Abstract
Previous studies using different techniques have shown that adenoviral-mediated gene transfer to different tissues, including the kidney, is more efficient in neonatal mice. In this study, we report a simple technique that allows an efficient and long term expression of β-galactosidase (β-gal) in the heart of newborn mice. Newborn and adult C57BL6/J mice were subjected to a single retro-orbital venous plexus injection of recombinant adenoviral vector (rAd) (2 × 109 particles/g body weight) carrying the lac Z gene. Seven days after the injection, positive perinuclear β-gal staining was systematically observed in the heart, lung, intestine, liver, kidney and spleen of newborn mice. However, only the heart showed persistent expression of β-gal one year after the initial injection. In contrast, adult mice showed only significant but transient β-gal expression mainly in the liver. In summary, we have found that a single retro-orbital intravenous injection can be used to establish a long-term adenoviral-mediated gene transfer to cardiac cells of newborn mice.
Keywords: Recombinant adenoviral vector, long term gene transfer, newborn mouse, heart, cardiomyocytes
INTRODUCTION
As a result of their ability to infect both dividing and non-dividing cells in almost all tissues, recombinant adenovirus (rAd) vectors have become one of the most widely used vectors for in vivo delivery of foreign genes. The bio-distribution of rAd following in vivo administration has been studied by a number of groups, and found to depend largely on the route of viral administration [1–3]. Direct injection of the virus into a target tissue usually results in localized transgene expression. A systemic injection, on the other hand, provides access to all major organs, however, the recombinant adenoviruses are rapidly cleared from the circulation by the liver and Kupffer cells [4], resulting in high levels of transgene expression in the liver but not in other tissues [2, 4–8].
In previous studies we developed two procedures, named “portal-clamping” [9] and “prolonged renal-infusion techniques” [10], to express rAd-vectors in the lung, intestine, and renal glomeruli of adult mice [9] and rats [10]. Both methods are based on the principle of bypassing the hepatic circulation to prevent the rapid clearance of rAd by liver cells [11]. Our results suggest that if the rAd vectors can be retained in the circulation, they will continue to pass through the lung, intestine and glomerular circulation for an extended period of time, and therefore will establish an efficient infection of these structures. We have previously found that new-born mice also have a delayed clearance or rAd vectors from the circulation and a more efficient transduction of glomerular cells after a single retro-orbital injection of rAd [6]. A greater susceptibility of neonates to rAd mediated gene transfer has also been reported by a number of groups [2, 12–13]. In the present study, we systematically evaluated the long term tissue distribution and expression of β-galactosidase (β-gal) in neonatal and adult mice following a single retro-orbital venous plexus injection of rAd carrying the lacZ gene. We found that this simple injection technique allows a transient expression of β-gal in glomerular cells and a long term transfer of the transgene to cardiac cells up to one year after the initial injection.
EXPERIMENTAL DESIGN
Adult C57BL6/J mice were purchased from the Jackson Laboratory (Bar Harbor, ME, USA) and were housed in a specific pathogen-free animal facility at the Children’s National Medical Center. All animal experiments were carried out according to institutional guidelines for animal use. The E1-deleted recombinant Ad carrying the Escherichia coli lacZ gene, encoding β-gal (designated rAd-lacZ), was generated as previously described [7]. LacZ gene expression was controlled by a cytomegalovirus (CMV) enhancer and chicken β-actin gene promoter. The virus was amplified in 293 cells, purified from a frozen cell lysate by two centrifugations in CsCl gradient, desalted on Bio-Gel p-6-DG column (Bio-Rad Laboratories, Hercules, CA), suspended in phosphate buffered saline (PBS) with 10% of glycerol, and stored at −80°C. The particle/plaque forming unit (pfu) ratio of the virus stcok used in the experiments was 100.
RETRO-ORBITAL INJECTION TECHIQUE
Newborn (1 day old) and adult (6 months old) mice were injected retro-orbitally with rAd-lacZ. Injection of newborn pups was performed with a 100 µl Hamilton syringe connected to a No. 0160832 needle (Hamilton, Reno, NE, USA). Ten µl of virus suspension (2× 109 particles/g body weight) were injected into the retro-orbital vein plexus of newborn mice. Adult mice were injected with 100 µl of virus suspension (2 × 109 particles/g body weight) retro-orbitally using a 0.5 insulin syringe (Becton Dickinson, Franklin Lakes, NJ, USA).
DETERMINATION OF β-GAL ACTIVITY IN TISSUE SAMPLES
To study the localization of β-gal expression, frozen tissue sections (10 µm) were fixed in 0.5% glutaraldehyde (Sigma, St. Louis, MO) at room temperature for 10 min, washed with PBS, and stained for 2h at 37°C in PBS containing 5mM K3Fe(CN)6, 5mM K4Fe(CN)6, 1mM MgCl2 (all from Sigma, St. Louis, MO), and 1mg/ml 5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside (X-gal, Boehringer Mannheim, Indianapolis, IN). The sections were then counter-stained with hematoxylin (Fisher Scientific, Pittsburgh, PA) and mounted for microscopic evaluation. Quantification was performed by counting β-gal positive cells or structures in ten randomly selected 20× microscopic fields in each section and mouse. A β-gal positive structure was defined as one that contains at least five β-gal-positive cells. For quantitative analysis of β-gal activity, one mg of tissue harvested from the mice was homogenized in 10 mM Tris-HCl buffer. The protein concentration was determined using the Bio-Rad Protein assay, and 0.1 mg protein was used for each sample. β-gal activity was measured using the β-gal enzyme assay system (Promega, Madison, WI, USA) according to the manufacturer’s instructions.
TISSUE DISTRIBUTION AND PERSISTENCE OF β-GAL EXPRESSION
C57BL6/J adult and newborn mice were injected retro-orbitally with rAd-lacZ at day 1 (n =11) and at 6 months of age n= 10). The animals were sacrificed 7 days following viral injection, and various tissues were analyzed for β–gal staining and activity. As shown in Table 1, the pattern of β–gal expression in different tissues changed dependent on the age of the mice. Injections in both newborn and adult mice resulted in high levels of expression of β–gal in the liver, however, only newborn mice showed a widespread distribution of β–gal in the heart, lung, intestine, spleen, and kidney (Table 1, Figs. 1A-B-C). β–gal expression in the heart was predominately detected in myocytes (Fig. 1A and Fig. 2 A–C), while in the lung (Fig. 1B), intestinal villi (Fig. 1C), and renal glomeruli (not shown), it was predominantly localized to capillaries, vessels, and perivascular structures. It should be noted that the quantification of β-gal expression in the kidney in both newborn and adult mice should take into consideration the fact that β-gal released into the circulation by the hepatocytes could be trapped almost exclusively in renal glomeruli [14]. Nevertheless, in a previous study we have shown that in addition to the accumulation of circulating β-gal, both lacZ and β-gal are produced in glomerular cells of newborn mice, but not those from adult mice [6]. Little or no β–gal expression (< 1 %) was observed in the brain, pancreas, or skeletal muscles of mice injected at any age (data not shown).
Table 1.
β-Gal Staining and Activity in Tissues Harvested Seven Days after the Initial Injection of rAd-lacZ in Newborn and Adult C57BL6/J Mice
| # of mice | age | % β-gal staining ( positive cells per high power field) | ||||
|---|---|---|---|---|---|---|
| Heart | Lung | Intestine | Kidney | Liver | ||
| A. β-gal staining | ||||||
| 11 | newborn | 32 ± 13 | 38 ± 6 | 37 ± 16 | 37 ± 8 | 92 ± 7 |
| 10 | adult | Non detectable | Non detectable | Non detectable | 35 ± 12 | 95 ± 6 |
| B. β-gal activity in tissue homogenates | ||||||
| 6 | newborn | 5.4 ± 1.1 | 1.3 ± 0.6 | 0.18 ± 0.05 | 1.46 ± 0.56 | 12 ± 2.4 |
| 6 | adult | 0.45 ± 0.04 | 0.09 ± 0.05 | 0.08 ± 0.03 | 1.44 ± 0.6 | 47 ± 13 |
Data are expressed in means ± SD.
Quantification of β-gal staining was made in ten randomly selected 20 × microscopic fields per tissue in each mouse as described in methods.
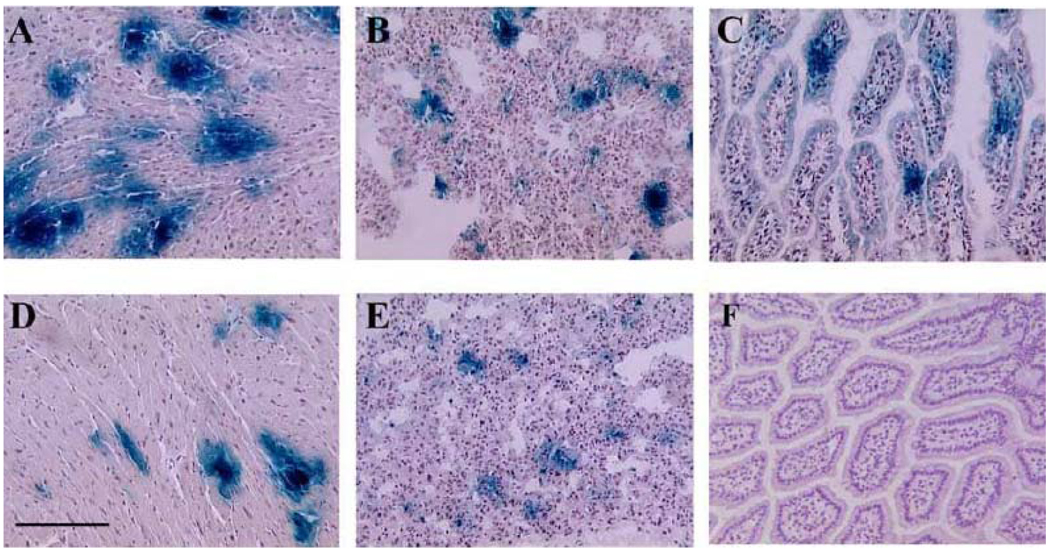
Fig. (1). β-gal expression after a single retro-orbital injection of rAd-lacZ in newborn mice.
One day old mice were injected retro-orbitally with rAd-lacZ (2 × 109 particles/gram), as described in methods. Subsequently, they were sacrificed at different time points post injection. Tissue samples were harvested and examined for β-gal expression by X-Gal staining (blue color). The panels show representative pictures demonstrating blue β-gal staining in: (A) heart, 7 days after injection; (B) lung, 7 days after injection., (C) intestine, 7 days after injection; (D) heart, 360 days after injection; (E) lung, 90 days after injection; and (F) intestine, 90 days after injection. Bar graph: 500 µm.
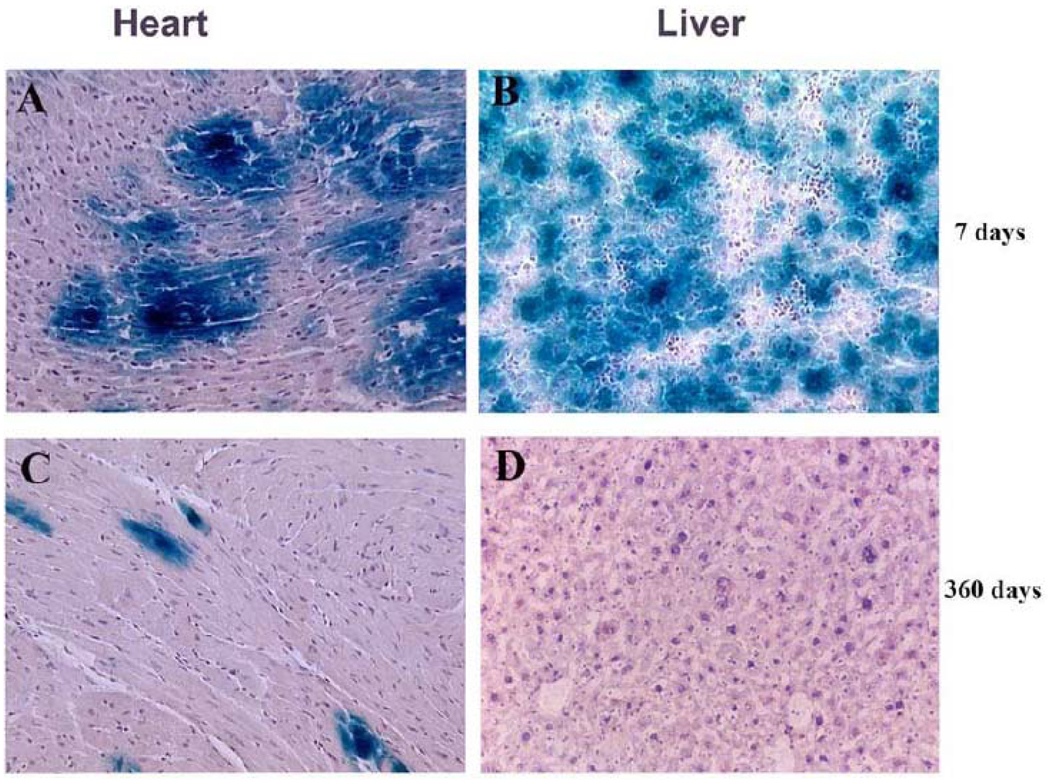
Fig. (2). Long-term expression of β-gal in the heart of mice injected via the retro-orbital venous plexus with rAd-lacZ.
Representative mouse heart and liver samples were taken from mice injected during the first day of life with rAd-lacZ (2 × 109 particles/gram). The samples shown in the panels were harvested at 7 and 360 days after the initial injection. Tissue samples were examined for β-gal expression by X-Gal staining (blue color). The panels show representative β-gal blue staining in the: (A) heart, 7 days after injection; (B) liver, 7 days after injection; (C) heart, 360 days after injection; (D) liver, 360 days after injection Bar graph: 500 µm.
We then examined the stability of virus-mediated β-gal expression in mice injected on the first day of life. In these experiments, 32 mice were injected with rAd-lacZ and sacrificed at 7, 21, 35, 90, 180 and 360 days post injection to asses the expression of β-gal in different tissues (Table 2). β-gal expression in the liver, intestine, and kidney peaked on day 7, but decreased significantly by day 21. β-gal expression in the lung lasted up to 90 days, while approximately 5 % of the cardiac cells remained positive for up to 360 days after the initial injection (Table 2, Fig. 1–Fig. 2).
Table 2.
Long-Term β-Gal Expression after a Retro-Orbital Injection of rAd-lacZ in Newborn Mice
| D % β-Gal Positive Cells ( Days p.i. # mice Heart Lung Intestine Liver Kidney Spleen | |||||||
|---|---|---|---|---|---|---|---|
| 7 | n = 11 | 29 ± 13 | 38 ± 6 | 37 ± 16 | 92 ± 7 | 37 ± 8 | <1 |
| 21 | n = 4 | 16 ± 10 | 18 ± 3 | - | 5 ± 2 | 12 ± 7 | <1 |
| 35 | n = 4 | 13 ± 5 | 10 ± 5 | - | <1 | 8 ± 5 | - |
| 90 | n = 4 | 16 ± 8 | 5 ± 3 | - | <1 | - | - |
| 180 | n = 4 | 6 ± 2 | - | - | - | - | - |
| 360 | n = 5 | 5 ± 3 | - | - | - | - | - |
Days p.i. = days after the initial injection of rAd-lacZ ; n = number of mice
Data are expressed in means ± SD. ( − ) = Non detectable
Quantification was made by counting β–gal positive cells in ten randomly selected 20× microscopic fields in each tissue per mouse.
DISCUSSION OF THE TECHNIQUE
In this study we have shown that a retro-orbital intravenous injection of rAd-lacZ to newborn mice leads to significant widespread β-gal expression in the heart, lung, intestine, liver, kidney, and spleen. The virus-mediated β-gal expression in the intestine, liver, kidney, and spleen of newborn mice was transient lasting approximately 7–30 days. In contrast, the expression of β-gal in lung and heart however, lasted much longer. Approximately 180 days after the initial injection, the heart remained the only organ showing significant β-gal activity. Alternatively, adult mice treated in a similar manner only showed high levels of β-gal in the liver and the kidney. However, based on the results of the current and previous studies, the β-gal staining in adult mouse kidney, unlike the β-gal staining in other tissues [6, 9–11, 15, 16, 17], was attributed to the trapping of circulating β-gal by renal glomeruli [14]. Overall, these findings show that a simple retro-orbital intravenous injection of adenoviral vectors can establish a long term gene transfer to the heart of newborn mice.
At the present time, the mechanisms responsible for the increased gene transfer efficiency of newborn mouse tissues are not clearly understood. Several factors might play an important role in this process. In a previous study we have demonstrated a prolonged retention of rAd-lacZ in the circulation of newborn mice [6]. We also showed that by increasing the retention of rAd in the circulation of adult mice using the “liver clamping technique”, the vascular structures of the lung, intestine, and renal glomeruli [9] can be transduced successfully. The liver clamping technique, however, did not enhance β-gal expression in the heart or skeletal muscles of adult mice [9]. These findings suggest that, in addition to the presence of high and persistent levels of rAd in the circulation, other factors are needed to facilitate the transduction of cardiac cells. Of interest, Christesen et al. [15] performed direct injections of adenovirus into the cardiac cavity, and showed a more efficient in vivo transduction of cardiac cells in one day old mice, when compared to mice injected 5 days after birth. They suggested that the adenovirus could be trapped in the trabecular network of ventricles and atria of developing newborn mice [15]. Thus, the presence of anatomical or physiological vector barriers in older mice may play a critical role in this process [18]. In our study, it could be assumed that the retro-orbital venous plexus injection will deliver rAd directly into the heart via the superior vena cava, and therefore bypassing the first liver passage, which plays a key role removing adenoviruses from the circulation [9]. This might explain the more efficient transduction of new-born hearts via the retro-orbital venous plexus, when compared to systemic intravenous injections in other sites [17]. Alternatively, when adenoviruses were injected directly into the thoracic cavity [2, 5] or pericardium [19–20], different results were obtained depending on the technique used to inject the rAd. One study showed higher levels of β-gal expression in the lungs and diaphragm [2], while other studies demonstrated efficient gene transfer to cardiac cells [16, 19–20]. Finally, a direct myocardial injection usually can establish a successful gene transfer over a limited cardiac region [21–22]. Taken together, these studies suggest that the age, site, and method of injection play critical roles in determining the efficiency of adenoviral-mediated gene transfer to mouse cardiac cells. All studies however, support that notion that the efficiency, reproducibility, and duration of gene expression are significantly increased in newborn mice.
Entry of adenovirus serotypes 2 or 5 into cells requires at least two receptors: a primary high affinity Coxsackievirus and adenovirus receptor (CAR) for attachment [23, 24], and a secondary cell surface integrin receptors for internalization [25, 26]. The integrins αvβ3 and αvβ5 are heterodimeric cell adhesion molecules that are differentially expressed during embryogenesis [27]. However, the relative importance of CAR and integrins in the infection of cardiac mouse cells is not well understood at the present moment. In addition, the distribution and expression of the genes transferred with rAd-vectors may be also impacted by the stage of maturity of the immune system in newborn mice [28, 29]. It is well known that B-cell and T-cell responses, as well as the generation of antibodies and cytotoxic immune responses are diminished in newborn mice, when compared to adult mice [30]. Overall, further studies will be required to determine the exact mechanism by which cardiac cells remain the only cells that continue to express β-gal throughout the first year of life.
CONCLUSION
We have described a simple and highly reproducible technique to establish a long-term gene transfer to the heart of newborn mice. It should be noted that when our technique is compared to the direct injection of adenoviruses into the cardiac cavity of newborn mice [15], it induces lower levels of gene expression, but it does not require special equipment, general anesthesia, and is not associated with high mortality rates. Our approach could become even more powerful when cardiac-specific promoters are used. We are confident that this simple adenoviral-mediated gene transfer technique will provide a valuable experimental tool to explore the role of different genes during mouse cardiac development and the pathogenesis of cardiac hypertrophy.
ACKNOWLEDGMENTS
This work was supported by National Institute of Health Grants R01 DK 049419, R01 HL 55605, R21-AT002278 (PER) and a grant from the National Kidney Foundation, Washington DC (MY).
REFERENCES
- 1.Huard JL, Lochmuller H, Acsadi G, Massie B, Karpati G. The route of administration is major determinant of the transduction efficiency of rat tissue by adenoviral recombinants. Gene Ther. 1995;2:107–115. [PubMed] [Google Scholar]
- 2.Kass-Eisler A, Falk-Pedersen E, Elfenbein DH, Alvira M, Buttrick PM, Leinwand LA. The impact of developmental stage, route of administration and immune system on adenovirus-mediated gene transfer. Gene Ther. 1994;1:395–402. [PubMed] [Google Scholar]
- 3.Wood M, Perrote P, Onishi E, Harper ME, Dinney C, Pagliaro L, Wilson DR. Biodistribution of an adenoviral vector carrying the luciferase reporter gene following intravesical or intravenous administration to a mouse. Cancer Gene Ther. 1999;6:367–372. doi: 10.1038/sj.cgt.7700090. [DOI] [PubMed] [Google Scholar]
- 4.Tao N, Gao GP, Parr M, Johnston J, Baradet T, Wilson JM, Barsoum J, Fawell SE. Sequestration of adenoviral vector by Kupffer cells leads to a nonlinear dose response of transduction in liver. Mol. Ther. 2000;3:28–35. doi: 10.1006/mthe.2000.0227. [DOI] [PubMed] [Google Scholar]
- 5.Jaffe HA, Daniel C, Longenecker G, Metzger M, Setoguchi Y, Rosenfeld MA, Gant TW, Thorgeirsson SS, Stratford-Perricaudet LD, Perricaudet M, Pavirani A, Lecocq J-P, Crystal RG. Adenovirus-mediated in vivo gene transfer and expression in normal rat liver. Nat. Genetics. 1992;1:372–378. doi: 10.1038/ng0892-372. [DOI] [PubMed] [Google Scholar]
- 6.Jerebtsova M, Liu X-H, Ye X, Ray PE. Adenovirus-mediated gene transfer to glomerular cells in newborn mice. Pediatr. Nephrol. 2005;20:1395–1400. doi: 10.1007/s00467-005-1882-0. [DOI] [PubMed] [Google Scholar]
- 7.Kozarsky K, Grossman M, Wilson JM. Adenovirus-mediated correction of the genetic defects in hepatocytes from patients with familial hypercholesterolemia. Somat. Cell Mol. Genet. 1993;19:449–458. doi: 10.1007/BF01233250. [DOI] [PubMed] [Google Scholar]
- 8.Li Q, Kay MA, Finegold M, Stratford-Perricaudet LD, Woo SL. Assessment of recombinant Ad vectors for hepatic gene therapy. Hum. Gene Ther. 1993;4:403–409. doi: 10.1089/hum.1993.4.4-403. [DOI] [PubMed] [Google Scholar]
- 9.Ye X, Jerebtsova M, Ray PE. Liver bypass significantly increases the transduction efficiency of recombinant adenoviral vectors in the lung, intestine and kidney. Human Gene Ther. 2000;11:621–627. doi: 10.1089/10430340050015806. [DOI] [PubMed] [Google Scholar]
- 10.Ye X, Liu X, Li Z, Ray PE. Efficient gene transfer to rat renal glomeruli with recombinant adenoviral vectors. Hum. Gene Ther. 2001;12:141–148. doi: 10.1089/104303401750061203. [DOI] [PubMed] [Google Scholar]
- 11.Ye X, Jerebtsova M, Liu X-H, Li Z, Ray PE. Adenovirus-mediated gene transfer to renal glomeruli in rodents. Kidney Int. 2002;61:16–23. doi: 10.1046/j.1523-1755.2002.0610s1016.x. [DOI] [PubMed] [Google Scholar]
- 12.Hamilton T, DeMatteo R, McClane S, Burke C, Rombeau J, Raper S. Adenoviral-mediated gene transfer to murine small intestine is more efficient in neonatal than adults. J. Pediatr. Surg. 1995;32:373–377. doi: 10.1016/s0022-3468(97)90214-1. [DOI] [PubMed] [Google Scholar]
- 13.Huard J, Lochmuller H, Acsadi G, Jani A, Holland P, Guerin C, Massie B, Karpati G. Differential short-term transduction efficiency of adults versus newborn mouse tissue by adenoviral recombinants. Exp. Mol. Pathol. 1995;62:1319–1438. doi: 10.1006/exmp.1995.1015. [DOI] [PubMed] [Google Scholar]
- 14.Zhu G, Nicolson AG, Zheng XX, Strong TB, Sukhatme VP. Adenovirus-mediated beta-galactosidase gene delivery to the liver leads to protein deposition in kidney glomeruli. Kidney Int. 1997;52:992–999. doi: 10.1038/ki.1997.421. [DOI] [PubMed] [Google Scholar]
- 15.Christensen G, Minamisawa S, Gruber P, Wang Y, Chien K. High-efficiency, long-term cardiac expression of foreign genes in living mouse embryos and neonates. Circulation. 2000;101:178–184. doi: 10.1161/01.cir.101.2.178. [DOI] [PubMed] [Google Scholar]
- 16.Ebelt H, Braun T. Optimized, highly efficient transfer of foreign genes into newborn mouse hearts in vivo. Biochem. Biophys. Res. Commun. 2003;310:1111–1116. doi: 10.1016/j.bbrc.2003.09.131. [DOI] [PubMed] [Google Scholar]
- 17.Stratford-Perricaudet LD, Makeh I, Perricaudet M, Briand P. Widespread long-term gene transfer to mouse skeletal muscle and heart. J. Clin. Invest. 1992;90:626–630. doi: 10.1172/JCI115902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fechner H, Haack A, Wang H, Wang X, Eizema K, Pauschinger M, Schoemaker R, Veghel R, Houtsmuller A, Schultheiss HP, Lamers J, Poller W. Expression of Coxsackie adenovirus receptor and alphav-integrin does not correlate with adenovector targeting in vivo indicating anatomical vector barriers. Gene Ther. 1999;6:1520–1535. doi: 10.1038/sj.gt.3301030. [DOI] [PubMed] [Google Scholar]
- 19.Zhang JC, Woo YJ, Chen JA, Swain JL, Sweeney HL. Efficient transmural cardiac gene transfer by intrapericardial injection in neonatal mice. J. Mol. Cell Cardiol. 1999;31:721–732. doi: 10.1006/jmcc.1998.0905. [DOI] [PubMed] [Google Scholar]
- 20.Fromes Y, Salmon A, Wang X, Collin H, Rouche A, Hagege A, Schwartz K, Fiszman MY. Gene delivery to the myocardium by intrapericardial injection. Gene Ther. 1999;4:683–688. doi: 10.1038/sj.gt.3300853. [DOI] [PubMed] [Google Scholar]
- 21.French B, Mazur W, Geske RS, Bolli R. Direct in vivo gene transfer into porcine myocardium using replication-deficient adenoviral vectors. Circulation. 1994;90:2414–2424. doi: 10.1161/01.cir.90.5.2414. [DOI] [PubMed] [Google Scholar]
- 22.Guzman RJ, Lemarchand P, Crystal RG, Epstein SE, Finkel T. Efficient gene transfer into myocardium by direct injection of adenovirus vectors. Cir. Res. 1993;73:1202–1207. doi: 10.1161/01.res.73.6.1202. [DOI] [PubMed] [Google Scholar]
- 23.Bergelson JM, Cunningham JA, Droguett G, Kurt-Jones EA, Krithivas A, Hong JS, Horwitz MS, Crowell RL, Finberg RW. Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5. Science. 1997;257:1320–1323. doi: 10.1126/science.275.5304.1320. [DOI] [PubMed] [Google Scholar]
- 24.Tomco RP, Xu R, Philipson L. HCAR and MCAR: the human and mouse cellular receptor for subgroup C adenoviruses and group B Coxsackie-viruses. Proc. Natl. Acad. Sci. USA. 1997;94:3352–3356. doi: 10.1073/pnas.94.7.3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davidson E, Diaz RM, Hart IR, Santis G, Marshall JF. Integrin alpha5- beta1-mediated adenovirus infection is enhanced by the integrin-activating antibody TS2/16. J. Virol. 1997;71:6204–6207. doi: 10.1128/jvi.71.8.6204-6207.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wickham TJ, Mathias P, Cheresh DA, Nemerow GR. Integrins alpha v beta 3 and alpha v beta 5 promote adenovirus internalization but not virus attachment. Cell. 1993;73:309–319. doi: 10.1016/0092-8674(93)90231-e. [DOI] [PubMed] [Google Scholar]
- 27.Yamada S, Brown KE, Yamada KM. Differential mRNA regulation of integrin subunits alpha V, beta1, beta 3, and beta 5 during mouse embryonic organogenesis. Cell Adhes. Commun. 1995;3:311–325. doi: 10.3109/15419069509081016. [DOI] [PubMed] [Google Scholar]
- 28.Barr D, Tubb J, Ferguson D, Scaria A, Lieber A, Wilson C, Perkins J, Kay MA. Strain related variation in adenovirally mediated transgene expression from mouse hepatocytes in vivo: Comparisons between immuno-competent and immunodeficient inbred strains. Gene Ther. 1995;2:151–155. [PubMed] [Google Scholar]
- 29.Michou AI, Santoro L, Christ M, Julliard V, Pavirani A, Mehtali M. Adenovirus-mediated gene transfer: Influence of transgene, mouse strain and type of immune response on persistence of transgene expression. Gene Ther. 1997;4:473–482. doi: 10.1038/sj.gt.3300412. [DOI] [PubMed] [Google Scholar]
- 30.Yang Y, Nunes FA, Berencsi K, Furth EE, Gonczol E, Wilson JM. Cellular immunity to viral antigens limits E-1-deleted adenoviruses for gene therapy. Proc. Natl. Acad. Sci. USA. 1994;91:4407–4411. doi: 10.1073/pnas.91.10.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]