Abstract
Aims
Cardiac L-type Ca2+-currents show distinct alterations in chronic heart failure, including increased single-channel activity and blunted adrenergic stimulation, but minor changes of whole-cell currents. Expression of L-type Ca2+-channel β2-subunits is enhanced in human failing hearts. In order to determine whether prolonged alteration of Ca2+-channel gating by β2-subunits contributes to heart failure pathogenesis, we generated and characterized transgenic mice with cardiac overexpression of a β2a-subunit or the pore Cav1.2 or both, respectively.
Methods and results
Four weeks induction of cardiac-specific overexpression of rat β2a-subunits shifted steady-state activation and inactivation of whole-cell currents towards more negative potentials, leading to increased Ca2+-current density at more negative test potentials. Activity of single Ca2+-channels was increased in myocytes isolated from β2a-transgenic mice. Ca2+-current stimulation by 8-Br-cAMP and okadaic acid was blunted in β2a-transgenic myocytes. In vivo investigation revealed hypotension and bradycardia upon Cav1.2-transgene expression but not in mice only overexpressing β2a. Double-transgenics showed cardiac arrhythmia. Interstitial fibrosis was aggravated by the β2a-transgene compared with Cav1.2-transgene expression alone. Overt cardiac hypertrophy was not observed in any model.
Conclusion
Cardiac overexpression of a Ca2+-channel β2a-subunit alone is sufficient to induce Ca2+-channel properties characteristic of chronic human heart failure. β2a-overexpression by itself did not induce cardiac hypertrophy or contractile dysfunction, but aggravated the development of arrhythmia and fibrosis in Cav1.2-transgenic mice.
Keywords: Arrhythmia, L-type calcium channel, Cardiac fibrosis, Hypertrophy, Heart failure, Transgenic mice
1. Introduction
Calcium entry through L-type channels, multimers of Cav1.2-, β-, and α2δ-subunits, triggers cardiac contraction. There is consensus that whole-cell current as readout of Ca2+-channel activity is largely unchanged in heart failure.1,2 However, our observation of a huge, counterintuitive increase of single-channel activity in ventricular myocytes from human failing heart led to a more cautious interpretation and a more complex view of Ca2+-channel remodelling in heart failure,3–5 involving perhaps regulatory or structural alterations which escape detection by standard whole-cell analysis.
β2-subunits confer the high-activity gating pattern typical for failing human heart,6 in rodent cardiac cells7 and recombinant systems,8,9 respectively. Recently, we provided proof-of-concept that the single-channel phenotype of human heart failure can be reproduced in mouse heart by short-term overexpression of a β2a-subunit (tgind β2a).9 Acute β2-subunit overexpression has been shown to cause cell death7 and apoptosis10 in isolated myocytes and life-long overexpression in a transgenic mouse model resulted in hypertrophy, failure, and premature death, albeit under conditions where whole-cell current density—in contrast to human heart failure—was vastly elevated.11 This finding may just confirm that chronic increases in calcium load through elevated L-type channel expression and function in the sarcolemma can lead to hypertrophy and failure.12 Conversely, and of more pathophysiological relevance, in vivo knockdown of β2-subunits in a rat model of aortic banding reduced Ca2+-current density and attenuated cardiac hypertrophy.13 Although considered to serve as a functional protein kinase A substrate,14 β2-subunit overexpression was recently reported to blunt adrenergic regulation of Ca2+-currents in cardiomyocytes,15 mimicking another feature of human heart failure.6,16,17
In order to further elucidate the putative role of β2-subunits in heart failure,4 we simulated human pathophysiology with respect to Ca2+-channel function, using a genetic mouse approach. Cardiac overexpression of β2a-subunits was induced in vivo for a period of 4 weeks in mice through continuous application of the selective inducing drug tebufenozide via osmotic pumps. Our aim was to elucidate whether the functional and structural Ca2+-channel remodelling—as it occurs in human heart failure—will by itself provoke pathophysiological consequences on cardiac morphology, contraction, or rhythm. To compare, contrast, and combine this strategy with a more conventional approach—an overt and long-term increase in Ca2+-current—we also examined the effects of stable cardiac overexpression of the Cav1.2 pore-subunit (tg Cav1.2), a model of calcium-dependent cardiac hypertrophy and failure.18,19 We investigated animals at an age of 4–5 months, i.e. at an age where tg Cav1.2 not yet develop significant hypertrophy or increased activity (‘heart-failure phenotype’) of ventricular L-type Ca2+-channels, respectively.9,19
2. Methods
2.1. Mouse models
All procedures complied with respective laws and local regulations (no IRB approval required) and conform with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996). The generation of transgenic mice with inducible, cardiac-specific expression of rat β2a Ca2+-channel subunits (tgind β2a, Figure 1A) has been described previously.9 Transgenic mice with cardiac expression of the human pore-forming Ca2+ subunit (tg CaV1.2) are described elsewhere.18,19 All strains were maintained on an FVB/N background with wild-type littermates serving as control. Experiments were performed with mice of both sexes at an age of 4–5 months. Animals (including wild-type mice) were used for experiments 26–28 days after implantation of osmotic minipumps, which contained the inducing drug tebufenozide (kind gift of Dow AgroSciences, Munich, Germany).
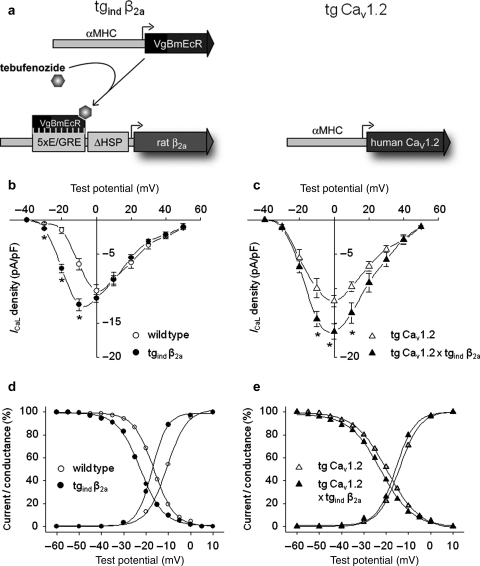
Figure 1.
Whole-cell Ca2+ current modulation by overexpression of cardiac β2-subunits. (A) Scheme of the binary, inducible β2a transgene (tgind β2a) and the cardiac-specific transgene for expression of the human CaV1.2 channel subunit (tg CaV1.2). Tebufenozide causes transgenic ecdysone receptors (VgBmEcR) to induce expression of the rat β2a-subunit. αMHC, α-myosin heavy-chain gene promoter; VgBmEcR, chimeric drosophila/bombyx ecdysone receptor; E/GRE, hybrid ecdysone/glucocorticoid response element; ΔHSP, minimal heat-shock promoter. (B and C) Induced overexpression of β2-subunits led to an increase in ventricular L-type Ca2+ peak current density in hearts with wild-type (B) or tg CaV1.2 genetic background (C), respectively. In addition, we observed a leftward shift of steady-state activation and inactivation (D and E). No significant changes of time-dependent inactivation (data not shown) due to overexpression of β2-subunits could be detected. Wild-type: n = 5–9, tgind β2a: n = 3–13, tg CaV1.2: n = 4–12, tg CaV1.2 × tgind β2a: n = 4–6; *P < 0.05.
2.2. Implantation of osmotic pumps
Mice were anaesthetized by intraperitoneal injection of S-ketamin (Ketanest S®, Pfizer; 125 mg/kg) and medetomidine (Domitor®, Pfizer; 250 µg/kg). Osmotic pumps (Alzet®, Model 2004) were prepared as described by the producer's instructions. During operation, pumps were placed subcutaneously at the animals’ back with a connected catheter placed intraperitoneally.
2.3. Isolation of ventricular myocytes
Single-ventricular myocytes were isolated from murine hearts by enzymatic dissociation using the method described earlier.9 In brief, hearts were perfused with a collagenase solution (Worthington type I and II, 75 U/L; CellSystems, St Katharinen, Germany) in a Langendorff setup at 37°C and subsequently cut into small chunks. Myocytes were harvested by pouring the suspension through cheesecloth. Viability of yielded cells did not differ between the several genotypes.
2.4. Electrophysiological investigation of single-ventricular myocytes
All experiments were performed at room temperature. Single-channel recordings were performed by using the cell-attached configuration of the patch-clamp technique.9 Cells were placed in disposable Petri-dishes containing 3 mL of a high-potassium solution (mM: 25 KCl, 120 K-glutamate, 2 MgCl2, 10 HEPES, 2 EGTA, 10 dextrose, 1 CaCl2, 1 Na2-ATP; pH 7.3 with KOH). Patch pipettes (borosilicate glass, 6–8 MΩ) were filled with pipette solution (mM: 70 BaCl2, 110 sucrose, and 10 HEPES; pH 7.4 with TEA-OH). Ba2+-currents were elicited by voltage steps (150 ms, 1.66 Hz) from −100 to +10 mV or +20 mV, respectively (≥180 sweeps per experiment). Data were sampled at 10 kHz and filtered at 2 kHz (3 dB, four-pole Bessel) by using an Axopatch 1D amplifier (Axon Instruments, Foster City, USA). PCLAMP software (CLAMPEX, FETCHAN, and PSTAT 6) was used for data acquisition and analysis (Axon Instruments). When necessary, analysis of single-channel recordings was performed with correction for multiple channels in a patch.6
Whole-cell recordings were obtained as described.20 We used an external solution containing (mM): 137 NaCl, 5.4 CsCl, 2 CaCl2, 1 MgCl2, 10 dextrose, 10 HEPES (pH 7.4 with NaOH). Pipettes (2–4 MΩ borosilicate glass) were filled with (mM): 120 CsCl, 1 MgCl2, 5 HEPES, 10 EGTA, 4 Mg-ATP (pH 7.2 with CsOH). Giga-Ohm seals (2–5 GΩ) were formed by gentle suction. Before series resistance compensation, membrane capacitance was measured by means of fast depolarizing ramp pulses (from −40 to −35 mV; 5 ms duration) at the beginning of each experiment. Membrane currents were low-pass filtered at 2 kHz. Starting from a holding potential of −80 mV cell membranes were depolarized to −40 mV for 50 ms to inactivate Na+- and T-type Ca2+-channels. To obtain current–voltage relationship Ca2+-currents were then elicited by 150 ms depolarizing pulses at 10 mV steps (range −30 to +50 mV). Analysis of whole-cell Ca2+-current kinetics was performed as described before.20 In brief, steady-state activation and inactivation curves were fitted using a Boltzmann equation (Microcal Origin® 6.0 software).
2.5. Haemodynamic measurements
Electrocardiograms in conscious, unrestrained mice were recorded by telemetry (DSI, Transoma Medical, USA, TA10EA-F20 transmitters) more than 7 days after implantation of transducers as previously described.21 Thirty seconds recordings were obtained every 10 min at a sampling frequency of 2 kHz. Periods when mice were at rest (activity = 0) were analysed using the ECG module of ADI Chart v5.5.5 (AD Instruments, Castle Hill, Australia). Aortic and left-ventricular catheterization was performed using a 1.4F pressure-volume catheter. Data were recorded and analysed with Chart v5.4 as described before.22 Mice were anaesthetized with isoflurane (2 vol% in O2) and their body temperature was kept at 37°C.
2.6. Quantitative real-time polymerase chain reaction
Quantitative real-time polymerase chain reaction (qPCR) was performed as described previously.23 Briefly, ventricles were rapidly removed from decapitated mice, separated from atria, and cut transversally. The ventricle basis was immediately frozen in liquid nitrogen. RNA was isolated using the RNeasy Mini Kit (Qiagen, Hilden, Germany). A total of 1 µg RNA was DNase treated and reverse transcribed according to the manufacturer's protocol by means of the QuantiTect Reverse Transcription Kit (Qiagen). qPCR reactions were run in triplicate on an MX3000P detector (Stratagene, Amsterdam, The Netherlands) using Quantitect SYBR Green mastermix (Qiagen). Cycling conditions: 15 min polymerase activation at 95°C and 40 cycles at 95°C for 15 s, at 58°C for 30 s, and at 72°C for 30 s. Gene expression was normalized to β-actin values.
2.7. Cardiac histology
After haemodynamic evaluation or ECG recording, hearts were fixed with 4% paraformaldehyde in phosphate-buffered saline, embedded in paraffin, cut in 3 µm slices, and stained either with haematoxylin–eosin or Sirius-red as described before.22 To allow for determination of myocyte cross-sectional area, heart sections were additionally stained with a green fluorescent dye coupled to wheat germ agglutinin (WGA, Alexa Fluor® 488 conjugate, Invitrogen) and nuclei were counterstained with propidium iodide. Left-ventricular myocyte cross-sectional areas were analysed by computer-assisted morphometry22 using a Zeiss AxioCam MRc camera on an Axiovert 200 inverted microscope with ×40 magnification and the AxioVision Rel. 4.5 software. Quantification of fibrosis revealed by Sirius-red staining was performed by measuring the extent of fibrotic area in heart cross-sections.
2.8. Data analyses and statistics
Data are presented as means±SEM. To evaluate effects of β2a overexpression, the following groups were compared using Student's t-tests unless otherwise indicated: tgind β2a vs. wild-type, tg CaV1.2×tgind β2a vs. tg CaV1.2, and tg CaV1.2 vs. wild-type. P-values <0.05 were considered significant.
3. Results
3.1. Transgenic mouse models
We studied three transgenic mouse-lines expressing distinct Ca2+-channel subunits specifically in cardiac myocytes in vivo9 (Figure 1A). Cardiac myocyte-specific transcription of a rat Ca2+-channel β2a subunit (tgind β2a) was induced by specific activation of a hybrid drosophila-bombyx ecdysone receptor by tebufenozide.9,24 The second mouse-line, tg CaV1.2, exhibits a constitutive cardiac overexpression of the human L-type Ca2+-channel pore (CaV1.2) and represents an established model of cardiac hypertrophy.18,19 Mice combining inducible cardiac expression of the rat β2a-subunit with constitutive cardiac overexpression of the human CaV1.2 subunit were obtained by cross-breeding (tg CaV1.2 x tgind β2a). Wild-type littermates from either breeding of FVB/N and tgind β2a or FvB/N and tg CaV1.2 mice served as controls.
For the present study, mice of all four genotypes received the ecdysone receptor agonist tebufenozide. Treatment for 4 weeks resulted in cardiac-specific overexpression of β2a subunits in tgind β2a at the mRNA level (see Supplementary material online, Figure S1A). In our previous study we had shown that β2a overexpression also occurred at the protein level after short-term induction.9 Human CaV1.2 mRNA was exclusively detected in tg CaV1.2 hearts (see Supplementary material online, Figure S1B). In order to test whether the transgenes affected expression of the endogenous Ca2+-channel subunits, mRNA from wild-type and transgenic hearts was analysed by quantitative RT-PCR using murine-specific primers (see Supplementary material online, Table S1). Importantly, expression of the endogenous CaV1.2- or β-subunits did not differ between genotypes (see Supplementary material online, Table S2).
3.2. Whole-cell Ca2+ currents
Cardiac overexpression of the β2a-subunit for 4 weeks led to an increase of the Ca2+-current density at its maximum (wild-type: −10.3 ± 0.9 pA/pF, n = 9 vs. tgind β2a: −12.7 ± 0.7 pA/pF, n = 13; P < 0.05) and at negative test voltages (Figure 1B), while current density at potentials positive to 0 mV suggested rather unchanged maximum conductance Gmax. A more pronounced elevation across the whole voltage range (Figure 1C) was observed on top of a constitutive cardiac overexpression of CaV1.2 (tg CaV1.2: −12.5 ± 1.0 pA/pF, n = 12 vs. tg CaV1.2 × tgind β2a: −16.6 ± 1.1 pA/pF, n = 6; P < 0.05). β2a-overexpression shifted I–V curves, derived steady-state activation curves (V0.5 values of activation for wildtype: −10.6 ± 1.0 mV, n = 12 vs. tgind β2a: −16.9 ± 1.0 mV, n = 14; P < 0.01; tg CaV1.2: −13.6 ± 1.1 mV, n = 13 vs. tg CaV1.2 x tgind β2a: −15.2 ± 1.0 mV, n = 6; P = 0.36, Figure 1D, E) and inactivation curves (V0.5 values of inactivation for wild-type: −16.8 ± 1.0 mV, n = 10 vs. tgind β2a: −23.2 ± 1.1 mV, n = 14; P < 0.01; tg CaV1.2: −20.4 ± 0.9 mV, n = 16 vs. tg CaV1.2 × tgind β2a: −23.4 ± 1.3 mV, n = 7; P = 0.06, Figure 1D, E) towards more negative membrane potentials. Cell capacitance was not different between genotypes (data not shown). Taken together, long-term overexpression of cardiac β2-subunits alone changed whole-cell Ca2+-currents in a manner resembling findings from human failing myocardium.1,16
3.3. Activity of single ventricular L-type Ca2+-channels
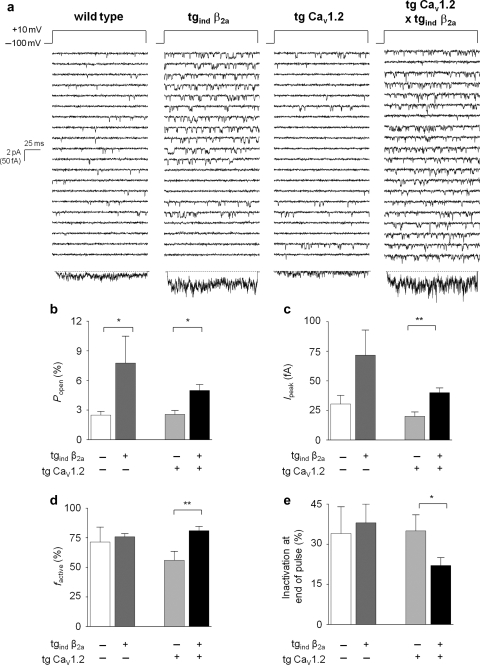
Single-channel recording revealed increased activity after maintained cardiac β2-overexpression for 4 weeks (Figure 2A). This was the case for β2-overexpression alone (wild-type: Ipeak = 30 ± 7 fA, n = 5 vs. tgind β2a: Ipeak = 72 ± 21 fA, n = 6; P = 0.08), and against the CaV1.2-transgenic background (tg CaV1.2: Ipeak = 20 ± 4 fA, n = 8 vs. tg CaV1.2 × tgind β2a: Ipeak = 40 ± 4 fA, n = 14; P < 0.01). This increase was mainly due to significantly enhanced open probability and fraction of active sweeps (Figure 2B, D). In accordance with the leftward shift of whole-cell current activation, effects of β2a-overexpression on single-channel gating were more pronounced at +10 mV compared with +20 mV (Figure 2B–E vs. Supplementary material online, Figure S2A–D). In summary, cardiac overexpression of β2-subunits caused L-type Ca2+-channel properties similar to those obtained with myocytes from human failing ventricle.6
Figure 2.
Increased activity of single ventricular L-type Ca2+-channels.(A) Exemplary traces show a significantly increased single-channel activity in ventricular myocytes where overexpression of β2-subunits was induced. This is mainly due to an increase of open probability and fraction of active sweeps (B–E). Wild-type: n = 5, tgind β2a: n = 6, tg CaV1.2: n = 8, tg CaV1.2 × tgind β2a: n = 14; *P < 0.05, **P < 0.01.
3.4. Effect of β2-subunit overexpression on cAMP-dependent modulation of whole-cell Ca2+ currents
To examine phosphorylation-dependent channel modulation, we tested the effects of 8-Br-cAMP (1 mM) and the phosphatase inhibitor Okadaic acid (1 µM) on whole-cell currents in ventricular myocytes from wild-type and tgind β2a mice (see Supplementary material online, Figure S3). Here, basal levels were only slightly higher in tgind β2a myocytes (−9.5 ± 2.9 pA/pF vs. wild-type −7.5 ± 1.6 pA/pF.; P > 0.05, n = 5–6). Significant cAMP-dependent stimulation was evident in wild-type myocytes only (wildtype: 158 ± 13%, n = 6, P < 0.05; tgind β2a: 116 ± 9%, n = 5, P = 0.1). Hence, β2-subunit expression resembled another important hallmark of human heart failure6,16 that also has been shown for Cav1.2-transgenic mice.12
3.5. Haemodynamic function of transgenic mice during anaesthesia
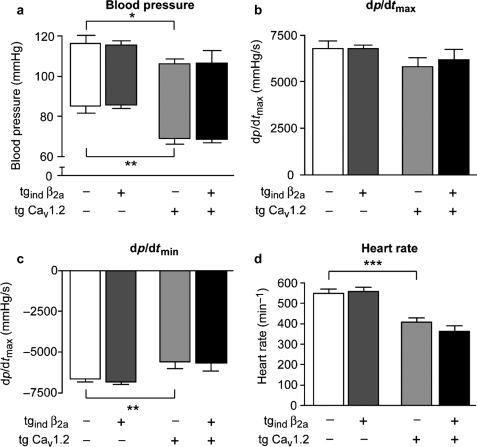
The effects of transgenic expression of β2a and CaV1.2 subunits on haemodynamic function were assessed by left-ventricular microtip catheterization during isoflurane anaesthesia (Figure 3). Mice with a single transgene expressing only the hybrid drosophila/bombyx ecdysone receptor (α-MHC VgBmEcR) did not differ in their haemodynamic function or cardiac structure from non-transgenic littermates (data not shown). Expression of the inducible β2a-subunit did not affect aortic blood pressure when compared with either wild-type or tg CaV1.2 animals (Figure 3A). Systolic and diastolic aortic pressures were significantly lower in CaV1.2-transgenic mice when compared with wild-type mice (Figure 3A). No significant differences in left-ventricular contractility (dp/dtmax) were detected between wild-type and transgenic mice (Figure 3B). However, LV relaxation (dp/dtmin) was significantly reduced in CaV1.2 transgenic mice (Figure 3C). Somewhat unexpected, resting heart rate was only 408 ± 20 min−1 in tg CaV1.2 mice as opposed to 551 ± 20 min−1 in wild-type mice (P < 0.01, Figure 3D). Moreover, during aortic and left-ventricular catheterization, irregularities in cardiac rhythm were observed in double-transgenic but not in control or single transgenic mice (Figure 4B, C). Aortic pressure recording revealed 35 ± 14 arrhythmic b.p.m. in double-transgenic mice as opposed to 0.2 arrhythmic b.p.m. in tg CaV1.2 mice (P < 0.01, Figure 4C).
Figure 3.
Haemodynamic function of Ca2+-channel β2a- and CaV1.2-subunit in transgenic mice. (A–D) Haemodynamic function was assessed by Millar microtip catheterization during isoflurane anaesthesia. Left ventricular (LV) systolic (A, upper limits of boxes), aortic diastolic pressure (A, lower limits), and LV relaxation (C, dp/dtmin) were significantly decreased by overexpression of CaV1.2. No effects of β2a subunit overexpression were observed in tgind β2a mice (compared with wild-type) or double-transgenic animals (compared with tg CaV1.2). LV contractility (B) did not differ significantly between strains. Heart rate (D) was significantly lower in tg CaV1.2 mice when compared with wild-type mice (n = 4–6 per genotype, *P < 0.05, **P < 0.01, ***P < 0.001).
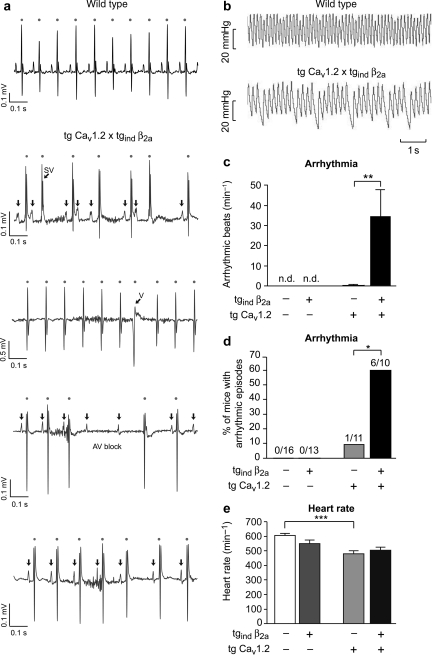
Figure 4.
Arrhythmia in transgenic mice expressing β2a and CaV1.2 Ca2+-channel subunits. (A and B) Original traces of recordings obtained by ECG telemetry in awake, freely moving mice (A) from wild-type (upper panel) and double-transgenic mice (lower panels) or by microtip catheterization in anaesthetized animals (B), respectively. (A) In case of wild-type mice (top row) we observed sinus rhythm as displayed. Tg CaV1.2 × tgind β2a mice showed several forms of rhythm disturbances including supraventricular extrasystolies (second row, SV), ventricular extrasystolies (third row, V), atrio-ventricular block (fourth row), and sinus pause/sinus bradycardia (bottom row). (B) Catheter data revealed the haemodynamic relevance of rhythm disturbances in double-transgenic mice. (C) The incidence of arrhythmic beats during microtip catheterization was significantly increased in tg CaV1.2 × tgind β2a mice (**P < 0.01). No arrhythmic beats were detected in wild-type or tgindβ2a mice. (D) Overall, arrhythmic episodes during ECG or catheterization were only seen in 1/11 (9.1%) tg CaV1.2, but in 6/10 (60%) double-transgenic mice (*P < 0.05; χ2 test). (E) Heart rate as determined by ECG telemetry in awake animals was significantly lowered in tg CaV1.2 mice (n = 5–9 per genotype, 3/5 examined tg CaV1.2 mice did not receive tebufenozide, *P < 0.05, **P < 0.01; ***P < 0.001).
3.6. Electrocardiography in awake animals
In order to test whether bradycardia and arrhythmia were also present in awake, freely moving animals, telemetric ECG transducers were implanted and electrocardiograms were recorded for successive days (Figure 4A, D, E). All four mouse strains showed expected diurnal heart-rate variations (data not shown) and tg CaV1.2 mice exhibited significant bradycardia during night and daytime (Figure 4E). Complementing our catheter data, episodes of arrhythmic beats were observed in 6/10 double-transgenic mice but only in 1/11 tg CaV1.2 animals (Figure 4D). No such rhythm disturbances were seen in wild-type or tgind β2a mice. Further ECG analysis revealed ventricular and supraventricular extrasystoles in tg CaV1.2 × tgind β2a mice (Figure 4A). Note, that none of these mice had atrial fibrillation and PR interval was unaffected (tg CaV1.2 × tgind β2a 32.1 ± 2.0 ms vs. wild-type 30.0 ± 0.9 ms, P = 0.35).
3.7. Cardiac histopathology and cardiac morphological parameters
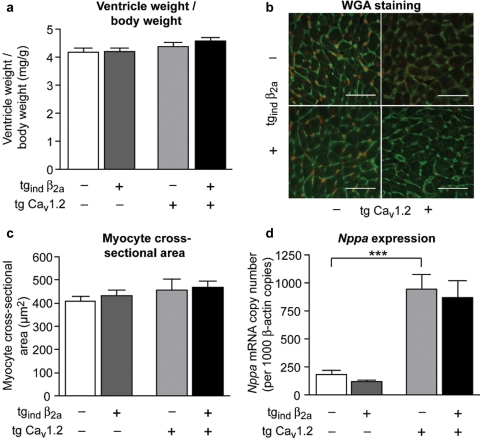
Transgenic mice overexpressing CaV1.2, β2a or both Ca2+-channel subunits did not display any signs of left-ventricular hypertrophy at 4–5 months of age (Figure 5A–C). Ventricle-to-body-weight (Figure 5A) or ventricle-weight-to-tibia-length ratios (data not shown) did not differ between transgenic strains and wild-type mice. Similarly, wheat germ agglutinin (WGA) staining of mid-ventricular transverse sections (Figure 5B) to determine single cardiomyocytes’ cross-sectional area did not reveal significant differences between groups (Figure 5C). Nppa (atrial natriuretic peptide) expression, however, which is a sensitive and early marker for cardiac load and myocyte hypertrophy, was significantly upregulated 5.0-fold and 4.4-fold in ventricles of tg CaV1.2 and tg CaV1.2 × tgind β2a mice compared with wild-type, respectively (Figure 5D).
Figure 5.
Heart weight, morphology, and atrial natriuretic peptide (Nppa) expression. (A) Ventricle-to-body-weight ratios did not differ between genotypes (n = 8–15 per genotype). (B and C) Cardiac myocyte cross-sectional area assessed by wheat germ agglutinin (WGA) staining of mid-ventricular transverse heart sections (B, representative pictures, bars: 50 µm) showed no differences between genotypes (n = 5–11 per genotype). (D) Nppa mRNA levels reached significant higher levels in tg CaV1.2 and tg CaV1.2 × tgind β2a mice when compared with wild-type or tgind β2a mice (n = 8–15 per genotype, ***P < 0.001).
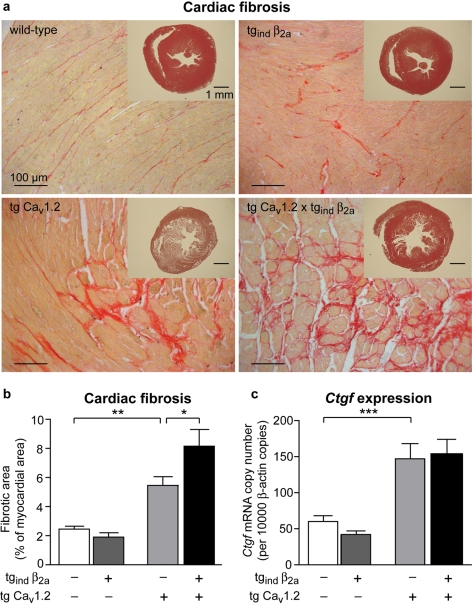
Furthermore, transgenic tg CaV1.2 and tg CaV1.2 × tgind β2a mice developed pronounced cardiac fibrosis (Figure 6). Interstitial fibrotic area in mid-ventricular transverse sections was increased by 120% in tg CaV1.2 and by 231% in tg CaV1.2 × tgind β2a compared with wild-types (Figure 6B). Left-ventricular fibrosis was significantly aggravated in double-transgenic mice when compared with tg CaV1.2 mice (tg CaV1.2 × tgind β2a 8.2 ± 1.2% vs. tg CaV1.2 5.4 ± 0.6%). However, no significant changes in fibrotic area were observed by overexpression of the β2a subunit alone (Figure 6B). mRNA expression of connective tissue growth factor (Ctgf), a marker of fibrosis, was significantly enhanced in both tg CaV1.2 and tg CaV1.2 × tgind β2a mice compared with wild-type or tgind β2a animals (Figure 6C), respectively.
Figure 6.
Cardiac fibrosis in transgenic mice overexpressing CaV1.2 Ca2+-channel subunits. (A) Sirius-red staining of mid-ventricular cardiac sections from wild-type mice and mice overexpressing the pore-forming subunit CaV1.2, the auxiliary subunit β2a or both subunits, respectively. Hearts of mice overexpressing CaV1.2 or additionally β2a (tg CaV1.2 × tgind β2a) displayed marked fibrosis. Inserts show heart slices stained with haematoxylin–eosin. (B) Interstitial fibrosis was determined by Sirius-red staining and is displayed as percentage of left ventricular cross-sectional area. Overexpression of CaV1.2 lead to significant increases in interstitial fibrous tissue (n = 8–16 per genotype, *P < 0.05, **P < 0.01). (C) mRNA expression of connective tissue growth factor (Ctgf) was quantified by qPCR. In cardiac ventricles of tg CaV1.2 and tg CaV1.2 × tgind β2a mice Ctgf expression was significantly increased (n = 8–15 per genotype, ***P < 0.001).
4. Discussion
Activity of single-ventricular L-type Ca2+-channels is significantly increased in human heart failure.6 Subtle alterations of Ca2+-current biophysics and pharmacology were also reported at the whole-cell level.16,17 These data are consistent with—though not proof of—the idea that Ca2+-current in human heart failure is carried by a reduced number of channels, each individually contributing more current at the expense of a reduced regulatory bandwidth. In order to further elucidate the pathophysiological relevance of the Ca2+-channel β2-subunit overexpression that we recently found in human heart failure,9 we now used an induction strategy with continuous administration of the inducing agent tebufenozide for 4 weeks, allowing for examination of long-term effects in vivo. It is important to emphasize that the continued induction of only the β2-subunit transgene was totally sufficient to resemble the Ca2+-channel phenotype of human heart failure:
little change (even unchanged at positive test potentials) of whole-cell current density,1,2
leftward shift of voltage-dependent activation,16
marked enhancement of single-channel activity,6 and
blunting of the Ca2+-current response towards phosphorylating conditions.6,16
These findings do not resolve the algebraic conundrum why in failing heart single channels—at least those located at the surface sarcolemma—are twice as active as would be predicted from largely unchanged whole-cell currents, with no direct evidence of a reduced expression of channel pore protein.9 Nor do they completely elucidate the mechanistic basis of hyperactivity of single channels (hyperphosphorylation, change in subunit isoform composition, or a combination of both). Our data do, however, prove that the entire spectrum of functional Ca2+-channel remodelling as it occurs in human heart failure can be reproduced by continuing overexpression of a β2-subunit alone. This serves as the basis to address the important question whether β2-subunit overexpression is an adaptive or maladaptive phenomenon, and—consequently—whether interference with structural remodelling would constitute not only a technically feasible13,25 but also a therapeutically promising approach.
In vivo overexpression of β2-subunits did not affect systolic contractile function, nor did we observe significant changes in tg CaV1.2 or double-transgenic hearts. This argues against a major adaptive role of β2-subunit overexpression at least in young animals (4–5 months) as studied here, i.e. during a stage where heart failure is not yet developed. Lower blood pressure in tg CaV1.2 and double-transgenic mice may be explained by the significant bradycardia, as left ventricular dp/dtmax was not altered in these mice. In this context, it is intriguing to speculate that reduced ventricular relaxation in CaV1.2 transgenic mice may be due to diastolic dysfunction associated with ventricular interstitial fibrosis. Based on these haemodynamic results it should be stressed that all the changes reported below are not indirectly mediated by a grossly distorted haemodynamic state.
Cardiac hypertrophy is not induced by β2-subunit overexpression as such. This is highlighted by gross and normalized heart weight, myocyte capacitance and morphometric size, and the lack of ANP increase. Hypertrophy is a known consequence of long-term and extensive increase of Ca2+-current density, regardless of whether induced by overexpression of CaV1.218 or β2-subunits.11 Our results indicate that cardiac hypertrophy is not an immediate, direct consequence of Ca2+-channel remodelling. It requires a timeframe of more than 4 weeks and/or a substantial increase of whole-cell Ca2+-current density, making indirect mechanisms much more likely.
β2-Subunit overexpression alone did not induce fibrosis, but Ca2+-channel remodelling led to morphological changes even within a 4 week-period in apparently healthy young animals: cardiac fibrosis developing with CaV1.2 overexpression19 aggravates by additional β2-subunit overexpression, along with a potentiated increase of the whole-cell current density. Thus, our data are consistent with the view that Ca2+-current density—and therefore cellular calcium load—rather than a particular subunit as such induces the morphological alterations found in this and other studies.11,18 Note that we cannot exclude that the differences we observed regarding the effects of overexpressing a rat β2a-subunit on an either (murine) wild-type or (human) Cav1.2-transgenic background, respectively, is due to combination of channel subunits from different species.
The one functional consequence that we can trace back directly and exclusively to long-term (or subacute) β2-subunit overexpression is the induction of resistance of channels towards cAMP-dependent stimulation. This finding deserves a detailed follow-up on its own, because a reduced bandwidth of—e.g. adrenergic—regulation is certainly an important aspect of heart-failure pathophysiology, and the Ca2+ channel may be a direct player here rather than just an innocent endpoint of a thoroughly altered signalling cascade.26 The findings of Miriyala et al.,15 together with our previous9 and present results provide a molecular basis to explain why individual Ca2+ channels in heart failure are hyperactive and resistant to stimulation by phosphorylation. It will be tempting to find out whether therapeutic reversal of Ca2+-channel remodelling can restitute adrenergic responses in heart-failure models.
The most prominent functional consequence of Ca2+-channel remodelling was observed with cardiac rhythm: CaV1.2 and double-transgenic animals revealed marked bradycardia under both anaesthetized (catheter data) and conscious (telemetry) conditions. Moreover, arrhythmic events were recorded when CaV1.2 (1/11 animals), but in particular when both transgenes were overexpressed (6/10 animals). Bradycardia was not associated with altered atrial morphology or atrio-ventricular conduction block and may be a consequence of an altered adrenergic response in vivo. On the other hand, it is tempting to speculate that heartbeat irregularities may have been triggered by the gross alterations of single-channel activity induced by β2-subunit overexpression leading to further enhanced Ca2+ influx via transgenic CaV1.2/β2a channels as a kind of last straw. The lack of pathology in the presence of β2-subunit overexpression alone indicates that the β2-subunit may be an essential but by itself not sufficient factor for disturbances in cardiac rhythm. It can be speculated that not (only) the amount of Ca2+-influx enhancement, but (also) the underlying mechanism (e.g. increased channel activity vs. increased channel activity plus increased channel density) is essential. The respective roles of single-channel behaviour vs. overall calcium load, as well as the cellular mechanism of arrhythmia need further study.
The short 4 week-period of intervention is likewise an important strength and limitation of our present study. Although our previous study has demonstrated that induction of the tgind β2a transgene in vivo occurs and takes effect within 48 h,9 many pathophysiological sequelae may take more time to develop, and therefore longer treatment and observation periods deserve to be studied. On the other hand, the molecular mechanisms of alterations will be hard to identify as soon as complex haemodynamic and morphological changes ensue in an animal model. In this situation, specific molecular interference (e.g. by siRNA) may be a strategy superior to the transgenic approach. Though the bradycardic and the arrhythmic phenotype of our mouse models are of considerable interest our study was limited to exploration by heart catheter and surface ECG. Thus in depth analysis is needed to reveal the kind and relevance of our observations. We believe that the consequences of Ca2+-channel remodelling on the complex electrical behaviour of the heart should be studied in other animal models. Lentiviral gene transfer may offer a means to do such studies in hearts much more similar to human regarding rate and rhythm, such as rabbit, pig, or dog.
In conclusion, cardiac β2-subunit overexpression in mice causes a Ca2+-channel phenotype typical of human heart failure. When present over 4 weeks, this phenotype causes resistance to cAMP-dependent stimulation, but no other direct functional or morphological defects. Cardiac fibrosis and arrhythmia as found in the context of CaV1.2 overexpression become potentiated by β2-subunit overexpression. Our data suggest that cardiac hypertrophy and contractile failure would require extended timeframes and/or an extent of overexpression of CaV1.2 and/or β2-subunit that are not pertinent to the human pathophysiology.
Supplementary material
Supplementary Material is available at Cardiovascular Research online.
Funding
This study was supported by the Deutsche Forschungsgemeinschaft (Excellence Cluster ‘Bioss’ EXC294 in support of L.H. and N.B.), by the Center of Molecular Medicine (A5 to S.H. and U.C.H.), and by the NIH (R01 HL 079599; A.S.).
Supplementary Material
Acknowledgements
The excellent technical help of Sigrid Kirchmann-Hecht and Jens Reifenrath is appreciated. We are grateful to Dr Roger Hullin for critical reading and discussion. We thank Dow AgroSciences for providing the drug tebufenozide.
Conflict of interest: none declared.
References
- 1.Mukherjee R, Spinale FG. L-type calcium channel abundance and function with cardiac hypertrophy and failure: a review. J Mol Cell Cardiol. 1998;30:1899–1916. doi: 10.1006/jmcc.1998.0755. [DOI] [PubMed] [Google Scholar]
- 2.Richard S, Leclercq F, Lemaire S, Piot C, Nargeot J. Ca2+ currents in compensated hypertrophy and heart failure. Cardiovasc Res. 1998;37:300–311. doi: 10.1016/s0008-6363(97)00273-3. [DOI] [PubMed] [Google Scholar]
- 3.Benitah JP, Gomez AM, Fauconnier J, Kerfant BG, Perrier E, Vassort G, et al. Voltage-gated Ca2+ currents in the human pathophysiologic heart: a review. Basic Res Cardiol. 2002;97(Suppl. 1):I11–I18. doi: 10.1007/s003950200023. [DOI] [PubMed] [Google Scholar]
- 4.Bodi I, Mikala G, Koch SE, Akhter SA, Schwartz A. The L-type calcium channel in the heart: the beat goes on. J Clin Invest. 2005;115:3306–3317. doi: 10.1172/JCI27167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sjaastad I, Wasserstrom JA, Sejersted OM. Heart failure—a challenge to our current concepts of excitation-contraction coupling. J Physiol. 2003;546:33–47. doi: 10.1113/jphysiol.2002.034728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schroder F, Handrock R, Beuckelmann DJ, Hirt S, Hullin R, Priebe L, et al. Increased availability and open probability of single L-type calcium channels from failing compared with nonfailing human ventricle. Circulation. 1998;98:969–976. doi: 10.1161/01.cir.98.10.969. [DOI] [PubMed] [Google Scholar]
- 7.Colecraft HM, Alseikhan B, Takahashi SX, Chaudhuri D, Mittman S, Yegnasubramanian V, et al. Novel functional properties of Ca(2+) channel beta subunits revealed by their expression in adult rat heart cells. J Physiol. 2002;541:435–452. doi: 10.1113/jphysiol.2002.018515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hullin R, Khan IF, Wirtz S, Mohacsi P, Varadi G, Schwartz A, et al. Cardiac L-type calcium channel beta-subunits expressed in human heart have differential effects on single channel characteristics. J Biol Chem. 2003;278:21623–21630. doi: 10.1074/jbc.M211164200. [DOI] [PubMed] [Google Scholar]
- 9.Hullin R, Matthes J, von Vietinghoff S, Bodi I, Rubio M, D'Souza K, et al. Increased expression of the auxiliary beta2-subunit of ventricular L-type Ca2+ channels leads to single-channel activity characteristic of heart failure. PLoS ONE. 2007;2:e292. doi: 10.1371/journal.pone.0000292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen X, Zhang X, Kubo H, Harris DM, Mills GD, Moyer J, et al. Ca2+ influx-induced sarcoplasmic reticulum Ca2+ overload causes mitochondrial-dependent apoptosis in ventricular myocytes. Circ Res. 2005;97:1009–1017. doi: 10.1161/01.RES.0000189270.72915.D1. [DOI] [PubMed] [Google Scholar]
- 11.Nakayama H, Chen X, Baines CP, Klevitsky R, Zhang X, Zhang H, et al. Ca2+- and mitochondrial-dependent cardiomyocyte necrosis as a primary mediator of heart failure. J Clin Invest. 2007;117:2431–2444. doi: 10.1172/JCI31060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Groner F, Rubio M, Schulte-Euler P, Matthes J, Khan IF, Bodi I, et al. Single-channel gating and regulation of human L-type calcium channels in cardiomyocytes of transgenic mice. Biochem Biophys Res Commun. 2004;314:878–884. doi: 10.1016/j.bbrc.2003.12.174. [DOI] [PubMed] [Google Scholar]
- 13.Cingolani E, Ramirez Correa GA, Kizana E, Murata M, Cho HC, Marban E. Gene therapy to inhibit the calcium channel beta subunit: physiological consequences and pathophysiological effects in models of cardiac hypertrophy. Circ Res. 2007;101:166–175. doi: 10.1161/CIRCRESAHA.107.155721. [DOI] [PubMed] [Google Scholar]
- 14.Bunemann M, Gerhardstein BL, Gao T, Hosey MM. Functional regulation of L-type calcium channels via protein kinase A-mediated phosphorylation of the beta(2) subunit. J Biol Chem. 1999;274:33851–33854. doi: 10.1074/jbc.274.48.33851. [DOI] [PubMed] [Google Scholar]
- 15.Miriyala J, Nguyen T, Yue DT, Colecraft HM. Role of CaVbeta subunits, and lack of functional reserve, in protein kinase A modulation of cardiac CaV1.2 channels. Circ Res. 2008;102:e54–e64. doi: 10.1161/CIRCRESAHA.108.171736. [DOI] [PubMed] [Google Scholar]
- 16.Chen X, Piacentino V, III, Furukawa S, Goldman B, Margulies KB, Houser SR. L-type Ca2+ channel density and regulation are altered in failing human ventricular myocytes and recover after support with mechanical assist devices. Circ Res. 2002;91:517–524. doi: 10.1161/01.res.0000033988.13062.7c. [DOI] [PubMed] [Google Scholar]
- 17.Chen X, Zhang X, Harris DM, Piacentino V, III, Berretta RM, Margulies KB, et al. Reduced effects of BAY K 8644 on L-type Ca2+ current in failing human cardiac myocytes are related to abnormal adrenergic regulation. Am J Physiol Heart Circ Physiol. 2008;294:H2257–H2267. doi: 10.1152/ajpheart.01335.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muth JN, Yamaguchi H, Mikala G, Grupp IL, Lewis W, Cheng H, et al. Cardiac-specific overexpression of the alpha(1) subunit of the L-type voltage-dependent Ca(2+) channel in transgenic mice. Loss of isoproterenol-induced contraction. J Biol Chem. 1999;274:21503–21506. doi: 10.1074/jbc.274.31.21503. [DOI] [PubMed] [Google Scholar]
- 19.Muth JN, Bodi I, Lewis W, Varadi G, Schwartz A. A Ca(2+)-dependent transgenic model of cardiac hypertrophy: A role for protein kinase Calpha. Circulation. 2001;103:140–147. doi: 10.1161/01.cir.103.1.140. [DOI] [PubMed] [Google Scholar]
- 20.Meszaros J, Coutinho JJ, Bryant SM, Ryder KO, Hart G. L-type calcium current in catecholamine-induced cardiac hypertrophy in the rat. Exp Physiol. 1997;82:71–83. doi: 10.1113/expphysiol.1997.sp004016. [DOI] [PubMed] [Google Scholar]
- 21.Knaus A, Zong X, Beetz N, Jahns R, Lohse MJ, Biel M, et al. Direct inhibition of cardiac hyperpolarization-activated cyclic nucleotide-gated pacemaker channels by clonidine. Circulation. 2007;115:872–880. doi: 10.1161/CIRCULATIONAHA.106.667675. [DOI] [PubMed] [Google Scholar]
- 22.Brede M, Wiesmann F, Jahns R, Hadamek K, Arnolt C, Neubauer S, et al. Feedback inhibition of catecholamine release by two different alpha2-adrenoceptor subtypes prevents progression of heart failure. Circulation. 2002;106:2491–2496. doi: 10.1161/01.cir.0000036600.39600.66. [DOI] [PubMed] [Google Scholar]
- 23.Gilsbach R, Brede M, Beetz N, Moura E, Muthig V, Gerstner C, et al. Heterozygous alpha 2C-adrenoceptor-deficient mice develop heart failure after transverse aortic constriction. Cardiovasc Res. 2007;75:728–737. doi: 10.1016/j.cardiores.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 24.Hoppe UC, Marban E, Johns DC. Adenovirus-mediated inducible gene expression in vivo by a hybrid ecdysone receptor. Mol Ther. 2000;1:159–164. doi: 10.1006/mthe.1999.0023. [DOI] [PubMed] [Google Scholar]
- 25.Murata M, Cingolani E, McDonald AD, Donahue JK, Marban E. Creation of a genetic calcium channel blocker by targeted gem gene transfer in the heart. Circ Res. 2004;95:398–405. doi: 10.1161/01.RES.0000138449.85324.c5. [DOI] [PubMed] [Google Scholar]
- 26.Tilley DG, Rockman HA. Role of beta-adrenergic receptor signaling and desensitization in heart failure: new concepts and prospects for treatment. Expert Rev Cardiovasc Ther. 2006;4:417–432. doi: 10.1586/14779072.4.3.417. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.