Abstract
ATP is a paracrine regulator of critical airway epithelial cell functions, but the mechanism of its release is poorly understood. Pannexin (Panx) proteins, related to invertebrate innexins, form channels (called pannexons) that are able to release ATP from several cell types. Thus, ATP release via pannexons was examined in airway epithelial cells. Quantitative RT-PCR showed Panx1 expression in normal human airway epithelial cells during redifferentiation at the air–liquid interface (ALI), at a level comparable to that of alveolar macrophages; Panx3 was not expressed. Immunohistochemistry showed Panx1 expression at the apical pole of airway epithelia. ALI cultures exposed to hypotonic stress released ATP to an estimated maximum of 255 (±64) nM within 1 minute after challenge (n = 6 cultures from three different lungs) or to approximately 1.5 (±0.4) μM, recalculated to a normal airway surface liquid volume. Using date- and culture-matched cells (each n ≥ 16 from 4 different lungs), the pannexon inhibitors carbenoxolone (10 μM) and probenecid (1 mM), but not the connexon inhibitor flufenamic acid (100 μM), inhibited ATP release by approximately 60%. The drugs affected Panx1 currents in Xenopus oocytes expressing exogenous Panx1 correspondingly. In addition, suppression of Panx1 expression using lentivirus-mediated production of shRNA in differentiated airway epithelial cells inhibited ATP release upon hypotonic stress by approximately 60% as well. These data not only show that Panx1 is expressed apically in differentiated airway epithelial cells but also that it contributes to ATP release in these cells.
Keywords: pannexin, ATP release, airway epithelia
CLINICAL RELEVANCE
Pannexin 1 contributes to release of ATP, an important paracrine regulator of mucociliary function, in airway epithelia. Given the regulation of pannexins, the findings described in this article may have important implications for the availability of ATP in the airway surface liquid in airway homeostasis and disease.
ATP release onto the surface of airway epithelia is important for the regulation of many cellular functions related to mucociliary clearance (1–7), and ATP concentrations at the apical surface can reach micromolar concentrations, sufficient for ATP signaling (8, 9). In addition to stimulated ATP release, resting ATP levels in the low nanomolar range on the apical surface reflect a steady state of ATP secretion/release and ATP metabolism. Constitutive ATP release has been calculated to be 20–200 fmol · min−1 · cells−6 (3, 10). A recent publication also provides a mathematical model of the influences of nucleotides on mucociliary clearance parameters that is based on measured data, again stressing the importance of this paracrine mechanism for airway homeostasis (11).
Despite its importance and the scrutiny placed on it in many cells and tissues, the mechanism of ATP release onto airway epithelia remains at least partially unknown. Two distinct pathways have been proposed: a vesicular- and a channel-mediated release. In support of a vesicular mechanism, ATP release has been shown to partially depend on intracellular calcium increases required for vesicular release. Further support for this mechanism comes from the fact that airway epithelial cells release uridine diphosphate–glucose (12), presumably from vesicles stemming from the endoplasmic reticulum where uridine diphosphate–glucose plays a role in glycosylation by providing glucose for glucosyl transferases. Although this vesicular mechanism may provide a vehicle for constitutive ATP release, recent work on airway epithelia shows that calcium is not needed for hypotonic stress–mediated ATP release (8).
As an alternative to vesicular ATP release, several membrane channels have been implicated. These include cystic fibrosis transmembrane regulator, connexins that form channels to the outside (instead of gap junctions between cells), including Cx43, Cx38, and Cx32, a volume-regulated channel, the purinergic receptor, P2X7, a voltage-dependent anion channel (VDAC), a volume expansion–sensing outward rectifier, and the Maxi anion channel. Published data make it unlikely that most of these act as ATP release channels—at least not alone. For example, multiple articles have ruled out cystic fibrosis transmembrane regulator as a direct ATP release channel (3, 8, 13, 14). Although VDAC-1 was found to contribute to ATP release from murine airway epithelial cells (15), the plasma membrane variant of the channel does not exist in human cells, and even the murine cells from VDAC-1–deficient animals still released significant amounts of ATP (15). Most of the properties of the Maxi anion channel, specifically its high ATP permeability, are consistent with an ATP release function (16, 17). However, its exclusive anion selectivity disfavors such a role, as dye uptake typically associated with ATP release is seen with positively charged dyes, such as YoPro or ethidiumbromide.
Channels formed by connexins to the outside of cells, called connexons, seem important, because gap junction inhibitors successfully interfere with ATP release (e.g., Ref. 18). In addition, conditions that induce ATP release are associated with uptake of extracellular dyes that test gap junction function (for review, see Ref. 19). Although connexins are unlikely to release ATP (see Discussion), vertebrates express not only connexins, but also orthologs of the invertebrate innexins, namely the pannexins (Panx). Unlike connexons, pannexons (i.e., channels to the outside of cells) open at resting membrane potentials in response to mechanical stress (20). When coexpressed with P2Y receptors, Panx1 channels open in response to extracellular ATP that signals via intracellular Ca2+ (21). Thus, Panx may participate in ATP release from airway epithelia. The data presented here reveal that Panx1 is expressed at the apical membrane of airway epithelia, and that pannexons contribute to ATP release onto the apical airway surface.
MATERIALS AND METHODS
Chemicals and Solutions
Unless stated otherwise, all materials were purchased from Sigma Chemical Co. (St. Louis, MO).
Cell Cultures and Alveolar Macrophages
Human airways were obtained from organ donors whose lungs were rejected for transplant. Consent for research was obtained by the Life Alliance Organ Recovery Agency of the University of Miami. From these lungs, airway epithelial cells were isolated and dedifferentiated through expansion and redifferentiated at an air–liquid interface (ALI) on 12-mm T-clear filters (Costar Corning, Corning, NY), as previously described (22–25). All consents were institutional review board–approved and conformed to the Declaration of Helsinki.
Human alveolar macrophages were purified from donor lungs using the method described by Mackenzie and colleagues (26), except that the cells were filtered through 70-μM sieves.
RNA Extraction and Quantitative RT-PCR
Total RNA was extracted from ALI-cultured human airway epithelial cells and from alveolar macrophages using the RNeasy Protect Mini Kit (Qiagen, Valencia, CA), subsequently treated with DNase (DNase I Amplification Grade; Invitrogen, Carlsbad, CA), and precipitated with ethanol. The quality of isolated RNA was confirmed with a NanoDrop (Thermo Fisher Scientific Inc., Waltham, MA) using an acceptable range of the 260:280 optical density ratio of between 1.9 and 2.0. Reverse transcription was done using the iScript cDNA synthesis kit (Bio-Rad Laboratories, Inc., Hercules, CA) with 1 μg of RNA according to the manufacturer's instructions.
Real-time quantitative PCR (qPCR) was performed using the IQ SYBR green supermix and the iCycler thermocycler iQ multicolor detection system (Bio-Rad Laboratories). Primer pairs were designed using BeaconDesigner (Premier Biosoft, Palo Alto, CA). The following primer sequences were used for PANX1, PANX2, and glyceraldehyde 3-phosphate dehydrogenase, respectively: forward, AGA GCG AGT CTG GAA ACC, reverse, CAA GTC TGA GCA AAT ATG AGG; forward, AAG CAG ATC CAG TCC AAG, reverse, GGG CTC TTC TCC TTC TCC; and forward, TGG TCT CCT CTG ACT TCA ACA G, reverse, TGC TGT AGC CAA ATT CGT TGT C. qPCR reactions were performed in a total reaction volume of 50 μl, containing 1 μl of template, 25 μl of 2× iQ SYBR green supermix (Bio-Rad Laboratories), 250 nM primers, and ddH2O. Thermal conditions were 95°C for 3 minutes, followed by 40 cycles of denaturation at 94°C for 15 seconds, annealing at 57°C for 30 seconds, and extension at 72°C for 30 seconds. In each qPCR run, a nontemplate control was included to monitor possible contamination. Data were evaluated using the iQ 5 software (Bio-Rad Laboratories).
qPCR for PANX3 mRNA was done using TaqMan primers (assay ID Hs00364808_m1; Applied Biosystems, Foster City, CA).
Immunohistochemistry
For immunohistochemistry, 8- to 10-μm paraffin-embedded sections were deparaffinized with 100% xylene and rehydrated through a graded alcohol series. After rinsing in PBS with 0.5% Tween-20 (PBS-T), the sections were heated to 85°C in 10 mM sodium citrate buffer (pH 6.0) for 25 minutes for antigen retrieval. Sections were allowed to cool in the citrate buffer at room temperature for 25 minutes before washing in PBS-T. Nonspecific labeling was blocked with preincubation serum (1:10 BlockHen in PBS-T) for 1 hour at room temperature (Aves Labs, Tigard, OR). The slides were then incubated with either nonimmune chicken serum or chicken α-PANX1 (#4515), diluted 1:175 in BlockHen:PBS-T (1:100) for 18 hours at 4°C. This antibody has been previously characterized to be specific for Panx1 (27), and is a polyclonal chicken immunoglobulin raised against the amino acid residues EKNSRQRLLNPS in the human Panx1 C terminus (27). The specificity of this antibody was characterized by Western blotting and immunohistochemistry in oocytes (27) and brain from Panx1 knockout mice (28).
After thorough washing with PBS-T, the sections were incubated with fluorescein-labeled goat α-chicken IgY (Aves Labs), at 1:1,000 for 1 hour at room temperature. Tissues were mounted in UltraCruz mounting media (Santa Cruz Biotechnology Inc., Santa Cruz, CA). Photomicrographs were examined on a Leica DMIRB epifluorescent inverted microscope (Leica, Bannockburn, IL) at the Analytical Imaging Core, University of Miami.
For immunolabeling of ALI cultures for confocal imaging, filters were washed three times with PBS and fixed in 4% paraformaldehyde (PFA) for 1 hour. After fixing, filters were washed with PBS before permeabilization in 100% MeOH for 5 minutes at −20°C. Next, filters were treated three times with 5 mg/ml sodium borohydride. Filters were washed three times with PBS before blocking in 1% BSA for 1 hour. Finally, filters were treated with anti-PANX1 (1:100) overnight at 4°C, followed by fluorescein-labeled goat α-chicken IgY (Aves Labs), at 1:1,000 for 1 hours. Sections were also probed with antiacetylated tubulin (1:10,000) for 1 hour at room temperature. Images were taken on a Zeiss LSM 510 confocal microscope (Zeiss, Thornwood, NY) at the Analytical Imaging Core, University of Miami.
Western Blotting
ALI-cultured human airway epithelial cells and alveolar macrophages were solubilized in RIPA buffer containing protease inhibitors, and briefly cleared from debris by centrifugation. Protein yield was measured by the BCA assay (Pierce, Rockford, IL). Proteins were separated on a 4–20% precast Ready Gel (Bio-Rad Laboratories) and electroblotted onto Immobilon-P membranes (Millipore, Billerica, MA). Load in each lane was 25 μg protein. Membranes were blocked with 1% gelatin in Tris-buffered saline (pH 7.4) with 0.05% Tween-20 (TTBS) for 1 hour at 4°C. Primary antibody was a rabbit anti-Panx1 antibody raised against a synthetic peptide derived from the C terminus of the mouse Panx1 protein (catalog no. 488,100; Zymed Laboratories, San Francisco, CA) and used at 1 μg/ml in 1% gelatin-supplemented TTBS for 1 hour at room temperature. Secondary antibody was an anti-rabbit, horseradish peroxidase–linked antibody (catalog no. 7074; Cell Signaling, Danvers, MA) used at 1:2,000 in TTBS for 1 hour at room temperature. Positive signals were visualized by chemiluminescence on a ChemiDoc XRS system (Bio-Rad Laboratories) and quantified using the QuantityOne software (Bio-Rad Laboratories).
Hypotonic Stress–Induced ATP Release Assays
Culture media in the basolateral compartment were replaced with media containing treatment or vehicle, and the culture was allowed to equilibrate for 2.5 hours at 37°C. Media were removed and the filters transferred to a Turner TD 20/20 luminometer (Turner Biosystems, Sunnyvale, CA), where 26 μl of a luciferin (150 μM) and luciferase (2.0 μg) solution, containing vehicle (0.01% DMSO or control) or treatment (100 μM flufenamic acid in DMSO, 10 μM carbenoxolone [CBX] in DMSO, or 1 mM probenecid), was gently added to the apical compartment. The used concentrations of these drugs did not influence the ATP measurements, and were additionally controlled by using comparison solutions with the same solvents (CBX versus flufenamic acid [FFA], probenecid versus no-solvent control). The culture was allowed to equilibrate for 10 minutes to re-establish basal conditions, at which point (Time 0), 15 μl of a hypotonic solution (H2O with 1 mM CaCl2 and 1 mM MgCl2), also containing vehicle or treatment, was gently added to the apical compartment. This addition of 15 μl to the 26 μl already present on the apical surface caused a hypotonic stress due to the solution now being 67% of normal osmolality (8). ATP was subsequently recorded in arbitrary light units (ALU) every 0.2 seconds for 5 minutes. ATP concentrations were estimated using standard curves with known ATP concentrations.
Electrophysiology
RNA for mouse Panx1 was prepared using the mMessage mMachine in vitro transcription kit (Invitrogen) (20). Oocytes were injected with 20–40 nl mRNA (1 μg/μl) and incubated for 18–48 hours at 18°C. Oocytes were tested using a two-electrode voltage clamp (Model OC725C; Warner Instruments, Hamden, CT) under constant perfusion according to the protocols described in the legend of Figure 5. Membrane conductance was determined using voltage pulses. Oocytes expressing Panx1 were held at −50 mV, and pulses to +50 mV were applied to transiently open the channels.
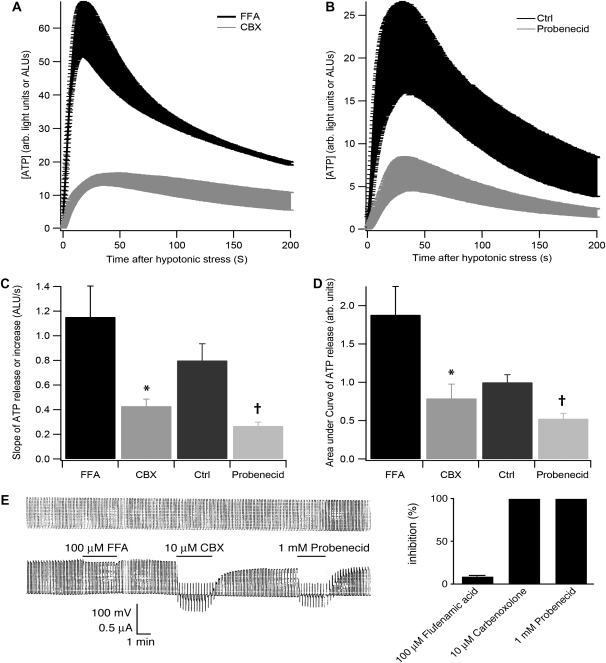
Figure 5.
ATP release by airway epithelial cells in response to hypotonic stress is decreased by Panx inhibitors. (A) ATP release in response to hypotonic stress is shown in the presence of 100 μM flufenamic acid (FFA) (black) or 10 μM carbenoxolone (CBX) (gray) with equal amounts of DMSO (solvent). CBX inhibits ATP release compared with FFA. Shown are data from triplicate measurements from one lung as mean (±SEM). (B) ATP release in response to hypotonic stress is shown in the absence (black) or presence of 1 mM probenecid (gray). Probenecid inhibits ATP release compared with control. Shown are data from triplicate measurements from one lung as mean (±SEM) Quantitative analysis of (C) slope of ATP release during the first 20 seconds and (D) area under curve (AUC) of ATP release for the first 200 seconds after hypotonic shock of differentiated airway epithelial cells in the absence or presence of FFA (n = 16 from at least four lungs), CBX (n = 18 from at least four lungs), or probenecid (n = 18 from at least four lungs). *Significant difference of CBX compared with FFA; †significant difference of probenecid to control. (E) Electrophysiology of Panx channels expressed in oocytes (see Materials and Methods) to confirm adequacy of inhibitors. Left panel: upper trace shows voltage pulses (from −50 to +50 mV) to open the channels, seen in the lower trace as current. Whereas 10 μM CBX (in DMSO) and 1 mM probenecid inhibited channel opening, 100 μM FFA (in DMSO) had a marginal effect. Right panel: quantification of triplicate measurements.
shRNA Lentiviruses
Five proviral plasmids (pLKO.1) with different anti-PANX1 shRNA sequences were purchased from Open Biosystems, Inc. (Huntsville, AL). The pLKO.1 lentiviral vector contains a selectable marker for resistance to puromycin. Replication-deficient lentiviruses were prepared by cotransfecting vector and packaging DNAs, pMDLg/pRRE#54 pRSV-Rev and pMDLgVSVG, into HEK 293T cells by calcium phosphate coprecipitation, as previously described (25). Viruses were collected daily for 3 days beginning 24 hours after removing the precipitates. Viruses were concentrated by precipitation with the addition of polyethylene glycol to 11% and centrifugation. Virus titers were estimated by measuring p24 by ELISA (Perkin-Elmer, Shelton, CT).
Undifferentiated normal human bronchial epithelial (NHBE) cells were infected as previously described (25). After infection, the cells were fed bronchial epithelial growth medium (BEGM) for 24 hours and then BEGM containing 1 μg/ml puromycin to select for infected cells.
Statistical Analysis
Results were compared by one-way ANOVA and, if a significant difference was found, by the Tukey-Kramer honestly significant difference test using JMP software (SAS Institute, Cary, NC) for multiple groups or a t test for single pairs. A P value of less than 0.05 was accepted as significant. Data are given as mean values (±SEM).
RESULTS
Panx Is Expressed in ALI Cultures and the Native Superficial Airway Epithelium
Panx1, -2, and -3 expression was examined by quantitative RT-PCR in a Bio-Rad iCycler iQ thermocycler and detection system using RNA from NHBE ALI cultures and primers designed with BeaconDesigner (Premier Biosoft) or TaqMan kits, as described in Materials and Methods. Denaturation curves and agarose gel electrophoresis confirmed that single products of the predicted sizes were amplified. Panx1 was expressed in all cultures examined (from nine individuals, each in triplicate; Figure 1A). There was no significant difference of expression between cells from smokers (n = 4) and nonsmokers (n = 5; P > 0.05; Figure 1). Panx1 expression was present in nondifferentiated cells plated on Transwell membranes 1 day after switching to ALI culture, and no significant changes in mRNA expression occurred throughout differentiation (Figure 1B), indicating that these genes are constitutively expressed. Even though panx2 does not form channels by itself (29), and the evidence on panx3 is mixed, with channel formation found only in cells overexpressing panx3 (30, 31), primary human airway epithelial cells were also found to express low levels of panx2 (Figure 1A), but not panx3 mRNA (data not shown).
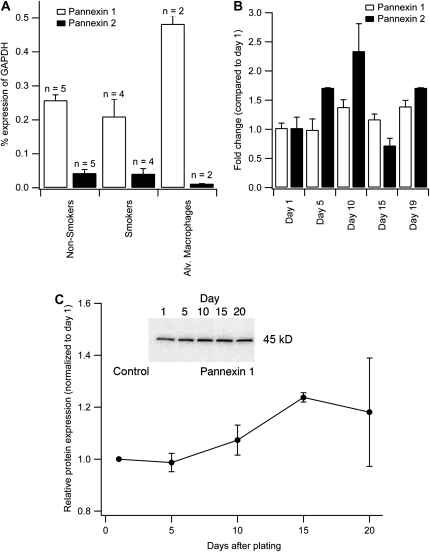
Figure 1.
Measurements of pannexin (Panx) 1 and 2 mRNA expression by quantitative RT-PCR. (A) Comparisons of Panx1 and -2 mRNA in airway epithelial cells cultured at an air–liquid interface (ALI) to full differentiation from nine different donors (five nonsmokers and four smokers) and alveolar macrophages (numbers above bar indicate number of experiments). mRNA amount is expressed as a percent of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) expression. There is no significant difference in mRNA levels between nonsmokers and smokers when cells are cultured at the ALI. Panx1 expression in alveolar macrophages is about twice as high as in airway epithelia cells (P < 0.05). Panx3 is not detected (data not shown). (B) Panx1 and -2 mRNA expression during differentiation of normal human bronchial epithelial cells from Day 1 on air to full differentiation (Day 19). The data are corrected for GAPDH expression and Day 1 results served as the reference. Values are averages of triplicate cultures from a single nonsmoking donor. (C) Western blot analysis of panx1 expression levels of airway epithelial cells during differentiation at the ALI (cultures from three donors), normalized to Day 1 on air. Panx1 protein expression increases during differentiation. Inserts show Western blots with positive band at the expected 45-kD size; ‘Control’ indicates nonimmune serum to show specificity of used antibody.
Alveolar macrophages also expressed panx1 mRNA, and this expression was about twice that found in human airway epithelial cells, as measured by qPCR. Here, we concentrate on panx1 expression and function in airway epithelial cells. Panx2 was expressed approximately 8- to 10-fold lower than panx1, and at similar levels in human airway epithelial cells and alveolar macrophages.
Panx1 Protein Expression during Airway Cell Differentiation at the ALI
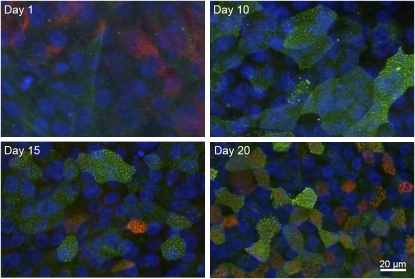
In contrast to the mRNA expression results, Western blot analysis of panx1 expression during airway epithelial cell differentiation at the ALI showed an approximately 20% increase in protein expression during differentiation (three experiments from three different lungs; Figure 1C). This was, however, consistent with the findings of panx1 expression using immunocytochemistry (Figure 2). During airway epithelial cell differentiation, panx1 expression was examined in ALI cultures by immunocytochemistry. Panx1 was diffusely detected in undifferentiated cells on Day 1 after switching the cultures to ALI conditions (Figure 2), but expression increased over time, consistent with the findings in the Western blots (Figure 1). After 10 days at the ALI, Panx1 was clearly detected at the apex (Figure 2), where ciliogenesis occurred, but was present there even before cilia were evident (no ciliary pattern seen with antiacetylated tubulin staining on Day 10).
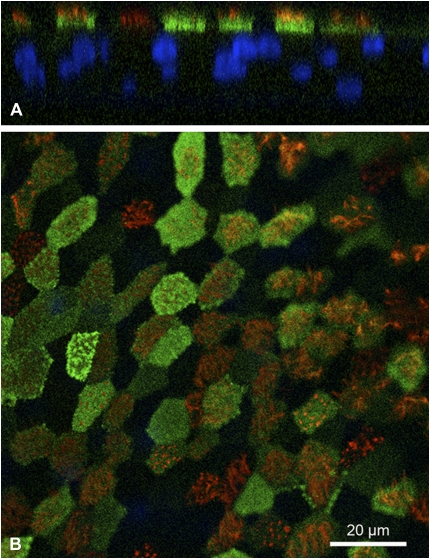
Figure 2.
Panx1 expression during airway epithelial cell differentiation at the ALI. ALI cultures 1, 10, 15, and 20 days after establishing ALI conditions were fixed and stained with anti-Panx1 (green) and antiacetylated tubulin (enriched in cilia, red). Panx1 localizes to the apical pole of cells upon differentiation, but before cilia are present (see Days 10 and 15 in upper right and lower left, respectively).
Localization of Panx1 in Airway Epithelial Cells
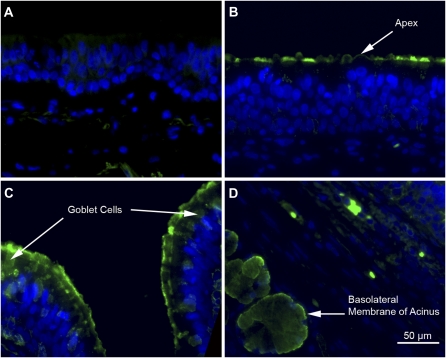
To determine the cellular location of Panx1 in the airway epithelium, immunohistochemistry was done using a chicken antibody against Panx1, previously characterized to be specific (27), and tracheal sections from three lung donors, as well as NHBE cell ALI cultures from the same individuals. Panx1 was observed at the apical pole of airway epithelia in tracheal sections (Figure 3). The expression did not seem restricted to ciliated cells, because goblet cells showed possible Panx1 staining (Figure 3C). In addition, submucosal glands express Panx1 preferentially at the basolateral aspect of the glandular acini (Figure 3D, arrow).
Figure 3.
Immunohistochemistry of Panx1 in tracheal sections. (A) Immunostaining of a human tracheal section using nonimmune IgY control. (B and C) Human tracheal epithelial cells in sections stained for Panx1 using an IgY-anti-Panx antibody (green) showing Panx1 at the apical pole of the airway epithelium in ciliated cells and in goblet cells. Arrows point to the labeled structures. (D) Human submucosal glands in tracheal sections stained with anti-Panx1 in the cytoplasm and basolateral membrane. Arrow indicates glandular acini cells. Also, some erythrocytes are labeled, because they are known to express Panx1. Cell nuclei are in blue (4′,6′-diamidino-2-phenylindole [DAPI]).
Confocal images from NHBE cell ALI cultures also revealed expression of Panx1 at the apical membrane (Figures 4A and 4B). Although most ciliated cells showed expression of Panx1 at the apical membrane (just below ciliary axonemes stained with antiacetylated tubulin in red), expression was not limited to these cells.
Figure 4.
Immunostaining for Panx1 expression in ALI cultures. (A and B) Confocal microscopy images of ALI cultures stained for Panx1 (green; IgY against Panx1), cilia (red; antiacetylated tubulin), and nuclei (blue; DAPI). Panx1 localizes to the apical pole of most ciliated cells. (A) Z axis reconstruction; (B) xy plane through apex.
The expression of panx1 at the apex upon differentiation makes pannexons suitable candidates for ATP release in airway epithelial cells.
Measurement of ATP Release upon Hypotonic Stress
Convincing evidence shows that pannexons mediate ATP release in several other cell types, including erythrocytes (20, 21, 27, 32). To examine whether pannexons are involved in ATP release from airway epithelial cell cultures, ALI cultures were exposed to hypotonic stress (200 mosM), as previously described (8). ATP release was measured in real time in the apical compartment of 24-mm diameter Transwell cultures with luciferin/luciferase in a luminometer (8). After initially adding inhibitors of ATP metabolizing enzymes, including 300 μM β-γ-methylene-ATP, 30 μM ebselen, and 10 mM levamisole (8), we abandoned the practice, as we did not observe any differences in the results with or without inhibitors (except that the sensitivity of the assay decreased with inhibitors present). Standard curves were created using known ATP concentrations. Hypotonic stress induced maximal ATP release within less than 1 minute after challenge, with a time course similar to the one described by others (8). The maximal ATP concentration reached was estimated at 255 (±64) nM using ATP standards (n = 6 ALI cultures from three different lungs). If the dilution of the added fluid to the apical surface is taken into account, this would translate to approximately 1.5 (±0.4) μM in a “normal” airway surface liquid volume (33).
Evaluation of Pannexon Inhibitors on ATP Release upon Hypotonic Stress
To assess whether or not pannexon inhibitors influence ATP release from cells fully differentiated at the ALI, experiments using hypotonic stress were repeated in the presence or absence of the pannexon inhibitors, CBX (31) and probenecid (34). Simple hypotonic stress was used as a control for probenecid (NaOH soluble and buffered), while FFA in DMSO was used as a control for CBX (soluble in DMSO). FFA is a good control here, because it exerts only a modest inhibitory effect on pannexons in contrast to CBX (31, 35). To analyze the curves, we calculated the slope of ATP increase over the first 20 seconds of hypotonic stress as an indication of the initial speed of ATP release, as well as the area under curve (AUC) for the first 200 seconds as a measure of overall ATP increase, similar to previous analyses (35).
In comparison to FFA, CBX significantly inhibited ATP release (Figure 5). The same was true for probenecid in comparison to control (Figure 5). The slope of ATP release and the AUC in response to hypotonic stress (the latter normalized to control) was 1.15 (±0.25) ALU/s (mean ± SE) and 1.8 (±0.4) arbitrary units, respectively (n = 16 from at least four different lungs). In contrast, both values were significantly reduced by 10 μM CBX, to 0.43 (±0.06) ALU/s for the slope and 0.79 (±0.2) arbitrary units for the AUC (n = 18 from at least four different lungs; for both values, P compared with FFA < 0.05). Probenecid (n = 18 from at least four different lungs; 1 mM) reduced the slope of ATP release from a control value of 0.80 (±0.14) to 0.27 (±0.03) ALU/s (P < 0.05), and the AUC from a control value of 1 (±0.14) (normalized) to 0.5 (±0.1) arbitrary units (P < 0.05).
To assure the adequacy of these inhibitors for pannexons formed by panx1, Xenopus oocytes expressing panx1 were used and panx1-related currents measured during application of appropriate concentrations of FFA, CBX, and probenecid in the whole cell patch configuration. Figure 5E shows that 100 μM FFA had no significant influence on pannexon-related currents, whereas both 10 μM CBX and 1 mM probenecid inhibited the channels almost completely.
Panx1 Knockdown with shRNA Reduces Panx1 Expression and ATP Release upon Hypotonic Stress
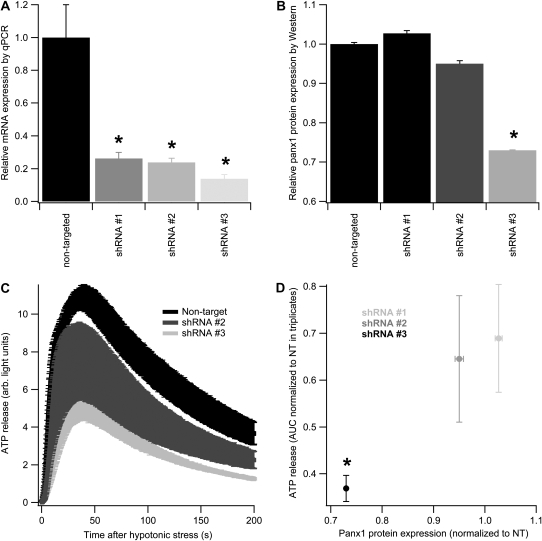
To confirm the results with inhibitors that suggested a role for Panx1 in hypotonic stress–induced ATP release, shRNA-expressing lentiviruses were used to specifically reduce Panx1 expression in airway epithelial cells. Five human immunodeficiency virus–pseudotyped lentiviruses expressing five different shRNAs targeted at Panx1 with a selectable puromycin resistance gene were evaluated for mRNA knockdown. Nondifferentiated cells were infected with individual shRNA-expressing lentiviruses, redifferentiated in the presence of puromycin, and tested for Panx1 mRNA (triplicate cultures for quantitative RT-PCR). Three of the lentivirus constructs showed significant knockdown, with one shRNA construct reducing Panx1 mRNA expression consistently to less than 15% of the nontargeted control (shRNA #3; Figure 6). By Western blot analysis, however, panx1 protein reduction using the three shRNAs was less dramatic. shRNA #1 did not reduce panx1 protein expression and shRNA #2 reduced it only slightly (95% of nontargeted sequence; duplicate experiments), whereas shRNA #3 reduced protein expression to 73 (±1)% (duplicate experiments) of nontargeted control (Figure 6).
Figure 6.
shRNA-mediated Panx1 knockdown reduces hypotonic stress–induced ATP release. (A) Panx1 mRNA levels in fully differentiated cells infected (in an undifferentiated state) with four different lentiviruses expressing three different shRNA sequences that target Panx1 or a nontargeted sequence. shRNA #3 shows the greatest knockdown of Panx1 mRNA. (B) Panx1 protein expression levels in the same fully differentiated cells as in (A). Only shRNA #3 reveals significant Panx1 protein reduction. (C) ATP release in response to hypotonic stress is shown in cells infected with nontargeted, shRNA #2, and shRNA #3 sequence–expressing lentiviruses (triplicate culture measurements). A significant reduction in ATP release is found in shRNA #3–expressing cells. (D) Relationship of protein expression and ATP release (quantified as AUC as in Figure 5) is shown in cultures expressing shRNA #1, shRNA #2, and shRNA #3 sequence–expressing lentiviruses. All points represent triplicate cultures. shRNA #3 reduces both Panx1 protein expression and ATP release. *P < 0.05.
To assess the relation between panx1 protein knockdown and ATP release, ALI cultures infected with each of these three constructs were also used for ATP release experiments (triplicate experiments from one lung, but from a different individual than any of the inhibitor experiments; Figure 6). Compared with a nontarget control, shRNA #1 reduced the AUC for ATP release from 1,397 (±140) to 962 (±197), shRNA #2 to 902 (±232), and shRNA #3 to 553 (±47) arbitrary units (latter, P < 0.05). The relationships between panx1 protein expression and ATP release for these experiments are shown in Figure 6D.
These results show that knockdown of Panx1 expression in fully differentiated airway epithelial cells using shRNA-expressing lentiviruses also reduces ATP release significantly, and was consistent with the results from the inhibitor experiments. Together, these data support ATP release via Panx1 in airway epithelial cells.
DISCUSSION
The importance of ATP release from airway epithelial cells for the regulation of many cellular functions has been recognized for some time. One of the most important airway host defense mechanisms governed by the airway epithelium is mucociliary clearance. Effective mucociliary clearance depends on many factors: in addition to mucus rheology, among these important factors are adequate airway surface liquid (ASL) volume and adequate ciliary activity. Both ASL volume and ciliary beat frequency are regulated by the paracrine action of ATP (e.g., Refs. 2, 5). In the absence of compensatory cAMP signaling, mucociliary clearance fails if this paracrine ATP system breaks down (33, 36).
Although the exact mechanism of ATP release remains unknown, it is quite likely that multiple mechanisms are involved. We know that airway epithelial cells release ATP upon increases in cytosolic Ca2+ concentration or upon mechanical stress. The evidence for vesicular release is covered in the introduction to this article. In addition, a recent publication also gives credence to the previously assumed release of ATP together with mucins in the airway, likely stemming from goblet cell vesicles (37). Whether the Panx1 expression in goblet cells is related to this phenomenon remains to be explored. However, there seem to be additional mechanisms at play. When focusing on literature describing mechanically activated calcium waves spreading through airway epithelial cell sheets, gap junctional proteins were implicated to play a role for the spread of the wave. Initially, the data were interpreted to mean that a messenger spreads through gap junctions to different cells (38–41). However, other data have surfaced implicating ATP release with paracrine action rather than messenger spreading through gap junction in calcium wave propagation (42), whereas others have reported data supporting both theories (43).
Multiple channels have been studied as candidates for ATP release, and many of them have been rejected as the main contributors, as discussed in the introduction. Given the data on calcium wave spreading through airway epithelia, there is a need to bring together the seemingly disparate results on the regulation of calcium wave propagation using only one paradigm.
Many studies have invoked “hemichannel”-forming Cx43 (channel to the outside of the cell, as opposed to a full gap junction channel between cells) as the mediator of ATP release, at least in experiments dealing with calcium wave propagation (e.g., Refs. 44–46). The key question is whether Cx43 could release ATP by forming channels to the extracellular space, even though the existence of such channels is not well substantiated. Before the discovery of Panx, three key observations led to the conclusion that connexons could be involved in ATP release: (1) ATP release in most cell types is correlated with the uptake of dyes typically used for testing intercellular communication through gap junction channels (45, 47); (2) gap junction blockers can attenuate ATP release (47); and (3) connexin mimetic peptides inhibit ATP release or surrogate measures of it (18, 48). On the other hand, there are weighty arguments against a connexon-mediated ATP release. No authentic connexin (hemichannel to the outside of cells) activity has been documented under physiological conditions. Cx43 hemichannel activity, for example, requires extreme polarization (> +50 mV), which can only be achieved by voltage clamp in a laboratory setting (49). Dye uptake and sensitivity to drugs is shared by connexin gap junction channels and pannexon channels (27, 31). Importantly, connexin mimetic peptides have been demonstrated to affect pannexon channels rather than connexin hemichannels (50, 51). Finally, ATP release can be observed in connexin-free environments. Erythrocytes and taste cells release ATP, but do not express connexins (27, 32). Furthermore, observations that calcium wave propagation in spinal cord astrocytes from Cx43 knockout mice are still mediated by extracellular diffusion of ATP argue against Cx43 connexon as the sole ATP release channel (52). In addition, an inhibitor of Cx43 (FFA; see also below) did not prevent ATP release upon shear stress from airway epithelial cells in media containing normal calcium levels (35). Finally, immunohistochemical studies on fully differentiated human airway epithelia do reveal gap junction formation by CX30 and CX31 between cells (53). Cx43, however, was down-regulated in fully differentiated cells compared with the undifferentiated state. No connexin expression was found at the apical membrane in human airway epithelial cells (53), and the same was true for mouse airway epithelial sections (53). Thus, substantial data exist that strongly argue against a role for connexins in release of ATP.
Erythrocytes release ATP in low-oxygen conditions and upon mechanical stress (54, 55). Erythrocytes express Panx1 at high levels, and a channel activity consistent with Panx1 can be recorded from membrane patches excised from erythrocytes (27). Furthermore, ATP release from erythrocytes can be attenuated by CBX (27). Thus, functions thought to be due to connexins may be provided by Panx instead, especially Panx1 that forms “pannexons” (i.e., channels to the outside of cells [and little, if any, evidence in fact supports formation of junctional channels between cells by Panx; see Refs. 56–58). In addition to being expressed in tissues that reveal ATP release, pannexons are mechanosensitive, gated by Ca2+, and, if open, highly permeable to ATP (20). Unlike connexons, pannexons open at resting membrane potentials in response to mechanical stress (20). When coexpressed with P2Y receptors, Panx1 channels open in response to extracellular ATP that signals via intracellular Ca2+ (21). Application of micromolar Ca2+ to the cytoplasmic side of pannexons in excised membrane patches activates the channels, in contrast to most connexons that are either closed or unaffected by Ca2+ (e.g., Ref. 59).
The data shown here indicate that Panx1 in fact contributes to ATP release in airway epithelial cells. Panx1 is expressed and correctly localized for ATP release, namely at the apex. In addition, CBX and probenecid, but not FFA, inhibited ATP release from airway epithelia upon hypotonic stress, consistent with the finding that FFA, in contrast to CBX and probenecid, exerts only a modest inhibitory effect on pannexons (31). Although CBX only poorly discriminates between connexin and Panx channels, probenecid inhibits Panx1 channels, but not connexin channels (34). Finally, suppressing Panx1 expression in fully differentiated airway epithelial cells using shRNA-expressing lentiviruses also inhibited ATP release upon hypotonic stress. Together, these data make a strong case for the involvement of Panx1 in ATP from airway epithelia. These data are also consistent with those of previous reports indicating that FFA was ineffective in inhibiting ATP release upon shear stress (35).
We also examined panx2 and panx3 expression by mRNA in airway epithelial cells. Panx2 mRNA is expressed at much lower levels than panx1, and, as indicated above, panx2 cannot form a functional channel itself (29). The data on panx3 are still inconclusive, with one article indicating doubt that panx3 itself is functional (31), and one other article showing channel formation when panx3 is overexpressed (30). However, human airway epithelial cells do not express panx3 mRNA, and, thus, this protein does not function as an ATP release channel in these cells.
If we accept that panx1 contributes to ATP release from airway epithelia, the question remains whether its inhibition will have a significant effect on airway epithelial cell function. Although experiments addressing this issue are beyond the scope of this article, the question brings up a previous criticism of ATP release measurements on cell surfaces: many measurements of ATP concentrations on cells indicate concentrations reaching less than the EC50 of approximately 1 μM required for signaling by naturally expressed P2Y2 receptors (e.g., Ref. 60). On the other hand, studies not measuring ATP directly, but evaluating its effects on airway epithelial cell function (e.g., Ref. 36), made a strong case for ATP release being an important paracrine mediator. A recent study showed that the discrepancy is due to the measurement system used and the inability to bring the luciferase close enough to its substrate ATP in sampling studies (8). Using very low measurement volumes or cell-attached luciferase solved the problem, and showed that hypotonic stress–induced ATP release onto airway surfaces can reach ATP concentrations in the micromolar range (8). In fact, we used small volume measurements, shown to yield similar results compared with cell-attached luciferase (8), and our estimations of ATP concentrations are consistent with this report (8): [ATP] in ASL reached > 1 μM. Assessing the contribution of Panx1 to this process then reveals that inhibiting Panx (here, ATP release was reduced by approximately 60% in the presence of Panx inhibitors) will bring this concentration below the threshold of 1 μM. This indicates that adequate Panx1 function may be crucial for maintaining normal airway epithelial cell function during stress.
Finally, other sources of ATP in the airway will have to be assessed. An evaluation of the potential for macrophages to contribute has been attempted here by looking at Panx expression in these cells as well. In fact, panx1 is expressed in alveolar macrophages, at least on an mRNA level. However, the number of macrophages in the airway under normal conditions is low, and, thus, the contribution of these cells to ATP availability will have to await further testing.
Acknowledgments
The authors thank the Analytical Imaging Core at the University of Miami, and Monica Valencia-Gattas for her help with the Western blots.
This work was supported by National Institutes of Health grants HL-60644 and HL-89399 (M.S.), HL-66125 (G.E.C.), GM-48610 (G.D.), and by the Flight Attendant Medical Research Institute.
Originally Published in Press as DOI: 10.1165/rcmb.2008-0367OC on February 12, 2009
Conflict of Interest Statement: M.S. has no financial interest in a commercial entity with an interest in the subject matter of this manuscript; he was on an advisory roundtable for Genentech regarding Pulmozyme. None of the other authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Morse DM, Smullen JL, Davis CW. Differential effects of UTP, ATP, and adenosine on ciliary activity of human nasal epithelial cells. Am J Physiol 2001;280:C1485–C1497. [DOI] [PubMed] [Google Scholar]
- 2.Lieb T, Wijkstrom Frei C, Frohock JI, Bookman RJ, Salathe M. Prolonged increase in ciliary beat frequency after short-term purinergic stimulation in human airway epithelial cells. J Physiol 2002;538:633–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Donaldson SH, Lazarowski ER, Picher M, Knowles MR, Stutts MJ, Boucher RC. Basal nucleotide levels, release, and metabolism in normal and cystic fibrosis airways. Mol Med 2000;6:969–982. [PMC free article] [PubMed] [Google Scholar]
- 4.Lazarowski ER, Tarran R, Grubb BR, van Heusden CA, Okada S, Boucher RC. Nucleotide release provides a mechanism for airway surface liquid homeostasis. J Biol Chem 2004;279:36855–36864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tarran R, Trout L, Donaldson SH, Boucher RC. Soluble mediators, not cilia, determine airway surface liquid volume in normal and cystic fibrosis superficial airway epithelia. J Gen Physiol 2006;127:591–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cobb BR, Ruiz F, King CM, Fortenberry J, Greer H, Kovacs T, Sorscher EJ, Clancy JP. A(2) adenosine receptors regulate CFTR through PKA and PLA(2). Am J Physiol Lung Cell Mol Physiol 2002;282:L12–L25. [DOI] [PubMed] [Google Scholar]
- 7.Huang P, Lazarowski ER, Tarran R, Milgram SL, Boucher RC, Stutts MJ. Compartmentalized autocrine signaling to cystic fibrosis transmembrane conductance regulator at the apical membrane of airway epithelial cells. Proc Natl Acad Sci USA 2001;98:14120–14125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Okada SF, Nicholas RA, Kreda SM, Lazarowski ER, Boucher RC. Physiological regulation of ATP release at the apical surface of human airway epithelia. J Biol Chem 2006;281:22992–23002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Button B, Boucher RC. Role of mechanical stress in regulating airway surface hydration and mucus clearance rates. Respir Physiol Neurobiol 2008;163:189–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lazarowski ER, Boucher RC, Harden TK. Constitutive release of ATP and evidence for major contribution of ecto-nucleotide pyrophosphatase and nucleoside diphosphokinase to extracellular nucleotide concentrations. J Biol Chem 2000;275:31061–31068. [DOI] [PubMed] [Google Scholar]
- 11.Zuo P, Picher M, Okada SF, Lazarowski ER, Button B, Boucher RC, Elston TC. Mathematical model of nucleotide regulation on airway epithelia: implications for airway homeostasis. J Biol Chem 2008;283:26805–26819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lazarowski ER, Shea DA, Boucher RC, Harden TK. Release of cellular UDP-glucose as a potential extracellular signaling molecule. Mol Pharmacol 2003;63:1190–1197. [DOI] [PubMed] [Google Scholar]
- 13.Grygorczyk R, Hanrahan JW. CFTR-independent ATP release from epithelial cells triggered by mechanical stimuli. Am J Physiol 1997;272:C1058–C1066. [DOI] [PubMed] [Google Scholar]
- 14.Grygorczyk R, Tabcharani JA, Hanrahan JW. CFTR channels expressed in CHO cells do not have detectable ATP conductance. J Membr Biol 1996;151:139–148. [DOI] [PubMed] [Google Scholar]
- 15.Okada SF, O'Neal WK, Huang P, Nicholas RA, Ostrowski LE, Craigen WJ, Lazarowski ER, Boucher RC. Voltage-dependent anion channel-1 (VDAC-1) contributes to ATP release and cell volume regulation in murine cells. J Gen Physiol 2004;124:513–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sabirov RZ, Okada Y. Wide nanoscopic pore of maxi-anion channel suits its function as an ATP-conductive pathway. Biophys J 2004;87:1672–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu HT, Toychiev AH, Takahashi N, Sabirov RZ, Okada Y. Maxi-anion channel as a candidate pathway for osmosensitive ATP release from mouse astrocytes in primary culture. Cell Res 2008;18:558–565. [DOI] [PubMed] [Google Scholar]
- 18.Evans WH, De Vuyst E, Leybaert L. The gap junction cellular internet: connexin hemichannels enter the signalling limelight. Biochem J 2006;397:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stout C, Goodenough DA, Paul DL. Connexins: functions without junctions. Curr Opin Cell Biol 2004;16:507–512. [DOI] [PubMed] [Google Scholar]
- 20.Bao L, Locovei S, Dahl G. Pannexin membrane channels are mechanosensitive conduits for ATP. FEBS Lett 2004;572:65–68. [DOI] [PubMed] [Google Scholar]
- 21.Locovei S, Wang J, Dahl G. Activation of pannexin 1 channels by ATP through P2Y receptors and by cytoplasmic calcium. FEBS Lett 2006;580:239–244. [DOI] [PubMed] [Google Scholar]
- 22.Bernacki SH, Nelson AL, Abdullah L, Sheehan JK, Harris A, William Davis C, Randell SH. Mucin gene expression during differentiation of human airway epithelia in vitro: muc4 and muc5b are strongly induced. Am J Respir Cell Mol Biol 1999;20:595–604. [DOI] [PubMed] [Google Scholar]
- 23.Nlend MC, Bookman RJ, Conner GE, Salathe M. Regulator of G-protein signaling protein 2 modulates purinergic calcium and ciliary beat frequency responses in airway epithelia. Am J Respir Cell Mol Biol 2002;27:436–445. [DOI] [PubMed] [Google Scholar]
- 24.Fragoso MA, Fernandez V, Forteza R, Randell SH, Salathe M, Conner GE. Transcellular thiocyanate transport by human airway epithelia. J Physiol 2004;561:183–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schmid A, Bai G, Schmid N, Zaccolo M, Ostrowski L, Conner G, Fregien N, Salathe M. Real-time analysis of cAMP-mediated regulation of ciliary motility in single primary human airway epithelial cells. J Cell Sci 2006;119:4176–4186. [DOI] [PubMed] [Google Scholar]
- 26.Mackenzie AB, Chirakkal H, North RA. Kv1.3 potassium channels in human alveolar macrophages. Am J Physiol Lung Cell Mol Physiol 2003;285:L862–L868. [DOI] [PubMed] [Google Scholar]
- 27.Locovei S, Bao L, Dahl G. Pannexin 1 in erythrocytes: function without a gap. Proc Natl Acad Sci USA 2006;103:7655–7659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zoidl G, Petrasch-Parwez E, Ray A, Meier C, Bunse S, Habbes HW, Dahl G, Dermietzel R. Localization of the pannexin1 protein at postsynaptic sites in the cerebral cortex and hippocampus. Neuroscience 2007;146:9–16. [DOI] [PubMed] [Google Scholar]
- 29.Bruzzone R, Hormuzdi SG, Barbe MT, Herb A, Monyer H. Pannexins, a family of gap junction proteins expressed in brain. Proc Natl Acad Sci USA 2003;100:13644–13649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Penuela S, Bhalla R, Gong XQ, Cowan KN, Celetti SJ, Cowan BJ, Bai D, Shao Q, Laird DW. Pannexin 1 and pannexin 3 are glycoproteins that exhibit many distinct characteristics from the connexin family of gap junction proteins. J Cell Sci 2007;120:3772–3783. [DOI] [PubMed] [Google Scholar]
- 31.Bruzzone R, Barbe MT, Jakob NJ, Monyer H. Pharmacological properties of homomeric and heteromeric pannexin hemichannels expressed in Xenopus oocytes. J Neurochem 2005;92:1033–1043. [DOI] [PubMed] [Google Scholar]
- 32.Huang YJ, Maruyama Y, Dvoryanchikov G, Pereira E, Chaudhari N, Roper SD. The role of pannexin 1 hemichannels in ATP release and cell–cell communication in mouse taste buds. Proc Natl Acad Sci USA 2007;104:6436–6441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tarran R, Button B, Boucher RC. Regulation of normal and cystic fibrosis airway surface liquid volume by phasic shear stress. Annu Rev Physiol 2006;68:543–561. [DOI] [PubMed] [Google Scholar]
- 34.Silverman W, Locovei S, Dahl GP. Probenecid, a gout remedy, inhibits pannexin 1 channels. Am J Physiol Cell Physiol 2008;295:C761–C767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guyot A, Hanrahan JW. ATP release from human airway epithelial cells studied using a capillary cell culture system. J Physiol 2002;545:199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tarran R, Button B, Picher M, Paradiso AM, Ribeiro CM, Lazarowski ER, Zhang L, Collins PL, Pickles RJ, Fredberg JJ, et al. Normal and cystic fibrosis airway surface liquid homeostasis: the effects of phasic shear stress and viral infections. J Biol Chem 2005;280:35751–35759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kreda SM, Okada SF, van Heusden CA, O'Neal W, Gabriel S, Abdullah L, Davis CW, Boucher RC, Lazarowski ER. Coordinated release of nucleotides and mucin from human airway epithelial Calu-3 cells. J Physiol 2007;584:245–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hansen M, Boitano S, Dirksen ER, Sanderson MJ. Intercellular calcium signaling induced by extracellular adenosine 5′-triphosphate and mechanical stimulation in airway epithelial cells. J Cell Sci 1993;106:995–1004. [DOI] [PubMed] [Google Scholar]
- 39.Sanderson MJ, Charles AC, Dirksen ER. Mechanical stimulation and intercellular communication increase intracellular calcium in epithelial cells. Cell Regul 1990;1:585–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boitano S, Dirksen ER, Sanderson MJ. Intercellular propagation of calcium waves mediated by inositol triphosphate. Science 1992;258:292–295. [DOI] [PubMed] [Google Scholar]
- 41.Boitano S, Dirksen ER, Evans WH. Sequence-specific antibodies to connexins block intercellular calcium signaling through gap junctions. Cell Calcium 1998;23:1–9. [DOI] [PubMed] [Google Scholar]
- 42.Homolya L, Steinberg TH, Boucher RC. Cell to cell communication in response to mechanical stress via bilateral release of ATP and UTP in polarized epithelia. J Cell Biol 2000;150:1349–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Isakson BE, Evans WH, Boitano S. Intercellular Ca2+ signaling in alveolar epithelial cells through gap junctions and by extracellular ATP. Am J Physiol Lung Cell Mol Physiol 2001;280:L221–L228. [DOI] [PubMed] [Google Scholar]
- 44.Cotrina ML, Lin JH, Lopez-Garcia JC, Naus CC, Nedergaard M. ATP-mediated glia signaling. J Neurosci 2000;20:2835–2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stout CE, Costantin JL, Naus CC, Charles AC. Intercellular calcium signaling in astrocytes via ATP release through connexin hemichannels. J Biol Chem 2002;277:10482–10488. [DOI] [PubMed] [Google Scholar]
- 46.Cotrina ML, Lin JH, Alves-Rodrigues A, Liu S, Li J, Azmi-Ghadimi H, Kang J, Naus CC, Nedergaard M. Connexins regulate calcium signaling by controlling ATP release. Proc Natl Acad Sci USA 1998;95:15735–15740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Braet K, Aspeslagh S, Vandamme W, Willecke K, Martin PE, Evans WH, Leybaert L. Pharmacological sensitivity of ATP release triggered by photoliberation of inositol-1,4,5-trisphosphate and zero extracellular calcium in brain endothelial cells. J Cell Physiol 2003;197:205–213. [DOI] [PubMed] [Google Scholar]
- 48.De Vuyst E, Decrock E, Cabooter L, Dubyak GR, Naus CC, Evans WH, Leybaert L. Intracellular calcium changes trigger connexin 32 hemichannel opening. EMBO J 2006;25:34–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Contreras JE, Saez JC, Bukauskas FF, Bennett MV. Gating and regulation of connexin 43 (Cx43) hemichannels. Proc Natl Acad Sci USA 2003;100:11388–11393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang J, Ma M, Locovei S, Keane RW, Dahl G. Modulation of membrane channel currents by gap junction protein mimetic peptides: size matters. Am J Physiol Cell Physiol 2007;293:C1112–C1119. [DOI] [PubMed] [Google Scholar]
- 51.Dahl G. Gap junction–mimetic peptides do work, but in unexpected ways. Cell Commun Adhes 2007;14:259–264. [DOI] [PubMed] [Google Scholar]
- 52.Scemes E, Suadicani SO, Spray DC. Intercellular communication in spinal cord astrocytes: fine tuning between gap junctions and P2 nucleotide receptors in calcium wave propagation. J Neurosci 2000;20:1435–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wiszniewski L, Sanz J, Scerri I, Gasparotto E, Dudez T, Lacroix JS, Suter S, Gallati S, Chanson M. Functional expression of connexin30 and connexin31 in the polarized human airway epithelium. Differentiation 2007;75:382–392. [DOI] [PubMed] [Google Scholar]
- 54.Bergfeld GR, Forrester T. Release of ATP from human erythrocytes in response to a brief period of hypoxia and hypercapnia. Cardiovasc Res 1992;26:40–47. [DOI] [PubMed] [Google Scholar]
- 55.Sprague RS, Ellsworth ML, Stephenson AH, Kleinhenz ME, Lonigro AJ. Deformation-induced ATP release from red blood cells requires CFTR activity. Am J Physiol 1998;275:H1726–H1732. [DOI] [PubMed] [Google Scholar]
- 56.Dahl G, Locovei S. Pannexin: to gap or not to gap, is that a question? IUBMB Life 2006;58:409–419. [DOI] [PubMed] [Google Scholar]
- 57.Boassa D, Ambrosi C, Qiu F, Dahl G, Gaietta G, Sosinsky G. Pannexin1 channels contain a glycosylation site that targets the hexamer to the plasma membrane. J Biol Chem 2007;282:31733–31743. [DOI] [PubMed] [Google Scholar]
- 58.Boassa D, Qiu F, Dahl G, Sosinsky G. Trafficking dynamics of glycosylated pannexin 1 proteins. Cell Commun Adhes 2008;15:119–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Loewenstein WR. Junctional intercellular communication: the cell-to-cell membrane channel. Physiol Rev 1981;61:829–913. [DOI] [PubMed] [Google Scholar]
- 60.Mason SJ, Paradiso AM, Boucher RC. Regulation of transepithelial ion transport and intracellular calcium by extracellular ATP in human normal and cystic fibrosis airway epithelium. Br J Pharmacol 1991;103:1649–1656. [DOI] [PMC free article] [PubMed] [Google Scholar]