Abstract
Background:
Autoimmune autonomic ganglionopathy is characterized by impairment of multiple autonomic domains of which sudomotor function is among the most common. Many patients with this disorder have difficulties with thermoregulation and anhidrosis. Our objective was to characterize the distribution and severity of sudomotor dysfunction in this disorder.
Methods:
Sudomotor function was analyzed in a cohort of 21 patients with ganglionic α3 nicotinic acetylcholine receptor (nAChR) antibody positive autoimmune autonomic ganglionopathy. Standard measurements of sudomotor function were used including the Thermoregulatory Sweat Test and Quantitative Sudomotor Axon Reflex Test.
Results:
The clinical presentation in all patients was characterized by widespread sudomotor dysfunction. Sudomotor impairment was predominantly postganglionic in 17 of the 21 patients studied. Higher ganglionic α3 nAChR antibody levels resulted in progressive postganglionic predominant dysfunction (postganglionic, r = 0.637, p = 0.002; mixed ganglionic, r = 0.709, p < 0.001). The pattern of anhidrosis on Thermoregulatory Sweat Testing was consistent with a ganglionopathy in the majority of patients (14 of 21) and a distal pattern in a minority of patients (8 of 21). These patterns of anhidrosis coupled with increasing postganglionic dysfunction in a proximal to distal pattern (foot > distal leg > proximal leg > forearm) indicate lesions at both the ganglia and distal axon of the postganglionic sudomotor sympathetic neuron.
Conclusions:
Our data characterize the unique sudomotor dysfunction in autoimmune autonomic ganglionopathy as widespread, predominantly postganglionic, and a result of lesions at both the ganglia and distal axon. This study provides important support to the hypothesis that this disorder represents a ganglionic neuropathy.
GLOSSARY
- AAG
= autoimmune autonomic ganglionopathy;
- CASS
= Composite Autonomic Severity Score;
- nAChR
= nicotinic acetylcholine receptor;
- QSART
= Quantitative Sudomotor Axon Reflex Testing;
- TST
= Thermoregulatory Sweat Test.
The clinical features of autoimmune autonomic ganglionopathy (AAG) include orthostatic hypotension, gastrointestinal hypomotility, and sudomotor dysfunction.1 Ganglionic α3 nicotinic acetylcholine receptor (nAChR) antibodies are found in serum of about half of reported cases, correlate to disease severity, and have been shown to be pathogenic.2–7 Accordingly, AAG has been shown to respond to immunotherapy with IV immunoglobulin, plasma exchange, and immunosuppressants.8
It has been reported that 74% of α3 nAChR patients have clinically significant issues with thermoregulation including reduced sweating and heat intolerance.4 However, the distribution and degree of sudomotor dysfunction has not been fully quantified in AAG. Standard autonomic testing allows for quantitation of the sweat response. The Thermoregulatory Sweat Test (TST) measures whole body sweating and requires an intact thermoregulatory pathway from the preoptic/anterior hypothalamus to the postganglionic sudomotor axon.9 Quantitative Sudomotor Axon Reflex Testing (QSART) measures only the integrity of the postganglionic sudomotor axon's contribution to the sweat response.9 As a result, a “preganglionic” lesion would be indicated by anhidrosis on TST but an intact QSART response. Conversely, an abnormal QSART response would be consistent with a “postganglionic” lesion, although a preganglionic lesion cannot absolutely be ruled out. Using both the TST and QSART, the pattern of sudomotor dysfunction in AAG can be determined.
In the present study, it was our objective to characterize the deficits in sudomotor function in ganglionic α3 antibody positive AAG patients using standard measures of autonomic dysfunction. Specifically, our aims were to 1) determine the distribution and degree of anhidrosis present in our cohort; 2) assess whether there is an association between α3 AChR antibody levels and the degree of sudomotor dysfunction; and 3) assess whether there is a correlation between α3 AChR antibody levels and the anatomic pattern of sudomotor dysfunction (preganglionic, postganglionic, or mixed ganglionic).
METHODS
Patients.
Patients with generalized or restricted autonomic dysfunction and ganglionic α3 nAChR antibody levels of >0.05 nmol/L (prior to treatment) were identified from prior records (Mayo Clinic, Rochester, MN). Patients with involvement of at least one autonomic domain (adrenergic, cardiovagal, or sudomotor) and a positive antibody level were considered to have antibody positive AAG. Patients were required to have undergone QSART and TST on at least one occasion to be included in the study. All patients had rigorous hematologic, biochemical, and serologic investigations to rule out other causes of autonomic dysfunction including but not limited to multiple system atrophy, diabetes, amyloidosis, rheumatologic disorders, and malignancy. Patients with alternate causes for their autonomic dysfunction were eliminated. Initially 32 patients were identified with positive α3 nAChR antibody levels. A total of 21 patients met the above stated inclusion criteria. All patients were free of any other neuronal autoantibody on standard paraneoplastic antibody panels (Mayo Clinic).
Standard protocol approvals, registrations, and patient consents.
This study was approved by the Mayo Clinic Institutional Review Board. All patients provided written permission to allow the use of their medical records for research purposes as per standard institutional research authorization protocols.
Autoantibody measurements.
Immunoprecipitation assays were used to detect autoantibodies binding to the neuronal α3 nAChR as previously described.2 The normal range in serum is <0.05 nmol/L.
Quantitative sudomotor axon reflex testing.
Postganglionic sympathetic sudomotor function was evaluated using the QSART and is a measure of the postganglionic sudomotor axon.10 The routine sweat response was recorded from standardized sites comprising the left forearm, proximal leg, distal leg, and foot. At all sites a 10% acetylcholine solution was iontophoresed for 5 minutes at 2 mA using standard multi-compartmented sweat cells. The resulting sweat response was recorded for a total of 10 minutes. The data obtained were compared to normative values derived from 223 healthy subjects aged 10–83 years.11
Thermoregulatory sweat testing.
The TST is a quantitative measure of total body sweating and has been described previously.12 Briefly, the TST was conducted in a sweat cabinet (43°C–46°C air temperature, 35%–40% relative humidity). Average skin temperature was kept between 38.5 and 39.5°C. Oral temperature was raised by 1.0°C or to 38.0°C (depending on which value was higher). Sweating was demonstrated by an indicator powder (alizarin red, cornstarch, and sodium carbonate). The percentage of anhidrosis on the anterior body surface was calculated from the distribution of total body sweating using standard digital imaging software (100% indicates complete anhidrosis).13
The various patterns of anhidrosis on TST were determined as previously described.13 Distal patterns affect the distal extremities in a length-dependent manner. Focal patterns are localized usually to small surface areas or to select sympathetic dermatomes or peripheral nerve territories. Segmental patterns involve large body surface areas, usually follow sympathetic dermatomes, and are bordered by areas of normal sweating. Regional patterns refer to larger regions of anhidrosis (<80%) that gradually blend into areas of normal sweating. Global anhidrosis refers to widespread anhidrosis greater than 80% of the total body surface area. Mixed patterns are a combination the above patterns. Normal patterns have no significant sweat loss, do not follow any of the above patterns, and have less than 5% anhidrosis based on prior normal control subjects. Segmental, regional, and global patterns of anhidrosis were considered to be consistent with a ganglionopathy presentation when they matched the known sweat distribution of relevant autonomic ganglia. Mixed patterns, including segmental, regional, and global patterns, were also considered to be consistent with a ganglionopathy if the distribution was concordant with ganglionic sudomotor distribution.
Preganglionic vs postganglionic determination.
Each QSART site (forearm, distal leg, proximal leg, foot) was compared to the corresponding region on TST. If the TST site showed anhidrosis but the associated QSART site was normal, then the lesion was considered preganglionic. If the QSART site was abnormal, then the lesion was considered postganglionic. The designation “postganglionic” was used as QSART measures the postganglionic sudomotor axon integrity only. However, an associated “preganglionic” lesion cannot be absolutely ruled out. In the same context, a postganglionic lesion will result in an abnormal TST finding as this test also requires an intact postganglionic sudomotor axon reflex. If both the TST and QSART were normal, then that site was considered not to have an underlying neurologic lesion. The use of QSART and TST to determine whether a lesion may be preganglionic or postganglionic in nature has been used in multiple disorders including pure autonomic failure and multiple systems atrophy.9,11
With regards to the individual patient, each was characterized as having a preganglionic, postganglionic, or a mixed lesion. If one or more QSART sites (forearm, distal leg, proximal leg, foot) were designated as preganglionic, then the patient was deemed to have a preganglionic pattern. If one or more sites were designated as postganglionic, then the patient was deemed to have a postganglionic pattern. If a patient had both lesion types, then they were designated as a mixed pattern. If all QSART and their corresponding TST sites were normal, the patient was considered not to have a sudomotor lesion.
Composite Autonomic Severity Score.
The Composite Autonomic Severity Score (CASS) is derived from the autonomic reflex screen as previously described.10 The CASS score provides a measure of the severity and distribution of autonomic failure. The 11-point CASS score is divided into 3 subscores: sudomotor, cardiovagal, and adrenergic. Each score is normalized for the confounding variables of age and gender.11 The CASS sudomotor subscore (range 0–3) was used in this study as a composite measure to quantify sudomotor dysfunction and is a direct reflection of the TST and QSART. Both the CASS score and the CASS sudomotor subscore have been previously validated.14
Statistical analyses.
Descriptive statistics are presented as percentages or mean ± SD. The Spearman rho (r) tested correlations among continuous variables. All tests were two-tailed. p < 0.05 was considered significant. All statistical analyses were performed using SPSS statistical software, version 15 for Windows (SPSS Inc., Chicago, IL).
RESULTS
Average age of our patient group was 55.1 ± 13.2 years with a female to male ratio of 2 to 1. There was a slight preponderance of patients with subacute onset vs those with more gradual disease progression (13 vs 8). Disease duration prior to evaluation with TST, QSART, and antibody measurements was on average 5.1 ± 9.0 years (range 0.5–18 years; see table e-1 on the Neurology® Web site at www.neurology.org).
The majority of patients had a regional, mixed, distal, or mixed plus distal pattern of anhidrosis on TST (table 1). Of the total 21 patients, TST results from 14 were compatible with a ganglionopathy presentation (table 1). Only a single patient had a normal TST. There was no correlation between antibody level and % anhidrosis on TST (r = 0.403, p = 0.071; figure 1A). There was an increase in CASS sudomotor scores with elevated ganglionic α3 nAChR antibody levels (r = 0.508, p = 0.019; figure 1B). Similar findings were seen for CASS total scores, including adrenergic and cardiovagal autonomic subdomains in addition to the CASS sudomotor score (r = 0684, p < 0.001; figure 1C).
Table 1 Thermoregulatory Sweat Test abnormalities by patient and those consistent with ganglionopathy
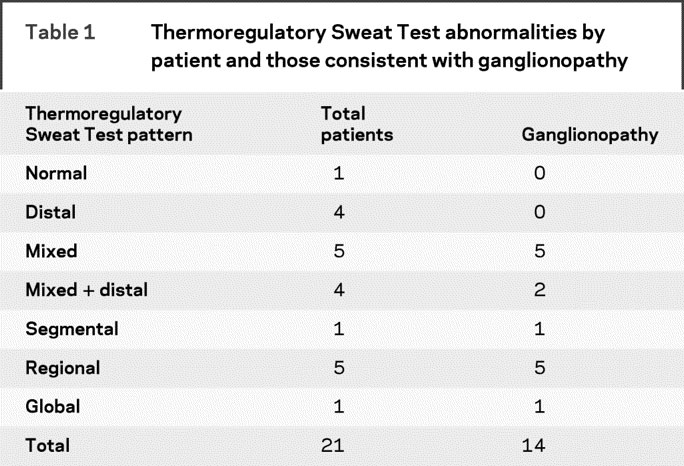
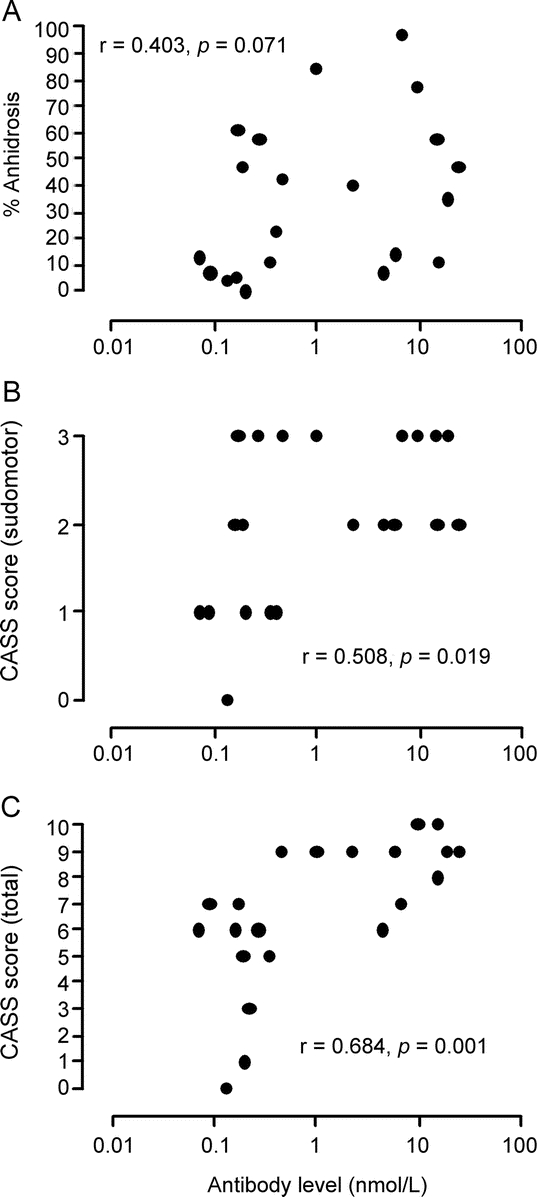
Figure 1 Elevated ganglionic antibody levels result in higher Composite Autonomic Severity Score (CASS) sudomotor scores but not % anhidrosis
Comparison of ganglionic α3 nAChR antibody levels to % anhidrosis on the Thermoregulatory Sweat Test (A), the sudomotor subdomain of the CASS score (B), and the CASS total score (C). There was no correlation with % anhidrosis and ganglionic α3 nAChR antibody levels. Increased antibody levels resulted in higher CASS sudomotor and CASS total scores. Each individual data point represents a single patient.
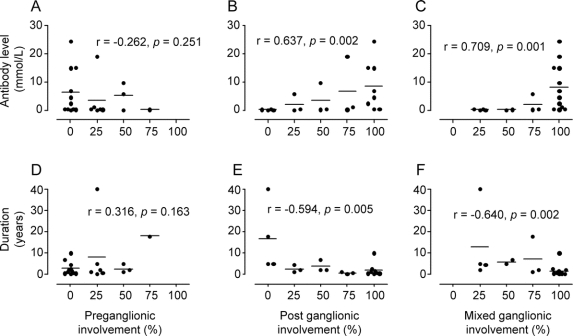
Disease duration did not correlate with the type of involvement (i.e., pre-, post-, and mixed ganglionic; figure 2, D–F). Higher ganglionic α3 nAChR antibody levels did result in greater postganglionic and mixed ganglionic but not preganglionic involvement (figure 2, A–C; postganglionic, r = 0.637, p = 0.002; mixed ganglionic, r = 0.709, p < 0.001; r = −0.262; p = 0.251). The relationship between elevated ganglionic α3 nAChR antibody levels and increased mixed ganglionic involvement is most consistent with postganglionic predominant dysfunction given the lack of an interaction between antibody level and preganglionic involvement (see Discussion).
Figure 2 Increased ganglionic antibody levels but not disease duration results in greater postganglionic sudomotor dysfunction
Higher ganglionic α3 nAChR antibody levels results in progressive postganglionic (B) and mixed ganglionic (C) patterns of sudomotor dysfunction. Conversely, there was no correlation with preganglionic sudomotor dysfunction (A). Preganglionic (D), postganglionic (E), and mixed ganglionic (F) sudomotor dysfunction did not correlate with disease duration. The Y axis indicates antibody levels (nmol/L; A–C) and disease duration (years; D–F). The X axis indicates the percentage of QSART sites (i.e., forearm, proximal leg, distal leg, foot) involved. For example, if all 4 sites were involved for a particular pattern (preganglionic, postganglionic, or mixed ganglionic), this would be equivalent to 100% on the X axis. Each point on the graph represents a single patient. The corresponding lines are equal to the mean ganglionic α3 nAChR antibody level (A–C) or duration in years (D–F).
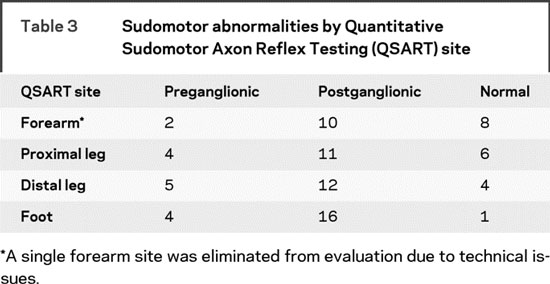
Sudomotor abnormalities in AAG patients were most consistent with postganglionic involvement (both postganglionic and mixed ganglionic patterns, n = 17, table 2) compared to patients with preganglionic patterns only (n = 4, table 2). The same trend was apparent when the separate ganglionic patterns were broken down by QSART site (i.e., forearm, proximal leg, distal leg, and foot; table 3). Postganglionic involvement was predominant at all 4 sites. Only a single forearm site from 1 patient was eliminated due to technical issues related to high skin resistance. Interestingly, there was a proximal to distal pattern with respect to postganglionic involvement. An increased number of sites showing postganglionic sudomotor dysfunction at all 4 QSART sites was observed in the following pattern: foot > distal leg > proximal leg > forearm (table 3).
Table 2 Sudomotor abnormalities by patient

Table 3 Sudomotor abnormalities by Quantitative Sudomotor Axon Reflex Testing (QSART) site
DISCUSSION
In this study, we have characterized the unique sudomotor dysfunction in patients with AAG. The key findings are as follows: 1) sudomotor dysfunction was generally severe and widespread; 2) the severity of sudomotor function is directly proportional to ganglionic α3 AChR antibody levels; 3) sudomotor dysfunction was predominantly postganglionic; and 4) higher ganglionic α3 AChR antibody levels are associated with greater postganglionic sudomotor dysfunction.
Ganglionic α3 nAChR antibody levels did not show a correlation with % anhidrosis on TST, in keeping with previous reports.3 However, CASS sudomotor scores did increase with increasing ganglionic antibody levels. Since the CASS sudomotor score is derived from both the TST and QSART, these findings more likely reflect the interaction between ganglionic α3 nAChR antibody levels and QSART as a measure of postganglionic sudomotor function, given the lack of a correlation with ganglionic α3 nAChR antibodies and TST findings. Therefore, this supports the conclusion that postganglionic dysfunction is the predominant type of sudomotor impairment in AAG and is directly proportional to ganglionic α3 nAChR antibody levels. Additionally, the increased severity of autonomic dysfunction as measured by the CASS total score (including all autonomic subdomains: sudomotor, adrenergic, and cardiovagal) was significant when compared to progressively elevated ganglionic α3 nAChR antibody levels as expected based on previous findings.3,15
The preganglionic impairment in a select number of patients (n = 4) likely represents a blockade of ganglionic transmission. Ganglionic antibodies interfere with acetylcholine (ACh) binding to receptors, crosslinking of nAChRs on the postganglionic cell surface membrane, and subsequent receptor internalization.6,16,17 This argument is supported by evidence that the α3 nAChR subunit is abundant in autonomic ganglia, located on postganglionic neurons, and α3 nAChR antibodies block ganglionic transmission in animal models.7,18
Progressive elevation in α3 nAChR antibody levels resulted in increased postganglionic but not preganglionic sudomotor dysfunction. A similar finding with ganglionic α3 nAChR antibody levels and progressive mixed ganglionic sudomotor dysfunction was also described. This finding was primarily based on the strong relationship of elevated antibody levels to overall postganglionic sudomotor dysfunction. Additionally there may be a small contribution due to ganglionic transmission impairment given the slightly greater statistical correlation with ganglionic antibodies and mixed ganglionic dysfunction vs pure postganglionic dysfunction (postganglionic, r = 0.637, p = 0.002, vs mixed ganglionic, r = 0.709, p < 0.001). This finding supports the idea of a lesion at both the ganglia and distal axon of the postganglionic neuron.
Whether this postganglionic dysfunction is a result of physiologic disturbance of the postganglionic neuron or a result of postganglionic neuron/fiber loss cannot be absolutely determined based on the evidence presented here. Abnormal QSART values could represent either mechanism. Models of experimental autoimmune autonomic ganglionopathy have shown that neurons in the autonomic ganglia are intact, arguing against neuronal/axon loss in the human condition.6 Evidence from fluoro-l-DOPA positron emission topography and I123 MIBG SPECT showing intact postganglionic sympathetic innervation to the heart in AAG patients supports this assertion.19,20
While it is difficult to determine the resulting pathology leading to postganglionic dysfunction, strong evidence points to lesions of both the ganglia and nerve fiber. The pattern of anhidrosis on TST was consistent with a ganglionopathy in 14 of the 21 patients examined. A smaller number of patients revealed a distal pattern of anhidrosis (8 of 21). These TST patterns coupled with predominant postganglionic dysfunction in 17 of the 21 patients studied indicate lesions of both the ganglia and postganglionic sudomotor axon at distal sites. A ganglionopathy plus distal neuropathy is further supported by the findings that the postganglionic impairment in our patients followed a proximal to distal pattern (foot > distal leg > proximal leg > forearm; see table 3). Our data show lesions at both the ganglia and distal axon and are indicative of a ganglionic neuropathy unique to AAG.
AUTHOR CONTRIBUTIONS
Statistical analysis was conducted by Drs. K. Kimpinski and V. Iodic.
DISCLOSURE
Dr. Kimpinski and Dr. Iodice report no disclosures. Dr. Sandroni serves on scientific boards for Cervelo Pharmaceuticals Inc. and Ono Pharmaceutical Co. Ltd. and receives research support from the NIH [P50 NS 32352-15 (Co-I), P01 NS 044233-1 (Co-I)]. Dr. Fealey directs a laboratory which conducts the Thermoregulatory Sweat Test (15%). Dr. Vernino has received honoraria for lectures or educational activities not funded by industry; serves as a consultant to Athena Diagnostics, Inc.; and receives research support from Chelsea Therapeutics and from the NIH [P50NS32352 (PI)] and from the Department of Veterans Affairs [VA549-P-0027 (PI) administered by the Dallas VA Medical Center]. Dr. Low reports no disclosures.
Supplementary Material
Address correspondence and reprint requests to Dr. Phillip A. Low, Mayo Clinic, 200 First Street SW, Rochester, MN 55905 low@mayo.edu
Supplemental data at www.neurology.org
Supported in part by the NIH (NS 32352, NS 44233, NS 22352, and NS 43364), Mayo CTSA (UL1 RR24150), and Mayo Funds. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders and Stroke or the National Institutes of Health.
Disclosure: Author disclosures are provided at the end of the article.
Received April 22, 2009. Accepted in final form July 27, 2009.
REFERENCES
- 1.Suarez GA, Fealey RD, Camilleri M, Low PA. Idiopathic autonomic neuropathy: clinical, neurophysiologic, and follow-up studies on 27 patients. Neurology 1994;44:1675–1682. [DOI] [PubMed] [Google Scholar]
- 2.Vernino S, Lennon VA. Neuronal ganglionic acetylcholine receptor autoimmunity. Ann NY Acad Sci 2003;998:211–214. [DOI] [PubMed] [Google Scholar]
- 3.Klein CM, Vernino S, Lennon VA, et al. The spectrum of autoimmune autonomic neuropathies. Ann Neurol 2003;53:752–758. [DOI] [PubMed] [Google Scholar]
- 4.Sandroni P, Vernino S, Klein CM, et al. Idiopathic autonomic neuropathy: comparison of cases seropositive and seronegative for ganglionic acetylcholine receptor antibody. Arch Neurol 2004;61:44–48. [DOI] [PubMed] [Google Scholar]
- 5.Lennon VA, Ermilov LG, Szurszewski JH, Vernino S. Immunization with neuronal nicotinic acetylcholine receptor induces neurological autoimmune disease. J Clin Invest 2003;111:907–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vernino S, Low PA, Lennon VA. Experimental autoimmune autonomic neuropathy. J Neurophysiol 2003;90:2053–2059. [DOI] [PubMed] [Google Scholar]
- 7.Vernino S, Ermilov LG, Sha L, Szurszewski JH, Low PA, Lennon VA. Passive transfer of autoimmune autonomic neuropathy to mice. J Neurosci 2004;24:7037–7042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iodice V, Kimpinski K, Vernino S, Sandroni P, Fealey RD, Low PA. Efficacy of immunotherapy in autonomic autoimmune ganglionopathy. Neurology 2009;72:2002–2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Low PA. Evaluation of sudomotor function. Clin Neurophysiol 2004;115:1506–1513. [DOI] [PubMed] [Google Scholar]
- 10.Low PA, Sletten DM. Laboratory evaluation of autonomic failure. In: Low PA, Benarroch EE, eds. Clinical Autonomic Disorders. 3rd ed. Philadelphia: Lippincott Williams & Wilkins; 2008:130–163. [Google Scholar]
- 11.Low PA, Denq JC, Opfer-Gehrking TL, Dyck PJ, O'Brien PC, Slezak JM. Effect of age and gender on sudomotor and cardiovagal function and blood pressure response to tilt in normal subjects. Muscle Nerve 1997;20:1561–1568. [DOI] [PubMed] [Google Scholar]
- 12.Fealey RD, Low PA, Thomas JE. Thermoregulatory sweating abnormalities in diabetes mellitus. Mayo Clin Proc 1989;64:617–628. [DOI] [PubMed] [Google Scholar]
- 13.Fealey RD. Thermoregulatory sweat test. In: Low PA, Benarroch EE. Clinical Autonomic Disorders. 3rd ed. Philadelphia: Lippincott Williams & Wilkins; 2008:244–263. [Google Scholar]
- 14.Low PA. Composite autonomic scoring scale for laboratory quantification of generalized autonomic failure. Mayo Clin Proc 1993;68:748–752. [DOI] [PubMed] [Google Scholar]
- 15.Vernino S, Low PA, Fealey RD, Stewart JD, Farrugia G, Lennon VA. Autoantibodies to ganglionic acetylcholine receptors in autoimmune autonomic neuropathies. N Engl J Med 2000;343:847–855. [DOI] [PubMed] [Google Scholar]
- 16.Drachman DB, Adams RN, Stanley EF, Pestronk A. Mechanisms of acetylcholine receptor loss in myasthenia gravis. J Neurol Neurosurg Psychiatry 1980;43:601–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Z, Low PA, Jordan J, et al. Autoimmune autonomic ganglionopathy: IgG effects on ganglionic acetylcholine receptor current. Neurology 2007;68:1917–1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Skok MV, Voitenko LP, Voitenko SV, et al. Alpha subunit composition of nicotinic acetylcholine receptors in the rat autonomic ganglia neurons as determined with subunit-specific anti-alpha (181-192) peptide antibodies. Neuroscience 1999;93:1427–1436. [DOI] [PubMed] [Google Scholar]
- 19.Goldstein DS, Holmes C, Dendi R, Li ST, Brentzel S, Vernino S. Pandysautonomia associated with impaired ganglionic neurotransmission and circulating antibody to the neuronal nicotinic receptor. Clin Auton Res 2002;12:281–285. [DOI] [PubMed] [Google Scholar]
- 20.Maki T, Nakamura M, Nakamura M, Suenaga T. A case of autoimmune autonomic neuropathy with marked orthostatic hypotension, decreased salivation, constipation, and Adie pupil. Rinsho Shinkeigaku Clin Neurol 2006;46:218–222. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.