Abstract
Genetic variation can be beneficial to one sex yet harmful when expressed in the other - a condition referred to as sexual antagonism. Because X chromosomes are transmitted from fathers to daughters, and sexually antagonistic fitness variation is predicted to often be X-linked, mates of relatively low-fitness males might produce high-fitness daughters, while mates of high-fitness males produce low-fitness daughters. Such fitness consequences have been predicted to influence the evolution of female mating biases and the offspring sex ratio. Females might evolve to prefer mates that provide good genes for daughters or might adjust offspring sex ratios in favor of the sex with the highest relative fitness. We test these possibilities in a lab-adapted population of Drosophila melanogaster, and find that females preferentially mate with males carrying genes that are deleterious for daughters. Preferred males produce equal numbers of sons and daughters, whereas unpreferred males produce female-biased sex ratios. As a consequence, mean offspring fitness of unpreferred males is higher than offspring fitness of preferred males. This observation has several interesting implications for sexual selection and the maintenance of population genetic variation for fitness.
The presence of sexually antagonistic variation - where alleles increasing male fitness are deleterious when expressed in female genomes - poses a dilemma for the evolution of female choice (Chippindale et al. 2001). Females face a tradeoff of producing relatively fit sons and unfit daughters, or relatively fit daughters and unfit sons. If sexually antagonistic variation is sufficiently abundant and X-linked, as suggested by theory and data (Rice 1984; Gibson et al. 2002; Pischedda & Chippindale 2006), there will be no benefit of mating with relatively fit males, but there can be a cost associated with the production of low-fitness daughters.
Two possible evolutionary responses are predicted when sexually antagonistic fitness variation predominates, and has a strong X-linked component. Albert & Otto (2005) argued that female mating biases will evolve to favor males providing good genes to daughters. Female choice might therefore reverse the direction of selection acting on males and resolve the sexual antagonism. Calsbeek & Sinervo (2004) proposed an alternative response, that females should modify the sex ratio of their offspring in order to minimize indirect costs of mating with relatively high-fitness males.
The fruit fly Drosophila melanogaster is a suitable species for testing these hypotheses. Lab-adapted populations of flies, where the environmental context of adaptation is known, are amenable to the measurement of traits closely associated with fitness. Previous studies show that sexually antagonistic genetic variation influences adult fitness variation (Chippindale et al. 2001; Long & Rice 2007), and much of this variation appears to be X-linked (Gibson et al. 2002; Pischedda & Chippindale 2006). Furthermore, females can adjust the sex ratio of their offspring (Mange 1970; Long & Pischedda 2005; Fuller & Mousseau 2007), suggesting that adaptive sex ratio adjustment with respect to sexually antagonistic variation is at least possible.
We conducted experiments using a lab-adapted population of fruit flies to address two questions:
Do female mating biases lead to sexually antagonistic fitness consequences for offspring?
Is the offspring sex ratio skewed in favor of the sex with the highest relative fitness?
METHODS
Drosophila stocks
Female choice and adult fitness components were estimated using the IV population, a lab-adapted population of Drosophila melanogaster that is described in Houle & Rowe (2003). The IV population and the competitor population, IVe - a lab-adapted population that is homozygous for the ebony mutation - were kindly provided by David Houle.
Mate choice trials
Female mating biases were ascertained by two approaches. A series of tournament-style mating trials were used to identify males differing in mating success (Fig. S1, Supplementary Material). Trials used two- and three-day-old virgin males and females, sampled from the IV population. Each trial was conducted with a pair of males and a single virgin female. The first male to successfully mate was designated as the winner. Winners or losers from each round of trials were arbitrarily paired with each other and retested. Males that lost or won three successive trials were used for sex ratio and offspring fitness assays.
Male-male competition, or intra-sexual selection, might influence the outcome of the tournament-style assays, and could potentially override female mating biases. To test whether females preferentially mate with males that perform well in the tournament setting, we conducted a series of female mating latency experiments. A single male and virgin female were placed in a vial and observed until copulation occurred. The relative success of each male was estimated by the time required for him to mate. Successful males from the tournament trials were able to achieve copulation more quickly than unsuccessful males (Fig. S2, Supplementary Material), indicating that the tournament trials capture information about female mating biases and are not driven by male-male competition. Females bias matings towards relatively successful (3 wins, 0 losses) and away from relatively unsuccessful (3 losses, 0 wins) males. Such males are hereafter referred to as “preferred” and “unpreferred”, respectively.
Offspring Sex Ratio & Adult Fitness Components
During the day after mating success trials were concluded, preferred and unpreferred males were assigned at random to virgin females from the IV population. Previous research using D. melanogaster indicates that male fertility quickly recovers during a lag period of this duration (Markow et al. 1978), and thus, seminal fluid limitation should not adversely affect the mates of preferred males. Mates of experimental males were then permitted to lay eggs in vials for 12 hours. From these eggs, sets of 40 to 50 eggs were transferred to 8 ounce bottles, each containing standard cornmeal medium, 20 adult ebony females, and 20 ebony males. This is a typical adult density for IV and IVe flies, creating a typical larval environment for this lab-adapted population. The relatively low density of introduced eggs per bottle also minimizes interactions between the experimental offspring.
Offspring sex ratio was examined in three independent experimental trials. The first two trials each followed offspring of 40 preferred and 40 unpreferred males. The third trial followed offspring of 70 preferred and 70 unpreferred males. Offspring from the third trial were used for fitness assays. Adult offspring were collected on the 14th day, consistent with the evolutionary history of the population, which has been continuously reared on a 14-day generation cycle since 1975, representing over 800 generations of adaptation to the lab environment (Houle & Rowe 2003).
Sex-spefic selection in Drosophila melanogaster may influence the evolution of juvenile growth traits (Prasad et al. 2007). Juvenile growth differences can therefore potentially underlie sexually antagonistic fitness effects that manifest at the adult stage. However, juvenile sex-specific selection does not appear to give rise to sexually antagonistic viability selection. Indeed, there is a strong, positive intersexual genetic correlation for Drosophila juvenile viability (e.g. Chippindale et al. 2001). Since our major concern here is with sexual antagonism, the results focus on adult fitness-related traits - female fecundity and male mating success - that are potentially influenced by sexually antagonistic variation. However, the overall conclusions do not rely upon an emphasis on adult-stage fitness. Estimates of egg-to-adult viability for preferred and unpreferred males reveal no mortality differences between treatments (unpreferred offspring: n = 1248 eggs, 70.8 % survival; preferred offspring: n = 1082 eggs, 69.8 % survival; two-tailed Fisher exact test p = 0.82).
Adult-stage female fitness was estimated by the number of eggs produced on the 14th day of the life cycle. Female offspring were placed in pairs, along with two randomly assigned ebony males, into vials containing standard cornmeal medium and were permitted to lay eggs for 24 hours. Houle & Rowe (2003) previously showed that this is the critical time period during which egg laying contributes to adult female fitness.
Male fitness was estimated by mating success experiments, conducted during the 14th day of the life cycle. Male offspring were individually transferred to mate competition vials, each containing a randomly selected (and unrelated) male and female. These females and competitor males were each heterozygous for an ebony allele, and expressed the wild-type pigmentation pattern. Each competition vial was observed until the female mated with one of the males. The female was then isolated and permitted to lay eggs in a fresh vial. Paternity was assigned 14 days later by the presence or absence of ebony-expressing offspring. This measure of male fitness eliminates postcopulatory sexual selection, but is not expected to bias the results because precopulatory mating success is positively correlated with sperm competition success (Bangham et al. 2002; Hosken et al. 2008; though the two traits have different genetic bases: Zhang et al. 2008). The measure also assumes that mating success on day 14 is correlated with overall male success, which will be a function of success on or before day 14. This assumption could potentially be violated if there is an extreme reversal in relative male mating success during the span of a couple of days, but there is no a priori reason to expect such an extreme reversal, nor is there any such precedent in Drosophila melanogaster.
Chi-square tests were used to detect sex ratio deviations from unity (1:1). Fisher exact tests were used to examine whether preferred and unpreferred fathers had sons with different degrees of mating success. Two-tailed t-tests were used to test whether egg production rates differed between daughters of preferred and unpreferred fathers.
RESULTS & DISCUSSION
Offspring Fitness Estimates
Preferred fathers had sons with slightly higher mating success, though the difference was small and not statistically significant (percent paternity unpreferred = 0.627, n = 193; percent paternity preferred = 0.647, n = 207; Fisher exact test p = 0.679). Daughters of preferred males had decreased fecundity compared to the daughters of unpreferred males (Fig. 1; unpreferred daughters produced a mean of 70.20 eggs per vial, n = 203 vials; preferred daughters produced 56.85 eggs per vial, n = 164 vials; two-tailed ttest p = 0.0000004). The estimated fitness gain of 3 percent to sons is substantially lower than the estimated fitness drop of 19 percent to daughters of preferred males.
Fig. 1.
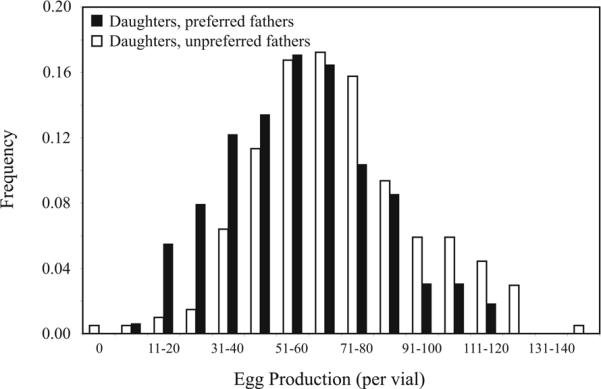
Paternal mating success influences daughter fecundity. The egg production distribution is based on egg counts per vial, each containing two experimental females and two randomly selected males (see methods for details).
A strong, negative fitness correlation between fathers and daughters, coupled with marginal father-son fitness heritability cannot be explained by autosome-linked sexually antagonistic variation, which predicts that costs and benefits will be symmetrical between sons and daughters (Kidwell et al. 1977). It is also possible that females differentially provision eggs fertilized by unpreferred males. However, a “paternal effect” such as this should reduce the fitness of both sons and daughters - this prediction is difficult to reconcile with the data. The offspring fitness pattern is consistent with prior theory and data suggesting that adult fitness traits are strongly influenced by X-linked sexually antagonistic variation (Rice 1984; Gibson et al. 2002; Pischedda & Chippindale 2006; Oneal et al. 2007).
Offspring Sex Ratio
Offspring sex ratios differed between experimental treatments, with preferred males producing sons and daughters at equal ratios, and unpreferred males producing daughter-biased sex ratios (Table 1). The single exception in trial 2 (equal sex ratio for preferred and unpreferred fathers) can be attributable to its markedly reduced sample size relative to sampling in trails 1 and 3. Indeed, the overall sex ratio reduction in unpreferred male broods is relatively strong and highly significant (male: female ratio = 0.874; p = 0.0056).
Table 1.
Male mating success and offspring sex ratios
| Males | Females | Sex Ratio | P1 | |
|---|---|---|---|---|
| Trial 1 | ||||
| unpreferred | 296 | 366 | 0.809 | 0.0065 |
| preferred | 255 | 260 | 0.981 | 0.83 |
| Trial 2 | ||||
| unpreferred | 78 | 72 | 1.08 | 0.62 |
| preferred | 87 | 86 | 1.01 | 0.94 |
| Trial 3 | ||||
| unpreferred | 417 | 467 | 0.893 | 0.093 |
| preferred | 377 | 378 | 0.997 | 0.97 |
| All Trials | ||||
| unpreferred | 791 | 905 | 0.874 | 0.0056 |
| preferred | 719 | 724 | 0.993 | 0.90 |
Significance based on chi-squared tests with 1 d.f.
There are three possible mechanistic explanations for the sex ratio biases observed here. The sex ratio might be equal among fertilized eggs, but viability selection might differentially remove males and females from the adult population. This mechanism would require that son and daughter viability be decoupled for unpreferred but not for preferred males. This scenario appears unlikely in D. melanogaster, where juvenile viability is strongly positively correlated between the sexes (Chippindale et al. 2001).
Females might adjust progeny sex ratios in response to their mate. This is a possibility in D. melanogaster, where females have been shown to adjust offspring sex ratio in a mating context-dependent manner (Mange 1970; Long & Pischedda 2005; Fuller & Mousseau 2007). When variation is sexually antagonistic, this hypothesis predicts that females mated to preferred males will produce male-biased offspring sex ratios, while unpreferred males will sire female-biased broods. Only part of this pattern is supported, as preferred male broods have a sex ratio near unity. Nevertheless, the direction of skew in offspring of unpreferred males is adaptive since it is biased towards the sex with highest relative fitness (daughters).
Males with low mating success might have sex ratio distorting X chromosomes, which are common in Drosophila populations (Jaenike 2001). Associations between mating success and male meiotic drive have been reported in studies using mice and stalk-eyed flies (Lenington 1991; Wilkinson et al. 1998), though it is not known whether “driving” X chromosomes are associated with sexually antagonistic variation in these species. Such linkage disequilibrium might be expected. Males carrying a driving X with female-beneficial alleles will have higher quality offspring than males carrying driving, female-detrimental X chromosomes. The effect could promote the development of linkage disequilibrium between sex ratio and sexually antagonistic alleles. This is an area of population genetics theory that has yet to be formally explored (see Burt & Trivers 2006 for verbal discussion of meiotic drive and sexual antagonism).
Conclusion and Future Directions
The results indicate that female mating biases in Drosophila might cause a net decrease to offspring fitness. This is not simply an artifact of the tournament mate-trial setting because mating latency trials independently confirm that females mate more readily with males designated as “preferred” (see Supplementary Fig. 2). Mating biases that reduce offspring fitness seem paradoxical, yet three factors could potentially explain the persistence of such a pattern of female choice. First, males might provide a direct benefit to their mates, which could potentially counteract any indirect costs (e.g. Oneal et al. 2007). Although current evidence from Drosophila melanogaster suggests that direct effects of female preferences are not beneficial and instead could be costly (Friberg & Arnqvist 2003), this scenario would be an interesting topic for future research. A second possibility is that Drosophila female mating biases are passive rather than active. For example, male mating success is at least partially a function of locomotor activity, with high-activity males encountering and consequently mating with more females than low-activity males. A passive female mating bias of this variety is not directly costly to females because there is no energetic cost of searching for a mate (Kokko et al. 2006; Kotiaho & Puurtinen 2007), and is likely to have sexually antagonistic fitness consequences for offspring (Long & Rice 2007). Furthermore, females are only expected to evolve to resist males carrying female-detrimental genes if the cost of active mate choice is less than the indirect costs of having less fit offspring. Lastly, multiple mating might mitigate the indirect fitness costs that we observe here, in single mating contexts. We estimated the fitness of offspring from females that were singly-mated to a preferred or unpreferred male. In natural contexts, Drosophila females mate multiply, which could help to eliminate sexually antagonistic consequences for offspring if females preferentially use X-bearing sperm from “unpreferred” males and Y-bearing sperm from “preferred” males.
The sex ratio bias observed for unpreferred fathers has adaptive consequences for both parents. Unpreferred males and their mates have high-fitness daughters and benefit by producing daughters in excess of sons. By producing offspring with a higher mean fitness, unpreferred fathers might make a greater long-term genetic contribution to the population than might be expected based on their relative mating success. In other words, male mating success variance might be considerably higher than the actual fitness variance among males.
Sexually antagonistic selection was largely ignored experimentally until the last several years, but steadily mounting evidence now indicates that it is an important mechanism maintaining population genetic variation for fitness (e.g. Chippindale et al. 2001; Foerster et al 2007; Cox & Calsbeek 2008). The potential ubiquity of sexually antagonistic variation, coupled with a variety of sex ratio distortion mechanisms (e.g. Clutton-Brock & Iason 1986; Jaenike 2001), suggests that the results reported here might commonly occur in nature. To our knowledge, only one other such report, in an Anolis lizard species (Calsbeek & Bonneaud 2008), has been published. Future work in additional animal species might benefit by integrating female choice assays with analyses of sex ratio modification and sex-specific offspring fitness.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to David Houle for providing fly stocks, to L. Lacey Knowles, Rhonda Snook, and two anonymous referees for very helpful comments on the manuscript, and to Akane Uesugi for help during part of the experiment. This work was supported by an NSF dissertation improvement grant (DEB 0709908) and an NIH Genome Training Grant (T32 HG00040) to TC.
REFERENCES
- Albert AYK, Otto SP. Sexual selection can resolve sex-linked sexual antagonism. Science. 2005;310:119–121. doi: 10.1126/science.1115328. [DOI] [PubMed] [Google Scholar]
- Bangham J, Chapman T, Partridge L. Effects of body size, accessory gland and testis size and pre-and postcopulatory success in Drosophila melanogaster. Animal Behavior. 2002;64:915–921. [Google Scholar]
- Burt A, Trivers R. Genes in Conflict: The Biology of Selfish Genetic Elements. Harvard University Press; Cambridge: 2006. [Google Scholar]
- Calsbeek R, Bonneaud C. Postcopulatory fertilization bias as a form of cryptic sexual selection. Evolution. 2008;62:1137–1148. doi: 10.1111/j.1558-5646.2008.00356.x. [DOI] [PubMed] [Google Scholar]
- Calsbeek R, Sinervo B. Within-clutch variation in offspring sex determined by differences in sire body size: cryptic mate choice in the wild. Journal of Evolutionary Biology. 2004;17:464–470. doi: 10.1046/j.1420-9101.2003.00665.x. [DOI] [PubMed] [Google Scholar]
- Chippindale AK, Gibson JR, Rice WR. Negative genetic correlation for adult fitness between sexes reveals ontogenetic conflict in Drosophila. Proceedings of the National Academy of Sciences USA. 2001;98:1671–1675. doi: 10.1073/pnas.041378098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clutton-Brock TH, Iason GR. Sex ratio variation in mammals. Quarterly Review of Biology. 1986;61:339–374. doi: 10.1086/415033. [DOI] [PubMed] [Google Scholar]
- Cox RM, Calsbeek R. Sexually antagonistic selection, sexual dimorphism, and the resolution of intralocus sexual conflict. The American Naturalist. 2009 doi: 10.1086/595841. in press. [DOI] [PubMed] [Google Scholar]
- Foerster K, Coulson T, Sheldon BC, Pemberton JM, Clutton-Brock TH, Kruuk LEB. Sexually antagonistic genetic variation for fitness in red deer. Nature. 2007;447:1107–1110. doi: 10.1038/nature05912. [DOI] [PubMed] [Google Scholar]
- Fuller BA, Moussseau TA. Precision in sex allocation is influenced by mate choice in Drosophila melanogaster. Journal of Evolutionary Biology. 2007;20:1700–1704. doi: 10.1111/j.1420-9101.2007.01400.x. [DOI] [PubMed] [Google Scholar]
- Friberg U, Arnqvist G. Fitness effects of female mate choice: preferred males are detrimental for Drosophila melanogaster females. Journal of Evolutionary Biology. 2003;16:797–811. doi: 10.1046/j.1420-9101.2003.00597.x. [DOI] [PubMed] [Google Scholar]
- Gibson JR, Chippindale AK, Rice WR. The X chromosome is a hot spot for sexually antagonistic fitness variation. Proceedings of the Royal Society of London B. 2002;269:499–505. doi: 10.1098/rspb.2001.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosken DJ, Taylor ML, Hoyle K, Higgins S, Wedell N. Attractive males have greater success in sperm competition. Current Biology. 2008;18:R553–R554. doi: 10.1016/j.cub.2008.04.028. [DOI] [PubMed] [Google Scholar]
- Houle D, Rowe L. Natural selection in a bottle. Am Nat. 2003;161:50–67. doi: 10.1086/345480. [DOI] [PubMed] [Google Scholar]
- Jaenike J. Sex chromosome meiotic drive. Annual Review of Ecology, Evolution and Systematics. 2001;32:25–49. [Google Scholar]
- Kokko H, Jennions MD, Brooks R. Unifying and testing models of sexual selection. Annual Review of Ecology, Evolution and Systematics. 2006;37:43–66. [Google Scholar]
- Kotiaho JS, Puurtinen M. Mate choice for indirect genetic benefits: scrutiny of the current paradigm. Functional Ecology. 2007;21:638–644. [Google Scholar]
- Lenington S. The t-complex: a story of genes, behavior, and populations. Advances in the Study of Behavior. 1991;20:51–86. [Google Scholar]
- Long TAF, Pischedda A. Do female Drosophila melanogaster adaptively bias offspring sex ratios in relation to the age of their mate? Proceedings of the Royal Society of London B. 2005;272:1781–1787. doi: 10.1098/rspb.2005.3165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long TAF, Rice WR. Adult locomotory activity mediates intralocus sexual conflict in a laboratory-adapted population of Drosophila melanogaster. Proceedings of the Royal Society of London B. 2007;274:3105–3112. doi: 10.1098/rspb.2007.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mange AP. Possible nonrandom utilization of X- and Y-bearing sperm in Drosophila melanogaster. Genetics. 1970;65:95–106. doi: 10.1093/genetics/65.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markow TA, Quaid M, Kerr S. Male mating experience and competitive courtship in Drosophila melanogaster. Nature. 1978;276:821–822. [Google Scholar]
- Oneal E, Connallon T, Knowles LL. Conflict between direct and indirect benefits of female choice in desert Drosophila. Biology Letters. 2007;3:29–32. doi: 10.1098/rsbl.2006.0565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pischedda A, Chippindale AK. Intralocus sexual conflict diminishes the benefits of sexual selection. PLoS Biology. 2006;4:2099–1203. doi: 10.1371/journal.pbio.0040356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad NG, Bedhomme S, Day T, Chippindale AK. An evolutionary cost of separate genders revealed by male-limited evolution. The American Naturalist. 2007;169:29–37. doi: 10.1086/509941. [DOI] [PubMed] [Google Scholar]
- Rice WR. Sex chromosomes and the evolution of sexual dimorphism. Evolution. 1984;38:735–742. doi: 10.1111/j.1558-5646.1984.tb00346.x. [DOI] [PubMed] [Google Scholar]
- Wilkinson GS, Presgraves DC, Crymes L. Male eye span in stalk-eyed flies indicates genetic quality by meiotic drive suppression. Nature. 1998;391:276–279. [Google Scholar]
- Zhang R, Amah L, Fiumera AC. Autosomal variation for male body size and sperm competition phenotypes is uncorrelated in Drosophila melanogaster. Biology Letters. 2008;4:500–503. doi: 10.1098/rsbl.2008.0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


