Abstract
Objective:
To investigate the incidence of and risk factors for cognitive impairment in a large, well-defined clinical trial cohort of patients with early Parkinson disease (PD).
Methods:
The Mini-Mental State Examination (MMSE) was administered periodically over a median follow-up period of 6.5 years to participants in the Deprenyl and Tocopherol Antioxidative Therapy of Parkinsonism trial and its extension studies. Cognitive impairment was defined as scoring 2 standard deviations below age- and education-adjusted MMSE norms.
Results:
Cumulative incidence of cognitive impairment in the 740 participants with clinically confirmed PD (baseline age 61.0 ± 9.6 years, Hoehn-Yahr stage 1–2.5) was 2.4% (95% confidence interval: 1.2%–3.5%) at 2 years and 5.8% (3.7%–7.7%) at 5 years. Subjects who developed cognitive impairment (n = 46) showed significant progressive decline on neuropsychological tests measuring verbal learning and memory, visuospatial working memory, visuomotor speed, and attention, while the performance of the nonimpaired subjects (n = 694) stayed stable. Cognitive impairment was associated with older age, hallucinations, male gender, increased symmetry of parkinsonism, increased severity of motor impairment (except for tremor), speech and swallowing impairments, dexterity loss, and presence of gastroenterologic/urologic disorders at baseline.
Conclusions:
The relatively low incidence of cognitive impairment in the Deprenyl and Tocopherol Antioxidative Therapy of Parkinsonism study may reflect recruitment bias inherent to clinical trial volunteers (e.g., younger age) or limitations of the Mini-Mental State Examination–based criterion. Besides confirming known risk factors for cognitive impairment, we identified potentially novel predictors such as bulbar dysfunction and gastroenterologic/urologic disorders (suggestive of autonomic dysfunction) early in the course of the disease.
GLOSSARY
- CI
= confidence interval;
- COWA
= Controlled Word Association task;
- DATATOP
= Deprenyl and Tocopherol Antioxidative Therapy of Parkinsonism;
- DLB
= dementia with Lewy bodies;
- DST
= Digit Span Test;
- MMSE
= Mini-Mental State Examination;
- NDT
= New Dot Task;
- OMO
= Odd Man Out test;
- PD
= Parkinson disease;
- PDD
= dementia in Parkinson disease;
- PIGD
= postural instability and gait difficulty;
- SDMT
= Symbol Digit Modalities Test;
- SRT
= Selective Reminding Test;
- UPDRS
= Unified Parkinson's Disease Rating Scale.
Cognitive impairment can be present in the early,1,2 even yet untreated3 stages of Parkinson disease (PD). The current criteria for dementia in PD (PDD) require cognitive impairment in more than one domain, representing a decline from premorbid level with deficits severe enough to impair daily life (social, occupational, or personal care).4 Depending on the baseline age, severity of parkinsonism, and cognitive function of the studied population, 20% to 83% of patients with PD develop dementia (PDD).5–11 Known risk factors for PDD include postural instability and gait difficulty (PIGD), hallucinations, advanced age, male gender, depression, and poor performance on baseline cognitive tests.4,6,7 The annual incidence of PDD ranges between 42.6 and 112.5 cases per 1,000 person-years depending on the baseline characteristics (e.g., age, disease severity) or setting (hospital vs community based).5,6,8–10 The only community-based incident PD study on PDD reported an annual incidence (95% confidence interval [CI]) of 30.0 (16.4–52.9) per 1,000 person-years, with 13 out of 126 patients (10%) having developed dementia when tested 3.5 ± 0.7 years after diagnosis.2
Identifying important risk factors for cognitive impairment in early PD would enable clinicians to target those at highest risk when a disease-modifying treatment for dementia becomes available. We investigated the incidence of and risk factors for cognitive impairment in the Deprenyl and Tocopherol Antioxidative Therapy of Parkinsonism (DATATOP) cohort, a large early PD cohort with frequent evaluations (every 3–6 months) and long-term follow-up (up to 7.6 years) starting with the untreated (pre-levodopa) phase.12–14
METHODS
Subjects.
The DATATOP study enrolled 800 subjects (30–80 years old) within 5 years of symptom onset, who were not yet requiring symptomatic therapy.15,16 Further information on DATATOP12 and its extension studies13 can be found on the Neurology® Web site at www.neurology.org. Subjects were considered to have clinically confirmed idiopathic PD if the investigator maintained ≥60% confidence in the PD diagnosis14 across all visits. There were 57 subjects for whom the diagnostic confidence level fell below 60% at any visit or for whom no confidence data were available; of these, 7 were subjects in whom the alternative diagnosis was dementia with Lewy bodies (DLB), but with the onset of dementia more than 1 year after the onset of parkinsonism. Therefore, these 7 subjects were considered as having maintained a diagnosis of idiopathic PD per DLB Consortium17 and PDD Task Force4 criteria and were included in the analyses. Of the 750 subjects who maintained a diagnosis of clinically confirmed idiopathic PD throughout the study, 6 were found to have met our criteria for cognitive impairment at baseline (see below) and 4 did not have any follow-up Mini-Mental State Examination (MMSE) data. Thus, the final analysis group consisted of 740 clinically confirmed patients with PD free of cognitive impairment at baseline and with longitudinal MMSE evaluations.
Standard protocol approvals, registrations, and patient consents.
The DATATOP study and its extension studies were approved by the Institutional Review Boards of the participating institutions. A written informed consent was obtained from all participants.
Potential risk factors for cognitive impairment.
Our statistical analyses considered numerous factors across 8 domains. 1) Demographics: age, age ≥65, gender, and years of education >12.2) Family history: family histories of Alzheimer disease, psychiatric illness, and depression. 3) PD history: Years since diagnosis, years since symptom onset, side of PD onset, and initial symptoms of gait and tremor. 4) Motor features: Unified Parkinson's Disease Rating Scale (UPDRS) factor scores based on parts I–III were obtained from a principal component factor analysis with orthogonal (Varimax) rotation using data from the DATATOP baseline visit. This analysis identified 8 factors that accounted for 60.8% of the total sample variance (table e-1). As factor 8 was essentially UPDRS part I (mental), whose items were considered as separate independent variables (see below), the other 7 factor scores were considered as independent variables: PIGD, bradykinesia, rigidity, left-side tremor, right-side tremor, bulbar dysfunction, and dexterity loss (difficulty with manual abilities such as handwriting or using utensils). We also considered an asymmetry score derived from the UPDRS Motor subscale as previously described.14 5) Cognition18,19: MMSE (every 3 months), Digit Span Test forward and backward (DST–attention), Symbol Digit Modalities Test (SDMT–attention and visuomotor speed), Selective Reminding Test Delayed and Total Recall (SRT–verbal learning and memory), Odd Man Out test (OMO—set shifting-executive functions) using total correct in trials 2 and 4, New Dot Task (NDT–visuospatial working memory), and the Controlled Word Association task (COWA–verbal fluency/language, executive functions). Neuropsychological tests were administered at 6-month intervals. However, DST, OMO, and COWA were not administered after the first 2 years. 6) Psychiatric features: depression (HAM-D18 score ≥1020), apathy (UPDRS part I, item 4 [motivation/initiative] ≥2), and hallucinations (UPDRS part I, item 2 [thought disorder] ≥2). 7) Systemic disorders: a comprehensive neurologic and general medical evaluation was performed at baseline including whether a disorder was present in each of 11 organ systems. Neither the specific disorder nor its severity or significance was recorded.14 8) Medication use: use (yes/no) of deprenyl, agonists, amantadine, or anticholinergics, and cumulative exposure to levodopa (expressed as 100 mg-years).
The variables with available longitudinal data (UPDRS, SDMT, SRT, NDT, psychiatric features [depression, apathy, and hallucinations], and medication use) were treated as time-dependent covariates in the statistical analyses. Baseline values were used for all other predictors.
Analysis.
The primary outcome variable was the time from randomization to the development of cognitive impairment. We defined cognitive impairment as performing at least 2 SD below the age- and education-adjusted population norms on the MMSE,21 as operationalized by others.22 We did not use the 3 longitudinally available cognitive tests for diagnosis because of potential confounding of SDMT results by motor dysfunction18 and unavailability of adequate norms for the NDT, leaving only the SRT as a suitable test with established norms.23
Follow-up times were censored at the last available evaluation for subjects who did not experience the event of cognitive impairment. A Kaplan-Meier curve was used to describe the cumulative incidence of cognitive impairment over time; 95% confidence intervals were calculated for event rates at selected times.
Cox proportional hazards regression models were used to examine associations between the potential risk factors (independent variables given above) and the time to cognitive impairment. Our basic strategy for analysis was to first perform separate analyses of the associations of each potential risk factor with outcome. We then constructed separate multivariate models that did and did not include the neuropsychological test results as covariates. All variables retained in the final models were significant using a liberal 20% significance level, with the exception of age (p = 0.58), which was forced to be included in the model that did not include neuropsychological test results.
To explore the consequences of cognitive impairment on daily life, we defined functional decline as a score of 2 or more on UPDRS part I, item 1 (intellectual function),24 or a score of 2 (marginal function, major assistance) or 3 (unable) on either the occupational capacity or the handling finances question on the DATATOP disability progression form.16
In exploratory analyses, mixed-effects regression models were used to describe the changes over time in cognitive test performance in subjects who eventually did or did not develop cognitive impairment. Details of all statistical analyses are provided on the Neurology® Web site at www.neurology.org.
RESULTS
The subjects in the analysis cohort (n = 740) were followed for a median of 6.5 years (interquartile range 1.9–7.1 years). At baseline, they had mild parkinsonism and scored in the low-normal range on neuropsychological tests (table 1). Of the 740 subjects, 117 reached the primary endpoint (requiring levodopa) before any follow-up studies for DATATOP were in place (early endpointers). These “early endpointers” were included in the analysis up to the point of their last follow-up evaluation.
Table 1 Baseline characteristics of the analysis cohort (n = 740)
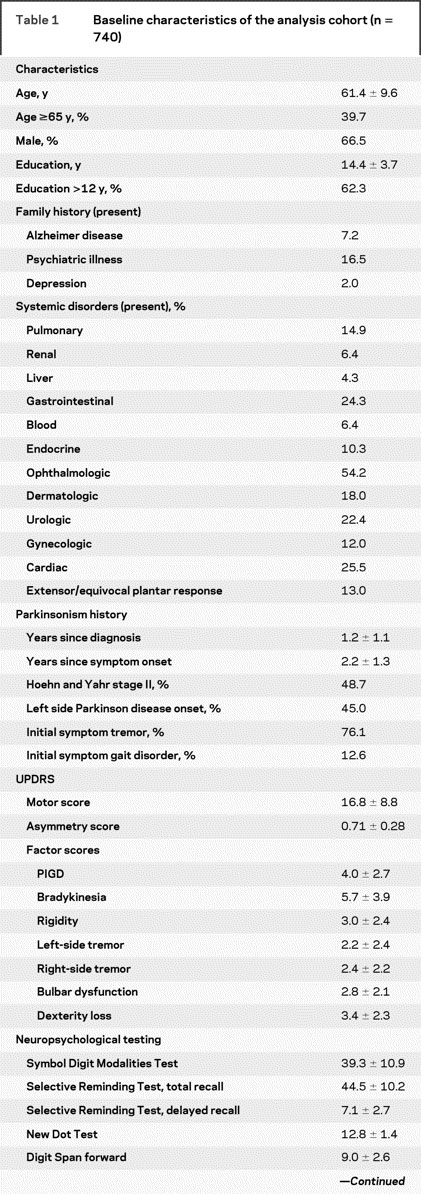
Table 1 Continued
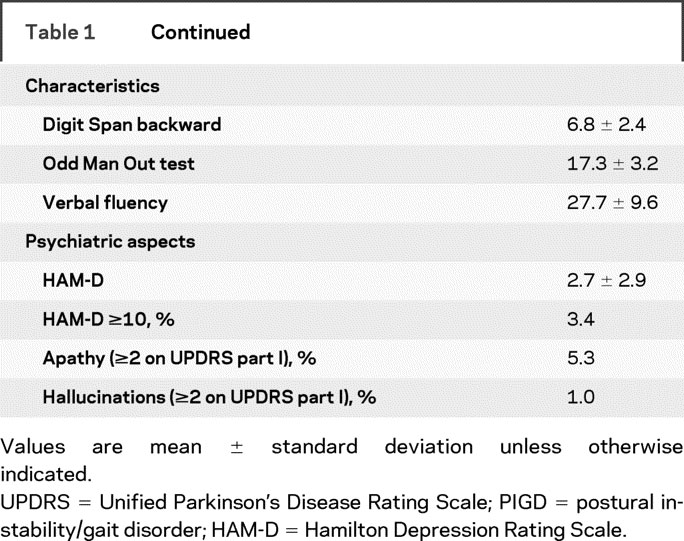
Compared to the remainder of the cohort who participated in the extension studies, the early endpointers had greater severity of parkinsonism, more symmetry at baseline, a larger proportion of male subjects, and a higher percentage with left-sided onset of parkinsonism (table e-2). There were no significant between-group differences in the scores of the neuropsychological tests, depression, apathy, hallucinations, comorbidities, age, and education.
Over the course of the study, 46 (6.2%) of 740 subjects reached the cognitive impairment endpoint at a mean age of 67.7 ± 10.2 years after a mean follow-up period of 3.3 ±2.5 years. The incidence (95% CI) of cognitive impairment was 2.4% (1.2%–3.5%) at 2 years and 5.8% (3.7%–7.7%) at 5 years (figure 1). The incidence rate was 12.7 (9.0–16.4)/1,000 person-years. There was a strong association between cognitive impairment and functional decline: although there were 366 subjects with functional decline (including 282 who showed limitations in occupational capacity) without cognitive impairment, all 46 subjects (100%) with cognitive impairment met functional decline criteria (p < 0.001, Fisher exact test).
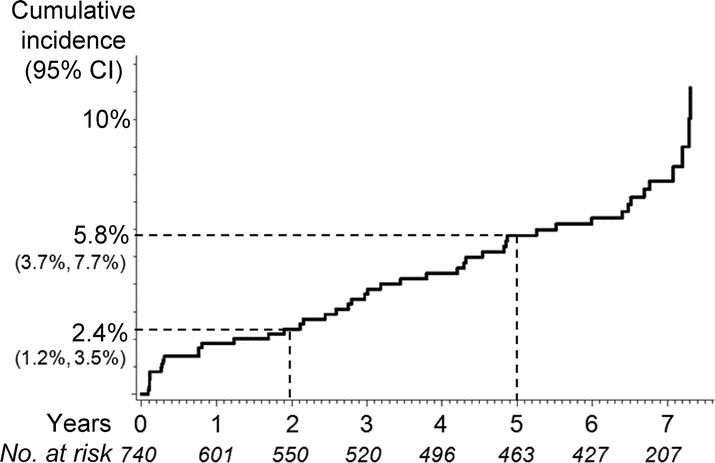
Figure 1 Kaplan-Meier curve showing the cumulative incidence of cognitive impairment in the Deprenyl and Tocopherol Antioxidative Therapy of Parkinsonism cohort
Values below the years indicate the numbers of subjects at risk for cognitive impairment.
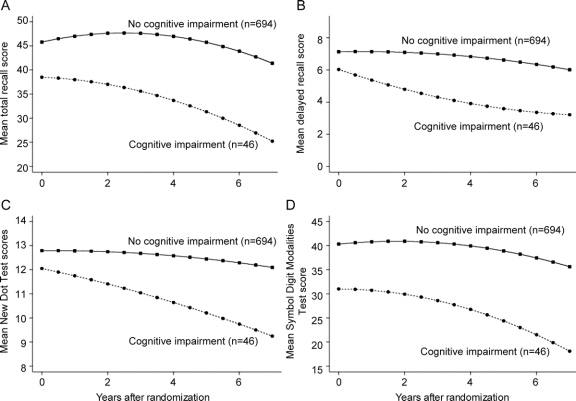
The evolution of performance on longitudinally available cognitive tests (SRT–verbal learning and memory, NDT–visuospatial working memory, SDMT–attention and visuomotor speed) is shown in figure 2. Subjects who eventually became cognitively impaired by the MMSE criterion (n = 46) had worse performance at baseline than those who did not become cognitively impaired (n = 694) (p < 0.003 for all tests) and also had greater decline over time on both verbal and nonverbal tests (p < 0.01 for SRT and NDT; p < 0.05 for SDMT), suggesting that our MMSE-based criterion was able to discriminate well between subjects with progressive cognitive decline on detailed, domain-specific cognitive tests and those with stable performance.
Figure 2 Evolution of cognitive test performances over time
The plotted values are adjusted group means derived from a mixed-effects regression model that included linear and quadratic terms for time. Subjects who eventually developed cognitive impairment (n = 46) had worse performance at baseline than those who did not develop cognitive impairment (n = 694) (p < 0.003 for all tests) and also had greater decline over time on both verbal and nonverbal tests (p < 0.01 for Selective Reminding Test and New Dot Task; p < 0.05 for Symbol Digit Modalities Test).
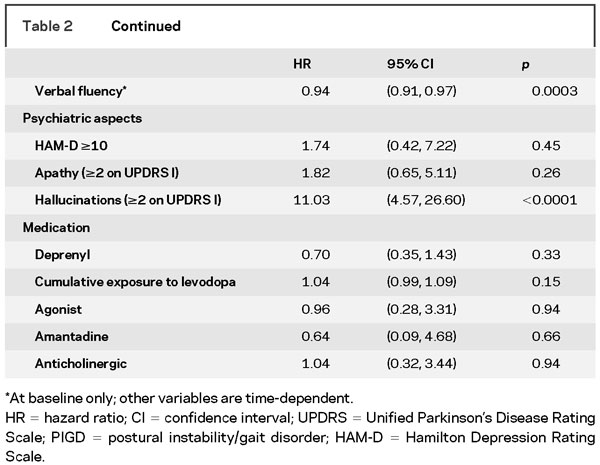
The baseline and time-dependent (longitudinal) univariate risk factors for time to cognitive impairment are shown in table 2. Variables that were individually associated with time to cognitive impairment included older age, male gender, severity of motor impairment (except for tremor, consistent with previous observations), increased symmetry of motor impairment, poor neuropsychological test performance, and hallucinations. Novel risk factors for cognitive impairment included presence of gastrointestinal and urologic disorders and UPDRS factors on bulbar dysfunction and dexterity loss.
Table 2 Associations between individual potential risk factors and time to cognitive impairment (n = 740)
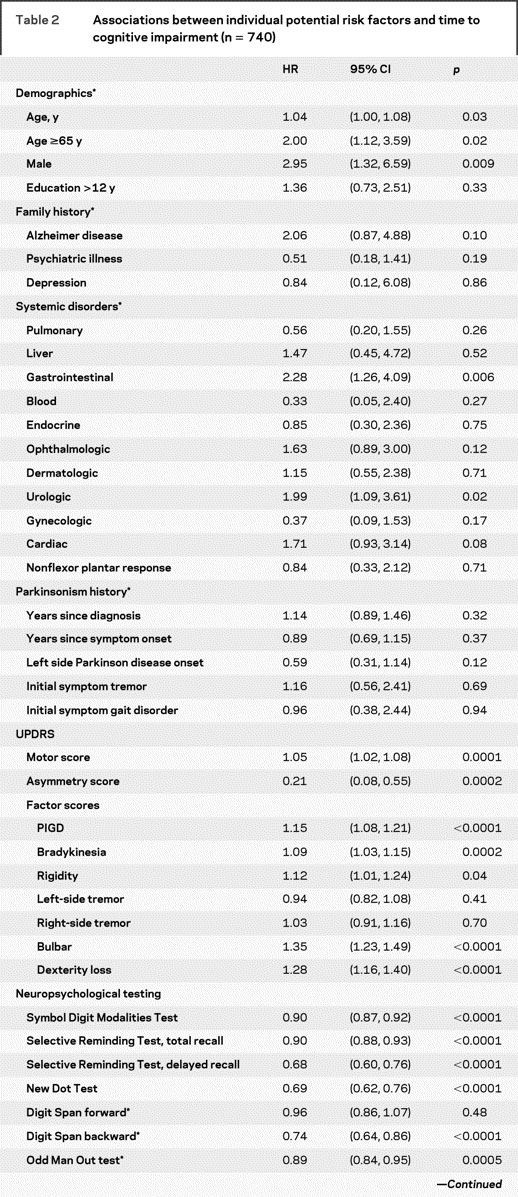
Table 2 Continued
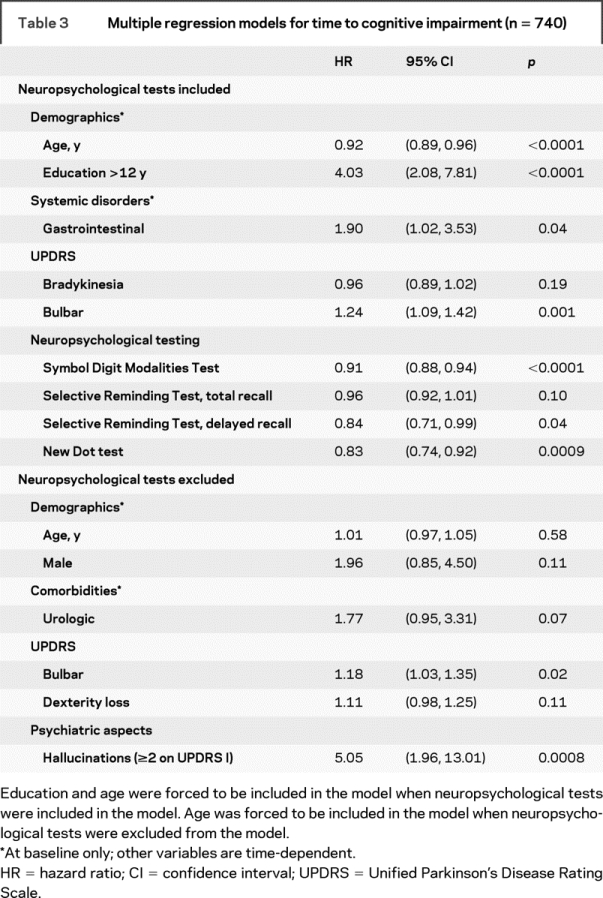
In the multiple regression analyses, when all variables, including the neuropsychological test results, were considered, the selected model included the SDMT (p < 0.0001), NDT (p < 0.001), SRT–delayed recall (p = 0.04), UPDRS–bulbar dysfunction factor (p = 0.001), and baseline gastrointestinal disorder (p = 0.04) as nominally significant risk factors (table 3). The findings in the multiple regression analyses of risk factors for cognitive impairment in table 3 (upper half: when neuropsychological tests were included) appear to counterintuitively suggest that older age and lesser education were “protective” against cognitive impairment. These results, however, need to be interpreted in the context of multiple regression analysis, i.e., holding the values of all other risk factors (e.g., scores on cognitive tests) constant. This result means that if 2 subjects have the same scores on neuropsychological tests, the one who is younger and better educated is at higher risk for cognitive impairment, as he or she would have been expected to score better than the older and less educated subject. Furthermore, in the univariate analyses (above), older age was significantly associated with cognitive impairment, as expected. Education was not significantly associated with cognitive impairment in this cohort in univariate analyses. When neuropsychological test results were excluded from consideration, hallucinations (p < 0.001) and UPDRS–bulbar dysfunction factor (p = 0.02) were the nominally significant risk factors (table 3, lower half).
Table 3 Multiple regression models for time to cognitive impairment (n = 740)
DISCUSSION
This study found that the annual incidence rate of cognitive impairment in the DATATOP study was 12.7 cases/1,000 person-years; Kaplan-Meier estimates were 2.4% at 2 years and 5.8% at 5 years. This incidence estimate of cognitive impairment is lower than reported dementia incidences from hospital5,8 or community-based6,9–11 cohorts of subjects with existing PD, as well as a community-based incident PD cohort.2 However, informal comparisons suggest that the annual incidence rate of cognitive impairment in the DATATOP study is higher than the observed incidence of dementia in comparable age brackets in several general population cohorts.25 For example, an Olmsted County, MN, cohort with similar demographic characteristics followed during approximately the same period as the DATATOP study showed lower incidences of dementia for the age groups of 65–69 (1.7 cases/1,000 person-years) and 70–74 (5.2 cases/1,000 person-years).26
Factors that may account for the relatively low incidence of cognitive impairment in the DATATOP cohort include recruitment bias inherent to clinical trial volunteers (e.g., younger age, higher education, better general health),14 shorter follow-up in the early endpointers (15.8% of the original analysis cohort, worse parkinsonism at baseline), the retrospective nature of our study, and potentially low sensitivity of the MMSE-based cognitive impairment criterion.27 The mean age at baseline in the DATATOP cohort was 61.0, similar to the mean baseline age in other clinical trials in patients with early PD (range 59–64),12,28–32 whereas the peak incidence of PD in the general population is between ages 70 and 79 years.33 In the only study of PDD in incident PD, the mean age at baseline was 69.6, with an annual incidence rate of PDD of 30.0 cases/1,000 person-years.2 While the generalizability of our incidence results may be limited, our study provides knowledge on the cognitive prognosis of patients with early PD who participate in clinical trials, which may have implications for future clinical trial design.
Choosing an outcome measure for this study was a challenge as DATATOP was not designed primarily as a study on the cognitive course of PD. Although cognitive impairment was significantly associated with functional decline, our ad hoc functional criteria were not suitable for dementia diagnosis as recent studies have shown that the intellectual impairment item of the UPDRS has low sensitivity34,35 and is not appropriate for screening or diagnostic purposes.34 Furthermore, the responses on the occupational capacity and handling finances questions on the DATATOP disability progression form16 can be confounded by motor dysfunction. The truncation of the DATATOP neuropsychological battery after the initial phase limited our options for the objective assessment of cognitive impairment. As we only had one reliable cognitive test that was longitudinally available (SRT–verbal learning and memory), we used an MMSE-based criterion for global cognitive impairment. The limitations of this approach included potential practice effects due to repeated administration of the MMSE despite using alternate versions of 3-word list during different visits, and heavy reliance of the MMSE on verbal abilities and low sensitivity to detect dementia, especially when the frequently used cutoff score for dementia (24/30) is applied to highly educated individuals.27,36
However, using age- and education-specific MMSE criterion21,22 for cognitive impairment, our MMSE cutoff score was ≤26 for the most common demographic group in DATATOP (ages 61–65, >12 years of education). This cutoff is consistent with the recommendations of a recent study which found that the ≤26 cutoff had a high sensitivity (0.89) and specificity (0.91) for detecting dementia in highly educated individuals with cognitive complaints screened with the MMSE and followed up with neuropsychological testing.36 Furthermore, our cognitive impairment criterion was able to discriminate well between subjects who showed progressive decline and those who remained stable on all longitudinally available neuropsychological tests (figure 2). Those who developed cognitive impairment started at a lower baseline level on average and showed progressive decline in performance on the SRT–total recall (verbal learning), SRT–delayed recall (verbal memory), SDMT (visuomotor speed and attention), and NDT (visuospatial working memory), while those who did not develop cognitive impairment displayed stable performance during follow-up.
We have confirmed a number of previously identified risk factors for PDD such as older age, male gender, poorer performance on neuropsychological tests, hallucinations, worse motor function, PIGD, and increased symmetry of motor severity. We found that the risk of cognitive impairment in early PD did not appear to be associated with the use of medications to treat PD. We also identified potentially novel risk factors such as bulbar dysfunction, early difficulties in dexterity, and gastrointestinal and genitourinary disorders at baseline, suggestive of early autonomic dysfunction. Alternatively, one could interpret the genitourinary disturbances as related to common disorders in this age population such as hypertrophy of the prostate or prolapse, and the gastrointestinal disturbances, specifically constipation, as an early prodromal symptom in PD.
Bulbar dysfunction appeared to be more strongly associated with cognitive impairment than PIGD, the most commonly reported motor risk factor for cognitive impairment.2,4,6 Both PIGD and bulbar impairment reflect axial dysfunction.6 Bulbar dysfunction has not been investigated as a separate entity because previous factor analyses of the UPDRS37,38 and UPDRS item groupings6 have combined items from the bulbar dysfunction and PIGD factors into a single axial dysfunction factor. The large sample size of DATATOP is a major strength of our factor analysis and might have enabled us to better separate the axial functions into 2 factors.
Gastrointestinal and genitourinary (and, to a lesser extent, cardiac) disorders at baseline were risk factors for cognitive impairment. We cannot say with certainty whether they represent specific diseases or just symptom complexes referable to these systems. Presence of these disorders as severe specific diseases is unlikely, however, as unstable medical comorbidity was among the exclusion criteria in the DATATOP study.16 Considering that symptoms such as constipation, difficulty with gastric emptying, urinary urgency, and erectile dysfunction are common nonmotor manifestations of PD,39 it is conceivable that these disorders might have represented autonomic symptoms. An association between autonomic dysfunction and time to cognitive impairment is compatible with results of cross-sectional studies showing increased frequency of autonomic dysfunction in patients with DLB or PDD compared to patients with PD without dementia.40
DISCLOSURE
Dr. Uc has received speaker honoraria for activities not sponsored by industry; has served as a grant reviewer for the Parkinson Study Group; and receives research support from the NIH [NINDS NS044930 (PI)], the US Department of Veterans Affairs [Merit Review from Rehabilitation R&D Branch; B6261R (PI) and 1 I01 RX000170 (PI)], and the Parkinson Disease Foundation. Dr. McDermott has received honoraria from the Michael J. Fox Foundation; has served as a consultant to the New York State Department of Health and the American Epilepsy Society; has received research support from Medivation, Inc., Boehringer Ingelheim, NeuroSearch Sweden AB, and Forest Laboratories, Inc.; and receives research support from the NIH [NS42372 (Co-I), HD44430 (Co-I), DE16280 (Co-I), NS52619 (PI), NS45686 (Co-I), NS46487 (Co-I), NS50095 (Co-I), NS49639 (Co-I), AR52274 (Co-I), NS50573 (Co-I), HL80107 (Co-I), RR24160 (Co-I), NS58259 (Co-I), and NS48843 (Co-I)], the US Food and Drug Administration, the Michael J. Fox Foundation, the Spinal Muscular Atrophy Foundation, and the Muscular Dystrophy Association. Dr. Marder serves on the editorial board of Neurology®; receives research support from Amarin Corporation, Boehringer Ingelheim, and NeuroSearch Sweden AB; and receives research support from the NIH [NS36630 (PI), 1UL1 RR024156-01 (Director PCIR), PO412196-G (Co-I), and PO412196-G (Co-I)], and from the Parkinson Disease Foundation, Huntington's Disease Society of America, and the Parkinson Study Group. Dr. Anderson reports no disclosures. Dr. Litvan served on a scientific advisory board for Neuropharma; serves as Co-Editor of Moving Along, and on editorial boards for Clinical Neurology News and Archivos de Neurología, Neurocirugía y Neuropsiquiatría; receives royalties from publishing Handbook of Clinical Neurology Dementias, volume 89 (Elsevier, 2008); has received speaker honoraria from Novartis, Teva Pharmaceutical Industries Ltd., Boehringer Ingelheim, the Movement Disorder Society, and the American Academy of Neurology; has served on speakers’ bureaus for Novartis, Boehringer Ingelheim, Teva Pharmaceutical Industries Ltd., and GlaxoSmithKline; has received research support from Kyowa Hakko Kirin Pharma, Inc., and Chiltern International Inc.; and receives research support from the NIH [NIA 5 R01 PAS-03-092 (PI), 1 R13 NS062643-01 (PI), and NINDS NPTUNE (Site PI)] and from the University of Louisville, the Center for Environmental Genomics and Integrative Biology, the Parkinson Support Center of Kentuckiana, the National Parkinson Foundation, Parkinson Study Group, Movement Disorder Society, and CurePSP. Dr. Como serves on a scientific review committee for the Parkinson Study Group; serves as a consultant to Prestwick Pharmaceuticals and Medivation, Inc.; and has received honoraria from the Michael J. Fox Foundation, Med Reviews, Inc., and Leerink Swann LLC. P. Auinger reports no disclosures. Dr. Chou receives royalties from publishing chapters (diagnosis of Parkinson disease, clinical manifestations of Parkinson disease, patient information: Parkinson disease symptoms and diagnosis) in UpToDate (2007); serves/has served on speakers’ bureaus for GlaxoSmithKline, Teva Pharmaceutical Industries Ltd., Boehringer Ingelheim, and Allergan, Inc.; and receives research support from the NIH [4 U10 NS44504–06 (PI)]. Dr. Growdon serves on a scientific advisory board for Neuroimmune Therapeutics AG; serves on editorial boards for Archives of Neurology and Neurodegenerative Diseases; receives royalties from publishing The Dementias (Elsevier, 2007); and receives research support from the NIH [NIA P50 AG05134 (Co-PI), NINDS P50 NS037372 (Co-PI), and NINDS R21 NS60310 (PI)] and from the Michael J. Fox Foundation.
Supplementary Material
Address correspondence and reprint requests to Dr. Ergun Y. Uc, Department of Neurology, University of Iowa, Carver College of Medicine, 200 Hawkins Drive-2RCP, Iowa City, IA 52242 ergun-uc@uiowa.edu
Supplemental data at www.neurology.org
*The list of DATATOP investigators/coordinators can be found in reference 12 as well as on the Neurology® Web site at www.neurology.org.
Supported by a grant from the Parkinson's Disease Foundation (E.Y.U.). DATATOP was supported by US Public Health Service grant NS 24778 (National Institute of Neurological Disorders and Stroke).
Disclosure: Author disclosures are provided at the end of the article.
Received April 9, 2009. Accepted in final form July 21, 2009.
REFERENCES
- 1.Uc EY, Rizzo M, Anderson SW, et al. Visual dysfunction in Parkinson disease without dementia. Neurology 2005;65:1907–1913. [DOI] [PubMed] [Google Scholar]
- 2.Williams-Gray CH, Foltynie T, Brayne CE, et al. Evolution of cognitive dysfunction in an incident Parkinson's disease cohort. Brain 2007;130:1787–1798. [DOI] [PubMed] [Google Scholar]
- 3.Aarsland D, Bronnick K, Larsen JP, et al. Cognitive impairment in incident, untreated Parkinson disease: The Norwegian ParkWest Study. Neurology 2009;72:1121–1126. [DOI] [PubMed] [Google Scholar]
- 4.Emre M, Aarsland D, Brown R, et al. Clinical diagnostic criteria for dementia associated with Parkinson's disease. Mov Disord 2007;22:1689–1707. [DOI] [PubMed] [Google Scholar]
- 5.Hughes TA, Ross HF, Musa S, et al. A 10-year study of the incidence of and factors predicting dementia in Parkinson's disease. Neurology 2000;54:1596–1602. [DOI] [PubMed] [Google Scholar]
- 6.Levy G, Schupf N, Tang MX, et al. Combined effect of age and severity on the risk of dementia in Parkinson's disease. Ann Neurol 2002;51:722–729. [DOI] [PubMed] [Google Scholar]
- 7.Aarsland D, Andersen K, Larsen JP, et al. Prevalence and characteristics of dementia in Parkinson disease: an 8-year prospective study. Arch Neurol 2003;60:387–392. [DOI] [PubMed] [Google Scholar]
- 8.Hely MA, Reid WG, Adena MA, et al. The Sydney multicenter study of Parkinson's disease: the inevitability of dementia at 20 years. Mov Disord 2008;23:837–844. [DOI] [PubMed] [Google Scholar]
- 9.Hobson P, Meara J. Risk and incidence of dementia in a cohort of older subjects with Parkinson's disease in the United Kingdom. Mov Disord 2004;19:1043–1049. [DOI] [PubMed] [Google Scholar]
- 10.Marder K, Tang MX, Cote L, et al. The frequency and associated risk factors for dementia in patients with Parkinson's disease. Arch Neurol 1995;52:695–701. [DOI] [PubMed] [Google Scholar]
- 11.Aarsland D, Andersen K, Larsen JP, et al. Risk of dementia in Parkinson's disease: a community-based, prospective study. Neurology 2001;56:730–736. [DOI] [PubMed] [Google Scholar]
- 12.Parkinson Study Group. Effects of tocopherol and deprenyl on the progression of disability in early Parkinson's disease. N Engl J Med 1993;328:176–183. [DOI] [PubMed] [Google Scholar]
- 13.Shoulson I, Oakes D, Fahn S, et al. Impact of sustained deprenyl (selegiline) in levodopa-treated Parkinson's disease: a randomized placebo-controlled extension of the deprenyl and tocopherol antioxidative therapy of parkinsonism trial. Ann Neurol 2002;51:604–612. [DOI] [PubMed] [Google Scholar]
- 14.Marras C, McDermott MP, Rochon PA, et al. Survival in Parkinson disease: thirteen-year follow-up of the DATATOP cohort. Neurology 2005;64:87–93. [DOI] [PubMed] [Google Scholar]
- 15.Parkinson Study Group. Effect of deprenyl on the progression of disability in early Parkinson's disease. N Engl J Med 1989;321:1364–1371. [DOI] [PubMed] [Google Scholar]
- 16.Parkinson Study Group. DATATOP: a multicenter controlled clinical trial in early Parkinson's disease. Arch Neurol 1989;46:1052–1060. [DOI] [PubMed] [Google Scholar]
- 17.McKeith IG, Dickson DW, Lowe J, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology 2005;65:1863–1872. [DOI] [PubMed] [Google Scholar]
- 18.Growdon JH, Kieburtz K, McDermott MP, et al. Levodopa improves motor function without impairing cognition in mild non-demented Parkinson's disease patients: Parkinson Study Group. Neurology 1998;50:1327–1331. [DOI] [PubMed] [Google Scholar]
- 19.Kieburtz K, McDermott M, Como P, et al. The effect of deprenyl and tocopherol on cognitive performance in early untreated Parkinson's disease. Parkinson Study Group Neurology 1994;44:1756–1759. [DOI] [PubMed] [Google Scholar]
- 20.Dissanayaka NN, Sellbach A, Matheson S, et al. Validity of Hamilton Depression Inventory in Parkinson's disease. Mov Disord 2007;22:399–403. [DOI] [PubMed] [Google Scholar]
- 21.Crum RM, Anthony JC, Bassett SS, et al. Population-based norms for the Mini-Mental State Examination by age and educational level. JAMA 1993;269:2386–2391. [PubMed] [Google Scholar]
- 22.Anderson SW, Todd MM, Hindman BJ, et al. Effects of intraoperative hypothermia on neuropsychological outcomes after intracranial aneurysm surgery. Ann Neurol 2006;60:518–527. [DOI] [PubMed] [Google Scholar]
- 23.Larrabee GJ, Trahan DE, Levin HS. Normative data for a six-trial administration of the Verbal Selective Reminding Test. Clin Neuropsychol 2000;14:110–118. [DOI] [PubMed] [Google Scholar]
- 24.Fahn S, Elton RL, members of the UPDRS Development Committee. Unified Parkinson's Disease Rating Scale. In: Fahn S, Marsden CD, Calne DB, et al, eds. Recent Developments in Parkinson's Disease. Florham Park, NJ: Macmillan Healthcare Information; 1987:153–163. [Google Scholar]
- 25.The Canadian Study of Health and Aging Working Group. The incidence of dementia in Canada. Neurology 2000;55:66–73. [PubMed] [Google Scholar]
- 26.Edland SD, Rocca WA, Petersen RC, et al. Dementia and Alzheimer disease incidence rates do not vary by sex in Rochester, Minn. Arch Neurol 2002;59:1589–1593. [DOI] [PubMed] [Google Scholar]
- 27.Riedel O, Klotsche J, Spottke A, et al. Cognitive impairment in 873 patients with idiopathic Parkinson's disease: results from the German Study on Epidemiology of Parkinson's Disease with Dementia (GEPAD). J Neurol 2008;255:255–264. [DOI] [PubMed] [Google Scholar]
- 28.Parkinson Study Group. Mixed lineage kinase inhibitor CEP-1347 fails to delay disability in early Parkinson disease. Neurology 2007;69:1480–1490. [DOI] [PubMed] [Google Scholar]
- 29.Fahn S, Oakes D, Shoulson I, et al. Levodopa and the progression of Parkinson's disease. N Engl J Med 2004;351:2498–2508. [DOI] [PubMed] [Google Scholar]
- 30.Rascol O, Brooks DJ, Korczyn AD, et al. A five-year study of the incidence of dyskinesia in patients with early Parkinson's disease who were treated with ropinirole or levodopa: 056 Study Group. N Engl J Med 2000;342:1484–1491. [DOI] [PubMed] [Google Scholar]
- 31.Parkinson's Disease Research Group in the United Kingdom. Comparisons of therapeutic effects of levodopa, levodopa and selegiline, and bromocriptine in patients with early, mild Parkinson's disease: three year interim report. BMJ 1993;307:469–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parkinson Study Group. Pramipexole vs levodopa as initial treatment for Parkinson disease: a randomized controlled trial. JAMA 2000;284:1931–1938. [DOI] [PubMed] [Google Scholar]
- 33.Twelves D, Perkins KS, Counsell C. Systematic review of incidence studies of Parkinson's disease. Mov Disord 2003;18:19–31. [DOI] [PubMed] [Google Scholar]
- 34.Holroyd S, Currie LJ, Wooten GF. Validity, sensitivity and specificity of the mentation, behavior and mood subscale of the UPDRS. Neurol Res 2008;30:493–496. [DOI] [PubMed] [Google Scholar]
- 35.Starkstein SE, Merello M. The Unified Parkinson's Disease Rating Scale: validation study of the mentation, behavior, and mood section. Mov Disord 2007;22:2156–2161. [DOI] [PubMed] [Google Scholar]
- 36.O’Bryant SE, Humphreys JD, Smith GE, et al. Detecting dementia with the mini-mental state examination in highly educated individuals. Arch Neurol 2008;65:963–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stochl J, Boomsma A, Ruzicka E, et al. On the structure of motor symptoms of Parkinson's disease. Mov Disord 2008;23:1307–1312. [DOI] [PubMed] [Google Scholar]
- 38.Stebbins GT, Goetz CG. Factor structure of the Unified Parkinson's Disease Rating Scale: motor examination section. Mov Disord 1998;13:633–636. [DOI] [PubMed] [Google Scholar]
- 39.Chaudhuri KR, Naidu Y. Early Parkinson's disease and non-motor issues. J Neurol 2008;255 suppl 5:33–38. [DOI] [PubMed] [Google Scholar]
- 40.Poewe W. Dysautonomia and cognitive dysfunction in Parkinson's disease. Mov Disord 2007;22:S374–S378. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.