Abstract
In the liver, adenosine triphosphate (ATP) is an extracellular signaling molecule that is released into bile and stimulates a biliary epithelial cell secretory response via engagement of apical P2 receptors. The molecular identities of the ion channels involved in ATP-mediated secretory responses have not been fully identified. Intermediate-conductance Ca2+-activated K+ channels (IK) have been identified in biliary epithelium, but functional data are lacking. The aim of these studies therefore was to determine the location, function, and regulation of IK channels in biliary epithelial cells and to determine their potential contribution to ATP-stimulated secretion. Expression of IK-1 mRNA was found in both human Mz-Cha-1 biliary cells and polarized normal rat cholangiocyte (NRC) monolayers, and immunostaining revealed membrane localization with a predominant basolateral signal. In single Mz-Cha-1 cells, exposure to ATP activated K+ currents, increasing current density from 1.6 ± 0.1 to 7.6 ± 0.8 pA/pF. Currents were dependent on intracellular Ca2+ and sensitive to clotrimazole and TRAM-34 (specific IK channel inhibitors). Single-channel recording demonstrated that clotrimazole-sensitive K+ currents had a unitary conductance of 46.2 ± 1.5 pS, consistent with IK channels. In separate studies, 1-EBIO (an IK activator) stimulated K+ currents in single cells that were inhibited by clotrimazole. In polarized NRC monolayers, ATP significantly increased transepithelial secretion which was inhibited by clotrimazole. Lastly, ATP-stimulated K+ currents were inhibited by the P2Y receptor antagonist suramin and by the inositol 1,4,5-triphosphate (IP3) receptor inhibitor 2-APB. Together these studies demonstrate that IK channels are present in biliary epithelial cells and contribute to ATP-stimulated secretion through a P2Y-IP3 receptor pathway.
Keywords: purinergic, P2Y receptor, ATP, KCa2+ channel, bile formation, liver
intrahepatic biliary epithelial cells, known as cholangiocytes, contribute to the volume and composition of bile through the regulated secretion of electrolytes and water (14). Although the factors that regulate cholangiocyte secretion are not fully known, secretion mediated by extracellular nucleotides (e.g., ATP) acting on purinergic (P2) receptors on the luminal membrane of biliary epithelial cells has emerged as functionally important (7, 8, 12, 28, 33). ATP is present in bile, and binding of ATP to P2 receptors increases Cl− efflux from isolated cholangiocytes (28) and dramatically increases transepithelial secretion in biliary epithelial monolayers (33). Furthermore, the magnitude of the secretory response to ATP is greater than that in response to increases in cAMP (via the classical secretin-stimulated pathway) in single human biliary epithelial cells or polarized rat monolayers (9). Although cAMP increases Cl− efflux through CFTR (3), the molecular identities of the channels responsible for mediating the secretory response to ATP and other extracellular nucleotides are not known.
Binding of ATP to P2 receptors results in rapid increases in intracellular Ca2+ concentration ([Ca2+]i) from inositol 1,4,5-triphosphate (IP3) receptor-mediated Ca2+ release with subsequent activation of membrane channels resulting in Cl− efflux from the apical cholangiocyte membrane (9). Although Cl− channels are thought to provide the driving force for biliary secretion, secretion also requires complementary actions of membrane K+ channels. In secretory epithelium it is felt that activation of K+ channels leads to membrane hyperpolarization to maintain the electrical driving force for continued Cl− efflux (6).
In the liver, several lines of evidence are beginning to emerge that suggest that membrane K+ channels are critically important for secretion and bile formation. First, in isolated bile duct units, fluid secretion is significantly diminished by the nonspecific K+ channel inhibitor Ba2+ (36). Second, in single cholangiocytes or polarized biliary epithelial preparations, inhibition of membrane K+ channels significantly diminishes membrane currents and transepithelial secretion in response to increases in [Ca2+]i, respectively (11). Third, we have previously identified a small-conductance Ca2+-activated K+ channel, SK2, in both human and rat biliary epithelial cells that is activated by increases in [Ca2+]i (11), metabolic stress (41), and cell swelling (11, 30). However, whereas SK2 is exquisitely sensitive to inhibition by apamin, the Ca2+-activated K+ currents in single biliary cells are only partially inhibited in the presence of this bee venom toxin (11), suggesting that apamin-insensitive K+ channels may contribute to Ca2+-stimulated secretion.
IK channels with an intermediate conductance of 25–50 pS, a calcium sensitivity of 100 nM–1 μM, and negligible apamin sensitivity (21, 26) remain potential candidates for the apamin-insensitive component of the Ca2+-activated K+ conductance mediating secretion. In other epithelial cells, such as enterocytes, IK channels have been shown to be functionally expressed on the basolateral membrane, where they are an essential component of the secretory response to increases in [Ca2+]i (6). Immunostaining of whole rat liver with anti-IK-1 antibody reveals a prominent signal along the intrahepatic bile ducts (37), although no functional studies have previously been performed. We hypothesized therefore that IK-1 channels are present in biliary epithelium, contribute to the apamin-insensitive component of Ca2+-activated K+ efflux, and complement the role of apical Cl− channels in mediating the secretory response to extracellular ATP. Thus the goal of these studies in both human and rat biliary epithelial cells was to determine the location, function, and regulation of IK-1 channels and to determine their potential contribution to ATP-stimulated secretion.
METHODS
Cell models.
Studies in isolated cells were performed using Mz-Cha-1 cells and in polarized monolayers were performed utilizing normal rat cholangiocytes (NRC). Mz-Cha-1 cells, originally isolated from human adenocarcinoma of the gallbladder (23), were cultured as previously described (13). Mz-Cha-1 cells exhibit phenotypic features of differentiated biliary epithelium (2, 23) and have been utilized as models for purinergic signaling (13) and Ca2+-dependent secretion (11, 31). NRC monolayers isolated from intrahepatic bile ducts (39) express phenotypic features of differentiated biliary epithelium including receptors, signaling pathways, and ion channels similar to those found in primary cells (32, 33). NRC monolayers were cultured on rat tail collagen slabs as previously described and passaged onto collagen-coated semipermeable (24-mm diameter, 0.4-μm pore) Transwell supports (Costar Corning, Acton, MA-01720) 7–10 days before electrophysiological and molecular studies. This protocol permits highly polarized cells and the development of a high transepithelial resistance (Rt > 1,000 Ω·cm2) (33). Lastly, hTERT-immortalized human hepatic stellate cells (HSC) were cultured in DMEM supplemented with 10% FBS as previously described (34). These cells were used as a negative control for RNA blots and immunostaining because they do not express IK-1 channels.
Reverse transcription/PCR.
RNA was isolated using TRIzol, and quantified spectrophotometrically. One microgram of RNA was reverse transcribed by using oligo(dT) primer and Superscript RNase H-reverse transcriptase per the manufacturer's directions (Invitrogen, Carlsbad, CA). PCR reactions were then set up by using primers specific for mouse IK-1 [IK-1 pair 1: sense, cgg gaa caa gtg aac tcc at; antisense, aag gcc tac cca ctt tag cc (expected product size = 406 bp). IK-1 pair 2 sense, gag agg cag gct gtt aat gc; antisense, gga gct tcc ggt gtt tca g (expected product size = 387 bp)] (25). The reaction mixtures (25 μl) contained 2 μl of cDNA, 1 μl of dNTP (10 mmol/l), 2.5 μl of 10 × buffer, 1.5 μl of MgCl2 (25 mmol/l), 2.5 U Taq polymerase (Invitrogen), and 50 pmol of each primer. The mixture was subjected to 30 cycles of amplification (94°C for 60 s; denaturation, 60°C for 60 s annealing, and 72°C for 60 s extension). PCR products (10 μl) were detected by gel electrophoresis (1.5% agarose), which included size markers.
Immunofluorescence microscopy.
NRC cells were grown on collagen-coated (8-well) tissue culture chamber slides (BD BioCoat) for 5–7 days until appropriate confluency. After a washing with PBS, cells were fixed in 4% paraformaldehyde (pH 7.2) for 15 min at room temperature. After being rinsed several times with PBS, cells were permeabilized with 1% Triton-X in PBS for 10 min. After a further PBS wash, cells were covered with 2% normal donkey serum for 20 min. There were two primary antibodies: a rabbit anti-IK-1 (Kca3.1, SK4, KCNN4, Alomone Labs) and the negative control that is the same antibody preincubated with the Kca3.1 (SK4) peptide. Both antibodies were diluted at 1:200 and incubated with cells for 1 h at room temperature. Specimens were again rinsed in PBS before the addition of the secondary antibody, FITC-conjugated donkey anti-rabbit (Jackson Immunoresearch), at 1:200 dilution at room temperature for 30 min. The slide was washed in PBS several times and was incubated at room temperature for 30 min with the addition of Alexa Fluor 555 phalloidin (Invitrogen/Molecular Probes) at a 1:40 dilution in 1% BSA/PBS. After several PBS rinses, the slide was coverslipped with Mowiol 4–88 (Sigma-Aldrich) with 2.5% DABCO (Sigma-Aldrich) in PBS.
Measurement of membrane Cl− currents.
Membrane currents were measured via whole cell and single-channel patch-clamp techniques. Cells on a coverslip were mounted in a chamber (volume ∼400 μl), and whole cell currents were measured with a standard extracellular solution containing (in mM) 140 NaCl, 4 KCl, 1 CaCl2, 2 MgCl2, 1 KH2PO4, 10 glucose, and 10 HEPES-NaOH (pH ∼7.40). The standard intracellular (pipette) solution for whole cell recordings contained (in mM) 130 KCl, 10 NaCl, 2 MgCl2, 10 HEPES-KOH, 0.5 CaCl2, 3 MgATP2−, and 1 EGTA (pH 7.3), corresponding to a free Ca2+ concentration of ∼100 nM. In selected studies, the concentration of Cl− was decreased by partial replacement with aspartate to minimize the contribution of Cl− currents to the observed response. The low Cl− intracellular solution contained (in mM) 130 K-aspartate, 20 NaCl, 2 MgCl2, 10 HEPES-KOH, 0.5 CaCl2, 3 MgATP2−, and 1 EGTA (pH 7.3). With these solutions the K+ equilibrium potential (EK) is −83 mV and outward currents at a test potential of 0 mV are carried by K+ ions (IK). For single-channel recording, bath solutions contained (in mM) 140 NaCl, 4 KCl, 1 CaCl2, 2 MgCl2, 1 KH2PO4,10 glucose, and 10 HEPES-NaOH (pH ∼7.40) and pipette solutions contained (in mM) 146 K-gluconate, 4 NaCl, 2 MgCl2, 10 HEPES-KOH, 1 CaCl2 (pH 7.3). In selected experiments K+ was replaced by tetraethyl ammonium (TEA+) as described in results. Patch pipettes were pulled from Corning 7052 glass and had a resistance of 2–5 or 7–10 MΩ for whole cell and single-channel recordings, respectively. Recordings were made with an Axopatch ID amplifier (Axon Instruments, Foster City, CA) and were filtered at 1–2 kHz and sampled at 4 kHz for storage on a computer and analyzed with pCLAMP version 10 (Axon Instruments, Burlingame, CA) as previously described (9, 13). Two voltage protocols were utilized: 1) holding potential −40 mV, with 100-ms steps to 0 mV and −80 mV at 10-s intervals (for real-time tracings), and 2) holding potential −40 mV, with 100-ms steps from −100 mV to +100 mV in 20-mV increments. Current-voltage (I-V) relations were generated from the “step” protocol. Pipette voltages (Vp) are referred to the bath. In the whole cell configuration, Vp corresponds to the membrane potential, and upward deflections of the current trace indicate outward membrane current. Results are compared with control studies measured on the same day to minimize any effects of day-to-day variability and reported as current density (pA/pF) to normalize for differences in cell size (9). In the cell-attached patch configuration, applied voltages represent negative Vp values. Average open probability (NPo) was determined from 10- to 20-s recordings and mean open times were determined with pCLAMP systems and OriginPro 7.0 software (MicroCal Software, Northampton, MA). The number of channels in each patch (n) was measured by use of an all-points amplitude histogram and calculated as the peak current divided by the unitary current. The slope conductance was calculated from currents at negative voltages, representing inward current. Liquid junction potential was corrected where necessary.
Transepithelial Cl− secretion.
NRC cells were utilized to study the relative contribution of IK channels involved in extracellular nucleotide-stimulated transepithelial secretion. Cells were grown to confluence on collagen-treated polycarbonate filters with a pore size of 0.4 μm (Costar, Cambridge, MA) until resistance was >1,000 Ω·cm2 (EVOHM; World Precision Instruments, Sarasota, FL). Cells were mounted in a Trans-24 miniperfusion system for tissue culture cups (Jim's Instrument Manufacturing, Iowa City, IA). All experiments were carried out at 37°C, and basolateral and apical (luminal) sides were bubbled with O2 through air-lift circulators. The standard extracellular buffer solution contained (in mM) 140 NaCl, 4 KCl, 1 KH2PO4, 2 MgCl2, 1 CaCl2, 5 glucose, and 10 HEPES-NaOH (pH 7.3). Transepithelial voltage (Vt) was clamped to 0 mV, and short-circuit current (Isc) was recorded either through agar bridges (3% agar in 1 M KCl) connected to Ag-AgCl electrodes (cartridge electrodes, WPI) or directly through the Ag-AgCl electrodes in the bath. The Isc represents the net sum of the transepithelial anion and cation fluxes and reflects the level of ion and fluid secretion (33). Studies included paired, same-day monolayers to minimize any potential effects of day-to-day variability.
Reagents.
The IP3 receptor inhibitor 2-APB was obtained from Calbiochem/EMD Biosciences (La Jolla, CA) and from MP Biomedicals (Solon, OH). It should be noted that, since 2-APB can affect gap junction channels and alter cell capacitance in other cell models, capacitance measurements were performed in the presence or absence of the reagent prior to the start of the studies, and no statistical differences were noted consistent with our previous findings (9). The specific Ca2+-activated K+ channel activator 1-EBIO was obtained from Tocris (Bristol, UK). All other reagents, including ATP, were obtained from Sigma-Aldrich (St. Louis, MO).
Statistics.
Results are presented as means ± SE, with n representing the number of culture plates or repetitions for each assay as indicated. Student's paired or unpaired t-test or ANOVA for multiple comparisons was used to assess statistical significance as indicated, and P values <0.05 were considered to be statistically significant.
RESULTS
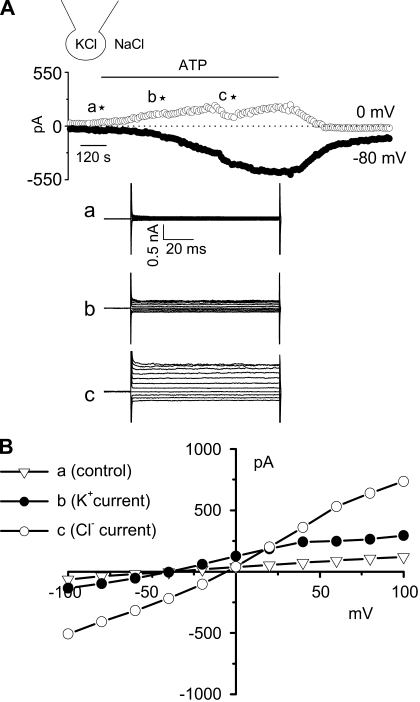
ATP activates clotrimazole-sensitive K+ channels in isolated biliary cells. To evaluate the potential functional properties of IK channels in isolated biliary cells, whole cell patch clamp studies were performed in Mz-Cha-1 cells in response to extracellular ATP, a potent agonist mediating biliary secretion (9). With standard extracellular and intracellular solutions, basal currents were small (Fig. 1). Exposure to ATP (50 μM) resulted in outward current secondary to K+ efflux (5.1 ± 0.4 pA/pF measured at 0 mV, n = 11), followed by sustained Cl− currents (−13.6 ± 1.2 pA/pF measured at −80 mV, n = 11). The ATP-stimulated K+ currents demonstrated an oscillatory pattern, reversal at −48 mV, and mild inward rectification, whereas the ATP-stimulated Cl− currents demonstrated outward rectification and reversal at 0 mV, consistent with previous studies (9, 11).
Fig. 1.
Characterization of whole cell ATP stimulated currents. Exposure to ATP stimulates both outward and inward currents in human Mz-Cha-1 biliary epithelial cells. Whole cell currents were measured during basal conditions and during exposure to ATP (50 μM) (methods). A: representative whole cell recording. Currents measured at −80 mV (●), representing ICl−, and at 0 mV (○), representing IK+, are shown. ATP exposure is indicated by the bar. A voltage-step protocol (test potentials between −100 mV and +100 mV in 20-mV increments) was obtained a★ (basal), b★ (maximal outward current response), and c★ (maximal inward current response) as indicated. The current-voltage (I-V) plot shown in B was generated from these protocols. B: I-V relationship of whole cell currents during basal (▿) and ATP-stimulated maximal outward current (●) and maximal inward current (○) conditions.
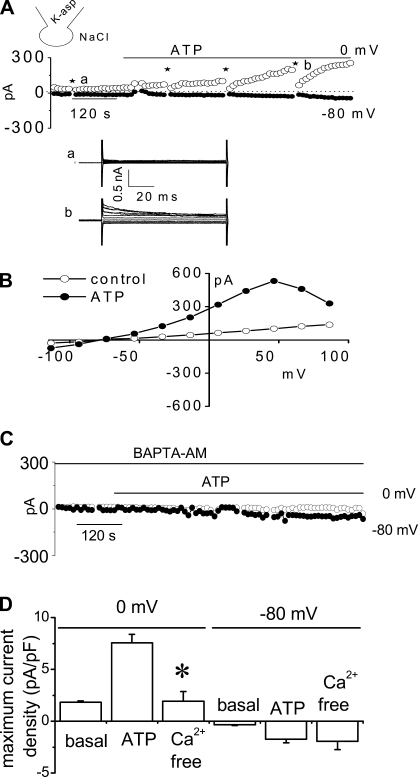
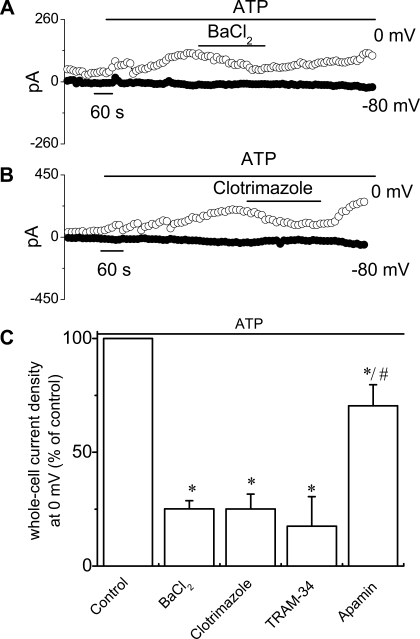
To isolate the K+ currents contributing to this response, the standard buffer solutions were replaced with low-Cl− solutions (methods). Under these conditions ATP (50 μM) increased current density from 1.6 ± 0.1 pA/pF to 7.6 ± 0.8 pA/pF at 0 mV (n = 18, P < 0.001), and the reversal potential shifted to −81.0 ± 0.7 mV (EK = −83 mV), without subsequent activation of Cl− currents (Fig. 2). The ATP-stimulated K+ currents were dependent on intracellular Ca2+ since chelation with BAPTA-AM completely abolished the currents (1.9 ± 0.8 pA/pF at 0 mV, n = 3, P < 0.05). In individual studies, the ATP-stimulated currents were significantly inhibited by selective pharmacological inhibitors of K+ channels (Fig. 3), including 1) the nonselective K+ channel blocker Ba2+ (5 mM), decreasing current density to 2.9 ± 0.4 pA/pF (a decrease of 74.9 ± 3.5%, n = 6, P < 0.05); 2) the specific IK channel inhibitor clotrimazole (10 μM), decreasing current density to 3.9 ± 0.9 pA/pF (a decrease of 74.9 ± 7.5%, n = 7, P < 0.05); and 3) the specific IK channel inhibitor TRAM-34 (20 μM), decreasing current density to 1.3 ± 0.9 pA/pF (a decrease of 82.5 ± 12.9%, n = 3, P < 0.05, Fig. 3). Conversely, the SK channel blocker apamin (50 nM) only partially inhibited ATP-stimulated currents 7.6 ± 1.4 pA/pF (a decrease of 30.0 ± 9.9%, n = 4, P < 0.05, Fig. 3C). Thus the pharmacological profile of the K+ currents activated by ATP is consistent with IK.
Fig. 2.
Characterization of whole cell ATP stimulated outward currents. Exposure to ATP stimulates outward currents in human Mz-Cha-1 biliary epithelial cells when intracellular KCl was replaced with K-aspartate (methods). Whole cell currents were measured during basal conditions and during exposure to ATP (50 μM) (methods). A: representative whole cell recording. Currents measured at 0 mV (○), representing IK+, and at −80 mV (●), representing ICl−, are shown. ATP exposure is indicated by the bar. A voltage-step protocol (test potentials between −100 mV and +100 mV in 20-mV increments) was obtained at a★ (basal) and b★ (maximal current response) as indicated. The I-V plot shown in 2B was generated from these protocols. B: I-V relationship of whole cell currents during basal (○) and ATP-stimulated (●) conditions. C: representative ATP-stimulated whole cell current tracings measured at 0 and −80 mV in the absence of intracellular Ca2+ (pretreated with BAPTA-AM 50 μM for 5–10 min and EGTA 2 mM in pipette solution). D: cumulative data demonstrating magnitude of ATP-stimulated currents in control conditions or after removal of intracellular Ca2+ (pretreated with BAPTA-AM 50 μM for 5–10 min and EGTA 2 mM in pipette solution). Values represent maximum current density measured at 0 and −80 mV (n = 3–10 each). *ATP-stimulated currents were significantly inhibited (P < 0.05 for each).
Fig. 3.
Pharmacological profile of ATP-stimulated whole cell currents. A and B: representative ATP-stimulated whole cell current tracings measured at 0 and −80 mV in the presence or absence of the nonspecific K+ channel blocker BaCl2 (5 mM) and the intermediate-conductance Ca2+-activated K+ (IK) channel inhibitor clotrimazole (10 μM) in the bath. Application of ATP and K+ channel inhibitors are indicated by bar. C: cumulative data demonstrating magnitude of ATP-stimulated currents in the presence or absence of the K+ channel inhibitors BaCl2 (5 mM), clotrimazole (10 μM), TRAM-34 (20 μM), and apamin (50 nM). Values represent % of maximum control current density (pA/pF) measured at 0 mV (n = 3–18). *ATP-stimulated currents were significantly inhibited vs. control (P < 0.05); #significantly different from Tram-34 (P < 0.05).
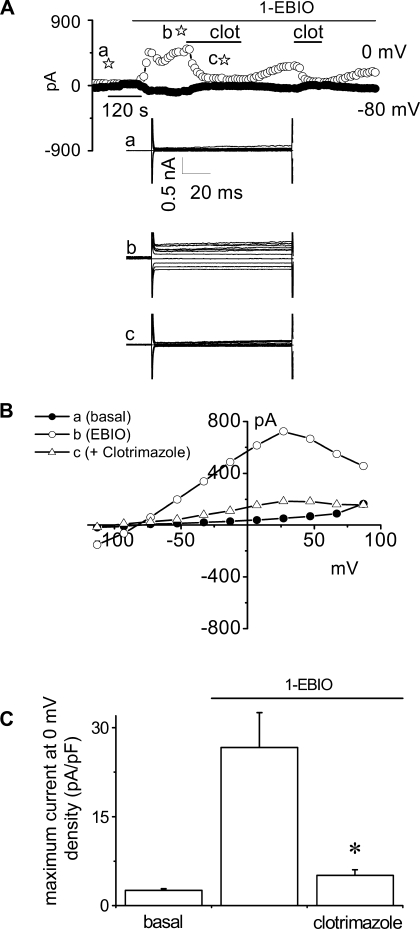
To further confirm the functional presence of IK channels in Mz-Cha-1 cells, whole cell currents were measured in response to the IK-1 channel enhancer activator, 1-EBIO (6). Exposure to 1-EBIO (600 μM) increased current density from 2.6 ± 0.3 pA/pF (basal) to 26.7 ± 5.8 pA/pF (n = 8, P < 0.01) with identical biophysical properties to the ATP-stimulated K+ currents (Fig. 4). The 1-EBIO-stimulated currents were reversibly inhibited by clotrimazole (10 μM, 5.1 ± 0.9 pA/pF, n = 7, P < 0.01). In summary, the pharmacological sensitivity and biophysical properties of the ATP-stimulated current response are consistent with IK channel activation.
Fig. 4.
1-EBIO activates K+ currents in human Mz-Cha-1 biliary cells. Whole cell currents were measured during basal conditions and during exposure to 1-EBIO (600 μM). A: representative whole cell recording. Currents measured at −80 mV (●), representing ICl−, and at 0 mV (○), representing IK+, are shown. Applications of 1-EBIO and clotrimazole (clot) are indicated by the bars. A voltage-step protocol (test potentials between −100 mV and +100 mV in 20-mV increments) was obtained at a☆ (basal), b☆ (maximal outward current response), and c☆ (current response after clotrimazole) as indicated. The I-V plot shown in B was generated from these protocols. B: I-V relationship of whole cell currents during basal (●), 1-EBIO-stimulated (○), and 1-EBIO-stimulated currents after exposure to clotrimazole (▵). C: cumulative data demonstrating maximum current density (pA/pF) measured at 0 mV under basal conditions and after exposure to 1-EBIO (600 μM) in the presence or absence of the IK-1 channel inhibitor clotrimazole (10 μM). Values represent maximum current density (pA/pF) measured at 0 mV (n = 7–8 each). *1-EBIO-stimulated currents were significantly inhibited (P < 0.01).
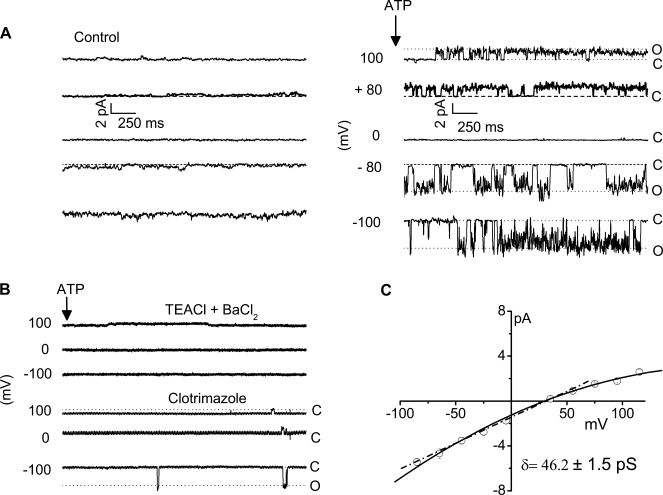
Single-channel characterization. To further characterize the channel types contributing to ATP-stimulated K+ currents, unitary currents were measured in the cell-attached configuration. Under basal conditions, few spontaneous openings were evident (Fig. 5A, left). Exposure to ATP (50 μM) activated currents (Fig. 5A, right), increasing NPo from 0.1 ± 0.0 to 0.6 ± 0.1 (n = 7, P < 0.001). Single-channel conductance was calculated at negative potentials and was 46.2 ± 1.5 pS (n = 18, Fig. 5C). Additional studies were performed to establish that the ATP-stimulated currents were mediated by K+. First, replacement of K+ with inclusion of TEA+ (146 mM) and BaCl2 (5 mM) in the patch pipette completely inhibited the ATP-activated currents, decreasing NPo to 0.1 ± 0.1 (n = 3, P < 0.05). Second, the IK-1 channel inhibitor clotrimazole (10 μM) decreased NPo to 0.1 ± 0.0 (n = 5, P < 0.01) when added to bath and pipette solutions (Fig. 5B). Together these studies demonstrate that ATP stimulates a 46.2 ± 1.5 pS, clotrimazole-sensitive K+ channel in single biliary cells. Thus the whole cell and single-channel studies functionally identify an intermediate-conductance Ca2+-activated K+ channel with biophysical and pharmacological properties identical to those reported for IK (21, 26).
Fig. 5.
ATP exposure activates unitary K+ currents. A: single ion channel currents were measured in the cell-attached configuration. Currents are shown at the pipette potentials (Vp, as indicated). Under basal conditions few spontaneous openings were measured (left). Exposure to ATP (50 μM) caused rapid appearance of single-channel events at different voltages (right). The channel opening and closing are indicated by O and C, respectively. B: replacement of the monovalent cations (Na+, K+) in the patch pipette with tetraethyl ammonium (TEA) and adding a K+ channel blocker BaCl2 completely inhibited ATP-stimulated currents (top traces); similarly, the specific IK-1 channel blocker clotrimazole also inhibited ATP-stimulated single-channel currents (bottom traces). C: current-voltage relationships for the ATP-stimulated K+ channel currents recorded in cell-attached patch. The dotted line represents the linear best fit at the negative potentials recorded with standard extracellular and K-gluconate-rich pipette solutions. The single-channel conductance was calculated from this slope and found to be 46.2 ± 1.5 pS.
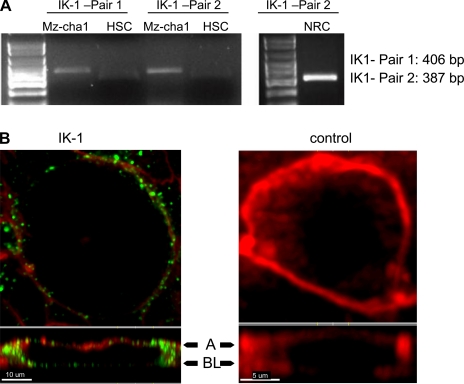
IK-1 channels are expressed in biliary epithelial cells. To confirm that the biliary cells used in these studies expressed IK-1 channels as shown by functional studies, we performed PCR to detect Mz-Cha-1 and NRC mRNA and examined polarized rat cholangiocyte by immunohistochemistry utilizing an IK-1 specific antibody (both as in methods). First, PCR products were detected by using primers directed against IK-1 channel mRNA extracted from Mz-Cha1, NRC, and HSC. The primer pairs yielded the predicted product size of 406 bp (1st pair) and 387 bp (2nd pair) in Mz-Cha-1 and NRC cells, but not in HSC, as expected (Fig. 6A). Additionally, IK-1 was identified utilizing a specific antibody to IK-1 (SK4, KCNN4) in polarized NRC monolayers. Cells were dual stained with F-actin to better highlight cell boundaries. In sequential confocal images, NRC staining with F-actin revealed individual cholangiocytes (Fig. 6B), and dual labeling (Fig. 6B) revealed IK-1 in the plasma membrane, with a prominent signal in basolateral compartments (Fig. 6B, bottom). These data provide definitive proof that both rat and human biliary preparations express IK-1 and further suggest that it is found predominantly in the plasma membrane, consistent with the functional studies in single cells.
Fig. 6.
Expression and localization of IK-1 protein in human MZ-cha-1 cells and normal rat cholangiocyte (NRC) monolayers. A: RT-PCR, as described in methods, was performed in Mz-cha-1 and NRC cells. The IK-1 primer pair 1 and the IK-1 primer pair 2 were used to detect IK-1 expression in Mz-cha cells (left), whereas IK-1 mRNA was identified in NRC cells by use of IK-1 primer pair 2 (right). A human hepatic stellate cell line, HSC, was used as a negative control, and a 100-bp DNA ladder was used to delineate the size of respective amplicons. B: membrane localization of IK-1 protein on NRC. Double staining for membrane F-actin (red) and IK-1 (green) in single NRC demonstrates presence of IK-1 channel protein along cell membrane, both apical and basolateral side (green) with predominance in lateral compartments (left). Single confocal image of control cell stained with same antibody after preincubation with IK-1 (SK4) peptide and stained for membrane F-actin (right). Bottom: z-axis view; arrowheads: A, apical, BL, basolateral membrane.
Transepithelial secretion.
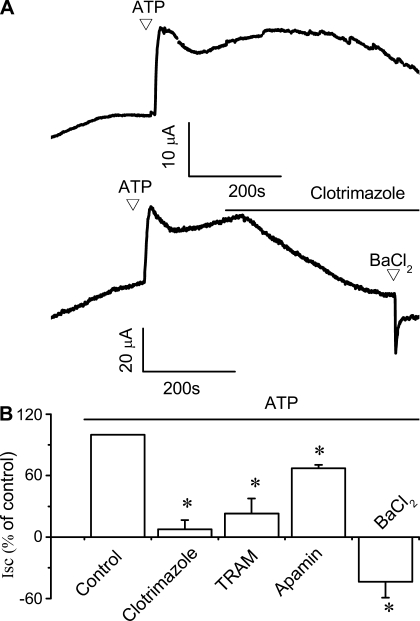
To determine the functional significance of IK-1 localization to ATP-stimulated secretory responses, polarized NRC monolayers were mounted in an Ussing chamber and Isc was measured in response to ATP in the presence or absence of pharmacological modulators of IK-1. We have previously shown that addition of ATP to the apical membrane of polarized NRC monolayers results in large increases in Isc, reflecting transepithelial secretion (9, 32). In control monolayers, addition of ATP to the apical membrane caused a relative increase in the Isc of 23.5 ± 2.8 μA/cm2 (n = 16, Fig. 7A, top trace). Addition of the IK inhibitor clotrimazole (20 μM) significantly decreased the magnitude of the ATP-induced Isc by 92.4 ± 9.0% (n = 7, P < 0.05, Fig. 7A, bottom). Likewise TRAM-34 (20 μM) also significantly decreased the magnitude of the ATP-stimulated Isc by 77.1 ± 14.9% (n = 5, P < 0.05). In contrast, addition of the SK2 inhibitor apamin decreased Isc by only 33.0 ± 3.4% (n = 4, P < 0.05, Fig. 7B). Addition of the nonspecific K+ channel blocker Ba2+ decreased the basal Isc and completely abolished the normal increase in Isc in response to ATP (Fig. 7), suggesting that K+ channels contribute to the basal Isc during unstimulated conditions as well as the ATP-stimulated response. Taken together, these studies in polarized biliary monolayers demonstrate that ATP-stimulated transepithelial secretion is dependent on IK channels.
Fig. 7.
Pharmacological inhibition of IK-1 inhibits ATP-stimulated transepithelial secretion in polarized cholangiocyte monolayers. Short-circuit current (Isc) across NRC monolayers was measured under voltage-clamp conditions in an Ussing chamber. In these representative recordings agonists are added to the apical chamber. A: addition of ATP (200 μM) resulted in significant increase in the magnitude of the Isc (top tracing). Addition of clotrimazole (20 μM) to apical and basolateral chambers significantly inhibited the ATP-stimulated Isc (bottom tracing). Addition of BaCl2 further decreased the Isc. B: cumulative data showing the average change in percentage of Isc after addition different K+ channel inhibitors, in the presence of ATP. The y-axis values are reported as percent of maximal control Isc. *Significantly inhibited the ATP-induced increase in Isc (P < 0.05). Each bar represents 4–12 trials.
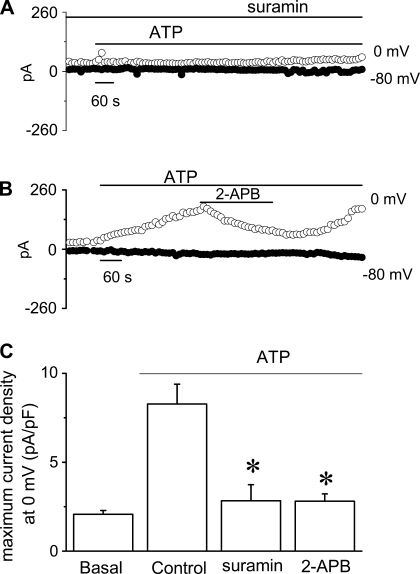
ATP responses are transduced through a P2Y-IP3 pathway. We have recently demonstrated that ATP stimulates membrane Cl− currents and transepithelial secretion via a P2Y-IP3 receptor pathway in biliary epithelium (9). P2Y receptors are G protein-coupled receptors that activate phospholipase C (PLC), hydrolyze the membrane phospholipid phosphatidylinositol 4,5-bisphosphate, forming diacylglycerol and IP3. In biliary epithelium, interaction of IP3 with IP3 receptors results in Ca2+ release from intracellular stores (17), activation of membrane Cl− currents, and transepithelial secretion (9). To determine whether similar pathways are responsible for Ca2+-activated K+ current activation, the effect of P2Y inhibition by suramin (100 μM) and IP3 receptor inhibition by 2-APB (100 μM) on ATP-stimulated K+ currents were assessed. In control cells, ATP (50 μM) activated K+ currents (8.3 ± 1.1 pA/pF, n = 10); however, preincubation with suramin (100 μM) significantly blocked current activation (2.8 ± 0.9 pA/pF, n = 4, P < 0.005), (Fig. 8, A and C). In separate studies, 2-APB also inhibited ATP-stimulated K+ currents (1.2 ± 0.4 pA/pF, n = 4, P < 0.05). Continued release from intracellular stores is necessary for continued ATP-stimulated K+ currents since addition of 2-APB during maximum current activation also decreased current magnitude significantly (2.8 ± 0.4 pA/pF, n = 9, P < 0.005, Fig. 8, B and C). Thus ATP-stimulated IK channels are regulated through a P2Y-IP3 receptor pathway, a pathway that parallels that of ATP-stimulated Cl− channel regulation (9).
Fig. 8.
Effects of pharmacological inhibition of P2Y- or inositol 1,4,5-triphosphate (IP3) receptors on ATP-stimulated K+ currents. Whole cell patch clamp studies were performed according to the protocol described in Fig. 1. A and B: representative recordings of ATP-stimulated (50 μM) currents in the presence of suramin (100 μM) (A) or 2-APB (100 μM) (B). C: cumulative data demonstrating effects of P2Y or IP3 inhibition on ATP-stimulated current density. *Suramin or 2-APB significantly (P < 0.05) inhibited ATP-stimulated currents. Values represent maximum current density (pA/pF) measured at 0 mV, respectively (n = 4 −10 each).
DISCUSSION
The principal findings of these studies, in human Mz-Cha-1 biliary epithelial cells and polarized rat cholangiocyte monolayers, are as follows: 1) ATP, a potent Ca2+-mobilizing agent, activates K+ currents in single cells and increases transepithelial secretion in polarized monolayers; 2) ATP-stimulated K+ currents have a unitary conductance of 46 pS and are dependent on intracellular Ca2+ and sensitive to the IK blockers clotrimazole and TRAM-34, but only partially inhibited by the SK inhibitor apamin; 3) IK-1 mRNA and protein were present in all biliary cells used in our study, and the protein was found predominantly in the basolateral portion of the plasma membrane; 4) IK channels are regulated through a P2Y-IP3 receptor pathway; and 5) 1-EBIO, an enhancer/activator of IK channels, stimulates K+ currents in single cells with identical properties to the ATP-stimulated currents including sensitivity to clotrimazole. Thus the biophysical, molecular, and pharmacological data are consistent with the functional presence of IK-1 in biliary epithelium and furthermore demonstrate that these channels represent an essential component of the secretory apparatus mediating the response to extracellular ATP. Accordingly, these studies represent the first functional characterization of IK-1 channels in biliary epithelium.
Calcium-activated K+ channels are classified into three groups on the basis of their unitary conductance: BK or large conductance, SK or small conductance, and IK or intermediate conductance, with conductances of ∼250, ∼5–20, and ∼40–50 pS, respectively (38). We have evaluated the potential contribution of these K+ channels to biliary secretion in several biliary models utilizing molecular, biophysical, and pharmacological approaches, and several important findings should be highlighted. First, the biophysical properties of the calcium-activated K+ channel described in the present studies are not consistent with those of BK, which express a much larger conductance (∼250 pS), has a different range of sensitivity to calcium, and exhibits different pharmacological sensitivity (35, 38). Additionally, we have found neither specific BK mRNA nor protein expression in any of the biliary epithelial cells tested (data not shown). Second, although SK2 channels have been identified in liver and biliary epithelium (27), in single biliary cells SK2 exhibits a unitary conductance of ∼17 pS and exquisite apamin sensitivity (Kd1/2 ∼[60 pM]) (41), again different from the properties of the K+ channel described here.
IK channels are 47-kDa proteins with a unitary conductance of 20–50 pS and a Ca2+ sensitivity of half maximal activation of ∼100 nM (21, 26). The cloned IK-1 channel (SK4, KCNN4) is only 40% homologous with SK channels (SK1,2,3) (21). Only one isoform (IK-1) exists, and the primary amino acid sequence predicts a serpentine architecture with six transmembrane-spanning domains, a reentrant loop between the fifth and sixth transmembrane domains forming the pore, and a long cytoplasmic COOH terminus that binds calmodulin and hence confers Ca2+ sensitivity (26). Unlike SK family members, IK-1 channels do not contain the two amino acids in the pore region that confer apamin sensitivity and hence are not sensitive to this bee venom toxin (4, 20). In contrast, IK-1 channels are inhibited by clotrimazole, TRAM-34, and charybdotoxin (4, 21, 26), all of which have negligible effects on SK channel family members (27). Additionally, the benzimidazolone 1-EBIO has been shown to be a strong enhancer of Ca2+-activated K+ channels and increases IK activity at significantly lower [Ca2+]i than SK channels, probably by interacting with the calmodulin binding domain at the COOH terminus (6, 15). IK-1 therefore has structural features that confer specific pharmacological parameters that can be harnessed to probe its relative functional properties as well as distinguish it from other Ca2+-activated K+ channels (19).
We have previously shown that the apamin-sensitive K+ channel, SK2, is present in biliary cells and contributes to secretion in response to increases in [Ca2+]i (11). However, in polarized NRC monolayers ∼40% of the calcium-activated secretory response is insensitive to apamin (11), suggesting the presence of other calcium-activated K+ channels. On the basis of the present studies, we propose that IK-1 channels account for this apamin-insensitive component of calcium-activated K+ secretion in biliary epithelium, because of the following: 1) the residual apamin-insensitive ATP-simulated whole cell currents in single cells, and the Isc in polarized preparations, were blocked by either clotrimazole or TRAM-34, specific IK inhibitors; 2) 1-EBIO activated K+ currents with identical biophysical and pharmacological properties to ATP-stimulated K+ currents, including inhibition by clotrimazole; and 3) single-channel characterization of the ATP-stimulated K+ currents revealed a unitary conductance of 46 pS, consistent with IK channels. These results, together with the molecular data demonstrating the presence and localization of IK-1 in biliary epithelium, provide collective evidence that IK-1 is present and functional in biliary epithelial cells and contributes to ATP-stimulated secretion. Additionally, IK-1 activation requires intracellular Ca2+ and intact PLC-IP3 signaling pathways, thus paralleling the regulation of Ca2+-activated Cl− channels by extracellular ATP (9). Therefore we propose a model in which IK-1 channels complement the function of SK2 channels and work in parallel with membrane Ca2+-activated Cl− channels to mediate secretion in response to extracellular nucleotides and/or other calcium mobilizing agonists (Fig. 9).
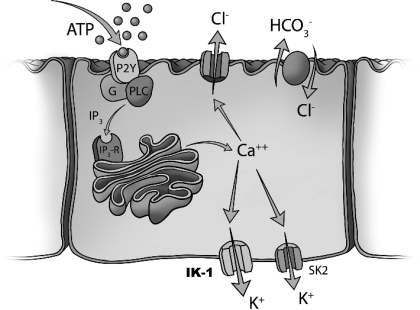
Fig. 9.
Proposed model of ATP-stimulated secretion in biliary epithelium. Extracellular ATP in bile binds purinergic (P2) receptors on the apical cholangiocyte membrane. P2Y receptors are G protein-linked receptors and generate IP3 through PLC activation. IP3 increases intracellular Ca2+ by release from intracellular stores. Increases in intracellular Ca2+ activate apical membrane Cl− channels that represent the driving force for biliary secretion. The increase in luminal Cl− drives Cl−/HCO3− exchange. Membrane Ca2+-activated K+ channels (IK-1 and SK2) function in a complementary manner and are responsible for continued secretion by hyperpolarizing the cell membrane. The molecular identity of the Ca2+-activated Cl− channel is unknown. IP3-R, IP3 receptor; PLC, phospholipase C; SK2, small-conductance Ca2+-activated K+ channel 2; IK-1, intermediate-conductance Ca2+-activated K+ channel 1.
Given the importance of calcium-activated secretory pathways to biliary secretion, identification of several Ca2+-activated K+ channels each with different regulatory and biophysical properties, is not surprising. In many tissues, SK and IK channels appear to work in a complementary manner to coordinate cellular response in response to increases in [Ca2+]i (16). Although IK-1 and SK2 channels exhibit similar EC50 values for Ca2+ activation, IK-1 has a significantly lower Hill coefficient (18) and therefore may be active at much lower [Ca2+]i. This suggests that differences exist between the gating mechanisms of IK-1 and SK2 channels despite their overall similarity in Ca2+ binding. Additionally, the finding that IK-1 and SK2 may localize to different subcellular compartments, as well as the identification of microdomains with varying Ca2+ concentrations within the cell (10, 17), suggests the presence of different subcellular functional “zones.” These considerations may allow cellular coupling of [Ca2+]i to membrane hyperpolarization over a broad range of Ca2+ concentrations to “fine tune” coordinated physiological responses. As an additional level of complexity, IK-1 channel expression may change during different stages of development (5) and, hence, alter the relative amounts of functional IK-1 vs. SK2 channels present in the membrane. Thus the presence of different Ca2+-activated K+ channels with different gating properties, regulation, and developmental expression may represent not simply redundancy but rather flexibility and adaptability to coordinate cellular responses to various physiological or pathological stimuli, although clearly much more work is required.
Although the present studies demonstrate a role of IK-1 in biliary secretion, the role of IK-1 in other hepatobiliary functions such as cell volume regulation, mechanotransduction, and metabolism is largely unknown. Interestingly, IK-1 channels play a role in the adaptive response to cell swelling in rat hepatoma cells (1), a process requiring ATP release and autocrine stimulation of P2 receptors (40). In other cell models, Ca2+-activated K+ channels have been shown to play an important role in mechanotransduction, translating mechanical stimuli to intracellular biochemical signals. For instance, in renal epithelium, bending of the primary cilium in response to shear activates IK-1 channels (29). Fluid flow and/or shear stress has recently been shown to modulate cellular ATP release in biliary epithelial cells and increases Cl− secretion through P2 stimulation and increases in [Ca2+]i (42). Thus the mechanical effect of fluid flow on the cholangiocyte apical membrane may represent an important modulator of biliary secretion and bile formation. Lastly, targeting IK-1 channels may have potential therapeutic benefit in prostate (24) and pancreatic (22) cancers and therefore, given the expression of IK-1 in Mz-Cha-1 cells, suggests that targeting these channels may be a potential therapeutic strategy for cholangiocarcinoma as well. The role of IK-1 in mechanosensitive liver functions such as flow-stimulated secretory responses and cell volume regulation, as well as in biliary epithelial cell growth and proliferation, may be exciting areas for future investigation.
In summary, the present studies represent the first functional characterization of IK-1 channels in biliary epithelium. Together with membrane Cl− channels, IK-1 contributes importantly to ATP-stimulated biliary secretion. In the future, targeting IK-1 channels may represent a potential therapeutic strategy to modulate biliary secretion and bile formation for the treatment of cholestatic liver disease.
GRANTS
This study was supported by the Cystic Fibrosis Foundation (FERANC08G0) and the National Institute of Diabetes, Digestive and Kidney Diseases (NIDDK) of the National Institute of Health grants RO1 DK 50574 (D. C. Rockey) and RO1 DK078587 (A. P. Feranchak).
ACKNOWLEDGMENTS
Special thanks to Richard Howdy for help with the artwork in Fig. 9.
REFERENCES
- 1.Barfod ET, Moore AL, Roe MW, Lidofsky SD. Ca2+-activated IK1 channels associate with lipid rafts upon cell swelling and mediate volume recovery. J Biol Chem 282: 8984–8993, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Basavappa S, Middleton JP, Mangel A, McGill J, Cohn JA, Fitz JG. Cl− and K+ transport in human biliary cell lines. Gastroenterology 104: 1796–1805, 1993 [DOI] [PubMed] [Google Scholar]
- 3.Cohn JA, Strong TA, Picciotto MA, Nairn AC, Collins FS, Francis S, Fitz JG. Localization of CFTR in human bile duct epithelial cells. Gastroenterology 105: 1857–1864, 1993 [DOI] [PubMed] [Google Scholar]
- 4.de-Allie FA, Bolsover SR, Nowicky AV, Strong PN. Characterization of Ca2+-activated 86Rb+ fluxes in rat C6 glioma cells: a system for identifying novel IKCa-channel toxins. Br J Pharmacol 117: 479–487, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deng XL, Lau CP, Lai K, Cheung KF, Lau GK, Li GR. Cell cycle-dependent expression of potassium channels and cell proliferation in rat mesenchymal stem cells from bone marrow. Cell Prolif 40: 656–670, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Devor DC, Singh AK, Frizzell RA, Bridges RJ. Modulation of Cl− secretion by benzimidazolones. I Direct activation of a Ca2+-dependent K+ channel. Am J Physiol Lung Cell Mol Physiol 271: L775–L784, 1996 [DOI] [PubMed] [Google Scholar]
- 7.Doctor RB, Matzakos T, McWilliams R, Johnson S, Feranchak AP, Fitz JG. Purinergic regulation of cholangiocyte secretion: identification of a novel role for P2X receptors. Am J Physiol Gastrointest Liver Physiol 288: G779–G786, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Dranoff JA, Masyuk AI, Kruglov EA, LaRusso NF, Nathanson MH. Polarized expression and function of P2Y ATP receptors in rat bile duct epithelia. Am J Physiol Gastrointest Liver Physiol 281: G1059–G1067, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Dutta AK, Woo K, Doctor RB, Fitz JG, Feranchak AP. Extracellular nucleotides stimulate Cl− currents in biliary epithelia through receptor-mediated IP3 and Ca2+ release. Am J Physiol Gastrointest Liver Physiol 295: G1004–G1015, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fakler B, Adelman JP. Control of K(Ca) channels by calcium nano/microdomains. Neuron 59: 873–881, 2008 [DOI] [PubMed] [Google Scholar]
- 11.Feranchak AP, Doctor RB, Troetsch M, Brookman K, Johnson SM, Fitz JG. Calcium-dependent regulation of secretion in biliary epithelial cells: the role of apamin-sensitive SK channels. Gastroenterology 127: 903–913, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Feranchak AP, Fitz JG. Adenosine triphosphate release and purinergic regulation of cholangiocyte transport. Semin Liver Dis 22: 251–262, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Feranchak AP, Roman RM, Doctor RB, Salter KD, Toker A, Fitz JG. The lipid products of phosphoinositide 3-kinase contribute to regulation of cholangiocyte ATP and chloride transport. J Biol Chem 274: 30979–30986, 1999 [DOI] [PubMed] [Google Scholar]
- 14.Fitz JG. Cellular mechanisms of bile secretion. In: Hepatology, edited by Zakim D, Boyer TD. Philadelphia, PA: Saunders, 1996, p. 362–376 [Google Scholar]
- 15.Frei E, Spindler I, Grissmer S, Jager H. Interactions of N-terminal and C-terminal parts of the small conductance Ca2+ activated K+ channel, hSK3. Cell Physiol Biochem 18: 165–176, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Hilgers RH, Webb RC. Reduced expression of SKCa and IKCa channel proteins in rat small mesenteric arteries during angiotensin II-induced hypertension. Am J Physiol Heart Circ Physiol 292: H2275–H2284, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Hirata K, Dufour JF, Shibao K, Knickelbein R, O'Neill AF, Bode HP, Cassio D, St-Pierre MV, LaRusso NF, Leite MF, Nathanson MH. Regulation of Ca2+ signaling in rat bile duct epithelia by inositol 1,4,5-trisphosphate receptor isoforms. Hepatology 36: 284–296, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hirschberg B, Maylie J, Adelman JP, Marrion NV. Gating of recombinant small-conductance Ca-activated K+ channels by calcium. J Gen Physiol 111: 565–581, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hougaard C, Eriksen BL, Jorgensen S, Johansen TH, Dyhring T, Madsen LS, Strobaek D, Christophersen P. Selective positive modulation of the SK3 and SK2 subtypes of small conductance Ca2+-activated K+ channels. Br J Pharmacol 151: 655–665, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ishii TM, Maylie J, Adelman JP. Determinants of apamin and d-tubocurarine block in SK potassium channels. J Biol Chem 272: 23195–23200, 1997 [DOI] [PubMed] [Google Scholar]
- 21.Ishii TM, Silvia C, Hirschberg B, Bond CT, Adelman JP, Maylie J. A human intermediate conductance calcium-activated potassium channel. Proc Natl Acad Sci USA 94: 11651–11656, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jager H, Dreker T, Buck A, Giehl K, Gress T, Grissmer S. Blockage of intermediate-conductance Ca2+-activated K+ channels inhibit human pancreatic cancer cell growth in vitro. Mol Pharmacol 65: 630–638, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Knuth A, Gabbert H, Dippold W, Klein O, Sachsse W, Bitter-Suermann D, Prellwitz W, Meyer zum Buschenfelde KH. Biliary adenocarcinoma. Characterisation of three new human tumor cell lines. J Hepatol 1: 579–596, 1985 [DOI] [PubMed] [Google Scholar]
- 24.Lallet-Daher H, Roudbaraki M, Bavencoffe A, Mariot P, Gackiere F, Bidaux G, Urbain R, Gosset P, Delcourt P, Fleurisse L, Slomianny C, Dewailly E, Mauroy B, Bonnal JL, Skryma R, Prevarskaya N. Intermediate-conductance Ca2+-activated K+ channels (IKCa1) regulate human prostate cancer cell proliferation through a close control of calcium entry. Oncogene 28: 1792–1806, 2009 [DOI] [PubMed] [Google Scholar]
- 25.Lee EL, Hasegawa Y, Shimizu T, Okada Y. IK1 channel activity contributes to cisplatin sensitivity of human epidermoid cancer cells. Am J Physiol Cell Physiol 294: C1398–C1406, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Logsdon NJ, Kang J, Togo JA, Christian EP, Aiyar J. A novel gene, hKCa4, encodes the calcium-activated potassium channel in human T lymphocytes. J Biol Chem 272: 32723–32726, 1997 [DOI] [PubMed] [Google Scholar]
- 27.Maylie J, Bond CT, Herson PS, Lee WS, Adelman JP. Small conductance Ca2+-activated K+ channels and calmodulin. J Physiol 554: 255–261, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McGill J, Basavappa S, Shimokura GH, Middleton JP, Fitz JG. Adenosine triphosphate activates ion permeabilities in biliary epithelial cells. Gastroenterology 107: 236–243, 1994 [DOI] [PubMed] [Google Scholar]
- 29.Praetorius HA, Frokiaer J, Nielsen S, Spring KR. Bending the primary cilium opens Ca2+-sensitive intermediate-conductance K+ channels in MDCK cells. J Membr Biol 191: 193–200, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Roman R, Feranchak AP, Troetsch M, Dunkelberg JC, Kilic G, Schlenker T, Schaack J, Fitz JG. Molecular characterization of volume-sensitive SKCa channels in human liver cell lines. Am J Physiol Gastrointest Liver Physiol 282: G116–G122, 2002 [DOI] [PubMed] [Google Scholar]
- 31.Roman RM, Wang Y, Fitz JG. Regulation of cell volume in a human biliary cell line: calcium-dependent activation of K+ and Cl− currents. Am J Physiol Gastrointest Liver Physiol 271: G239–G248, 1996 [DOI] [PubMed] [Google Scholar]
- 32.Salter KD, Fitz JG, Roman RM. Domain-specific purinergic signaling in polarized rat cholangiocytes. Am J Physiol Gastrointest Liver Physiol 278: G492–G500, 2000 [DOI] [PubMed] [Google Scholar]
- 33.Schlenker T, Romac JMJ, Sharara A, Roman RM, Kim S, LaRusso N, Liddle R, Fitz JG. Regulation of biliary secretion through apical purinergic receptors in cultured rat cholangiocytes. Am J Physiol Gastrointest Liver Physiol 273: G1108–G1117, 1997 [DOI] [PubMed] [Google Scholar]
- 34.Schnabl B, Choi YH, Olsen JC, Hagedorn CH, Brenner DA. Immortal activated human hepatic stellate cells generated by ectopic telomerase expression. Lab Invest 82: 323–333, 2002 [DOI] [PubMed] [Google Scholar]
- 35.Schreiber M, Salkoff L. A novel calcium-sensing domain in the BK channel. Biophys J 73: 1355–1363, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Singh SK, Mennone A, Gigliozzi A, Fraioli F, Boyer JL. Cl−-dependent secretory mechanisms in isolated rat bile duct epithelial units. Am J Physiol Gastrointest Liver Physiol 281: G438–G446, 2001 [DOI] [PubMed] [Google Scholar]
- 37.Thompson-Vest N, Shimizu Y, Hunne B, Furness JB. The distribution of intermediate-conductance, calcium-activated, potassium (IK) channels in epithelial cells. J Anat 208: 219–229, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vergara C, Latorre R, Marrion NV, Adelman JP. Calcium-activated potassium channels. Curr Opin Neurobiol 8: 321–329, 1998 [DOI] [PubMed] [Google Scholar]
- 39.Vroman B, LaRusso N. Development and characterization of polarized primary cultures of rat intrahepatic bile duct epithelial cells. Lab Invest 74: 303–313, 1996 [PubMed] [Google Scholar]
- 40.Wang Y, Roman RM, Lidofsky SD, Fitz JG. Autocrine signaling through ATP release represents a novel mechanism for cell volume regulation. Proc Natl Acad Sci USA 93: 12020–12025, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang Y, Roman RM, Schlenker T, Hannun YA, Raymond JR, Fitz JG. Cytosolic calcium and protein kinase C(alpha) couple cellular metabolism to membrane K+ permeability in a human biliary cell line. J Clin Invest 99: 2890–2897, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Woo K, Dutta AK, Patel V, Kresge C, Feranchak AP. Fluid flow induces mechanosensitive ATP release, calcium signalling and Cl− transport in biliary epithelial cells through a PKCzeta-dependent pathway. J Physiol 586: 2779–2798, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]