Abstract
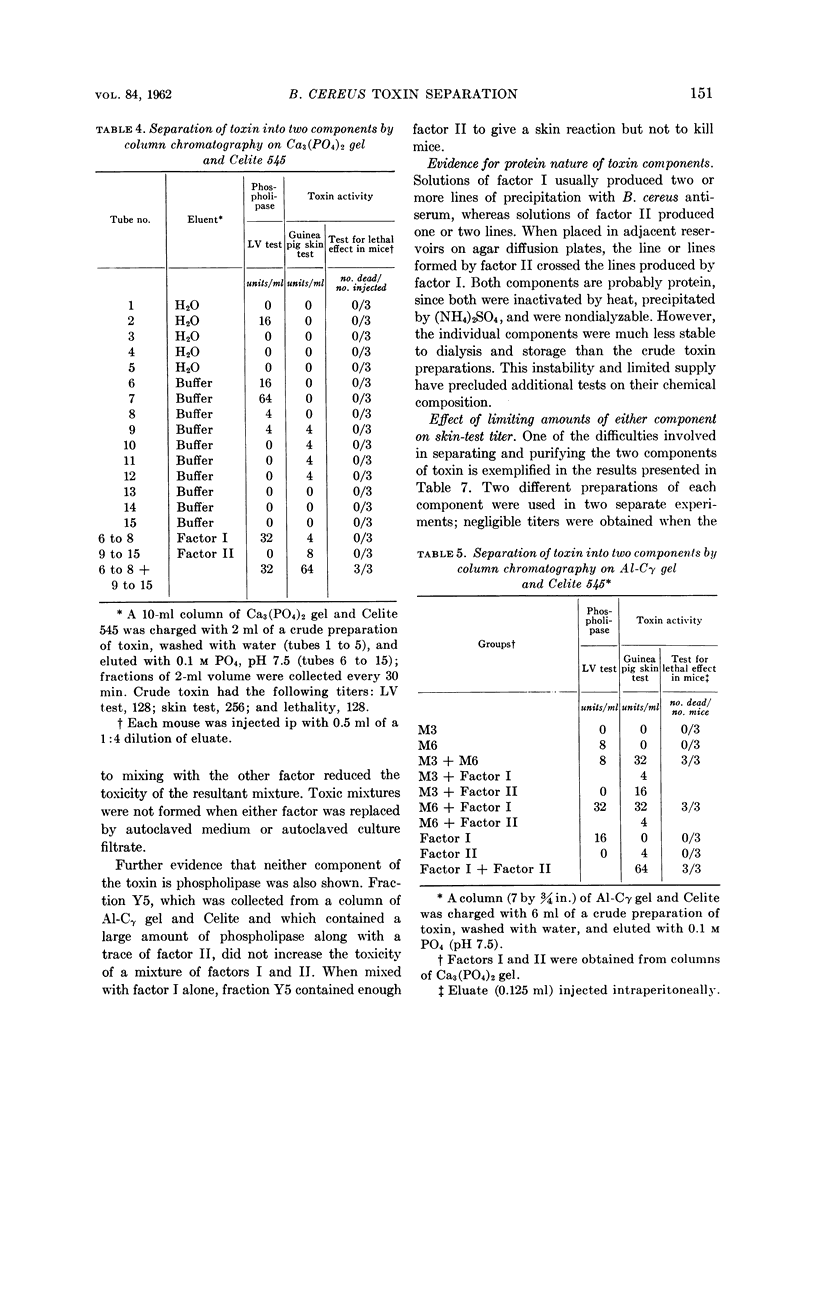
Molnar, Dorothy M. (U.S. Army Chemical Corps, Fort Detrick, Frederick, Md.). Separation of the toxin of Bacillus cereus into two components and nonidentity of the toxin with phospholipase. J. Bacteriol. 84:147–153. 1962—Bacillus cereus produced toxin in a Casamino acids medium without added serum or other protein. The toxin was separated into two components by adsorption on columns of calcium phosphate gel followed by elution with phosphate buffer (pH 7.5). The component eluted first has been called factor I and the component eluted later factor II. When tested alone each component was relatively nontoxic, but when combined they formed a toxic mixture as evidenced by skin reactions in guinea pigs and tests for lethal effects in mice.
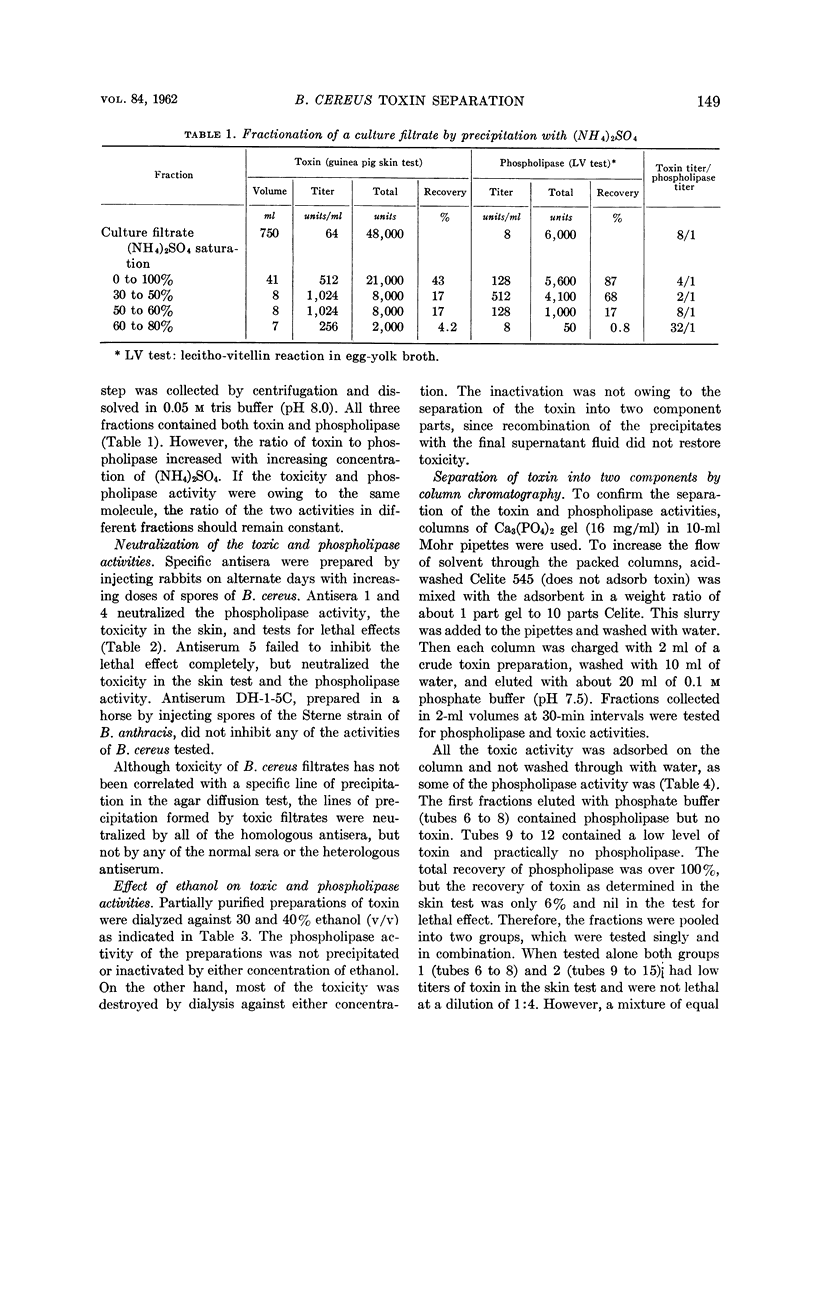
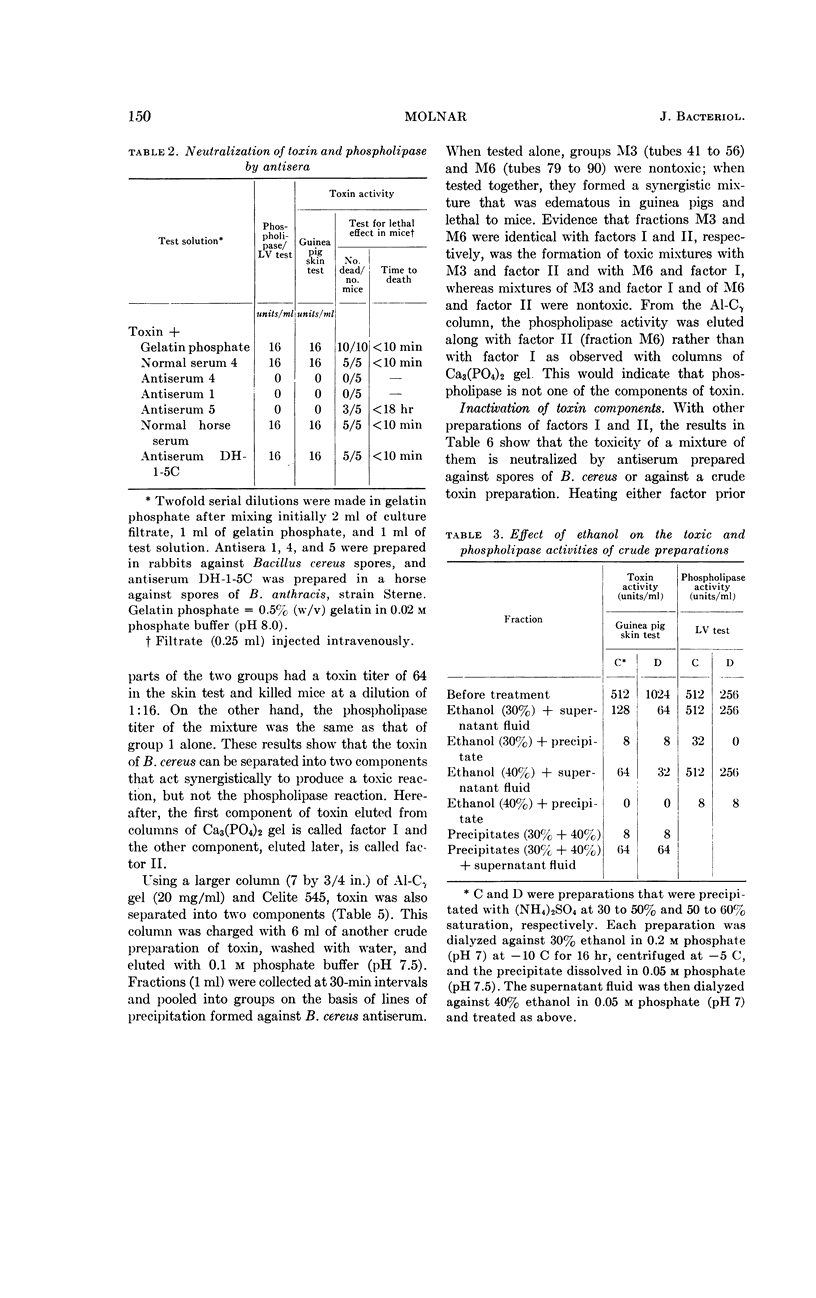
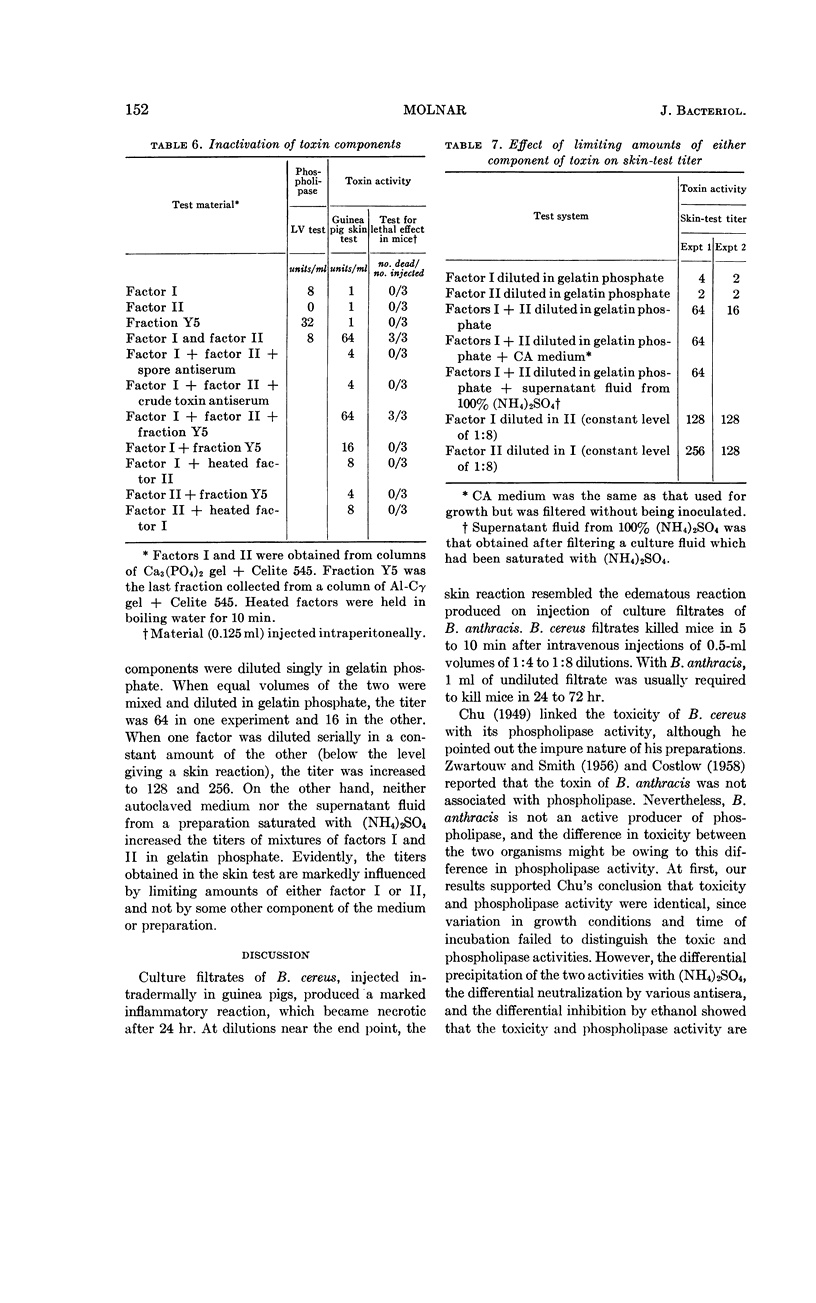
The phospholipase activity, determined by the egg-yolk reaction, was found in the fraction containing factor I. However, using columns of alumina-Cγ gel the phospholipase activity was found in the fraction containing factor II. This suggests that the phospholipase is not the same chemical entity as either factor I or II. The following are further evidence for the non-identity of the toxin and phospholipase: (i) differential precipitation of the two activities by (NH4)2SO4; (ii) differential neutralization by various antisera; and (iii) differential inhibition with ethanol.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- COSTLOW R. D. Lecithinase from Bacillus anthracis. J Bacteriol. 1958 Sep;76(3):317–325. doi: 10.1128/jb.76.3.317-325.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EVANS D. G., WARDLAW A. C. In vitro and in vivo properties of culture filtrates of Bacillus subtilis with high gelatinase activity. J Gen Microbiol. 1952 Nov;7(3-4):397–408. doi: 10.1099/00221287-7-3-4-397. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- SMITH H., ZWARTOUW H. T. Non-identity of the phospholipase of Bacillus anthracis with the anthrax toxin. J Gen Microbiol. 1956 Oct;15(2):261–265. doi: 10.1099/00221287-15-2-261. [DOI] [PubMed] [Google Scholar]
- THORNE C. B., BELTON F. C. An agar-diffusion method for titrating Bacillus anthracis immunizing antigen and its application to a study of antigen production. J Gen Microbiol. 1957 Oct;17(2):505–516. doi: 10.1099/00221287-17-2-505. [DOI] [PubMed] [Google Scholar]
- THORNE C. B., MOLNAR D. M., STRANGE R. E. Production of toxin in vitro by Bacillus anthracis and its spearation into two components. J Bacteriol. 1960 Mar;79:450–455. doi: 10.1128/jb.79.3.450-455.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]


