270: 27348–27357, 1995. [DOI] [PubMed] [Google Scholar]
- 61. Wakefield AJ, Sawyerr AM, Dhillon AP, Pittilo RM, Rowles PM, Lewis AA, Pounder RE. Pathogenesis of Crohn's disease: multifocal gastrointestinal infarction. Lancet 2: 1057–1062, 1989. [DOI] [PubMed] [Google Scholar]
- 62. Wirtz S, Neufert C, Weigmann B, Neurath MF. Chemically induced mouse models of intestinal inflammation. Nat Protoc 2: 541–546, 2007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.