Abstract
Genital tubercle (GT) initiation and outgrowth involve coordinated morphogenesis of surface ectoderm, cloacal mesoderm and hindgut endoderm. GT development appears to mirror that of the limb. Although Shh is essential for the development of both appendages, its role in GT development is much less clear than in the limb. Here, by removing Shh at different stages during GT development in mice, we demonstrate a continuous requirement for Shh in GT initiation and subsequent androgen-independent GT growth. Moreover, we investigated the Hh responsiveness of different tissue layers by removing or activating its signal transducer Smo with tissue-specific Cre lines, and established GT mesenchyme as the primary target tissue of Shh signaling. Lastly, we showed that Shh is required for the maintenance of the GT signaling center distal urethral epithelium (dUE). By restoring Wnt-Fgf8 signaling in Shh-/- cloacal endoderm genetically, we revealed that Shh relays its signal partly through the dUE, but regulates Hoxa13 and Hoxd13 expression independently of dUE signaling. Altogether, we propose that Shh plays a central role in GT development by simultaneously regulating patterning of the cloacal field and supporting an outgrowth signal.
Keywords: Shh, Genital tubercle, Cloaca, Hox, Mouse
INTRODUCTION
External genitalia (the penis in males and clitoris in females) are reproductive organs specialized for internal fertilization. In mice, the early development of the embryonic anlage of the external genitalia, the genital tubercle (GT), is androgen-independent and presumably regulated by the same genetic program in both sexes. The GT emerges as paired swellings on either side of the cloaca. These swellings, together with a third dorsal swelling, then merge to form a single GT and continue to grow distally (Perriton et al., 2002). Up to E15.5, GTs of male and female mice are morphologically identical (Suzuki et al., 2002).
Epithelial-mesenchymal interactions are crucial for GT development (Kurzrock et al., 1999b; Murakami and Mizuno, 1986). The cloacal endoderm-derived urethral epithelium (UE) plays an instructive role, as grafting it to the chick limb bud results in a GT-like patterning (Perriton et al., 2002). GT development has been suggested to mirror that of the limb bud as similar developmental regulators, including Fgf (Haraguchi et al., 2000), Hedgehog (Haraguchi et al., 2001; Perriton et al., 2002), Wnt (Lin et al., 2008) and Hox (Morgan et al., 2003; Warot et al., 1997) genes show similar expression patterns and/or function in both processes (Cohn, 2004; Yamada et al., 2006). Notably, the Fgf8-expressing distal UE (dUE) has been shown to have a growth-promoting function, both in vitro and in vivo, similar to the Fgf8-expressing apical ectodermal ridge (AER) in the developing limb bud (Haraguchi et al., 2000; Lin et al., 2008). However, one of the most dramatic differences between limb and GT development is the tubular genesis of the UE, which is derived from the most caudal part of cloacal endoderm, within the GT. Previous work proposed that GT development has to be placed in the context of cloaca morphogenesis (Seifert et al., 2008). Indeed, severe GT malformations are often accompanied by a persistent cloaca (Haraguchi et al., 2001; Haraguchi et al., 2007; Perriton et al., 2002; Warot et al., 1997).
In the mouse, Shh expression can be detected as early as the 15-somite stage in the hindgut (Echelard et al., 1993), and its expression is maintained in the endodermal epithelial lining of the cloaca and, later, the urogenital sinus, including the UE of the GT. Two groups independently reported GT agenesis with persistent cloaca in Shh- knockout mice (Haraguchi et al., 2001; Perriton et al., 2002). Dysregulated gene expression in cloacal endoderm and para-cloacal mesenchyme, as well as altered cell survival and proliferation, were also reported in Shh-/- mutants. However, the underlying mechanism by which Shh exerts its function in GT development is far from clear. Both the temporal requirement and the primary target tissue of Shh signaling remain unknown. Downregulation of the dUE marker Fgf8 was particularly noted in both reports, as the dUE plays an obligatory role in directing GT outgrowth. However, to what extent Shh relays its signal through the dUE remains obscure.
In this study, we used spatially and temporally controlled Cre/rtTA transgenic lines to manipulate the expression of Shh and its signal executor smoothened (Smo) to further investigate the function of Shh signaling in the early androgen-independent phase of GT development. We report that Shh function is required not only during GT initiation, but also throughout androgen-independent GT morphogenesis. The primary target tissue of Hh signaling is the GT mesenchyme, rather than the UE. Last, but not least, we restored genital outgrowth in Shh mutant mice by ectopically activating dUE signaling in cloacal endoderm, and revealed dUE-dependent and -independent events downstream of Shh in GT development.
MATERIALS AND METHODS
Animal maintenance and treatments
ShhCregfp (Harfe et al., 2004), ShhCreesr (Harfe et al., 2004), Shhc/c, Smoc/c (Gritli-Linde et al., 2002) and R26-SmoM2 (Jeong et al., 2004) strains were purchased from the Jackson Laboratory (Bar Harbor, MN, USA). The ShhCregfp strain expresses an EGFP-Cre fusion protein in endogenous Shh-expressing domains. The ShhCreesr strain expresses a fusion protein between Cre and a mutated human estrogen receptor α ligand-binding domain, which allows conditional activation of Cre activity upon Tamoxifen administration. Shhc/c and Smoc/c are conditional knockout alleles for Shh and Smo, respectively. The R26-SmoM2 allele contains a constitutively active mouse Smo (W539L) fused to the enhanced yellow fluorescent protein under the control of the endogenous R26 ubiquitous promoter. However, expression of SmoM2 is normally blocked by a loxP-flanked stop cassette, which can be removed by Cre expression, allowing tissue-specific SmoM2 expression. The Msx2-rtTA strain is a BAC transgenic line that expresses the reverse tetracycline-controlled transactivator (rtTA) in many Msx2-expressing domains (Lin et al., 2009). The tetO-Cre strain (Perl et al., 2002), a gift from Dr Feng Chen, expresses Cre recombinase upon rtTA transactivation. Tamoxifen (Sigma-Aldrich, St Louis, MO, USA) was dissolved in corn oil and delivered to pregnant females by oral gavage at a dose of 0.2 g/kg body weight. Doxycycline (Sigma-Aldrich) was dissolved in water and delivered to animals by oral gavage at 0.1 g/kg body weight. For studies involving embryos of E15.5 and older, only males are presented.
Histology and immunofluorescence
Bouin-fixed paraffin-embedded samples were cut as 6 μm sections using a microtome. Hematoxylin and Eosin (H&E) staining, TUNEL analysis and immunofluorescence studies were carried out as previously described (Yin et al., 2006). For polyclonal K14 antibody (Covance, Princeton, NJ, USA) staining, a 1:1000 dilution was used. For phospho-histone H3 (PHH3) immunofluorescence, a rabbit polyclonal antibody against PHH3 (Millipore, Billerica, MA, USA) was used at 1:300.
Cell death analysis
GTs were collected, rinsed three times in PBS and stained with 500 ng/ml Acridine Orange (Sigma-Aldrich) at 37°C for 30 minutes. Samples were then rinsed briefly in PBS, followed by fluorescence microscopy.
Scanning electron microscopy
For scanning electron microscopy (SEM), samples were fixed in a solution of 3% glutaraldehyde and 4% paraformaldehyde (PFA) in PBS. SEM analysis was performed as previously described (Lin et al., 2008).
Whole-mount and radioactive in situ hybridizations
Samples were fixed in 4% PFA overnight followed by dehydration through a graded series of methanol (in PBS) solutions. Whole-mount analysis was performed according to a standard protocol (Wilkinson, 1992). Probes for Fgf8, Shh, Ptch1, Bmp4, Wnt5a, Msx2, Hoxa13 and Hoxd13 were as described (Lin et al., 2008). Probes for gremlin and noggin were kindly provided by Dr Yiping Chen (Tulane University, New Orleans, LA, USA).
β-galactosidase staining
For whole-mount β-galactosidase (β-gal) staining, embryos were collected, briefly fixed with 4% PFA and stained with 0.1% X-Gal in PBS containing 2 mM MgCl2, 5 mM potassium ferrocyanide [K4Fe(CN)6 3H20; Sigma P-9287] and 5 mM potassium ferricyanide [K3Fe(CN)6; Sigma P-8131] overnight at 37°C.
Statistics
Data were analyzed by unpaired Student's t-test. The number of independent experiments is specified in the Results.
RESULTS
Continuous requirement for Shh signaling in GT development
The GT agenesis phenotype in Shh-/- mice is consistent with a function of Shh in the cloacal endoderm and para-cloacal mesenchyme when GT growth begins. It is equally likely, however, that because Shh expression can be detected in both the hindgut and notochord long before GT formation begins, the lack of Hh signaling in those earlier structures could potentially compromise the responsiveness of progenitor cells to genital inductive signals, which could also contribute to GT agenesis. To address this question and to investigate the temporal requirement of Hh signaling throughout GT development, we conditionally removed Shh at later stages during genital development. Males carrying a Tamoxifen (Tm)-inducible Cre allele at the Shh locus (ShhCreesr/+) (Harfe et al., 2004) were mated to females carrying two floxed Shh alleles (Shhc/c) (Lewis et al., 2001). Embryos with one floxed allele and one ShhCreesr knock-in allele (also a null allele) at the Shh locus (ShhCreesr/c) lose Shh expression upon Tm treatment and are hereafter referred to as Shh-cKOs (conditional knockouts).
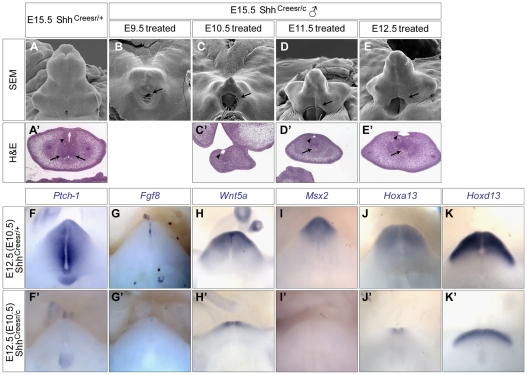
We treated pregnant females with a single shot of Tm at successive time points from 9.5 to 12.5 days post-coitus (dpc) by oral gavage and examined genital phenotype at embryonic day (E) 15.5. We previously showed that Cre-mediated recombination occurs as early as 12 hours after TM treatment and is homogeneous throughout the UE 24 hours after treatment (Lin et al., 2008). Thus, E9.5 treatment would abolish Shh expression at E10.5, when the GT initiates, whereas E12.5 treatment deletes Shh by E13.5, when GT outgrowth is underway. GT outgrowth in all Shh-cKOs was clearly defective, as evidenced by both scanning electron microscopy (SEM) and histological analysis (Fig. 1B-E). Tm treatment at E9.5 completely abrogated GT formation (Fig. 1B), whereas later treatments resulted in progressively less severe GT undergrowth (Fig. 1C-E). Furthermore, all Shh-cKOs exhibited proximal hypospadias, which showed an abnormal urethral opening on the ventral side of the GT (arrows in Fig. 1B-E). Histological analysis revealed either no (Fig. 1C′) or reduced (arrows in Fig. 1D′,E′ compared with 1A′) mesenchymal condensation, as well as defective urethral epithelia (arrowheads in Fig. 1C′-E′) in the mutant GTs.
Fig. 1.
Phenotypic and gene expression analysis of Shh conditional knockout GTs. (A-E) Scanning electron microscopy (SEM) analyses of E15.5 male genital tubercles (GTs) of control (A) and Shh-cKO (B-E) mice. The timing of Tamoxifen (Tm) treatment is indicated. Proximal hypospadias is indicated by arrows in B-E. (A′,C′-E′). Hematoxylin and Eosin (H&E)-stained cross-sections of wild-type (A′) and late Shh-cKO (C′-E′) GTs. Urethral epithelium and mesenchymal condensations are indicated by arrowheads and arrows, respectively. (F-K′) Whole-mount in situ analysis on E12.5 GTs from Shh-cKOs (F′-K′) and littermate controls (F-K). All embryos were exposed to Tm at E10.5.
We next investigated the molecular effectors downstream of Shh signaling. Previous reports demonstrated downregulation of GT-specific genes in Shh-/- embryos (Haraguchi et al., 2001; Perriton et al., 2002). However, as the GT is completely absent in Shh-/- embryos, it is difficult to rule out the possibility that those gene alterations were due to loss of GT cells. Moreover, although Hoxd13 expression was shown to persist in the presumptive GT region of Shh-/- mutants (Perriton et al., 2002), the identity of those Hoxd13-expressing cells was unclear. Thus, we re-examined the expression of GT regulatory genes at E12.5 in Shh-cKOs treated with TM at E10.5, which still retain a considerable GT structure. The Ptch1 transcript was undetectable in the mesenchyme of the mutant GT (Fig. 1F′), indicating a complete loss of Shh signaling. Fgf8 was markedly downregulated (Fig. 1G′ compared with 1G) in the dUE of the mutant GT. Consistent with previous reports, Msx2 and Wnt5a, presumed downstream targets of dUE signaling (Lin et al., 2008), were also downregulated in the distal mesenchyme of the Shh-cKO GTs (Fig. 1H′,I′ compared with 1H,I). Unexpectedly, we also uncovered a clear reduction in the expression of both Hoxa13 and Hoxd13. Whereas Hoxa13 expression is normally detected throughout the entire GT (Fig. 1J), it was limited to the distal GT in the mutants (Fig. 1J′). Similarly, Hoxd13 was downregulated in Shh-cKOs when compared with the control (Fig. 1K versus 1K′ and see Fig. S1A versus S1B in the supplementary material; note the comparable expression in the limb but reduced expression in the GT). To further verify our findings, we performed real-time RT-PCR analysis on E12.5 Shh-cKO GTs (Tm treated at E10.5). A 70% reduction in Hoxa13 expression (n=5, P=0.001) and a 50% reduction in Hoxd13 expression (n=4, P=0.008) was found in these mutant GTs (see Fig. S2C,D in the supplementary material). Altogether, these data revealed a continuous and genital-specific role of Shh signaling in maintaining GT-specific gene expression. We also found that cloacal septation in these mutant embryos was incomplete. Control E12.5 embryos showed complete cloacal separation (see Fig. S2A in the supplementary material), whereas the urogenital sinus was still connected to the anal channel in E10.5 Tm-treated Shh-cKO embryos (see Fig. S2B in the supplementary material, white arrow).
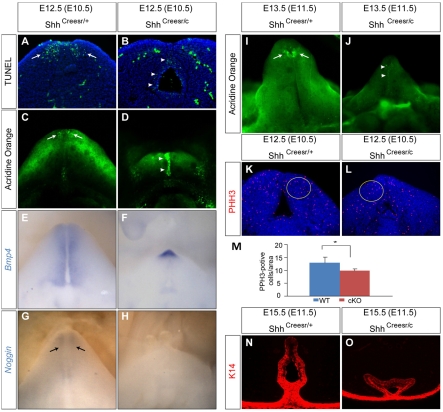
To investigate the cellular mechanisms underlying GT dysplasia in Shh-cKOs, we examined apoptosis by TUNEL assay (Fig. 2A,B) and Acridine Orange staining at E12.5 (Fig. 2C,D) and E13.5 (Fig. 2I,J). We found a notable increase in UE cell death at E12.5 and E13.5 (arrowheads in Fig. 2B,D,J), but a lack of normal mesenchymal apoptosis (arrows in Fig. 2A,C,I) in the mutants. As Bmp signaling has been implicated in the regulation of apoptosis during GT development (Suzuki et al., 2003), we examined the expression of Bmp4 and its antagonist noggin (Nog) in Shh-cKO GTs. Nog expression was clearly downregulated in the distal GT mesenchyme (compare Fig. 2H with 2G). By contrast, Bmp4 expression was upregulated in the distal GT (compare Fig. 2E with 2F). The altered expression of Bmp4 and Nog correlates well with the increased apoptosis observed in Shh-cKOs, suggesting a causal relationship. Interestingly, despite increased apoptosis at earlier stages, the progenitor cell population characterized by keratin 14 (K14) expression was maintained in the mutant UE at E15.5 (Fig. 2N versus 2O). Next, we examined cell proliferation by immunofluorescence staining using a polyclonal antibody against the mitosis marker phospho-histone H3 (PHH3). We scored the number of PHH3-positive cells in a fixed-sized area in the GT mesenchyme, and revealed a 25% reduction in PHH3-positive cells in the GT mesenchyme of Shh-cKOs (n=8, P=0.0027; for each sample, one circled region on four different sections was counted; Fig. 2K-M). Both the increased UE cell death and the lack of mesenchymal cell proliferation are likely to contribute to the GT dysplasia in Shh-cKO mice.
Fig. 2.
Cell death and proliferation analyses in Shh-cKOs. (A,B) TUNEL analysis on coronal sections of GTs (Tm treatment at E10.5) showing distal mesenchymal apoptosis in control (A, arrows) and excessive urethral epithelium (UE) staining in Shh-cKO (B, arrowheads) GT. (C,D) Whole-mount Acridine Orange (AO) staining showing patterned mesenchymal cell death in control (C, arrows), and a drastically augmented UE staining in Shh-cKOs (D, arrowheads). (E-H) Whole-mount in situ hybridization on E12.5 GTs from Shh-cKOs (F,H) and littermate controls (E,G) using the probes indicated. Bmp4 expression is normally detected in the genital mesenchyme (E), and is increased in the distal GT of the mutant (F). Nog is expressed in the distal mesenchyme (arrows in G), and is undetectable in the mutant (H). (I,J) AO staining of E13.5 control (I) and Shh-cKO (J) GT (both were treated with Tm at E11.5) showing a normal mesenchymal staining pattern in the control (I, arrows), and a UE-restricted staining in the Shh-cKO (J, arrowheads). (K-M) Phospho-histone H3 (PHH3) staining on coronal sections of Shh-cKO (L) and controls (K) showing a 20% reduction (M) in PHH3-positive cells in the yellow-circled region. n=8, *P=0.0027. (N,O) Indirect immunofluorescence using a K14 antibody on E15.5 control (N) and Shh-cKO (O) male GT (TM treated at E11.5).
GT mesenchyme is responsive to Shh signaling
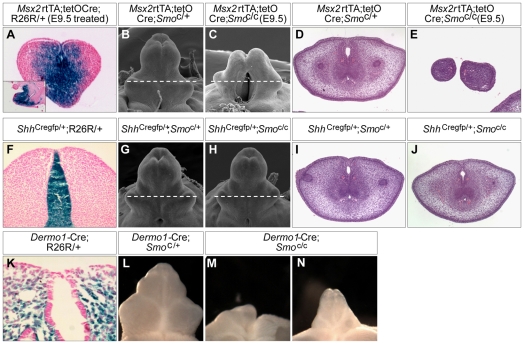
To distinguish the roles of Shh in the UE versus the mesenchyme, we manipulated the activities of the Shh signal executor smoothened (Smo) in a tissue-specific manner. In the first experiment, we removed Smo from both UE and mesenchyme using an Msx2-rtTA;tetO-Cre system, which confers Cre activity in both tissues (Lin et al., 2009). In this system, rtTA (reverse tetracycline-controlled transactivator) expression is under the control of the Msx2 promoter in a BAC transgenic line. Upon doxycycline (Dox) treatment, rtTA is activated and binds to the tetO sequence to activate Cre expression. Cre activity is monitored by the R26R allele (Soriano, 1999) (Fig. 3A). After verifying the activation pattern of the Msx2-rtTA;tetO-Cre system, we mated Msx2-rtTA;tetO-Cre;Smoc/+ males to female Smoc/c mice. This mating generates one in every eight embryos with the desired Msx2-rtTA;tetO-Cre;Smoc/c genotype. These embryos, upon Dox treatment, lose functional Smo in the Cre-expressing urethra and genital mesenchyme and thus are termed UEMes-Smo-cKOs. E9.5 Dox-treated UEmes-Smo-cKO embryos exhibited GT outgrowth defects as well as proximal hypospadias (Fig. 3C). Later Dox administrations led to less severe GT underdevelopment (data not shown). Histological analysis of an E9.5 Dox-treated UEMes-Smo-cKO embryo showed a completely split GT with two separate lateral growth areas that developed from the initial lateral swellings (Fig. 3E). Consistent with the strong activity of this transgenic system in this region (Fig. 3A), the dorsal GT mesenchyme, which normally originates from the dorsal GT swelling, was completely missing in the mutant embryo (Fig. 3C,E).
Fig. 3.
Phenotypic analyses of conditional knockout Smo mutant GTs. (A) β-gal staining on cross-section or sagittal section (inset) of an E12.5 Msx2-rtTA;tetO-Cre;R26R/+ GT (Dox treatment at E9.5) showing strong Cre expression in UE and mesenchyme, especially the dorsal GT mesenchyme. (B,C) SEM analysis of E15.5 male UEMes-Smo-cKO (C) and littermate control (B) GT. (D,E) H&E staining of UEMes-Smo-cKO (E) and littermate control (D) GT. The planes of sections are indicated in B and C (dashed lines). (F) β-gal staining on cross-sections of a E12.5 ShhCregfp/+;R26R/+ GT demonstrating Cre expression in UE. (G-J) SEM and histological analyses of UE-Smo-cKOs (H,J) and littermate controls (G,I). (K) β-gal staining on cross-sections of a E10.5 Dermo1-Cre;R26R/+ GT showing that the expression was exclusively in the cloacal mesenchyme. (L-N) Light microscopy of Mes-Smo-cKOs (M,N) and control (L). GTs from E15.5 male mouse embryos were analyzed in B-E,G-J,L-N.
Expression studies showed that similar to Shh-cKOs, UEMes-Smo-cKOs also exhibited downregulation of Fgf8, Hoxd13, Hoxa13 and Wnt5a expression (compare Fig. S3K-N with S3G-J in the supplementary material). We next removed Smo using the UE-specific Shh-Cregfp (UE-Smo-cKOs) (Lin et al., 2008), or the GT mesenchyme-specific Dermo1-Cre (mes-Smo-cKOs) (Lin et al., 2008) (Dermo1 is also known as Twist2 - Mouse Genome Informatics). Importantly, the UE-Smo-cKOs exhibited normal GT development (Fig. 3G,J), with no major changes in the expression of Fgf8, Hoxd13 and Hoxa13 (compare Fig. S3D-F with S3A-C in the supplementary material). Thus, Shh signaling in the UE is dispensable for GT development. By contrast, GT outgrowth in Mes-Smo-cKOs was consistently retarded (Fig. 3M,N compared with 3L), although the severity varied among different embryos. The most severely affected individual showed GT agenesis similar to that observed in Shh-cKOs treated with Tm at E9.5 (Fig. 3M compared with Fig. 1B), whereas others exhibited moderate underdevelopment (Fig. 3N). The differential expressivity of the phenotype is likely to have resulted from the patchy deletion pattern of this Cre line, as previously reported (Lin et al., 2008). We also found that similar to Shh-cKOs, the urogenital sinus and anal channel failed to completely separate in the Mes-Smo-cKOs (see Fig. S2D in the supplementary material, white arrow). By contrast, UE-Smo-cKOs showed normal cloacal patterning (see Fig. S2C in the supplementary material). Overall, these results demonstrate that the primary target tissue of Shh signaling in genital development is the GT mesenchyme.
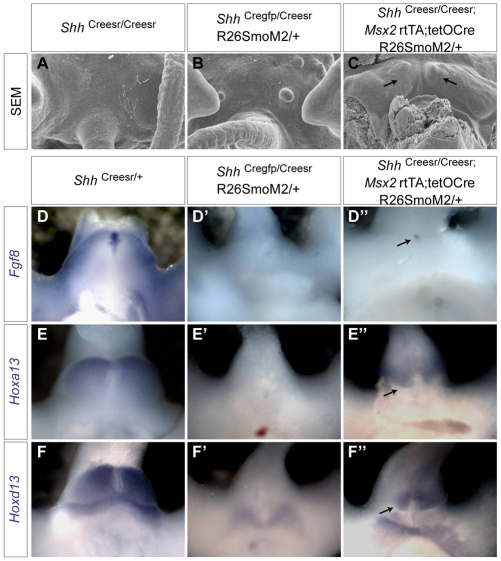
To further test our hypothesis, we sought to restore Hh responsiveness in different tissues to rescue the Shh-/- phenotype. We used R26-SmoM2 (Jeong et al., 2004), a previously described conditional constitutively active Smo allele. This allele was made by knocking a constitutively active Smo (W539L) into the ubiquitous R26 promoter, with a floxed transcription stop cassette. SmoM2 will not be expressed unless the stop cassette is removed by Cre recombinase. Crossing of this strain together with ShhCregfp, Dermo1-Cre or the Msx2-rtTA;tetO-Cre system back into an Shh-null background will restore Shh responsiveness in the UE, mesenchyme or both, respectively, in the absence of the Shh ligand. Dermo1-Cre;R26-SmoM2/+;Shh-/- embryos died at ~E10.5-11, with hemorrhages throughout the body, precluding further investigation. ShhCregfp/Creesr;R26-SmoM2/+ (ShhCregfp and ShhCreesr are both Shh-null alleles) did not show any phenotypic rescue in the genital region (compare Fig. 4B with 4A). By contrast, Msx2- rtTA;tetO-Cre;R26-SmoM2/+;ShhCreesr/Creesr embryos showed bilateral genital growth in the cloaca region upon Dox treatment at E9.5 (Fig. 4C, arrows). However, the genital structure appeared smaller than in littermate controls.
Fig. 4.
Rescuing GT agenesis in Shh-/- embryos by restoring Hh responsiveness. (A-C) SEM analysis of E12.5 genital region of Shh-/- mouse embryos (A) and Shh-/- embryos with Hh responsiveness restored in UE (B) or in UE and mesenchyme together (C). Bilateral growth in the UEmes-rescued line is indicated by arrows in C. (D-F″) Whole-mount in situ hybridization on E11.5 UE-rescued Shh-/- (D′,E′,F′), UEmes-rescued Shh-/- (D″,E″,F″) and ShhCreesr/+ littermate control (D,E,F) GT. The rescued expression of Fgf8, Hoxa13 and Hoxd13 is indicated by arrows.
Further expression analysis revealed that Fgf8, Hoxa13 and Hoxd13 (Fig. 4D″,E″,F″) were partially rescued in the Msx2-rtTA-mediated, but not in the ShhCregfp-mediated, lines (Fig. 4D′,E′,F′). Expression of these genes was detected in the rescued genital structure, which was localized more posteriorly than in controls. The Fgf8 expression level was reduced and its expression domain was clearly smaller in the rescued GTs (Fig. 4D″). Hoxa13 and Hoxd13 were strongly expressed in the mesenchyme of the rescued GT surrounding the UE (Fig. 4E″,F″). Even though their expression levels appeared to be comparable to those of controls, their expression domains were smaller in the rescued embryos. This reduction is consistent with the smaller size of the rescued GT structure (Fig. 4C). The incomplete rescue, both at the morphological and at the molecular level, might be caused by reduced Msx2 promoter activity in Shh-null mutant GT. Alternatively, ectopically sustained Smo activity throughout the GT mesenchyme might also affect normal GT patterning. Nonetheless, these results indicate that restoring Hh responsiveness in the mesenchyme, but not the UE, is crucial for rescuing GT growth defects in Shh mutants. Thus, both loss- and gain-of-function studies indicate that Shh primarily signals to the mesenchyme to promote GT growth.
Shh relays its signal though dUE in GT initiation
It is well established that the development of the limb bud relies on a positive-feedback loop between two signaling centers: the AER marked by Wnt signaling activity and Fgf8 expression, and the Shh-expressing zone of polarizing activity (ZPA) located in the posterior limb mesenchyme. It is proposed that Shh relays its growth-promoting signal through maintenance of the AER (Allard and Tabin, 2009). The similarities between limb and GT development prompted us to hypothesize that Shh might also signal through the dUE, the AER-equivalent signaling center in the GT. To test this, we employed a floxed gain-of-function (GOF) β-catenin (Ctnnb1) allele (βCatex3) (Harada et al., 1999), which has been shown to induce ectopic dUE signaling when activated in cloacal endoderm (Lin et al., 2008). Specifically, we generated ShhCreesr/Creesr;βCatex3/+ embryos (hereafter referred to as dUE-rescued embryos) and induced Cre activity by Tm injection at 9.5 dpc. To verify the establishment of dUE signals in dUE-rescued embryos, the expression of Fgf8 and Bmp7, both dUE markers, was examined. Transcription of these genes was completely abolished in the Shh mutant dUE (Fig. 6A′,B′); however, expression of both was restored in the dUE-rescued embryos (Fig. 6A″,B″). The expression domain of dUE markers appeared to be expanded and disorganized in the dUE-rescued GT compared with controls (Fig. 6A″,B″). This change is likely to reflect an expansion of the UE under ectopic Wnt activity, as also observed when β-catenin is overexpressed in the UE of Shh+/- embryos (Lin et al., 2008). As a consequence of dUE rescue, the initial GT outgrowth was restored in these rescued embryos at E11.5 as revealed by SEM (Fig. 5C compared with 5B) and as evidenced by the comparable size of wild-type and rescued embryos (Fig. 5C compared with 5A). However, GTs in the dUE-rescued embryos failed to sustain their growth as development progressed, as indicated by their much smaller size at E12.5 as compared with controls (Fig. 5F versus 5D). Moreover, dUE-rescued GT tilted caudally (Fig. 5F) in contrast to a rostral tilt in controls (Fig. 5D). Histological analysis of rescued embryos showed that although GT growth was prominent on the dorsal side (arrows in Fig. 5I), the urethra failed to extend to the distal GT (the distal-most part of UE is indicated by an arrowhead in Fig. 5I), and the cloaca remained unseptated (Fig. 5I). Despite the incomplete rescue, these data indicate that Shh promotes GT initiation at least in part through β-catenin signaling to the dUE.
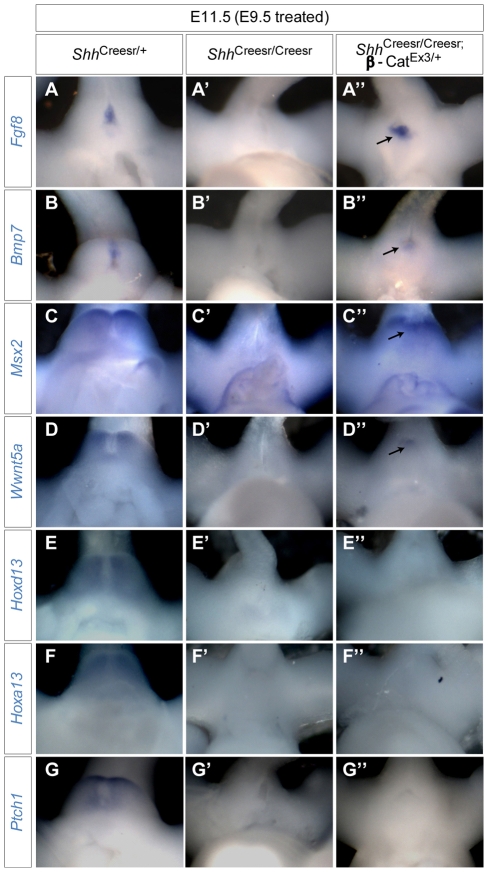
Fig. 6.
Gene expression analysis of dUE-rescued Shh-/- GTs. (A-G″) Mouse embryos were treated with Tm at E9.5 and collected on E11.5 for in situ hybridization with the probes indicated. The expected expression pattern of Fgf8, Bmp7, Msx2 and Wnt5a was observed in the controls (A-D), was absent in Shh-/- GTs (A′-D′), but was fully (A″,B″, arrows) or partially (C″,D″, arrows) restored in dUE-rescued Shh-/- GTs. Expression of Hoxd13 and Hoxa13 was either severely reduced (E′,F′) or undetectable (E″,F″) in both Shh-/- and dUE-rescued Shh-/- GTs. Ptch1 expression was not detected in either Shh-/- or dUE-rescued GTs (G-G″).
Fig. 5.
Phenotypic analysis of dUE-rescued Shh-/- GTs. (A-C) SEM analysis of E11.5 control (A), Shh-/- (B) and dUE-rescued Shh-/- (C) mouse embryos showing agenesis of the GT in Shh-/- (B) and prominent GT growth in the rescued Shh-/- (arrow in C). (D-F) SEM analysis of E12.5 embryos of the genotypes indicated showing that the rescued GT (F) was much smaller than in the wild type (D). (G-I) H&E staining on mid-sagittal sections of E12.5 embryos showing a considerable dorsal growth (arrows in I) and a persistent cloaca in the dUE-rescued Shh-/-. The distal end of the UE is indicated by arrowheads in G,I. gt, genital tubercle; us, urogenital sinus; r, rectum; c, cloaca; t, tail.
We next examined whether forced activation of β-catenin signaling in the dUE could replace Shh to activate downstream gene expression. We compared gene expression in genital regions between Shh-/- and dUE-rescued Shh-/- embryos. Msx2 and Wnt5a expression was partially rescued (Fig. 6C″,D″ compared with 6C′,D′). Msx2 expression was detected in the distal genital mesenchyme, similar to its normal expression pattern (Fig. 6C″ compared with 6C). By contrast, the Wnt5a expression domain was smaller and distally restricted in the dUE-rescued GT. This is consistent with our previous finding that both genes can be induced by ectopic Wnt-Fgf8 signaling (Lin et al., 2008). However, Hoxa13 and Hoxd13 expression was not rescued (Fig. 6E″,F″ compared with 6E′,F′). This is also consistent with the fact that the expression of neither gene is altered in β-catenin GOF and loss-of function (LOF) mutants (Lin et al., 2008). Expression of Ptch1 was not detectable in Shh mutant and dUE-rescued embryos, indicating that restoring dUE signaling did not rescue Shh responsiveness in the genital mesenchyme (Fig. 6G-G″). To rule out the possibility that the reduced cell number in Shh-/- GTs could affect the outcome of gene expression analyses, we mated the same GOF β-catenin allele into the Shh-cKO background (ShhCreesr/c;βCatex3/+) and re-examined gene expression. Again, we found that the GOF β-catenin allele rescued expression of the dUE target gene Msx2, but not of Hoxa13 and Hoxd13, in the Shh-cKO background (data not shown). Thus, Shh appears to regulate Hox genes independently of dUE signaling, and the failure to restore Hox gene expression could contribute to the lack of cloacal septation and failure of continuous GT growth in the dUE-rescued GT. These data strongly suggest that dUE and Shh signaling coordinately regulate GT growth, although each has an indispensable function that cannot be compensated for by the other.
DISCUSSION
We have analyzed the function of Shh during early GT development in mice, identified its target tissue and studied its interaction with the dUE. We showed that Shh plays a crucial role during GT initiation and subsequent growth. Both LOF and rescue experiments indicated that the primary target tissue of Shh is the genital mesenchyme. Shh exerts its function in part through maintenance of the dUE, and this regulation is essential for the formation of the GT primordia. We also uncovered a dUE-independent role for Shh in patterning the cloaca field and sustaining GT growth, possibly through regulating Hox gene expression. These findings highlight a dynamic role for Shh in coordinating GT outgrowth and cloaca patterning.
Shh in genital development
By removing Shh from cloacal endoderm and, later, from the UE, at successive stages during GT development, we demonstrated an obligatory and continuous role for Shh in establishing and maintaining GT growth. Ablating Shh before GT development completely abrogated GT initiation, whereas loss of Shh after the GT primordia had formed still resulted in a drastic reduction in GT size. In this sense, Shh plays a more profound role in GT development than in the limb, as limb outgrowth of Shh-/- embryos is only moderately affected, as evidenced by the presence of digit 1 and some proximal skeletal elements (Kraus et al., 2001). Moreover, a short pulse of Shh signaling appears to be sufficient for continuous limb outgrowth, as Hoxb6-Creesr;Shhc/c and ShhCregfp/c limbs exhibit digit loss but near-normal proximal skeletal structures (Scherz et al., 2007; Zhu et al., 2008).
Shh can operate either intra-epithelially (Gritli-Linde et al., 2002; Gritli-Linde et al., 2007) or non-autonomously through epithelial-mesenchymal interactions (Jeong et al., 2004; Vokes et al., 2004). By conditionally removing its receptor smoothened in different tissue layers, we identified GT mesenchyme as the primary Shh-responsive tissue layer in GT development. Mes-Smo-cKO and UEMes-Smo-cKO embryos showed a similar undergrowth phenotype and downregulated gene expression compared with Shh-cKO, whereas UE-Smo-cKO embryos showed a wild-type GT morphology. In addition, the Shh-/- GT agenesis phenotype can be partially rescued when Hh responsiveness is restored in both epithelium and mesenchyme, but not in epithelium alone. Together, these results strongly argue for a non-autonomous action of Shh signaling through the GT mesenchyme. Our data do not exclude genital ectoderm, especially the midline ectoderm positioned directly above the endodermal urethra (Suzuki et al., 2003), as a Shh-responsive tissue, although none of the Shh reporter lines marks this tissue layer (Haraguchi et al., 2007). It is also noteworthy that Hh can mediate cell cycle progression by regulating cyclin gene expression through non-canonical Hh signaling independently of the canonical Smo-Gli pathway (Barnes et al., 2001; Jenkins et al., 2007). Thus, manipulating Smo expression does not necessarily recapitulate all Hh activity. Future analyses from these perspectives should be highly informative.
Our data also revealed that Shh maintains dUE signaling through reciprocal interactions with the GT mesenchyme. Our previous work demonstrated the downregulation of Shh expression in a β-catenin LOF mutant in which the dUE is abolished or reduced (Lin et al., 2008). This positive-feedback loop appears to be similar in both limb and GT. The ectopic activation of dUE signaling in the absence of Shh restored the initiation of GT growth, indicating that part of the function of Shh in the GT is to maintain dUE. Altogether, these findings demonstrated that outgrowth programs in both limb and GT are governed by a similar genetic cassette. However, in the dUE-rescued embryos, although initial growth of the GT was restored, the cloaca remained unseptated and, instead of extending to the distal GT, the cloacal endoderm remained trapped in the body, resulting in a patterning failure and growth arrest (Fig. 5I). Consistently, Hoxd13 and Hoxa13 expression was not restored in the dUE-rescued embryos. These data indicate that the continuous growth of the GT has to be coordinated with the proper morphogenesis of the cloacal endoderm and para-cloacal mesoderm. Shh orchestrates this process by promoting GT outgrowth and regulating cloaca patterning. The regulation of Hox genes by Shh is of particular interest in this process. Our data demonstrating that Hh signaling is essential for both Hoxa13 and Hoxd13 expression are consistent with an inductive role reported for Shh in regulating chicken gut patterning (Roberts et al., 1995), and also agree with previous findings that ectopic Hh signaling can stimulate Hoxd13 expression in mouse GT culture (Haraguchi et al., 2001). As Hox genes are good candidates for determining the anatomical boundary of gut derivatives, acquiring Hoxa13 and Hoxd13 expression might be important in establishing GT identity at the caudal end of the cloaca. In support of this notion, mice with mutations in both genes exhibit profound malformation in the urogenital sinus and its derivatives (Warot et al., 1997). Other than the failure to rescue Hox gene expression, uncontrolled β-catenin signaling might also have negative effects on GT formation in the absence of Hh signaling.
Another interesting question is how Shh signaling maintains dUE. This regulation has to go through the genital mesenchyme as we demonstrated above; Shh does not function autonomously within the UE. One of the candidate pathways that might participate in this reciprocal regulation is Bmp signaling. It is known that in the limb, Shh maintains the AER by activating gremlin (Grem) expression in the anterior mesenchyme (Harfe et al., 2004). The finding that the expression of Bmp4 was augmented, whereas Nog expression was reduced, in the distal GT of Shh-cKO embryos fits well with the speculation that Shh maintains dUE in a similar manner in the GT as it does in the limb, i.e. by antagonizing the activity of the pro-apoptotic Bmp pathway. However, definitive evidence has to come from future genetic experiments in which either Nog is conditionally overexpressed or Bmp4 is conditionally knocked out in the Shh-deficient GT.
Shh, dUE and genital evolution
The finding that Shh plays a similar, but obviously more complicated role in GT development than in limb development, raises questions regarding the evolution of both appendages. A similar genetic regulatory innovation was proposed to play a role in the evolution of digits and GTs (Kondo et al., 1997). Although Shh is expressed in both appendages, its expression in the ZPA and that in the hindgut are regulated by different enhancers. Hindgut Shh expression relies on DNA elements that are not conserved between mammals and the teleost fish medaka (Sagai et al., 2009), whereas those controlling ZPA Shh expression are highly conserved (Lettice et al., 2008; Sagai et al., 2005). These findings suggest a differential acquisition of Shh expression in the evolution of the two appendages. Recruiting Shh expression is considered an innovation of teleost fish to develop a proximal-distal limb axis and autopod elements in the existing fin bud (Tanaka et al., 2002), whereas Shh expression in the cloaca is rather ancestral and can be detected in species with no genital eminence (Freitas et al., 2007). These observations suggest a divergent function for Shh in the evolution of limbs and genitalia.
Furthermore, the concept of a median unpaired fin-to-genitalia transition is not supported by recent genetic analyses. If the GT forms by imposing modifications on a pre-existing fin structure, one would expect the instructive signaling epithelium to be ectodermal in origin, like the AER, or apical ectodermal fold (AEF) in fish. But instead, GT growth is directed by the Fgf8-expressing dUE, which is completely endodermal in origin (Kurzrock et al., 1999a; Lin et al., 2008; Seifert et al., 2008). However, a similar outgrowth mechanism appears to function in the development of both appendages. In our genetic analysis of GOF and LOF β-catenin mutants, a conserved regulation of Fgf8 by the Wnt-β-catenin pathway was found in both the AER and dUE (Lin et al., 2008). The role of Shh in maintaining the distal signaling center also appears to be similar in both appendages. Thus, we propose a model in which the GT evolved through a mechanism whereby the caudal end of the cloaca adopted an AER-like outgrowth genetic cassette, and in so doing established a novel growth field around the cloacal membrane. Our finding that the regulation of Hox genes by Shh is not mediated through dUE signaling, together with the fact that cloacal expression of Shh and Hox genes occurs in species with no phallus growth (Freitas et al., 2007), suggest that Shh and Hox genes are likely to function as permissive factors in this process. In this sense, the establishment of dUE signaling should represent the most critical event in GT evolution (summarized in Fig. 7). This model is consistent with the previously proposed hypothesis that the evolution of the genital organ might be achieved by receiving a proliferative cue in the cloaca region of those species that have already gained Hox and Shh expression (Freitas et al., 2007).
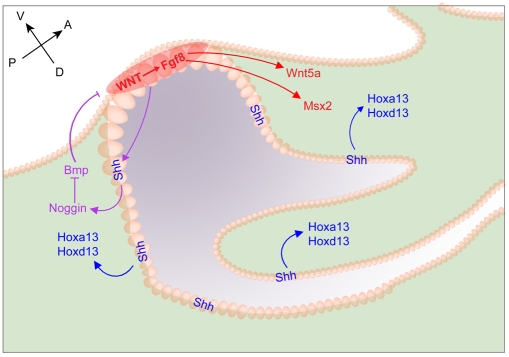
Fig. 7.
Coordination of Shh and dUE signaling in patterning the cloaca and in formation of the mouse GT. Shh signals (dark-blue arrows) to adjacent cloacal mesenchyme to activate Hox gene expression and to coordinate patterning and growth of the whole cloacal field. The addition of dUE signaling (red), activated by Wnt signaling, functions as an AER-like outgrowth genetic cassette, triggering the distal growth of the mesenchymal cells and resulting in the formation of the genital primordia (red arrows). Shh might also signal to genital mesenchyme to activate noggin expression, which in turn inhibits Bmp activity and thus maintains dUE signaling (purple arrows).
Supplementary Material
Acknowledgements
We thank Drs Gen Yamada and Martin Cohn for exchanging progress before submission; Dr Feng Chen for tetO-Cre animals; Jaclynn Lett and the Research Center for Auditory and Vestibular Studies, Department of Otolaryngology at Washington University for technical assistance with electron microscopy; and Dr Yiping Chen for in situ probes. This work was funded by NIH grants ES014482 and ES01659701A1. Deposited in PMC for release after 12 months.
Footnotes
Supplementary material
Supplementary material for this article is available at http://dev.biologists.org/cgi/content/full/136/23/3959/DC1
References
- Allard P., Tabin C. J. (2009). Achieving bilateral symmetry during vertebrate limb development. Semin. Cell Dev. Biol. 20, 479-484 [DOI] [PubMed] [Google Scholar]
- Barnes E. A., Kong M., Ollendorff V., Donoghue D. J. (2001). Patched1 interacts with cyclin B1 to regulate cell cycle progression. EMBO J. 20, 2214-2223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn M. J. (2004). Developmental genetics of the external genitalia. Adv. Exp. Med. Biol. 545, 149-157 [DOI] [PubMed] [Google Scholar]
- Echelard Y., Epstein D. J., St-Jacques B., Shen L., Mohler J., McMahon J. A., McMahon A. P. (1993). Sonic hedgehog, a member of a family of putative signaling molecules, is implicated in the regulation of CNS polarity. Cell 75, 1417-1430 [DOI] [PubMed] [Google Scholar]
- Freitas R., Zhang G., Cohn M. J. (2007). Biphasic Hoxd gene expression in shark paired fins reveals an ancient origin of the distal limb domain. PLoS ONE 2, e754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gritli-Linde A., Bei M., Maas R., Zhang X. M., Linde A., McMahon A. P. (2002). Shh signaling within the dental epithelium is necessary for cell proliferation, growth and polarization. Development 129, 5323-5337 [DOI] [PubMed] [Google Scholar]
- Gritli-Linde A., Hallberg K., Harfe B. D., Reyahi A., Kannius-Janson M., Nilsson J., Cobourne M. T., Sharpe P. T., McMahon A. P., Linde A. (2007). Abnormal hair development and apparent follicular transformation to mammary gland in the absence of hedgehog signaling. Dev. Cell 12, 99-112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada N., Tamai Y., Ishikawa T., Sauer B., Takaku K., Oshima M., Taketo M. M. (1999). Intestinal polyposis in mice with a dominant stable mutation of the beta-catenin gene. EMBO J. 18, 5931-5942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haraguchi R., Suzuki K., Murakami R., Sakai M., Kamikawa M., Kengaku M., Sekine K., Kawano H., Kato S., Ueno N., et al. (2000). Molecular analysis of external genitalia formation: the role of fibroblast growth factor (Fgf) genes during genital tubercle formation. Development 127, 2471-2479 [DOI] [PubMed] [Google Scholar]
- Haraguchi R., Mo R., Hui C., Motoyama J., Makino S., Shiroishi T., Gaffield W., Yamada G. (2001). Unique functions of Sonic hedgehog signaling during external genitalia development. Development 128, 4241-4250 [DOI] [PubMed] [Google Scholar]
- Haraguchi R., Motoyama J., Sasaki H., Satoh Y., Miyagawa S., Nakagata N., Moon A., Yamada G. (2007). Molecular analysis of coordinated bladder and urogenital organ formation by Hedgehog signaling. Development 134, 525-533 [DOI] [PubMed] [Google Scholar]
- Harfe B. D., Scherz P. J., Nissim S., Tian H., McMahon A. P., Tabin C. J. (2004). Evidence for an expansion-based temporal Shh gradient in specifying vertebrate digit identities. Cell 118, 517-528 [DOI] [PubMed] [Google Scholar]
- Jenkins D., Winyard P. J., Woolf A. S. (2007). Immunohistochemical analysis of Sonic hedgehog signalling in normal human urinary tract development. J. Anat. 211, 620-629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong J., Mao J., Tenzen T., Kottmann A. H., McMahon A. P. (2004). Hedgehog signaling in the neural crest cells regulates the patterning and growth of facial primordia. Genes Dev. 18, 937-951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo T., Zakany J., Innis J. W., Duboule D. (1997). Of fingers, toes and penises. Nature 390, 29 [DOI] [PubMed] [Google Scholar]
- Kraus P., Fraidenraich D., Loomis C. A. (2001). Some distal limb structures develop in mice lacking Sonic hedgehog signaling. Mech. Dev. 100, 45-58 [DOI] [PubMed] [Google Scholar]
- Kurzrock E. A., Baskin L. S., Cunha G. R. (1999a). Ontogeny of the male urethra: theory of endodermal differentiation. Differentiation 64, 115-122 [DOI] [PubMed] [Google Scholar]
- Kurzrock E. A., Baskin L. S., Li Y., Cunha G. R. (1999b). Epithelial-mesenchymal interactions in development of the mouse fetal genital tubercle. Cells Tissues Organs 164, 125-130 [DOI] [PubMed] [Google Scholar]
- Lettice L. A., Hill A. E., Devenney P. S., Hill R. E. (2008). Point mutations in a distant sonic hedgehog cis-regulator generate a variable regulatory output responsible for preaxial polydactyly. Hum. Mol. Genet. 17, 978-985 [DOI] [PubMed] [Google Scholar]
- Lewis P. M., Dunn M. P., McMahon J. A., Logan M., Martin J. F., St-Jacques B., McMahon A. P. (2001). Cholesterol modification of sonic hedgehog is required for long-range signaling activity and effective modulation of signaling by Ptc1. Cell 105, 599-612 [DOI] [PubMed] [Google Scholar]
- Lin C., Yin Y., Long F., Ma L. (2008). Tissue-specific requirements of beta-catenin in external genitalia development. Development 135, 2815-2825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C., Yin Y., Chen H., Fisher A. V., Chen F., Rauchman M., Ma L. (2009). Construction and characterization of a doxycycline-inducible transgenic system in Msx2 expressing cells. Genesis 5, 352-359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan E. A., Nguyen S. B., Scott V., Stadler H. S. (2003). Loss of Bmp7 and Fgf8 signaling in Hoxa13-mutant mice causes hypospadia. Development 130, 3095-3109 [DOI] [PubMed] [Google Scholar]
- Murakami R., Mizuno T. (1986). Proximal-distal sequence of development of the skeletal tissues in the penis of rat and the inductive effect of epithelium. J. Embryol. Exp. Morphol. 92, 133-143 [PubMed] [Google Scholar]
- Perl A. K., Wert S. E., Nagy A., Lobe C. G., Whitsett J. A. (2002). Early restriction of peripheral and proximal cell lineages during formation of the lung. Proc. Natl. Acad. Sci. USA 99, 10482-10487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perriton C. L., Powles N., Chiang C., Maconochie M. K., Cohn M. J. (2002). Sonic hedgehog signaling from the urethral epithelium controls external genital development. Dev. Biol. 247, 26-46 [DOI] [PubMed] [Google Scholar]
- Roberts D. J., Johnson R. L., Burke A. C., Nelson C. E., Morgan B. A., Tabin C. (1995). Sonic hedgehog is an endodermal signal inducing Bmp-4 and Hox genes during induction and regionalization of the chick hindgut. Development 121, 3163-3174 [DOI] [PubMed] [Google Scholar]
- Sagai T., Hosoya M., Mizushina Y., Tamura M., Shiroishi T. (2005). Elimination of a long-range cis-regulatory module causes complete loss of limb-specific Shh expression and truncation of the mouse limb. Development 132, 797-803 [DOI] [PubMed] [Google Scholar]
- Sagai T., Amano T., Tamura M., Mizushina Y., Sumiyama K., Shiroishi T. (2009). A cluster of three long-range enhancers directs regional Shh expression in the epithelial linings. Development 136, 1665-1674 [DOI] [PubMed] [Google Scholar]
- Scherz P. J., McGlinn E., Nissim S., Tabin C. J. (2007). Extended exposure to Sonic hedgehog is required for patterning the posterior digits of the vertebrate limb. Dev. Biol. 308, 343-354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert A. W., Harfe B. D., Cohn M. J. (2008). Cell lineage analysis demonstrates an endodermal origin of the distal urethra and perineum. Dev. Biol. 318, 143-152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano P. (1999). Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat. Genet. 21, 70-71 [DOI] [PubMed] [Google Scholar]
- Suzuki K., Bachiller D., Chen Y. P., Kamikawa M., Ogi H., Haraguchi R., Ogino Y., Minami Y., Mishina Y., Ahn K., et al. (2003). Regulation of outgrowth and apoptosis for the terminal appendage: external genitalia development by concerted actions of BMP signaling [corrected]. Development 130, 6209-6220 [DOI] [PubMed] [Google Scholar]
- Suzuki K., Ogino Y., Murakami R., Satoh Y., Bachiller D., Yamada G. (2002). Embryonic development of mouse external genitalia: insights into a unique mode of organogenesis. Evol. Dev. 4, 133-141 [DOI] [PubMed] [Google Scholar]
- Tanaka M., Munsterberg A., Anderson W. G., Prescott A. R., Hazon N., Tickle C. (2002). Fin development in a cartilaginous fish and the origin of vertebrate limbs. Nature 416, 527-531 [DOI] [PubMed] [Google Scholar]
- Vokes S. A., Yatskievych T. A., Heimark R. L., McMahon J., McMahon A. P., Antin P. B., Krieg P. A. (2004). Hedgehog signaling is essential for endothelial tube formation during vasculogenesis. Development 131, 4371-4380 [DOI] [PubMed] [Google Scholar]
- Warot X., Fromental-Ramain C., Fraulob V., Chambon P., Dolle P. (1997). Gene dosage-dependent effects of the Hoxa-13 and Hoxd-13 mutations on morphogenesis of the terminal parts of the digestive and urogenital tracts. Development 124, 4781-4791 [DOI] [PubMed] [Google Scholar]
- Wilkinson D. (1992). In Situ Hybridization; a Practical Approach London: Oxford University Press; [Google Scholar]
- Yamada G., Suzuki K., Haraguchi R., Miyagawa S., Satoh Y., Kamimura M., Nakagata N., Kataoka H., Kuroiwa A., Chen Y. (2006). Molecular genetic cascades for external genitalia formation: an emerging organogenesis program. Dev. Dyn. 235, 1738-1752 [DOI] [PubMed] [Google Scholar]
- Yin Y., Lin C., Ma L. (2006). MSX2 promotes vaginal epithelial differentiation and wolffian duct regression and dampens the vaginal response to diethylstilbestrol. Mol. Endocrinol. 20, 1535-1546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J., Nakamura E., Nguyen M. T., Bao X., Akiyama H., Mackem S. (2008). Uncoupling Sonic hedgehog control of pattern and expansion of the developing limb bud. Dev. Cell 14, 624-632 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.