Abstract
Liver fibrosis results from chronic liver injury due to hepatitis B and C, excessive alcohol ingestion, and metal ion overload. Fibrosis culminates in cirrhosis and results in liver failure. Therefore, a potent antifibrotic therapy is in urgent need to reverse scarring and eliminate progression to cirrhosis. Although activated hepatic stellate cells (HSCs) remains the principle cell type responsible for liver fibrosis, perivascular fibroblasts of portal and central veins as well as periductular fibroblasts are other sources of fibrogenic cells. This review will critically discuss various treatment strategies for liver fibrosis, including prevention of liver injury, reduction of inflammation, inhibition of HSC activation, degradation of scar matrix, and inhibition of aberrant collagen synthesis. Oligonucleotides (ODNs) are short, single-stranded nucleic acids, which disrupt expression of target protein by binding to complementary mRNA or forming triplex with genomic DNA. Triplex forming oligonucleotides (TFOs) provide an attractive strategy for treating liver fibrosis. A series of TFOs have been developed for inhibiting the transcription of α1(I) collagen gene, which opens a new area for antifibrotic drugs. There will be in depth discussion on the use of TFOs and how different bioconjugation strategies can be utilized for their site-specific delivery to HSCs or hepatocytes for enhanced antifibrotic activities. Various insights developed in individual strategy and the need for multipronged approaches will also be discussed.
1. INTRODUCTION
Fibrosis is characterized by an excessive production of extracellular matrix (ECM) components, in the interstitial space of an organ which if not controlled can result in organ dysfunctional (1). Liver fibrosis occurs in response to a variety of insults, including viral hepatitis (especially hepatitis B and C), excessive alcohol ingestion, drugs, metabolic diseases due to overload of iron or copper, autoimmune attack of hepatocytes or bile duct epithelium, or congenital abnormalities. Cirrhosis is an advanced stage of liver fibrosis, characterized by the formation of regenerative nodules of liver parenchyma separated by fibrotic septa. Cirrhosis is one of the leading causes of death in the United States, accounting for more than 25,000 deaths in 2000 (2). Major clinical complications of cirrhosis include ascites, renal failure, hepatic encephalopathy, and variceal bleeding. (3). Furthermore, cirrhosis is largely associated with primary liver cancer, with a further increase in the relative mortality rate (4, 5). Cirrhosis is divided into compensated and decompensated cirrhosis. Patients with compensated cirrhosis may remain free of major complications for several years, while decompensated cirrhosis is associated with short survival (6).
Regardless of extensive efforts, liver transplantation is currently the only curative approach for treating cirrhosis (7). However, the limited number of donor organs available and the condition of the potential recipient limit the applicability of this technique. Furthermore, in patients with hepatitis C virus (HCV)-induced cirrhosis, viral infection recurrence after transplantation leads to aggressive chronic hepatitis and progression to cirrhosis. Traditionally, liver fibrosis is considered reversible. However, reversibility of advanced liver fibrosis/cirrhosis may also be possible. In current view, death of parenchymal cells (hepatocytes) followed by inflammatory response in the injured liver initiates the liver repair process; besides the recruited leukocytes, Kupffer cells (KCs) as well as sinusoidal endothelial cells (SEC) are involved in the inflammation; and activated hepatic stellate cells (HSCs) are the key fibrogenic cells responsible for the excessive production of fibrillar collagens (Type I and III collagens) and other sources of fibrogenic cells are also possible (Figure 1 & 2). The interaction of these cells as well as many others results in the progression of liver fibrosis after chronic liver injury. Therefore, there is a considerable interest in developing antifibrotic strategies for treating liver fibrosis. Better understanding of pathophysiology of liver fibrosis is essential for developing antifibrotic treatment strategies. Current efforts in developing antifibrotic drugs are heavily relying on these basic understandings. Activated HSCs become the main focus. Different treatment strategies in development are discussed.
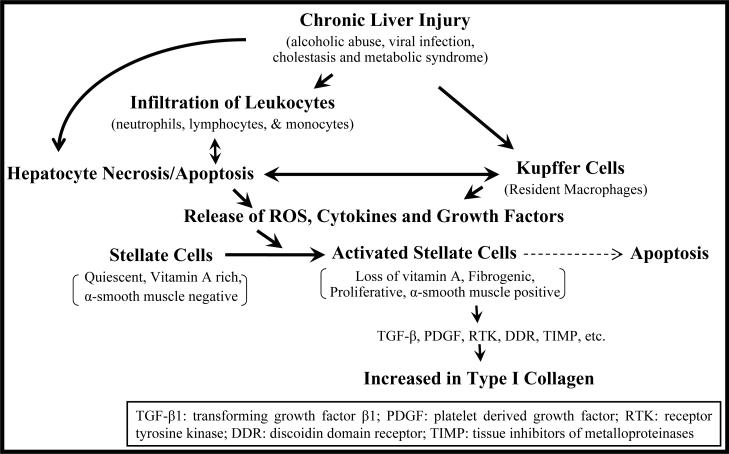
Figure 1. Events leading to liver fibrosis.
During liver injury, infiltrating leukocytes (neutrophils, lymphocytes and monocytes) along with resident macrophages (Kupffer cells) release reactive oxygen species (ROS), growth factors and inflammatory cytokines, leading to activation of hepatic stellate cells (HSCs) into actively proliferating, α-smooth muscle actin-containing myofibroblast-like cells. The activated HSCs are the source of cytokines, chemotatics, and also secrete large amounts of type I collagen and other extracellular matrix (ECM) components. Apoptosis of activated HSCs is implicated in the spontaneous resolution of liver fibrosis.
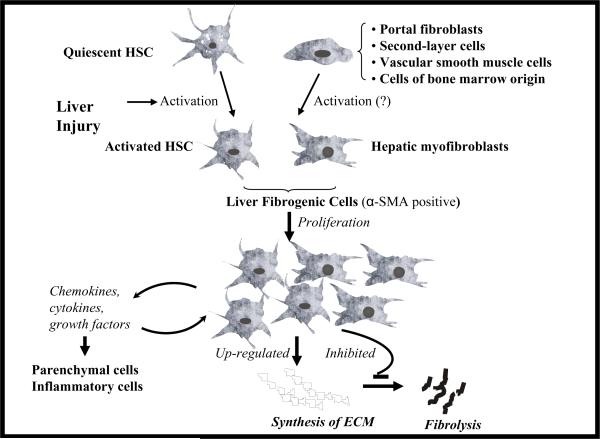
Figure 2. Different liver fibrogenic cells.
Due to liver injury, hepatic stellate cells (HSCs) undergo transformational change into myofibroblast-like activated HSCs, which are depleted of vitamin A, but rich in α-smooth muscle actin (α-SMA). There is considerable evidence supporting that HSCs are a major source of fibrogenic cells in the injured liver. However, contributions from other cell types including portal fibroblasts, second-layer cells located around centrolobular veins (CLVs), vascular smooth muscle cells and cells of bone marrow origin are also possible. These liver fibrogenic cells proliferate at the sites of liver injury, produce a variety of proinflammatory cytokines, chemokines and growth factors, synthesize extracellular matrix (ECM) proteins and inhibit their degradation, leading to fibrosis.
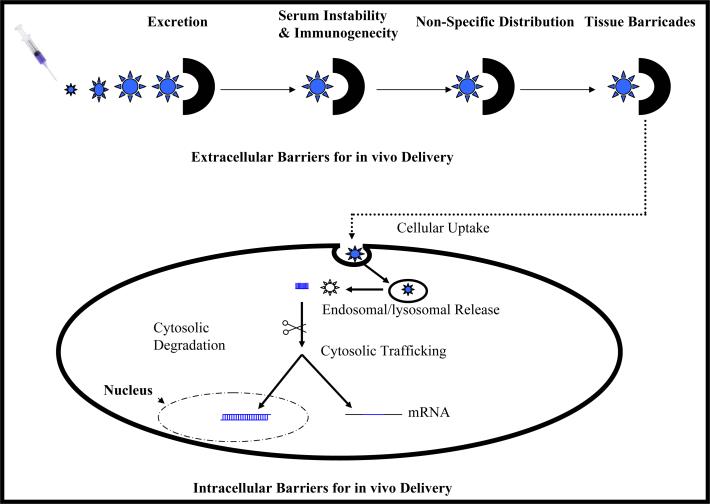
Emergence of various gene silencing technologies has boosted the possible use of nucleic acids (plasmid DNA, antisense oligonucleotides (ODNs), triplex forming oligonucleotides (TFOs), small interference RNA (siRNA)) for treating liver fibrosis. TFOs may confer some advantages over others due to their act on DNA rather than mRNA. Introduction of exogenous genes or specifically gene silencing provide attractive ways to regulate the function of key modulators in liver fibrosis. Therefore, efforts in developing TFOs as gene silencing therapeutics will be summarized with implications for treating liver fibrosis. However, there are many barriers for the application of nucleic acids in vivo, such as instability against nucleases, poor cellular uptake, and non-specific biodistribution. Therefore, the lack of efficient and targeted delivery after systemic administration of nucleic acids has impaired their use as therapeutics. Even though studies have shown that liver is the disposition site for phosphorothioate modified ODNs (PS-ODNs) after their systemic administration, there have still not been many studies for chemically modified as well as G-rich ODNs, which may have different biodistribution profiles. In addition, due to the potent gene silencing ability of ODNs and TFOs, a site-specific delivery is required to avoid toxic side effects in the body and maximize their efficiency in vivo.
Among different delivery strategies for ODN-based therapeutics, bioconjugation on ODNs is very attractive compared to other delivery strategies, such as cationic liposomes and cationic polymers, especially for the conditions of liver fibrosis. The pharmacological activity and pharmacokinetics of ODNs can be significantly improved by simple conjugation chemistry with lipids, sugars polyethylene glycol (PEG) and peptides among others. Moreover, conjugation with polymers due to their versatility and biocompatibility presents a beautiful scene in ODN delivery, in which many different components aimed at overcoming barriers for in vivo delivery of ODNs can be assembled. Carriers with high efficiency and safety can be constructed with a multicomponent polymer for ODNs. Several promising strategies for delivery of nucleic acids to the liver will be discussed.
Naturally, the liver is involved in the disposition of a variety of macromolecules including ODNs by phagocytotic processes as well as receptor-mediated endocytosis and followed by their degradation in KCs, HSCs, SECs and hepatocytes. For targeting different liver cells, receptor-mediated endocytosis in various cell types has been utilized extensively (8). On hepatocytes, asialoglycoprotein receptor (ASGPR) recognizes galactose- and N-acetyl-galactosamine (GalNAc)-terminated glycoproteins (<10 nm); on KCs, there is a mannose/GalNAc/fucose receptor. In addition, a galactose-particle receptor also exists on KCs, recognizing particles exposing galactose larger than 10nm. Moreover, KCs exhibit receptors (scavenger receptors) for positively charged as well as negatively charged particles. Furthermore, these scavenger receptors are also present on SECs as well as HSCs. Therefore, modifications of cargoes with these different ligands present attractive specific targeting strategies for drug delivery to different liver cell types for varieties of purposes. For example, glycoproteins can be recognized by sugar-specific receptors or scavenger receptors in various cell types in the body and have been extensively used for drug delivery to the liver. Onto these glycoproteins, ODNs can be conjugated for their delivery to the liver cells. Specifically, a summary of targeted delivery of drugs to HSCs for treating liver fibrosis will be presented. We proposed a targeted delivery system for TFOs specific for α1(I) collagen to HSCs for treating liver fibrosis. Finally, we conclude with an outlook for the future and the strategies holding promise for treating liver fibrosis.
2. STRUCTURE AND FUNCTION OF THE LIVER
In the liver, there is rapid exchange of blood constituents with the tissue, which is related to both the impressive blood flow (25% of the cardiac output) as well as the fenestrated endothelium that allows direct contact of blood components with the very large surface of the villous plasma membranes of hepatocytes. It performs a large number of tasks that impact our body systems, such as detoxifying, manufacturing proteins, synthesizing, storing, and processing of fats, metabolizing and storing of carbohydrates, forming and secreting bile and eliminating the potentially harmful biochemical products from the body.
2.1. Types and Functions of Liver Cells
Liver contains several cell types, including hepatocytes, SECs, KCs, and HSCs, which contribute to 78%, 2.8%, 2.1%, and 1.4% of total liver volume and 92.5%, 3.3%, 2.5%, and 1.7% of the total liver cell volume, respectively (8). However, other cell types including pit cells, monocytes, T- and B-lymphocytes, bile duct epithelial cells (cholangiocytes), vascular smooth muscle cells, second-layer cells and portal fibroblasts are also involved in the different liver functions and may play important roles in liver fibrosis.
2.1.1. Hepatocytes
Hepatocytes are the major liver epithelial cells responsible for most of liver functions. They are metabolically active cells and contain a vast array of organelles. Their strategic position between two different environments, the blood and the bile, makes hepatocytes unique compared to other parenchymal cells. The hepatocyte plasma membrane is differentiated into sinusoidal, contiguous, and canalicular domains, whose relative surface areas are 77%, 15%, and 13%, respectively. The sinusoidal domain is the site of the sodium pump and organic ion and drug transporters. Receptors for glycoproteins, immunoglobulin A, asialoglycoprotein, various peptides and hormones, and growth factors are also located in this domain.
2.1.2. Sinusoidal Endothelial Cells
Liver SECs are regarded as: (a) “selective sieves” for substances passing from the blood to hepatocytes and HSCs, and vice versa, (b) a “scavenger system” which clears the blood from many different macromolecular waste products that originate from turnover processes in different tissues. These cells express a variety of adhesion molecules and have a capacity to secrete cytokines. In addition, SECs are also responsible for producing type IV collagen in normal liver (9).
The main structural characteristic of SECs is the fenestrae of 50–150 nm in diameter. Fenestrae filter fluids, solutes and particles that are exchanged between the sinusoidal lumen and the space of Disse, allowing only particles smaller than the fenestrae to reach the parenchymal cells or to leave the space of Disse. Molecules with a molecular weight exceeding 250 kD cannot pass through the pores (10), and therefore do not interact with hepatocytes. Furthermore, the fenestrae controls the interchange between blood and perisinusoidal space, and its diameter is influenced by endobiotics (hormones and neurotransmitters) and xenobiotics (ethanol and drugs) (10).
2.1.3. Kupffer Cells
KCs are part of the reticuloendothelial system (RES) and represent 80–90% of all the resident liver macrophages (8). They attach to the sinusoidal wall, but possibly migrating along the lumen (11). KCs are the first cells in the liver to be exposed to materials absorbed from the gastrointestinal tract. Their ability to eliminate and detoxify microorganisms, endotoxins, degenerated cells, immune complexes, and toxic agents (e.g., ethanol) is an important physiological function. Upon activation, they secrete a number of products with potent biologic effects, including oxygen-derived free radicals, proteases and cytokines with influence on parenchymal and other sinusoidal lining cells (8).
2.1.4. Hepatic Stellate Cells
HSCs are also called para- or perisinusoidal cells, Ito cells, fat storing cells, vitamin A storing cells, lipocytes, and arachnocytes. HSCs occupy a perisinusoidal location, with cytoplasmic extensions wrapped around the sinusoidal endothelial lining, comparable to pericytes in other locations and they have spindle-shaped cell bodies with oval or elongated nuclei. They have moderately developed rough endoplasmic reticulum, juxtanuclear small Golgi complex, and prominent dendritic cytoplasmic processes. These subendothelial processes extend beneath endothelial cells and wrap around sinusoids. A single HSC usually surrounds more than two nearby sinusoids. On the luminal side, multiple processes extend across the space of Disse to make contact with hepatocytes and this intimate contact between HSCs and neighboring cell types may facilitate intercellular transport of soluble mediators and cytokines. In addition, HSCs have direct connections with nerve ending, which may be important for neurally mediated vasoregulation.
HSCs are the principle ECM producing cells in the liver (12). They play an important role in the maintenance of the ECM by synthesis and secretion of its normal components and their degradation by proteases. During the fibrotic process they can be transformed to myofibroblasts to increase the portal resistance in periportal areas and slow down portal blood flow (10). Besides, HSCs also have other major functions in a normal liver: (a) control of microvascular tone; (b) storage of retinoids; and (c) a role in the control of regeneration in the normal liver and in response to necrosis. In addition, HSCs represent an important source of cytokines in the liver, including acidic fibroblast growth factor (aFGF), connective tissue growth factor (CTGF), cytokine-induced neutrophil chemoattractant (CINC), endothelin-1 (ET-1), epidermal growth factor (EGF), insulin growth factors I and II (IGF-I and II) and their receptors, interleukin-6 (IL-6), interleukin-10 (IL-10), intercellular adhesion molecule 1 (ICAM-1), neural cell adhesion molecule (NCAM), platelet-derived growth factor (PDGF), platelet-activating factor (PAF), transforming growth factor α (TGF-α), transforming growth factor β (TGF-β), vascular cell adhesion molecule (VCAM) and others (13).
2.2. Pathogenesis of Liver Fibrosis
The abnormal deposition of ECM is the common characteristic of liver fibrosis regardless of etiology of the liver injury. Liver fibrosis results from the interplay among the injury, inflammation and fibrogenesis of the liver, which implicates sophisticated interactions among different liver cell types (14, 15). Normally, liver injury leads to damage of hepatocytes, which is followed by activation of KCs and infiltration of inflammatory cells. Reactive oxygen species (ROS), inflammatory cytokines and growth factors are released, leading to activation of HSCs, which are responsible for the producing excess amount of ECM and progression of liver fibrosis (Figure 1).
2.2.1. Injury to Parenchymal Cells
Injury to hepatocytes is the common consequence of many liver diseases including alcoholic hepatitis, nonalcoholic steatohepatitis (NASH), viral hepatitis, and cholestatic liver diseases (16, 17). However, intermediates that lead to hepatocyte injury are different. Retention of bile salts is a major cause of liver damage in cholestatic liver disease (17), while viral infections are associated with cytotoxic T lymphocytes (18). T lymphocytes have been shown to induce KCs to release TNF-α (19) or directly destroy hepatocytes (20). Excessive alcoholic ingestion increases oxidative stress by producing ROS, which directly mediate damage to hepatocytes (21). On the other hand, in alcoholic liver injury, ischemia/reperfusion and viral infections, interaction between KCs and lipopolysaccharide (LPS) is the initiating event leading to hepatotoxicity (22–24). Therefore, activation of KCs may happen before or after hepatocyte damage. Activated KCs are a major source of inflammatory mediators and demonstrate increased cytotoxicity and chemotaxis (25–28). Proinflammatory cytokines such as interferon γ (IFN-γ) followed by tumor necrosis factor-α (TNF-α) are released by both KCs and NK lymphocytes (29, 30), which leads to expression of ICAM-1 in ECs, allowing the recruitment and sinusoidal transmigration of inflammatory cells including T lymphocytes, macrophages, neutrophils, NK cells and mast cells among others (31).
Liver injury leads to death of hepatocytes either by necrosis or apoptosis (32). Necrosis is most often the consequence of metabolic injury leading to ATP depletion (33), while apoptosis may predominate in cholestatic liver disease (34) as well as NASH (35). Controversies still exist regarding which mode of death predominates in various forms of liver injury. These two events may represent alternate outcomes of cell death mediated by mitochondrial permeabilization (32). For apoptosis of hepatocytes, there are two mechanisms. The extrinsic pathway of apoptosis is signaled through cell surface death receptors, including Fas, TNF-α-receptor-1, and TNF-α-related-apoptosis-inducing-ligand (TRAIL) receptors 1 and 2 (36). Examples of this extrinsic pathway of apoptosis include antoimmune hepatitis, viral hepatitis, chronic alcohol consumption, D-galactosamine (GalN) plus LPS-induced acute liver injury, and ischemia/reperfusion-associated liver injury (37). In contrast, the intrinsic pathway of apoptosis is based on the damage or dysfunction of intracellular organelles, such as lysosomes, endoplasmic reticulum, nucleus and mitochondria (38). It is often seen in drug toxicity or hepatotoxin-induced liver injury, such as acetaminophen overdose and alcohol toxicity (37, 39).
Death of hepatocytes amplifies the inflammation by release of ROS and fibrogenic mediators, recruiting inflammatory cells or directly activating HSCs to synthesize ECM (40). Apoptosis of hepatocytes has now been considered as the nexus of liver fibrosis (41–43). When the magnitude of apoptosis overcomes the capacity to clear cellular debris, apoptotic bodies undergo spontaneous disruption and release their contents, which elicits an inflammatory response (44). Furthermore, engulfment of apoptotic bodies by KCs can induce expression of death ligands, especially Fas, thereby accelerating apoptosis of hepatocytes (45). Phagocytosis of apoptotic bodies by quiescent HSCs has been shown to stimulate their activation via increased intracellular oxidative stress (41, 43, 46). Other cells including epithelial cells and fibroblasts may also have phagocytic functions (47, 48). One of the important consequences when cells phagocytose apoptotic bodies is the generation of TFG-β (49, 50), which is the most important fibrogenic mediator in liver fibrosis and leads to up-regulation of collagen gene expression in activated HSCs.
2.2.2. Hepatic Stellate Cells, the Principle Fibrogenic Cells
2.2.2.1. Initiation of HSC activation
The consequence of liver damage is the generation of large amount of soluble mediators and increased oxidative stress, which result in activation of HSCs. In some liver diseases, some reagents, such as alcohol metabolites, ferritin, and bile acids may also directly act on HSCs leading to their activation without induction of inflammatory responses (51–53). Initiation of activation represents the rapid early changes in gene expression and phenotype responsive to liver injury (54). HSCs transdifferentiate into myofibroblast-like cells, acquiring contractile, proinflammatory, and fibrogenic properties (54, 55) under the direction of growth factors, oxidants, and additional stimuli released from injured hepatocytes and cholangiocytes, KCs, SECs or other recruited inflammatory cells (56–58). Hepatocytes and KCs promote HSC activation by producing ROS leading to oxidative stress (59, 60). Cytokines released by damaged neighboring cells activate HSCs. These include TGF-β1 (61), PDGF (62) and ET-1 (63), which stimulate transcription factors such as Sp1, c-myb, nuclear factor κB (NF-κB), c-jun/AP1, STAT-1, and SMAD proteins that regulate gene expression in activated HSCs. Injury to SECs stimulates the production of type IV collagen, fibronectin, proteoglycans, and urokinase-type plasminogen activator (uPA), which contribute to activation of HSCs through the activation of latent TGF-β1 (64–67).
2.2.2.2. Perpetuation of HSC activation
Amplification of activated phenotype of HSCs is called perpetuation, which involves several discrete changes in cell behavior (54): i) proliferation, ii) chemotaxis, iii) fibrogenesis, iv) contractility, v) matrix degradation, vi) retinoid loss and vii) chemoattractant and cytokine release.
Loss of intracellular vitamin A is a notable feature of HSC activation. However, its relationship with HSC activation remains unknown. Minor metabolites of retinoic acid (RA), 9-cis RA and 9, 13-di-cis RA can stimulate the activation of latent TGF-β1 implying a direct link to fibrogenesis (68). Activated HSCs have increased expression of cell membrane receptors including integrins and receptor tyrosine kinases (RTKs) (69). Increased cytokine effects and remodeling of ECM perpetuate the activation of HSCs (70). Activated HSCs also secrete TGF-β1 and have up-regulated level of its receptors, which perpetuates their own activation through autocrine loops (71, 72). It is suggested that TGF-β1 is not required for initiating HSC activation (73), which emphasizes the fibrogenic property of TGF-β1.
HSCs accumulate at the injured sites via migration and proliferation. PDGF is the most powerful growth factor for HSCs, but EGF, FGF, or IGF also results in increased HSC proliferation (62). The expression of these growth factors has been markedly increased in hepatic tissue after both acute and chronic liver injury (74, 75). PDGF and monocyte chemotactic protein-1 (MCP-1) are the chmoattractants for activated HSCs (76–78).
Vasoactive substances also regulate HSC growth. Vasoconstrictors including thrombin (79), arginine-vasopressin (80) and angiotensin-II (AngII) (81), exert a mitogenic effect on activated HSCs, whereas vasodilators tend to inhibit cell proliferation, which include prostaglandin E2 (82) and nitric oxide (83). The role of ET-1, a vasoconstrictor, in liver fibrosis remains disputed. Both profibrogenic and antifibrogenic action of ET-1 have been suggested in the process of liver fibrosis (63, 84–86). It induces fibrogenic gene expression in quiescent HSCs, but inhibits proliferation of activated HSCs. ET-1 stimulates the contractility of activated HSCs, which represents an important mechanism underlying increased portal resistance during liver injury (63).
2.2.2.3. Activation of HSCs and inflammation
HSCs play an active role in hepatic inflammation (87). Activated HSCs migrate in response to cytokines released by monocytes and secrete a number of proinflammatory cytokines and chemokines including colony-stimulating factor and MCP-1 that could participate in the activation of lymphocytes and the recruitment of white blood cells (neutrophil and monocyte), thus amplifying the inflammatory response (78, 88). HSCs can take up and process antigens and, under stimulation with cytokines, express the cell machinery required for antigen presentation and thereby modulate the growth of lymphocytes (89). A direct link between the inflammatory and fibrogenic properties of HSCs is that they express cluster of differentiation (CD)-40, a receptor whose ligand is present on immune effector cells (90). Therefore, a loop in which inflammatory and fibrogenic cells stimulate each other is likely to occur (91), which results in amplified fibrogenesis.
2.2.2.4. Survival of activated HSCs
Apoptosis represents a default pathway for HSCs, and thus appropriate anti-apoptotic signals are needed for their survival (92). The survival of activated HSCs is dependent on soluble growth factors including IGF-1 (93–95), cytokines including TNF-α and TGF-β (96), and components of the fibrotic ECM (97). Unlike IGF-1, these cytokines may act via the Fas/Fas-ligand (Fas-L) system (96). In contrast to IGF-1, PDGF has relatively little anti-apoptotic activity. The separate regulation of proliferation and survival in HSCs provides a further control on HSC numbers. Indeed, the expression of a single growth factor with both mitogenic and antiapoptotic activities for HSCs might lead to an uncontrolled and damaging increase in cell numbers during injury. TIMP-1 may also act as a survival factor and is known to be antiapoptotic for HSCs in an autocrine manner, which is independent of its ability to inhibit MMP activity (98).
2.2.2.5. Increased production of ECM
Progression of liver fibrosis is a remodeling process of ECM, by degradation of normal ECM and substitution with scar matrix (99, 100). Activated HSCs are the principle fibrogenic cells responsible for producing interstitial collagens type I and III, which are the two main components of ECM in liver fibrosis (12, 101–103). Matrix metalloproteinases (MMPs), which are responsible for degradation of ECM components, include MMP-1 in human and MMP-13 in rats and mice (104–106) as well as MMP-2 and MMP-14. HSCs and KCs are the major sources of MMPs (104, 107, 108). MMP inhibitors, tissue inhibitors of metalloproteinases (TIMP) 1 and 2 are also expressed by HSCs (30, 109). Activated HSCs produce MMP-2 that degrades basement-membrane ECM (especially type IV collagen) (110). Degradation of normal ECM hastens its replacement by fibril-forming collagen. In addition, degradation of type IV collagen has been shown to facilitate HSC activation (25, 100). This combination of enzymes and their inhibitors provides a mechanism for HSCs to degrade normal and fibrotic matrix in the remodeling process and deposit excess amount of ECM during progression of liver fibrosis.
2.2.3. Existence of Other Fibrogenic Cells
In most cases, activated HSCs are responsible for liver fibrosis (111) and they may take part in the repair process by migration and proliferation (112, 113). However, HSCs themselves are not homogenous cell type and different subtypes are present in the liver parenchyma (114). On the other hand, other potential fibrogenic cell types in liver fibrosis should also be taken into account (Figure 2). Portal myofibroblasts, second-layer cells located around centrolobular veins (CLVs), vascular smooth muscle cells and cells of bone marrow origin have been shown to exhibit fibrogenic potential, probably by modulation to myofibroblastic cells (115–120). In addition, it is not clear what type of fibroblastic cells is involved in fibrosis induced by the intraperitoneal injection of pig serum in rats (121). Compared with activated HSCs, little is known about the factors leading to their proliferation and matrix synthesis (117). For these liver fibrogenic cells, differences may exist in the mechanisms of differentiation, activation and deactivation.
Relative importance of different fibrogenic cells in liver fibrogenesis depends on the origin of liver injury, since they are distributed differently in the hepatic lobule: HSCs are located along the sinusoids in the space of Disse; whereas the portal fibroblasts are in the portal tract connective tissue around portal structures. As a consequence, the distribution of fibrous ECM varies in different liver injuries. In chronic viral hepatitis and chronic cholestatic disorders, the fibrotic ECM is initially located around portal tracts, while in alcohol-induced liver diseases, it is located in the pericentral and perisinusoidal areas (103). However, in one disease status, different types of fibrogenic cells may be involved. For example, in biliary liver fibrosis, the involvement of portal fibroblasts around biliary structures have been demonstrated, at least for the early stages of fibrosis and HSCs may be involved later (118, 122, 123).
Phenotypic and functional properties of hepatic myofibroblasts are similar to those of activated HSCs. However, several phenotypic markers can be used to distinguish them, including selective expression of fibulin-2 and IL-6 by hepatic myofibroblasts and protease P100 and reelin by activated HSCs (124–126). With increased amount of evidence for existence of hepatic myofibroblasts involved in liver fibrosis, the concern is that liver fibroblastic cells other than typical HSCs might have been analyzed since myofibroblasts proliferate better than HSCs in vitro (127). Therefore, clear classification of the liver fibrogenic cells is necessary and care should be taken when extrapolating data from in vitro studies to pathological situations.
2.2.4. Deposition of Extracellular Matrix
In normal liver, ECM may be interstitial or basement membrane-like (59). Interstitial ECM is in the connective tissue of the fibrous external capsule, septa, periductal and perivascular areas and portal tracts, rich in collagen types I, III, V and fibronectin, while basement membrane-like ECM is rich in types IV, VI together with laminin and fibronectin in the space of Disse. Fibroblasts are the cells essentially responsible for ECM production in interstitial space, while the ECM in the space of Disse is mainly dependent on HSCs.
In an acute liver injury, hepatocytes regenerate and replace the necrotic or apoptotic ones in response to inflammatory response, leading to limited deposition of ECM, especially type III collagen (128). However, this process is restricted in chronic injuries to the liver. In advanced liver fibrosis/cirrhosis, fibrotic septa separate regenerative nodules in liver parenchyma, which leads to liver dysfunction. Although these major morphological changes represent the most commonly observed form of scarring, it is actually the early deposition of fibrillar ECM in the subendothelial space of Disse that is more directly responsible for the progressive reduction of liver function (129).
Regardless of etiology of liver injury, ECM deposition is the common characteristic at the fibrotic stage, which changes both the quantity and composition of normal ECM (130). In normal liver, collagens (Types I, III, V, and XI) are largely confined to the capsule, the area around large vessels and the portal triad, with only scattered fibrils containing types I and III in the subendothelial space (128). In advanced fibrosis, the liver contains approximately 6 times more ECM, including collagens (I, III, and IV), fibronectin, undulin, elastin, laminin, haluronan, dermatan and proteoglycans (131–133). Among them, type I collagen increases the most (131, 134–136). Excessive deposition of ECM results from both increased synthesis and decreased degradation of collagens (99). Expression of type I collagen is regulated at both the transcriptional and posttranscriptional levels (137). Posttranscriptional regulation results in up to 20-fold increase in the half life of collagen α1(I) mRNA (138, 139). The production of type I collagen can be increased ~70 fold by activated HSCs (137). In addition, the degradation of ECM is down-regulated. Although mRNA for interstitial collagenases, such as MMP-1 and MMP-13 remains unaltered as fibrosis develops (105, 106, 140), their activities are inhibited by TIMP-1 and TIMP-2 (104–106). Furthermore, MMPs are released as inactive pro-enzymes, which needs cleavage of the inhibitory N-terminal peptide to confer activity (141). The means of pro-enzyme activation varies between different MMPs and the protease plasmin is required for efficient activation of pro-MMP-1 (142). Plasmin synthesis can be inhibited by plasminogen activator inhibitor-1 (PAI-1), which is produced by activated HSCs (143, 144).
ECM has been shown to actively participate in the progression of liver fibrosis by providing survival factors for activated HSCs. It is a reservoir for growth factors and MMPs (145). Replacement of the low-density matrix of basement membrane by high-density interstitial matrix leads to capillarization of the sinusoids with formation of a continuous endothelium. This contributes to alterations in the phenotype of HSCs, sinusoidal endothelial cells, and hepatocytes (146–149). ECM rich in fibril-forming collagen accelerates activation of HSCs through interactions with integrins, the classic ECM receptors and RTKs such as discoidin domain receptor (DDRs) (69, 150).
2.2.5. Different Patterns of Fibrosis
Different liver diseases induce different types of liver injuries, resulting in different patterns of liver fibrosis during disease progression (151). This includes portal-portal septa surrounding liver nodules in which the central vein and its connections with the portal tract are preserved until late stages; portal-central (vein) septa, central-central septa and that in which the deposition of fibrillar matrix is concentrated around the sinusoids and groups of hepatocytes.
In both alcohol-induced liver disease and NASH, early changes are concentrated in the area of CLV: (i) around the sinusoids with capillarization of the sinusoids, (ii) around groups of hepatocytes, and (iii) around the central vein (117). The fibrogenic cells in the center of the lobule are recruited: in situ activated HSCs, second-layer myofibroblasts around the CLV, and smooth muscle cells from the CLV wall (125). Infection with HCV can cause different injuries (152). In some cases, it may have portal inflammation, with additional damage to bile ducts and portal vessels. Interface hepatitis is defined as inflammation and damage to the hepatocyte plates surrounding the portal tract, whereas lobular hepatitis is characterized by hepatocyte damage with or without inflammatory cells, either dispersed throughout the lobules or confluent, leading to bridging necrosis (often from central vein to portal tract and from central vein to central vein). Therefore, ECM deposition is contributed by the fibrogenic myofibroblasts located in portal spaces and the fibrogenic cells recruited at the interface between the fibrous septa and the parenchyma. HSCs activated in situ may also be involved in interface hepatitis damage repair and/or may migrate towards the portal tract (78). In primary biliary cirrhosis (PBC), T lymphocytes and cytokines mediate persistent bile duct damage (115). Biliary cells secrete fibrogenic mediators activating neighboring portal myofibroblasts to secrete ECM. At the end, perisinusoidal HSCs become activated and fibrotic bands develop.
2.2.6. Resolution of Liver Fibrosis
In contrast with the traditional view, evidences indicate that even advanced fibrosis/cirrhosis is reversible (153–155). Spontaneous resolution of liver fibrosis has been observed in humans after successful treatment of the underlying diseases (153, 156–161). However, resolution of advanced liver fibrosis is not always possible and may be limited due to ECM cross-linking and resistance to apoptosis of activated HSCs (130, 162). To understand the mechanisms of resolution of liver fibrosis, establishing and analyzing a model of spontaneous recovery from liver fibrosis is needed. Elimination of activated HSCs plays key role in resolution of liver fibrosis (163). However, since it is not easy to clearly define the fate of activated HSCs during resolution of fibrosis in human (164), most of conclusions are based on studies in animal models of liver fibrosis.
Apoptosis is one mechanism for diminishment of activated HSCs. In both bile duct ligated (93) and carbon tetrachloride (CCl4)-induced liver fibrosis models (165, 166), apoptosis of activated HSCs has been observed in resolution process, which decreases the production of both ECM and TIMPs. Apoptosis is a default pathway for HSCs. When partial degradation of fibrillar collagen occurs, the altered interaction between activated HSCs and ECM favors apoptosis (154). Death ligands such as Fas-L, and TRAIL-2 and -5, and their receptors are up-regulated in activated HSCs (166–168). The low-affinity NGF receptor/P75, another member of the TNF receptor superfamily may also be involved in apoptosis of activated HSCs (169). In addition, mast cells, which accumulate during fibrotic injury in areas adjacent to fibrosis, are a potent source of NGF (170), suggesting that incoming inflammatory and immune cells may also modulate HSC numbers through the control of apoptosis. Another possibility for the decreased number of activated HSCs during recovery from liver fibrosis is the conversion of activated HSCs to quiescent status (171, 172). A basement membrane-like ECM has been suggested to induce the deactivation of HSCs (171). However, this process is trivial, because there is no evidence of increased number of perisinusoidal cells after resolution (165).
Accompanying with the loss of activated HSCs is the increase in collagenolytic activity (130). During fibrosis resolution, the activity of MMPs was found to increase due to a rapid decrease in TIMP-1 and -2 expression (153, 165). Since expression of MMP-13 in rats is constant despite reduction in HSC numbers (92), other cell types including KCs or inflammatory cells are the potential sources of MMP-13 and other types of MMPs including MMP-2 and MMP-14 might also contribute the increased collagenase activity. In addition, IL-10 produced by HSCs themselves down-regulates inflammation and increases interstitial collagenase activity, which also contribute to the resolution of liver fibrosis (173, 174).
3. THERAPEUTIC APPROACHES TO LIVER FIBROSIS
Substantial progress has been made in understanding the pathogenesis of liver fibrosis, which has yielded potential new therapeutic targets. Antifibrotic therapies to regulate fibrosis independent of liver injury are likely to benefit all patients with fibrotic liver (158). The hope is that if antifibrotic therapy can reconstitute the normal microenvironment of liver, normal function can be restored and clinical manifestations may regress. Current and evolving approaches primarily target the activated HSCs to inhibit their activation, proliferation, and products (175). Gene therapy and gene silencing technologies may provide more opportunities. Although experimental studies have revealed targets to prevent fibrosis progression in rodents, the efficacy of most treatments has not been proven in humans (176).
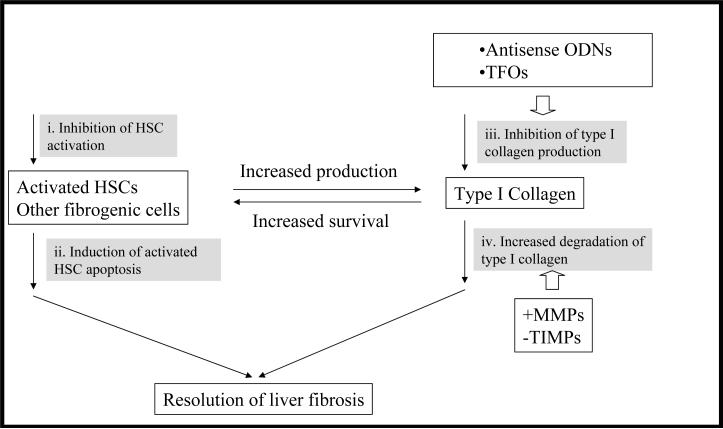
Treatment strategies for liver fibrosis includes i) prevention of liver injury, ii) reduction of inflammation or host immune response, iii) inhibition of HSC activation, iv) degradation of scar matrix, v) induction of HSC apoptosis, vi) stimulation of liver regeneration, vii) removal of initial fibrotic stimulus, and viii) inhibition of collagen synthesis (Figure 3).
Figure 3. Treatment strategies for liver fibrosis.
The common characteristics of liver fibrosis is that activated HSCs and other fibrogenic cells produce excess amount of type I collagen. Accumulation of type I collagen provides survival signal for activated HSCs. Therefore, strategies focusing on fibrogenic cells themselves as well as type I collagen regardless of etiology of liver injuries are attractive. Inhibition of the activation of HSCs (i) and induction of their apoptosis (ii) have attracted lots of attention. Gene silencing technologies have been utilized to directly inhibit the production of type I collagen (iii) at gene level. Inducing degradation of type I collagen (iv) can be realized by increasing the activity of matrix metalloproteinases (+MMPs) and inhibiting the activity of tissue inhibitor of metalloproteinases (−TIMPs). Decreased disposition of type I collagen and remodeling of extracellular matrix (ECM) accelerate apoptosis of activated HSCs.
3.1. Cure the Primary Disease
The most effective way to treat liver fibrosis is to clear underlying causes of liver diseases. Treatment of chronic HCV infection is of particular interest since it is the most common cause of cirrhosis in Western countries. IFN-α has been suggested to have antifibrotic effect in addition to its antiviral effect (177, 178). Using IFN-α plus ribavirin (RBV), the persistent clearance of viral infection can be achieved with the resolution of hepatic inflammation and liver fibrosis/cirrhosis (179–182). Furthermore, Pegylated IFN may provide a sustained virologic response and has been approved for treating chronic HCV infection (183, 184). Even though the treatment is better tolerated in patients with chronic hepatitis C and up to moderate fibrosis (185), it is poorly tolerated by patients with advanced-stage recurrent HCV (186). In CCl4-induced rat liver fibrosis, pegylated IFN-α-2b plus ursodeoxycholic acid (UDCA) has been shown to improve regression of liver fibrosis (187).
3.2. Inhibition of Hepatocyte Apoptosis
There is direct link between hepatocyte apoptosis and liver fibrogenesis. Fas-deficient lymphoproliferation (lpr) mice show decreased inflammation and fibrosis following bile duct ligation (43). IDN-6556, a general inhibitor of caspases has been shown to reduce hepatocyte apoptosis and fibrosis (188, 189) and is currently undergoing phase II clinical trials (190).
Nucleic acid-based gene silencing to interrupt signaling pathways (e.g., Fas/Fas-L system and other intracellular mediators) in hepatocyte apoptosis is a promising strategy for treating liver fibrosis. An antisense ODN, specific for mouse Fas receptor, reduced Fas mRNA and protein expression by 90%, and therefore prevented hepatocyte apoptosis in the liver (191). In another study, using Fas-specific siRNA, long-term inhibition of Fas expression was achieved and liver fibrosis induced by repeated concanavalin A adminstration is inhibited (192). Bcl-2 family is the regulators of apoptosis, which block cytochrome C release during apoptosis signaling (193). Bid, which can promote apoptosis, is one member of this family. Using an ODN against Bid caused an 80% decrease in Bid expression and protected hepatocytes from Fas-mediated apoptosis in mice (194). siRNA against caspase 8 has been successfully used to prevent hepatocyte apoptosis in mice (195). However, the effect of agents on liver fibrosis and safety still remains to be confirmed. In addition, adminstration of molecules interfering with hepatocyte apoptotic pathways carry a high risk of carcinogenesis, particularly at the cirrhotic stage. Therefore, this option should be considered at early stages of chronic liver diseases.
3.3. Reduction of Inflammation and Immune Response
Corticosteroids are indicated for treating hepatic fibrosis in patients with autoimmune hepatitis and acute alcoholic hepatitis (156). In autoimmune hepatitis, patients responding to corticosteroids progress more slowly to fibrosis and cirrhosis (196). However, it is not effective in autoimmune cholestatic diseases such as primary biliary cirrhosis (PBC) and primary sclerosing cholangitis (PSC) (197, 198). Malotilate is another agent with anti-inflammatory effects that is under investigation for treating liver fibrosis. It has been shown to prevent collagen deposition by inhibiting cytochrome P450 activity in animal models (199). However, this drug failed to improve liver histology and survival of patients with PBC or alcoholic liver disease (200).
Proinflammatory cytokines and their receptors present targets for inhibiting liver inflammation and therefore liver fibrosis. Antagonists of IL-1 receptor (201) or soluble TNF-α receptors (202) are associated with diminished hepatocyte necrosis and inflammation in the liver. IL-10 has been shown to have a negative auto-regulatory effect on collagen production by HSCs (173). IL-10 deficient mice have been shown to develop greater inflammation and fibrosis than wild-type mice (174, 203). Even though a pilot study on IL-10 had suggested its therapeutic potential in patients with chronic hepatitis C, who did not respond to interferon-based therapy (204), the subsequently long-term study showed no beneficial effects (205).
3.4. HSC as a Target of Antifibrotic Drugs
3.4.1. Modulation of Key Cytokine Production and Signaling Pathways
The most extensively studied strategy relates to the inhibition of TGF-β signaling pathways. TGF-β favors the transition of HSCs to myofibroblast-like cells, stimulates the synthesis of ECM proteins, and inhibits its degradation (206). TGF-β is synthesized in a latent form linked to a glycoprotein (latent TGF-β binding protein, LTBP), which serves as an anchor in the ECM. Proteolytic cleavage of LTBP is a prerequisite for the release and generation of bioactive TGF-β. Binding of active TGF-β to its receptors results in the phosphorylation and activation of the Smad family of intracellular signaling proteins. Phosphorylated Smad2 and Smad3 recruit the common mediator Smad4 and translocate into the nucleus, regulating gene transcription. On the other hand, several nuclear oncoproteins, such as Ski and SnoN, as well as Smad7, repress the activity of Smad proteins and limit the biological actions of TGF-β (207).
Strategies aimed at disrupting TGF-β synthesis or signaling pathways have decreased liver fibrosis in experimental models. Camostat mesilate, a serine protease inhibitor that reduces the cleavage of LTBP and thus the release of active TGF-β attenuated serum-induced liver fibrosis in rats (208). Approaches used to prevent the binding of TGF-β to its receptors include the use of a dominant-negative type II TGF-β receptor, the expression of the ectodomain of type II receptor fused to the Fc portion of human IgG, the expression of a truncated type II receptor, and the construction of a soluble type II receptor (209–212). These strategies utilized gene delivery to express specific protein products and showed successful prevention of liver fibrosis. Inhibition of intracellular signaling steps in TGF-β can also prevent liver fibrosis. Using an adenovirus expressing Smad 7, HSC activation and experimental fibrogenesis were inhibited (213). Directly silencing of TGF-β1 is another way to interrupt its signaling pathway and thus prevent progression of liver fibrosis. Kim et al. (214) used siRNA, which is against TGF-β1 mRNA and expressed from a plasmid vector, to silence TGF-β1 expression in CCl4-treated mice. TGF-β1 gene silencing was accompanied with reduced production of type I collagen and prevented progression of liver fibrogenesis. However, there is concern about the safety of a prolonged inhibition of TGF-β activity in humans, because this cytokine plays a role in defense against cancer, modulates the immune response, and inhibits inflammation.
TNF-α is mainly produced by activated KCs during liver injury and play a crucial role in HSC activation and hepatocyte regeneration besides its involvement in hepatocyte apoptosis. An antisense ODN against TNF-α has been shown to prevent liver damage induced by ethanol and LPS with the help of a liposomal formulation (215, 216).
CTGF is a highly profibrogenic molecule implicated in liver fibrogenesis. Intraportal vein injection of CTGF siRNA into CCl4-treated rats markedly attenuated the induction of CTGF, type I and III collagen, TGF-β1 genes, as well as activation of HSCs (217). The staging of liver fibrosis was also significantly decreased.
IFN-α and IFN-γ markedly decreases HSC activation and collagen synthesis (177, 218) and attenuates ECM deposition in experimental liver fibrosis (219–221). Studies in patients with chronic hepatitis C suggest that IFN-α may inhibit the progression of liver fibrosis irrespective of virological response (222, 223). A randomized open-labeled multicenter trial demonstrated the antifibrotic activity of IFN-γ in HBV infection (224). Recently, it was demonstrated that IFN-γ displays antifibrotic effects via STAT-1 phosphorylation, up-regulation of Smad7 expression and impaired TGF-β signaling (225). However, high dose or repeated administration of these cytokines needed for treating liver fibrosis may cause systemic toxicity.
In activated HSC, the most potent proliferative factor is PDGF that binds to its tyrosine kinase receptor and is up-regulated upon HSC activation. Glivec, a targeted tyrosine kinase receptor antagonist has attenuated the fibrotic response in fibrotic animal models (162). Pentoxifylline, a phosphodiesterase inhibitor, decreases HSC proliferation in vitro and in vivo by inhibiting PDGF-related signaling (226–228). Amiloride, an Na+/H+ exchanger inhibitor, decreases PDGF-induced proliferation and modulates the fibrogenic effect of oxidative stress in HSCs (229, 230) and has also been shown to be effective in experimental liver fibrosis (231). S-farnesylthiosalicylic acid, a ras antagonist, inhibits proliferation and migration of HSCs and reduces thioacetamide-induced liver fibrosis in rats (232). The semisynthetic analogue of fumagillin, TNP-470, inhibits HSC proliferation by blocking the cell cycle transition from G1 to S, prevents HSC activation, and attenuates the progression of liver fibrosis (233).
3.4.2. Reduction of Oxidative Stress
Oxidative stress stimulates HSC activation, and thus reducing oxidative stress is a possible antifibrotic target. They are particularly effective for alcohol-induced liver fibrosis, in which oxidative stress plays a key role (234), but have also shown some benefit in other experimental models such as CCl4 and iron overload (235, 236). Antioxidant compounds not only inhibit the activation of both HSCs and KCs, but also protect hepatocytes from undergoing apoptosis.
Vitamin E (α-tocopherol) decreases lipid peroxidation and has beneficial effects in CCl4- and iron overload-induced liver fibrosis (237). However, its efficacy in human liver diseases has not been confirmed (238, 239). Phosphatidylcholine (PC), a polyunsaturated phospholipid extract from soybeans has been studied as a hepatoprotective and antifibrogenic substance (240) and probably reduces oxidative stress (241) and therefore inhibits the activation of HSCs (242). A silybin-PC-Vitamin E complex has been demonstrated to be effective in prevention of liver fibrosis in experimental models (243). S-adenosyl-L-methionine (SAMe) is a substrate of glutathione synthesis that has hepatoprotective and antioxidant properties (244). It attenuates liver fibrosis in alcohol, biliary obstruction, and CCl4 models (245, 246). SAMe is currently used in several human liver diseases, such as alcoholic liver disease, primary biliary cirrhosis, drug-induced liver disease, and cholestasis of pregnancy. In alcoholic patients, it improves survival and delays the need for liver transplantation (247). Other substances with antioxidant properties that also inhibit experimental liver fibrosis include retinoids (retinyl palmitate) and natural phenolic compounds (resveratrol and quercetin) (248, 249). Dietary saturated fatty acids have been shown to attenuate liver fibrosis in vivo (250). This dietary supplementation reduces endotoxinemia, lipid peroxidation, and TNF-α level in rats with ethanol-induced liver disease. Another dietary supplement with antifibrotic action is zinc, a molecule involved in collagen synthesis and degradation (251, 252).
3.4.3. Modulation of Vasoactive Substances
Vasoactive substances regulate HSC growth. These include vasoconstrictors (AngII, aldosterone, and ET-1) and vasodilators (prostaglandins and nitric oxide). AngII mediates key biological actions in liver fibrosis, including myofibroblast proliferation, infiltration of inflammatory cells and collagen synthesis. Activated HSCs secrete AngII, which induces contraction and proliferation of activated HSCs via AngII type 1 (AT-1) receptor through the activation of NADPH oxidase (81, 253), while AngII has anti-fibrogenic effect by interaction with AT-2 receptors in oxidative stress-induced liver fibrosis (254). AT-1 receptor antagonists including captopril, candesartan and losartan inhibit liver fibrosis in bile duct-ligated and CCl4 treated rats (255–257). In human studies, it is suggested that AT-1 receptor antagonist can decrease the number of activated HSCs (258). A controlled pilot study in hepatitis C showed that losartan reduces liver fibrosis as compared to untreated controls (259)
ET-1 is another vasoconstrictor that merits study. Both ET-1 and its receptors are markedly induced in activated HSCs during liver fibrosis (260, 261). ET-1 displays dual pro- and antifibrogenic effects in the liver according to receptor subtypes. In early phases of hepatic fibrogenesis, the expression of ET-A receptors is predominant over ET-B receptors; therefore, ET-1 could play a profibrogenic role (262). In contrast, as liver fibrosis progresses, there is a marked up-regulation of ET-B receptors in the diseased liver, which could prevent progression of liver fibrosis by inhibiting HSC proliferation and collagen synthesis, in which a mechanism involving the sequential generation of sphingosine-1-phosphate (S1P), cyclooxygenase-2 (COX-2)-derived prostaglandins, and elevation of cAMP is suggested (84, 85, 263, 264). In light of these results, selective ET-A antagonists may be a proper strategy to treat liver fibrosis. A report has shown that the administration of a selective ET-A antagonist markedly prevents liver fibrosis development in bile duct-ligated rats (265), whereas treatment with a non-selective antagonist accelerates liver fibrosis in CCl4 treated rats (266). Several vasodilators, including prostaglandin E1 (PGE1) (267, 268), PGE2 (269) and nitric oxide donors (83), also inhibit proliferation of HSCs and exert antifibrotic activity. Enisoprost, a PGE1 analog was found to suppress type I collagen gene expression (267). The hepatoprotective effect of PGE2 has been related to its antioxidative capacity (269). Because the intrahepatic synthesis of nitric oxide is markedly decreased in advanced human and rat liver fibrosis, it is suggested that nitric oxide also plays a role in the progression of liver fibrosis (270). Other vasoactive substances with some antifibrotic potential are octreotide and adenosine (271, 272).
3.4.4. Induction of HSC/Hepatic Myofibroblast Apoptosis
The apoptosis of activated HSCs is a key step in the resolution of liver fibrosis, which is accompanied by a restoration of the collagenolytic capacities of MMP-1 and MMP-2 in the liver, subsequent to a decrease in TIMP-1 and TIMP-2 expression, which allows progressive matrix degradation. Gliotoxin, which provokes selective apoptosis of HSCs in cell culture and in vivo, leading to reduced fibrosis in both CCl4- and thioacetamide-induced fibrosis (273, 274). A COX-2 derived prostaglandin, 15-deoxy Δ12, 14 prostaglandin J2 (15-D-PGJ2) induces apoptosis of hepatic myofibroblasts, which might involve oxidative stress (275). Sphingomyelinase metabolites including ceramide, sphingosine, and S1P also induce apoptosis of hepatic myofibroblasts (276). However, the cell selectivity is a major issue of a proapoptotic strategy, which may result in life-threatening side effects, such as severe or fulminant hepatitis.
3.4.5. Inhibition of Other Signaling Pathways
Leptin is a 16kDa protein hormone produced by activated HSCs and may have direct effect on matrix synthesis via up-regulation of TGF-β in SECs and KCs (277–279). In mice with leptin deficiency or bearing mutations in leptin receptor, liver fibrosis is reduced, supporting a profibrogenic role of leptin (277, 278). These observations suggest that antagonists of leptin receptors should be investigated as antifibrotic agents.
Adiponectin, a major insulin-sensitizing hormone, has also antifibrogenic role by suppressing proliferation, migration and matrix synthesis of activated HSCs. In mice knocked-out for adiponectin enhanced liver fibrosis following treatment with CCl4 was observed, whereas pretreatment with an adenovirus encoding adiponectin prevents liver fibrogenesis (280). Adiponectin has been suggested to counteract progression of fibrosis in advanced stages since its serum level is elevated in patients with cirrhosis (281). Therefore, agonists of adiponectin receptors may have antifibrotic properties.
The cannabinoid (CB) Δ9-tetra-hydrocannabinol (THC) exerts a wide array of effects via tow G protein-coupled receptors, CB1 and CB2. However, the cannabinoid system may be important in liver fibrogenesis. CB1 and CB2 receptors are up-regulated in cirrhotic liver and interestingly, they display opposite effects on liver fibrogenesis (282, 283). Activation of CB-2 receptor induces antifibrogenic effects by growth inhibition and apoptosis of activated HSCs (282). It is further suggested that growth inhibition involves COX-2, and apoptosis results from oxidative stress. On the other hand, activation of CB1 receptors promotes progression of fibrosis. A CB1 receptor antagonist SR141716A inhibits progression of fibrosis after chronic liver injury (283). These promising results obviously warrant investigation of the effects of pharmacological antagonists of CB1 receptors and of selective agonists of CB2 receptors.
3.5. Inhibition of ECM Deposition
Because the collagen biosynthetic pathway has multiple steps involving transcription, mRNA stability, translation, as well as co-translational and posttranslational modifications, there are many potential targets for intervention.
Prolyl-4 hydroxylase catalyzes the synthesis of hydroxyproline residues, which are critical for the stability of the collagen triple helix. Inhibitors of prolyl-4 hydroxylase include HOE077 and S4682. In rat models of liver fibrosis induced by a choline-deficient L-amino acid-defined diet, CCl4 and pig serum, administration of HOE077 inhibited hydroxyproline content and histological liver fibrosis (284–286). However, HOE077 inhibits HSC activation in vitro and in vivo, which might be the predominant mechanism for its antifibrotic effect (287). S4682 decreases liver fibrosis in the CCl4 rat model, with a concomitant decrease in morbidity as documented by improved liver function tests and decreased ascites formation (288). The antimicrobial compound halofuginone, a potent inhibitor of α1(I) collagen gene expression, prevents the development of liver cirrhosis in dimethyl nitrosamine (DMN)-treated rats (289).
Type I collagen is the major structural protein of the ECM during liver fibrosis, which makes it an ideal target of silencing technologies. Type I collagen consists of two α1(I) and one α2(I) polypeptide chains encoded by the α1(I) and α2(I) genes, respectively, and synthesized at a 2:1 ratio. As mentioned before, the enhanced expression of type I collagen by activated HSCs is regulated at both the transcriptional and posttranscriptional levels (137). The production and stability of α1(I) collagen mRNA are highly increased in liver fibrosis. Wu et al. designed several sequences of antisense ODN against α1(I) and α2(I) mRNA and tested their silencing efficiencies in cell culture using asialoorosomucoid (AsOR) modified poly (L-lysine) (PLL) as a carrier (290). The α1(I) and α2(I) mRNA levels were reduced by 67% and 73%, respectively, which led to significant reduction in collagen synthesis. In another study by Laptev et al. (291), a series of antisense ODNs were tested to define the best target sites within an RNA transcript of collagen for effective inhibition of expression. Mouse NIH 3T3 fibroblasts were stably transfected with a human α1(I) collagen gene so that the cells simultaneously synthesized full-length mouse α1(I) collagen chains and internally deleted human α1(I) collagen chains. It was demonstrated that antisense ODNs could be designed to specifically inhibit production of α1(I) collagen from the human gene without affecting the levels of α1(I) collagen chains from the mouse endogenous gene. The most effective ODNs reduced the human α1(I) collagen chains to 37–67% of the controls. Moreover, the most effective ODNs were those targeted to sequences that were predicted to form clustered double-stranded structures in RNA transcripts.
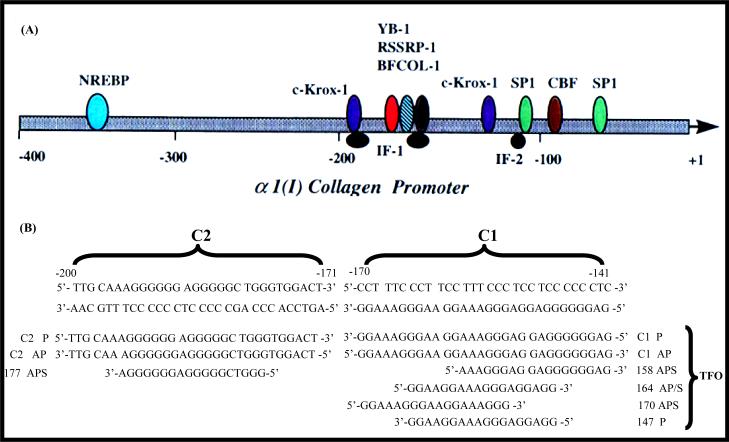
In type α1(I) procollagen promoters, two highly conserved polypurine-polypyrimidine tracts are present contiguous in the region from −140 to −200 (292). The polypurine sequence at −141 to −170, which we called C1, is localized on the non-coding strand, whereas the polypurine sequence from −171 to −200, which is denoted as C2, is present on the coding strand. As illustrated in Figure 4(A), several transcription factors are known to bind to the promoter sequence, which includes C1 and C2 regions (293). These regions play a key role for the cis-acting elements, and hence makes them an ideal target for developing antigene-based antifibrotic agents (293, 294). Different TFO sequences have been designed to target these regions as shown in Figure 4B (292, 295, 296). These TFOs formed stable triplex with high efficiency with target double-stranded DNA (dsDNA) and demonstrated inhibition of the α1(I) procollagen promoter activity, which was cloned into a plasmid vector. Since the production of type I collagen is the end step in the process of liver fibrosis, using antifibrotic agents directly targeting to this step has many advantages. We are currently evaluating the therapeutic application of TFO-based agents in liver fibrosis.
Figure 4. Sequence of the rat α1(I) collagen promoter showing duplex targets C1 and C2 and the TFOs.
(A) The schematic illustration of rat α1(I) collagen promoter region. Many transcription factors have been shown binding to this region and play key roles in regulation of the gene expression. (B) Phosphodiester and phosphorothioate triplex forming oligonucleotides (TFOs) are designed with different starting position corresponding to the type α1(I) collagen promoter sequence. These TFOs can be parallel or antiparallel to the target sequence. They are 18mer or 30mer. The ability of triplex formation can be different. P: parallel; AP: antiparallel; APS: antiparallel phosphorothioate. Reproduced with permission from Joseph et al. (1998) Nucleic Acids Res. 25(11):2182–8.
Little progress has been made to date in modulating collagen degradation. Potential targets include the modulation of MMP activity and its regulators such as plasminogen activator or TIMPs. uPA, an initiator of the matrix proteolysis cascade, has been examined in a rat model of cirrhosis (297). A single intravenous administration of a replication-deficient adenoviral vector encoding a non-secreted form of human uPA resulted in high production of functional uPA protein in the liver. This led to the induction of MMP expression and reversal of fibrosis with subsequent hepatocyte regeneration and improved liver function. In another study, a single intravenous injection of an adenoviral vector expressing human MMP-1 reversed stable liver fibrosis with a return to normal liver histology in thioacetamide-induced cirrhosis in rats (298).
4. TRIPLEX FORMING OLIGONUCLEOTIDES AS POTENTIAL ANTIFIBROTIC AGENTS
Advances in ODN-based therapeutics offer great hope in sequence-specific inhibition of aberrant gene expression. ODNs (299) as well as more recently developed siRNA/siRNAs (300) silence gene expression at translation levels via inducing degradation of target mRNA or inhibiting translation process, whereas those act at transcriptional levels by TFOs (301–306), called anti-gene strategy. In this section, some relevant issues regarding the application of TFOs for gene silencing and will be discussed.
Transcriptional inhibition via triplex formation presents an attractive application of TFOs and has several advantages over other gene silencing technologies (307, 308). There are only two copies (two alleles) of the target gene, whereas there may be thousands of copies of an mRNA. In addition, blocking translation of mRNA does not prevent mRNA from re-production. In contrast, inhibition of gene transcription is expected to bring down the mRNA concentration in a more efficient and long-lasting way, depending on the residence time of TFO on its target sequence and its life-time determined by its nuclease sensitivity.
However, besides the delivery issue, which is common for most ODN-based therapeutics, the disadvantages of TFO-based therapeutics include instability of triplex, limited availability of target sequences and accessibility of the target sequence in the nucleus. Chemical modifications on TFOs provide promising strategies to assure their potential applications in vivo. TFOs have to get into nucleus and bind to their target DNA in the chromatin structures. T he binding of TFOs may be affected by many cellular factors. Detection of triplex formation in vivo is another important issue for developing TFOs as therapeutics since observed gene regulation may not be always correlated with triplex formation. Therefore, many studies are focusing on developing molecular tools for detection of triplex formation inside living cells and it is worth a summary on the progress in this field.
4.1. Rules for Triplex Formation
Since bases already engaged in Watson-Crick hydrogen bonding, only purines are able to further establish two Hoogsteen (or reverse-Hoogsteen) hydrogen bonds in the major groove of DNA, the optimal target sequences require consecutive purines on the same strand for stable binding (307, 309). Therefore, triplex formation must follow precise rules to minimize energy, which on the other hand bring its specificity. It is suggested that at least 15–20 bases are needed for a TFO sequence to bind its target site with sufficient affinity to have biochemical effects.
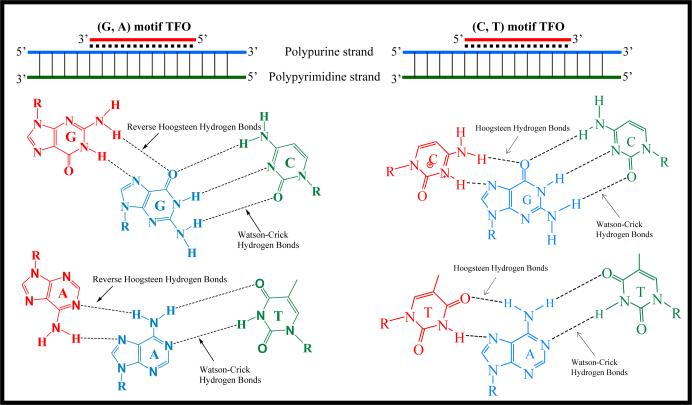
Even though TFO can only bind to polypurine strand of target DNA, TFO itself can be consisted of purines and pymidines, depending on the nature of the target sequences (Figure 5). The (C, T)-motif involves the formation of C•G×C and T•A×T base triplets (• stands for Watson-Crick hydrogen bond; × stands for Hoogsteen/reverse-Hoogsteen hydrogen bond), upon binding of a (C, T)-containing TFO with a parallel orientation with respect to the purine strand (Hoogsteen hydrogen bonds) (310). The (G, A)-motif involves the formation of C•G×G and T•A×A triplets, upon binding of a (G, A)-containing TFO in an antiparallel orientation with respect to the purine strand (reverse-Hoogsteen hydrogen bonds) (311). In addition, a (G, T)-motif TFO is also permitted. The (G, T)-motif involves binding of a (G, T)-containing TFO, whose orientation depends on both the number of GpT or TpG steps in the third strand and on the length of G and T tracts. Because of these intrinsic rules for TFOs, the repertoire of their target sequences in the genome is limited to only polypurine/polypyrimidine sequences.
Figure 5. Rules of triplex formation.
A third polynucleotide sequence can bind to double-stranded DNA at the major grove to form triplex structure via formation of Hoogsteen/reverse Hoogsteen hydrogen bonds. TFOs can only bind to polypurine strand of target DNA. TFOs can be either (G, A)-motif or (C, T)-motif. The (C, T)-motif involves the formation of C•G×C and T•A×T base triplets (• stands for Watson-Crick hydrogen bond; × stands for Hoogsteen hydrogen bond), upon binding of a (C, T)-containing TFO with a parallel orientation with respect to the purine strand (Hoogsteen hydrogen bonds). The (G, A)-motif involves the formation of C•G×G and T•A×A triplets, upon binding of a (G, A)-containing TFO in an antiparallel orientation with respect to the purine strand (reverse-Hoogsteen hydrogen bonds). A (G, T)-motif TFO is also permitted. The (G, T)-motif involves binding of a (G, T)-containing TFO, whose orientation depends on both the number of GpT or TpG steps in the third strand and on the length of G and T tracts.
The stability of a triplex is challenged by many factors. First, the stability of TFO against nucleases is a concern, which is universal for all ODN-based technologies. Triplex formation involves conformational changes on TFO sequence and some distortion of the underlying dsDNA (312, 313), which leads to the intrinsic instability of triplex structures. Triplex formation with (C, T)-motif TFOs is pH dependent because cytosines must be protonated at the N3 position in slightly acidic media (pH<6) to form two hydrogen bonds with G (314). Triplex formation is dependent on ionic strength. Typically, 5~10 mM of Mg2+ ions are required for forming stable triplex (301). This is because triplex formation involves the binding of a negatively charged third strand to a double negatively charged duplex and neutralization of charge repulsion can be achieved with Mg2+. However, this concentration of Mg2+ is much higher than that available inside cells (315). TFO can be made unavailable to target DNA by sequestering in self-associated stable structures. G-rich TFOs can easily form intra- or inter-molecular four-stranded structures involving G-quartets (316). Other intermolecular structures, such as parallel homoduplexes involving AA, GG and GA base pairs can also be formed (317). In addition, monovalent cations (Na+, K+) at physiological concentrations (140 mM) favor G-quartet formation and thus inhibit triplex formation (318). All these factors impose kinetic barriers on triplex formation and reduce the stability of triplexes once formed, which results in that most triplexes, even under optimal conditions in vitro, are less stable than the corresponding dsDNA.
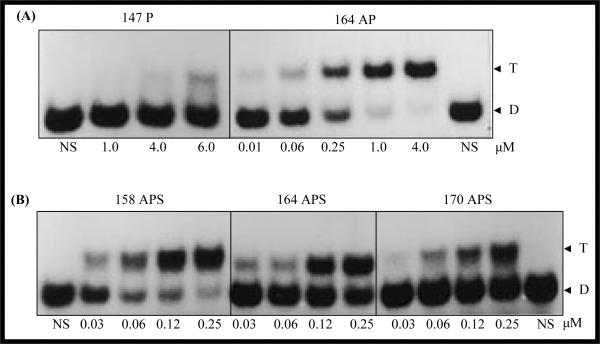
However, for a DNA target sequence, many different TFO sequences can be designed binding to different regions along the target DNA sequence. The triplex formation ability can be very different depending on the selection of TFO sequences. As shown in Figure 6, with different starting position, TFOs targeting α1(I) collagen promoter region can have varied triplex forming kinetics based on the electrophoretic mobility assays (292). Therefore, careful design and selection of TFO sequences for further studies are necessary for achieving high bioactivity.
Figure 6. Electrophoretic mobility shift assays showing triplex formation with parallel and antiparallel phosphodiester and phosphorothioate triplex forming oligonucleotides (TFOs) with C1 duplex.
(A) Comparison between the TFOs with different directions relative to C1 duplex; (B) Comparison between TFOs with different start positions on the C1 duplex. C1 represents the sequence from −141 to −170 of α1(I) gene promoter, in which a polypurine sequence exists on the non-coding strand. Duplex concentration, 2 nM; TFO concentration (μM) in each triplex forming reaction is shown below the corresponding lane. T, triplex; D, duplex; P, parallel; AP, antiparrallel; APS, antiparrallel phosphorothioate; 164 AP/APS, TFO sequence corresponding to the region from −164 to −147; 147 P, TFO sequence corresponding to the region from −147 to −164; 158 APS, TFO sequence corresponding the region from −158 to −141; 170 APS, TFO sequence corresponding to the region from −170 to −153. Reproduced with permission from Joseph et al. (1998) Nucleic Acids Res. 25(11):2182–8.
4.2. Chemical Modifications on TFOs
Considerable efforts have been made to extend the repertoire of potential target DNA sequences and increase the triplex stability while keeping its specificity via chemical modification on TFOs (307, 319). To extend the repertoire of target DNA sequences, most efforts focus on synthesis of new nucleobase analogues to avoid the requirement of a polypurine target sequence for TFO binding, i.e., to recognize a pyrimidine which interrupts an oligopurine sequence. For a TA base pair inversion, which means a thymidine interrupting an oligopurine sequence, an additional problem is the projection into the major groove by the 5-methyl group of thymine resulting in steric hindrance for TFO binding to the major groove, which further disturbs the triplex structures. A nucleoside analogue with a phenylimidazole derivative was shown to tolerate TA interruption and form stable triplexes (320). Two other nucleobase analogues for exact recognition of thymine in the TA base pair were also reported (321, 322). To recognize cytosine in the GC base pair, a novel thymine nucleobase analogue 4HT, which lacks a carbonyl oxygen at the 4-position, was reported (323). Several N4-substituted cytosine derivatives with side-chain extensions were shown to form hydrogen bonds with both the cytosine and the guanine in the inverted GC base pair and therefore enhance the stability of triplex structures (324). Furthermore, this approach is attractive because the formation of hydrogen bonds with both bases should enhance the specificity with the intended base pair.
To overcome pH dependence of the (C, T) motif, a cytosine analogue, 5-methylcytosine was shown to have potential use (325). Other analogues for substitution of cytosine include 8-oxoadenine (326), pseudoisocytidine (327), and a 6-keto derivative of 5-methylcytydine (328) and 2-aminopyridine (329, 330). However, much work still needs to be done on nucleobase analogues regarding their biological applications.
Different backbone modifications have been investigated to enhance both the nuclease resistance and binding affinity of TFOs (Figure 7). Phosphorothioate (PS) modification is the most extensively studied chemical modification on ODN and is known to be nuclease stable. However, triplexes with PS-TFO are as stable as native phosphodiester (PO) TFOs in the purine motif and is greatly reduced in the pyrimidine motif (307, 331). Replacement of the phosphate linkage with methylthiourea improves TFO binding (332), which is because this modification brings positive charges to the backbone of a TFO, and thus partially minimizes the charge repulsion. Similarly, a positive charge on a thymidine analogue (333), or attachment of positively charged moieties to TFOs (334, 335) have also enhanced triplex stability. Replacement of a non-bridging oxygen atom in the backbone with a charged amine enhanced the stability of triplexes, which also partially resulted from reduction of the likelihood of self-structure formation of purine TFOs in physiological K+ concentration (336–338).
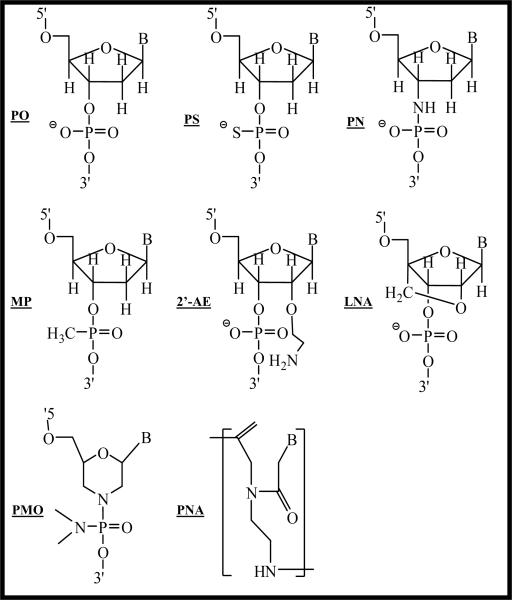
Figure 7. Modifications of the TFOs.
Different modifications have been developed to increase the stability and binding affinity of TFOs. Modifications can be on backbone and sugar moieties. More radical modification is to substitute the whole sugar structure, such as phosphorodiamidate morpholino oligonucleotides (PMOs) and peptide nuclei acids (PNAs). PO: phosphodiester; PS: phosphorothioate modification; PN: N3'→P5' phosphoramidate modification; MP: methyl phosphodiester; 2'-AE: 2'-O-aminoethyl (2'-AE); LNA: Locked nucleic acid (LNA).
Phosphoramidates (PN) analogues containing N3'→ P5' phosphoramidate linkages stabilize triplexes formed with (C, T)- and (G, T)-motif TFOs in a parallel orientation with respect to the oligopurine sequence, but not in an antiparallel orientation (339). A conformational change to N type in the sugar moiety might also contribute to this enhanced triplex-forming ability (319). It is suggested that the formation of a duplex or triplex is entropically unfavorable due to the restriction in conformational freedom of the furanose ring. Therefore, “pre-organization” of the sugar conformation in a suitable form to prevent the loss of entropy would be a promising strategy for stabilizing triplex structures.
Locked nucleic acid (LNA) is designed to have this “pre-organization” by introducing a bridge between the O2' and C4' atoms, the sugar conformation of which is restricted to the N type (319). As a result, triplex can be formed 300-fold more stable compared with the corresponding one without modification. Substitution of deoxyribose with ribose in the third strand of the triplex and this resulted in stabilization of triplexes for the (C, U) or (G, U)-motif with a parallel orientation of the third strand with respect to the purine target sequence (340–342). This inspired the use of RNA analogues, i.e., 2'-O-alkyl analogues (342, 343). The beneficial effects are probably due to the shift in the conformational equilibrium of the sugar to C3' endo configuration. This conformation imposes minimal distortion on the underlying duplex (313) and thus provide “pre-organization”, which has entropic benefits (344). In 2'-O-aminoethyl (2'-AE) modification, both a positive charge and a “pre-organized” sugar conformation can be provided (343). TFOs carrying this substitution show enhanced kinetics of triplex formation and greater stability of the resultant complex at physiological pH and low Mg2+ concentration
To minimize the G-quartet formation, G in the sequence was substituted with 6-thioguanine (345) or 7-deazaguanine (346). However, these modifications reduce the overall binding affinity. TFOs can be conjugated to a secondary structure that will impair the formation of self-associated structures but not triplex formation. For example, clamp or circular ODNs are less prone to self-association (347). An additional ODN helper forming a short duplex can be added at either the 3' or 5' end of the TFO, thereby reducing its propensity to self-association (348). Furthermore, a short hairpin structure has been designed incorporated at one end of TFO to prevent self-association (349).
The two most radical modifications are morpholino analogue, in which the sugars are replaced with morpholine rings, and peptide nuclei acids (PNAs). Replacement of ribose with a morpholino analogue reduces the Mg2+ dependence (350). In PNA modification, the deoxyribose phosphate backbone is replaced by a homomorphous, achiral and uncharged backbone based on N-(2-amino-ethyl)glycine (351). PNAs are not degraded by nucleases or proteases. PNAs hybridize virtually independently of the salt concentration. However, binding of PNAs to their target sequences is dependent on pH, with more stable binding occurring at the neutral to slightly acidic pH. In addition, for PNA, the anti-parallel binding is favored over the parallel one. Furthermore, PNA binding is more affected by base mismatches than native DNA, which means more specificity of PNA. One of the striking properties of PNAs is that they lead to strand displacement in double-stranded DNA, forming a 2:1 complex with the purine target sequence, in which the complementary pyrimidine sequence is displaced, thus resulting in the formation of a P-loop structure (352). In these triplexes, one PNA strand hybridizes to DNA through standard Watson-Crick base pairing rules, while the other PNA strand binds to DNA through Hoogsteen hydrogen bonds.
Conjugation of TFOs with intercalating agents, which may interact with DNA target leading to either covalent modification or cleavage of backbone, can definitely increase binding affinity and obtain more durable biological effect. For example, psoralen- and orthophenanthroline-TFO derivatives are remarkable by inducing triplex-mediated cross-linking and cleavage reactions, respectively, on a duplex target, such that a durable biological effect can be obtained (353). Intercalating agents can be conjugated to the 5'- or 3'-end or to internal positions, even though the preferential site of intercalation is formed at the triplex-duplex junction (307). Incorporation of acridine internally into TFOs, not only increased the stability of triplexes, but also enabled triplex formation with target DNA containing base pair inversions (354). However, only some of the chemicals have been found to specifically stabilize triplexes other than dsDNA (307).
4.3. Gene Regulation by Triplex Formation
4.3.1. Transcriptional Inhibition by Triplex Formation
TFOs bind to target DNA and lead to specific gene silencing. Transcription inhibition by triplex formation was first shown by Morgan and Wells (355). Binding of TFOs to target DNA sequences can compete with protein binding, therefore triplex formation can be designed to interfere with gene transcription at different levels depending on the target sites. Triplex formation has been shown to disturb chromatin structure (356), binding of transcription factors (308, 357) and RNA polymerase binding (358, 359). In addition, elongation process of DNA polymerase and RNA polymerase has also been shown to be interfered by triplex formation (331, 360, 361). Studies have shown that durable inhibition of transcription elongation can be further achieved by attachment of intercalating agents (e.g., acridine and psoralen) on TFOs (362, 363). Since PNA binding to duplex DNA leads to strand displacement, they could stop RNA polymerases only when hybridized to the transcribed strand (364–366). Furthermore, PNA binding to DNA target is promoted by transcription process, leading to the concept of suicidal transcription (367).
The mechanism of action of TFOs inside the live cells remains to be confirmed. Very few studies provides unambiguous evidence that transcriptional inhibition is due to triplex formation (307). Furthermore, sequence-specific effects can also be obtained by mechanisms other than triplex formation. For example, G-rich TFO can adopt structural conformations, which can be recognized by specific proteins (368–370). An oligothymidylate–acridine conjugate directed to the SV40 replication origin proved to be efficient at inhibiting viral replication in CV1 cells (371), but this effect may be due to the mechanisms other than triple helix formation on the target sequence. In lymphocytes, HIV-1 transcription was inhibited by (G, T)-rich 38-mer TFOs directed against the transcription initiation or nuclear factor Sp1 binding sites (372), This effect may be due to a direct interaction between the HIV-1 integrase and TFOs (307). In many studies, gene inhibition of the c-myc oncogene by TFOs is ascribed to triplex formation (373–375). However, other mechanism may also be possible since titration of the transcription activator CNBP by the purine-rich ODN could fully account for the observed decrease in c-myc transcription (376, 377). In addition, if two potential targets are constituted by both genomic DNA and the corresponding mRNA, then TFO may exert its effect by Watson–Crick base pairing to the RNA target (364, 378–380). The respective contribution of antigene, antisense or other effects is not easy to evaluate.
Because mechanisms other than triplex formation may also be involved in TFO-induced gene silencing, controls of studies regarding TFO induced gene silencing are very important for elucidating action of TFOs. Scrambled or mutant ODNs and irrelevant targets sequences are generally used as controls and definitive demonstration of a triplex-mediated mechanism is generally lacking (307). However, the best control experiment consists of using the same TFO but a mutated target sequence affecting triplex stability but not gene expression (381). One such example is the studies of transcription inhibition of the gene coding for the alpha subunit of interleukin-2 receptor (IL-2Rα). In this study, 15 mer-(C, T) TFOs with acridine or psoralen modification were used for triplex formation with genomic DNA; intracellular covalent triplex was measured in parallel to determine whether there is any correlation between triplex formation and transcription inhibition (381–383). A control plasmid mutated in the triplex site was unable to bind TFO and there was little effect on the transcription activity and regulatory protein binding.
4.3.2. G-rich Oligonucleotides
For (G, A)-motif TFOs, there are very high chances to have continuous guanine in the sequence. G-quartets are structural motifs formed by the planar arrangement of four guanine bases that interact with each of the two adjacent guanines through Hoogsteen-like hydrogen bonds (384). ODNs possessing contiguous G bases are assumed to have a tendency to form thermodynamically stable quadruplex structures, i.e., through vertical stacking of two or more G-quartets, which may confer them biological activities other than triplex formation induced gene regulation (385). In fact, these quadruplex structures have been observed involved in various biological processes in important genomic locations such as chromosomal telomeres, gene promoter regions, immunoglobulin switch regions and recombination sites, its function in cells is not well understood (384–386). In the regulatory region of genes, they are suggested to act as transcriptional regulators that may block or enhance DNA synthesis (387, 388). The potential of quadruplex DNA as targets for therapeutic intervention and as therapeutics is suggested (320).
An aptamer is RNA or DNA molecule that binds to a specific molecular target and the binding is related its quadruplex structures. The sequence GGTTGGTGTGGTTGG was the DNA aptamer with the highest affinity for thrombin (320). This DNA aptamer significantly increases the thrombin catalyzed clotting times of both purified fibrinogen and human plasma. Specific 16-mer and 17-mer ODNs with sequences consistent with the formation of quadruplex structures have been found to be potent inhibitors of HIV-1 integrase (389). Wyatt et al. reported that a certain G4 forming ODN bound to HIV-1 gp120 protein inhibiting in this way both cell-to-cell and virus-to-cell infection (194).
Several studies have shown that G-rich ODNs exhibit antiproliferative activities. For example, the G-quadruplex ODN (GQ-ODN) T40214, which forms a G-quadruplex structure intracellularly, has been shown to be potent inhibitors of Stat3 DNA binding activity, inhibit IL-6-stimulated Stat3 and suppress the Stat3-mediated upregulation of Bcl-XL and Mcl-1 gene expression (390) Another group has shown that G-rich ODNs have antiproliferative activity against a number of cancer cell lines (391). Again, the antiproliferative activity of these G-rich ODNs is associated with their ability to form stable G-quartet-containing structures and their potential binding to a specific cellular protein, nucleolin.
4.3.3. Accessibility of Genomic DNA to TFOs in Cells
TFOs have to reach the nucleus and compete with all nuclear proteins for binding to genomic DNA. Compared to antisense ODNs, an additional restriction to potential target sequences for triplex formation is linked to chromatin structure, a parameter which is largely unknown for most of potential target sequences. Endogenous gene silencing by TFOs has been demonstrated in many studies (372, 374, 375, 392, 393), which suggests the accessibility of nuclear DNA for TFOs. However, in these studies, triplex formation with DNA in native chromatin structure was not unambiguous confirmed. In addition, some in vitro experiments indicate that chromatin structure is incompatible with triplex formation (356, 394, 395). Therefore, to clarify the accessibility of target sites in the context of chromatin is crucial for developing antigene strategies.
To determine the accessibility of DNA in intact chromatin structure, most studies are using permeabilized cells or physical transfection technologies to enhance TFO accumulation in intact nuclei. These studies confirm the accessibility of target sequences in intact chromatin structures. The accessibility is dependent on the sites of target sequences and TFOs used. In a pioneer study, Giovannangeli et al. demonstrated triplex formation of psoralen modified 15-mer PO- or PN-TFOs with genomic DNA within permeabilized cells chronically infected with viral HIV-1 (396). PN-TFO showed higher efficiency in triplex formation than PO analogue. In another study, endogenous gene sequence of the chemokine receptor CCR5 was a target (397). A 12-mer (G, A)-motif TFO with G substituted by 8-aza-7-deazaguanine was modified with a phenylacetate mustard on the 5' end for alkylation with DNA sequence. TFO accessibility to target DNA in the cells permeabilized by streptolysin O treatment was revealed by showing that up to 24% of covalently modified target. Site-specific mutations induced by TFO-psoralen conjugates in the Hprt gene of CHO cells after electroporation have further confirmed the DNA accessibility in the cell nucleus (353).
The cellular environment has great effects on triplex formation. However, targeting of chromosomal sites by TFOs is limited by nuclear environment rather than cellular context (398). A LNA modified TFO was used to study the effect of nuclear environment on the triplex formation efficiency (398). When the target sequence was located in transcription region, the triplex formation efficiency varied from one gene to another and was not correlated with the status of gene expression. However, different degrees of triplex formation were observed when HIV-1 genome was integrated into different sites of the host genome (396). Since transcriptional activation can change chromatin organization including nucleosome disruption or displacement in several systems (399, 400), the transcription status of target DNA sequences must affect the triplex formation efficiency. Therefore, it is suggested that transcriptionally active genes might be accessible to triplex-mediated regulation, especially when the target sequences are located in the same DNA domain as transcription factor binding sites. It is still debated whether actively transcribed gene sequences can have higher accessibility to TFOs. Induction of transcription activation was shown to enhance triplex formation (398, 401). However, there was little effect on triplex formation when the cells were treated with phorbol-12 myristate-13 acetate (PMA), which is known to induce transcription activation of HIV-1 genes (396). Oh et al. did not see noticeably effect of transcription activation on triplex formation (402). This apparent contradiction may be because i) beyond certain transcription activity, an additional increase may have no effect on targeting efficiency by TFOs; and ii) difference exists for TFO targeting sites including promoter region or in transcription region (398).
4.3.4. Detection and Quantification of Triplex Formation
Most studies to demonstrate the efficiency and specificity of triplex formation are relying on more traditional molecular techniques including electrophoretic mobility shift assay (EMSA) (292), restriction enzyme protection assay (403, 404) and dimethyl sulfate (DMS) footprinting (348). However, these methods are not quantitative. New methods have been developed based on PCR, including competitive PCR (396), linear amplified primer extension (382), single-strand ligation PCR (402), and real-time PCR-based methods (398, 405, 406). Currently, interests are focused on the detection and quantification of triplex formation in the native chromatin structures rather than on isolated DNA targets and plasmids. Direct demonstrations of triplex formation in cell nuclei and/or permealized cells have been reported (353, 406, 407). In many studies, several detection strategies are used in combination.
Psoralen is a bifunctional photoactive agent that has been used as probes of nucleic acid structure and function, and is minimally harmful to cellular constituents (408). Conjugation of psoralen to the 5'end of a TFO allows photo-induced cross-linking reaction of the psoralen at the specific sequence where the TFO binds to duplex DNA. To detect the triplex formation, psoralen conjugated TFO has been used extensively (382, 406, 409). Upon UV irradiation, psoralen introduces a covalent crosslink into the target DNA sequence which can effectively block transcription (272, 382, 409, 410). This characteristic of psoralen is the basis of many PCR-based quantification methods.
4.3.4.1. Electrophoretic mobility shift assay
EMSA is used extensively for studying the kinetics of triplex formation with synthetic duplex DNA targets (292, 296, 352). In this method, either one strand of synthetic duplex DNA targets (411) or two strands (296) can be radiolabeled for detecting triplex formation using autoradiography after polyarylamide gel electrophoresis (PAGE) depending on the purpose of study. For example, the interaction between psoralen and duplex DNA after triplex formation was studied in details by Takasugi et al with changing of radiolabeled strand in the duplex DNA (411). Initial selection of potent TFO sequences as well as studies of effect of different factors on triplex formation (412) can be easily achieved by this method. In a study, the triplex formation ability of daunomycin-conjugated TFOs was studied using EMSA (413). Triplex formation using LNA modified TFOs has also been studied by EMSA (414).
4.3.4.2. Restriction enzyme protection assay
In restriction enzyme protection assay (REPA), a restriction enzyme is selected to have a recognition site within the triplex formation sequence. Formation of triplex structure at this recognition site prevents the restriction digestion by enzymes, thus giving different digestion pattern from those without triplex structures upon separation by gel electrophoresis (403). By utilizing this method, the efficiency and specificity of triplex formation can be established, mostly in targets cloned in plasmids (381, 404, 409). It is suggested that without psoralen modification on TFOs, the protection from restriction enzymes by triplex formation was only transient since the triplex formation is kinetically in equilibrium with duplex DNA targets (404). Using REPA, the persistence of preformed triplexes between a psoralen modified TFO with a plasmid after transfection into cells for 72 h has been established (409). When this method is used to detect the triplex structures isolated from cells, Southern blot is often used in combination for detecting expected DNA fragments to increase the sensitivity of detection (381, 409). This is especially true for the targets in the genome context extracted from nuclei or cells, since the amount of the expected fragmental DNA is extremely low. In one study, the triplex forming sequence was in HIV-1 genome, which was integrated into cellular genome (396). TFOs used were PN analogue with psoralen modification. After forming triplex, the DNA was digested with DraI and the DNA fragments were detected by DNA probes. The specificity of triplex formation was confirmed by the lack of protection of the two neighboring DraI sites. Up to 30% of the target sequence in the nuclei of permealized cells was shown to be protected from digestion. Macris and Glazer also showed around 30% of triplex formation in permealized cells at target site which is also integrated into genome of human 293 cells by REPA (401). Similar DraI protection assay was also used in another study with LNA modified TFOs by Brunet et al (398). However, only triplex formation with naked genome DNA was reported.
4.3.4.3. Competitive PCR
Giovannangeli et al applied competitive PCR to quantify the triplex formation of TFO modified with psoralen with isolated genomic DNA (396). In this method, a competitor DNA fragment was constructed for quantifying unknown amount of genomic DNA by co-amplification with PCR. For quantification purpose, two sets of primers were used as well, one to amplify a fragment (denoted as RT) overlapping the triplex formation site and another for a fragment (denoted as RC) outside the triplex formation region. Each primer set consists of (i) two external primers (PE1 and PE2) to amplify fragment T or C, and (ii) one internal primer (PI). The internal primer was designed to containing identical sequence of PE1 at the 5' end, linked to a sequence complementary to a region within T or C at its 3' end. Competitors were obtained by successive PCR amplification of isolated genomic DNA first using primers PI and PE2, and then using the external primers PE1 and PE2. Therefore, the competitor DNA fragments were similar to fragment T or C, but lacking several base pairs at one extremity.
For quantification, a fixed amount of genomic DNA of interest is treated with TFOs modified with psoralen and then mixed with increasing amounts of competitor for each primer set (396). The two fragments in the same test tube were then co-amplified using one external primer set. The ratio between the two DNA fragments (competitor DNA fragment and T or C) remains unchanged during the amplification process based on the competitive PCR principle. Therefore, the amount of fragment RT or RC can be calculated. For the same genomic DNA sample treated with TFOs, amplification of RT will be inhibited and that of RC will not. The difference between the amounts of RT and RC is the amount of genomic DNA containing triplex-induced psoralen modification. This method was used to quantify triplex formation with isolated genomic DNA and showed similar results as DraI protection assay (396), even though slightly lower degree of triplex formation was determined. This may be explained by the fact that the PCR-based assay measures the amount of triplex-induced cross-links, whereas the DraI cleavage assay reflects the amount of cross-links and mono-adducts.
4.3.4.4. Linear amplified primer extension
Linear amplified primer extension relies on the radiolabeled primers and is also based on the principle that TFO directed psoralen modification of target DNA sequences can block DNA polymerases. With covalent TFO bound on the target DNA sequences, the primer extension will give truncated product compared to no modification of target DNA sequences (402). On a plasmid, Guieysse et al. studied the triplex formation when transfected into cells using this method (382). After triplex formation either in vitro or inside cells, the plasmid was digested downstream of the triplex formation region and then subjected to primer extension. The cross-link by psoralen may lead to underestimation of the expected truncated product, since it favors the renaturation of two strands of DNA during primer hybridization. This is confirmed by the fact that when under conditions favoring cross-link formation, such as irradiation at λ > 310 nm, 65% of truncated product formed and 85% for condition favoring monoadduct formation (irradiation at λ > 390) (382).
4.3.4.5. Single-strand ligation PCR
Oh et al. developed a single-stranded ligation PCR for quantifying triplex formation (402). In this method, a ligation ODN was ligated to PCR intermediate product to enable simultaneous amplification of the DNA fragments, which resulted from a prior primer extension for DNA samples with or without TFO-directed crosslinking by psoralen. Genomic DNA of interest was first digested downstream of triplex formation region to release a free end. The DNA was then amplified by primer extension using a 5'-end biotinylated primer to produce either a truncated product or a `full length' product. The biotinylated PCR products were captured on streptavidin-coated magnetic beads. An ODN was ligated to the 3' end of the product. This ligation product was subjected to exponential PCR using a primer partially nested relative to the biotinylated primer and a ligation primer complementary to ligation ODN. Finally, a primer extension with radiolabeled primer was performed for detection on PAGE. Fraction of triplex formation on genomic DNA in live cells was determined to be 15% (402).
4.3.4.6. Real-time PCR-based strategies
More recently, many studies have developed different real-time PCR-based methods for quantifying triplex formation, with focus on genomic DNA in native chromatin structures and in live cells. Real-time PCR can be more quantitative and time-saving. Similar to the idea in the competitive PCR, by comparing the PCR amplification of two regions, one overlapping the triplex formation region (T) and another outside the triplex formation region (C), the degree of triplex formation can be determined. Normally a standard curve (Ct versus amount of DNA of interest) is drawn for quantification (398). The level of inhibition in the PCR amplification directly reflected the level of triplex-induced adducts. The degree of triplex formation was 0% to 50% in permealized cells depending on the genes of interest and gene expression status (398). Besch et al. developed a real-time PCR-based method in combination with DNA capture using magnetic beads (405, 406). In this method, TFO was modified with psoralen at one end and biotin at the other end for capture. The idea is that only genomic DNA bound with biotin modified TFOs could be captured with streptavidin-coated magnetic beads. The captured genomic DNA is then subjected to real-time PCR and the number of captured DNA sequences was determined by comparing to standard curve. However, in live cells, less than 1% of triplex formation was detected (406). This degree of triplex formation was very low, which may be due to many factors including the target gene of interest, cell types and sequences of TFOs. We proposed a much simpler real-time PCR-based strategy for detection of triplex formation in HSCs cells as schematic illustration in Figure 8 (unpublished data). An explicit purification step is added into the procedure aimed at removing unreacted TFO molecules.
Figure 8. Strategy for detecting triplex formation with genomic DNA using real-time PCR.
Triplex formation can be detected using a real-time PCR based method. TFO is modified with psoralen at the 3' end for forming covalent attachment at triplex formation site. The triplex formation can be done with isolated genomic DNA or genomic DNA in isolated nuclei or live cells. After triplex formation reaction (i) and UVA irradiation at 366nm for 10 at ~3.8 mW/cm2 from 5 cm distance (ii), the DNA samples are subjected to a agarose gel electrophoresis (0.5%) and extracted for the gel (iii). The aliquots of extracted DNA samples are subjected to real-time PCR (iv). Two sets of primers are used: one set of primer for amplification of a control region and another for target region overlapping triplex forming site. The DNA with psoralen covalent modification is not substrate of PCR reaction. Inhibition of PCR reaction at the triplex formation site relates to the triplex formation.
5. SITE-SPECIFIC DELIVERY OF OLIGONUCLEOTICES
Barriers to systemic delivery of ODNs have been extensively described in many excellent reviews (415–417). These barriers include limited stability, poor cellular uptake, unfavorable subcellular trafficking, lack of nuclear targeting especially for antigene strategies, and non-specific tissue distribution (Figure 9). Synthetic carriers including polymers or lipids are attractive owing to the flexibility in design, ability to be chemically or biochemically functionalized and tunable toxicity properties. These properties of synthetic carriers in combination with increased understanding of pathophysiology in ODN delivery are important in turning ODNs into therapeutics.
Figure 9. Barriers to systemic delivery of ODNs.
For ODNs to reach target cells from the site of administration there are four major barriers to overcome: instability against nucleases, non-specific tissue distribution, poor cellular uptake, and uncontrolled subcellular trafficking. ODNs are polyanions and have a relatively small molecular weight with around 10kDa. They are subjected to quick urine excretion. After uptake by cells, they are inefficient to escape from endosome/lysosomes to cytosol. For antisense ODNs, they need to be in cytosol and bind to mRNA; while TFOs have to enter nucleus and bind to genomic DNA.
5.1. Cellular Uptake and Subcellular Trafficking
Cellular uptake of naked ODNs is an active endocytotic process via adsorption and varies in efficiency depending on the cell type, cellular conditions, concentration as well as chemistry and length of ODNs (418–421). The uptake mechanism appears to be at least partially concentration dependent and that below a concentration of 1 μM, the uptake of ODNs is predominantly via a receptor-like mechanism, while at higher concentrations a fluid-phase endocytosis mechanism appears to predominate. These processes lead to ODNs ending up endosome/lysosomes, with only small fraction diffusing into cytoplasm as well as nuclei. Degradation of ODNs has largely been observed, which results in insufficient concentrations for achieving biological effects. Nuclear translocation seems not be a rate-limiting step, since both microinjection and permeabilization lead to readily nuclear localization of ODNs (422–425). However, studies on the mechanisms involved are still needed, even though it has been suggested that nuclear entry can be accomplished by translocation across the nuclear pores (416). Regardless of these, subcellular distribution of PS-ODNs has been mostly studied in liver cells after parenteral adminstration in rats (426), since the subcellular location of ODNs are important for their function. Furthermore, the subcellular distribution can be modulated accordingly for the purpose of gene silencing by delivery strategies. It was found that in liver cells, including hepatocytes, KCs and SECs, cytosol associated with highest PS-ODNs (426). One interesting observation was that at low dose nuclear association was not seen in hepatocytes, while it could be observed with much high dose. Subcellular distribution of ODNs in hepatocytes is species dependent (426). Much higher nuclear association was observed in human hepatocytes compared to those of rats and mice.
Cho et al. (427) has reported the cellular uptake as well as subcellular distribution of a 29mer purine-rich TFO molecule in different cell types. In this study, TFO molecule was labeled with 111In. The total cellular uptake ranged from 20% to 55% of dose depending on the cell types at 24h incubation at 37 °C. In addition, cancer cell lines had higher TFO uptake than normal cell lines. For TFOs, they have to enter nuclei to bind to their target DNA sequences in the chromatin structures. Therefore, nuclear localization of the TFO was also determined. The wide variation in nuclear accumulation was also observed in different cell types ranging from 7% to 30% of the total cellular uptake. We have also studied the cellular uptake and subcellular distribution of a 25mer purine-rich TFO targeting to rat α1(I) collagen promoter (428). Around 12% of the dose was found to be associated with cells in HSC-T6 cell line, in which 30% was associated with nuclear structures. We further studied the intracellular distribution in vivo by isolating nuclear structures from freshly isolated liver cells after i.v. injection of radiolabeled TFOs, and found that 2–6% of total liver uptake was associated with nuclear structures (Figure 10C&D) (429).
Figure 10. Hepatic cellular localization and subcellular distribution of 33P-TFO after intravenous administration in rats at doses of 0.2 and 1 mg/kg.
Different liver cells were isolated at 30 min post-injection by liver perfusion with a mixture of 0.5 mg/ml collagenase and 0.1 mg/ml pronase and fractionation on Nycodenz gradient. Fibrotic rats were induced by dimethyl nitrosamine (DMN). The amount of the TFO in each cell type is given as ng/mg cell protein (A) and % of the total liver recovery (B). Subcellular distribution of 33P-TFO in the liver at 2 and 4 h after intravenous administration in rats at doses of 1mg/kg. Highly purified nuclei were isolated from cytoplasm and cell debris using the sucrose gradient separation method. The purity and number of isolated nuclei were determined under microscopy by dilution in trypan blue solution (C). TFO distributions in the nuclei, cytoplasm and cell debris were given as % of the total liver recovery (D). Data are presented as the mean ± SD (n = 4). Reproduced with permission from Cheng et al. (2005) Mol Pharm. 2(3):206–217.
Chemical modifications have been introduced with intention to increase the stability and binding affinity of ODNs and may also have effect on their cellular uptake. While PS-ODN has been shown to have similar uptake mechanism as native ODNs (430), methylphosphonate (MP) ODNs are uncharged molecules that enter cells via passive diffusion (417), although the rate of diffusion across the lipid bilayer membrane is extremely slow (431).
To achieve the efficient cellular uptake of ODNs for therapeutic effects, cationic polymers such as polyethyleneimine (PEI) have been used to enhance the cellular uptake and endosomal escape of ODNs (432, 433). By forming complexes with cationic polymers, the internalization usually follows clathrin-mediated endocytosis pathway, which means the deposition of complexes inside acidic endosome/lysosome vesicles; therefore enhancing endosomal escape is necessary for avoiding degradation of nucleic acids and achieving high biological effects. Non-cationic polymers with endosomal lysis ability have also been developed for improved intracellular delivery of ODNs (434).
To avoid entrapment in endosome/lysosomes, other endocytosis mechanisms (caveolae-mediated endocytosis or macropinocytosis) not involving formation of endosome/lysosomes or nonendocytic delivery have been exploited for nucleic acid delivery (433). A class of cationic peptides, the protein transduction domains (PTDs), such as the TAT, penetratin, and VP22 peptides, may have the ability to be taken up by cells without endocytosis events (435–439). Therefore, incorporation of the TAT peptide into delivery systems has been shown to enhance the transfection efficiency (440, 441), even though Kang et al. (442) did not achieve any improvements on existing carriers by incorporation of TAT peptide.
Even though the translocation of naked ODNs into nuclei is not a problem, it is needed to clarify whether the delivery system associated with ODNs can get entry into nuclei or ODNs need to dissociate from the delivery system prior to their nuclear translocation. Cationic lipids have been observed to be dissociated from ODNs and they were not associated with nuclear membranes (443–445), while PEI/ODN complexes could reach the nuclei where ODNs were dissociated (446, 447). However, there are reports suggesting the dissociation of ODNs from the complexes before nuclear entry of ODNs (432). In fact, unpacking of ODNs is required for their biological activities inside the cells. Synthetic carriers can be tuned to have appropriate balance in the packing and unpacking of nucleic acids (448, 449). Displacement of nucleic acids by intracellular polyanions is likely to result in their unpacking for cationic lipids or polymers (449). The reducing environment or acidic conditions inside endosome/lysosomes within the cells have been exploited to provide extracellular stability to the nucleic acids, while the carrier molecules should be eliminated from the cell after internalization (450–452). For example, degradable PEI has been synthesized using low molecular weight PEI (1.8kDa), which has much lower cytotoxicity and nucleic acid condensing capability compared to large molecular weight PEI (25kDa) (452).
5.2. Biodistribution
For systemic delivery, ODNs still need to overcome many other biological barriers including rapid elimination from the circulation, interactions with biological fluids and extracellular matrix, nonspecific accumulation in organs, and rapid degradation. Biodistribution of naked ODNs has been studied intensively in animals and humans, especially for PS-ODNs due to their relatively high stability compared to native phosphodiester-ODNs (PO-ODNs) (453–455). The pharmacokinetics of PS-ODN is generally sequence independent (453). Following intravenous administration, PS-ODNs are rapidly cleared from plasma with their distribution half-lives of about 30–80 min and much slower elimination half-lives from 5 to 75 h depending on the isotope used to label the ODN, which might actually be related to the slow clearance of radiolabel from the distributed tissue as well as the low-molecular-weight metabolites of ODNs (453). In addition, it has been shown that plasma clearance rates are largely species independent in rat, rabbit, dog and monkey. ODNs are widely distributed to most peripheral tissues, with liver and kidney, spleen and lymph nodes accumulating the most. The residence time of PS-ODNs in tissues has been shown to be relatively long around days (453).
PO-ODNs have been shown to have less than 20% of hepatic uptake, while PS-ODNs have 40% of hepatic uptake (456). In addition, PS-ODNs, due to the extensive binding to the plasma proteins, accumulate much less in the kidney compared to PO-ODNs. Takakura et al. (457) examined the hepatic disposition characteristics of 20 mer PO-ODNs and the partially (PS3-ODNs, in which three internucleotide linkage at the 3'-end are phosphorothioated) and fully phosphorothioated derivatives in the isolated rat liver perfusion system after bolus injection into the portal vein. The magnitude of the hepatic interaction of ODNs increased as the extent of PS modification in the molecules increased: About 20%, 36% and 52% of the injected dose was taken up by the liver during a single passage after bolus injection of PO, PS3 and PS, respectively. Urinary excretion is mainly dependent on the molecular weight of a compound, while its apparent charge affects both the hepatic uptake and renal excretion. Therefore, the disposition of ODNs at the whole body level can essentially be represented by hepatic uptake and urinary clearances. More favorable pharmacokinetic characteristics can be achieved using 2'-ribose alkoxy modification of PS-ODNs (453). This modification gives much longer elimination half-lives due to the improved stability of these derivatives.
Fibrosis results in the deposition of excess ECM proteins in the subendothelial space of Disse and loss of sinusoidal fenestrae. This, in turn, results in decrease free exchange flow between hepatocytes and sinusoidal blood. Furthermore, most biodistribution studies used PS-ODNs, which are not G-rich. PS-ODNs containing four or more consecutive guanine residues have tendency to form G-quartet structures and may show non-specific antiproliferative activity due to their binding the cellular nucleolin. Our studies have shown that in the DMN-induced liver fibrotic rats, the hepatic accumulation of a G-rich TFO decreased from 43% to 33% at 30 min and 38% to 27% at 4 h post-injection (429).
Suborgan distribution of ODNs focus on liver tissue in most studies, since liver is the major tissue to take up ODNs after intravenous adminstration. In the liver, most of ODNs could be detected in nonparenchymal cells (NPCs) including KCs and SECs; lower levels were localized in the cytoplasm of hepatocytes (458, 459). Graham et al. (460) reported that almost 80% of injected PS-ODNs were taken up by KCs and SECs equivalently using nonlabeling method, capillary gel electrophoresis (CGE). The remaining 20% of the dose was taken up by the hepatocytes. On the other hand, Bijsterbosch et al. (461) studied suborgan distribution of a parenterally administered 3H labeled PS-ODN specific for murine ICAM-1. They found PS-ODNs to be taken up by SECs and hepatocytes, with 56.1% and 39.6%, respectively, while KCs accumulated only 4.3% of the hepatic uptake. However, the concern with using radiolabeled ODNs in suborgan fractionation is that the redistribution or contamination often occurs during tissue homogenization (462). Furthermore, suggestions that PS-ODNs are taken up in liver principally by scavenger receptors on endothelial cells and that these compounds are not localized to KCs, are controversial since in a scavenger receptor knockout mice, PS-ODNs gave a similar distribution as to wild-type mice (463). In addition, in all these studies, the authors did not isolate HSCs and thus the reported uptake of PS-ODNs by KCs and SECs may be overestimated. We first showed that in the liver HSCs, KCs and SECs showed the highest uptake accounting for approximate 65–70% of the liver uptake (Figure 10B). At 1 mg/kg dose, the TFO concentration at different cells was in the following order: 1342, 1433 and 100 ng/mg of cell protein for HSCs, KCs & SECs, and hepatocytes, respectively (Figure 910) (429). Again, the scavenger receptors were also suggested to be involved in the cellular uptake of ODNs in liver.
5.3. Bioconjugation
The in vivo use of cationic polymers and cationic liposomes has many problems. The electrostatic nature of the self-assembly between cationic vectors and anionic nucleic acids has the potential to be unstable in an environment with high ionic strength, such as blood, leading to rapid aggregation. Furthermore, these charged complexes may fail to reach their target cells intact, owing to nonspecific interactions with serum proteins, ECM proteins and cell surface proteins. The reticuloendothelial system (RES) of organs, such as liver, spleen, kidney and lungs represent most likely effective clearance sites, following systemic administration of ODN complexes. Accordingly, directing ODNs to non-RES organs still remains a challenge. One example comes from a study of the disposition characteristics of glycosylated poly(L-lysine) (Gal-PLL)/ODN complexes targeting hepatocytes in mice in relation to their physicochemical properties (456). Although the uptake of Gal-PLL/PS-ODN complexes by hepatocytes was significantly higher than that of naked PS-ODNs, the specificity was not completely achieved. Due to the negative zeta potential (−30~−40 mV) and wide particle size distribution (150±70 nm), a part of the complexes is likely to be recognized by KCs and by SECs via scavenger receptors. Thus, formulations are typically prepared with a minimum of net charge and are modified with hydrophilic, neutral polymers (PEG) (464–466), N-(2-hydroxypropyl)methacrylamine polymer (pHPMA) (467, 468)) that help to shield the charges and confer long circulation time in blood.
Delivery of ODNs relying on bioconjugation rather than complex formation provides an attractive strategy to avoid some problems associated with complexes. Bioconjugates are smaller in size compared to the particulate systems (such as liposomes, polyplexes and nanoparticles). Even though relatively low loading capacity may be one disadvantage compared to particulate systems, this will not be a problem for high potent nucleic acid-based therapeutics. However, one of the advantages is that bioconjugates can easily leave the systemic circulation. Consequently, they can be used to target drugs to cells that are not in direct contact with the blood, including hepatocytes and HSCs. Construction of a delivery system with multifunctional components greatly depends on the bioconjugate chemistry. Modification of existing carrier molecules as well as direct conjugation on ODNs has been developed. The most common strategy for active targeting of delivery systems is to exploit a unique cell surface receptor to achieve receptor-mediated endocytosis. A ligand (a small molecule, protein or antibody) is incorporated within the delivery system. For liver targeting, asialoglycoprotein, galactose, mannose, and many other carbohydrate derivatives are extensively used ligands. To achieve efficient cellular uptake and subcellular distribution, peptides including protein transduction domains (PTDs), fusogenic peptides and nuclear targeting signal have also been conjugated with either carrier or ODN itself. PEGylation of ODNs can elongate their blood circulation time. Further modification of PEGylated ODNs with targeting ligands presents a targeted delivery strategy. In addition, in most cases, conjugation on ODNs confers some stability against nucleases. Polymers such as synthetic polymers, antibodies, and serum proteins may present an ideal platform for construction of multicomponent carriers for delivery of ODNs.
5.3.1. Conjugation with Lipophilic Molecules
The hydrophilic character and anionic backbone of the ODNs reduces their uptake by cells as well as pharmacokinetics after systemic delivery. Therefore, various lipophilic molecules have been conjugated to ODNs to confer them more `drug-like' properties (469). Many lipophilic molecules have been used to modify ODNs (470, 471). However, among them, cholesterol is perhaps the best characterized and extensively studied in vivo (472–474). Unlike other lipophilic molecules, it has been shown that cholesteryl conjugation confers ODNs enhanced binding to lipoproteins and thereby improved cellular association and transport especially into liver tissue. Therefore, liver targeting is the interest for most of studies involving cholesteryl conjugated ODNs.
Cholesterol can be conjugated to ODNs at 3'- or 5'-end. Conjugation with cholesterol can be integrated into the synthesis of ODNs using nucleoside derivatives (472, 475, 476). However, cholesterol can also be conjugated to post-synthetic ODNs with functionalities at ends. Alefelder et al. (477) reported modification of postsynthetic PS-ODNs with either α-(bromoacetamido)-3-cholesterol or 2-(5'-nitropyridyl)-3-cholesterol disulfide. We conjugated thiocholesterol to TFOs containing sulfhydryl group at 3'-end via activation by bis-(5-nitro-2-pyridyl)-disulfide (Figure 11A) (428). The disulfide bond formation between cholesterol and TFO has advantage over other non-cleavable bonds in releasing TFO molecules within target cells.
Figure 11.
(A) Synthesis of TFO-Chol using thiocholesterol and bis-(5-nitro-2-pyridyl)-disulphide and bioconjugation via disulfide bond formation Reagent and conditions: (1) 5 ml of pyridine, stirring at room temperature for 2 h; and 2, 1500 μL of dimethylformamide (DMF), stirring under N2 protection at 40°C for 24 h. (B) Biodistribution of TFO-Chol after systematic administration of a mixture of 33P-TFO-Chol and TFO-Chol or a mixture of 33P-TFO and TFO at a dose of 0.2 mg/kg. At 30 min and 4 h after injection, blood was collected by cardiac puncture, and urine was collected from the bladder. The rats were sacrificed, tissues were collected, washed, and weighted, 150 mg of tissue was digested, and radioactivity was determined using a scintillation counter. Data are represented as the mean ± S.D. (n = 4). Reproduced with permission from Cheng et al. (2006) J Pharmacol Exp Ther. 317(2):797–805.
Cholesterol conjugation did not affect the binding affinity of ODNs to their targets (469). Under certain conditions, cholesterol conjugation to ODNs could even increase the binding affinity to mRNA targets as well as forming more stable triplex structures, possibly through intercholesteryl hydrophobic interaction (478). In the mean time, the sequence specificity is also increased. The stability against nuclease attack is also increased by 3'-cholesteryl conjugation, but not by 5'-end conjugation (470). This is because the 3'-hydroxyl group is involved in the nucleophilic attack of the adjacent phosphate bond by the exonuclease enzyme.
Cellular uptake of a 3'-cholesterol-conjugated ODN was studied using a real-time confocal laser microscopy (479). It was observed that cytosolic uptake of cholesterol conjugate was increased 5-fold of that of PS-ODNs and 2-fold in nuclear uptake. Positions of conjugation on cholesterol (3, 7, or 22 positions of cholesterol attached to 3'-end of ODNs) have effects on the cellular uptake due to different hydrophobicity of the conjugates (480). It was found that cholesterol-derivatized ODNs at position 3 or 7 gave more favorable cellular binding and subcellular distribution. Furthermore, it is suggested that the interaction with specific proteins on cell surfaces contribute to the enhanced entry of cholesterol conjugates into cells (470).
The increased stability and enhanced cellular uptake result in the increased bioactivity of cholesterol conjugate. A PS-ODN specific for the 3' untranslated region of ICAM-1 mRNA was incorporated with a cholesterol at the 5'-end and shown to have increased antisense effect in cell culture (476). By conjugation with cholesterol at the 5'-end, a PS-ODN against mRNA multiple drug resistance-1 (MDR-1) gene has also shown increased silencing effects in vitro (481). Furthermore, it was found that conjugating cholesterol to both ends of a PS-ODN could confer more activity as compared to only one-end modification (482).
Cholesterol conjugation to PS-ODNs resulted in increased retention in plasma as well as their accumulation in the liver (470, 483). When a 3'-cholesteryl-conjugated PS-ODN was administrated into rats intravenously, 63.7% of the dose was found in the liver, which was almost 2-fold higher compared to naked PS-ODNs (484). The plasma half life was 49.9 min, which is at the level of naked PS-ODNs. SECs, KCs and hepatocytes accounted for 45.7%, 33.0% and 21.3% of the liver uptake, respectively. A 3',5'-bis-cholesteryl-conjugated ODN with two cholesteryl moieties increased hepatic uptake to 83% (485). The hepatic uptake of occurred mainly by SECs (51.9% of the liver uptake). Hepatocytes and KCs were responsible for 24.9% and 23.3%, respectively. The increased retention in plasma is possibly due to the increased serum protein binding (486). However, it is not clear whether the increased hepatic uptake is due to an active transport of the lipophilic conjugates or whether the effects were simply due to the changes in lipophilicity. Nonetheless, this prompts using of cholesterol-conjugated ODNs for treating liver related diseases. The therapeutic effect of 5'-cholesterol-conjugated PS-ODNs against ICAM-1 mRNA in mouse liver in vivo was significantly increased over unmodified PS-ODNs because of the increased hepatic uptake (470).
We have also conjugated cholesterol to a TFO molecule against α1(I) collagen gene promoter at 3' end via disulfide bond formation (428). Around 4-fold increase in the cellular uptake and enhanced bioactivity in inhibition of α1(I) collagen gene transcription were observed in HSC-T6 cell culture. After i.v. injection into rats, significant increase in hepatic uptake both in normal rats and fibrotic rats were achieved (Figure 11B). However, no significant change in the suborgan distribution in comparison with free TFOs was seen after separation of different liver cells (35%, 49% and 16% for hepatocytes, HSCs and KCs & SECs, respectively).
5.3.2. PEGylation of Oligonucleotides
PEG plays important roles in the pharmacokinetic profiles of small molecules as well as macromolecules (487). PEG is nontoxic and very soluble, reduces immunogenic reactions. By direct conjugation of PEG to ODNs, many advantages have been implicated: longer half-life of ODNs, higher stability against exonucleases and increased cellular uptake. In some cases, the solubility of ODNs can be increased, especially for some chemically modified ODNs. Furthermore, short PEG conjugation on ODNs is used as a spacer for additional conjugation to happen, since PEG itself can be made bifunctional. The higher molecular weight of PEG conjugates guarantees the longer circulation time by decreasing renal clearance. For PEG, the high water coordination of the polymer increases its hydrodynamic volume up to 3–5 times that of a globular protein having the same molecular weight and a glomerular filtration threshold is around 30–45 kDa.
PEG can be introduced to an ODN at the 3' end or 5' end or internal positions in the solid-phase synthesis of ODNs (488–490). However, these synthesis strategies do not allow easy upscaling. Subsequently, the use of the liquid-phase approach utilizing the PEG polymer as support became possible (491, 492) and has been used for the large scale synthesis of PEGylated ODNs. PEG is used as a soluble, inert synthetic support, onto which nucleosides are added sequentially using phosphoramidite-based procedure. Normally, PEG is monomethoxy at one end (mPEG). The hydroxyl group at the distal end of mPEG is functionalized with the first nucleoside using similar chemistry for elongation of ODN chain, which results in the formation of a phosphate bond between PEG and ODN. PEG with a molecular mass between 5 and 20 kDa has been successfully incorporated with ODNs of up to 20 monomers. Normally, a phosphate bond between PEG and ODN is formed. However, different chemistries in the linker between ODN and PEG can also been introduced (493–495), which may confer better properties to the conjugates. PEG can be introduced after the synthesis of ODNs, which contain reactive groups, such as amines or thiols. For example, ODNs can be easily introduced with a 5' or 3' aminohexyl functionality. The formation of carbamate between ODN and PEG enables the release ability of ODN (494).
PEGylation of ODNs can increase their stability. PEG coupling to both the 3' and 5' terminal positions of an antisense ODN produced more than 10-fold increase in exonuclease stability, while having no effect on the ability of hybridization (488). Furthermore, the cellular uptake of ODNs can also benefit from PEGylation (496). This is possibly due to the introduction of hydrophobicity and shielding of negative charges of ODNs by PEG. Rapozzi et al. demonstrated that PEGylation (9 kDa) of a 13-mer (A, G) motif TFO targeting the 5' flanking region of the human bcr/abl oncogene could enhance cellular uptake (497). The triplex formation ability of the TFO was not affected in vitro. The stability was increased while PEGylated TFO less inclined to self-associate into multistrand structures. Therefore, the PEGylated TFO showed enhanced downregulation of the transcription of bcr/abl mRNA. In contrast, Manoharan et al. demonstrated that conjugation of ODNs against human ICAM-1 to a series of PEG esters of average molecular weight 550, 2200, and 5000 interfered with the cellular permeation of ODNs in vitro (470).
Different structures of PEG conjugated can confer different biological properties of PEGylated ODNs. Bonora et al. studied two different conjugates of an anti-HIV 12-mer ODN with a linear PEG and a branched PEG (498). It was found that only the conjugate with linear PEG showed anti-HIV activity. Furthermore, the number and attachment sites (at the 5', 3' or both 5' and 3' termini) of the conjugated PEG greatly influence the hydrophobicity of the conjugates (488).
Prolonged plasma retention is one of the major advantages of PEGylated ODNs compared to naked ODNs in vivo. Watson et al. (499) studied the pharmacokinetic parameters and in vivo activity of a PEGylated (40kDa) 2'F pyrimidine 2'OH purine RNA anti-human L-selectin aptamer. They found that the pharmacokinetic parameters were significantly improved by PEGylation. However, further improvement of in vivo activity is still needed. An aptamer directed against TGF-β2 was also PEGylated (20 kDa and 40 kDa) (500). Following single bilateral subconjunctival administration to Dutch-belted rabbits, the PEGylated aptamer showed prolonged residence in the subconjunctival space and aqueous compartment (tmax = 6 and 12 h, respectively, in plasma) compared to naked ones. These properties warranted the further pharmacological evaluation of these conjugates.
An 18-mer PS-ODN targeting the initiation codon region of the anti-apoptotic gene Bcl-2 mRNA was conjugated to a PEG (20 kDa) at 5' end via releasable linkers (501). ODNs could be continuously released from the conjugate. Surprisingly, the researchers found that in 518A2 melanoma cells in vitro, PEGylation resulted in greatly diminished cellular uptake and therefore, bioactivity in cell cultures. In contrast, they demonstrated the pharmacokinetic profile of the conjugate was significantly improved (e.g., the dramatically increased AUC). However, only similar anti-tumor effects were observed for the conjugate and naked ODNs. In addition, this anti-tumor activity is observed accompanied with minimal immunostimulation, which may be due to the shielding effects of PEG for CpG motifs embedded in the ODN sequence.
However, in most cases, PEGylated ODNs themselves are used in combination with other delivery carriers, which further enhances the bioactivity. They can form micellar structures by forming complexes with cationic polymers such as PEI (502). The ODN/PEI polyelectrolyte complex forms an inner core while PEG chains surround it as a shell. Different linkage (glutathione-sensitive SS linkage, pH-sensitive linkage and thiomaleimide linkage) in the conjugates also substantially affected biological effects of the micelles (451, 503). The use of PEGylated ODNs in combination with lipids could significantly improve the stability of complexes against serum (504). Furthermore, different targeting ligands such as folic acids can be attached at the distal terminus of PEG to have receptor-mediated targeted delivery of ODNs (503, 505, 506).
5.3.3. Carbohydrate Conjugates for Targeted Delivery
Delivery of ODNs to tissues does not mean that ODNs have been localized to target cells within tissues (453). ODNs can also be found at extracellular spaces. Therefore, to have efficient cellular uptake is one of the objectives for delivery of ODNs. Furthermore, specifically for liver tissue, ODNs nonspecifically distribute more to NPC compared to hepatocytes. Therefore, targeted delivery of ODNs to hepatocytes becomes the focus of most studies using direct carbohydrate conjugates with ODNs.
Carbohydrate conjugates, termed `glycotargeting', provide novel strategies for targeted delivery, since different liver cells may express specific receptors recognizing different carbohydrate clusters. Glycotargeting exploits the highly specific interactions of endogenous lectins with carbohydrates (often with multiple carbohydrates). In particular, small molecule drugs including ODNs, no matter how heavily glycosylated will always have the potential to pass into the kidneys, through glomerular filtration, and be rapidly cleared. Consequently, carbohydrate in combination with PEGylation provides an attractive alternative option.
The ASGPR is expressed in liver by hepatocytes, which recognizes specifically glycoproteins that possess exposed terminal galactose or lactose residues (507, 508). In the case of lactose, the glucose moiety functions as a tether and the galactose serves as the ligand. The ligands are internalized via receptor-mediated endocytosis (509) and transferred to lysosomes (510). Therefore, galactose and its derivatives have been exploited for targeted delivery of ODNs by direct conjugation with them.
Cluster of galactose or its derivatives is believed to have more binding affinity to ASGPR than monovalent galacosides, which led to the term `cluster effect' (511, 512). The binding strength of a cluster ligand depended mainly on two factors: (1) the maximum spatial inter-galactose distances and (2) the flexibility of the arm connecting galactosyl residues and the branch points (513, 514). A glycotripeptide, N-acetylgalactosamine neoglycopeptide, Tyr-Glu-Glu-(aminohexyl GalNAc)3, YEE(ahGalNAc)3, was shown to bind to Gal/GalNAc receptor on hepatocytes with Kd of subnanomoles (515). Hangeland et al. (516) conjugated a 7-mer MP-ODN with YEE(ahGalNAc)3 via a metabolically stable thioether in HepG2 cells. The cellular uptake was ligand-specific and was increased 20- to 40-fold. However, the bioactivity was not tested. Subsequent studies of this glycoconjugate in mice also showed that hepatic uptake of this conjugate was 70% of the injected dose (517). In another study, when a PS-ODN was conjugated to this ligand, >90% inhibition of the integrated HBV via expression in hepatoma cells could be achieved (518).
A tetra-antennary cluster galactoside is N2-(N2-(N2,N6-bis[N-[p-(β-D-galactopyranosyloxy)-anilino]thiocarbamyl]-L-lysyl)-N6-(N-[p-(β-D-galactopyranosyloxy)-anilino]thiocarbamyl]-L-lysyl)-N6-(N-[p-(β-D-galactopyranosyloxy)-anilino]thiocarbamyl)]-L-lysine (L3G4) with Kd of 6.5 nM (519, 520). When attached to a 20-mer PO-ODN with a 3'-capped amine (L3G4-ODN), it was shown that in rats hepatic uptake was greatly enhanced from 19% for unconjugated ODN to 77% of the injected does for L3G4-ODN. Furthermore, the uptake of L3G4-ODN could be mainly contributed by hepatocytes (75% of the total liver uptake).
Maier et al. (521) designed and synthesized another cluster galactoside based on structural features of known high affinity ligands for the ASGPR. It involves three β-aminogalactosyl residues attached to a rigid cholane scaffold via ε-aminocapramide linkers. The rigid steroid scaffold conferred more affinity upon binding to the receptor via a proper preorganization of the galactose residues. This ligand has been conjugated with different ODNs. However, no biological testing is available.
5.3.4. Conjugation of Peptides
Peptide conjugates of ODNs can be used to change pharmacokinetics as well as enhance cellular uptake and nuclear targeting. The hybridization properties of ODNs may also be improved in some cases. In contrast to the use of complexes, such covalent conjugates of peptides and ODN are discrete chemical entities of known stoichiometry. The efficiency of such peptides is generally high and, hence, very low concentrations of the peptides are needed to observe any biological effects.
5.3.4.1. Conjugation with Cell Penetrating Peptides
Cell penetrating peptides (CPPs), also called PTDs are small motifs in proteins having been shown to carry nucleic acids such as ODNs, siRNAs, and even plasmid DNA across cell membranes (522–524). Representatives of these peptides are the TAT protein from HIV-1 virus (525), Drosophila homeotic Antennapedia protein (Antp, marketed as penetratin) (526), and the herpes simplex virus type-1 (HSV-1) VP-22 transcription factor (527). The domains responsible for the transduction have been identified for these CPPs and the commonality between each PTD is the presence of basic amino acids (arginine and lysine), which might be important for penetration of the membrane (524). Other peptides with similar properties include transportan (528) and MPG (529) and have also been developed for delivery of ODNs. One of the advantages of CPPs is that they are taken up by cells efficiently via caveolae-mediated endocytosis as well as macropinocytosis, which do not lead to lysosomal degradation of cargos, or nonendocytic pathways (433), even though the mechanisms are still controversial and may depend on cell type, conjugated cargo and other factors (530). Therefore, these peptides provide an efficient strategy for enhancing cellular uptake of ODNs and improving their intracellular delivery to cytosol as well. In most cases, the covalent linkage between CPPs and ODNs is via disulfide bond formation, which facilitates the release of cargoes inside cells.
The most extensive applied CPPs for delivery of ONDs are TAT and Antp peptides. The minimal TAT transduction domain is the basic residues 47–57 (435) and the third alpha helix (residues 43–58) of the Antp homeodomain are required for transduction (526). Astriab-Fisher et al. (531) used TAT and Antp conjugated to a PS-ODN targeting a site flanking the AUG of mRNA of the MDR1 gene, which encodes P-glycoprotein. Both peptides were able to efficiently deliver the ODN to cells in culture. Effective inhibition of P-glycoprotein expression was attained with sumicromolar concentrations of the conjugates. One striking feature of the peptide–ODN conjugates is that they inhibited P-glycoprotein expression more effectively in the presence of serum than in the absence of serum. Another study from the same group also showed the successful transportation of 2'-O-methyl PS-ODNs conjugated to TAT and Antp peptides to the cytoplasm as well as nucleus, leading to an increased pharmacological activity without compromising the specificity of antisense ODNs (531). In addition, a retro-inverso form of Ant was a peptidomimetic modification to confer more stability but still retain its activity and can rapidly be internalized and distribute throughout the cytoplasm and even the cell nucleus (532). When a PNA specific for the AUG translation inhibition region of prepro-oxytocin mRNA was coupled to this retro-inverso peptide, the conjugate could be quickly taken up by cultured cerebral cortex neurons (533). PNA was also able to enter the cells, but more slowly. However, in this study, both PNA-peptide and PNA itself gave similar effects on gene inhibition.
Other peptides may also have similar properties. A chimeric peptide MPG consisting of a hydrophobic fusion domain derived from HIV gp41 and a hydrophilic nuclear localization signal derived from the SV40 T-antigen was constructed to have membrane translocation property (529, 534). This peptide was conjugated to a PS-ODN via a disulfide bond and greatly increased the cellular uptake of the ODN (534). Moulton et al. (535) studied the effect of conjugation of arginine-rich peptides to PMO. Different factors including the number of arginines in the peptide, the choice of linker, the peptide conjugation position, the length of the PMO, and the cell culture conditions, were investigated to optimize the efficiency of peptide-PMOs. PMO was used to correct missplicing of exogenous luciferase pre-mRNA. A peptide containing 9 arginines was found to be best and even more efficient than Tat and Antp. Following study confirmed the effectiveness of the PMO conjugated with one arginine-rich peptide, (RXR)4XB, where R = arginine, X = 6-aminohexanoic acid and B = beta-alanine in correcting the endogenous gene mutation in vitro (536) as well as in human muscle explants (537).
Only few reports demonstrated the in vivo use of these peptide carriers for ODNs. A 21-mer PNA specific for the human type 1 galanin receptor was conjugated to cell permeation peptides via disulfide bonds and the bioactivity in vivo was demonstrated in rats (538). The peptides used in this study were transportan and Antp. The conjugates improved internalization and down-regulation of the human galanin receptor in vitro in Bowes cell line. After intrathecal administration of the conjugates, the galanin binding was decreased in the dorsal horn.
The stability of CPP-ODN conjugate is one concern for their in vitro and in vivo applications. A recent study investigated the effects of several structural features of arginine-rich CPPs on the metabolic stability of CPP conjugated to phosphorodiamidate morpholino oligonucleotides (PMOs) in human serum and in cells (539). Those structural features include amino acid configurations (d or l), incorporation of non-alpha-amino acids, peptide sequences, and types of linkages between CPPs and PMOs. It was found that d-configuration CPPs were completely stable, while l-CPPs were degraded in both serum and HeLa cells. Insertions of 6-aminohexanoic acid residues (X) into an R8 peptide failed to increase intracellular stability, while insertions of β-alanines (B) increased intracellular stability. An amide or a maleimide linkage was stable in both serum and cells; however, an unhindered disulfide linkage was not stable in either, which is contrary to other studies.
Furthermore, there are several concerns for covalent attachment of CPPs with ODNs. It is known that the dense cationic charge of CPPs is critical for their interaction with negative charged phospholipids and proteoglycans on the cell surface prior to the induction of cell internalization (540). Upon linkage of CPPs with 6–8 positive charges to ODNs with ~20 negative charges, the CPPs are almost neutralized and lose the ability to bind the cell surface (541). Furthermore, the high tendency of cationic peptides to condense ODNs makes covalent attachment of CPPs to ODNs difficult. In fact, CPP mediated delivery of ODNs has been unsuccessful in several instances (542–544), which suggests that further understanding of the mechanisms of the cellular uptake of CPPs and careful design of CPP-ODN conjugates are needed. Using uncharged ODNs such as PMO-ODN and PNA may avoid this problem.
5.3.4.2. Fusogenic Peptide Conjugation
Peptides derived from the N-terminal sequence of the influenza virus hemagglutinin subunit HA-2, or prepared synthetically, such as GALA or KALA are pH-sensitive fusogenic, which can use the acidic pH of the endosome to induce their rupture (545, 546). These peptides change conformation at acidic pH and destabilize the endosomal membrane resulting in an increased cytoplasmic delivery of ODNs. Bongartz et al. (545) conjugated a peptide derived from HA-2 to an antisense PO-ODN targeting the AUG initiation site of the HIV TAT protein via a disulfide or thioether bond and observed as 5 to 10 fold improvement of the anti HIV activities, respectively. However, no sequence specificity was obtained and the fusogenic peptide possessed some antiviral activities on its own (IC50: 6 μM). Very few following work exists regarding fusogenic peptide conjugated ODNs. Shadidi et al. (547) explored the potential of the cancer cell binding peptide LTVSPWY to specifically deliver antisense ODN. The PS-ODN against the ErbB2 receptor was conjugated to the peptide via a disulfide bond. The conjugate increased the internalization of PS-ODN by cancer cell line SKBR3 and resulted in ~60% inhibition of ErbB2, which was probably due to the fusogenic property of the LTVSPWY peptide.
5.3.4.3. Conjugation with Nuclear Targeting Peptides
Chaloin et al. (534) designed a serials of peptides with a nuclear localization signal (PKKKRKV) fused with different peptides containing a hydrophobic motif. A PS-ODN was conjugated to these peptides through a disulfide bridge. The cellular up was found to be an efficient, with a little effect of temperature on internalization, suggesting the involvement of non-endocytic pathway in the cellular uptake. Another signal peptide KEDL was shown to carry ODNs to the endoplasmic reticulum and from there to the cytosol and the nucleus where their targets are located (548). When a 5', 3'-modified pentacosanucleotide, complementary to the translation initiation region of the gag mRNA of HIV was coupled to a bromoacetyldodecapeptide containing a KDEL signal sequence, the anti-HIV activity was improved. Furthermore, it was also found that a thioether bridge for the peptide conjugate had a higher activity over that with a disulfide bond.
5.3.5. Polymer-based Conjugates
Using polymer-based conjugates, it becomes possible to present viral mimetic properties to the carriers for ODNs such that several functionalities can be integrated into a multi-component carrier with many objectives: i) targeting by receptor-mediated binding to surfaces of recipient cells and endosomal uptake into these cells; ii) provision of an activity for disruption of endosomal membranes and for the release of the drug carrier system into the cytoplasm; iii) a cleavable linkage for the release of the drug from the carrier system; iv) a prolonged circulation through protection by PEG against degradation activities; and v) attachment of a fluorescent reporter for the localization of the carrier system. For a polymeric carrier to be efficient in delivering ODNs to the target cells, it has to overcome many extracellular and intracellular barriers as mentioned above. Although polymeric carriers have witnessed great advancements, a polymeric carrier, that is capable of addressing all these challenges, is still yet to be achieved. Furthermore, regarding so many barriers in ODN delivery, different design elements in developing polymeric carriers may affect different barriers to varying extents, such that a polymeric carrier that is rationally designed to overcome certain barriers may alter the cellular processing of the carrier, dramatically increasing the importance of other barriers.
5.3.5.1. Hydroxypropyl methacrylate (HPMA)-based polymers
HPMA-based polymers have been extensively used as carriers for drug delivery including delivery of ODNs (549). HPMA homopolymer is biologically inert and exhibits no antibody response after intraperitoneal injection into mice (550). They can be easily modified to incorporate various functionalities by forming different HPMA copolymers and these copolymers may present some immunogenecity (550). Normally, HPMA polymers of molecular weight less than 50kDa are used to permit glomerular filtration and eventual excretion. Targeting to a specific cell population by attaching moieties such as carbohydrates or antibodies can be achieved. However, even though HPMA-based polymers have been studied in vivo for delivery of anticancer drugs, few studies on the delivery of ODNs using HPMA polymers are reported.
HPMA-based polymer-drug conjugates normally are lysosomotrophic (551), leading to possible degradation of drugs, especially for nucleic acids. Jensen et al. (552) conjugated an antisense PS-ODN to HPMA copolymer via lysosomal cleavable glycylphenylalanylleucylglycine (GFLG) peptide linker and studied the subcellular fate of the conjugate in HepG2 cells (Figure 12A). Two different conjugates were synthesized. One is PS-ODN attached to the HPMA polymer via non-cleavable spacer, glycylglycine (GG), and another is PS-ODN attached to the HPMA polymer via GFLG peptide linker. In the latter conjugate, the HPMA polymer and the PS-ODN were labeled with different fluorescence dyes for separate tracking under confocal microscopy. With the non-cleavable spacer, the conjugate was entrapped in the vesicular compartments. In contrast, the conjugate with cleavable linker showed separation of two different colors after 2~4 h incubation, which suggested the release of PS-ODN from HPMA polymer, and significant amount of PS-ODN distributed into cytoplasm and nuclei compared to free PS-ODN. Furthermore, the biological activity of the PS-ODN was increased compared to free PS-ODN.
Figure 12. Synthetic polymer-based multicomponent carriers for delivery of ODNs.
(A) Hydroxypropyl methacrylate (HPMA)-based copolymers. A phosphorothioate oligonucleotides (PS-ODN) was covalently linked to the HPMA copolymer via the degradable GFLG linker. The ODNs was labeled with fluorescein on the 3' end. The polymer backbone was labeled with Lissamine rhodamine B to allow independent visualization of the polymer and the ODN. Reproduced with permission from Jensen et al. (2002) Bioconjug Chem., 13(5), 975–984. (B) Polycefin, poly(β-L-malic acid) based copolymer. Percent values refer to the number of malyl moieties of PMLA that are conjugated with a given module (100%) total malyl content). The distribution of conjugates along the scaffold is assumed to be random. Abbreviations are: PMLA, poly(β-L-malic acid); PMLA-NHS, N-hydroxysuccinimidyl ester at pending carboxyl groups of PMLA; mPEG5000, methoxy poly(ethylene glycol) (5kDa); 2-MEA, 2-mercaptoethylamine; mAb OX-26, mouse monoclonal antibody to rat transferrin receptor; maleimide-PEG3400-maleimide, a bifunctional maleimide derivative of PEG (3400 Da); MORPH-AON-1, morpholino antisense oligonucleotide to laminin α4 chain; MORPH-AON-2, morpholino antisense oligonucleotide to laminin β1 chain. Reproduced with permission from Lee et al. (2006) Bioconjug Chem., 17(2), 317–326.
The versatility of HPMA polymers can be further demonstrated in another study. Wang et al. (553) synthesized a HPMA copolymer containing an activated sulfide group, onto which a PS-ODN modified with sulfhydryl group and/or Antp peptide, were attached via disulfide bond formation. Antp peptide was also included to increase cellular uptake (173). The successful synthesis of the conjugates including HPMA polymer-Antp, HPMA polymer-PS-ODN, and HPMA polymer-Antp-PS-ODN was demonstrated. However, the conjugate was found to be no improvement for cellular uptake of PS-ODN, which may be because the peptide formed an intermolecular electrolyte complex with ODNs leading to its incapability of translocating through the membranes.
5.3.5.2. Poly(L-malic acid)
Lee et al developed poly(β-L-malic acid) (PLMA) based copolymer, Polycefin, as a platform for drug delivery (Figure 12B) (214). PLMA can be prepared from the myxomycete Physarum polycephalum with high purity (554). PLMA resembles HPMA polymers in carrying abundant carboxyl groups. However, unlike HPMA polymers, it is biodegradable since it is a polyester. In addition, it is highly water-soluble. These properties make PLMAs ideal drug carriers.
Polycefin was synthesized as a multicomponent prototype for targeted delivery of ODNs to brain tumors (214), which contained: i) biodegradable, nonimmunogenic, nontoxic PLMA as backbone; ii) morpholino ODN targeting α4 and β1 chains of laminin-8, which is specifically overexpressed in glial brain tumors via disulfide bonds; iii) monoclonal anti-transferrin receptor antibody for specific tissue targeting; iv) L-valine containing, pH-sensitive membrane disrupting unit(s), v) PEG. Polycefin was extensively characterized (214). The different functionalities were statistically distributed over PLMA backbone. On the basis of a Mw of 50kDa (approximately 500 malyl residues), Polycefin molecules contain on average 1 molecule of the monoclonal antibody (mAb), 11 molecules of either morpholino ODN, 22 molecules of mPEG (5kDa), 210–220 molecules of L-valine (membrane disrupting module), and 140–150 pending carboxylate residues. The composition accounted for a molecular mass of approximately 550kDa.
Polycefin was shown to be taken up by two human glioma cell lines U87MG and T98G cells via a receptor mediated endocytosis and was able to inhibit the synthesis of α4 and β1 chains of laminin-8. After i.v. inject into mice, the accumulation of Polycefin in brain tumor tissue was via the antibody-targeted transferrin receptor-mediated pathway along with a less efficient mechanism of enhanced permeation and retention (EPR). Furthermore, Polycefin was shown to be nontoxic. The accumulation of Polycefin to other major tissues such as liver and kidney was to much less extent (555). And, it was preferably accumulated into xenografted human MDA-MB 468 breast tumor in mice after 60min (555).
5.3.5.3. Proteins as carriers
Besides as carriers, proteins can be served as ligands, such as glycoproteins and antibodies of specific receptors. Synthetic polypeptides have also been developed for delivery of ODNs.
ASGPs recognize their receptors (ASGPR) on hepatocytes. Rajur et al. conjugated ODNs to ASGP by a disulfide linkage (556). Six ODN molecules could be conjugated to each ASGP molecule. The ODN was designed to be complementary to the mRNA of the IL-6 signal transduction protein (gp130) and be delivered to HepG2 cells in vitro. These conjugates inhibited the cytokine-stimulated up-regulation of the acute-phase protein haptoglobin.
Glycoproteins are also attractive objects for targeting liver. Plasma proteins such as albumin can be randomly derivatized with sugar groups. For example, in bovine serum albumin (BSA), sugars can be reacted with ε-NH2 of lysines. Galacotose, mannose and mannose 6-phosphate (M6P) have been used to modify BSA for drug delivery to hepatocytes, KCs and HSCs, respectively (557, 558). The efficiency of endocytosis and further degradation of glycoproteins can be largely influenced by the number, density, and geometric clustering of the sugar groups on the protein molecules (559, 560). Even though many drugs have been targeted to liver using glycoproteins, few reports focused on the delivery of ODNs as well as siRNAs.
Specific antibodies for receptors on target cell surface can be used as carrier molecules besides being used as targeting ligands. Walker et al. (561) used a transferring receptor antibody to deliver ODNs. A maleimide-derivatized IgG was reacted with a 5'-thiol-modified ODN. Human glioblasoma cell line U87-MG and the human endothelial cell line ECV304, which express the transferring receptor on their surfaces, bound and internalized the antibody-ODN conjugates three-fold more effectively than naked ODNs. In another study, a rat transferring receptor antibody (OX-26) was conjugated with ODNs and could enhance the cellular uptake into an immortalized cell line called RBE4 as an in vitro model of the blood-brain barrier (562). The subcellular trafficking was studied. It was found that the OX-26 conjugates showed altered subcellular distribution from those for naked ODNs or a nonspecific IgG conjugate.
5.3.5.4. Targeted drug delivery for treating liver fibrosis
Antifibrotic drugs, which are used predominantly in animal models and cell cultures but not humans, are neither liver- nor fibrosis-specific. Therefore, their clinical application necessitated the development of site-directed delivery systems affecting quite selectively the effector cells during liver fibrosis. However, particles larger than 100–150 nm cannot pass the fenestrae in the endothelial lining. This is true for most particulate systems and they can only reach KCs, pit and SECs. This situation is even worse in the case of liver fibrosis, where the loss of fenestrae becomes apparent.
Efforts to targeted delivery to HSCs were first contributed by Beljaars et al. (558). In their study, a human serum albumin (HSA)-based bioconjugate modified with M6P was reported to be specifically taken up by activated HSCs in experimental liver fibrosis models. The M6P/insulin-like growth factor II (M6P/IGF-II) receptor, which is expressed in particular upon HSC during fibrosis, can be utilized for targeted delivery to activated HSCs (563, 564). The M6P/IGF-II receptor has been characterized as a 275 kDa Mr receptor which bind M6P even in absence of cation is a membrane lectin involved both in lysosomal enzyme trafficking and in the endocytic pathway (565) and can bind diverse M6P- and non M6P-containing ligands such as TGF-β, proliferin, IGF-II, RA and uPA receptor (566). In the fibrotic liver M6P/IGF-II receptors facilitate the activation of latent TGF-β (567, 568). In fact, Bonfils et al. (569) had conjugated 19mer ODNs to M6P-BSA via disulfide bond and demonstrated 2-fold higher cellular uptake by macrophages (J774 cells) as compared to fibroblast-like BHK cells and 30-fold higher than monocytes (U937 cells). However, these authors did not determine the biodistribution and uptake of M6P-BSA-ODN conjugate by different liver cells.
HSA modified with M6P (M6P-HSA) was synthesized with different numbers of M6P (2, 4, 10, and 28) (558). The hepatic uptake of M6P-HSA was found to increase with increasing numbers of M6P in the conjugates. With 2, 4 and 10, the conjugates accumulated for 9% or less of injected dose in fibrotic rat livers, while with 28, 59% was in the liver. Intrahepatic distribution of M6P-HSA was determined as well using double-immunostaining techniques (staining for HSA and HSCs). The number of M6P in the conjugates also affected their accumulation in HSCs, with increased substitution of M6P associated with an increased accumulation in HSCs and 70% ±11% of the intrahepatic staining for M6P28-HSA was found in HSCs. In addition, M6P-BSA accumulates in slices of normal and cirrhotic human livers. After incubation of this neoglycoprotein with human tissue, the protein is found in nonparenchymal liver cells. Furthermore, an extensive study in vitro and in human liver slices confirmed the specific uptake of M6P28-HSA by activated HSCs, but not quiescent HSCs (570). In addition, a lysosomal pathway for cellular uptake was suggested for the conjugate. These studies created new opportunities for the pharmacological intervention of liver fibrosis, since no delivery strategies had been developed for HSCs before. Following these, many antifibrotic drugs have been delivered to HSCs by conjugation with M6P-BSA/HSA, such as glycyrrhetin (277), antiproliferative drugs including mycophenolic acid (571), pentoxifylline (571) and doxorubicin (571), apoptosis-inducing drug 15-D-PGJ2 (572), a tyrosine kinase inhibitor, 4-chloro-N-[4-methyl-3-(4-pyridin-3-yl-pyrimidin-2-ylamino)-phenyl]-benzamide (573) and 18beta-glycyrrhetinic acid (574). These attempts to selectively deliver antifibrotic drugs to HSCs in experimental liver fibrosis have demonstrated enhanced disposition in activated HSCs. However, the efficacy in preventing liver fibrosis still needs to be improved.
Few studies have attempted to delivery ODNs to activated HSCs. The high potency of ODN-based gene silencing therapeutics such as TFOs grantees them high potential as antifibrotic agents. Based on the above mentioned delivery strategy for HSCs, we proposed M6P-BSA conjugates with TFOs to treat liver fibrosis (Figure 13). Bovine serum albumin is used as the backbone, onto which targeting ligand M6P can be attached with the NH2 of lysines. In addition, TFO molecules modified sulfhydryl functionalities can be conjugated with BSA via disulfide bond formation. Disulfide bonds enable the intracellular release of TFOs upon taken up by HSCs. TFOs used are specific for type α1(I) collagen gene promoter. Therefore, this conjugate has potential to be used as antifibrotic drugs.
Figure 13. Schematic of a propose conjugate for site-specific delivery of TFOs to activated HSCs for treating liver fibrosis.
Bovine serum albumin is used as the backbone, onto which targeting ligands, mannose 6-phosphate (M6P) can be attached with the NH2 of lysines. In addition, TFO molecules modified sulfhydryl functionalities can be conjugated with BSA via disulfide bond formation. The M6P/insulin-like growth factor II (M6P/IGF-II) receptor, which is expressed in particular upon HSCs during fibrosis, can be utilized for targeted delivery to activated HSCs. Disulfide bonds enable the intracellular release of TFOs upon taken up by HSCs. TFOs used are specific for type α1(I) collagen gene promoter and can inhibit the gene transcription. This conjugate has potential to be used as antifibrotic drugs.
6. CONCLUDING REMARKS
Thorough understanding of liver pathophysiology is essential to devise strategies for treating liver fibrosis. Injury to hepatocytes leads to the release of ROS, inflammatory cytokines and growth factor, which in turn activate HSCs. Therefore, prevention of apoptosis of hepatocytes and induction of apoptosis of activated HSCs are attracting more attention. Although many different organic compounds are being developed as antifibrotic drugs, none of them are liver- and fibrosis-specific. Therefore, strategies for site-specific delivery strategies are urgently needed.
ODN-based gene silencing technologies provide an efficient and specific way to interrupt the signaling pathways and prevent the production of key factors promoting the progression of liver fibrosis. However, ODNs are polyanions and poorly taken up by target cells. Moreover, deposition of excess scar matrix and the disappearance of sinusoidal gaps do not allow efficient delivery of liposomal and nanoparticulate systems. Further, complex formation with cationic lipids and polymers causes non-specific interaction with plasma proteins and non-target cells. Therefore, bioconjugation of ODNs with non-charged polymer containing ligands has great potential for enhance delivery of ODNs to specific liver cells. Since M6P receptors present on the surface of HSCs are upregulated during liver fibrosis, bioconjugation of TFOs specific for α1(I) collagen gene promoter with M6P-BSA is efficiently taken up by activated HSCs and M6P-BSA-TFO conjugates have potential for treating liver fibrosis.
ACKNOWLEDGEMENTS
This work was supported by grants RO1 DK 064366, EB003922 and USPHS AR47379 from the NIH.
ABBREVIATION
- 2'-AE
2'-O-aminoethyl
- aFGF
Acidic Fibroblast Growth Factor
- AngII
Angiotensin-II
- ASGP
Asialoglycoprotein
- ASGPR
Asialoglycoprotein Receptor
- AsOR
Asialoorosomucoid
- AT-1
Angiotensin-II Type 1 receptor
- AT-2
Angiotensin-II Type 2 receptor
- BSA
Bovine Serum Albumin
- CB
Cannabinoid
- CCl4
Carbon Tetrachloride
- CPPs
Cell Penetrating Peptides
- CD
Cluster of Differentiation
- CGE
Capillary Gel Electrophoresis
- CINC
Cytokine-Induced Neutrophil Chemoattractant
- CLV
Centrolobular Vein
- COX-2
Cyclooxygenase-2
- CTGF
Connective Tissue Growth Factor
- DDR
Discoidin Domain Receptor
- DMF
Dimethylformamide
- DMN
Dimethyl Nitrosamine
- DMS
Dimethyl Sulfate
- 15-D-PGJ2
15-Deoxy Δ12, 14 Prostaglandin J2
- dsDNA
Double-Stranded DNA
- ECM
Extracellular Matrix
- EGF
Epidermal Growth Factor
- EMSA
Electrophoretic Mobility Shift Assay
- EPR
Enhanced Permeation and Retention
- ET-1
Endothelin-1
- Fas-L
Fas Ligand
- FGF
Fibroblast Growth Factor
- GalN
D-Galactosamine
- GalNAc
N-Acetylgalactosamine
- GLAP
Glial Fibrillary Acidic Protein
- HCV
Hepatitis C Virus
- HGF
Hepatocyte Growth Factor
- HSA
Human Serum Albumin
- HSC
Hepatic Stellate Cell
- HSV-1
Herpes Simplex Virus Type-1
- ICAM-1
Intracellular Adhesion Molecule-1
- IGF-I
Insulin Growth Factors I
- IGF-II
Insulin Growth Factors II
- IFN-
γ Interferon γ
- IL-6
Interleukin-6
- IL-10
Interleukin-10
- IL-2R
α Interleukin-2 Receptor
- KC
Kupffer Cell
- L3G4
N2-(N2-(N2,N6-bis[N-[p-(β-D-galactopyranosyloxy)-anilino]thiocarbamyl]-L-lysyl)-N6-(N-[p-(β-D-galactopyranosyloxy)-anilino]thiocarbamyl]-L-lysyl)-N6-(N-[p=(β-D-galactopyranosyloxy)-anilino]thiocarbamyl)]-L-lysine
- LNA
Locked Nucleic Acid
- lpr
Lymphoproliferation
- LPS
Lipopolysaccharide
- LTBP
Latent TGF-β-Binding Protein
- mAb
Monoclonal Antibody
- M6P
Mannose 6-phosphate
- M6P/IGF-II
Mannose 6-phosphate/Insulin-like Growth Factor II
- MCP-1
Monocyte Chemotactic Protein-1
- 2-MEA
2-Mercaptoethylamine
- MMP
Matrix Metalloproteinase
- MP
Methylphosphonate
- mPEG
Poly(ethylene glycol) with Monomethoxy at one end
- mRNA
Messenger RNA
- NASH
Nonalcoholic Steatohepatitis
- NCAM
Neural Cell Adhesion Molecule
- NF-kB
Nuclear Factor κB
- NGF
Nerve Growth Factor
- NHS
N-Hydroxysuccinimidyl Ester
- NK
Natural Killer
- NPCs
Nonparenchymal cells
- ODN
Oligonucleotide
- PAF
Platelet-Activating Factor
- PAGE
Polyacrylamide Gel Electrophoresis
- PAI-1
Plasminogen Activator Inhibitor-1
- PBC
Primary Biliary Cirrhosis
- PC
Phosphatidylcholine
- PDGF
Platelet-Derived Growth Factor
- PEG
Poly(ethylene glycol)
- PEI
Poly(ethyleneimine)
- PGE
Prostaglandin E
- PLL
Poly(L-lysine)
- PLMA
poly(β-L-malic acid)
- PMA
Phorbol-12 myristate-13 acetate
- PMOs
Phosphorodiamidate Morpholino Oligonucleotides
- PN
Phosphoramidates
- PNAs
Peptide nuclei acids
- PO
Phosphodiester
- PPAR
Peroxisome Proliferator-Activated Receptor
- PS
Phosphorothioate
- PTDs
Protein Transduction Domains
- REPA
Restriction Enzyme Protection Assay
- RA
Retinoic Acid
- RBV
Ribavirin
- RES
Reticuloendothelial System
- ROS
Reactive Oxygen Species
- RTK
Receptor Tyrosine Kinase
- SAMe
S-Adenosyl-L-Methionine
- SEC
Sinusoidal Endothelial Cell
- S1P
Sphingosine-1-phosphate
- siRNA
Small Interference RNA
- TFO
Triplex Forming Oligonucleotides
- TGF-
β Transforming Growth Factor-β
- THC
Δ9-Tetra-Hydrocannabinol
- TIMP
Tissue Inhibitor of Metalloproteinase
- TNF-
α Tumor Necrosis Factor-α
- TRAIL-R
TNF-α-Related-Apoptosis-Inducing-Ligand Receptor
- UDCA
Ursodeoxycholic Acid
- uPA
Urokinase-Type Plasminogen Activator
- VCAM
Vascular Cell Adhesion Molecule
REFERENCES
- (1).Raghow R. The role of extracellular matrix in postinflammatory wound healing and fibrosis. Faseb J. 1994;8:823–31. doi: 10.1096/fasebj.8.11.8070631. [DOI] [PubMed] [Google Scholar]
- (2).Anderson RN. Deaths: leading causes for 2000. Natl Vital Stat Rep. 2002;50:1–85. [PubMed] [Google Scholar]
- (3).Gines P, Cardenas A, Arroyo V, Rodes J. Management of cirrhosis and ascites. N Engl J Med. 2004;350:1646–54. doi: 10.1056/NEJMra035021. [DOI] [PubMed] [Google Scholar]
- (4).El-Serag HB. Hepatocellular carcinoma and hepatitis C in the United States. Hepatology. 2002;36:S74–83. doi: 10.1053/jhep.2002.36807. [DOI] [PubMed] [Google Scholar]
- (5).Befeler AS, Di Bisceglie AM. Hepatocellular carcinoma: diagnosis and treatment. Gastroenterology. 2002;122:1609–19. doi: 10.1053/gast.2002.33411. [DOI] [PubMed] [Google Scholar]
- (6).Davis GL, Albright JE, Cook SF, Rosenberg DM. Projecting future complications of chronic hepatitis C in the United States. Liver Transpl. 2003;9:331–8. doi: 10.1053/jlts.2003.50073. [DOI] [PubMed] [Google Scholar]
- (7).Berenguer M. Management of hepatitis C virus in the transplant patient. Clin Liver Dis. 2007;11:355–76. doi: 10.1016/j.cld.2007.04.010. [DOI] [PubMed] [Google Scholar]
- (8).Meijer DK, Molema G. Targeting of drugs to the liver. Semin Liver Dis. 1995;15:202–56. doi: 10.1055/s-2007-1007278. [DOI] [PubMed] [Google Scholar]
- (9).Irving MG, Roll FJ, Huang S, Bissell DM. Characterization and culture of sinusoidal endothelium from normal rat liver: lipoprotein uptake and collagen phenotype. Gastroenterology. 1984;87:1233–47. [PubMed] [Google Scholar]
- (10).Ballet F. Hepatic circulation: potential for therapeutic intervention. Pharmacol Ther. 1990;47:281–328. doi: 10.1016/0163-7258(90)90091-F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).MacPhee PJ, Schmidt EE, Groom AC. Evidence for Kupffer cell migration along liver sinusoids, from high-resolution in vivo microscopy. Am J Physiol. 1992;263:G17–23. doi: 10.1152/ajpgi.1992.263.1.G17. [DOI] [PubMed] [Google Scholar]
- (12).Friedman SL, Roll FJ, Boyles J, Bissell DM. Hepatic lipocytes: the principal collagen-producing cells of normal rat liver. Proc Natl Acad Sci U S A. 1985;82:8681–5. doi: 10.1073/pnas.82.24.8681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Bissell DM, Maher JJ. Hepatic Fibrosis and Cirrhosis. In: Zakim D, Boyer TD, editors. Hepatology: a Textbook of Liver Disease. WB Saunders; Philadelphia: 2003. pp. 395–416. [Google Scholar]
- (14).Kmiec Z. Cooperation of liver cells in health and disease. Adv Anat Embryol Cell Biol. 2001;161:III–XIII. 1–151. doi: 10.1007/978-3-642-56553-3. [DOI] [PubMed] [Google Scholar]
- (15).McCaughan GW, Gorrell MD, Bishop GA, Abbott CA, Shackel NA, McGuinness PH, Levy MT, Sharland AF, Bowen DG, Yu D, Slaitini L, Church WB, Napoli J. Molecular pathogenesis of liver disease: an approach to hepatic inflammation, cirrhosis and liver transplant tolerance. Immunol Rev. 2000;174:172–91. doi: 10.1034/j.1600-0528.2002.017420.x. [DOI] [PubMed] [Google Scholar]
- (16).Higuchi H, Gores GJ. Mechanisms of liver injury: an overview. Curr Mol Med. 2003;3:483–90. doi: 10.2174/1566524033479528. [DOI] [PubMed] [Google Scholar]
- (17).Rust C, Gores GJ. Apoptosis and liver disease. Am J Med. 2000;108:567–74. doi: 10.1016/s0002-9343(00)00370-3. [DOI] [PubMed] [Google Scholar]
- (18).Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115:209–18. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Schumann J, Wolf D, Pahl A, Brune K, Papadopoulos T, van Rooijen N, Tiegs G. Importance of Kupffer cells for T-cell-dependent liver injury in mice. Am J Pathol. 2000;157:1671–83. doi: 10.1016/S0002-9440(10)64804-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Ramadori G, Moebius U, Dienes HP, Meuer S, Meyer zum Buschenfelde KH. Lymphocytes from hepatic inflammatory infiltrate kill rat hepatocytes in primary culture. Comparison with peripheral blood lymphocytes. Virchows Arch B Cell Pathol Incl Mol Pathol. 1990;59:263–70. doi: 10.1007/BF02899413. [DOI] [PubMed] [Google Scholar]
- (21).Hug H, Strand S, Grambihler A, Galle J, Hack V, Stremmel W, Krammer PH, Galle PR. Reactive oxygen intermediates are involved in the induction of CD95 ligand mRNA expression by cytostatic drugs in hepatoma cells. J Biol Chem. 1997;272:28191–3. doi: 10.1074/jbc.272.45.28191. [DOI] [PubMed] [Google Scholar]
- (22).Gregory SH, Wing EJ. Neutrophil-Kupffer cell interaction: a critical component of host defenses to systemic bacterial infections. J Leukoc Biol. 2002;72:239–48. [PubMed] [Google Scholar]
- (23).Su GL. Lipopolysaccharides in liver injury: molecular mechanisms of Kupffer cell activation. Am J Physiol Gastrointest Liver Physiol. 2002;283:G256–65. doi: 10.1152/ajpgi.00550.2001. [DOI] [PubMed] [Google Scholar]
- (24).Adams DH, Hubscher SG. Systemic viral infections and collateral damage in the liver. Am J Pathol. 2006;168:1057–9. doi: 10.2353/ajpath.2006.051296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Winwood PJ, Arthur MJ. Kupffer cells: their activation and role in animal models of liver injury and human liver disease. Semin Liver Dis. 1993;13:50–9. doi: 10.1055/s-2007-1007337. [DOI] [PubMed] [Google Scholar]
- (26).Laskin DL. Nonparenchymal cells and hepatotoxicity. Semin Liver Dis. 1990;10:293–304. doi: 10.1055/s-2008-1040485. [DOI] [PubMed] [Google Scholar]
- (27).Valatas V, Kolios G, Manousou P, Notas G, Xidakis C, Diamantis I, Kouroumalis E. Octreotide regulates CC but not CXC LPS-induced chemokine secretion in rat Kupffer cells. Br J Pharmacol. 2004;141:477–87. doi: 10.1038/sj.bjp.0705633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Valatas V, Kolios G, Manousou P, Xidakis C, Notas G, Ljumovic D, Kouroumalis EA. Secretion of inflammatory mediators by isolated rat Kupffer cells: the effect of octreotide. Regul Pept. 2004;120:215–25. doi: 10.1016/j.regpep.2004.03.009. [DOI] [PubMed] [Google Scholar]
- (29).Batusic DS, Armbrust T, Saile B, Ramadori G. Induction of Mx-2 in rat liver by toxic injury. J Hepatol. 2004;40:446–53. doi: 10.1016/j.jhep.2003.11.031. [DOI] [PubMed] [Google Scholar]
- (30).Knittel T, Mehde M, Kobold D, Saile B, Dinter C, Ramadori G. Expression patterns of matrix metalloproteinases and their inhibitors in parenchymal and non-parenchymal cells of rat liver: regulation by TNF-alpha and TGF-beta1. J Hepatol. 1999;30:48–60. doi: 10.1016/s0168-8278(99)80007-5. [DOI] [PubMed] [Google Scholar]
- (31).Baldus SE, Zirbes TK, Weidner IC, Flucke U, Dittmar E, Thiele J, Dienes HP. Comparative quantitative analysis of macrophage populations defined by CD68 and carbohydrate antigens in normal and pathologically altered human liver tissue. Anal Cell Pathol. 1998;16:141–50. doi: 10.1155/1998/192975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Malhi H, Gores GJ, Lemasters JJ. Apoptosis and necrosis in the liver: a tale of two deaths? Hepatology. 2006;43:S31–44. doi: 10.1002/hep.21062. [DOI] [PubMed] [Google Scholar]
- (33).Lemasters JJ, Ji S, Thurman RG. Centrilobular injury following hypoxia in isolated, perfused rat liver. Science. 1981;213:661–3. doi: 10.1126/science.7256265. [DOI] [PubMed] [Google Scholar]
- (34).Faubion WA, Guicciardi ME, Miyoshi H, Bronk SF, Roberts PJ, Svingen PA, Kaufmann SH, Gores GJ. Toxic bile salts induce rodent hepatocyte apoptosis via direct activation of Fas. J Clin Invest. 1999;103:137–45. doi: 10.1172/JCI4765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Feldstein AE, Canbay A, Angulo P, Taniai M, Burgart LJ, Lindor KD, Gores GJ. Hepatocyte apoptosis and fas expression are prominent features of human nonalcoholic steatohepatitis. Gastroenterology. 2003;125:437–43. doi: 10.1016/s0016-5085(03)00907-7. [DOI] [PubMed] [Google Scholar]
- (36).Locksley RM, Killeen N, Lenardo MJ. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell. 2001;104:487–501. doi: 10.1016/s0092-8674(01)00237-9. [DOI] [PubMed] [Google Scholar]
- (37).Jaeschke H, Gujral JS, Bajt ML. Apoptosis and necrosis in liver disease. Liver Int. 2004;24:85–9. doi: 10.1111/j.1478-3231.2004.0906.x. [DOI] [PubMed] [Google Scholar]
- (38).Green DR, Reed JC. Mitochondria and apoptosis. Science. 1998;281:1309–12. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- (39).Wu J, Danielsson A, Zern MA. Toxicity of hepatotoxins: new insights into mechanisms and therapy. Expert Opin Investig Drugs. 1999;8:585–607. doi: 10.1517/13543784.8.5.585. [DOI] [PubMed] [Google Scholar]
- (40).Casini A, Ceni E, Salzano R, Biondi P, Parola M, Galli A, Foschi M, Caligiuri A, Pinzani M, Surrenti C. Neutrophil-derived superoxide anion induces lipid peroxidation and stimulates collagen synthesis in human hepatic stellate cells: role of nitric oxide. Hepatology. 1997;25:361–7. doi: 10.1053/jhep.1997.v25.pm0009021948. [DOI] [PubMed] [Google Scholar]
- (41).Canbay A, Taimr P, Torok N, Higuchi H, Friedman S, Gores GJ. Apoptotic body engulfment by a human stellate cell line is profibrogenic. Lab Invest. 2003;83:655–63. doi: 10.1097/01.lab.0000069036.63405.5c. [DOI] [PubMed] [Google Scholar]
- (42).Canbay A, Friedman S, Gores GJ. Apoptosis: the nexus of liver injury and fibrosis. Hepatology. 2004;39:273–8. doi: 10.1002/hep.20051. [DOI] [PubMed] [Google Scholar]
- (43).Canbay A, Higuchi H, Bronk SF, Taniai M, Sebo TJ, Gores GJ. Fas enhances fibrogenesis in the bile duct ligated mouse: a link between apoptosis and fibrosis. Gastroenterology. 2002;123:1323–30. doi: 10.1053/gast.2002.35953. [DOI] [PubMed] [Google Scholar]
- (44).Patel T, Roberts LR, Jones BA, Gores GJ. Dysregulation of apoptosis as a mechanism of liver disease: an overview. Semin Liver Dis. 1998;18:105–14. doi: 10.1055/s-2007-1007147. [DOI] [PubMed] [Google Scholar]
- (45).Canbay A, Feldstein AE, Higuchi H, Werneburg N, Grambihler A, Bronk SF, Gores GJ. Kupffer cell engulfment of apoptotic bodies stimulates death ligand and cytokine expression. Hepatology. 2003;38:1188–98. doi: 10.1053/jhep.2003.50472. [DOI] [PubMed] [Google Scholar]
- (46).Zhan SS, Jiang JX, Wu J, Halsted C, Friedman SL, Zern MA, Torok NJ. Phagocytosis of apoptotic bodies by hepatic stellate cells induces NADPH oxidase and is associated with liver fibrosis in vivo. Hepatology. 2006;43:435–43. doi: 10.1002/hep.21093. [DOI] [PubMed] [Google Scholar]
- (47).Savill J, Fadok V. Corpse clearance defines the meaning of cell death. Nature. 2000;407:784–8. doi: 10.1038/35037722. [DOI] [PubMed] [Google Scholar]
- (48).Parnaik R, Raff MC, Scholes J. Differences between the clearance of apoptotic cells by professional and non-professional phagocytes. Curr Biol. 2000;10:857–60. doi: 10.1016/s0960-9822(00)00598-4. [DOI] [PubMed] [Google Scholar]
- (49).Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J Clin Invest. 1998;101:890–8. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Kurosaka K, Watanabe N, Kobayashi Y. Production of proinflammatory cytokines by resident tissue macrophages after phagocytosis of apoptotic cells. Cell Immunol. 2001;211:1–7. doi: 10.1006/cimm.2001.1824. [DOI] [PubMed] [Google Scholar]
- (51).Nieto N, Greenwel P, Friedman SL, Zhang F, Dannenberg AJ, Cederbaum AI. Ethanol and arachidonic acid increase alpha 2(I) collagen expression in rat hepatic stellate cells overexpressing cytochrome P450 2E1. Role of H2O2 and cyclooxygenase-2. J Biol Chem. 2000;275:20136–45. doi: 10.1074/jbc.M001422200. [DOI] [PubMed] [Google Scholar]
- (52).Pietrangelo A, Gualdi R, Casalgrandi G, Montosi G, Ventura E. Molecular and cellular aspects of iron-induced hepatic cirrhosis in rodents. J Clin Invest. 1995;95:1824–31. doi: 10.1172/JCI117861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Brady LM, Beno DW, Davis BH. Bile acid stimulation of early growth response gene and mitogen-activated protein kinase is protein kinase C-dependent. Biochem J. 1996;316(Pt 3):765–9. doi: 10.1042/bj3160765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Friedman SL. Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J Biol Chem. 2000;275:2247–50. doi: 10.1074/jbc.275.4.2247. [DOI] [PubMed] [Google Scholar]
- (55).Eng FJ, Friedman SL. Fibrogenesis I. New insights into hepatic stellate cell activation: the simple becomes complex. Am J Physiol Gastrointest Liver Physiol. 2000;279:G7–G11. doi: 10.1152/ajpgi.2000.279.1.G7. [DOI] [PubMed] [Google Scholar]
- (56).Friedman SL, Arthur MJ. Activation of cultured rat hepatic lipocytes by Kupffer cell conditioned medium. Direct enhancement of matrix synthesis and stimulation of cell proliferation via induction of platelet-derived growth factor receptors. J Clin Invest. 1989;84:1780–5. doi: 10.1172/JCI114362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Maher JJ. Leukocytes as modulators of stellate cell activation. Alcohol Clin Exp Res. 1999;23:917–21. [PubMed] [Google Scholar]
- (58).Gressner AM, Lahme B, Brenzel A. Molecular dissection of the mitogenic effect of hepatocytes on cultured hepatic stellate cells. Hepatology. 1995;22:1507–18. [PubMed] [Google Scholar]
- (59).Poli G. Pathogenesis of liver fibrosis: role of oxidative stress. Mol Aspects Med. 2000;21:49–98. doi: 10.1016/s0098-2997(00)00004-2. [DOI] [PubMed] [Google Scholar]
- (60).Britton RS, Bacon BR. Intracellular signaling pathways in stellate cell activation. Alcohol Clin Exp Res. 1999;23:922–5. [PubMed] [Google Scholar]
- (61).Reimann T, Hempel U, Krautwald S, Axmann A, Scheibe R, Seidel D, Wenzel KW. Transforming growth factor-beta1 induces activation of Ras, Raf-1, MEK and MAPK in rat hepatic stellate cells. FEBS Lett. 1997;403:57–60. doi: 10.1016/s0014-5793(97)00024-0. [DOI] [PubMed] [Google Scholar]
- (62).Pinzani M, Gesualdo L, Sabbah GM, Abboud HE. Effects of platelet-derived growth factor and other polypeptide mitogens on DNA synthesis and growth of cultured rat liver fat-storing cells. J Clin Invest. 1989;84:1786–93. doi: 10.1172/JCI114363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (63).Rockey DC, Fouassier L, Chung JJ, Carayon A, Vallee P, Rey C, Housset C. Cellular localization of endothelin-1 and increased production in liver injury in the rat: potential for autocrine and paracrine effects on stellate cells. Hepatology. 1998;27:472–80. doi: 10.1002/hep.510270222. [DOI] [PubMed] [Google Scholar]
- (64).Neubauer K, Saile B, Ramadori G. Liver fibrosis and altered matrix synthesis. Can J Gastroenterol. 2001;15:187–93. doi: 10.1155/2001/870205. [DOI] [PubMed] [Google Scholar]
- (65).Jarnagin WR, Rockey DC, Koteliansky VE, Wang SS, Bissell DM. Expression of variant fibronectins in wound healing: cellular source and biological activity of the EIIIA segment in rat hepatic fibrogenesis. J Cell Biol. 1994;127:2037–48. doi: 10.1083/jcb.127.6.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (66).George J, Wang SS, Sevcsik AM, Sanicola M, Cate RL, Koteliansky VE, Bissell DM. Transforming growth factor-beta initiates wound repair in rat liver through induction of the EIIIA-fibronectin splice isoform. Am J Pathol. 2000;156:115–24. doi: 10.1016/s0002-9440(10)64711-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (67).Breitkopf K, Lahme B, Tag CG, Gressner AM. Expression and matrix deposition of latent transforming growth factor beta binding proteins in normal and fibrotic rat liver and transdifferentiating hepatic stellate cells in culture. Hepatology. 2001;33:387–96. doi: 10.1053/jhep.2001.21996. [DOI] [PubMed] [Google Scholar]
- (68).Okuno M, Sato T, Kitamoto T, Imai S, Kawada N, Suzuki Y, Yoshimura H, Moriwaki H, Onuki K, Masushige S, Muto Y, Friedman SL, Kato S, Kojima S. Increased 9,13-di-cis-retinoic acid in rat hepatic fibrosis: implication for a potential link between retinoid loss and TGF-beta mediated fibrogenesis in vivo. J Hepatol. 1999;30:1073–80. doi: 10.1016/s0168-8278(99)80262-1. [DOI] [PubMed] [Google Scholar]
- (69).Pinzani M, Marra F, Carloni V. Signal transduction in hepatic stellate cells. Liver. 1998;18:2–13. doi: 10.1111/j.1600-0676.1998.tb00120.x. [DOI] [PubMed] [Google Scholar]
- (70).Friedman SL. Cytokines and fibrogenesis. Semin Liver Dis. 1999;19:129–40. doi: 10.1055/s-2007-1007105. [DOI] [PubMed] [Google Scholar]
- (71).Friedman SL, Yamasaki G, Wong L. Modulation of transforming growth factor beta receptors of rat lipocytes during the hepatic wound healing response. Enhanced binding and reduced gene expression accompany cellular activation in culture and in vivo. J Biol Chem. 1994;269:10551–8. [PubMed] [Google Scholar]
- (72).Kim Y, Ratziu V, Choi SG, Lalazar A, Theiss G, Dang Q, Kim SJ, Friedman SL. Transcriptional activation of transforming growth factor beta1 and its receptors by the Kruppel-like factor Zf9/core promoter-binding protein and Sp1. Potential mechanisms for autocrine fibrogenesis in response to injury. J Biol Chem. 1998;273:33750–8. doi: 10.1074/jbc.273.50.33750. [DOI] [PubMed] [Google Scholar]
- (73).Hellerbrand C, Stefanovic B, Giordano F, Burchardt ER, Brenner DA. The role of TGFbeta1 in initiating hepatic stellate cell activation in vivo. J Hepatol. 1999;30:77–87. doi: 10.1016/s0168-8278(99)80010-5. [DOI] [PubMed] [Google Scholar]
- (74).Pinzani M, Milani S, Grappone C, Weber FL, Jr., Gentilini P, Abboud HE. Expression of platelet-derived growth factor in a model of acute liver injury. Hepatology. 1994;19:701–7. doi: 10.1002/hep.1840190323. [DOI] [PubMed] [Google Scholar]
- (75).Pinzani M, Milani S, Herbst H, DeFranco R, Grappone C, Gentilini A, Caligiuri A, Pellegrini G, Ngo DV, Romanelli RG, Gentilini P. Expression of platelet-derived growth factor and its receptors in normal human liver and during active hepatic fibrogenesis. Am J Pathol. 1996;148:785–800. [PMC free article] [PubMed] [Google Scholar]
- (76).Ikeda K, Wakahara T, Wang YQ, Kadoya H, Kawada N, Kaneda K. In vitro migratory potential of rat quiescent hepatic stellate cells and its augmentation by cell activation. Hepatology. 1999;29:1760–7. doi: 10.1002/hep.510290640. [DOI] [PubMed] [Google Scholar]
- (77).Marra F, Gentilini A, Pinzani M, Choudhury GG, Parola M, Herbst H, Dianzani MU, Laffi G, Abboud HE, Gentilini P. Phosphatidylinositol 3-kinase is required for platelet-derived growth factor's actions on hepatic stellate cells. Gastroenterology. 1997;112:1297–306. doi: 10.1016/s0016-5085(97)70144-6. [DOI] [PubMed] [Google Scholar]
- (78).Marra F, Romanelli RG, Giannini C, Failli P, Pastacaldi S, Arrighi MC, Pinzani M, Laffi G, Montalto P, Gentilini P. Monocyte chemotactic protein-1 as a chemoattractant for human hepatic stellate cells. Hepatology. 1999;29:140–8. doi: 10.1002/hep.510290107. [DOI] [PubMed] [Google Scholar]
- (79).Marra F, Grandaliano G, Valente AJ, Abboud HE. Thrombin stimulates proliferation of liver fat-storing cells and expression of monocyte chemotactic protein-1: potential role in liver injury. Hepatology. 1995;22:780–7. [PubMed] [Google Scholar]
- (80).Bataller R, Nicolas JM, Gines P, Esteve A, Nieves Gorbig M, Garcia-Ramallo E, Pinzani M, Ros J, Jimenez W, Thomas AP, Arroyo V, Rodes J. Arginine vasopressin induces contraction and stimulates growth of cultured human hepatic stellate cells. Gastroenterology. 1997;113:615–24. doi: 10.1053/gast.1997.v113.pm9247484. [DOI] [PubMed] [Google Scholar]
- (81).Bataller R, Gines P, Nicolas JM, Gorbig MN, Garcia-Ramallo E, Gasull X, Bosch J, Arroyo V, Rodes J. Angiotensin II induces contraction and proliferation of human hepatic stellate cells. Gastroenterology. 2000;118:1149–56. doi: 10.1016/s0016-5085(00)70368-4. [DOI] [PubMed] [Google Scholar]
- (82).Davaille J, Gallois C, Habib A, Li L, Mallat A, Tao J, Levade T, Lotersztajn S. Antiproliferative properties of sphingosine 1-phosphate in human hepatic myofibroblasts. A cyclooxygenase-2 mediated pathway. J Biol Chem. 2000;275:34628–33. doi: 10.1074/jbc.M006393200. [DOI] [PubMed] [Google Scholar]
- (83).Failli P, De FR, Caligiuri A, Gentilini A, Romanelli RG, Marra F, Batignani G, Guerra CT, Laffi G, Gentilini P, Pinzani M. Nitrovasodilators inhibit platelet-derived growth factor-induced proliferation and migration of activated human hepatic stellate cells. Gastroenterology. 2000;119:479–92. doi: 10.1053/gast.2000.9354. [DOI] [PubMed] [Google Scholar]
- (84).Mallat A, Fouassier L, Preaux AM, Gal CS, Raufaste D, Rosenbaum J, Dhumeaux D, Jouneaux C, Mavier P, Lotersztajn S. Growth inhibitory properties of endothelin-1 in human hepatic myofibroblastic Ito cells. An endothelin B receptor-mediated pathway. J Clin Invest. 1995;96:42–9. doi: 10.1172/JCI118052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (85).Gallois C, Habib A, Tao J, Moulin S, Maclouf J, Mallat A, Lotersztajn S. Role of NF-kappaB in the antiproliferative effect of endothelin-1 and tumor necrosis factor-alpha in human hepatic stellate cells. Involvement of cyclooxygenase-2. J Biol Chem. 1998;273:23183–90. doi: 10.1074/jbc.273.36.23183. [DOI] [PubMed] [Google Scholar]
- (86).Koda M, Bauer M, Krebs A, Hahn EG, Schuppan D, Murawaki Y. Endothelin-1 enhances fibrogenic gene expression, but does not promote DNA synthesis or apoptosis in hepatic stellate cells. Comp Hepatol. 2006;5:5. doi: 10.1186/1476-5926-5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (87).Marra F. Hepatic stellate cells and the regulation of liver inflammation. J Hepatol. 1999;31:1120–30. doi: 10.1016/s0168-8278(99)80327-4. [DOI] [PubMed] [Google Scholar]
- (88).Marra F, DeFranco R, Grappone C, Milani S, Pastacaldi S, Pinzani M, Romanelli RG, Laffi G, Gentilini P. Increased expression of monocyte chemotactic protein-1 during active hepatic fibrogenesis: correlation with monocyte infiltration. Am J Pathol. 1998;152:423–30. [PMC free article] [PubMed] [Google Scholar]
- (89).Vinas O, Bataller R, Sancho-Bru P, Gines P, Berenguer C, Enrich C, Nicolas JM, Ercilla G, Gallart T, Vives J, Arroyo V, Rodes J. Human hepatic stellate cells show features of antigen-presenting cells and stimulate lymphocyte proliferation. Hepatology. 2003;38:919–29. doi: 10.1053/jhep.2003.50392. [DOI] [PubMed] [Google Scholar]
- (90).Schwabe RF, Schnabl B, Kweon YO, Brenner DA. CD40 activates NF-kappa B and c-Jun N-terminal kinase and enhances chemokine secretion on activated human hepatic stellate cells. J Immunol. 2001;166:6812–9. doi: 10.4049/jimmunol.166.11.6812. [DOI] [PubMed] [Google Scholar]
- (91).Maher JJ. Interactions between hepatic stellate cells and the immune system. Semin Liver Dis. 2001;21:417–26. doi: 10.1055/s-2001-17555. [DOI] [PubMed] [Google Scholar]
- (92).Iredale JP. Hepatic stellate cell behavior during resolution of liver injury. Semin Liver Dis. 2001;21:427–36. doi: 10.1055/s-2001-17557. [DOI] [PubMed] [Google Scholar]
- (93).Issa R, Williams E, Trim N, Kendall T, Arthur MJ, Reichen J, Benyon RC, Iredale JP. Apoptosis of hepatic stellate cells: involvement in resolution of biliary fibrosis and regulation by soluble growth factors. Gut. 2001;48:548–57. doi: 10.1136/gut.48.4.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (94).Brenzel A, Gressner AM. Characterization of insulin-like growth factor (IGF)-I-receptor binding sites during in vitro transformation of rat hepatic stellate cells to myofibroblasts. Eur J Clin Chem Clin Biochem. 1996;34:401–9. doi: 10.1515/cclm.1996.34.5.401. [DOI] [PubMed] [Google Scholar]
- (95).Pinzani M, Abboud HE, Aron DC. Secretion of insulin-like growth factor-I and binding proteins by rat liver fat-storing cells: regulatory role of platelet-derived growth factor. Endocrinology. 1990;127:2343–9. doi: 10.1210/endo-127-5-2343. [DOI] [PubMed] [Google Scholar]
- (96).Saile B, Matthes N, Knittel T, Ramadori G. Transforming growth factor beta and tumor necrosis factor alpha inhibit both apoptosis and proliferation of activated rat hepatic stellate cells. Hepatology. 1999;30:196–202. doi: 10.1002/hep.510300144. [DOI] [PubMed] [Google Scholar]
- (97).Issa R, Zhou X, Trim N, Millward-Sadler H, Krane S, Benyon C, Iredale J. Mutation in collagen-1 that confers resistance to the action of collagenase results in failure of recovery from CCl4-induced liver fibrosis, persistence of activated hepatic stellate cells, and diminished hepatocyte regeneration. Faseb J. 2003;17:47–9. doi: 10.1096/fj.02-0494fje. [DOI] [PubMed] [Google Scholar]
- (98).Guedez L, Stetler-Stevenson WG, Wolff L, Wang J, Fukushima P, Mansoor A, Stetler-Stevenson M. In vitro suppression of programmed cell death of B cells by tissue inhibitor of metalloproteinases-1. J Clin Invest. 1998;102:2002–10. doi: 10.1172/JCI2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (99).Arthur MJ. Fibrogenesis II. Metalloproteinases and their inhibitors in liver fibrosis. Am J Physiol Gastrointest Liver Physiol. 2000;279:G245–9. doi: 10.1152/ajpgi.2000.279.2.G245. [DOI] [PubMed] [Google Scholar]
- (100).Benyon RC, Hovell CJ, Da Gaca M, Jones EH, Iredale JP, Arthur MJ. Progelatinase A is produced and activated by rat hepatic stellate cells and promotes their proliferation. Hepatology. 1999;30:977–86. doi: 10.1002/hep.510300431. [DOI] [PubMed] [Google Scholar]
- (101).Friedman SL. Seminars in medicine of the Beth Israel Hospital, Boston. The cellular basis of hepatic fibrosis. Mechanisms and treatment strategies. N Engl J Med. 1993;328:1828–35. doi: 10.1056/NEJM199306243282508. [DOI] [PubMed] [Google Scholar]
- (102).Maher JJ, McGuire RF. Extracellular matrix gene expression increases preferentially in rat lipocytes and sinusoidal endothelial cells during hepatic fibrosis in vivo. J Clin Invest. 1990;86:1641–8. doi: 10.1172/JCI114886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (103).Pinzani M. Liver fibrosis. Springer Semin Immunopathol. 1999;21:475–90. doi: 10.1007/s002810000037. [DOI] [PubMed] [Google Scholar]
- (104).Iredale JP, Goddard S, Murphy G, Benyon RC, Arthur MJ. Tissue inhibitor of metalloproteinase-I and interstitial collagenase expression in autoimmune chronic active hepatitis and activated human hepatic lipocytes. Clin Sci (Lond) 1995;89:75–81. doi: 10.1042/cs0890075. [DOI] [PubMed] [Google Scholar]
- (105).Iredale JP, Benyon RC, Arthur MJ, Ferris WF, Alcolado R, Winwood PJ, Clark N, Murphy G. Tissue inhibitor of metalloproteinase-1 messenger RNA expression is enhanced relative to interstitial collagenase messenger RNA in experimental liver injury and fibrosis. Hepatology. 1996;24:176–84. doi: 10.1002/hep.510240129. [DOI] [PubMed] [Google Scholar]
- (106).Benyon RC, Iredale JP, Goddard S, Winwood PJ, Arthur MJ. Expression of tissue inhibitor of metalloproteinases 1 and 2 is increased in fibrotic human liver. Gastroenterology. 1996;110:821–31. doi: 10.1053/gast.1996.v110.pm8608892. [DOI] [PubMed] [Google Scholar]
- (107).Winwood PJ, Schuppan D, Iredale JP, Kawser CA, Docherty AJ, Arthur MJ. Kupffer cell-derived 95-kd type IV collagenase/gelatinase B: characterization and expression in cultured cells. Hepatology. 1995;22:304–15. [PubMed] [Google Scholar]
- (108).Emonard H, Guillouzo A, Lapiere CM, Grimaud JA. Human liver fibroblast capacity for synthesizing interstitial collagenase in vitro. Cell Mol Biol. 1990;36:461–7. [PubMed] [Google Scholar]
- (109).Herbst H, Wege T, Milani S, Pellegrini G, Orzechowski HD, Bechstein WO, Neuhaus P, Gressner AM, Schuppan D. Tissue inhibitor of metalloproteinase-1 and -2 RNA expression in rat and human liver fibrosis. Am J Pathol. 1997;150:1647–59. [PMC free article] [PubMed] [Google Scholar]
- (110).Arthur MJ, Friedman SL, Roll FJ, Bissell DM. Lipocytes from normal rat liver release a neutral metalloproteinase that degrades basement membrane (type IV) collagen. J Clin Invest. 1989;84:1076–85. doi: 10.1172/JCI114270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (111).Reeves HL, Friedman SL. Activation of hepatic stellate cells--a key issue in liver fibrosis. Front Biosci. 2002;7:d808–26. doi: 10.2741/reeves. [DOI] [PubMed] [Google Scholar]
- (112).Kinnman N, Hultcrantz R, Barbu V, Rey C, Wendum D, Poupon R, Housset C. PDGF-mediated chemoattraction of hepatic stellate cells by bile duct segments in cholestatic liver injury. Lab Invest. 2000;80:697–707. doi: 10.1038/labinvest.3780073. [DOI] [PubMed] [Google Scholar]
- (113).Kinnman N, Goria O, Wendum D, Gendron MC, Rey C, Poupon R, Housset C. Hepatic stellate cell proliferation is an early platelet-derived growth factor-mediated cellular event in rat cholestatic liver injury. Lab Invest. 2001;81:1709–16. doi: 10.1038/labinvest.3780384. [DOI] [PubMed] [Google Scholar]
- (114).Friedman SL. Stellate cells: a moving target in hepatic fibrogenesis. Hepatology. 2004;40:1041–3. doi: 10.1002/hep.20476. [DOI] [PubMed] [Google Scholar]
- (115).Ramadori G, Saile B. Portal tract fibrogenesis in the liver. Lab Invest. 2004;84:153–9. doi: 10.1038/labinvest.3700030. [DOI] [PubMed] [Google Scholar]
- (116).Forbes SJ, Russo FP, Rey V, Burra P, Rugge M, Wright NA, Alison MR. A significant proportion of myofibroblasts are of bone marrow origin in human liver fibrosis. Gastroenterology. 2004;126:955–63. doi: 10.1053/j.gastro.2004.02.025. [DOI] [PubMed] [Google Scholar]
- (117).Guyot C, Lepreux S, Combe C, Doudnikoff E, Bioulac-Sage P, Balabaud C, Desmouliere A. Hepatic fibrosis and cirrhosis: the (myo)fibroblastic cell subpopulations involved. Int J Biochem Cell Biol. 2006;38:135–51. doi: 10.1016/j.biocel.2005.08.021. [DOI] [PubMed] [Google Scholar]
- (118).Kinnman N, Housset C. Peribiliary myofibroblasts in biliary type liver fibrosis. Front Biosci. 2002;7:d496–503. doi: 10.2741/A790. [DOI] [PubMed] [Google Scholar]
- (119).Magness ST, Bataller R, Yang L, Brenner DA. A dual reporter gene transgenic mouse demonstrates heterogeneity in hepatic fibrogenic cell populations. Hepatology. 2004;40:1151–9. doi: 10.1002/hep.20427. [DOI] [PubMed] [Google Scholar]
- (120).Andrade ZA, Guerret S, Fernandes AL. Myofibroblasts in schistosomal portal fibrosis of man. Mem Inst Oswaldo Cruz. 1999;94:87–93. doi: 10.1590/s0074-02761999000100018. [DOI] [PubMed] [Google Scholar]
- (121).Bhunchet E, Wake K. Role of mesenchymal cell populations in porcine serum-induced rat liver fibrosis. Hepatology. 1992;16:1452–73. doi: 10.1002/hep.1840160623. [DOI] [PubMed] [Google Scholar]
- (122).Tuchweber B, Desmouliere A, Bochaton-Piallat ML, Rubbia-Brandt L, Gabbiani G. Proliferation and phenotypic modulation of portal fibroblasts in the early stages of cholestatic fibrosis in the rat. Lab Invest. 1996;74:265–78. [PubMed] [Google Scholar]
- (123).Kinnman N, Francoz C, Barbu V, Wendum D, Rey C, Hultcrantz R, Poupon R, Housset C. The myofibroblastic conversion of peribiliary fibrogenic cells distinct from hepatic stellate cells is stimulated by platelet-derived growth factor during liver fibrogenesis. Lab Invest. 2003;83:163–73. doi: 10.1097/01.lab.0000054178.01162.e4. [DOI] [PubMed] [Google Scholar]
- (124).Knittel T, Kobold D, Saile B, Grundmann A, Neubauer K, Piscaglia F, Ramadori G. Rat liver myofibroblasts and hepatic stellate cells: different cell populations of the fibroblast lineage with fibrogenic potential. Gastroenterology. 1999;117:1205–21. doi: 10.1016/s0016-5085(99)70407-5. [DOI] [PubMed] [Google Scholar]
- (125).Cassiman D, Libbrecht L, Desmet V, Denef C, Roskams T. Hepatic stellate cell/myofibroblast subpopulations in fibrotic human and rat livers. J Hepatol. 2002;36:200–9. doi: 10.1016/s0168-8278(01)00260-4. [DOI] [PubMed] [Google Scholar]
- (126).Kobold D, Grundmann A, Piscaglia F, Eisenbach C, Neubauer K, Steffgen J, Ramadori G, Knittel T. Expression of reelin in hepatic stellate cells and during hepatic tissue repair: a novel marker for the differentiation of HSC from other liver myofibroblasts. J Hepatol. 2002;36:607–13. doi: 10.1016/s0168-8278(02)00050-8. [DOI] [PubMed] [Google Scholar]
- (127).Kim WH, Matsumoto K, Bessho K, Nakamura T. Growth inhibition and apoptosis in liver myofibroblasts promoted by hepatocyte growth factor leads to resolution from liver cirrhosis. Am J Pathol. 2005;166:1017–28. doi: 10.1016/S0002-9440(10)62323-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (128).D. Montgomery Bissell JJM. Hepatic Fibrosis and Cirrhosis. In: David Zakim TDB, editor. Hepatology: a Textbook of Liver Disease. WB Saunders; Philadelphia: 2003. pp. 395–416. [Google Scholar]
- (129).Friedman SL. Liver fibrosis -- from bench to bedside. J Hepatol. 2003;38(Suppl 1):S38–53. doi: 10.1016/s0168-8278(02)00429-4. [DOI] [PubMed] [Google Scholar]
- (130).Benyon RC, Iredale JP. Is liver fibrosis reversible? Gut. 2000;46:443–6. doi: 10.1136/gut.46.4.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (131).Burt AD, Griffiths MR, Schuppan D, Voss B, MacSween RN. Ultrastructural localization of extracellular matrix proteins in liver biopsies using ultracryomicrotomy and immuno-gold labelling. Histopathology. 1990;16:53–8. doi: 10.1111/j.1365-2559.1990.tb01060.x. [DOI] [PubMed] [Google Scholar]
- (132).Schuppan D. Structure of the extracellular matrix in normal and fibrotic liver: collagens and glycoproteins. Semin Liver Dis. 1990;10:1–10. doi: 10.1055/s-2008-1040452. [DOI] [PubMed] [Google Scholar]
- (133).Gressner AM, Haarmann R. Hyaluronic acid synthesis and secretion by rat liver fat storing cells (perisinusoidal lipocytes) in culture. Biochem Biophys Res Commun. 1988;151:222–9. doi: 10.1016/0006-291x(88)90582-7. [DOI] [PubMed] [Google Scholar]
- (134).Hahn E, Wick G, Pencev D, Timpl R. Distribution of basement membrane proteins in normal and fibrotic human liver: collagen type IV, laminin, and fibronectin. Gut. 1980;21:63–71. doi: 10.1136/gut.21.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (135).Rojkind M, Giambrone MA, Biempica L. Collagen types in normal and cirrhotic liver. Gastroenterology. 1979;76:710–9. [PubMed] [Google Scholar]
- (136).Seyer JM, Hutcheson ET, Kang AH. Collagen polymorphism in normal and cirrhotic human liver. J Clin Invest. 1977;59:241–8. doi: 10.1172/JCI108634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (137).Lindquist JN, Marzluff WF, Stefanovic B. Fibrogenesis. III. Posttranscriptional regulation of type I collagen. Am J Physiol Gastrointest Liver Physiol. 2000;279:G471–6. doi: 10.1152/ajpgi.2000.279.3.G471. [DOI] [PubMed] [Google Scholar]
- (138).Lindquist JN, Parsons CJ, Stefanovic B, Brenner DA. Regulation of alpha1(I) collagen messenger RNA decay by interactions with alphaCP at the 3'-untranslated region. J Biol Chem. 2004;279:23822–9. doi: 10.1074/jbc.M314060200. [DOI] [PubMed] [Google Scholar]
- (139).Stefanovic B, Hellerbrand C, Holcik M, Briendl M, Aliebhaber S, Brenner DA. Posttranscriptional regulation of collagen alpha1(I) mRNA in hepatic stellate cells. Mol Cell Biol. 1997;17:5201–9. doi: 10.1128/mcb.17.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (140).Milani S, Herbst H, Schuppan D, Grappone C, Pellegrini G, Pinzani M, Casini A, Calabro A, Ciancio G, Stefanini F, et al. Differential expression of matrix-metalloproteinase-1 and -2 genes in normal and fibrotic human liver. Am J Pathol. 1994;144:528–37. [PMC free article] [PubMed] [Google Scholar]
- (141).Murphy G, Stanton H, Cowell S, Butler G, Knauper V, Atkinson S, Gavrilovic J. Mechanisms for pro matrix metalloproteinase activation. Apmis. 1999;107:38–44. doi: 10.1111/j.1699-0463.1999.tb01524.x. [DOI] [PubMed] [Google Scholar]
- (142).Matrisian LM. The matrix-degrading metalloproteinases. Bioessays. 1992;14:455–63. doi: 10.1002/bies.950140705. [DOI] [PubMed] [Google Scholar]
- (143).Leyland H, Gentry J, Arthur MJ, Benyon RC. The plasminogen-activating system in hepatic stellate cells. Hepatology. 1996;24:1172–8. doi: 10.1002/hep.510240532. [DOI] [PubMed] [Google Scholar]
- (144).Knittel T, Fellmer P, Ramadori G. Gene expression and regulation of plasminogen activator inhibitor type I in hepatic stellate cells of rat liver. Gastroenterology. 1996;111:745–54. doi: 10.1053/gast.1996.v111.pm8780581. [DOI] [PubMed] [Google Scholar]
- (145).Olaso E, Ikeda K, Eng FJ, Xu L, Wang LH, Lin HC, Friedman SL. DDR2 receptor promotes MMP-2-mediated proliferation and invasion by hepatic stellate cells. J Clin Invest. 2001;108:1369–78. doi: 10.1172/JCI12373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (146).McGuire RF, Bissell DM, Boyles J, Roll FJ. Role of extracellular matrix in regulating fenestrations of sinusoidal endothelial cells isolated from normal rat liver. Hepatology. 1992;15:989–97. doi: 10.1002/hep.1840150603. [DOI] [PubMed] [Google Scholar]
- (147).Bissell DM, Caron JM, Babiss LE, Friedman JM. Transcriptional regulation of the albumin gene in cultured rat hepatocytes. Role of basement-membrane matrix. Mol Biol Med. 1990;7:187–97. [PubMed] [Google Scholar]
- (148).Bissell DM, Arenson DM, Maher JJ, Roll FJ. Support of cultured hepatocytes by a laminin-rich gel. Evidence for a functionally significant subendothelial matrix in normal rat liver. J Clin Invest. 1987;79:801–12. doi: 10.1172/JCI112887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (149).Friedman SL, Roll FJ, Boyles J, Arenson DM, Bissell DM. Maintenance of differentiated phenotype of cultured rat hepatic lipocytes by basement membrane matrix. J Biol Chem. 1989;264:10756–62. [PubMed] [Google Scholar]
- (150).Shrivastava A, Radziejewski C, Campbell E, Kovac L, McGlynn M, Ryan TE, Davis S, Goldfarb MP, Glass DJ, Lemke G, Yancopoulos GD. An orphan receptor tyrosine kinase family whose members serve as nonintegrin collagen receptors. Mol Cell. 1997;1:25–34. doi: 10.1016/s1097-2765(00)80004-0. [DOI] [PubMed] [Google Scholar]
- (151).Cassiman D, Roskams T. Beauty is in the eye of the beholder: emerging concepts and pitfalls in hepatic stellate cell research. J Hepatol. 2002;37:527–35. doi: 10.1016/s0168-8278(02)00263-5. [DOI] [PubMed] [Google Scholar]
- (152).Hoofring A, Boitnott J, Torbenson M. Three-dimensional reconstruction of hepatic bridging fibrosis in chronic hepatitis C viral infection. J Hepatol. 2003;39:738–41. doi: 10.1016/s0168-8278(03)00413-6. [DOI] [PubMed] [Google Scholar]
- (153).Arthur MJ. Reversibility of liver fibrosis and cirrhosis following treatment for hepatitis C. Gastroenterology. 2002;122:1525–8. doi: 10.1053/gast.2002.33367. [DOI] [PubMed] [Google Scholar]
- (154).Issa R, Zhou X, Constandinou CM, Fallowfield J, Millward-Sadler H, Gaca MD, Sands E, Suliman I, Trim N, Knorr A, Arthur MJ, Benyon RC, Iredale JP. Spontaneous recovery from micronodular cirrhosis: evidence for incomplete resolution associated with matrix cross-linking. Gastroenterology. 2004;126:1795–808. doi: 10.1053/j.gastro.2004.03.009. [DOI] [PubMed] [Google Scholar]
- (155).Wanless IR, Nakashima E, Sherman M. Regression of human cirrhosis. Morphologic features and the genesis of incomplete septal cirrhosis. Arch Pathol Lab Med. 2000;124:1599–607. doi: 10.5858/2000-124-1599-ROHC. [DOI] [PubMed] [Google Scholar]
- (156).Czaja AJ, Carpenter HA. Decreased fibrosis during corticosteroid therapy of autoimmune hepatitis. J Hepatol. 2004;40:646–52. doi: 10.1016/j.jhep.2004.01.009. [DOI] [PubMed] [Google Scholar]
- (157).Dixon JB, Bhathal PS, Hughes NR, O'Brien PE. Nonalcoholic fatty liver disease: Improvement in liver histological analysis with weight loss. Hepatology. 2004;39:1647–54. doi: 10.1002/hep.20251. [DOI] [PubMed] [Google Scholar]
- (158).Hammel P, Couvelard A, O'Toole D, Ratouis A, Sauvanet A, Flejou JF, Degott C, Belghiti J, Bernades P, Valla D, Ruszniewski P, Levy P. Regression of liver fibrosis after biliary drainage in patients with chronic pancreatitis and stenosis of the common bile duct. N Engl J Med. 2001;344:418–23. doi: 10.1056/NEJM200102083440604. [DOI] [PubMed] [Google Scholar]
- (159).Kweon YO, Goodman ZD, Dienstag JL, Schiff ER, Brown NA, Burchardt E, Schoonhoven R, Brenner DA, Fried MW. Decreasing fibrogenesis: an immunohistochemical study of paired liver biopsies following lamivudine therapy for chronic hepatitis B. J Hepatol. 2001;35:749–55. doi: 10.1016/s0168-8278(01)00218-5. [DOI] [PubMed] [Google Scholar]
- (160).Pares A, Caballeria J, Bruguera M, Torres M, Rodes J. Histological course of alcoholic hepatitis. Influence of abstinence, sex and extent of hepatic damage. J Hepatol. 1986;2:33–42. doi: 10.1016/s0168-8278(86)80006-x. [DOI] [PubMed] [Google Scholar]
- (161).Poynard T, McHutchison J, Manns M, Trepo C, Lindsay K, Goodman Z, Ling MH, Albrecht J. Impact of pegylated interferon alfa-2b and ribavirin on liver fibrosis in patients with chronic hepatitis C. Gastroenterology. 2002;122:1303–13. doi: 10.1053/gast.2002.33023. [DOI] [PubMed] [Google Scholar]
- (162).Albanis E, Friedman SL. Antifibrotic agents for liver disease. Am J Transplant. 2006;6:12–9. doi: 10.1111/j.1600-6143.2005.01143.x. [DOI] [PubMed] [Google Scholar]
- (163).Mathew J, Hines JE, James OF, Burt AD. Non-parenchymal cell responses in paracetamol (acetaminophen)-induced liver injury. J Hepatol. 1994;20:537–41. doi: 10.1016/s0168-8278(05)80502-1. [DOI] [PubMed] [Google Scholar]
- (164).Mathew J, Hines JE, Toole K, Johnson SJ, James OF, Burt AD. Quantitative analysis of macrophages and perisinusoidal cells in primary biliary cirrhosis. Histopathology. 1994;25:65–70. doi: 10.1111/j.1365-2559.1994.tb00599.x. [DOI] [PubMed] [Google Scholar]
- (165).Iredale JP, Benyon RC, Pickering J, McCullen M, Northrop M, Pawley S, Hovell C, Arthur MJ. Mechanisms of spontaneous resolution of rat liver fibrosis. Hepatic stellate cell apoptosis and reduced hepatic expression of metalloproteinase inhibitors. J Clin Invest. 1998;102:538–49. doi: 10.1172/JCI1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (166).Saile B, Knittel T, Matthes N, Schott P, Ramadori G. CD95/CD95L-mediated apoptosis of the hepatic stellate cell. A mechanism terminating uncontrolled hepatic stellate cell proliferation during hepatic tissue repair. Am J Pathol. 1997;151:1265–72. [PMC free article] [PubMed] [Google Scholar]
- (167).Gong W, Pecci A, Roth S, Lahme B, Beato M, Gressner AM. Transformation-dependent susceptibility of rat hepatic stellate cells to apoptosis induced by soluble Fas ligand. Hepatology. 1998;28:492–502. doi: 10.1002/hep.510280229. [DOI] [PubMed] [Google Scholar]
- (168).Taimr P, Higuchi H, Kocova E, Rippe RA, Friedman S, Gores GJ. Activated stellate cells express the TRAIL receptor-2/death receptor-5 and undergo TRAIL-mediated apoptosis. Hepatology. 2003;37:87–95. doi: 10.1053/jhep.2003.50002. [DOI] [PubMed] [Google Scholar]
- (169).Trim N, Morgan S, Evans M, Issa R, Fine D, Afford S, Wilkins B, Iredale J. Hepatic stellate cells express the low affinity nerve growth factor receptor p75 and undergo apoptosis in response to nerve growth factor stimulation. Am J Pathol. 2000;156:1235–43. doi: 10.1016/S0002-9440(10)64994-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (170).Leon A, Buriani A, Dal Toso R, Fabris M, Romanello S, Aloe L, Levi-Montalcini R. Mast cells synthesize, store, and release nerve growth factor. Proc Natl Acad Sci U S A. 1994;91:3739–43. doi: 10.1073/pnas.91.9.3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (171).Gaca MD, Zhou X, Issa R, Kiriella K, Iredale JP, Benyon RC. Basement membrane-like matrix inhibits proliferation and collagen synthesis by activated rat hepatic stellate cells: evidence for matrix-dependent deactivation of stellate cells. Matrix Biol. 2003;22:229–39. doi: 10.1016/s0945-053x(03)00017-9. [DOI] [PubMed] [Google Scholar]
- (172).Sohara N, Znoyko I, Levy MT, Trojanowska M, Reuben A. Reversal of activation of human myofibroblast-like cells by culture on a basement membrane-like substrate. J Hepatol. 2002;37:214–21. doi: 10.1016/s0168-8278(02)00103-4. [DOI] [PubMed] [Google Scholar]
- (173).Wang SC, Ohata M, Schrum L, Rippe RA, Tsukamoto H. Expression of interleukin-10 by in vitro and in vivo activated hepatic stellate cells. J Biol Chem. 1998;273:302–8. doi: 10.1074/jbc.273.1.302. [DOI] [PubMed] [Google Scholar]
- (174).Louis H, Van Laethem JL, Wu W, Quertinmont E, Degraef C, Van den Berg K, Demols A, Goldman M, Le Moine O, Geerts A, Deviere J. Interleukin-10 controls neutrophilic infiltration, hepatocyte proliferation, and liver fibrosis induced by carbon tetrachloride in mice. Hepatology. 1998;28:1607–15. doi: 10.1002/hep.510280621. [DOI] [PubMed] [Google Scholar]
- (175).Wu J, Zern MA. Hepatic stellate cells: a target for the treatment of liver fibrosis. J Gastroenterol. 2000;35:665–72. doi: 10.1007/s005350070045. [DOI] [PubMed] [Google Scholar]
- (176).Bataller R, Brenner DA. Hepatic stellate cells as a target for the treatment of liver fibrosis. Semin Liver Dis. 2001;21:437–51. doi: 10.1055/s-2001-17558. [DOI] [PubMed] [Google Scholar]
- (177).Mallat A, Preaux AM, Blazejewski S, Rosenbaum J, Dhumeaux D, Mavier P. Interferon alfa and gamma inhibit proliferation and collagen synthesis of human Ito cells in culture. Hepatology. 1995;21:1003–10. [PubMed] [Google Scholar]
- (178).Bueno MR, Daneri A, Armendariz-Borunda J. Cholestasis-induced fibrosis is reduced by interferon alpha-2a and is associated with elevated liver metalloprotease activity. J Hepatol. 2000;33:915–25. doi: 10.1016/s0168-8278(00)80123-3. [DOI] [PubMed] [Google Scholar]
- (179).Poynard T, McHutchison J, Davis GL, Esteban-Mur R, Goodman Z, Bedossa P, Albrecht J. Impact of interferon alfa-2b and ribavirin on progression of liver fibrosis in patients with chronic hepatitis C. Hepatology. 2000;32:1131–7. doi: 10.1053/jhep.2000.19347. [DOI] [PubMed] [Google Scholar]
- (180).Davis GL, Esteban-Mur R, Rustgi V, Hoefs J, Gordon SC, Trepo C, Shiffman ML, Zeuzem S, Craxi A, Ling MH, Albrecht J. Interferon alfa-2b alone or in combination with ribavirin for the treatment of relapse of chronic hepatitis C. International Hepatitis Interventional Therapy Group. N Engl J Med. 1998;339:1493–9. doi: 10.1056/NEJM199811193392102. [DOI] [PubMed] [Google Scholar]
- (181).Poynard T, Moussali J, Ratziu V, Regimbeau C, Opolon P. Effects of interferon therapy in “non responder” patients with chronic hepatitis C. J Hepatol. 1999;31(Suppl 1):178–83. doi: 10.1016/s0168-8278(99)80397-3. [DOI] [PubMed] [Google Scholar]
- (182).Guerret S, Desmouliere A, Chossegros P, Costa AM, Badid C, Trepo C, Grimaud JA, Chevallier M. Long-term administration of interferon-alpha in non-responder patients with chronic hepatitis C: follow-up of liver fibrosis over 5 years. J Viral Hepat. 1999;6:125–33. doi: 10.1046/j.1365-2893.1999.00148.x. [DOI] [PubMed] [Google Scholar]
- (183).Baker DE. Pegylated interferon plus ribavirin for the treatment of chronic hepatitis C. Rev Gastroenterol Disord. 2003;3:93–109. [PubMed] [Google Scholar]
- (184).Matthews SJ, McCoy C. Peginterferon alfa-2a: a review of approved and investigational uses. Clin Ther. 2004;26:991–1025. doi: 10.1016/s0149-2918(04)90173-7. [DOI] [PubMed] [Google Scholar]
- (185).Meyer-Wyss B, Rich P, Egger H, Helbling B, Mullhaupt B, Rammert C, Gonvers JJ, Oneta C, Criblez D, Rossi L, Borovicka J, Meyenberger C, Arn M, Renner EL. Comparison of two PEG-interferon alpha-2b doses (1.0 or 1.5 microg/kg) combined with ribavirin in interferon-naive patients with chronic hepatitis C and up to moderate fibrosis. J Viral Hepat. 2006;13:457–65. doi: 10.1111/j.1365-2893.2005.00709.x. [DOI] [PubMed] [Google Scholar]
- (186).Babatin M, Schindel L, Burak KW. Pegylated-interferon alpha 2b and ribavirin for recurrent hepatitis C after liver transplantation: From a Canadian experience to recommendations for therapy. Can J Gastroenterol. 2005;19:359–65. doi: 10.1155/2005/745197. [DOI] [PubMed] [Google Scholar]
- (187).Tasci I, Mas MR, Vural SA, Comert B, Alcigir G, Serdar M, Mas N, Isik AT, Ates Y. Rat liver fibrosis regresses better with pegylated interferon alpha2b and ursodeoxycholic acid treatments than spontaneous recovery. Liver Int. 2006;26:261–8. doi: 10.1111/j.1478-3231.2005.01210.x. [DOI] [PubMed] [Google Scholar]
- (188).Canbay A, Feldstein A, Baskin-Bey E, Bronk SF, Gores GJ. The caspase inhibitor IDN-6556 attenuates hepatic injury and fibrosis in the bile duct ligated mouse. J Pharmacol Exp Ther. 2004;308:1191–6. doi: 10.1124/jpet.103.060129. [DOI] [PubMed] [Google Scholar]
- (189).Hoglen NC, Anselmo DM, Katori M, Kaldas M, Shen XD, Valentino KL, Lassman C, Busuttil RW, Kupiec-Weglinski JW, Farmer DG. A caspase inhibitor, IDN-6556, ameliorates early hepatic injury in an ex vivo rat model of warm and cold ischemia. Liver Transpl. 2007;13:361–6. doi: 10.1002/lt.21016. [DOI] [PubMed] [Google Scholar]
- (190).Poordad FF. IDN-6556 Idun Pharmaceuticals Inc. Curr Opin Investig Drugs. 2004;5:1198–204. [PubMed] [Google Scholar]
- (191).Zhang H, Cook J, Nickel J, Yu R, Stecker K, Myers K, Dean NM. Reduction of liver Fas expression by an antisense oligonucleotide protects mice from fulminant hepatitis. Nat Biotechnol. 2000;18:862–7. doi: 10.1038/78475. [DOI] [PubMed] [Google Scholar]
- (192).Song E, Lee SK, Wang J, Ince N, Ouyang N, Min J, Chen J, Shankar P, Lieberman J. RNA interference targeting Fas protects mice from fulminant hepatitis. Nat Med. 2003;9:347–51. doi: 10.1038/nm828. [DOI] [PubMed] [Google Scholar]
- (193).Pellegrini M, Strasser A. A portrait of the Bcl-2 protein family: life, death, and the whole picture. J Clin Immunol. 1999;19:365–77. doi: 10.1023/a:1020598632068. [DOI] [PubMed] [Google Scholar]
- (194).Zhang H, Taylor J, Luther D, Johnston J, Murray S, Wyatt JR, Watt AT, Koo S, York-DeFalco C, Stecker K, Dean NM. Antisense oligonucleotide inhibition of Bcl-xL and Bid expression in liver regulates responses in a mouse model of Fas-induced fulminant hepatitis. J Pharmacol Exp Ther. 2003;307:24–33. doi: 10.1124/jpet.103.050435. [DOI] [PubMed] [Google Scholar]
- (195).Zender L, Hutker S, Liedtke C, Tillmann HL, Zender S, Mundt B, Waltemathe M, Gosling T, Flemming P, Malek NP, Trautwein C, Manns MP, Kuhnel F, Kubicka S. Caspase 8 small interfering RNA prevents acute liver failure in mice. Proc Natl Acad Sci U S A. 2003;100:7797–802. doi: 10.1073/pnas.1330920100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (196).Dufour JF, DeLellis R, Kaplan MM. Reversibility of hepatic fibrosis in autoimmune hepatitis. Ann Intern Med. 1997;127:981–5. doi: 10.7326/0003-4819-127-11-199712010-00006. [DOI] [PubMed] [Google Scholar]
- (197).Mitchison HC, Palmer JM, Bassendine MF, Watson AJ, Record CO, James OF. A controlled trial of prednisolone treatment in primary biliary cirrhosis. Three-year results. J Hepatol. 1992;15:336–44. doi: 10.1016/0168-8278(92)90065-w. [DOI] [PubMed] [Google Scholar]
- (198).Lindor KD, Wiesner RH, Colwell LJ, Steiner B, Beaver S, LaRusso NF. The combination of prednisone and colchicine in patients with primary sclerosing cholangitis. Am J Gastroenterol. 1991;86:57–61. [PubMed] [Google Scholar]
- (199).Ryhanen L, Stenback F, Ala-Kokko L, Savolainen ER. The effect of malotilate on type III and type IV collagen, laminin and fibronectin metabolism in dimethylnitrosamine-induced liver fibrosis in the rat. J Hepatol. 1996;24:238–45. doi: 10.1016/s0168-8278(96)80035-3. [DOI] [PubMed] [Google Scholar]
- (200).Keiding S, Badsberg JH, Becker U, Bentsen KD, Bonnevie O, Caballeria J, Eriksen J, Hardt F, Keiding N, Morgan M, et al. The prognosis of patients with alcoholic liver disease. An international randomized, placebo-controlled trial on the effect of malotilate on survival. J Hepatol. 1994;20:454–60. doi: 10.1016/s0168-8278(05)80489-1. [DOI] [PubMed] [Google Scholar]
- (201).Mancini R, Benedetti A, Jezequel AM. An interleukin-1 receptor antagonist decreases fibrosis induced by dimethylnitrosamine in rat liver. Virchows Arch. 1994;424:25–31. doi: 10.1007/BF00197389. [DOI] [PubMed] [Google Scholar]
- (202).Bruck R, Hershkoviz R, Lider O, Shirin H, Aeed H, Halpern Z. The use of synthetic analogues of Arg-Gly-Asp (RGD) and soluble receptor of tumor necrosis factor to prevent acute and chronic experimental liver injury. Yale J Biol Med. 1997;70:391–402. [PMC free article] [PubMed] [Google Scholar]
- (203).Thompson K, Maltby J, Fallowfield J, McAulay M, Millward-Sadler H, Sheron N. Interleukin-10 expression and function in experimental murine liver inflammation and fibrosis. Hepatology. 1998;28:1597–606. doi: 10.1002/hep.510280620. [DOI] [PubMed] [Google Scholar]
- (204).Nelson DR, Lauwers GY, Lau JY, Davis GL. Interleukin 10 treatment reduces fibrosis in patients with chronic hepatitis C: a pilot trial of interferon nonresponders. Gastroenterology. 2000;118:655–60. doi: 10.1016/s0016-5085(00)70134-x. [DOI] [PubMed] [Google Scholar]
- (205).Nelson DR, Tu Z, Soldevila-Pico C, Abdelmalek M, Zhu H, Xu YL, Cabrera R, Liu C, Davis GL. Long-term interleukin 10 therapy in chronic hepatitis C patients has a proviral and anti-inflammatory effect. Hepatology. 2003;38:859–68. doi: 10.1053/jhep.2003.50427. [DOI] [PubMed] [Google Scholar]
- (206).Gressner AM, Weiskirchen R, Breitkopf K, Dooley S. Roles of TGF-beta in hepatic fibrosis. Front Biosci. 2002;7:d793–807. doi: 10.2741/A812. [DOI] [PubMed] [Google Scholar]
- (207).Wells RG. Fibrogenesis. V. TGF-beta signaling pathways. Am J Physiol Gastrointest Liver Physiol. 2000;279:G845–50. doi: 10.1152/ajpgi.2000.279.5.G845. [DOI] [PubMed] [Google Scholar]
- (208).Okuno M, Moriwaki H, Muto Y, Kojima S. Protease inhibitors suppress TGF-beta generation by hepatic stellate cells. J Hepatol. 1998;29:1031–2. doi: 10.1016/s0168-8278(98)80136-0. [DOI] [PubMed] [Google Scholar]
- (209).Nakamura T, Sakata R, Ueno T, Sata M, Ueno H. Inhibition of transforming growth factor beta prevents progression of liver fibrosis and enhances hepatocyte regeneration in dimethylnitrosamine-treated rats. Hepatology. 2000;32:247–55. doi: 10.1053/jhep.2000.9109. [DOI] [PubMed] [Google Scholar]
- (210).George J, Roulot D, Koteliansky VE, Bissell DM. In vivo inhibition of rat stellate cell activation by soluble transforming growth factor beta type II receptor: a potential new therapy for hepatic fibrosis. Proc Natl Acad Sci U S A. 1999;96:12719–24. doi: 10.1073/pnas.96.22.12719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (211).Qi Z, Atsuchi N, Ooshima A, Takeshita A, Ueno H. Blockade of type beta transforming growth factor signaling prevents liver fibrosis and dysfunction in the rat. Proc Natl Acad Sci U S A. 1999;96:2345–9. doi: 10.1073/pnas.96.5.2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (212).Yata Y, Gotwals P, Koteliansky V, Rockey DC. Dose-dependent inhibition of hepatic fibrosis in mice by a TGF-beta soluble receptor: implications for antifibrotic therapy. Hepatology. 2002;35:1022–30. doi: 10.1053/jhep.2002.32673. [DOI] [PubMed] [Google Scholar]
- (213).Dooley S, Hamzavi J, Breitkopf K, Wiercinska E, Said HM, Lorenzen J, Ten Dijke P, Gressner AM. Smad7 prevents activation of hepatic stellate cells and liver fibrosis in rats. Gastroenterology. 2003;125:178–91. doi: 10.1016/s0016-5085(03)00666-8. [DOI] [PubMed] [Google Scholar]
- (214).Kim KH, Kim HC, Hwang MY, Oh HK, Lee TS, Chang YC, Song HJ, Won NH, Park KK. The antifibrotic effect of TGF-beta1 siRNAs in murine model of liver cirrhosis. Biochem Biophys Res Commun. 2006;343:1072–8. doi: 10.1016/j.bbrc.2006.03.087. [DOI] [PubMed] [Google Scholar]
- (215).Ponnappa BC, Dey I, Tu GC, Zhou F, Aini M, Cao QN, Israel Y. In vivo delivery of antisense oligonucleotides in pH-sensitive liposomes inhibits lipopolysaccharide-induced production of tumor necrosis factor-alpha in rats. J Pharmacol Exp Ther. 2001;297:1129–36. [PubMed] [Google Scholar]
- (216).Ponnappa BC, Israel Y. Targeting Kupffer cells with antisense oligonucleotides. Front Biosci. 2002;7:e223–33. doi: 10.2741/A918. [DOI] [PubMed] [Google Scholar]
- (217).Li G, Xie Q, Shi Y, Li D, Zhang M, Jiang S, Zhou H, Lu H, Jin Y. Inhibition of connective tissue growth factor by siRNA prevents liver fibrosis in rats. J Gene Med. 2006;8:889–900. doi: 10.1002/jgm.894. [DOI] [PubMed] [Google Scholar]
- (218).Rockey DC, Maher JJ, Jarnagin WR, Gabbiani G, Friedman SL. Inhibition of rat hepatic lipocyte activation in culture by interferon-gamma. Hepatology. 1992;16:776–84. doi: 10.1002/hep.1840160325. [DOI] [PubMed] [Google Scholar]
- (219).Rockey DC, Chung JJ. Interferon gamma inhibits lipocyte activation and extracellular matrix mRNA expression during experimental liver injury: implications for treatment of hepatic fibrosis. J Investig Med. 1994;42:660–70. [PubMed] [Google Scholar]
- (220).Baroni GS, D'Ambrosio L, Curto P, Casini A, Mancini R, Jezequel AM, Benedetti A. Interferon gamma decreases hepatic stellate cell activation and extracellular matrix deposition in rat liver fibrosis. Hepatology. 1996;23:1189–99. doi: 10.1002/hep.510230538. [DOI] [PubMed] [Google Scholar]
- (221).Inagaki Y, Nemoto T, Kushida M, Sheng Y, Higashi K, Ikeda K, Kawada N, Shirasaki F, Takehara K, Sugiyama K, Fujii M, Yamauchi H, Nakao A, de Crombrugghe B, Watanabe T, Okazaki I. Interferon alfa down-regulates collagen gene transcription and suppresses experimental hepatic fibrosis in mice. Hepatology. 2003;38:890–9. doi: 10.1053/jhep.2003.50408. [DOI] [PubMed] [Google Scholar]
- (222).Manabe N, Chevallier M, Chossegros P, Causse X, Guerret S, Trepo C, Grimaud JA. Interferon-alpha 2b therapy reduces liver fibrosis in chronic non-A, non-B hepatitis: a quantitative histological evaluation. Hepatology. 1993;18:1344–9. [PubMed] [Google Scholar]
- (223).Duchatelle V, Marcellin P, Giostra E, Bregeaud L, Pouteau M, Boyer N, Auperin A, Guerret S, Erlinger S, Henin D, Degott C. Changes in liver fibrosis at the end of alpha interferon therapy and 6 to 18 months later in patients with chronic hepatitis C: quantitative assessment by a morphometric method. J Hepatol. 1998;29:20–8. doi: 10.1016/s0168-8278(98)80174-8. [DOI] [PubMed] [Google Scholar]
- (224).Weng HL, Wang BE, Jia JD, Wu WF, Xian JZ, Mertens PR, Cai WM, Dooley S. Effect of interferon-gamma on hepatic fibrosis in chronic hepatitis B virus infection: a randomized controlled study. Clin Gastroenterol Hepatol. 2005;3:819–28. doi: 10.1016/s1542-3565(05)00404-0. [DOI] [PubMed] [Google Scholar]
- (225).Weng H, Mertens PR, Gressner AM, Dooley S. IFN-gamma abrogates profibrogenic TGF-beta signaling in liver by targeting expression of inhibitory and receptor Smads. J Hepatol. 2007;46:295–303. doi: 10.1016/j.jhep.2006.09.014. [DOI] [PubMed] [Google Scholar]
- (226).Preaux AM, Mallat A, Rosenbaum J, Zafrani ES, Mavier P. Pentoxifylline inhibits growth and collagen synthesis of cultured human hepatic myofibroblast-like cells. Hepatology. 1997;26:315–22. doi: 10.1002/hep.510260210. [DOI] [PubMed] [Google Scholar]
- (227).Pinzani M, Marra F, Caligiuri A, DeFranco R, Gentilini A, Failli P, Gentilini P. Inhibition by pentoxifylline of extracellular signal-regulated kinase activation by platelet-derived growth factor in hepatic stellate cells. Br J Pharmacol. 1996;119:1117–24. doi: 10.1111/j.1476-5381.1996.tb16012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (228).Windmeier C, Gressner AM. Pharmacological aspects of pentoxifylline with emphasis on its inhibitory actions on hepatic fibrogenesis. Gen Pharmacol. 1997;29:181–96. doi: 10.1016/s0306-3623(96)00314-x. [DOI] [PubMed] [Google Scholar]
- (229).Di Sario A, Bendia E, Svegliati Baroni G, Ridolfi F, Bolognini L, Feliciangeli G, Jezequel AM, Orlandi F, Benedetti A. Intracellular pathways mediating Na+/H+ exchange activation by platelet-derived growth factor in rat hepatic stellate cells. Gastroenterology. 1999;116:1155–66. doi: 10.1016/s0016-5085(99)70019-3. [DOI] [PubMed] [Google Scholar]
- (230).Svegliati-Baroni G, Di Sario A, Casini A, Ferretti G, D'Ambrosio L, Ridolfi F, Bolognini L, Salzano R, Orlandi F, Benedetti A. The Na+/H+ exchanger modulates the fibrogenic effect of oxidative stress in rat hepatic stellate cells. J Hepatol. 1999;30:868–75. doi: 10.1016/s0168-8278(99)80141-x. [DOI] [PubMed] [Google Scholar]
- (231).Benedetti A, Di Sario A, Casini A, Ridolfi F, Bendia E, Pigini P, Tonnini C, D'Ambrosio L, Feliciangeli G, Macarri G, Svegliati-Baroni G. Inhibition of the NA(+)/H(+) exchanger reduces rat hepatic stellate cell activity and liver fibrosis: an in vitro and in vivo study. Gastroenterology. 2001;120:545–56. doi: 10.1053/gast.2001.21203. [DOI] [PubMed] [Google Scholar]
- (232).Reif S, Weis B, Aeed H, Gana-Weis M, Zaidel L, Avni Y, Romanelli RG, Pinzani M, Kloog Y, Bruck R. The Ras antagonist, farnesylthiosalicylic acid (FTS), inhibits experimentally-induced liver cirrhosis in rats. J Hepatol. 1999;31:1053–61. doi: 10.1016/s0168-8278(99)80318-3. [DOI] [PubMed] [Google Scholar]
- (233).Wang YQ, Ikeda K, Ikebe T, Hirakawa K, Sowa M, Nakatani K, Kawada N, Kaneda K. Inhibition of hepatic stellate cell proliferation and activation by the semisynthetic analogue of fumagillin TNP-470 in rats. Hepatology. 2000;32:980–9. doi: 10.1053/jhep.2000.18658. [DOI] [PubMed] [Google Scholar]
- (234).Lieber CS. Alcoholic liver disease: new insights in pathogenesis lead to new treatments. J Hepatol. 2000;32:113–28. doi: 10.1016/s0168-8278(00)80420-1. [DOI] [PubMed] [Google Scholar]
- (235).Deulofeu R, Pares A, Rubio M, Gasso M, Roman J, Gimenez A, Varela-Moreiras G, Caballeria J, Ballesta AM, Mato JM, Rodes J. S-adenosylmethionine prevents hepatic tocopherol depletion in carbon tetrachloride-injured rats. Clin Sci (Lond) 2000;99:315–20. [PubMed] [Google Scholar]
- (236).Sokol RJ. Antioxidant defenses in metal-induced liver damage. Semin Liver Dis. 1996;16:39–46. doi: 10.1055/s-2007-1007217. [DOI] [PubMed] [Google Scholar]
- (237).Parola M, Leonarduzzi G, Biasi F, Albano E, Biocca ME, Poli G, Dianzani MU. Vitamin E dietary supplementation protects against carbon tetrachloride-induced chronic liver damage and cirrhosis. Hepatology. 1992;16:1014–21. doi: 10.1002/hep.1840160426. [DOI] [PubMed] [Google Scholar]
- (238).de la Maza MP, Petermann M, Bunout D, Hirsch S. Effects of long-term vitamin E supplementation in alcoholic cirrhotics. J Am Coll Nutr. 1995;14:192–6. doi: 10.1080/07315724.1995.10718493. [DOI] [PubMed] [Google Scholar]
- (239).Houglum K, Venkataramani A, Lyche K, Chojkier M. A pilot study of the effects of d-alpha-tocopherol on hepatic stellate cell activation in chronic hepatitis C. Gastroenterology. 1997;113:1069–73. doi: 10.1053/gast.1997.v113.pm9322499. [DOI] [PubMed] [Google Scholar]
- (240).Ma X, Zhao J, Lieber CS. Polyenylphosphatidylcholine attenuates non-alcoholic hepatic fibrosis and accelerates its regression. J Hepatol. 1996;24:604–13. doi: 10.1016/s0168-8278(96)80147-4. [DOI] [PubMed] [Google Scholar]
- (241).Aleynik SI, Leo MA, Ma X, Aleynik MK, Lieber CS. Polyenylphosphatidylcholine prevents carbon tetrachloride-induced lipid peroxidation while it attenuates liver fibrosis. J Hepatol. 1997;27:554–61. doi: 10.1016/s0168-8278(97)80361-3. [DOI] [PubMed] [Google Scholar]
- (242).Poniachik J, Baraona E, Zhao J, Lieber CS. Dilinoleoylphosphatidylcholine decreases hepatic stellate cell activation. J Lab Clin Med. 1999;133:342–8. doi: 10.1016/s0022-2143(99)90064-1. [DOI] [PubMed] [Google Scholar]
- (243).Di Sario A, Bendia E, Taffetani S, Omenetti A, Candelaresi C, Marzioni M, De Minicis S, Benedetti A. Hepatoprotective and antifibrotic effect of a new silybin-phosphatidylcholine-Vitamin E complex in rats. Dig Liver Dis. 2005;37:869–76. doi: 10.1016/j.dld.2005.05.011. [DOI] [PubMed] [Google Scholar]
- (244).Lieber CS. Role of S-adenosyl-L-methionine in the treatment of liver diseases. J Hepatol. 1999;30:1155–9. doi: 10.1016/s0168-8278(99)80274-8. [DOI] [PubMed] [Google Scholar]
- (245).Cutrin C, Menino MJ, Otero X, Miguez J, Perez-Becerra E, Barrio E. Effect of nifedipine and S-adenosylmethionine in the liver of rats treated with CCl4 and ethanol for one month. Life Sci. 1992;51:PL113–8. doi: 10.1016/0024-3205(92)90494-a. [DOI] [PubMed] [Google Scholar]
- (246).Muriel P, Castro V. Effects of S-adenosyl-L-methionine and interferon-alpha2b on liver damage induced by bile duct ligation in rats. J Appl Toxicol. 1998;18:143–7. doi: 10.1002/(sici)1099-1263(199803/04)18:2<143::aid-jat485>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- (247).Mato JM, Camara J, Fernandez de Paz J, Caballeria L, Coll S, Caballero A, Garcia-Buey L, Beltran J, Benita V, Caballeria J, Sola R, Moreno-Otero R, Barrao F, Martin-Duce A, Correa JA, Pares A, Barrao E, Garcia-Magaz I, Puerta JL, Moreno J, Boissard G, Ortiz P, Rodes J. S-adenosylmethionine in alcoholic liver cirrhosis: a randomized, placebo-controlled, double-blind, multicenter clinical trial. J Hepatol. 1999;30:1081–9. doi: 10.1016/s0168-8278(99)80263-3. [DOI] [PubMed] [Google Scholar]
- (248).Mizobuchi Y, Shimizu I, Yasuda M, Hori H, Shono M, Ito S. Retinyl palmitate reduces hepatic fibrosis in rats induced by dimethylnitrosamine or pig serum. J Hepatol. 1998;29:933–43. doi: 10.1016/s0168-8278(98)80121-9. [DOI] [PubMed] [Google Scholar]
- (249).Godichaud S, Krisa S, Couronne B, Dubuisson L, Merillon JM, Desmouliere A, Rosenbaum J. Deactivation of cultured human liver myofibroblasts by trans-resveratrol, a grapevine-derived polyphenol. Hepatology. 2000;31:922–31. doi: 10.1053/he.2000.5848. [DOI] [PubMed] [Google Scholar]
- (250).Nanji AA, Zakim D, Rahemtulla A, Daly T, Miao L, Zhao S, Khwaja S, Tahan SR, Dannenberg AJ. Dietary saturated fatty acids down-regulate cyclooxygenase-2 and tumor necrosis factor alfa and reverse fibrosis in alcohol-induced liver disease in the rat. Hepatology. 1997;26:1538–45. doi: 10.1002/hep.510260622. [DOI] [PubMed] [Google Scholar]
- (251).Gimenez A, Caballeria J, Pares A, Alie S, Deulofeu R, Andreu H, Rodes J. Influence of dietary zinc on hepatic collagen and prolyl hydroxylase activity in alcoholic rats. Hepatology. 1992;16:815–9. doi: 10.1002/hep.1840160331. [DOI] [PubMed] [Google Scholar]
- (252).Gimenez A, Pares A, Alie S, Camps J, Deulofeu R, Caballeria J, Rodes J. Fibrogenic and collagenolytic activity in carbon-tetrachloride-injured rats: beneficial effects of zinc administration. J Hepatol. 1994;21:292–8. doi: 10.1016/s0168-8278(05)80304-6. [DOI] [PubMed] [Google Scholar]
- (253).Bataller R, Sancho-Bru P, Gines P, Brenner DA. Liver fibrogenesis: a new role for the renin-angiotensin system. Antioxid Redox Signal. 2005;7:1346–55. doi: 10.1089/ars.2005.7.1346. [DOI] [PubMed] [Google Scholar]
- (254).Nabeshima Y, Tazuma S, Kanno K, Hyogo H, Iwai M, Horiuchi M, Chayama K. Anti-fibrogenic function of angiotensin II type 2 receptor in CCl4-induced liver fibrosis. Biochem Biophys Res Commun. 2006;346:658–64. doi: 10.1016/j.bbrc.2006.05.183. [DOI] [PubMed] [Google Scholar]
- (255).Jonsson JR, Clouston AD, Ando Y, Kelemen LI, Horn MJ, Adamson MD, Purdie DM, Powell EE. Angiotensin-converting enzyme inhibition attenuates the progression of rat hepatic fibrosis. Gastroenterology. 2001;121:148–55. doi: 10.1053/gast.2001.25480. [DOI] [PubMed] [Google Scholar]
- (256).Ueki M, Koda M, Yamamoto S, Matsunaga Y, Murawaki Y. Preventive and therapeutic effects of angiotensin II type 1 receptor blocker on hepatic fibrosis induced by bile duct ligation in rats. J Gastroenterol. 2006;41:996–1004. doi: 10.1007/s00535-006-1891-1. [DOI] [PubMed] [Google Scholar]
- (257).Croquet V, Moal F, Veal N, Wang J, Oberti F, Roux J, Vuillemin E, Gallois Y, Douay O, Chappard D, Cales P. Hemodynamic and antifibrotic effects of losartan in rats with liver fibrosis and/or portal hypertension. J Hepatol. 2002;37:773–80. doi: 10.1016/s0168-8278(02)00307-0. [DOI] [PubMed] [Google Scholar]
- (258).Yokohama S, Tokusashi Y, Nakamura K, Tamaki Y, Okamoto S, Okada M, Aso K, Hasegawa T, Aoshima M, Miyokawa N, Haneda M, Yoneda M. Inhibitory effect of angiotensin II receptor antagonist on hepatic stellate cell activation in non-alcoholic steatohepatitis. World J Gastroenterol. 2006;12:322–6. doi: 10.3748/wjg.v12.i2.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (259).Terui Y, Saito T, Watanabe H, Togashi H, Kawata S, Kamada Y, Sakuta S. Effect of angiotensin receptor antagonist on liver fibrosis in early stages of chronic hepatitis C. Hepatology. 2002;36:1022. doi: 10.1053/jhep.2002.32679. [DOI] [PubMed] [Google Scholar]
- (260).Pinzani M, Milani S, De Franco R, Grappone C, Caligiuri A, Gentilini A, Tosti-Guerra C, Maggi M, Failli P, Ruocco C, Gentilini P. Endothelin 1 is overexpressed in human cirrhotic liver and exerts multiple effects on activated hepatic stellate cells. Gastroenterology. 1996;110:534–48. doi: 10.1053/gast.1996.v110.pm8566602. [DOI] [PubMed] [Google Scholar]
- (261).Leivas A, Jimenez W, Bruix J, Boix L, Bosch J, Arroyo V, Rivera F, Rodes J. Gene expression of endothelin-1 and ET(A) and ET(B) receptors in human cirrhosis: relationship with hepatic hemodynamics. J Vasc Res. 1998;35:186–93. doi: 10.1159/000025583. [DOI] [PubMed] [Google Scholar]
- (262).Rockey DC. Vasoactive agents in intrahepatic portal hypertension and fibrogenesis: implications for therapy. Gastroenterology. 2000;118:1261–5. doi: 10.1016/s0016-5085(00)70379-9. [DOI] [PubMed] [Google Scholar]
- (263).Mallat A, Preaux AM, Serradeil-Le Gal C, Raufaste D, Gallois C, Brenner DA, Bradham C, Maclouf J, Iourgenko V, Fouassier L, Dhumeaux D, Mavier P, Lotersztajn S. Growth inhibitory properties of endothelin-1 in activated human hepatic stellate cells: a cyclic adenosine monophosphate-mediated pathway. Inhibition of both extracellular signal-regulated kinase and c-Jun kinase and upregulation of endothelin B receptors. J Clin Invest. 1996;98:2771–8. doi: 10.1172/JCI119103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (264).Gallois C, Davaille J, Habib A, Mallat A, Tao J, Levade T, Lotersztajn S. Endothelin-1 stimulates sphingosine kinase in human hepatic stellate cells. A novel role for sphingosine-1-P as a mediator of growth inhibition. Ann N Y Acad Sci. 2000;905:311–4. doi: 10.1111/j.1749-6632.2000.tb06568.x. [DOI] [PubMed] [Google Scholar]
- (265).Cho JJ, Hocher B, Herbst H, Jia JD, Ruehl M, Hahn EG, Riecken EO, Schuppan D. An oral endothelin-A receptor antagonist blocks collagen synthesis and deposition in advanced rat liver fibrosis. Gastroenterology. 2000;118:1169–78. doi: 10.1016/s0016-5085(00)70370-2. [DOI] [PubMed] [Google Scholar]
- (266).Poo JL, Jimenez W, Maria Munoz R, Bosch-Marce M, Bordas N, Morales-Ruiz M, Perez M, Deulofeu R, Sole M, Arroyo V, Rodes J. Chronic blockade of endothelin receptors in cirrhotic rats: hepatic and hemodynamic effects. Gastroenterology. 1999;116:161–7. doi: 10.1016/s0016-5085(99)70240-4. [DOI] [PubMed] [Google Scholar]
- (267).Beno DW, Espinal R, Edelstein BM, Davis BH. Administration of prostaglandin E1 analog reduces rat hepatic and Ito cell collagen gene expression and collagen accumulation after bile duct ligation injury. Hepatology. 1993;17:707–14. doi: 10.1002/hep.1840170427. [DOI] [PubMed] [Google Scholar]
- (268).Beno DW, Davis BH. Prostaglandin E Suppresses Hepatic Fibrosis: Section I. The In Vivo Approach; Section II. The In Vitro Approach. Am J Ther. 1995;2:687–705. doi: 10.1097/00045391-199509000-00018. [DOI] [PubMed] [Google Scholar]
- (269).Buko V, Lukivskaya O, Nikitin V, Kuryan A, Dargel R. Antioxidative effect of prostaglandin E2 in thioacetamide-induced liver cirrhosis. Exp Toxicol Pathol. 1997;49:141–6. doi: 10.1016/S0940-2993(97)80087-5. [DOI] [PubMed] [Google Scholar]
- (270).Rockey DC, Chung JJ. Reduced nitric oxide production by endothelial cells in cirrhotic rat liver: endothelial dysfunction in portal hypertension. Gastroenterology. 1998;114:344–51. doi: 10.1016/s0016-5085(98)70487-1. [DOI] [PubMed] [Google Scholar]
- (271).Fort J, Oberti F, Pilette C, Veal N, Gallois Y, Douay O, Rousselet MC, Rosenbaum J, Cales P. Antifibrotic and hemodynamic effects of the early and chronic administration of octreotide in two models of liver fibrosis in rats. Hepatology. 1998;28:1525–31. doi: 10.1002/hep.510280612. [DOI] [PubMed] [Google Scholar]
- (272).Hernandez-Munoz R, Diaz-Munoz M, Lopez V, Lopez-Barrera F, Yanez L, Vidrio S, Aranda-Fraustro A, Chagoya de Sanchez V. Balance between oxidative damage and proliferative potential in an experimental rat model of CCl4-induced cirrhosis: protective role of adenosine administration. Hepatology. 1997;26:1100–10. doi: 10.1002/hep.510260503. [DOI] [PubMed] [Google Scholar]
- (273).Wright MC, Issa R, Smart DE, Trim N, Murray GI, Primrose JN, Arthur MJ, Iredale JP, Mann DA. Gliotoxin stimulates the apoptosis of human and rat hepatic stellate cells and enhances the resolution of liver fibrosis in rats. Gastroenterology. 2001;121:685–98. doi: 10.1053/gast.2001.27188. [DOI] [PubMed] [Google Scholar]
- (274).Dekel R, Zvibel I, Brill S, Brazovsky E, Halpern Z, Oren R. Gliotoxin ameliorates development of fibrosis and cirrhosis in a thioacetamide rat model. Dig Dis Sci. 2003;48:1642–7. doi: 10.1023/a:1024792529601. [DOI] [PubMed] [Google Scholar]
- (275).Li L, Tao J, Davaille J, Feral C, Mallat A, Rieusset J, Vidal H, Lotersztajn S. 15-deoxy-Delta 12,14-prostaglandin J2 induces apoptosis of human hepatic myofibroblasts. A pathway involving oxidative stress independently of peroxisome-proliferator-activated receptors. J Biol Chem. 2001;276:38152–8. doi: 10.1074/jbc.M101980200. [DOI] [PubMed] [Google Scholar]
- (276).Davaille J, Li L, Mallat A, Lotersztajn S. Sphingosine 1-phosphate triggers both apoptotic and survival signals for human hepatic myofibroblasts. J Biol Chem. 2002;277:37323–30. doi: 10.1074/jbc.M202798200. [DOI] [PubMed] [Google Scholar]
- (277).Ikejima K, Takei Y, Honda H, Hirose M, Yoshikawa M, Zhang YJ, Lang T, Fukuda T, Yamashina S, Kitamura T, Sato N. Leptin receptor-mediated signaling regulates hepatic fibrogenesis and remodeling of extracellular matrix in the rat. Gastroenterology. 2002;122:1399–410. doi: 10.1053/gast.2002.32995. [DOI] [PubMed] [Google Scholar]
- (278).Saxena NK, Ikeda K, Rockey DC, Friedman SL, Anania FA. Leptin in hepatic fibrosis: evidence for increased collagen production in stellate cells and lean littermates of ob/ob mice. Hepatology. 2002;35:762–71. doi: 10.1053/jhep.2002.32029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (279).Piche T, Vandenbos F, Abakar-Mahamat A, Vanbiervliet G, Barjoan EM, Calle G, Giudicelli J, Ferrua B, Laffont C, Benzaken S, Tran A. The severity of liver fibrosis is associated with high leptin levels in chronic hepatitis C. J Viral Hepat. 2004;11:91–6. doi: 10.1046/j.1365-2893.2003.00483.x. [DOI] [PubMed] [Google Scholar]
- (280).Kamada Y, Tamura S, Kiso S, Matsumoto H, Saji Y, Yoshida Y, Fukui K, Maeda N, Nishizawa H, Nagaretani H, Okamoto Y, Kihara S, Miyagawa J, Shinomura Y, Funahashi T, Matsuzawa Y. Enhanced carbon tetrachloride-induced liver fibrosis in mice lacking adiponectin. Gastroenterology. 2003;125:1796–807. doi: 10.1053/j.gastro.2003.08.029. [DOI] [PubMed] [Google Scholar]
- (281).Tietge UJ, Boker KH, Manns MP, Bahr MJ. Elevated circulating adiponectin levels in liver cirrhosis are associated with reduced liver function and altered hepatic hemodynamics. Am J Physiol Endocrinol Metab. 2004;287:E82–9. doi: 10.1152/ajpendo.00494.2003. [DOI] [PubMed] [Google Scholar]
- (282).Julien B, Grenard P, Teixeira-Clerc F, Van Nhieu JT, Li L, Karsak M, Zimmer A, Mallat A, Lotersztajn S. Antifibrogenic role of the cannabinoid receptor CB2 in the liver. Gastroenterology. 2005;128:742–55. doi: 10.1053/j.gastro.2004.12.050. [DOI] [PubMed] [Google Scholar]
- (283).Teixeira-Clerc F, Julien B, Grenard P, Tran Van Nhieu J, Deveaux V, Li L, Serriere-Lanneau V, Ledent C, Mallat A, Lotersztajn S. CB1 cannabinoid receptor antagonism: a new strategy for the treatment of liver fibrosis. Nat Med. 2006;12:671–6. doi: 10.1038/nm1421. [DOI] [PubMed] [Google Scholar]
- (284).Sakaida I, Uchida K, Hironaka K, Okita K. Prolyl 4-hydroxylase inhibitor (HOE 077) prevents TIMP-1 gene expression in rat liver fibrosis. J Gastroenterol. 1999;34:376–7. doi: 10.1007/s005350050277. [DOI] [PubMed] [Google Scholar]
- (285).Wang YJ, Wang SS, Bickel M, Guenzler V, Gerl M, Bissell DM. Two novel antifibrotics, HOE 077 and Safironil, modulate stellate cell activation in rat liver injury: differential effects in males and females. Am J Pathol. 1998;152:279–87. [PMC free article] [PubMed] [Google Scholar]
- (286).Sakaida I, Matsumura Y, Kubota M, Kayano K, Takenaka K, Okita K. The prolyl 4-hydroxylase inhibitor HOE 077 prevents activation of Ito cells, reducing procollagen gene expression in rat liver fibrosis induced by choline-deficient L-amino acid-defined diet. Hepatology. 1996;23:755–63. doi: 10.1053/jhep.1996.v23.pm0008666329. [DOI] [PubMed] [Google Scholar]
- (287).Matsumura Y, Sakaida I, Uchida K, Kimura T, Ishihara T, Okita K. Prolyl 4-hydroxylase inhibitor (HOE 077) inhibits pig serum-induced rat liver fibrosis by preventing stellate cell activation. J Hepatol. 1997;27:185–92. doi: 10.1016/s0168-8278(97)80300-5. [DOI] [PubMed] [Google Scholar]
- (288).Bickel M, Baringhaus KH, Gerl M, Gunzler V, Kanta J, Schmidts L, Stapf M, Tschank G, Weidmann K, Werner U. Selective inhibition of hepatic collagen accumulation in experimental liver fibrosis in rats by a new prolyl 4-hydroxylase inhibitor. Hepatology. 1998;28:404–11. doi: 10.1002/hep.510280217. [DOI] [PubMed] [Google Scholar]
- (289).Pines M, Knopov V, Genina O, Lavelin I, Nagler A. Halofuginone, a specific inhibitor of collagen type I synthesis, prevents dimethylnitrosamine-induced liver cirrhosis. J Hepatol. 1997;27:391–8. doi: 10.1016/s0168-8278(97)80186-9. [DOI] [PubMed] [Google Scholar]
- (290).Wu CH, Walton CM, Wu GY. Targeted inhibition of type I procollagen synthesis by antisense DNA oligonucleotides. Gene Therapy and Regulation. 2000;1:193–205. [Google Scholar]
- (291).Laptev AV, Lu Z, Colige A, Prockop DJ. Specific inhibition of expression of a human collagen gene (COL1A1) with modified antisense oligonucleotides. The most effective target sites are clustered in double-stranded regions of the predicted secondary structure for the mRNA. Biochemistry. 1994;33:11033–9. doi: 10.1021/bi00202a024. [DOI] [PubMed] [Google Scholar]
- (292).Joseph J, Kandala JC, Veerapanane D, Weber KT, Guntaka RV. Antiparallel polypurine phosphorothioate oligonucleotides form stable triplexes with the rat alpha1(I) collagen gene promoter and inhibit transcription in cultured rat fibroblasts. Nucleic Acids Res. 1997;25:2182–8. doi: 10.1093/nar/25.11.2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (293).Weber KT, Swamynathan SK, Guntaka RV, Sun Y. Angiotensin II and extracellular matrix homeostasis. Int J Biochem Cell Biol. 1999;31:395–403. doi: 10.1016/s1357-2725(98)00125-3. [DOI] [PubMed] [Google Scholar]
- (294).Dhalla AK, Kandala JC, Weber KT, Guntaka RV. Identification of negative and positive regulatory elements in the rat alpha 1(I) collagen gene promoter. Int J Biochem Cell Biol. 1997;29:143–51. doi: 10.1016/s1357-2725(96)00126-4. [DOI] [PubMed] [Google Scholar]
- (295).Kovacs A, Kandala JC, Weber KT, Guntaka RV. Triple helix-forming oligonucleotide corresponding to the polypyrimidine sequence in the rat alpha 1(I) collagen promoter specifically inhibits factor binding and transcription. J Biol Chem. 1996;271:1805–12. doi: 10.1074/jbc.271.3.1805. [DOI] [PubMed] [Google Scholar]
- (296).Nakanishi M, Weber KT, Guntaka RV. Triple helix formation with the promoter of human alpha1(I) procollagen gene by an antiparallel triplex-forming oligodeoxyribonucleotide. Nucleic Acids Res. 1998;26:5218–22. doi: 10.1093/nar/26.22.5218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (297).Salgado S, Garcia J, Vera J, Siller F, Bueno M, Miranda A, Segura A, Grijalva G, Segura J, Orozco H, Hernandez-Pando R, Fafutis M, Aguilar LK, Aguilar-Cordova E, Armendariz-Borunda J. Liver cirrhosis is reverted by urokinase-type plasminogen activator gene therapy. Mol Ther. 2000;2:545–51. doi: 10.1006/mthe.2000.0210. [DOI] [PubMed] [Google Scholar]
- (298).Iimuro Y, Nishio T, Morimoto T, Nitta T, Stefanovic B, Choi SK, Brenner DA, Yamaoka Y. Delivery of matrix metalloproteinase-1 attenuates established liver fibrosis in the rat. Gastroenterology. 2003;124:445–58. doi: 10.1053/gast.2003.50063. [DOI] [PubMed] [Google Scholar]
- (299).Opalinska JB, Gewirtz AM. Nucleic-acid therapeutics: basic principles and recent applications. Nat Rev Drug Discov. 2002;1:503–14. doi: 10.1038/nrd837. [DOI] [PubMed] [Google Scholar]
- (300).Wall NR, Shi Y. Small RNA: can RNA interference be exploited for therapy? Lancet. 2003;362:1401–3. doi: 10.1016/S0140-6736(03)14637-5. [DOI] [PubMed] [Google Scholar]
- (301).Seidman MM, Glazer PM. The potential for gene repair via triple helix formation. J Clin Invest. 2003;112:487–94. doi: 10.1172/JCI19552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (302).Vasquez KM, Glazer PM. Triplex-forming oligonucleotides: principles and applications. Q Rev Biophys. 2002;35:89–107. doi: 10.1017/s0033583502003773. [DOI] [PubMed] [Google Scholar]
- (303).Guntaka RV, Varma BR, Weber KT. Triplex-forming oligonucleotides as modulators of gene expression. Int J Biochem Cell Biol. 2003;35:22–31. doi: 10.1016/s1357-2725(02)00165-6. [DOI] [PubMed] [Google Scholar]
- (304).Felsenfeld G, Davies DR, Rich A. Formation of a 3-Stranded Polynucleotide Molecule. Journal of the American Chemical Society. 1957;79:2023–2024. [Google Scholar]
- (305).Frank-Kamenetskii MD, Mirkin SM. Triplex DNA structures. Annu Rev Biochem. 1995;64:65–95. doi: 10.1146/annurev.bi.64.070195.000433. [DOI] [PubMed] [Google Scholar]
- (306).Thuong NT, Helene C. Sequence-Specific Recognition and Modification of Double-Helical DNA by Oligonucleotides. Angewandte Chemie-International Edition in English. 1993;32:666–690. [Google Scholar]
- (307).Praseuth D, Guieysse AL, Helene C. Triple helix formation and the antigene strategy for sequence-specific control of gene expression. Biochim Biophys Acta. 1999;1489:181–206. doi: 10.1016/s0167-4781(99)00149-9. [DOI] [PubMed] [Google Scholar]
- (308).Maher LJ., 3rd. Prospects for the therapeutic use of antigene oligonucleotides. Cancer Invest. 1996;14:66–82. doi: 10.3109/07357909609018437. [DOI] [PubMed] [Google Scholar]
- (309).Radhakrishnan I, Patel DJ. DNA triplexes: solution structures, hydration sites, energetics, interactions, and function. Biochemistry. 1994;33:11405–16. doi: 10.1021/bi00204a001. [DOI] [PubMed] [Google Scholar]
- (310).Fossella JA, Kim YJ, Shih H, Richards EG, Fresco JR. Relative specificities in binding of Watson-Crick base pairs by third strand residues in a DNA pyrimidine triplex motif. Nucleic Acids Res. 1993;21:4511–5. doi: 10.1093/nar/21.19.4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (311).Letai AG, Palladino MA, Fromm E, Rizzo V, Fresco JR. Specificity in formation of triple-stranded nucleic acid helical complexes: studies with agarose-linked polyribonucleotide affinity columns. Biochemistry. 1988;27:9108–12. doi: 10.1021/bi00426a007. [DOI] [PubMed] [Google Scholar]
- (312).Gilbert DE, Feigon J. Multistranded DNA structures. Curr Opin Struct Biol. 1999;9:305–14. doi: 10.1016/S0959-440X(99)80041-4. [DOI] [PubMed] [Google Scholar]
- (313).Asensio JL, Carr R, Brown T, Lane AN. Conformational and thermodynamic properties of parallel intramolecular triple helices containing a DNA, RNA, or 2 '-OMeDNA third strand. Journal of the American Chemical Society. 1999;121:11063–11070. [Google Scholar]
- (314).Asensio JL, Lane AN, Dhesi J, Bergqvist S, Brown T. The contribution of cytosine protonation to the stability of parallel DNA triple helices. J Mol Biol. 1998;275:811–22. doi: 10.1006/jmbi.1997.1520. [DOI] [PubMed] [Google Scholar]
- (315).Pesco J, Salmon JM, Vigo J, Viallet P. Mag-indo1 affinity for Ca(2+), compartmentalization and binding to proteins: the challenge of measuring Mg(2+) concentrations in living cells. Anal Biochem. 2001;290:221–31. doi: 10.1006/abio.2000.4983. [DOI] [PubMed] [Google Scholar]
- (316).Arimondo PB, Garestier T, Helene C, Sun JS. Detection of competing DNA structures by thermal gradient gel electrophoresis: from self-association to triple helix formation by (G,A)-containing oligonucleotides. Nucleic Acids Res. 2001;29:E15. doi: 10.1093/nar/29.4.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (317).Noonberg SB, Francois JC, Garestier T, Helene C. Effect of competing self-structure on triplex formation with purine-rich oligodeoxynucleotides containing GA repeats. Nucleic Acids Res. 1995;23:1956–63. doi: 10.1093/nar/23.11.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (318).Olivas WM, Maher LJ., 3rd. Competitive triplex/quadruplex equilibria involving guanine-rich oligonucleotides. Biochemistry. 1995;34:278–84. doi: 10.1021/bi00001a034. [DOI] [PubMed] [Google Scholar]
- (319).Obika S. Development of bridged nucleic acid analogues for antigene technology. Chem Pharm Bull (Tokyo) 2004;52:1399–404. doi: 10.1248/cpb.52.1399. [DOI] [PubMed] [Google Scholar]
- (320).Griffin LC, Kiessling LL, Beal PA, Gillespie P, Dervan PB. Recognition of All 4 Base-Pairs of Double-Helical DNA by Triple-Helix Formation - Design of Nonnatural Deoxyribonucleosides for Pyrimidine.Purine Base Pair Binding. Journal of the American Chemical Society. 1992;114:7976–7982. [Google Scholar]
- (321).Guianvarc'h D, Benhida R, Fourrey JL, Maurisse R, Sun JS. Incorporation of a novel nucleobase allows stable oligonucleotide-directed triple helix formation at the target sequence containing a purine center dot pyrimidine interruption. Chemical Communications. 2001:1814–1815. doi: 10.1039/b103743a. [DOI] [PubMed] [Google Scholar]
- (322).Guianvarc'h D, Fourrey JL, Maurisse R, Sun JS, Benhida R. Synthesis, incorporation into triplex-forming oligonucleotide, and binding properties of a novel 2 '-deoxy-C-nucleoside featuring a 6-(thiazolyl-5)benzimidazole nucleobase. Organic Letters. 2002;4:4209–4212. doi: 10.1021/ol026609h. [DOI] [PubMed] [Google Scholar]
- (323).Prevot-Halter I, Leumann CJ. Selective recognition of a C-G base-pair in the parallel DNA triple-helical binding motif. Bioorganic & Medicinal Chemistry Letters. 1999;9:2657–2660. doi: 10.1016/s0960-894x(99)00451-5. [DOI] [PubMed] [Google Scholar]
- (324).Huang CY, Bi G, Miller PS. Triplex formation by oligonucleotides containing novel deoxycytidine derivatives. Nucleic Acids Res. 1996;24:2606–13. doi: 10.1093/nar/24.13.2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (325).Lee JS, Woodsworth ML, Latimer LJ, Morgan AR. Poly(pyrimidine). poly(purine) synthetic DNAs containing 5-methylcytosine form stable triplexes at neutral pH. Nucleic Acids Res. 1984;12:6603–14. doi: 10.1093/nar/12.16.6603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (326).Miller PS, Bi G, Kipp SA, Fok V, DeLong RK. Triplex formation by a psoralen-conjugated oligodeoxyribonucleotide containing the base analog 8-oxo-adenine. Nucleic Acids Res. 1996;24:730–6. doi: 10.1093/nar/24.4.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (327).Ono A, Tso POP, Kan LS. Triplex Formation of Oligonucleotides Containing 2'-O-Methylpseudoisocytidine in Substitution for 2'-Deoxycytidine. Journal of the American Chemical Society. 1991;113:4032–4033. [Google Scholar]
- (328).Xiang GB, McLaughlin LW. A cytosine analogue containing a conformationally flexible acyclic linker for triplex formation at sites with contiguous G-C base pair. Tetrahedron. 1998;54:375–392. [Google Scholar]
- (329).Cassidy SA, Slickers P, Trent JO, Capaldi DC, Roselt PD, Reese CB, Neidle S, Fox KR. Recognition of GC base pairs by triplex forming oligonucleotides containing nucleosides derived from 2-aminopyridine. Nucleic Acids Res. 1997;25:4891–8. doi: 10.1093/nar/25.24.4891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (330).Hildbrand S, Blaser A, Parel SP, Leumann CJ. 5-substituted 2-aminopyridine C-nucleosides as protonated cytidine equivalents: Increasing efficiency and selectivity in DNA triple-helix formation. Journal of the American Chemical Society. 1997;119:5499–5511. [Google Scholar]
- (331).Hacia JG, Dervan PB, Wold BJ. Inhibition of Klenow fragment DNA polymerase on double-helical templates by oligonucleotide-directed triple-helix formation. Biochemistry. 1994;33:6192–200. doi: 10.1021/bi00186a019. [DOI] [PubMed] [Google Scholar]
- (332).Arya DP, Bruice TC. Triple-helix formation of DNA oligomers with methylthiourea-linked nucleosides (DNmt): a kinetic and thermodynamic analysis. Proc Natl Acad Sci U S A. 1999;96:4384–9. doi: 10.1073/pnas.96.8.4384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (333).Bijapur J, Keppler MD, Bergqvist S, Brown T, Fox KR. 5-(1-propargylamino)-2'-deoxyuridine (UP): a novel thymidine analogue for generating DNA triplexes with increased stability. Nucleic Acids Res. 1999;27:1802–9. doi: 10.1093/nar/27.8.1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (334).Rajeev KG, Jadhav VR, Ganesh KN. Triplex formation at physiological pH: comparative studies on DNA triplexes containing 5-Me-dC tethered at N4 with spermine and tetraethyleneoxyamine. Nucleic Acids Res. 1997;25:4187–93. doi: 10.1093/nar/25.21.4187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (335).Sund C, Puri N, Chattopadhyaya J. Synthesis of C-branched spermine tethered oligo-DNA and the thermal stability of the duplexes and triplexes. Tetrahedron. 1996;52:12275–12290. [Google Scholar]
- (336).Dagle JM, Weeks DL. Positively charged oligonucleotides overcome potassium-mediated inhibition of triplex DNA formation. Nucleic Acids Res. 1996;24:2143–9. doi: 10.1093/nar/24.11.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (337).Ehrenmann F, Vasseur JJ, Debart F. Alpha-oligonucleotides with anionic phosphodiester and cationic phosphoramidate linkages enhanced stability of DNA triple helix. Nucleosides Nucleotides Nucleic Acids. 2001;20:797–9. doi: 10.1081/NCN-100002432. [DOI] [PubMed] [Google Scholar]
- (338).Vasquez KM, Dagle JM, Weeks DL, Glazer PM. Chromosome targeting at short polypurine sites by cationic triplex-forming oligonucleotides. J Biol Chem. 2001;276:38536–41. doi: 10.1074/jbc.M101797200. [DOI] [PubMed] [Google Scholar]
- (339).Gryaznov SM, Lloyd DH, Chen JK, Schultz RG, DeDionisio LA, Ratmeyer L, Wilson WD. Oligonucleotide N3'-->P5' phosphoramidates. Proc Natl Acad Sci U S A. 1995;92:5798–802. doi: 10.1073/pnas.92.13.5798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (340).Sun JS, Garestier T, Helene C. Oligonucleotide directed triple helix formation. Curr Opin Struct Biol. 1996;6:327–33. doi: 10.1016/s0959-440x(96)80051-0. [DOI] [PubMed] [Google Scholar]
- (341).Roberts RW, Crothers DM. Stability and properties of double and triple helices: dramatic effects of RNA or DNA backbone composition. Science. 1992;258:1463–6. doi: 10.1126/science.1279808. [DOI] [PubMed] [Google Scholar]
- (342).Escude C, Sun JS, Rougee M, Garestier T, Helene C. Stable triple helices are formed upon binding of RNA oligonucleotides and their 2'-O-methyl derivatives to double-helical DNA. C R Acad Sci III. 1992;315:521–5. [PubMed] [Google Scholar]
- (343).Cuenoud B, Casset F, Husken D, Natt F, Wolf RM, Altmann KH, Martin P, Moser HE. Dual recognition of double-stranded DNA by 2 '-aminoethoxy-modified oligonucleotides. Angewandte Chemie-International Edition. 1998;37:1288–1291. doi: 10.1002/(SICI)1521-3773(19980518)37:9<1288::AID-ANIE1288>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- (344).Shimizu M, Konishi A, Shimada Y, Inoue H, Ohtsuka E. Oligo(2'-O-methyl)ribonucleotides. Effective probes for duplex DNA. FEBS Lett. 1992;302:155–8. doi: 10.1016/0014-5793(92)80428-j. [DOI] [PubMed] [Google Scholar]
- (345).Olivas WM, Maher LJ., 3rd. Overcoming potassium-mediated triplex inhibition. Nucleic Acids Res. 1995;23:1936–41. doi: 10.1093/nar/23.11.1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (346).Faruqi AF, Krawczyk SH, Matteucci MD, Glazer PM. Potassium-resistant triple helix formation and improved intracellular gene targeting by oligodeoxyribonucleotides containing 7-deazaxanthine. Nucleic Acids Res. 1997;25:633–40. doi: 10.1093/nar/25.3.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (347).Vo T, Wang S, Kool ET. Targeting pyrimidine single strands by triplex formation: structural optimization of binding. Nucleic Acids Res. 1995;23:2937–44. doi: 10.1093/nar/23.15.2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (348).Svinarchuk F, Debin A, Bertrand JR, Malvy C. Investigation of the intracellular stability and formation of a triple helix formed with a short purine oligonucleotide targeted to the murine c-pim-1 proto-oncogene promotor. Nucleic Acids Res. 1996;24:295–302. doi: 10.1093/nar/24.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (349).Svinarchuk F, Nagibneva I, Cherny D, Ait-Si-Ali S, Pritchard LL, Robin P, Malvy C, Harel-Bellan A, Chern D. Recruitment of transcription factors to the target site by triplex-forming oligonucleotides. Nucleic Acids Res. 1997;25:3459–64. doi: 10.1093/nar/25.17.3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (350).Lacroix L, Arimondo PB, Takasugi M, Helene C, Mergny JL. Pyrimidine morpholino oligonucleotides form a stable triple helix in the absence of magnesium ions. Biochem Biophys Res Commun. 2000;270:363–9. doi: 10.1006/bbrc.2000.2438. [DOI] [PubMed] [Google Scholar]
- (351).Nielsen PE, Egholm M. An introduction to peptide nucleic acid. Curr Issues Mol Biol. 1999;1:89–104. [PubMed] [Google Scholar]
- (352).Demidov VV, Yavnilovich MV, Belotserkovskii BP, Frank-Kamenetskii MD, Nielsen PE. Kinetics and mechanism of polyamide ("peptide") nucleic acid binding to duplex DNA. Proc Natl Acad Sci U S A. 1995;92:2637–41. doi: 10.1073/pnas.92.7.2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (353).Majumdar A, Puri N, McCollum N, Richards S, Cuenoud B, Miller P, Seidman MM. Gene targeting by triple helix-forming oligonucleotides. Ann N Y Acad Sci. 2003;1002:141–53. doi: 10.1196/annals.1281.013. [DOI] [PubMed] [Google Scholar]
- (354).Kukreti S, Sun JS, Garestier T, Helene C. Extension of the range of DNA sequences available for triple helix formation: stabilization of mismatched triplexes by acridine-containing oligonucleotides. Nucleic Acids Res. 1997;25:4264–70. doi: 10.1093/nar/25.21.4264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (355).Morgan AR, Wells RD. Specificity of the three-stranded complex formation between double-stranded DNA and single-stranded RNA containing repeating nucleotide sequences. J Mol Biol. 1968;37:63–80. doi: 10.1016/0022-2836(68)90073-9. [DOI] [PubMed] [Google Scholar]
- (356).Westin L, Blomquist P, Milligan JF, Wrange O. Triple helix DNA alters nucleosomal histone-DNA interactions and acts as a nucleosome barrier. Nucleic Acids Res. 1995;23:2184–91. doi: 10.1093/nar/23.12.2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (357).Giovannangeli C, Helene C. Progress in developments of triplex-based strategies. Antisense Nucleic Acid Drug Dev. 1997;7:413–21. doi: 10.1089/oli.1.1997.7.413. [DOI] [PubMed] [Google Scholar]
- (358).Skoog JU, Maher LJ., 3rd. Repression of bacteriophage promoters by DNA and RNA oligonucleotides. Nucleic Acids Res. 1993;21:2131–8. doi: 10.1093/nar/21.9.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (359).Duval-Valentin G, Thuong NT, Helene C. Specific inhibition of transcription by triple helix-forming oligonucleotides. Proc Natl Acad Sci U S A. 1992;89:504–8. doi: 10.1073/pnas.89.2.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (360).Rando RF, DePaolis L, Durland RH, Jayaraman K, Kessler DJ, Hogan ME. Inhibition of T7 and T3 RNA polymerase directed transcription elongation in vitro. Nucleic Acids Res. 1994;22:678–85. doi: 10.1093/nar/22.4.678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (361).Alunni-Fabbroni M, Manfioletti G, Manzini G, Xodo LE. Inhibition of T7 RNA polymerase transcription by phosphate and phosphorothioate triplex-forming oligonucleotides targeted to a R.Y site downstream from the promoter. Eur J Biochem. 1994;226:831–9. doi: 10.1111/j.1432-1033.1994.00831.x. [DOI] [PubMed] [Google Scholar]
- (362).Giovannangeli C, Perrouault L, Escude C, Nguyen T, Helene C. Specific inhibition of in vitro transcription elongation by triplex-forming oligonucleotide-intercalator conjugates targeted to HIV proviral DNA. Biochemistry. 1996;35:10539–48. doi: 10.1021/bi952993x. [DOI] [PubMed] [Google Scholar]
- (363).Wang Z, Rana TM. DNA damage-dependent transcriptional arrest and termination of RNA polymerase II elongation complexes in DNA template containing HIV-1 promoter. Proc Natl Acad Sci U S A. 1997;94:6688–93. doi: 10.1073/pnas.94.13.6688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (364).Hanvey JC, Peffer NJ, Bisi JE, Thomson SA, Cadilla R, Josey JA, Ricca DJ, Hassman CF, Bonham MA, Au KG, et al. Antisense and antigene properties of peptide nucleic acids. Science. 1992;258:1481–5. doi: 10.1126/science.1279811. [DOI] [PubMed] [Google Scholar]
- (365).Nielsen PE, Egholm M, Buchardt O. Sequence-specific transcription arrest by peptide nucleic acid bound to the DNA template strand. Gene. 1994;149:139–45. doi: 10.1016/0378-1119(94)90422-7. [DOI] [PubMed] [Google Scholar]
- (366).Gambacorti-Passerini C, Mologni L, Bertazzoli C, le Coutre P, Marchesi E, Grignani F, Nielsen PE. In vitro transcription and translation inhibition by anti-promyelocytic leukemia (PML)/retinoic acid receptor alpha and anti-PML peptide nucleic acid. Blood. 1996;88:1411–7. [PubMed] [Google Scholar]
- (367).Larsen HJ, Nielsen PE. Transcription-mediated binding of peptide nucleic acid (PNA) to double-stranded DNA: sequence-specific suicide transcription. Nucleic Acids Res. 1996;24:458–63. doi: 10.1093/nar/24.3.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (368).Rando RF, Ojwang J, Elbaggari A, Reyes GR, Tinder R, McGrath MS, Hogan ME. Suppression of human immunodeficiency virus type 1 activity in vitro by oligonucleotides which form intramolecular tetrads. J Biol Chem. 1995;270:1754–60. doi: 10.1074/jbc.270.4.1754. [DOI] [PubMed] [Google Scholar]
- (369).Xu X, Hamhouyia F, Thomas SD, Burke TJ, Girvan AC, McGregor WG, Trent JO, Miller DM, Bates PJ. Inhibition of DNA replication and induction of S phase cell cycle arrest by G-rich oligonucleotides. J Biol Chem. 2001;276:43221–30. doi: 10.1074/jbc.M104446200. [DOI] [PubMed] [Google Scholar]
- (370).Skogen M, Roth J, Yerkes S, Parekh-Olmedo H, Kmiec E. Short G-rich oligonucleotides as a potential therapeutic for Huntington's Disease. BMC Neurosci. 2006;7:65. doi: 10.1186/1471-2202-7-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (371).Birg F, Praseuth D, Zerial A, Thuong NT, Asseline U, Le Doan T, Helene C. Inhibition of simian virus 40 DNA replication in CV-1 cells by an oligodeoxynucleotide covalently linked to an intercalating agent. Nucleic Acids Res. 1990;18:2901–8. doi: 10.1093/nar/18.10.2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (372).McShan WM, Rossen RD, Laughter AH, Trial J, Kessler DJ, Zendegui JG, Hogan ME, Orson FM. Inhibition of transcription of HIV-1 in infected human cells by oligodeoxynucleotides designed to form DNA triple helices. J Biol Chem. 1992;267:5712–21. [PubMed] [Google Scholar]
- (373).Cooney M, Czernuszewicz G, Postel EH, Flint SJ, Hogan ME. Site-specific oligonucleotide binding represses transcription of the human c-myc gene in vitro. Science. 1988;241:456–9. doi: 10.1126/science.3293213. [DOI] [PubMed] [Google Scholar]
- (374).Postel EH, Flint SJ, Kessler DJ, Hogan ME. Evidence that a triplex-forming oligodeoxyribonucleotide binds to the c-myc promoter in HeLa cells, thereby reducing c-myc mRNA levels. Proc Natl Acad Sci U S A. 1991;88:8227–31. doi: 10.1073/pnas.88.18.8227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (375).Thomas TJ, Faaland CA, Gallo MA, Thomas T. Suppression of c-myc oncogene expression by a polyamine-complexed triplex forming oligonucleotide in MCF-7 breast cancer cells. Nucleic Acids Res. 1995;23:3594–9. doi: 10.1093/nar/23.17.3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (376).Michelotti EF, Tomonaga T, Krutzsch H, Levens D. Cellular nucleic acid binding protein regulates the CT element of the human c-myc protooncogene. J Biol Chem. 1995;270:9494–9. doi: 10.1074/jbc.270.16.9494. [DOI] [PubMed] [Google Scholar]
- (377).Kinniburgh AJ, Firulli AB, Kolluri R. DNA triplexes and regulation of the c-myc gene. Gene. 1994;149:93–100. doi: 10.1016/0378-1119(94)90416-2. [DOI] [PubMed] [Google Scholar]
- (378).Macaulay VM, Bates PJ, McLean MJ, Rowlands MG, Jenkins TC, Ashworth A, Neidle S. Inhibition of aromatase expression by a psoralen-linked triplex-forming oligonucleotide targeted to a coding sequence. FEBS Lett. 1995;372:222–8. doi: 10.1016/0014-5793(95)00987-k. [DOI] [PubMed] [Google Scholar]
- (379).Bonham MA, Brown S, Boyd AL, Brown PH, Bruckenstein DA, Hanvey JC, Thomson SA, Pipe A, Hassman F, Bisi JE, et al. An assessment of the antisense properties of RNase H-competent and steric-blocking oligomers. Nucleic Acids Res. 1995;23:1197–203. doi: 10.1093/nar/23.7.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (380).Scaggiante B, Morassutti C, Tolazzi G, Michelutti A, Baccarani M, Quadrifoglio F. Effect of unmodified triple helix-forming oligodeoxyribonucleotide targeted to human multidrug-resistance gene mdr1 in MDR cancer cells. FEBS Lett. 1994;352:380–4. doi: 10.1016/0014-5793(94)00995-3. [DOI] [PubMed] [Google Scholar]
- (381).Grigoriev M, Praseuth D, Guieysse AL, Robin P, Thuong NT, Helene C, Harel-Bellan A. Inhibition of gene expression by triple helix-directed DNA cross-linking at specific sites. Proc Natl Acad Sci U S A. 1993;90:3501–5. doi: 10.1073/pnas.90.8.3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (382).Guieysse AL, Praseuth D, Grigoriev M, Harel-Bellan A, Helene C. Detection of covalent triplex within human cells. Nucleic Acids Res. 1996;24:4210–6. doi: 10.1093/nar/24.21.4210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (383).Grigoriev M, Praseuth D, Robin P, Hemar A, Saison-Behmoaras T, Dautry-Varsat A, Thuong NT, Helene C, Harel-Bellan A. A triple helix-forming oligonucleotide-intercalator conjugate acts as a transcriptional repressor via inhibition of NF kappa B binding to interleukin-2 receptor alpha-regulatory sequence. J Biol Chem. 1992;267:3389–95. [PubMed] [Google Scholar]
- (384).Sen D, Gilbert W. Formation of parallel four-stranded complexes by guanine-rich motifs in DNA and its implications for meiosis. Nature. 1988;334:364–6. doi: 10.1038/334364a0. [DOI] [PubMed] [Google Scholar]
- (385).Simonsson T, Pecinka P, Kubista M. DNA tetraplex formation in the control region of c-myc. Nucleic Acids Res. 1998;26:1167–72. doi: 10.1093/nar/26.5.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (386).Blackburn EH. Structure and function of telomeres. Nature. 1991;350:569–73. doi: 10.1038/350569a0. [DOI] [PubMed] [Google Scholar]
- (387).Reddy MS, Hardin SH. Features in short guanine-rich sequences that stimulate DNA polymerization in vitro. Biochemistry. 2003;42:350–62. doi: 10.1021/bi020380w. [DOI] [PubMed] [Google Scholar]
- (388).Han H, Hurley LH, Salazar M. A DNA polymerase stop assay for G-quadruplex-interactive compounds. Nucleic Acids Res. 1999;27:537–42. doi: 10.1093/nar/27.2.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (389).Jing N, Rando RF, Pommier Y, Hogan ME. Ion selective folding of loop domains in a potent anti-HIV oligonucleotide. Biochemistry. 1997;36:12498–505. doi: 10.1021/bi962798y. [DOI] [PubMed] [Google Scholar]
- (390).Jing N, Li Y, Xu X, Sha W, Li P, Feng L, Tweardy DJ. Targeting Stat3 with G-quartet oligodeoxynucleotides in human cancer cells. DNA Cell Biol. 2003;22:685–96. doi: 10.1089/104454903770946665. [DOI] [PubMed] [Google Scholar]
- (391).Bates PJ, Kahlon JB, Thomas SD, Trent JO, Miller DM. Antiproliferative activity of G-rich oligonucleotides correlates with protein binding. J Biol Chem. 1999;274:26369–77. doi: 10.1074/jbc.274.37.26369. [DOI] [PubMed] [Google Scholar]
- (392).Orson FM, Thomas DW, McShan WM, Kessler DJ, Hogan ME. Oligonucleotide inhibition of IL2R alpha mRNA transcription by promoter region collinear triplex formation in lymphocytes. Nucleic Acids Res. 1991;19:3435–41. doi: 10.1093/nar/19.12.3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (393).Porumb H, Gousset H, Letellier R, Salle V, Briane D, Vassy J, Amor-Gueret M, Israel L, Taillandier E. Temporary ex vivo inhibition of the expression of the human oncogene HER2 (NEU) by a triple helix-forming oligonucleotide. Cancer Res. 1996;56:515–22. [PubMed] [Google Scholar]
- (394).Espinas ML, Jimenez-Garcia E, Martinez-Balbas A, Azorin F. Formation of triple-stranded DNA at d(GA.TC)n sequences prevents nucleosome assembly and is hindered by nucleosomes. J Biol Chem. 1996;271:31807–12. doi: 10.1074/jbc.271.50.31807. [DOI] [PubMed] [Google Scholar]
- (395).Brown PM, Fox KR. Nucleosome core particles inhibit DNA triple helix formation. Biochem J. 1996;319(Pt 2):607–11. doi: 10.1042/bj3190607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (396).Giovannangeli C, Diviacco S, Labrousse V, Gryaznov S, Charneau P, Helene C. Accessibility of nuclear DNA to triplex-forming oligonucleotides: the integrated HIV-1 provirus as a target. Proc Natl Acad Sci U S A. 1997;94:79–84. doi: 10.1073/pnas.94.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (397).Belousov ES, Afonina IA, Kutyavin IV, Gall AA, Reed MW, Gamper HB, Wydro RM, Meyer RB. Triplex targeting of a native gene in permeabilized intact cells: covalent modification of the gene for the chemokine receptor CCR5. Nucleic Acids Res. 1998;26:1324–8. doi: 10.1093/nar/26.5.1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (398).Brunet E, Corgnali M, Cannata F, Perrouault L, Giovannangeli C. Targeting chromosomal sites with locked nucleic acid-modified triplex-forming oligonucleotides: study of efficiency dependence on DNA nuclear environment. Nucleic Acids Res. 2006;34:4546–53. doi: 10.1093/nar/gkl630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (399).Struhl K. Chromatin structure and RNA polymerase II connection: implications for transcription. Cell. 1996;84:179–82. doi: 10.1016/s0092-8674(00)80970-8. [DOI] [PubMed] [Google Scholar]
- (400).Wolffe AP. Transcription: in tune with the histones. Cell. 1994;77:13–6. doi: 10.1016/0092-8674(94)90229-1. [DOI] [PubMed] [Google Scholar]
- (401).Macris MA, Glazer PM. Transcription dependence of chromosomal gene targeting by triplex-forming oligonucleotides. J Biol Chem. 2003;278:3357–62. doi: 10.1074/jbc.M206542200. [DOI] [PubMed] [Google Scholar]
- (402).Oh DH, Hanawalt PC. Triple helix-forming oligonucleotides target psoralen adducts to specific chromosomal sequences in human cells. Nucleic Acids Res. 1999;27:4734–42. doi: 10.1093/nar/27.24.4734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (403).Hanvey JC, Shimizu M, Wells RD. Site-specific inhibition of EcoRI restriction/modification enzymes by a DNA triple helix. Nucleic Acids Res. 1990;18:157–61. doi: 10.1093/nar/18.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (404).Giovannangeli C, Thuong NT, Helene C. Oligodeoxynucleotide-directed photo-induced cross-linking of HIV proviral DNA via triple-helix formation. Nucleic Acids Res. 1992;20:4275–81. doi: 10.1093/nar/20.16.4275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (405).Besch R, Giovannangeli C, Kammerbauer C, Degitz K. Specific inhibition of ICAM-1 expression mediated by gene targeting with Triplex-forming oligonucleotides. J Biol Chem. 2002;277:32473–9. doi: 10.1074/jbc.M203311200. [DOI] [PubMed] [Google Scholar]
- (406).Besch R, Giovannangeli C, Schuh T, Kammerbauer C, Degitz K. Characterization and quantification of triple helix formation in chromosomal DNA. J Mol Biol. 2004;341:979–89. doi: 10.1016/j.jmb.2004.05.079. [DOI] [PubMed] [Google Scholar]
- (407).Shahid KA, Majumdar A, Alam R, Liu ST, Kuan JY, Sui X, Cuenoud B, Glazer PM, Miller PS, Seidman MM. Targeted cross-linking of the human beta-globin gene in living cells mediated by a triple helix forming oligonucleotide. Biochemistry. 2006;45:1970–8. doi: 10.1021/bi0520986. [DOI] [PubMed] [Google Scholar]
- (408).Li H, Broughton-Head VJ, Peng G, Powers VE, Ovens MJ, Fox KR, Brown T. Triplex staples: DNA double-strand cross-linking at internal and terminal sites using psoralen-containing triplex-forming oligonucleotides. Bioconjug Chem. 2006;17:1561–7. doi: 10.1021/bc0601875. [DOI] [PubMed] [Google Scholar]
- (409).Musso M, Wang JC, Van Dyke MW. In vivo persistence of DNA triple helices containing psoralen-conjugated oligodeoxyribonucleotides. Nucleic Acids Res. 1996;24:4924–32. doi: 10.1093/nar/24.24.4924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (410).Giovannangeli C, Thuong NT, Helene C. Oligonucleotide clamps arrest DNA synthesis on a single-stranded DNA target. Proc Natl Acad Sci U S A. 1993;90:10013–7. doi: 10.1073/pnas.90.21.10013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (411).Takasugi M, Guendouz A, Chassignol M, Decout JL, Lhomme J, Thuong NT, Helene C. Sequence-specific photo-induced cross-linking of the two strands of double-helical DNA by a psoralen covalently linked to a triple helix-forming oligonucleotide. Proc Natl Acad Sci U S A. 1991;88:5602–6. doi: 10.1073/pnas.88.13.5602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (412).Bates PJ, Macaulay VM, McLean MJ, Jenkins TC, Reszka AP, Laughton CA, Neidle S. Characteristics of triplex-directed photoadduct formation by psoralen-linked oligodeoxynucleotides. Nucleic Acids Res. 1995;23:4283–9. doi: 10.1093/nar/23.21.4283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (413).Carbone GM, McGuffie E, Napoli S, Flanagan CE, Dembech C, Negri U, Arcamone F, Capobianco ML, Catapano CV. DNA binding and antigene activity of a daunomycin-conjugated triplex-forming oligonucleotide targeting the P2 promoter of the human c-myc gene. Nucleic Acids Res. 2004;32:2396–410. doi: 10.1093/nar/gkh527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (414).Brunet E, Corgnali M, Perrouault L, Roig V, Asseline U, Sorensen MD, Babu BR, Wengel J, Giovannangeli C. Intercalator conjugates of pyrimidine locked nucleic acid-modified triplex-forming oligonucleotides: improving DNA binding properties and reaching cellular activities. Nucleic Acids Res. 2005;33:4223–34. doi: 10.1093/nar/gki726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (415).Chan JH, Lim S, Wong WS. Antisense oligonucleotides: from design to therapeutic application. Clin Exp Pharmacol Physiol. 2006;33:533–40. doi: 10.1111/j.1440-1681.2006.04403.x. [DOI] [PubMed] [Google Scholar]
- (416).Shi F, Hoekstra D. Effective intracellular delivery of oligonucleotides in order to make sense of antisense. J Control Release. 2004;97:189–209. doi: 10.1016/j.jconrel.2004.03.016. [DOI] [PubMed] [Google Scholar]
- (417).Shoji Y, Nakashima H. Current status of delivery systems to improve target efficacy of oligonucleotides. Curr Pharm Des. 2004;10:785–96. doi: 10.2174/1381612043453009. [DOI] [PubMed] [Google Scholar]
- (418).Yakubov LA, Deeva EA, Zarytova VF, Ivanova EM, Ryte AS, Yurchenko LV, Vlassov VV. Mechanism of oligonucleotide uptake by cells: involvement of specific receptors? Proc Natl Acad Sci U S A. 1989;86:6454–8. doi: 10.1073/pnas.86.17.6454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (419).Loke SL, Stein CA, Zhang XH, Mori K, Nakanishi M, Subasinghe C, Cohen JS, Neckers LM. Characterization of oligonucleotide transport into living cells. Proc Natl Acad Sci U S A. 1989;86:3474–8. doi: 10.1073/pnas.86.10.3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (420).Wu-Pong S, Bard J, Huffman J, Jimerson J. Oligonucleotide biological activity: relationship to the cell cycle and nuclear transport. Biol Cell. 1997;89:257–61. [PubMed] [Google Scholar]
- (421).Hanss B, Leal-Pinto E, Bruggeman LA, Copeland TD, Klotman PE. Identification and characterization of a cell membrane nucleic acid channel. Proc Natl Acad Sci U S A. 1998;95:1921–6. doi: 10.1073/pnas.95.4.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (422).Leonetti JP, Mechti N, Degols G, Gagnor C, Lebleu B. Intracellular distribution of microinjected antisense oligonucleotides. Proc Natl Acad Sci U S A. 1991;88:2702–6. doi: 10.1073/pnas.88.7.2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (423).Fisher TL, Terhorst T, Cao X, Wagner RW. Intracellular disposition and metabolism of fluorescently-labeled unmodified and modified oligonucleotides microinjected into mammalian cells. Nucleic Acids Res. 1993;21:3857–65. doi: 10.1093/nar/21.16.3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (424).Giles RV, Spiller DG, Tidd DM. Detection of ribonuclease H-generated mRNA fragments in human leukemia cells following reversible membrane permeabilization in the presence of antisense oligodeoxynucleotides. Antisense Res Dev. 1995;5:23–31. doi: 10.1089/ard.1995.5.23. [DOI] [PubMed] [Google Scholar]
- (425).Spiller DG, Tidd DM. Nuclear delivery of antisense oligodeoxynucleotides through reversible permeabilization of human leukemia cells with streptolysin O. Antisense Res Dev. 1995;5:13–21. doi: 10.1089/ard.1995.5.13. [DOI] [PubMed] [Google Scholar]
- (426).Crooke RM, Graham MJ. Suborgan Pharmacokinetics. In: Crooke RM, editor. Antisene Drug Technology. Marcel Dekker, Inc.; New York: 2001. pp. 155–182. [Google Scholar]
- (427).Cho JG, Kim MK, Oh EJ, Yoon EJ, Sohn J, Park MK, Park GH. Pharmacokinetics of (111)In-labeled triplex-forming oligonucleotide targeting human N-myc gene. Mol Cells. 2002;14:93–100. [PubMed] [Google Scholar]
- (428).Cheng K, Ye Z, Guntaka RV, Mahato RI. Enhanced hepatic uptake and bioactivity of type alpha1(I) collagen gene promoter-specific triplex-forming oligonucleotides after conjugation with cholesterol. J Pharmacol Exp Ther. 2006;317:797–805. doi: 10.1124/jpet.105.100347. [DOI] [PubMed] [Google Scholar]
- (429).Cheng K, Ye Z, Guntaka RV, Mahato RI. Biodistribution and hepatic uptake of triplex-forming oligonucleotides against type alpha1(I) collagen gene promoter in normal and fibrotic rats. Mol Pharm. 2005;2:206–17. doi: 10.1021/mp050012x. [DOI] [PubMed] [Google Scholar]
- (430).Beltinger C, Saragovi HU, Smith RM, LeSauteur L, Shah N, DeDionisio L, Christensen L, Raible A, Jarett L, Gewirtz AM. Binding, uptake, and intracellular trafficking of phosphorothioate-modified oligodeoxynucleotides. J Clin Invest. 1995;95:1814–23. doi: 10.1172/JCI117860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (431).Hughes MD, Hussain M, Nawaz Q, Sayyed P, Akhtar S. The cellular delivery of antisense oligonucleotides and ribozymes. Drug Discov Today. 2001;6:303–315. doi: 10.1016/s1359-6446(00)00326-3. [DOI] [PubMed] [Google Scholar]
- (432).Sirsi SR, Williams JH, Lutz GJ. Poly(ethylene imine)-poly(ethylene glycol) copolymers facilitate efficient delivery of antisense oligonucleotides to nuclei of mature muscle cells of mdx mice. Hum Gene Ther. 2005;16:1307–17. doi: 10.1089/hum.2005.16.1307. [DOI] [PubMed] [Google Scholar]
- (433).Khalil IA, Kogure K, Akita H, Harashima H. Uptake pathways and subsequent intracellular trafficking in nonviral gene delivery. Pharmacol Rev. 2006;58:32–45. doi: 10.1124/pr.58.1.8. [DOI] [PubMed] [Google Scholar]
- (434).Murthy N, Campbell J, Fausto N, Hoffman AS, Stayton PS. Design and synthesis of pH-responsive polymeric carriers that target uptake and enhance the intracellular delivery of oligonucleotides. J Control Release. 2003;89:365–74. doi: 10.1016/s0168-3659(03)00099-3. [DOI] [PubMed] [Google Scholar]
- (435).Vives E, Brodin P, Lebleu B. A truncated HIV-1 Tat protein basic domain rapidly translocates through the plasma membrane and accumulates in the cell nucleus. J Biol Chem. 1997;272:16010–7. doi: 10.1074/jbc.272.25.16010. [DOI] [PubMed] [Google Scholar]
- (436).Thoren PE, Persson D, Isakson P, Goksor M, Onfelt A, Norden B. Uptake of analogs of penetratin, Tat(48–60) and oligoarginine in live cells. Biochem Biophys Res Commun. 2003;307:100–7. doi: 10.1016/s0006-291x(03)01135-5. [DOI] [PubMed] [Google Scholar]
- (437).Nakase I, Niwa M, Takeuchi T, Sonomura K, Kawabata N, Koike Y, Takehashi M, Tanaka S, Ueda K, Simpson JC, Jones AT, Sugiura Y, Futaki S. Cellular uptake of arginine-rich peptides: roles for macropinocytosis and actin rearrangement. Mol Ther. 2004;10:1011–22. doi: 10.1016/j.ymthe.2004.08.010. [DOI] [PubMed] [Google Scholar]
- (438).Brooks H, Lebleu B, Vives E. Tat peptide-mediated cellular delivery: back to basics. Adv Drug Deliv Rev. 2005;57:559–77. doi: 10.1016/j.addr.2004.12.001. [DOI] [PubMed] [Google Scholar]
- (439).Gupta B, Levchenko TS, Torchilin VP. Intracellular delivery of large molecules and small particles by cell-penetrating proteins and peptides. Adv Drug Deliv Rev. 2005;57:637–51. doi: 10.1016/j.addr.2004.10.007. [DOI] [PubMed] [Google Scholar]
- (440).Kleemann E, Neu M, Jekel N, Fink L, Schmehl T, Gessler T, Seeger W, Kissel T. Nano-carriers for DNA delivery to the lung based upon a TAT-derived peptide covalently coupled to PEG-PEI. J Control Release. 2005;109:299–316. doi: 10.1016/j.jconrel.2005.09.036. [DOI] [PubMed] [Google Scholar]
- (441).Nori A, Jensen KD, Tijerina M, Kopeckova P, Kopecek J. Tat-conjugated synthetic macromolecules facilitate cytoplasmic drug delivery to human ovarian carcinoma cells. Bioconjug Chem. 2003;14:44–50. doi: 10.1021/bc0255900. [DOI] [PubMed] [Google Scholar]
- (442).Kang H, DeLong R, Fisher MH, Juliano RL. Tat-conjugated PAMAM dendrimers as delivery agents for antisense and siRNA oligonucleotides. Pharm Res. 2005;22:2099–106. doi: 10.1007/s11095-005-8330-5. [DOI] [PubMed] [Google Scholar]
- (443).Shi F, Nomden A, Oberle V, Engberts JB, Hoekstra D. Efficient cationic lipid-mediated delivery of antisense oligonucleotides into eukaryotic cells: down-regulation of the corticotropin-releasing factor receptor. Nucleic Acids Res. 2001;29:2079–87. doi: 10.1093/nar/29.10.2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (444).Shi F, Visser WH, de Jong NM, Liem RS, Ronken E, Hoekstra D. Antisense oligonucleotides reach mRNA targets via the RNA matrix: downregulation of the 5-HT1A receptor. Exp Cell Res. 2003;291:313–25. doi: 10.1016/j.yexcr.2003.07.003. [DOI] [PubMed] [Google Scholar]
- (445).Marcusson EG, Bhat B, Manoharan M, Bennett CF, Dean NM. Phosphorothioate oligodeoxyribonucleotides dissociate from cationic lipids before entering the nucleus. Nucleic Acids Res. 1998;26:2016–23. doi: 10.1093/nar/26.8.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (446).Brunner S, Furtbauer E, Sauer T, Kursa M, Wagner E. Overcoming the nuclear barrier: cell cycle independent nonviral gene transfer with linear polyethylenimine or electroporation. Mol Ther. 2002;5:80–6. doi: 10.1006/mthe.2001.0509. [DOI] [PubMed] [Google Scholar]
- (447).Godbey WT, Wu KK, Mikos AG. Tracking the intracellular path of poly(ethylenimine)/DNA complexes for gene delivery. Proc Natl Acad Sci U S A. 1999;96:5177–81. doi: 10.1073/pnas.96.9.5177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (448).Schaffer DV, Fidelman NA, Dan N, Lauffenburger DA. Vector unpacking as a potential barrier for receptor-mediated polyplex gene delivery. Biotechnol Bioeng. 2000;67:598–606. doi: 10.1002/(sici)1097-0290(20000305)67:5<598::aid-bit10>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- (449).Hwang SJ, Bellocq NC, Davis ME. Effects of structure of beta-cyclodextrin-containing polymers on gene delivery. Bioconjug Chem. 2001;12:280–90. doi: 10.1021/bc0001084. [DOI] [PubMed] [Google Scholar]
- (450).Read ML, Singh S, Ahmed Z, Stevenson M, Briggs SS, Oupicky D, Barrett LB, Spice R, Kendall M, Berry M, Preece JA, Logan A, Seymour LW. A versatile reducible polycation-based system for efficient delivery of a broad range of nucleic acids. Nucleic Acids Res. 2005;33:e86. doi: 10.1093/nar/gni085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (451).Oishi M, Hayama T, Akiyama Y, Takae S, Harada A, Yamasaki Y, Nagatsugi F, Sasaki S, Nagasaki Y, Kataoka K. Supramolecular assemblies for the cytoplasmic delivery of antisense oligodeoxynucleotide: polyion complex (PIC) micelles based on poly(ethylene glycol)-SS-oligodeoxynucleotide conjugate. Biomacromolecules. 2005;6:2449–54. doi: 10.1021/bm050370l. [DOI] [PubMed] [Google Scholar]
- (452).Kim YH, Park JH, Lee M, Kim YH, Park TG, Kim SW. Polyethylenimine with acid-labile linkages as a biodegradable gene carrier. J Control Release. 2005;103:209–19. doi: 10.1016/j.jconrel.2004.11.008. [DOI] [PubMed] [Google Scholar]
- (453).Geary RS, Yu RZ, Leeds JM, Wwtanabe TA, Henry SP, Levin AA, Templin MV. Pharmacokinetic Properties in Animals. In: Crooke ST, editor. Antisense Drug Technology. Marcel Dekker, Inc.; New York: 2001. pp. 119–154. [Google Scholar]
- (454).Yu RZ, Geary RS, Watanabe TA, Levin AA, Schoenfeld SL. Pharmacokinetic Properties in Humans. In: Crooke ST, editor. Antisense Drug Technology. Marcel Dekker, Inc.; New York: 2001. pp. 183–200. [Google Scholar]
- (455).Sands H, Gorey-Feret LJ, Cocuzza AJ, Hobbs FW, Chidester D, Trainor GL. Biodistribution and metabolism of internally 3H-labeled oligonucleotides. I. Comparison of a phosphodiester and a phosphorothioate. Mol Pharmacol. 1994;45:932–43. [PubMed] [Google Scholar]
- (456).Mahato RI, Takemura S, Akamatsu K, Nishikawa M, Takakura Y, Hashida M. Physicochemical and disposition characteristics of antisense oligonucleotides complexed with glycosylated poly(L-lysine) Biochem Pharmacol. 1997;53:887–95. doi: 10.1016/s0006-2952(96)00880-5. [DOI] [PubMed] [Google Scholar]
- (457).Takakura Y, Mahato RI, Yoshida M, Kanamaru T, Hashida M. Uptake characteristics of oligonucleotides in the isolated rat liver perfusion system. Antisense Nucleic Acid Drug Dev. 1996;6:177–83. doi: 10.1089/oli.1.1996.6.177. [DOI] [PubMed] [Google Scholar]
- (458).Nicklin PL, Craig SJ, Phillips JA. Pharmacokinetic properties of phosphorothioates in animals-adsorption, distribution, metabolism and eliminatino. In: Crooke ST, editor. Antisense Research and Application. Handbook of Experimental Pharmacology. Springer-Verlag; Berlin: 1998. pp. 141–168. [Google Scholar]
- (459).Plenat F, Klein-Monhoven N, Marie B, Vignaud JM, Duprez A. Cell and tissue distribution of synthetic oligonucleotides in healthy and tumor-bearing nude mice. An autoradiographic, immunohistological, and direct fluorescence microscopy study. Am J Pathol. 1995;147:124–35. [PMC free article] [PubMed] [Google Scholar]
- (460).Graham MJ, Crooke ST, Monteith DK, Cooper SR, Lemonidis KM, Stecker KK, Martin MJ, Crooke RM. In vivo distribution and metabolism of a phosphorothioate oligonucleotide within rat liver after intravenous administration. J Pharmacol Exp Ther. 1998;286:447–58. [PubMed] [Google Scholar]
- (461).Bijsterbosch MK, Manoharan M, Rump ET, De Vrueh RL, van Veghel R, Tivel KL, Biessen EA, Bennett CF, Cook PD, van Berkel TJ. In vivo fate of phosphorothioate antisense oligodeoxynucleotides: predominant uptake by scavenger receptors on endothelial liver cells. Nucleic Acids Res. 1997;25:3290–6. doi: 10.1093/nar/25.16.3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (462).Crooke RM, Graham MJ, Cooke ME, Crooke ST. In vitro pharmacokinetics of phosphorothioate antisense oligonucleotides. J Pharmacol Exp Ther. 1995;275:462–73. [PubMed] [Google Scholar]
- (463).Butler M, Crooke RM, Graham MJ, Lemonidis KM, Lougheed M, Murray SF, Witchell D, Steinbrecher U, Bennett CF. Phosphorothioate oligodeoxynucleotides distribute similarly in class A scavenger receptor knockout and wild-type mice. J Pharmacol Exp Ther. 2000;292:489–96. [PubMed] [Google Scholar]
- (464).Kursa M, Walker GF, Roessler V, Ogris M, Roedl W, Kircheis R, Wagner E. Novel shielded transferrin-polyethylene glycol-polyethylenimine/DNA complexes for systemic tumor-targeted gene transfer. Bioconjug Chem. 2003;14:222–31. doi: 10.1021/bc0256087. [DOI] [PubMed] [Google Scholar]
- (465).Kwok KY, McKenzie DL, Evers DL, Rice KG. Formulation of highly soluble poly(ethylene glycol)-peptide DNA condensates. J Pharm Sci. 1999;88:996–1003. doi: 10.1021/js990072s. [DOI] [PubMed] [Google Scholar]
- (466).Ogris M, Brunner S, Schuller S, Kircheis R, Wagner E. PEGylated DNA/transferrin-PEI complexes: reduced interaction with blood components, extended circulation in blood and potential for systemic gene delivery. Gene Ther. 1999;6:595–605. doi: 10.1038/sj.gt.3300900. [DOI] [PubMed] [Google Scholar]
- (467).Green NK, Herbert CW, Hale SJ, Hale AB, Mautner V, Harkins R, Hermiston T, Ulbrich K, Fisher KD, Seymour LW. Extended plasma circulation time and decreased toxicity of polymer-coated adenovirus. Gene Ther. 2004;11:1256–63. doi: 10.1038/sj.gt.3302295. [DOI] [PubMed] [Google Scholar]
- (468).Oupicky D, Ogris M, Howard KA, Dash PR, Ulbrich K, Seymour LW. Importance of lateral and steric stabilization of polyelectrolyte gene delivery vectors for extended systemic circulation. Mol Ther. 2002;5:463–72. doi: 10.1006/mthe.2002.0568. [DOI] [PubMed] [Google Scholar]
- (469).Crooke ST, Graham MJ, Zuckerman JE, Brooks D, Conklin BS, Cummins LL, Greig MJ, Guinosso CJ, Kornbrust D, Manoharan M, Sasmor HM, Schleich T, Tivel KL, Griffey RH. Pharmacokinetic properties of several novel oligonucleotide analogs in mice. J Pharmacol Exp Ther. 1996;277:923–37. [PubMed] [Google Scholar]
- (470).Manoharan M. Oligonucleotide Conjugates in Antisense Technology. In: Crooke RM, editor. Antisense Drug Technology. Marcel Dekker, Inc.; New York: 2001. pp. 391–469. [Google Scholar]
- (471).MacKellar C, Graham D, Will DW, Burgess S, Brown T. Synthesis and physical properties of anti-HIV antisense oligonucleotides bearing terminal lipophilic groups. Nucleic Acids Res. 1992;20:3411–7. doi: 10.1093/nar/20.13.3411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (472).Letsinger RL, Zhang GR, Sun DK, Ikeuchi T, Sarin PS. Cholesteryl-conjugated oligonucleotides: synthesis, properties, and activity as inhibitors of replication of human immunodeficiency virus in cell culture. Proc Natl Acad Sci U S A. 1989;86:6553–6. doi: 10.1073/pnas.86.17.6553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (473).de Smidt PC, Le Doan T, de Falco S, van Berkel TJ. Association of antisense oligonucleotides with lipoproteins prolongs the plasma half-life and modifies the tissue distribution. Nucleic Acids Res. 1991;19:4695–700. doi: 10.1093/nar/19.17.4695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (474).Krieg AM, Tonkinson J, Matson S, Zhao Q, Saxon M, Zhang LM, Bhanja U, Yakubov L, Stein CA. Modification of antisense phosphodiester oligodeoxynucleotides by a 5' cholesteryl moiety increases cellular association and improves efficacy. Proc Natl Acad Sci U S A. 1993;90:1048–52. doi: 10.1073/pnas.90.3.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (475).Manoharan M, Tivel KL, Cook PD. Lipidic Nucleic-Acids. Tetrahedron Letters. 1995;36:3651–3654. [Google Scholar]
- (476).Manoharan M, Tivel KL, Andrade LK, Mohan V, Condon TP, Bennett CF, Cook PD. Oligonucleotide Conjugates - Alteration of the Pharmacokinetic Properties of Antisense Agents. Nucleosides & Nucleotides. 1995;14:969–973. [Google Scholar]
- (477).Alefelder S, Patel BK, Eckstein F. Incorporation of terminal phosphorothioates into oligonucleotides. Nucleic Acids Res. 1998;26:4983–8. doi: 10.1093/nar/26.21.4983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (478).Gryaznov SM, Lloyd DH. Nucleic Acids Res. 1993:5909–15. doi: 10.1093/nar/21.25.5909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (479).Hanaki K, Ino M, Taniguchi T, Nishihara T, Nozaki C, Honda E, Hayashi M, Yamamoto K. Bioimaging analysis of cellular uptake and intracellular distribution of oligonucleotide. Bioimages. 1997;5:71–75. [Google Scholar]
- (480).LeDoan T, Etore F, Tenu JP, Letourneux Y, Agrawal S. Cell binding, uptake and cytosolic partition of HIV anti-gag phosphodiester oligonucleotides 3'-linked to cholesterol derivatives in macrophages. Bioorg Med Chem. 1999;7:2263–9. doi: 10.1016/s0968-0896(99)00115-7. [DOI] [PubMed] [Google Scholar]
- (481).Alahari SK, Dean NM, Fisher MH, Delong R, Manoharan M, Tivel KL, Juliano RL. Inhibition of expression of the multidrug resistance-associated P-glycoprotein of by phosphorothioate and 5' cholesterol-conjugated phosphorothioate antisense oligonucleotides. Mol Pharmacol. 1996;50:808–19. [PubMed] [Google Scholar]
- (482).Epa WR, Rong P, Bartlett PF, Coulson EJ, Barrett GL. Enhanced downregulation of the p75 nerve growth factor receptor by cholesteryl and bis-cholesteryl antisense oligonucleotides. Antisense Nucleic Acid Drug Dev. 1998;8:489–98. doi: 10.1089/oli.1.1998.8.489. [DOI] [PubMed] [Google Scholar]
- (483).Desjardins J, Mata J, Brown T, Graham D, Zon G, Iversen P. Cholesteryl-conjugated phosphorothioate oligodeoxynucleotides modulate CYP2B1 expression in vivo. J Drug Target. 1995;2:477–85. doi: 10.3109/10611869509015917. [DOI] [PubMed] [Google Scholar]
- (484).Bijsterbosch MK, Rump ET, De Vrueh RL, Dorland R, van Veghel R, Tivel KL, Biessen EA, van Berkel TJ, Manoharan M. Modulation of plasma protein binding and in vivo liver cell uptake of phosphorothioate oligodeoxynucleotides by cholesterol conjugation. Nucleic Acids Res. 2000;28:2717–25. doi: 10.1093/nar/28.14.2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (485).Bijsterbosch MK, Manoharan M, Dorland R, Van Veghel R, Biessen EA, Van Berkel TJ. bis-Cholesteryl-conjugated phosphorothioate oligodeoxynucleotides are highly selectively taken up by the liver. J Pharmacol Exp Ther. 2002;302:619–26. doi: 10.1124/jpet.302.2.619. [DOI] [PubMed] [Google Scholar]
- (486).Srinivasan SK, Tewary HK, Iversen PL. Characterization of binding sites, extent of binding, and drug interactions of oligonucleotides with albumin. Antisense Res Dev. 1995;5:131–9. doi: 10.1089/ard.1995.5.131. [DOI] [PubMed] [Google Scholar]
- (487).Veronese FM, Pasut G. PEGylation, successful approach to drug delivery. Drug Discov Today. 2005;10:1451–8. doi: 10.1016/S1359-6446(05)03575-0. [DOI] [PubMed] [Google Scholar]
- (488).Jaschke A, Furste JP, Nordhoff E, Hillenkamp F, Cech D, Erdmann VA. Synthesis and properties of oligodeoxyribonucleotide-polyethylene glycol conjugates. Nucleic Acids Res. 1994;22:4810–7. doi: 10.1093/nar/22.22.4810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (489).Efimov VA, Kalinkina AL, Chakhmakhcheva OG. Dipentafluorophenyl carbonate--a reagent for the synthesis of oligonucleotides and their conjugates. Nucleic Acids Res. 1993;21:5337–44. doi: 10.1093/nar/21.23.5337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (490).Tarasow TM, Tinnermeier D, Zyzniewski C. Characterization of oligodeoxyribonucleotide-polyethylene glycol conjugates by electrospray mass spectrometry. Bioconjug Chem. 1997;8:89–93. doi: 10.1021/bc960082+. [DOI] [PubMed] [Google Scholar]
- (491).Bonora GM, Scremin CL, Colonna FP, Garbesi A. HELP (high efficiency liquid phase) new oligonucleotide synthesis on soluble polymeric support. Nucleic Acids Res. 1990;18:3155–9. doi: 10.1093/nar/18.11.3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (492).Bonora GM, Ivanova E, Zarytova V, Burcovich B, Veronese FM. Synthesis and characterization of high-molecular mass polyethylene glycol-conjugated oligonucleotides. Bioconjug Chem. 1997;8:793–7. doi: 10.1021/bc970082p. [DOI] [PubMed] [Google Scholar]
- (493).Kawaguchi T, Asakawa H, Tashiro Y, Juni K, Sueishi T. Stability, specific binding activity, and plasma concentration in mice of an oligodeoxynucleotide modified at 5'-terminal with poly(ethylene glycol) Biol Pharm Bull. 1995;18:474–6. doi: 10.1248/bpb.18.474. [DOI] [PubMed] [Google Scholar]
- (494).Zhao H, Greenwald RB, Reddy P, Xia J, Peng P. A new platform for oligonucleotide delivery utilizing the PEG prodrug approach. Bioconjug Chem. 2005;16:758–66. doi: 10.1021/bc049804k. [DOI] [PubMed] [Google Scholar]
- (495).Jones DS, Hachmann JP, Osgood SA, Hayag MS, Barstad PA, Iverson GM, Coutts SM. Conjugates of double-stranded oligonucleotides with poly(ethylene glycol) and keyhole limpet hemocyanin: a model for treating systemic lupus erythematosus. Bioconjug Chem. 1994;5:390–9. doi: 10.1021/bc00029a003. [DOI] [PubMed] [Google Scholar]
- (496).Chirila TV, Rakoczy PE, Garrett KL, Lou X, Constable IJ. The use of synthetic polymers for delivery of therapeutic antisense oligodeoxynucleotides. Biomaterials. 2002;23:321–42. doi: 10.1016/S0142-9612(01)00125-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (497).Rapozzi V, Cogoi S, Spessotto P, Risso A, Bonora GM, Quadrifoglio F, Xodo LE. Antigene effect in K562 cells of a PEG-conjugated triplex-forming oligonucleotide targeted to the bcr/abl oncogene. Biochemistry. 2002;41:502–10. doi: 10.1021/bi011314h. [DOI] [PubMed] [Google Scholar]
- (498).Bonora GM, Tocco G, Zaramella S, Veronese FM, Pliasunova O, Pokrovsky A, Ivanova E, Zarytova V. Antisense activity of an anti-HIV oligonucleotide conjugated to linear and branched high molecular weight polyethylene glycols. Farmaco. 1998;53:634–7. doi: 10.1016/s0014-827x(98)00078-0. [DOI] [PubMed] [Google Scholar]
- (499).Watson SR, Chang YF, O'Connell D, Weigand L, Ringquist S, Parma DH. Anti-L-selectin aptamers: binding characteristics, pharmacokinetic parameters, and activity against an intravascular target in vivo. Antisense Nucleic Acid Drug Dev. 2000;10:63–75. doi: 10.1089/oli.1.2000.10.63. [DOI] [PubMed] [Google Scholar]
- (500).McCauley TG, Kurz JC, Merlino PG, Lewis SD, Gilbert M, Epstein DM, Marsh HN. Pharmacologic and pharmacokinetic assessment of anti-TGFbeta2 aptamers in rabbit plasma and aqueous humor. Pharm Res. 2006;23:303–11. doi: 10.1007/s11095-005-9305-2. [DOI] [PubMed] [Google Scholar]
- (501).Zhao H, Peng P, Longley C, Zhang Y, Borowski V, Mehlig M, Reddy P, Xia J, Borchard G, Lipman J, Benimetskaya L, Stein CA. Delivery of G3139 using releasable PEG-linkers: impact on pharmacokinetic profile and anti-tumor efficacy. J Control Release. 2007;119:143–52. doi: 10.1016/j.jconrel.2006.12.021. [DOI] [PubMed] [Google Scholar]
- (502).Kataoka K, Itaka K, Nishiyama N, Yamasaki Y, Oishi M, Nagasaki Y. Smart polymeric micelles as nanocarriers for oligonucleotides and siRNA delivery. Nucleic Acids Symp Ser (Oxf) 2005:17–8. doi: 10.1093/nass/49.1.17. [DOI] [PubMed] [Google Scholar]
- (503).Oishi M, Nagatsugi F, Sasaki S, Nagasaki Y, Kataoka K. Smart polyion complex micelles for targeted intracellular delivery of PEGylated antisense oligonucleotides containing acid-labile linkages. Chembiochem. 2005;6:718–25. doi: 10.1002/cbic.200400334. [DOI] [PubMed] [Google Scholar]
- (504).Jeong JH, Kim SH, Kim SW, Park TG. Intracellular delivery of poly(ethylene glycol) conjugated antisense oligonucleotide using cationic lipids by formation of self-assembled polyelectrolyte complex micelles. J Nanosci Nanotechnol. 2006;6:2790–5. doi: 10.1166/jnn.2006.414. [DOI] [PubMed] [Google Scholar]
- (505).Kim SH, Jeong JH, Mok H, Lee SH, Kim SW, Park TG. Folate Receptor Targeted Delivery of Polyelectrolyte Complex Micelles Prepared from ODN-PEG-Folate Conjugate and Cationic Lipids. Biotechnol Prog. 2007;23:232–237. doi: 10.1021/bp060243g. [DOI] [PubMed] [Google Scholar]
- (506).Jeong JH, Kim SH, Kim SW, Park TG. In vivo tumor targeting of ODN-PEG-folic acid/PEI polyelectrolyte complex micelles. J Biomater Sci Polym Ed. 2005;16:1409–19. doi: 10.1163/156856205774472335. [DOI] [PubMed] [Google Scholar]
- (507).Pricer WE, Jr., Ashwell G. The binding of desialylated glycoproteins by plasma membranes of rat liver. J Biol Chem. 1971;246:4825–33. [PubMed] [Google Scholar]
- (508).Ashwell G, Harford J. Carbohydrate-specific receptors of the liver. Annu Rev Biochem. 1982;51:531–54. doi: 10.1146/annurev.bi.51.070182.002531. [DOI] [PubMed] [Google Scholar]
- (509).Ashwell G, Morell AG. The role of surface carbohydrates in the hepatic recognition and transport of circulating glycoproteins. Adv Enzymol Relat Areas Mol Biol. 1974;41:99–128. doi: 10.1002/9780470122860.ch3. [DOI] [PubMed] [Google Scholar]
- (510).Schwartz AL, Fridovich SE, Lodish HF. Kinetics of internalization and recycling of the asialoglycoprotein receptor in a hepatoma cell line. J Biol Chem. 1982;257:4230–7. [PubMed] [Google Scholar]
- (511).Lee YC, Townsend RR, Hardy MR, Lonngren J, Arnarp J, Haraldsson M, Lonn H. Binding of synthetic oligosaccharides to the hepatic Gal/GalNAc lectin. Dependence on fine structural features. J Biol Chem. 1983;258:199–202. [PubMed] [Google Scholar]
- (512).Connolly DT, Townsend RR, Kawaguchi K, Bell WR, Lee YC. Binding and endocytosis of cluster glycosides by rabbit hepatocytes. Evidence for a short-circuit pathway that does not lead to degradation. J Biol Chem. 1982;257:939–45. [PubMed] [Google Scholar]
- (513).Lee RT, Lin P, Lee YC. New synthetic cluster ligands for galactose/N-acetylgalactosamine-specific lectin of mammalian liver. Biochemistry. 1984;23:4255–61. doi: 10.1021/bi00313a037. [DOI] [PubMed] [Google Scholar]
- (514).Biessen EA, Beuting DM, Roelen HC, van de Marel GA, van Boom JH, van Berkel TJ. Synthesis of cluster galactosides with high affinity for the hepatic asialoglycoprotein receptor. J Med Chem. 1995;38:1538–46. doi: 10.1021/jm00009a014. [DOI] [PubMed] [Google Scholar]
- (515).Lee RT, Lee YC. Preparation of cluster glycosides ofN-acetylgalactosamine that have subnanomolar binding constants towards the mammalian hepatic Gal/GalNAc-specific receptor. Glycoconjugate Journal. 1987;4:317–328. [Google Scholar]
- (516).Hangeland JJ, Levis JT, Lee YC, Ts'o PO. Cell-type specific and ligand specific enhancement of cellular uptake of oligodeoxynucleoside methylphosphonates covalently linked with a neoglycopeptide, YEE(ah-GalNAc)3. Bioconjug Chem. 1995;6:695–701. doi: 10.1021/bc00036a006. [DOI] [PubMed] [Google Scholar]
- (517).Hangeland JJ, Flesher JE, Deamond SF, Lee YC, Ts OP, Frost JJ. Tissue distribution and metabolism of the [32P]-labeled oligodeoxynucleoside methylphosphonate-neoglycopeptide conjugate, [YEE(ah-GalNAc)3]-SMCC-AET-pUmpT7, in the mouse. Antisense Nucleic Acid Drug Dev. 1997;7:141–9. doi: 10.1089/oli.1.1997.7.141. [DOI] [PubMed] [Google Scholar]
- (518).Duff RJ, Deamond SF, Roby C, Zhou Y, Ts'o PO. Intrabody tissue-specific delivery of antisense conjugates in animals: ligand-linker-antisense oligomer conjugates. Methods Enzymol. 2000;313:297–321. doi: 10.1016/s0076-6879(00)13019-8. [DOI] [PubMed] [Google Scholar]
- (519).Biessen EA, Vietsch H, Rump ET, Fluiter K, Bijsterbosch MK, van Berkel TJ. Targeted delivery of antisense oligonucleotides to parenchymal liver cells in vivo. Methods Enzymol. 2000;314:324–42. doi: 10.1016/s0076-6879(99)14113-2. [DOI] [PubMed] [Google Scholar]
- (520).Biessen EA, Vietsch H, Rump ET, Fluiter K, Kuiper J, Bijsterbosch MK, van Berkel TJ. Targeted delivery of oligodeoxynucleotides to parenchymal liver cells in vivo. Biochem J. 1999;340(Pt 3):783–92. [PMC free article] [PubMed] [Google Scholar]
- (521).Maier MA, Yannopoulos CG, Mohamed N, Roland A, Fritz H, Mohan V, Just G, Manoharan M. Synthesis of antisense oligonucleotides conjugated to a multivalent carbohydrate cluster for cellular targeting. Bioconjug Chem. 2003;14:18–29. doi: 10.1021/bc020028v. [DOI] [PubMed] [Google Scholar]
- (522).Gait MJ. Peptide-mediated cellular delivery of antisense oligonucleotides and their analogues. Cell Mol Life Sci. 2003;60:844–53. doi: 10.1007/s00018-003-3044-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (523).Meade BR, Dowdy SF. Exogenous siRNA delivery using peptide transduction domains/cell penetrating peptides. Adv Drug Deliv Rev. 2007;59:134–40. doi: 10.1016/j.addr.2007.03.004. [DOI] [PubMed] [Google Scholar]
- (524).Schwarze SR, Dowdy SF. In vivo protein transduction: intracellular delivery of biologically active proteins, compounds and DNA. Trends Pharmacol Sci. 2000;21:45–8. doi: 10.1016/s0165-6147(99)01429-7. [DOI] [PubMed] [Google Scholar]
- (525).Frankel AD, Pabo CO. Cellular uptake of the tat protein from human immunodeficiency virus. Cell. 1988;55:1189–93. doi: 10.1016/0092-8674(88)90263-2. [DOI] [PubMed] [Google Scholar]
- (526).Derossi D, Joliot AH, Chassaing G, Prochiantz A. The third helix of the Antennapedia homeodomain translocates through biological membranes. J Biol Chem. 1994;269:10444–50. [PubMed] [Google Scholar]
- (527).Elliott G, O'Hare P. Intercellular trafficking and protein delivery by a herpesvirus structural protein. Cell. 1997;88:223–33. doi: 10.1016/s0092-8674(00)81843-7. [DOI] [PubMed] [Google Scholar]
- (528).Muratovska A, Eccles MR. Conjugate for efficient delivery of short interfering RNA (siRNA) into mammalian cells. FEBS Lett. 2004;558:63–8. doi: 10.1016/S0014-5793(03)01505-9. [DOI] [PubMed] [Google Scholar]
- (529).Morris MC, Vidal P, Chaloin L, Heitz F, Divita G. A new peptide vector for efficient delivery of oligonucleotides into mammalian cells. Nucleic Acids Res. 1997;25:2730–6. doi: 10.1093/nar/25.14.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (530).Pujals S, Fernandez-Carneado J, Lopez-Iglesias C, Kogan MJ, Giralt E. Mechanistic aspects of CPP-mediated intracellular drug delivery: relevance of CPP self-assembly. Biochim Biophys Acta. 2006;1758:264–79. doi: 10.1016/j.bbamem.2006.01.006. [DOI] [PubMed] [Google Scholar]
- (531).Astriab-Fisher A, Sergueev DS, Fisher M, Shaw BR, Juliano RL. Antisense inhibition of P-glycoprotein expression using peptide-oligonucleotide conjugates. Biochem Pharmacol. 2000;60:83–90. doi: 10.1016/s0006-2952(00)00310-5. [DOI] [PubMed] [Google Scholar]
- (532).Brugidou J, Legrand C, Mery J, Rabie A. The retro-inverso form of a homeobox-derived short peptide is rapidly internalised by cultured neurones: a new basis for an efficient intracellular delivery system. Biochem Biophys Res Commun. 1995;214:685–93. doi: 10.1006/bbrc.1995.2340. [DOI] [PubMed] [Google Scholar]
- (533).Aldrian-Herrada G, Desarmenien MG, Orcel H, Boissin-Agasse L, Mery J, Brugidou J, Rabie A. A peptide nucleic acid (PNA) is more rapidly internalized in cultured neurons when coupled to a retro-inverso delivery peptide. The antisense activity depresses the target mRNA and protein in magnocellular oxytocin neurons. Nucleic Acids Res. 1998;26:4910–6. doi: 10.1093/nar/26.21.4910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (534).Chaloin L, Vidal P, Lory P, Mery J, Lautredou N, Divita G, Heitz F. Design of carrier peptide-oligonucleotide conjugates with rapid membrane translocation and nuclear localization properties. Biochem Biophys Res Commun. 1998;243:601–8. doi: 10.1006/bbrc.1997.8050. [DOI] [PubMed] [Google Scholar]
- (535).Moulton HM, Nelson MH, Hatlevig SA, Reddy MT, Iversen PL. Cellular uptake of antisense morpholino oligomers conjugated to arginine-rich peptides. Bioconjug Chem. 2004;15:290–9. doi: 10.1021/bc034221g. [DOI] [PubMed] [Google Scholar]
- (536).McClorey G, Moulton HM, Iversen PL, Fletcher S, Wilton SD. Antisense oligonucleotide-induced exon skipping restores dystrophin expression in vitro in a canine model of DMD. Gene Ther. 2006;13:1373–81. doi: 10.1038/sj.gt.3302800. [DOI] [PubMed] [Google Scholar]
- (537).McClorey G, Fall AM, Moulton HM, Iversen PL, Rasko JE, Ryan M, Fletcher S, Wilton SD. Induced dystrophin exon skipping in human muscle explants. Neuromuscul Disord. 2006;16:583–90. doi: 10.1016/j.nmd.2006.05.017. [DOI] [PubMed] [Google Scholar]
- (538).Pooga M, Soomets U, Hallbrink M, Valkna A, Saar K, Rezaei K, Kahl U, Hao JX, Xu XJ, Wiesenfeld-Hallin Z, Hokfelt T, Bartfai T, Langel U. Cell penetrating PNA constructs regulate galanin receptor levels and modify pain transmission in vivo. Nat Biotechnol. 1998;16:857–61. doi: 10.1038/nbt0998-857. [DOI] [PubMed] [Google Scholar]
- (539).Youngblood DS, Hatlevig SA, Hassinger JN, Iversen PL, Moulton HM. Stability of cell-penetrating peptide-morpholino oligomer conjugates in human serum and in cells. Bioconjug Chem. 2007;18:50–60. doi: 10.1021/bc060138s. [DOI] [PubMed] [Google Scholar]
- (540).Goncalves E, Kitas E, Seelig J. Binding of oligoarginine to membrane lipids and heparan sulfate: structural and thermodynamic characterization of a cell-penetrating peptide. Biochemistry. 2005;44:2692–702. doi: 10.1021/bi048046i. [DOI] [PubMed] [Google Scholar]
- (541).Jiang T, Olson ES, Nguyen QT, Roy M, Jennings PA, Tsien RY. Tumor imaging by means of proteolytic activation of cell-penetrating peptides. Proc Natl Acad Sci U S A. 2004;101:17867–72. doi: 10.1073/pnas.0408191101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (542).Turner JJ, Arzumanov AA, Gait MJ. Synthesis, cellular uptake and HIV-1 Tat-dependent trans-activation inhibition activity of oligonucleotide analogues disulphide-conjugated to cell-penetrating peptides. Nucleic Acids Res. 2005;33:27–42. doi: 10.1093/nar/gki142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (543).Kang SH, Cho MJ, Kole R. Up-regulation of luciferase gene expression with antisense oligonucleotides: implications and applications in functional assay development. Biochemistry. 1998;37:6235–9. doi: 10.1021/bi980300h. [DOI] [PubMed] [Google Scholar]
- (544).Abes S, Moulton H, Turner J, Clair P, Richard JP, Iversen P, Gait MJ, Lebleu B. Peptide-based delivery of nucleic acids: design, mechanism of uptake and applications to splice-correcting oligonucleotides. Biochem Soc Trans. 2007;35:53–5. doi: 10.1042/BST0350053. [DOI] [PubMed] [Google Scholar]
- (545).Bongartz JP, Aubertin AM, Milhaud PG, Lebleu B. Improved biological activity of antisense oligonucleotides conjugated to a fusogenic peptide. Nucleic Acids Res. 1994;22:4681–8. doi: 10.1093/nar/22.22.4681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (546).Li W, Nicol F, Szoka FC., Jr. GALA: a designed synthetic pH-responsive amphipathic peptide with applications in drug and gene delivery. Adv Drug Deliv Rev. 2004;56:967–85. doi: 10.1016/j.addr.2003.10.041. [DOI] [PubMed] [Google Scholar]
- (547).Shadidi M, Sioud M. Identification of novel carrier peptides for the specific delivery of therapeutics into cancer cells. Faseb J. 2003;17:256–8. doi: 10.1096/fj.02-0280fje. [DOI] [PubMed] [Google Scholar]
- (548).Arar K, Aubertin AM, Roche AC, Monsigny M, Mayer R. Synthesis and antiviral activity of peptide-oligonucleotide conjugates prepared by using N alpha-(bromoacetyl)peptides. Bioconjug Chem. 1995;6:573–7. doi: 10.1021/bc00035a011. [DOI] [PubMed] [Google Scholar]
- (549).Peterson CM, Shiah JG, Sun Y, Kopeckova P, Minko T, Straight RC, Kopecek J. HPMA copolymer delivery of chemotherapy and photodynamic therapy in ovarian cancer. Adv Exp Med Biol. 2003;519:101–23. doi: 10.1007/0-306-47932-X_7. [DOI] [PubMed] [Google Scholar]
- (550).Rihova B, Ulbrich K, Kopecek J, Mancal P. Immunogenicity of N-(2-hydroxypropyl)-methacrylamide copolymers--potential hapten or drug carriers. Folia Microbiol (Praha) 1983;28:217–27. doi: 10.1007/BF02884085. [DOI] [PubMed] [Google Scholar]
- (551).Kopecek J, Kopeckova P, Minko T, Lu Z. HPMA copolymer-anticancer drug conjugates: design, activity, and mechanism of action. Eur J Pharm Biopharm. 2000;50:61–81. doi: 10.1016/s0939-6411(00)00075-8. [DOI] [PubMed] [Google Scholar]
- (552).Jensen KD, Kopeckova P, Kopecek J. Antisense oligonucleotides delivered to the lysosome escape and actively inhibit the hepatitis B virus. Bioconjug Chem. 2002;13:975–84. doi: 10.1021/bc025559y. [DOI] [PubMed] [Google Scholar]
- (553).Wang L, Kristensen J, Ruffner DE. Delivery of antisense oligonucleotides using HPMA polymer: synthesis of A thiol polymer and its conjugation to water-soluble molecules. Bioconjug Chem. 1998;9:749–57. doi: 10.1021/bc980034k. [DOI] [PubMed] [Google Scholar]
- (554).Lee BS, Vert M, Holler E. Water-soluble aliphatic polyesters: poly(malic acid)s. In: Doi Y, Steinbuechel A, editors. Biopolymers. Wiley-VCH; New York: 2002. pp. 75–103. [Google Scholar]
- (555).Ljubimova JY, Fujita M, Khazenzon NM, Lee BS, Wachsmann-Hogiu S, Farkas DL, Black KL, Holler E. Nanoconjugate based on polymalic acid for tumor targeting. Chem Biol Interact. 2007 doi: 10.1016/j.cbi.2007.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (556).Rajur SB, Roth CM, Morgan JR, Yarmush ML. Covalent protein-oligonucleotide conjugates for efficient delivery of antisense molecules. Bioconjug Chem. 1997;8:935–40. doi: 10.1021/bc970172u. [DOI] [PubMed] [Google Scholar]
- (557).Hashida M, Nishikawa M, Yamashita F, Takakura Y. Cell-specific delivery of genes with glycosylated carriers. Adv Drug Deliv Rev. 2001;52:187–96. doi: 10.1016/s0169-409x(01)00209-5. [DOI] [PubMed] [Google Scholar]
- (558).Beljaars L, Molema G, Weert B, Bonnema H, Olinga P, Groothuis GM, Meijer DK, Poelstra K. Albumin modified with mannose 6-phosphate: A potential carrier for selective delivery of antifibrotic drugs to rat and human hepatic stellate cells. Hepatology. 1999;29:1486–93. doi: 10.1002/hep.510290526. [DOI] [PubMed] [Google Scholar]
- (559).Nishikawa M, Hirabayashi H, Takakura Y, Hashida M. Design for cell-specific targeting of proteins utilizing sugar-recognition mechanism: effect of molecular weight of proteins on targeting efficiency. Pharm Res. 1995;12:209–14. doi: 10.1023/a:1016222808484. [DOI] [PubMed] [Google Scholar]
- (560).Monsigny M, Midoux P, Mayer R, Roche AC. Glycotargeting: influence of the sugar moiety on both the uptake and the intracellular trafficking of nucleic acid carried by glycosylated polymers. Biosci Rep. 1999;19:125–32. doi: 10.1023/a:1020114611517. [DOI] [PubMed] [Google Scholar]
- (561).Walker I, Irwin WJ, Akhtar S. Improved cellular delivery of antisense oligonucleotides using transferrin receptor antibody-oligonucleotide conjugates. Pharm Res. 1995;12:1548–53. doi: 10.1023/a:1016260110049. [DOI] [PubMed] [Google Scholar]
- (562).Quetard C, Bourgerie S, Normand-Sdiqui N, Mayer R, Strecker G, Midoux P, Roche AC, Monsigny M. Novel glycosynthons for glycoconjugate preparation: oligosaccharylpyroglutamylanilide derivatives. Bioconjug Chem. 1998;9:268–76. doi: 10.1021/bc970122p. [DOI] [PubMed] [Google Scholar]
- (563).de Bleser PJ, Jannes P, van Buul-Offers SC, Hoogerbrugge CM, van Schravendijk CF, Niki T, Rogiers V, van den Brande JL, Wisse E, Geerts A. Insulinlike growth factor-II/mannose 6-phosphate receptor is expressed on CCl4-exposed rat fat-storing cells and facilitates activation of latent transforming growth factor-beta in cocultures with sinusoidal endothelial cells. Hepatology. 1995;21:1429–37. doi: 10.1002/hep.1840210529. [DOI] [PubMed] [Google Scholar]
- (564).Weiner JA, Chen A, Davis BH. E-box-binding repressor is down-regulated in hepatic stellate cells during up-regulation of mannose 6-phosphate/insulin-like growth factor-II receptor expression in early hepatic fibrogenesis. J Biol Chem. 1998;273:15913–9. doi: 10.1074/jbc.273.26.15913. [DOI] [PubMed] [Google Scholar]
- (565).Kornfeld S. Trafficking of lysosomal enzymes. Faseb J. 1987;1:462–8. doi: 10.1096/fasebj.1.6.3315809. [DOI] [PubMed] [Google Scholar]
- (566).Dahms NM, Hancock MK. P-type lectins. Biochim Biophys Acta. 2002;1572:317–40. doi: 10.1016/s0304-4165(02)00317-3. [DOI] [PubMed] [Google Scholar]
- (567).Dennis PA, Rifkin DB. Cellular activation of latent transforming growth factor beta requires binding to the cation-independent mannose 6-phosphate/insulin-like growth factor type II receptor. Proc Natl Acad Sci U S A. 1991;88:580–4. doi: 10.1073/pnas.88.2.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (568).Godar S, Horejsi V, Weidle UH, Binder BR, Hansmann C, Stockinger H. M6P/IGFII-receptor complexes urokinase receptor and plasminogen for activation of transforming growth factor-beta1. Eur J Immunol. 1999;29:1004–13. doi: 10.1002/(SICI)1521-4141(199903)29:03<1004::AID-IMMU1004>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- (569).Bonfils E, Depierreux C, Midoux P, Thuong NT, Monsigny M, Roche AC. Drug targeting: synthesis and endocytosis of oligonucleotide-neoglycoprotein conjugates. Nucleic Acids Res. 1992;20:4621–9. doi: 10.1093/nar/20.17.4621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (570).Beljaars L, Olinga P, Molema G, de Bleser P, Geerts A, Groothuis GM, Meijer DK, Poelstra K. Characteristics of the hepatic stellate cell-selective carrier mannose 6-phosphate modified albumin (M6P(28)-HSA) Liver. 2001;21:320–8. doi: 10.1034/j.1600-0676.2001.210504.x. [DOI] [PubMed] [Google Scholar]
- (571).Gonzalo T, Talman EG, van de Ven A, Temming K, Greupink R, Beljaars L, Reker-Smit C, Meijer DK, Molema G, Poelstra K, Kok RJ. Selective targeting of pentoxifylline to hepatic stellate cells using a novel platinum-based linker technology. J Control Release. 2006;111:193–203. doi: 10.1016/j.jconrel.2005.12.010. [DOI] [PubMed] [Google Scholar]
- (572).Hagens WI, Mattos A, Greupink R, de Jager-Krikken A, Reker-Smit C, van Loenen-Weemaes A, Gouw IA, Poelstra K, Beljaars L. Targeting 15d-prostaglandin J2 to hepatic stellate cells: two options evaluated. Pharm Res. 2007;24:566–74. doi: 10.1007/s11095-006-9175-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (573).Gonzalo T, Beljaars L, van de Bovenkamp M, Temming K, van Loenen AM, Reker-Smit C, Meijer DK, Lacombe M, Opdam F, Keri G, Orfi L, Poelstra K, Kok RJ. Local inhibition of liver fibrosis by specific delivery of a platelet-derived growth factor kinase inhibitor to hepatic stellate cells. J Pharmacol Exp Ther. 2007;321:856–65. doi: 10.1124/jpet.106.114496. [DOI] [PubMed] [Google Scholar]
- (574).Luk JM, Zhang QS, Lee NP, Wo JY, Leung PP, Liu LX, Hu MY, Cheung KF, Hui CK, Lau GK, Fan ST. Hepatic stellate cell-targeted delivery of M6P-HSA-glycyrrhetinic acid attenuates hepatic fibrogenesis in a bile duct ligation rat model. Liver Int. 2007;27:548–57. doi: 10.1111/j.1478-3231.2007.01452.x. [DOI] [PubMed] [Google Scholar]