Abstract
Contributing to the obesity epidemic, there is increasing evidence that overconsumption of high-fat foods may be analogous to drug addiction in that the palatability of these foods is associated with activation of specific reward pathways in the brain. With this perspective, we report that mice lacking the G protein γ3-subunit (Gng3−/− mice) show resistance to high-fat diet-induced weight gain over the course of a 12-wk study. Compared with Gng3+/+ controls, female Gng3−/− mice exhibit a 40% reduction in weight gain and a 53% decrease in fat pad mass, whereas male Gng3−/− mice display an 18% reduction in weight gain and no significant decrease in fat pad mass. The basis for the lowered weight gain is related to reduced food consumption for female and male Gng3−/− mice of 13% and 14%, respectively. Female Gng3−/− mice also show a lesser preference for high-fat chow than their female Gng3+/+ littermates, suggesting an attenuated effect on a reward pathway associated with overconsumption of fat. One possible candidate is the μ-opioid receptor (Oprm1) signaling cascade. Supporting a defect in this signaling pathway, Gng3−/− mice show marked reductions in both acute and chronic morphine responsiveness, as well as increases in endogenous opioid mRNA levels in reward-related regions of the brain. Taken together, these data suggest that the decreased weight gain of Gng3−/− mice may be related to a reduced rewarding effect of the high-fat diet resulting from a defect in Oprm1 signaling and loss of the G protein γ3-subunit.
Keywords: lean, reward, G protein-coupled receptor
a major factor driving the current obesity epidemic is the overconsumption of palatable foods rich in fats or carbohydrates (14, 17, 51). Analogous to drugs of abuse, such foods promote weight gain by activating reward pathways in the brain that override the normal homeostatic mechanisms controlling appetite (9, 24). In fact, several articles (10, 11, 19, 24) and a recent conference (30) highlight the parallels between obesity and drug addiction. Thus, a better understanding of how these reward pathways operate could provide new targets for the prevention and treatment of obesity in the developed world.
Activation of μ-opioid receptor (Oprm1) signaling is critical in mediating the rewarding effects of palatable foods (26). Physiological studies have shown that consumption of fatty food increases the levels of endogenous opioids and μ-opioid receptor binding in discrete regions of the brain (6, 36, 44). Moreover, pharmacological studies have demonstrated that stereotactic injection of a μ-opioid agonist into the nucleus accumbens preferentially stimulates the intake of high-fat food (48, 50, 52), whereas an antagonist selectively blocks this response (8, 48). Finally, gene-targeting studies have reported that mice lacking Oprm1 show increased weight gain on standard chow (12), reduced weight gain on a high-fat diet (38), diminished food anticipatory activity (15), decreased motivation to seek food (28), and attenuated psychomotor sensitization in response to morphine (49).
As a member of the G protein-coupled receptor superfamily, Oprm1 signals through one or more Gi/o proteins (5), whose roles are determined by their specific αβγ-subunit combinations in different brain regions (31). From the 16 α, 5 β, and 12 γ subtypes that have been identified in the mammalian genome (3), there is the potential to generate several hundred distinct G protein heterotrimers. Historically, the assumption has been that the functions of G protein heterotrimers are specified by their diverse α-subtypes. However, the recent discovery of similarly diverse γ-subtypes that direct the assembly, and hence, the functions of specific G protein heterotrimers has called this assumption into question (13, 22, 33). Nonetheless, more than 15 years after their cloning, the physiological functions of most of the 12 known γ-subtypes are still unknown. Using a gene-targeting strategy to abrogate Gng3 function in mice, we present evidence suggesting the G protein γ3-subtype acts downstream of Oprm1 to mediate the rewarding effects of a high-fat diet, particularly in females.
MATERIALS AND METHODS
Animals.
The Weis Center Animal Facility is fully accredited by American Association for Laboratory Animal Care International. All protocols involving animals were approved by the Geisinger Health System, Institutional Animal Care and Use Committee. Mice were housed in ventilated racks (Thoren Caging Systems, Hazelton, PA) on a 14:10-h light-dark cycle, and they were given free access to water and standard chow (Mouse Diet 9F; Purina Mills, St. Louis, MO) except as noted otherwise.
Targeted disruption of Gng3 was described previously (34). Gng3+/− mice were maintained by backcrosses to C57BL/6J obtained from Jackson Laboratory (Bar Harbor, ME). To look for an effect of maternal deficiency of the γ3-subtype on the offspring, the mice studied on a high-fat diet were generated by crossing Gng3+/− sires with either Gng3−/− dams or Gng3+/+ dams, at the N10F2 backcross. However, there were no significant differences in body weight, adiposity, energy expenditure, or caloric intake of Gng3+/− offspring of Gng3−/− dams compared with those of Gng3+/+ dams (not shown). Therefore, all other mice studied were generated by crossing Gng3+/− sires and Gng3+/− dams, at the N10 backcross or later. Genotypes were identified by PCR analysis of tail biopsy DNA with three primers (GAGTAGAAGGTGCTTGGAGT, ACGCAAGATGGTGGAACAGC, and TCTGGGCAGAACTTAAGCTG) that amplified a 629-bp fragment from the wild-type allele, or a 755-bp fragment from the deleted allele.
Body weight and food consumption.
At 3 wk of age, mice were weaned and group housed with littermates of the same sex. At 4 to 5 wk of age, mice were individually housed and given a weighed amount of high-fat chow, which provided 45% of calories from fat (Test Diet 58V8, Purina). The amount of food remaining in the animal's cage was weighed at weekly intervals. As assessed by visual inspection of the bedding, food spillage was noted in only 2 of the 536 weekly observations.
Necropsies were performed at 18 wk of age. Fat was dissected from four regions of each mouse: inguinal fat was collected as the intraperitoneal fat surrounding the gonads; mesenteric fat was collected from the small intestine; retroperitoneal fat was collected from around the kidneys; and interscapular brown fat was collected from the back. We noted that the interscapular fat pads were infiltrated with white fat, particularly in males. Dissected fat pads and other tissues were weighed, frozen in liquid N2, and stored at −80°C.
Feed preference.
Mice that had been maintained on standard chow (Mouse Diet 9F, Purina) were switched to a free choice paradigm with simultaneous access to weighed amounts of a high-fat chow (45% calories from fat; Test Diet 58V8, Purina) and a low-fat chow (10% calories from fat; Test Diet 58Y8, Purina). The two chows were presented in a slanted wire hopper in the top of the cage with the low-fat chow on the bottom (closest to the mouse) and the high-fat chow on the top (furthest from the mouse). Thus, the mice had adequate access to both diets and still preferred the high-fat diet even though it was further away. For each mouse, the amount of each chow consumed was calculated by weighing the amount of food remaining after the indicated intervals.
RT-PCR analysis.
Training in the mouse brain micropunch dissection method (18) was provided by Dr. M. F. Miles (Virginia Commonwealth University, Richmond, VA). Briefly, a vertical slice was made 0.5–1 mm caudal to the olfactory bulbs, and a second vertical slice was made just rostral to the optic chiasm. The intervening section was placed with the caudal face up, and the nuclei accumbens were dissected with a 1-mm micropunch (Fine Science Tools, Foster City, CA) centered over each anterior commissure. The caudate nuclei were dissected with a 2-mm micropunch inferior to the corpus callosum, bilaterally. After removal of the hypothalamus with tweezers, the caudal portion of the brain was placed dorsal side up. The cerebellum and pons were removed by a vertical slice between the superior and inferior colliculi. A slice was then made at a 45° angle from the dorsal caudal end down toward the ventral rostral end. The cortical regions of the ventral portion were peeled away. Finally, the ventral midbrain was isolated from the remaining ventral portion, by trimming the dorsal midbrain with a transverse slice. Dissections were completed 5 to 10 min from the time of death. Brain regions were placed in individual tubes, frozen immediately with liquid nitrogen, and stored at −80°C until isolation of total RNA.
RNA was isolated from dissected brain regions using TRIzol (Invitrogen, Carlsbad, CA). First-strand cDNA was prepared from 2 μg of total RNA primed with random hexamers using MMLV reverse transcriptase (Promega, Madison, WI). Primers were synthesized (Integrated DNA Technologies, Coralville, IA) for Pomc (CTCCTGCTTCAGACCTCCATAGAT; TTCATCTCCGTTGCCAGGAAACAC), Penk1 (AACCTTGTCAGAGACAGAACGGGT; AGATCCTTGCAGGTCTCCCAGATT), Pdyn (TGCCCTCTAATGTTATGGCGGACT; ATCTTCCAAGTCATCCTTGCCACG) and Eef1a1 (GGAATGGTGACAACATGCTG; CGTTGAAGCCTACATTGTCC). For amplification and detection of products, real-time PCR was performed on an iCycler (Bio-Rad, Hercules, CA), using iQ SYBRGreen Supermix (Bio-Rad). Conditions were as follows: 40 cycles of denaturation at 95°C for 30 s, annealing and extension at 63°C for 30 s, and detection at 79°C for 10 s.
A housekeeping gene, eukaryotic elongation factor (Eef1a1), was used to correct for variations in RNA concentrations and cDNA yield. A change in threshold cycle (ΔCt) was calculated by subtracting the threshold cycle for Eef1a1 for a particular cDNA sample from the threshold cycle of each primer pair (Penk1, Pdyn, and Pomc1) for that cDNA sample. For each primer pair, the level of expression in each cDNA sample was then calculated relative to the mean ΔCt for all of the wild-type samples: Relative Expression = 2−(sample ΔCt − mean ΔCt).
Energy expenditure, basal locomotor activity, and locomotor response to morphine.
Energy expenditure was measured by indirect calorimetry on high-fat fed mice between the ages of 8 and 14 wk. For this purpose, mice were housed for 3 days in comprehensive lab animal monitoring system (CLAMS) cages (Columbus Instruments, Columbus, OH). The cages measured 20 cm × 10 cm × 12.5 cm, allowed free access to food and water, and simultaneously measured CO2 production (V̇co2), O2 consumption (V̇o2), food intake, and locomotor activity. The room housing the CLAMS cages was maintained at 75–79°F to minimize heat loss. Energy expenditure (Heat) was calculated as 3.815 × V̇o2 + 1.232 × V̇co2 (7). Basal locomotor activity was quantified as consecutive photobeam breaks along the long (Xamb) and short (Yamb) axes of these same CLAMS cages. Mice were allowed to acclimate to the cages overnight. The values were then averaged (Heat) or summed (XYamb) over 14- or 10-h intervals, representing the light or dark phases, respectively. Data are presented as the average of two full days or two full nights. A resting energy expenditure was also calculated for each mouse by averaging the 10 intervals with the lowest locomotor activity.
In a separate series of experiments, locomotor responses to acute and chronic morphine administration were assessed in the CLAMS cages for a 5-h period each day. On day 1, mice were acclimated to the CLAMS cages. On day 2, mice were placed in the cages for 2 h before receiving an intraperitoneal injection of saline. On day 3, mice were placed in the cages for 2 h before administering an injection of morphine (5 mg/kg ip). On days 6, 8, 10, and 14, mice were placed in the cages for 2 h before receiving a injection of morphine (10 mg/kg ip). Morphine-induced locomotor activity was measured as consecutive photobeam breaks (Xamb + Yamb), summed over 15-min intervals, and then corrected for saline values obtained on day 2. The timing and dosage required for morphine-induced locomotor sensitization were based on a published study (21).
Data analysis.
Sample statistics and two-tailed Student's t-tests were calculated with Excel 2003 (Microsoft). Data are presented as means ± SE. Repeated-measures ANOVA and linear regressions were computed using JMP ver. 4.04 (SAS Institute, Cary, NC).
RESULTS
Gng3−/− mice are resistant to diet-induced obesity.
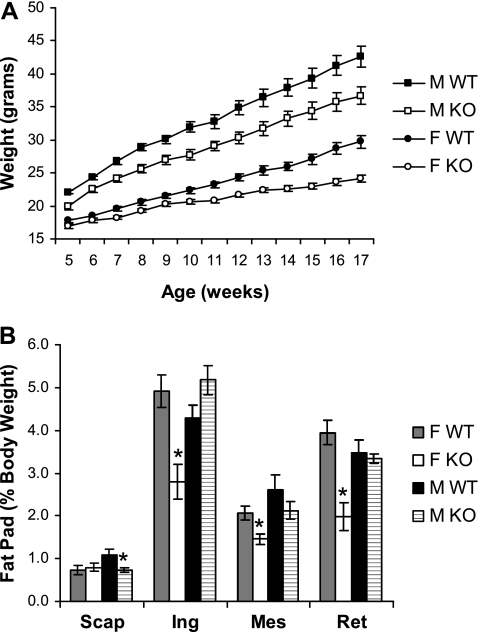
Our preliminary analysis of gene-targeted mice lacking the G protein γ3-subtype (Gng3−/− mice) revealed a trend toward reduced body weight, decreased fat pad mass, and reduced serum leptin levels that only reached statistical significance in female knockout animals on standard chow (20% of calories from fat) for more than one year (34). To further investigate the role of the G-protein γ3-subtype in the regulation of body weight, we extended this analysis to include Gng3−/− mice maintained on high-fat chow (45% of calories from fat). Because a diet rich in fat is a major risk factor for obesity in C57BL/6J mice (1), we hypothesized that any effect on body weight resulting from loss of the γ3-subtype might be exacerbated under this condition. Accordingly, we recorded the body weights of Gng3−/− mice and wild-type controls fed high-fat chow at weekly intervals. Over the course of this study, Gng3−/− mice gained less weight than the wild-type controls (Fig. 1A). The difference was significant for both sexes, with male and female Gng3−/− mice gaining 18% (16.8 ± 1.0 g vs. 20.5 ± 1.3 g, P = 0.03) and 40% (7.3 ± 0.9 g vs. 12.0 ± 0.7 g, P = 0.0004) less weight, respectively.
Fig. 1.
Effect of a high-fat diet on body and fat pad weights of Gng3+/+ (WT) and Gng3−/− mice (KO). A: time course of body weight gain in age-matched WT and KO mice fed high-fat chow from week 5 through week 17 of age. Both female (open circles, n = 10) and male KO mice (open squares, n = 14) weighed significantly less than female (solid circles, n = 17) and male WT mice (solid squares, n = 8), respectively. In a repeated-measures multivariate ANOVA, there was a significant effect of genotype for both females (F1,25 = 14.7, P = 0.0008) and males (F1,20 = 9.4, P = 0.006), and there was a trend toward an interaction between genotype and time for females (F12,14 = 2.3, P = 0.07). B: effect of the 12-wk high-fat diet on fat deposition in the brown adipose tissue of the interscapular (Scap) area and the white adipose tissue of the inguinal (Ing), mesenteric (Mes), and retroperitoneal (Ret) areas. Fat pad weight is expressed as a percentage of total body weight (% BW). Data are from the mice shown in A and killed at week 18. All three white adipose tissue fat pads from female KO mice weighed significantly less than those from female WT mice. *P < 0.01 for comparison to WT of the same sex by Student's t-test.
To determine whether the reduced weight gain was associated with decreased adiposity, we compared their dissected fat pad masses at the end of the study. Female Gng3−/− mice showed a dramatic 53% reduction in white adipose tissue mass (sum of inguinal, mesenteric, and retroperitoneal fat) compared with the wild-type controls (1.8 ± 0.4 g vs. 3.8 ± 0.3 g, P = 0.001), although the amount of brown adipose tissue (interscapular fat) was not changed. This difference in white adipose tissue mass remained statistically significant, even when expressed as a percentage of total body weight (Fig. 1B). In contrast, male Gng3−/− mice showed no reduction in white adipose tissue mass, although the amount of brown adipose tissue was significantly reduced (0.74 ± 0.05 g vs. 1.10 ± 0.13 g, P = 0.01). Thus, the absence of the G protein γ3-subtype produced greater resistance to diet-induced obesity in females than males. Similar sex-specific differences in adiposity have been noted previously (29, 35, 46).
Gng3−/− mice show reduced food intake.
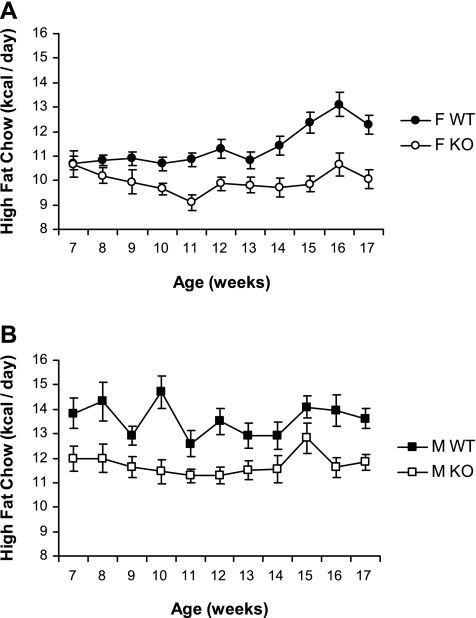
To analyze the mechanisms contributing to the lean phenotype of Gng3−/− mice, we measured food intake in these mice. Accordingly, each singly housed mouse was given a known amount of high-fat chow, and the amount of chow remaining at the end of each week was weighed. The difference was used to calculate the average amount of chow consumed on a daily basis over the course of the 12-wk study. Notably, the daily food intake of Gng3−/− mice was significantly reduced compared with wild-type controls (Fig. 2). On average, female Gng3−/− mice ate 13% less (2.14 ± 0.06 g/day vs. 2.45 ± 0.04 g/day, P = 0.0003), while male Gng3−/− mice ate 14% less (2.52 ± 0.06 g/day vs. 2.92 ± 0.07 g/day, P = 0.0003) than their respective wild-type controls. Thus, reduced caloric intake appears to contribute to the reduced weight gains observed for both male and female Gng3−/− mice.
Fig. 2.
Feed consumption of Gng3+/+ (WT) and Gng3−/− mice (KO) fed high-fat chow. Feed consumption was measured at weekly intervals over a 10-wk period and is expressed as caloric intake per day (kcal/day) A: female KO mice (open circles, n = 10) consumed significantly less feed than the female WT mice (solid circles, n = 17) when presented with high-fat chow (F1,25 = 17.1, P = 0.0003). B: male KO mice (open squares, n = 14) consumed significantly less feed than the male WT mice (solid squares, n = 8) when presented with high-fat chow (F1,20 = 16.8, P = 0.0006).
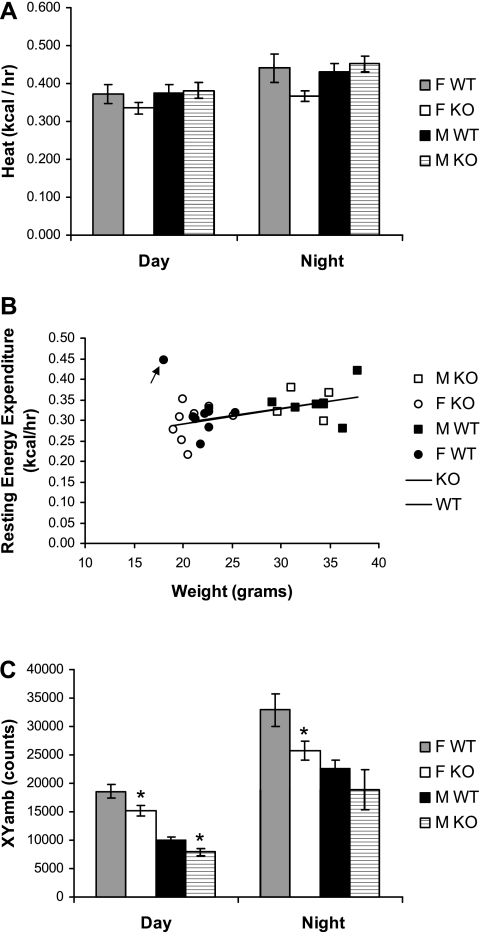
To further investigate the underlying mechanisms, we also measured energy expenditure and locomotor activity in these mice. Indirect calorimetry was used to estimate energy expenditure early in the study before substantial differences in body weight between the two genotypes developed. Notably, the diurnal energy expenditure of Gng3−/− mice was similar (male knockouts) or even slightly reduced (female knockouts) compared with control mice (Fig. 3A). Nevertheless, to account for any small differences in body weight, we also plotted regression of energy expenditure vs. body weight (2). Note that with the exception of one outlier (arrow; female control), all mice clustered along similar regression lines (Fig. 3B). By comparing male and female mice, it is apparent that the heavier male mice had greater resting energy expenditures. Moreover, by comparing knockout and wild-type mice, it is evident that the lighter female knockout mice exhibited slightly lower resting energy expenditures. In any case, it is clear that the Gng3−/− mice did not display excessively high-energy expenditures for their body weights that could account for their lean phenotypes. In the same experiments, locomotor activity was assessed by the number of consecutive photobeam breaks in the x- and y-directions (XYamb). Similar to energy expenditure, the diurnal activity of Gng3−/− mice was slightly (male knockouts) or significantly decreased (female knockouts), compared with wild-type controls (Fig. 3C). Taken together, these data indicate that the decreased weight gains recorded for Gng3−/− mice fed a high-fat diet are largely due to reduced energy intake rather than increased energy expenditure.
Fig. 3.
Comparison of energy expenditure and the locomotor activity of female (F) and male (M), Gng3+/+ mice (WT) and Gng3−/− mice (KO). Mice (9 F WT, 8 F KO, 6 M WT, and 5 M KO) were placed in CLAMS cages between 8 and 14 wk of age. Data were collected as described in materials and methods. A: no significant differences were found between sex-matched WT and KO mice during the 14 h of light (day) or 10 h of dark (night). B: relationship of resting energy expenditure (average of 10 intervals with the lowest locomotor activity) to body weight, in these same mice. Note that with the exception of one female WT mouse (arrow), all mice cluster around similar regression lines, with slopes of 0.0036 ± 0.0015 and 0.0037 ± 0.0018 and intercepts of 0.22 ± 0.04 and 0.22 ± 0.05 for WT and KO mice, respectively. C: locomotor activity (XYamb) was significantly lower in female KO mice relative to female WT mice during both day and night, and in male KO mice relative to male WT mice during the day. *P < 0.05 for comparison to WT of same sex by Student's t-test.
Gng3−/− mice exhibit a reduced preference for high-fat chow.
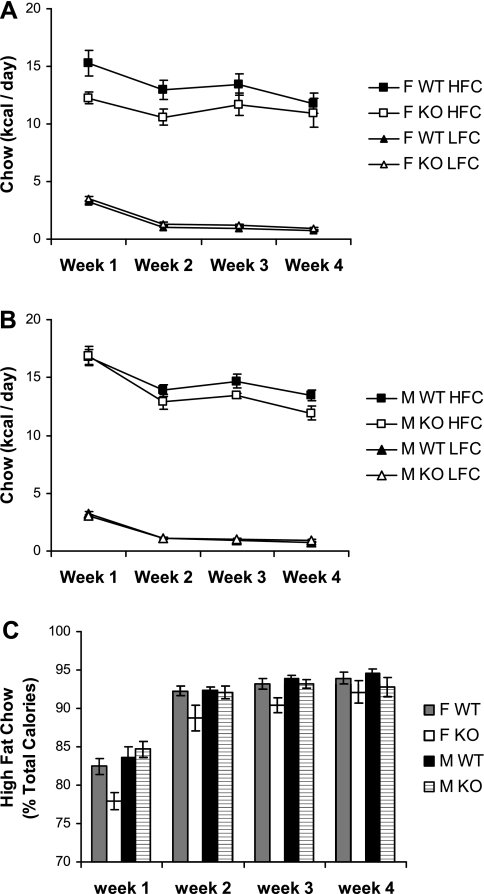
C57BL/6J mice show a preference for a high-fat diet (1). To determine whether the decreased food intake of Gng3−/− mice reflects a reduced preference for the high-fat content of the diet, we compared the intake of high-fat chow relative to low-fat chow in these mice. Briefly, mice maintained on standard chow (20% of calories from fat) were presented with a choice between high-fat chow (45% of calories from fat) and low-fat chow (10% of calories from fat). The average daily intake of each chow was then plotted at weekly intervals for females (Fig. 4A) and males (Fig. 4B). Initially, all groups of mice showed higher consumption of the two chows together than mice maintained on high-fat chow alone (compare Fig. 4, A and B and Fig. 2, A and B). Presumably, this excessive consumption reflects a response to the novelty of the two chows. Consistent with this possibility, all groups of mice displayed progressively lower consumption of the combined chows that approached the daily consumption of the high fat chow alone by the end of the study. Moreover, all groups of mice showed a strong preference for the high-fat chow over the low-fat chow. However, by comparing knockout and wild-type mice, it is evident that female Gng3−/− mice, in particular, tended to consume less of the high fat chow and more of the low fat chow. To examine this finding in more detail, the data were plotted as a preference (% of total calories from high fat chow) at weekly intervals over the course of the study (Fig. 4C). Consistent with a possible “rewarding” effect of the high-fat chow, all groups of mice showed a progressively increased preference for the high-fat chow over the course of this study. Notably, however, the female Gng3−/− mice showed a significantly reduced preference for the high-fat chow relative to female controls (Fig. 4C; F1,8 = 7.8, P = 0.02). At later time points, male Gng3−/− mice exhibited a similar trend that did not reach statistical significance. Taken together, these data suggest that deficiency of the G protein γ3-subtype may have disrupted a signaling pathway responsible for the rewarding effects of the high fat chow.
Fig. 4.
Feeding behavior of Gng3+/+ (WT) and Gng3−/− littermates (KO) provided a choice between high-fat chow (HFC) and low-fat chow (LFC) over a 4-wk period. A: female KO mice (n = 5) tended to eat less high-fat chow than female WT mice (n = 6), but the difference was not statistically significant (F1,9 = 3.5, P = 0.09). However, female KO mice did eat significantly more low-fat chow than female WT mice (F1,8 = 9.3, P = 0.02). [Note that one measurement was excluded from the analysis as an outlier, a value 1.54 g/day of low-fat chow for a female wild-type mouse during week 2, compared with 0.28 ± 0.03 g/day for all other female wild-type mice.] B: male KO mice (n = 6) also tended to eat less high-fat chow than male WT mice (n = 6), but the difference was not statistically significant (F1,10 = 3.1, P = 0.11). Moreover, there was no difference in consumption of low-fat chow (F1,10 = 0.3, P = 0.86). C: female KO mice showed a significantly lower preference (% of total calories) for high-fat chow than female WT mice (F1,8 = 7.8, P = 0.02), but male KO mice did not show a significantly lower preference for high-fat chow (F1,10 = 0.3, P = 0.59).
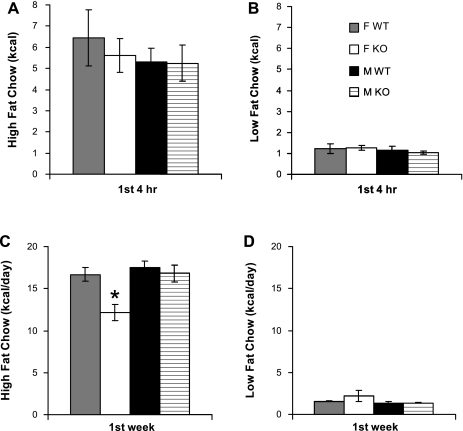
This possibility was confirmed in a second group of mice that examined the immediate response to the novelty of the two chows. During the first 4 h, all groups of mice showed excessive consumption of both chows together (Fig. 5, A and B). If calculated as a daily rate, each mouse would have consumed, on average, 40.9 ± 2.7 kcal/day, which greatly exceeds the rate for mice fed high-fat chow alone (Fig. 2) or maintained on a free choice diet (Fig. 4). Importantly, during the first 4 h, the Gng3−/− mice displayed no differences in consumption of either chow compared with their wild-type littermates (Fig. 5, A and B), indicating no impairment in their ability to sense the novelty of the two chows and no defect in their ability to sense the enhanced palatability of the high-fat chow. Nevertheless, by the end of the first week of the study (Fig. 5, C and D), female Gng3−/− mice again showed a reduced consumption of the high-fat chow compared with their wild-type littermates, suggesting a reduced ability to respond to the rewarding properties of the high-fat chow.
Fig. 5.
Initial response of female (F) and male (M), Gng3+/+ (WT) and Gng3−/− littermates (KO), 4 mice in each group, to a choice between HFC and LFC. During the first 4 h of exposure to the novel diets, there was no significant difference in the consumption of high-fat chow (A) or low-fat chow (B) between any of the groups. However, during the first week of exposure to the novel diets, female KO mice ate significantly less high-fat chow (C) than female WT mice, whereas consumption of low-fat chow was not significantly different during the first week (D). *P < 0.05 for comparison to WT of the same sex, by Student's t-test.
Gng3−/− mice are resistant to morphine.
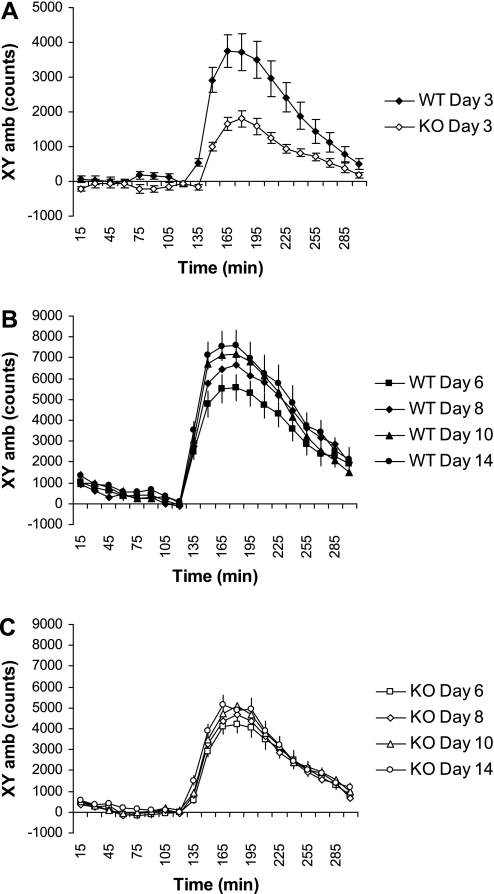
Opioids promote the overconsumption of fat (47, 50, 51), and Oprm1 has been strongly implicated in this process (8, 47, 48, 50, 52). To determine whether the reduced fat preference of knockout mice was associated with a defect in μ-opioid signaling, we assessed the behavioral responsiveness of Gng3−/− mice and their wild-type littermates to morphine, an opioid agonist that acts on the Oprm1 subtype (23, 25, 32, 37, 39). Morphine (5 mg/kg ip) produced a strong locomotor stimulatory effect in wild-type mice (Fig. 6A). Moreover, consistent with the literature, the stimulatory action of morphine (10 mg/kg ip) was markedly enhanced following repeated, intermittent injections in wild-type mice (Fig. 6B; compare day 6 vs. day 14). In contrast, both the acute (Fig. 6A) and sensitized (Fig. 6C compare day 6 vs. day 14) locomotor responses to morphine were markedly attenuated in knockout mice. These results indicate that Gng3−/− mice had a defect in Oprm1 signaling. Moreover, because the progressive enhancement of locomotor activity (locomotor sensitization) observed upon repeated morphine treatment is often used as a behavioral correlate of drug addiction (40), these results further suggest that defective Oprm1 signaling may explain the reduced response to the rewarding properties of both morphine and palatable food observed in knockout mice.
Fig. 6.
Effect of morphine on locomotor activity in Gng3+/+ (WT) and Gng3−/− littermates (KO). Mice were monitored in CLAMS cages for 5 h, and given one of the following injections after 2 h: none on day 1, saline on day 2, morphine (5 mg/kg) on day 3, or morphine (10 mg/kg) on days 6, 8, 10, and 14. Locomotor activity (XYamb) was averaged over 15-min intervals and corrected for activity in response to saline. A: in response to morphine (5 mg/kg), KO mice (n = 18) showed a significantly attenuated morphine-stimulated locomotor activity compared with WT littermates (n = 17) (F1,34 = 20.5, P = 0.001). B: in response to repeated, intermittent doses of morphine (10 mg/kg), WT mice show a significant increase in morphine-stimulated locomotor activity (F1,32 = 4.2, P = 0.05, for day 6 vs. day 14). C: in contrast, KO mice did not show a significant increase in morphine-stimulated locomotor activity following repeated, intermittent doses of morphine (10 mg/kg) (F1,36 = 1.9, P = 0.17, for day 6 vs. day 14).
Penk expression is increased in the midbrain of the Gng3−/− mice.
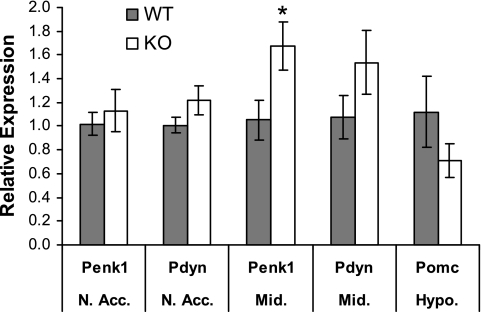
Through their binding to Oprm1, endogenous opioids act at different sites in the central nervous system to stimulate feeding behavior (20). To determine whether endogenous opioid levels are upregulated in an attempt to overcome the defective Oprm1 signaling observed in Gng3−/− mice, we used real-time PCR analysis to compare the mRNA levels for the precursors of enkephalin (Penk), dynorphin (Pdyn), and β-endorphin (Pomc) in various brain regions from control and knockout mice (Fig. 7). Notably, mRNA levels of Penk and Pdyn tended to increase in reward-related regions from Gng3−/− brains, with the rise in Penk mRNA reaching statistical significance in the midbrain. On the other hand, the mRNA level of Pomc was not significantly different in a nonreward-related region of knockout brains. Such differences may be explained by a preferential role for the G protein γ3-subtype acting downstream of a subset of Oprm1 receptors to modulate the hedonic value of food in the midbrain.
Fig. 7.
Expression levels of endogenous opioids in the nucleus accumbens (N.Acc.), ventral midbrain (Mid.), and the hypothalamus (Hypo.) in female Gng3+/+ (WT), and Gng3−/− mice (KO). Expression levels of preproenkephalin (Penk1), prodynorphin (Pdyn), and proopiomelanocortin (Pomc) were determined by real-time RT-PCR. Data are presented as relative expression in female KO mice (n = 6) compared with female WT mice (n = 6). Relative expression was calculated as described in materials and methods. *P < 0.05 by Student's t-test.
DISCUSSION
This study reveals a novel role for the Gng3 gene encoding the G protein γ3-subunit in the response to a high-fat diet. Compared with wild-type mice, Gng3−/− mice show resistance to obesity resulting from the overconsumption of fatty food. Because significant sex differences in the prevalence of obesity and eating disorders exist (16), males and females from both genotypes were compared. Notably, the resistance to the high-fat diet was most striking in female knockouts, which showed a 40% reduction in weight gain and a 53% decrease in fat pad mass compared with wild-type controls. Although there was a correlation between reduced weight gain and decreased fat accrual in female knockout animals, this was not observed in male knockout mice in accord with a recent study reporting little association between weight gain and fat pad mass in male C57BL/6J mice (1). Although the reason for this sex-specific difference is not clear, other studies have observed that males and females respond differently to a high-fat diet (29, 35, 46).
The reduced weight gain observed for Gng3−/− mice could result from a change in food intake and/or energy expenditure. Increased energy expenditure is not the causative factor, since knockout animals showed a reduction in locomotor activity and comparable rate energy expenditure (Heat) to their wild-type littermates. However, a decrease in food consumption is clearly a contributing factor since knockout mice exhibited a lower caloric intake, resulting from a reduced preference for high-fat chow compared with their wild-type controls. When plotted as the ratio of high-fat chow to low-fat chow consumed, the wild type mice preferred the high-fat chow at a 4:1 ratio at the start of the experiment, and the ratio progressively increased until they were eating the high-fat chow almost exclusively by the end of the 4th week. Although showing similar trends, the Gng3−/− mice showed a reduced ratio of high-fat chow to low-fat chow consumed over the course of the study compared with their wild-type littermates. Again, the effect was most noticeable in female knockout animals, although male knockout mice showed a similar trend by the end of the experiment.
The observation that mice show a progressive increase in fat consumption shows a striking resemblance to the locomotor sensitization that occurs with repeated administration of morphine (21). Indeed, a recent study suggests a common reward pathway is involved in the response to both palatable food and drugs of abuse (19). By extension then, our finding that the Gng3−/− mice show reduced weight gain, decreased adiposity, reduced food intake, and attenuated fat preference is consistent with these animals harboring a defect in a reward pathway that responds to the pleasurable properties of palatable food. Craving for palatable food is considered as a form of addictive behavior (10, 11, 19, 24, 30), with the Oprm1 being strongly implicated in this process (8, 47, 48, 50, 52). Because Oprm1 represents the major molecular target of morphine in vivo (23, 25, 32, 37, 39), the attenuated responsiveness of Gng3−/− mice to morphine is indicative of defective Oprm1 signaling. Consistent with a localized role for the G protein γ3-subtype acting downstream of a subset of Oprm1 receptors, the mRNA levels of endogenous opioids were increased in the midbrain that responds to the reward value of food but not in the hypothalamus that senses the caloric value of food.
What is the nature of the requirement for the G protein γ3-subtype in this reward pathway? Previous studies have shown that Oprm1 receptors couple preferentially to G proteins containing either the αi- or αo-subtypes (5), but the identities of the βγ-subunits are not known. Accumulating evidence points to a critical role for the γ-subtype in directing the assembly of specific G protein heterotrimers (22, 33). Therefore, a defect in morphine responsiveness could have resulted from loss of a specific G protein αi/oβγ3-heterotrimer acting downstream of Oprm1 subtype. Moreover, a recent study by Bonacci et al. (4) has underscored the importance of an unknown βγ-dimer in mediating the downstream actions of morphine. Thus, a defect in morphine responsiveness could reflect an additional requirement for the activated βγ-dimer in effector regulation. Using a gene knockout approach, we could not distinguish between these two possibilities since blocking the assembly of the G protein heterotrimer abolishes both receptor interaction and effector regulation. Nonetheless, the finding that the G protein γ3-subtype is required for morphine action emphasizes the importance of the γ-subunit composition in translating the functional specificity of the G protein to the upstream receptor.
How does our finding that Gng3−/− mice exhibit defective Oprm1 signaling explain their resistance to diet-induced obesity? Numerous pharmacologic studies have shown that μ-opioid agonists promote feeding behaviors related to the palatability and quality of the food (47, 50, 52). Moreover, studies with an irreversible μ-opioid antagonist have demonstrated that μ-opioid receptors in the nucleus accumbens play a role in hedonically driven eating (42), whereas those in the parabrachial nucleus are involved in the control of homeostatic eating (43). However, genetic studies targeting Oprm1 have not been nearly as uniform. In some cases, the gene-targeting studies have contradicted the preponderance of pharmacological studies showing the ability of μ-opioid agonists to stimulate fat intake. This discrepancy has been most often ascribed to compensatory changes resulting from chronic inactivation of the Oprm1 gene that is not observed upon acute injection of μ-opioid ligands (12). In other cases, gene-targeting studies using different Oprm1 mutant mice have yielded conflicting results. These differences have been variously ascribed to the background strain or experimental paradigm under study. However, our analysis of the literature suggests the apparent discrepancies may reflect the global nature and type of genetic disruption. In this regard, five different Oprm1−/− mice have been generated (23, 25, 32, 37, 39). Although all are global knockouts, the five groups each used different targeting strategies to produce their Oprm1−/− mice. The Oprm1 gene is encoded by at least 18 exons that produce at least 19 alternatively spliced forms (27). The effect of the gene-targeting strategy on the production of these variants has not been carefully assessed. When viewed retrospectively, there seems to be a clear segregation of body weight results based on the nature of gene-targeting event. In this regard, Oprm1−/− mice with disruption of exon 1 displayed increased body weights compared with controls (12), even though they showed diminished food anticipatory activity (15). In contrast, male Oprm1−/− mice with disruption of exon 2 exhibited reduced body weights when maintained on a high-fat diet (38), as well as decreased motivation to seek food (28), similar to the phenotype of the Gng3−/− mice. Finally, Oprm1−/− mice with disruption of both exons 2 and 3 were reported to be heavier when fed standard chow, but to gain less weight and accrue less body fat when given a sweet, high-fat diet (53). It is striking that loss of exon 1 vs. exon 2 produced opposite phenotypes. If related to the production of splicing variants, these results suggest that different forms of Oprm1 may play opposing roles in body weight regulation by acting in different tissues or brain regions. In this regard, a recent study (45) concludes that the increased body weight observed for exon 1-disrupted mice was due to defective Oprm1 signaling in the pancreas rather than its better-studied role in brain. These results indicate that the ability to selectively inactivate the G protein γ3-subtype in specific tissues or brain regions will be critical in establishing its hypothetical role acting downstream of a particular Oprm1 subtype responsible for mediating the rewarding properties of high-fat food. Although creation of floxed Oprm1 mice has not been reported, our development of floxed Gng3 mice will allow us to pursue this question.
Perspectives and Significance
Finally, how do these findings impact on the obesity epidemic? Overconsumption of fat results when reward pathways override the homeostatic mechanisms controlling appetite (9, 24). When maintained on a high-fat diet, Gng3−/− mice lacking the G-protein γ3-subtype exhibit reduced weight gain, decreased fat intake, and defective Oprm1 signaling. Therefore, further study of this novel mouse model is likely to identify new targets for drugs to combat obesity and other addictive disorders. An important component of future studies will be to explore the role of gender in the response to a high-fat diet. Although both male and female Gng3−/− mice show reduced weight gain and decreased food intake compared with their controls, the magnitude of this effect was much more dramatic in the females. This is consistent with a growing body of evidence that indicates females show a higher prevalence of obesity and eating disorders (16) and a lower ability to suppress hunger at a cognitive level (41). Females need high-fat foods not only for survival, but also for reproduction (29). During pregnancy, both the body weight and adiposity of the mother increase due to hyperphagia. Although the underlying mechanisms are not well understood, it could be theorized that females exhibit a greater dependence on the rewarding properties of high-fat foods to be more successful in maintaining the pregnancy and maximizing infant survival. Because most studies of Oprm1 signaling and fat preference have focused on males, additional studies will be needed to determine whether this is the case.
GRANTS
This work was supported by National Institutes of Health Grant GM39867 awarded to J. D. Robishaw.
ACKNOWLEDGMENTS
The authors thank Dr. Carl Hansen for critical reading of this manuscript and are grateful for the invaluable assistance provided by Cindy Rhone, Gail Gregory, and Shannon Wydra, the excellent technicians in our animal facility.
REFERENCES
- 1.Alexander J, Chang GQ, Dourmashkin JT, Leibowitz SF. Distinct phenotypes of obesity-prone AKR/J, DBA2J, and C57BL/6J mice compared to control strains. Int J Obes (Lond) 30: 50–59, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Arch JR, Hislop D, Wang SJ, Speakman JR. Some mathematical and technical issues in the measurement and interpretation of open-circuit indirect calorimetry in small animals. Int J Obes (Lond) 30: 1322–1331, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Birnbaumer L. Expansion of signal transduction by G proteins. The second 15 years or so: from 3 to 16α subunits plus βγ dimers. Biochim Biophys Acta 1768: 772–793, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonacci TM, Mathews JL, Yuan C, Lehmann DM, Malik S, Wu D, Font JL, Bidlack JM, Smrcka AV. Differential targeting of Gβγ-subunit signaling with small molecules. Science 312: 443–446, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Burford NT, Wang D, Sadée W. G-protein coupling of μ-opioid receptors (OP3): elevated basal signalling activity. Biochem J 348: 531–537, 2000 [PMC free article] [PubMed] [Google Scholar]
- 6.Chang GQ, Karatayev O, Ahsan R, Gaysinskaya V, Marwil Z, Leibowitz SF. Dietary fat stimulates endogenous enkephalin and dynorphin in the paraventricular nucleus: role of circulating triglycerides. Am J Physiol Endocrinol Metab 292: E561–E570, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Columbus Instruments Equations for Energy Expenditure [Online]. Columbus Instruments. http://colinst.com/equations.pdf [27 May 2009].
- 8.Echo JA, Lamonte N, Christian G, Znamensky V, Ackerman TF, Bodnar RJ. Excitatory amino acid receptor subtype agonists induce feeding in the nucleus accumbens shell in rats: opioid antagonist actions and interactions with μ-opioid agonists. Brain Res 921: 86–97, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Epstein LH, Leddy JJ, Temple JL, Faith MS. Food reinforcement and eating: a multilevel analysis. Psychol Bull 133: 884–906, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Geiger BM, Behr GG, Frank LE, Caldera-Siu AD, Beinfeld MC, Kokkotou EG, Pothos EN. Evidence for defective mesolimbic dopamine exocytosis in obesity-prone rats. FASEB J 22: 2740–2746, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Geiger BM, Haburcak M, Avena NM, Moyer MC, Hoebel BG, Pothos EN. Deficits of mesolimbic dopamine neurotransmission in rat dietary obesity. Neuroscience 159: 1193–1199, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Han W, Hata H, Imbe H, Liu QR, Takamatsu Y, Koizumi M, Murphy NP, Senba E, Uhl GR, Sora I, Ikeda K. Increased body weight in mice lacking μ-opioid receptors. Neuroreport 17: 941–944, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Hansen CA, Schwindinger WF, Robishaw JD. Specificity of G-protein βγ-dimer signaling. In: Handbook of Cell Signaling, 2nd ed., edited by Bradshaw RA, Dennis EA.San Diego, CA: Elsevier, In press. [Google Scholar]
- 14.Hill JO, Peters JC. Environmental contributions to the obesity epidemic. Science 280: 1371–1374, 1998 [DOI] [PubMed] [Google Scholar]
- 15.Kas MJ, van den Bos R, Baars AM, Lubbers M, Lesscher HM, Hillebrand JJ, Schuller AG, Pintar JE, Spruijt BM. Mu-opioid receptor knockout mice show diminished food-anticipatory activity. Eur J Neurosci 20: 1624–1632, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Keel PK, Baxter MG, Heatherton TF, Joiner TE., Jr A 20-year longitudinal study of body weight, dieting, and eating disorder symptoms. J Abnorm Psychol 116: 422–432, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Kelley AE, Baldo BA, Pratt WE, Will MJ. Corticostriatal-hypothalamic circuitry and food motivation: integration of energy, action and reward. Physiol Behav 86: 773–795, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Kerns RT, Ravindranathan A, Hassan S, Cage MP, York T, Sikela JM, Williams RW, Miles MF. Ethanol-responsive brain region expression networks: implications for behavioral responses to acute ethanol in DBA/2J versus C57BL/6J mice. J Neurosci 25: 2255–2266, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Le Merrer J, Stephens DN. Food-induced behavioral sensitization, its cross-sensitization to cocaine and morphine, pharmacological blockade, and effect on food intake. J Neurosci 26: 7163–7171, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leibowitz SF. Overconsumption of dietary fat and alcohol: mechanisms involving lipids and hypothalamic peptides. Physiol Behav 91: 513–521, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leite-Morris KA, Fukudome EY, Shoeb MH, Kaplan GB. GABAB receptor activation in the ventral tegmental area inhibits the acquisition and expression of opiate-induced motor sensitization. J Pharmacol Exp Ther 308: 667–678, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Lobanova ES, Finkelstein S, Herrmann R, Chen YM, Kessler C, Michaud NA, Trieu LH, Strissel KJ, Burns ME, Arshavsky VY. Transducin γ-subunit sets expression levels of α- and β-subunits and is crucial for rod viability. J Neurosci 28: 3510–3520, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loh HH, Liu HC, Cavalli A, Yang W, Chen YF, Wei LN. μ Opioid receptor knockout in mice: effects on ligand-induced analgesia and morphine lethality. Brain Res Mol Brain Res 54: 321–326, 1998 [DOI] [PubMed] [Google Scholar]
- 24.Lutter M, Nestler EJ. Homeostatic and hedonic signals interact in the regulation of food intake. J Nutr 139: 629–632, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matthes HW, Maldonado R, Simonin F, Valverde O, Slowe S, Kitchen I, Befort K, Dierich A, Le Meur M, Dollé P, Tzavara E, Hanoune J, Roques BP, Kieffer BL. Loss of morphine-induced analgesia, reward effect and withdrawal symptoms in mice lacking the μ-opioid-receptor gene. Nature 383: 819–823, 1996 [DOI] [PubMed] [Google Scholar]
- 26.Naleid AM, Grace MK, Chimukangara M, Billington CJ, Levine AS. Paraventricular opioids alter intake of high-fat but not high-sucrose diet depending on diet preference in a binge model of feeding. Am J Physiol Regul Integr Comp Physiol 293: R99–R105, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Pan YX, Xu J, Xu M, Rossi GC, Matulonis JE, Pasternak GW. Involvement of exon 11-associated variants of the mu opioid receptor MOR-1 in heroin, but not morphine, actions. Proc Natl Acad Sci USA 106: 4917–4922, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Papaleo F, Kieffer BL, Tabarin A, Contarino A. Decreased motivation to eat in μ-opioid receptor-deficient mice. Eur J Neurosci 25: 3398–3405, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Power ML, Schulkin J. Sex differences in fat storage, fat metabolism, and the health risks from obesity: possible evolutionary origins. Br J Nutr 99: 931–940, 2008 [DOI] [PubMed] [Google Scholar]
- 30.Rapaka R, Schnur P, Shurtleff D. Obesity and addiction: common neurological mechanisms and drug development. Physiol Behav 95: 2–9, 2008 [DOI] [PubMed] [Google Scholar]
- 31.Saidak Z, Blake-Palmer K, Hay DL, Northup JK, Glass M. Differential activation of G-proteins by μ-opioid receptor agonists. Br J Pharmacol 147: 671–680, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schuller AG, King MA, Zhang J, Bolan E, Pan YX, Morgan DJ, Chang A, Czick ME, Unterwald EM, Pasternak GW, Pintar JE. Retention of heroin and morphine-6 β-glucuronide analgesia in a new line of mice lacking exon 1 of MOR-1. Nat Neurosci 2: 151–156, 1999 [DOI] [PubMed] [Google Scholar]
- 33.Schwindinger WF, Betz KS, Giger KE, Sabol A, Bronson SK, Robishaw JD. Loss of G-protein γ7 alters behavior and reduces striatal αolf level and cAMP production. J Biol Chem 278: 6575–6579, 2003 [DOI] [PubMed] [Google Scholar]
- 34.Schwindinger WF, Giger KE, Betz KS, Stauffer AM, Sunderlin EM, Sim-Selley LJ, Selley DE, Bronson SK, Robishaw JD. Mice with deficiency of G-protein γ3 are lean and have seizures. Mol Cell Biol 24: 7758–7768, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shi H, Strader AD, Sorrell JE, Chambers JB, Woods SC, Seeley RJ. Sexually different actions of leptin in proopiomelanocortin neurons to regulate glucose homeostasis. Am J Physiol Endocrinol Metab 294: E630–E639, 2008 [DOI] [PubMed] [Google Scholar]
- 36.Smith SL, Harrold JA, Williams G. Diet-induced obesity increases μ opioid receptor binding in specific regions of the rat brain. Brain Res 953: 215–222, 2002 [DOI] [PubMed] [Google Scholar]
- 37.Sora I, Takahashi N, Funada M, Ujike H, Revay RS, Donovan DM, Miner LL, Uhl GR. Opiate receptor knockout mice define μ receptor roles in endogenous nociceptive responses and morphine-induced analgesia. Proc Natl Acad Sci USA 94: 1544–1549, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tabarin A, Diz-Chaves Y, Carmona Mdel C, Catargi B, Zorrilla EP, Roberts AJ, Coscina DV, Rousset S, Redonnet A, Parker GC, Inoue K, Ricquier D, Pénicaud L, Kieffer BL, Koob GF. Resistance to diet-induced obesity in μ-opioid receptor-deficient mice: evidence for a “thrifty gene”. Diabetes 54: 3510–3516, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Tian M, Broxmeyer HE, Fan Y, Lai Z, Zhang S, Aronica S, Cooper S, Bigsby RM, Steinmetz R, Engle SJ, Mestek A, Pollock JD, Lehman MN, Jansen HT, Ying M, Stambrook PJ, Tischfield JA, Yu L. Altered hematopoiesis, behavior, and sexual function in μ opioid receptor-deficient mice. J Exp Med 185: 1517–1522, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vanderschuren LJ, Kalivas PW. Alterations in dopaminergic and glutamatergic transmission in the induction and expression of behavioral sensitization: a critical review of preclinical studies. Psychopharmacology (Berl) 151: 99–120, 2000 [DOI] [PubMed] [Google Scholar]
- 41.Wang GJ, Volkow ND, Telang F, Jayne M, Ma Y, Pradhan K, Zhu W, Wong CT, Thanos PK, Geliebter A, Biegon A, Fowler JS. Evidence of gender differences in the ability to inhibit brain activation elicited by food stimulation. Proc Natl Acad Sci USA 106: 1249–1254, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ward HG, Nicklous DM, Aloyo VJ, Simansky KJ. Mu-opioid receptor cellular function in the nucleus accumbens is essential for hedonically driven eating. Eur J Neurosci 23: 1605–1613, 2006 [DOI] [PubMed] [Google Scholar]
- 43.Ward HG, Simansky KJ. Chronic prevention of μ-opioid receptor (MOR) G-protein coupling in the pontine parabrachial nucleus persistently decreases consumption of standard but not palatable food. Psychopharmacology (Berl) 187: 435–446, 2006 [DOI] [PubMed] [Google Scholar]
- 44.Welch CC, Kim EM, Grace MK, Billington CJ, Levine AS. Palatability-induced hyperphagia increases hypothalamic Dynorphin peptide and mRNA levels. Brain Res 721: 126–131, 1996 [DOI] [PubMed] [Google Scholar]
- 45.Wen T, Peng B, Pintar JE. The MOR-1 opioid receptor regulates glucose homeostasis by modulating insulin secretion. Mol Endocrinol 23: 671–678, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.West DB, York B. Dietary fat, genetic predisposition, and obesity: lessons from animal models. Am J Clin Nutr 67Suppl505S–512S, 1998 [DOI] [PubMed] [Google Scholar]
- 47.Will MJ, Franzblau EB, Kelley AE. Nucleus accumbens μ-opioids regulate intake of a high-fat diet via activation of a distributed brain network. J Neurosci 23: 2882–2888, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Will MJ, Pratt WE, Kelley AE. Pharmacological characterization of high-fat feeding induced by opioid stimulation of the ventral striatum. Physiol Behav 89: 226–234, 2006 [DOI] [PubMed] [Google Scholar]
- 49.Yoo JH, Yang EM, Lee SY, Loh HH, Ho IK, Jang CG. Differential effects of morphine and cocaine on locomotor activity and sensitization in μ-opioid receptor knockout mice. Neurosci Lett 344: 37–40, 2003 [DOI] [PubMed] [Google Scholar]
- 50.Zhang M, Gosnell BA, Kelley AE. Intake of high-fat food is selectively enhanced by μ opioid receptor stimulation within the nucleus accumbens. J Pharmacol Exp Ther 285: 908–914, 1998 [PubMed] [Google Scholar]
- 51.Zheng H, Berthoud HR. Eating for pleasure or calories. Curr Opin Pharmacol 7: 607–612, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zheng H, Patterson LM, Berthoud HR. Orexin signaling in the ventral tegmental area is required for high-fat appetite induced by opioid stimulation of the nucleus accumbens. J Neurosci 27: 11075–11082, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zuberi AR, Townsend L, Patterson L, Zheng H, Berthoud HR. Increased adiposity on normal diet, but decreased susceptibility to diet-induced obesity in μ-opioid receptor-deficient mice. Eur J Pharmacol 585: 14–23, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]