Abstract
Background
Among patients with acute myocardial infarction (AMI), depression is both common and under-recognized. The association of different manifestations of depression, somatic and cognitive, with depression recognition and long-term prognosis is poorly understood.
Methods and Results
Depression was confirmed in 481 AMI patients enrolled from 21 sites during their index hospitalization with a Patient Health Questionnaire (PHQ-9) score ≥10. Within the PHQ-9, separate somatic and cognitive symptom scores were derived and the independent association between these domains and the clinical recognition of depression, as documented in the medical records, was evaluated. In a separate multisite AMI registry of 2,347 patients, the association between somatic and cognitive depressive symptoms and 4-year all-cause mortality and 1-year all-cause rehospitalization was evaluated. Depression was clinically recognized in 29% (n=140) of patients. Cognitive depressive symptoms (Relative Risk [RR] per Standard Deviation [SD] increase=1.14; 95% confidence interval [CI] 1.03–1.26; p=0.01) were independently associated with depression recognition, while the association for somatic symptoms and recognition (RR=1.04; 95% CI 0.87–1.26; p=0.66) was not significant. However, unadjusted Cox regression analyses found that only somatic depressive symptoms were associated with 4-year mortality (Hazard Ratio [HR] per SD increase=1.22; 95% confidence interval [CI] 1.08–1.39) or 1-year rehospitalization (HR=1.22; 95%CI 1.11–1.33) while cognitive manifestations were not (HR for mortality=1.01; 95%CI 0.89–1.14; HR for rehospitalization=1.01; 95%CI 0.93–1.11). After multivariable adjustment, the association between somatic symptoms and rehospitalization persisted (HR=1.16; 95% CI:1.06–1.27; p=0.01) but was attenuated for mortality (HR=1.07; 95% CI:0.94–1.21; p=0.30).
Conclusions
Depression after AMI was recognized in fewer than 1 in 3 patients. Although cognitive symptoms were associated with recognition of depression, somatic symptoms were associated with long-term outcomes. Comprehensive screening and treatment of both somatic and cognitive symptoms may be necessary to optimize depression recognition and treatment in AMI patients.
Keywords: Depressive symptoms, myocardial infarction, treatment, mortality, rehospitalization
BACKGROUND
Depression after acute myocardial infarction (AMI) is prevalent and associated with both worse quality of life1 and higher rates of mortality and rehospitalization.2–5 Despite efforts to promote systematic depression screening and facilitate its treatment in AMI patients6–8, depression often goes unrecognized9–11 and its treatment after AMI with pharmacologic and behavioral interventions have not resulted in lower rates of mortality or rehospitalization.12 While depression is a common comorbidity that warrants treatment in its own right and can be useful in identifying high-risk patients for more aggressive treatment of coronary artery disease, a better understanding of which symptoms of depression are prognostic of long-term clinical outcomes would inform therapeutic strategies for future efficacy trials.
Depression is a complex disease characterized by both somatic and cognitive manifestations.13–15 Somatic depressive symptoms (e.g., fatigue, loss of energy, and sleep disturbances) are often masked by the physical symptoms of cardiovascular disease.16–18 Consequently, clinicians may be more likely to recognize and focus treatment of depression among those patients who manifest significant cognitive symptoms, such as sadness, pessimism, and anhedonia. While clinical trials of depression after AMI have largely relied on therapies that primarily target cognitive depressive symptoms,12, 19 preliminary studies suggest that somatic depressive symptoms have equal, if not greater, importance for prognosis in cardiac disease than do cognitive symptoms.20–23
An improved understanding of the cognitive and somatic depressive symptom dimensions would be a critical step in improving detection of and targeting interventions for depression in AMI patients. This is particularly important if there is discordance between the symptom dimensions that are associated with recognition and prognosis. Accordingly, we evaluated whether cognitive or somatic depressive symptoms facilitate recognition of depression in patients hospitalized with AMI and the extent to which each symptom domain was associated with long-term mortality and rehospitalization.
METHODS
Participants and study design
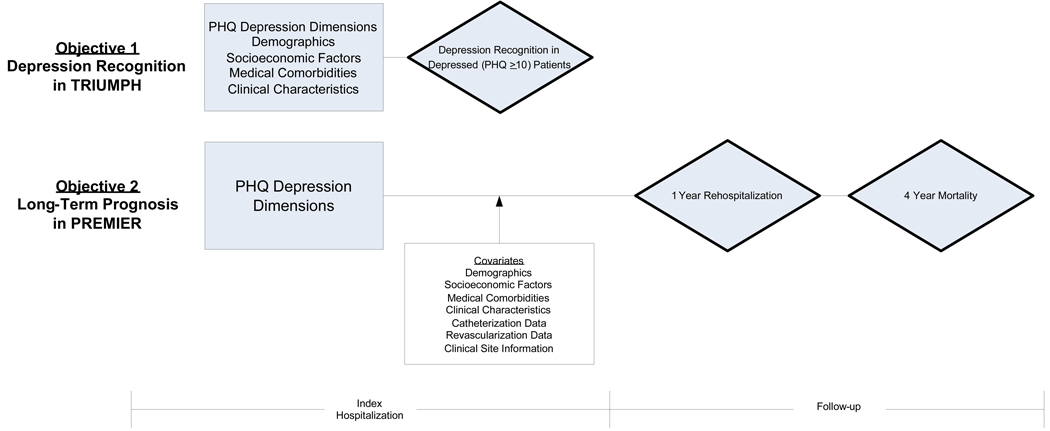
Data from two similar AMI registries—the Translational Research Investigating Underlying disparities in acute Myocardial infarction Patients’ Health Status (TRIUMPH) study and the Prospective Registry Evaluating outcomes after Myocardial Infarctions: Events and Recovery (PREMIER) study—were used to conduct this study. Both studies are large, prospective, geographically diverse, multi-site registries of academic and non-academic institutions within the U.S. Patients for the TRIUMPH study were enrolled from 21 hospitals between April 11, 2005 and December 31, 2008, while patients within PREMIER were enrolled from 19 hospitals between January 1, 2003, and June 28, 2004. The TRIUMPH study prospectively collected data on depression recognition and completed enrollment in December 2008. In contrast, the PREMIER study provided data on 1-year hospitalization rates and 4-year mortality but did not prospectively assess depression recognition. Therefore, we assessed depression recognition within TRIUMPH but evaluated long-term outcomes within PREMIER. The overview of both study designs is provided in Figure 1.
Figure 1. Overview of Study Design and Objectives.
Abbreviations: AMI, acute myocardial infarction; PHQ, patient health questionnaire; PREMIER, prospective registry evaluating outcomes after myocardial infarctions: events and recovery; TRIUMPH, translational research investigating underlying disparities in acute myocardial infarction patients’ health Status.
Both TRIUMPH and PREMIER had similar inclusion criteria and common enrollment sites, and the study design of PREMIER has been previously described.24 In both registries, patients 18 years of age or older with biomarker evidence of myocardial injury (a positive troponin or elevated creatinine kinase-MB fraction within 24 hours of hospital admission) and supporting evidence of an AMI (ischemic signs or symptoms for >20 minutes or electrocardiographic ST changes) were enrolled. Patients were excluded if they were incarcerated or had biomarker elevations after elective coronary revascularization. Additionally, because we were interested in evaluating the association between depressive symptoms with recognition and prognosis, we excluded patients (n=142 [5.5%] in TRIUMPH and n=127 [5.4%] in PREMIER) without a baseline assessment of depressive symptoms with the Patient Health Questionnaire (PHQ-9). All participants provided written informed consent and the study protocols were approved by the institutional review board at each participating site.
Data Collection
Detailed data in both TRIUMPH and PREMIER were collected through chart abstraction (on clinical comorbidities, admission medications, presenting electrocardiogram, and treatments during the first 24 hours) and standardized in-depth interviews by trained hospital research staff between 24 and 72 hours after AMI admission (depressive symptoms, tobacco use, demographics, socioeconomic factors). Finally, at the time of discharge, angiographic data, in-hospital treatment of AMI, discharge recommendations, discharge medications, and discharge diagnoses were also documented by chart abstraction. Patient data included demographics (age, sex, and race), social and economic factors (marital status, education, access to health insurance, and employment status), and clinical variables (hypercholesterolemia, hypertension, peripheral arterial disease, diabetes mellitus, prior AMI, prior angina, prior coronary artery bypass surgery [CABG] or percutaneous coronary intervention [PCI], prior stroke, chronic renal failure, chronic lung disease, chronic heart failure, non-skin cancer, smoking, body mass index, family history of coronary artery disease, and history of depression or current treatment for depression). In addition, data were obtained on AMI severity (ST elevation vs. non-ST elevation AMI, left ventricular ejection fraction <40%, Killip class, number of coronary arteries with ≥75% stenosis, and systolic blood pressure and heart rate at AMI presentation). Finally, treatment information (coronary angiography, PCI, and CABG), patient instructions at discharge (cardiac rehabilitation, diet counselling, exercise counselling, follow-up lipid assessment, and smoking cessation), and data on the percent and number of the Joint Commission on Accreditation of Healthcare Organizations’ quality of care indicators received at hospital discharge (e.g., appropriate use of aspirin, beta-blockers, thienopyridines, and other medications, median time to primary PCI, and lipid assessment during index hospitalization) were collected.25
Assessment of depressive symptoms
The PHQ-9, a validated tool for depression screening, was used to assess symptoms of depression.26 The PHQ-9 quantifies the frequency, over the past 2 weeks, of each of 9 Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition27 criteria on a 4-point Likert scale ranging from “0” (not at all) to “3” (nearly every day). Responses are summed to create a score between 0 and 27 points. A PHQ score of ≥10 has been recommended to screen for the diagnosis of major depression and has been shown to have a sensitivity and specificity of 88%.28–30
In this study, we were interested in examining the somatic and cognitive dimensions of depression. Based on prior work,20 4 PHQ-9 items related to problems with sleep, fatigability, appetite, and psychomotor agitation/retardation were classified as somatic depressive symptoms, whereas 5 items, related to lack of interest, depressed mood, negative feelings about self, concentration problems and suicidal ideation, were classified as cognitive depressive symptoms. These depression dimension classifications have been previously validated, with high Cronbach’s alpha statistics for both somatic depressive symptoms (0.77) and cognitive depressive symptoms (0.84).20 We accordingly calculated sum scores of the two dimensions for our analyses.20 Correlations between both dimensions among patients with a PHQ-9 ≥ 10 ranged from r=0.04 in TRIUMPH to r=0.23 in PREMIER.
Depression recognition
The primary endpoint for the first objective of this study was the recognition of depression at the time of AMI hospitalization in the TRIUMPH study. Patients were classified as depressed if they had a PHQ-9 score of ≥10. Clinicians, blinded to the results of the PHQ-9, had to make a diagnosis of depression in the hospital chart, assign a diagnosis of depression at hospital discharge, prescribe active depression treatment (antidepressant medication, counselling, or psychiatric consultation), or refer patients for depression management at discharge in order for patients to be classified as ‘clinically recognized’. To ensure that we did not misclassify the use of antidepressive medications as indicating depression recognition, the clinical indications for such medications were reviewed. Patients with a PHQ-9 ≥10 and taking antidepressant medications solely for the purposes of smoking cessation (n=6 for buproprion) or neuropathic pain (n=5 for tricyclic antidepressants or serotonin-norepinephrine reuptake inhibitors) were not classified as having recognized depression.
Mortality and Rehospitalization
The endpoints for the second objective of this study were 4-year all-cause mortality and 1-year all-cause rehospitalization among patients enrolled in the PREMIER study. Mortality was determined using the Social Security Death Master File, and hospitalization data were determined from phone interviews at 1 month, 6 months, and 1 year.
Statistical Analysis
There were two main objectives in this study. We first evaluated whether somatic or cognitive depressive symptoms were associated with clinical recognition of depression in TRIUMPH. Next, we evaluated whether somatic or cognitive depressive symptoms were associated with long-term outcomes in PREMIER.
Depression Recognition in TRIUMPH
To assess predictors of depression recognition, baseline characteristics of patients with recognized and unrecognized depression were compared using Student’s t-tests and the Wilcoxon tests for continuous variables and Chi-square or Fisher’s Exact tests for categorical variables, as appropriate. Hierarchical modified Poisson regression models, which adjust for clustering at the hospital level, were then constructed to assess the unadjusted and adjusted relationship between somatic and cognitive depression scores and clinical recognition of depression (binary dependent variable) by entering both dimensions simultaneously in the model as independent variables. Non-linear relationships between the depression dimensions and recognition were assessed with restricted cubic spline terms with 3 knots for curvature in the multivariable models.31 Because of the high event rate, a modified Poisson regression model (i.e., Poisson regression with a robust error variance) was used to derive relative risks, as odds ratios would overestimate the strengths of associations.32
The multivariable model adjusted for all variables with a significant association in bivariate analysis as described in Table 1, along with the following clinically important variables regardless of statistical significance: age, gender, marital status, insurance status, left ventricular ejection fraction <40%, history of chronic heart failure, and ST elevation AMI. Somatic and cognitive depression symptom scores were evaluated as continuous variables and were interpreted using a standard deviation increase for each measure (3 points for both). As a sensitivity analysis, we repeated the analyses when the cohort was restricted to patients without a history of depression.
Table 1.
Characteristics of Patients with Recognized and Unrecognized Depression Within TRIUMPH.*
| Depression | |||
|---|---|---|---|
| Recognized n=140 |
Unrecognized n=341 |
P Value | |
| Demographics, No. (%) | |||
| Age, years | 55.6±11.7 | 56.5±12.1 | .44 |
| Female sex | 68 (48.6) | 151 (44.3) | .39 |
| Race | |||
| White/Caucasian | 103 (73.6) | 219 (64.2) | .05 |
| Black/African American | 24 (17.1) | 95 (27.9) | |
| Other | 13 (9.3) | 27 (7.9) | |
| Socioeconomic factors, No. (%) | |||
| Married | 54 (38.6) | 144 (42.4) | .44 |
| Greater than high school education | 77 (55.0) | 145 (42.6) | .01 |
| No medical insurance | 28 (20.0) | 90 (26.4) | .20 |
| Working full- or part-time | 42 (30.0) | 142 (41.7) | .05 |
| Medical history, No. (%) | |||
| Hypercholesterolemia | 79 (56.4) | 158 (46.3) | .04 |
| Hypertension | 109 (77.9) | 240 (70.4) | .10 |
| Peripheral arterial disease | 9 (6.4) | 21 (6.2) | .91 |
| Diabetes mellitus | 63 (45.0) | 126 (37.0) | .10 |
| Prior AMI | 31 (22.1) | 71 (20.8) | .75 |
| Prior angina | 24 (17.1) | 41 (12.0) | .14 |
| Prior CABG | 27 (19.3) | 34 (10.0) | .01 |
| Prior PCI | 42 (30.0) | 73 (21.4) | .05 |
| Prior stroke | 10 (7.1) | 15 (4.4) | .22 |
| Chronic renal failure | 13 (9.3) | 23 (6.7) | .34 |
| Chronic lung disease | 17 (12.1) | 38 (11.1) | .75 |
| Chronic heart failure | 19 (13.6) | 34 (10.0) | .25 |
| Cancer (other than skin) | 9 (6.4) | 26 (7.6) | .65 |
| Smoked within last 30 days | 70 (50.0) | 170 (50.3) | .95 |
| Body Mass Index | 30.9±7.1 | 30.5±7.0 | .60 |
| Family history of CAD | 107 (77.0) | 262 (77.5) | .90 |
| History of depression | 57 (40.7) | 18 (5.3) | <.001 |
| Clinical characteristics index MI admission, No. (%) | |||
| ST-elevation MI | 51 (36.4) | 132 (38.7) | .64 |
| Ejection fraction <40% | 28 (20.0) | 71 (20.9) | .83 |
| Killip class | .91 | ||
| I (No heart failure) | 113 (81.9) | 281 (84.1) | |
| II (Heart failure) | 22 (15.9) | 45 (13.5) | |
| III (Pulmonary edema) | 2 (1.4) | 5 (1.5) | |
| IV (Cardiogenic shock) | 1 (0.7) | 3 (0.9) | |
| Systolic blood pressure, mm Hg | 137.0±32.1 | 143.2±30.0 | .05 |
| Heart rate, beats per minute | 87.0±24.8 | 83.8±21.9 | .16 |
Plus-minus values are means ± standard deviation.
Abbreviations: AMI, acute myocardial infarction; BMI, body mass index (kilograms/meters2); CABG, coronary artery bypass grafting; CAD, coronary artery disease; PCI, percutaneous coronary interventions.
Mortality and Rehospitalization in PREMIER
For descriptive purposes only, we categorized patients in the upper quartile of somatic and cognitive depressive symptom scores as having significant somatic or cognitive depressive symptoms. Baseline comparisons between those with and without significant somatic depressive symptoms were compared using Student’s t-tests and the Wilcoxon test for continuous variables and the Chi-square or Fisher’s Exact test for categorical variables, as appropriate. Similarly, baseline characteristics were compared between those with and without significant cognitive depressive symptoms.
The association of a PHQ-9 score ≥10 with higher mortality and rehospitalization risk, as demonstrated in prior studies 5, 33, was first validated in our study cohort with unadjusted Kaplan-Meier plots and multivariable Cox proportional hazards regression analyses stratified by site of care. Next, unadjusted and multivariable Cox proportional hazard regression models stratified by site were constructed to jointly evaluate the association of both somatic and cognitive depressive symptom scores (as continuous variables in the model) with mortality and rehospitalization in separate models. Multivariable models adjusted for demographic (age, sex, race), clinical (diabetes mellitus, prior coronary artery disease (prior MI, prior PCI or prior CABG), stroke, chronic renal failure, chronic lung disease, chronic heart failure, non-skin cancer, current smoking, body mass index) socioeconomic (marital status, education, insurance status and working status), AMI severity (ST elevation AMI, left ventricular ejection fraction <40%, heart rate), and treatment (angiography, revascularization, percent and number of quality of care indicators received) variables. Somatic and cognitive depression symptom scores were interpreted using a standard deviation increase in each measure (3 point increase). Non-linear relationships of depression dimensions and outcomes were assessed using restricted cubic spline terms with 3 knots for curvature in the multivariable models.31
Missing Data
Model covariates in TRIUMPH were missing for at least 1 study covariate in 7 patients (1.5% of cohort), with no study covariate having >1% missing data. Data in PREMIER were missing for at least 1 study covariate in 326 patients (13.9% of cohort), with no study covariate having >6% missing data. In both datasets, missing data were assumed to be missing at random and imputed as a single imputation dataset using IVEWARE software.34 Additionally, data on mortality was 100% complete in PREMIER, but follow-up interviews on rehospitalization were missing in 9% (n=197) of surviving patients. Based on prior work,35 bias attributable to those without follow-up interviews was assessed by creating a non-parsimonious model for the propensity to miss the 1-year follow-up interview. The reciprocal of this probability was then used to weight the associations among responders in the multivariable Cox regression model for rehospitalization to adjust for potential observable bias from lost follow-up.35 For both sets of analyses, results with and without weighting were comparable, so only the weighted are presented.
All tests for significance were two-tailed with an alpha level of 0.05 and were conducted with SAS Version 9.1.3 (SAS Institute, Cary, NC) and R version 2.6.0.36 The authors independently designed the study, collected and analyzed the data, and drafted and revised the manuscript. Drs. Smolderen and Chan had full access to all of the data and take full responsibility for the integrity of the data and the accuracy of the data analysis.
RESULTS
Depression recognition in the TRIUMPH registry
Of 2,573 patients screened with the PHQ-9 in TRIUMPH, depression was present in 481 (19%) patients. Of these, depression was unrecognized in 341 (71%) and recognized in 140 (29%) patients. Among those with recognized depression, antidepressants were prescribed at discharge in 118 (84.3%) patients and depression counselling or a recommendation for further follow-up was prescribed in 72 (51.4%) patients. Depression was less likely to be recognized in patients who were black, had attained a lower educational level, and who were employed (Table 1). Depression was more likely to be recognized among patients with a prior history of hypercholesterolemia, CABG, PCI, and depression. Patients with recognized depression, compared to those with unrecognized depression, had higher scores on the PHQ-9 for cognitive symptoms (7.5 ± 3.3 vs. 6.3 ± 2.9; p<0.001) but similar scores for somatic symptoms (7.7 ± 2.0 vs. 7.6 ± 2.0; p=0.59).
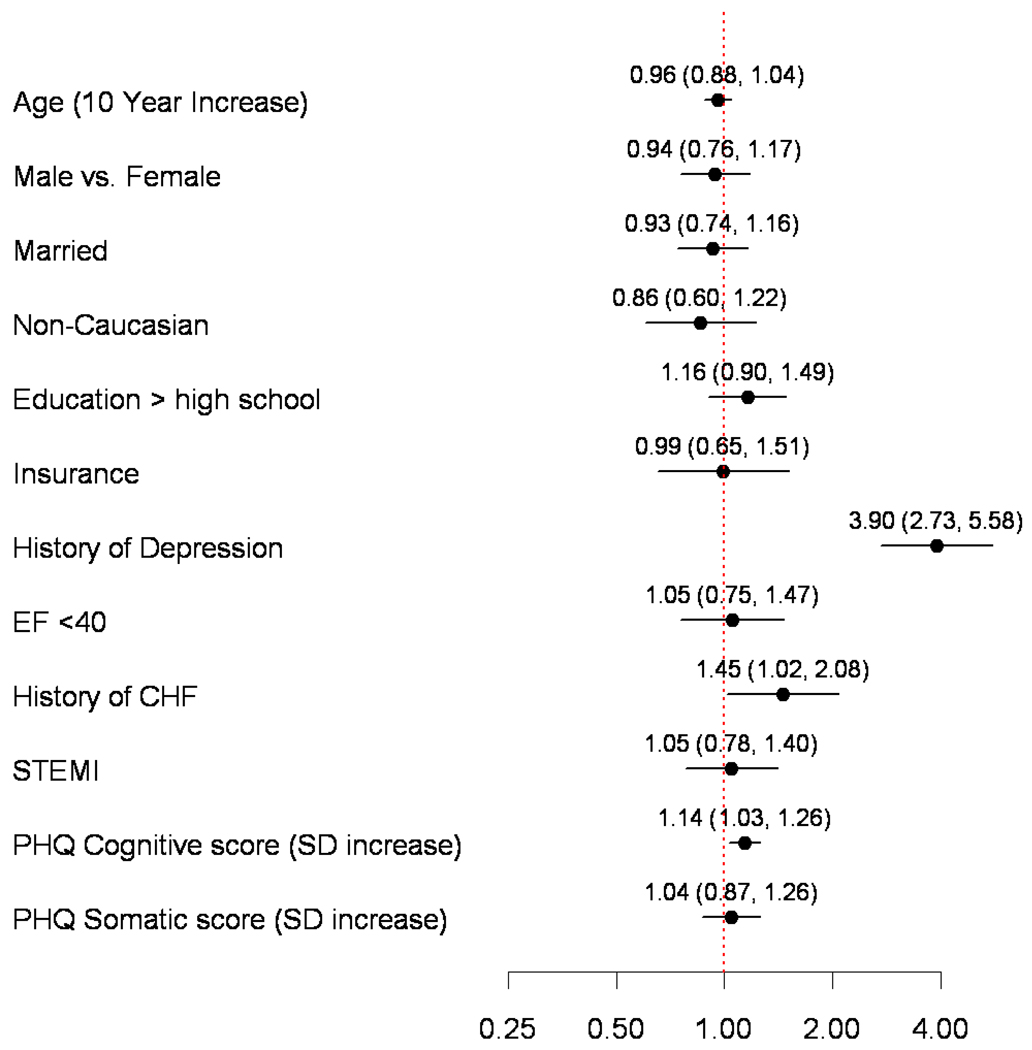
Adjusting for demographic, and clinical factors, cognitive depressive symptoms were associated with depression recognition (adjusted Risk Ratio [RR] per Standard Deviation [SD] increase=1.14; 95% CI, 1.03–1.26; p=0.01), but no significant association was observed for somatic depressive symptoms (adjusted RR per SD increase=1.04; 95% CI, 0.87–1.26; p=0.66) (Figure 2). There was no evidence of non-linearity (p-value >0.25). Results were not different when we restricted the cohort to only those patients without a history of depression. Other predictors of depression recognition included a history of depression (adjusted RR=3.90; 95% CI, 2.73–5.58; p<0.001) and chronic heart failure (adjusted RR=1.45; 95% CI, 1.02–2.08; p=0.04). Race, education, and employment status were not independently associated with depression recognition. The final model showed good discrimination (C-statistic=0.78).
Figure 2. Independent Predictors of Depression Recognition During Index AMI Hospitalization.
Model estimates are presented as Relative Risks with 95% Confidence Intervals. Abbreviations: AMI, acute myocardial infarction; CHF, chronic heart failure; EF, ejection fraction; PHQ, patient health questionnaire; SD, Standard Deviation; STEMI, ST-elevation myocardial infarction.
Mortality and rehospitalization in the PREMIER registry
Of 2,347 patients within PREMIER, 624 (26.6%) were classified as having significant somatic depressive symptoms (upper quartile of somatic depressive symptom score ≥6) and 590 (25.1%) were categorized as having significant cognitive depressive symptoms (upper quartile of cognitive depressive symptom dimension score ≥4).
Baseline comparisons between those with and without significant somatic depressive symptoms and those with and without significant cognitive depressive symptoms are presented in Table 2. In both comparisons, patients with significant depressive symptoms were younger; were more likely to be female and African American, and were less likely to be married. Depressed patients were also less likely to have completed post-secondary education and to be employed. Moreover, patients with either significant somatic or cognitive symptoms of depression, compared to those without, had higher frequencies of comorbidities (history of hypertension, diabetes mellitus, AMI, angina, PCI, chronic lung disease, and chronic heart failure) and worse disease severity at the time of their AMI (higher Killip class and heart rate). However, they were less likely to undergo coronary angiography, to undergo revascularization with either PCI or CABG, or to receive referrals for cardiac rehabilitation or exercise counselling at discharge. While patients in all groups were eligible for the same number of quality-of-care indicators (including medications) at discharge, patients with significant somatic depressive symptoms received fewer of these treatments at discharge.
Table 2.
| Significant PHQ Somatic Symptoms | Significant PHQ Cognitive Symptoms | |||||
|---|---|---|---|---|---|---|
| Yes n=624 |
No n=1723 |
P Value | Yes n=590 |
No n=1757 |
P Value | |
| Demographics, No. (%) | ||||||
| Age, years | 59.1±12.6 | 61.4±13.0 | <.001 | 58.0±11.7 | 61.7±13.1 | <.001 |
| Female sex | 256 (41.0) | 497 (28.8) | <.001 | 233 (39.5) | 520 (29.6) | <.001 |
| Race | <.001 | <.001 | ||||
| White/Caucasian | 427 (68.6) | 1307 (76.3) | 395 (67.3) | 1339 (76.6) | ||
| Black/African American | 162 (26.0) | 330 (19.3) | 161 (27.4) | 331 (18.9) | ||
| Other | 33 (5.3) | 75 (4.4) | 31 (5.3) | 77 (4.4) | ||
| Socioeconomic factors, No. (%) | ||||||
| Married | 335 (54.3) | 1066 (62.7) | <.001 | 307 (53.1) | 1094 (62.9) | .001 |
| Greater than high school education | 259 (42.2) | 864 (50.8) | <.001 | 244 (42.1) | 879 (50.6) | <.001 |
| Having no insurance | 81 (13.8) | 194 (11.8) | .22 | 91 (16.2) | 184 (11.0) | .001 |
| Working full- or part-time | 231 (37.2) | 793 (46.4) | <.001 | 211 (36.0) | 813 (46.6) | <.001 |
| Medical history, No. (%) | ||||||
| Hypercholesterolemia | 323 (51.8) | 822 (47.7) | .08 | 303 (51.4) | 842 (47.9) | .15 |
| Hypertension | 426 (68.3) | 1061 (61.6) | .01 | 410 (69.5) | 1077 (61.3) | <.001 |
| Peripheral arterial disease | 58 (9.3) | 128 (7.4) | .14 | 46 (7.8) | 140 (8.0) | .89 |
| Diabetes mellitus | 219 (35.1) | 453 (26.3) | <.001 | 201 (34.1) | 471 (26.8) | <.001 |
| Prior AMI | 164 (26.3) | 335 (19.4) | <.001 | 163 (27.6) | 336 (19.1) | <.001 |
| Prior angina | 132 (21.2) | 271 (15.7) | .01 | 128 (21.7) | 275 (15.7) | <.001 |
| Prior CABG | 90 (14.4) | 206 (12.0) | .11 | 85 (14.4) | 211 (12.0) | .13 |
| Prior PCI | 129 (20.7) | 279 (16.2) | .01 | 120 (20.3) | 288 (16.4) | .03 |
| Prior stroke | 45 (7.2) | 109 (6.3) | .44 | 49 (8.3) | 105 (6.0) | .05 |
| Chronic renal failure | 76 (12.2) | 155 (9.0) | .02 | 62 (10.5) | 169 (9.6) | .53 |
| Chronic lung disease | 122 (19.6) | 184 (10.7) | <.001 | 100 (16.9) | 206 (11.7) | .001 |
| Chronic heart failure | 108 (17.3) | 175 (10.2) | <.001 | 98 (16.6) | 185 (10.5) | <.001 |
| Cancer (other than skin) | 46 (7.4) | 150 (8.7) | .30 | 42 (7.1) | 154 (8.8) | .21 |
| Smoked within last 30 days | 233 (37.6) | 567 (33.0) | .04 | 244 (41.7) | 556 (31.7) | <.001 |
| Body Mass Index | 29.7±6.6 | 29.0±6.3 | .03 | 29.6±6.4 | 29.1±6.4 | .10 |
| Family history of CAD | 228 (36.5) | 574 (33.3) | .15 | 218 (36.9) | 584 (33.2) | .10 |
| Currently receiving treatment for depression | 150 (24.2) | 147 (8.6) | <.001 | 167 (28.5) | 130 (7.4) | <.001 |
| Clinical characteristics index MI admission, No. (%) | ||||||
| ST-elevation MI | 249 (39.9) | 768 (44.6) | .04 | 235 (39.8) | 782 (44.5) | .05 |
| Ejection fraction <40% | 181 (29.0) | 427 (24.9) | .04 | 159 (26.9) | 449 (25.6) | .53 |
| Killip class | <.001 | <.001 | ||||
| I (No heart failure) | 400 (76.0) | 1252 (85.1) | 391 (77.0) | 1261 (84.7) | ||
| II (Heart failure) | 87 (16.5) | 169 (11.5) | 83 (16.3) | 173 (11.6) | ||
| III (Pulmonary edema) | 28 (5.3) | 24 (1.6) | 23 (4.5) | 29 (1.9) | ||
| IV (Cardiogenic shock) | 11 (2.1) | 26 (1.8) | 11 (2.2) | 26 (1.7) | ||
| Diseased vessels (>75% stenosis) | .12 | .07 | ||||
| 0 | 61 (11.7) | 131 (8.6) | 58 (11.8) | 134 (8.6) | ||
| 1 | 268 (51.2) | 763 (50.3) | 227 (46.3) | 804 (51.8) | ||
| 2 | 101(19.3) | 340 (22.4) | 113 (23.1) | 328 (21.1) | ||
| 3 | 93 (17.8) | 284 (18.7) | 92 (18.8) | 285 (18.4) | ||
| Systolic blood pressure, mm Hg | 140.2±32.8 | 138.9±30.8 | .39 | 140.0±32.5 | 139.0±31.0 | .53 |
| Heart rate, beats per minute | 83.4±20.0 | 80.5±22.1 | .01 | 83.8±21.1 | 80.4±21.7 | <.001 |
| Catheterization and revascularization data, No. (%) | ||||||
| Primary or other PCI | 356 (57.1) | 1069 (62.0) | .03 | 324 (54.9) | 1101 (62.7) | <.001 |
| Coronary angiography (catheterization, PCI, CABG) |
523 (83.8) | 1518 (88.1) | .01 | 490 (83.1) | 1551 (88.3) | .001 |
| Revascularization (PCI, CABG, thrombolytic) |
421 (67.5) | 1268 (73.6) | .01 | 387 (65.6) | 1302 (74.1) | <.001 |
| Patient instructions at discharge, No. (%) | ||||||
| Cardiac rehabilitation | 263 (42.1) | 870 (50.5) | <.001 | 240 (40.7) | 893 (50.8) | <.001 |
| Diet counselling | 485 (77.7) | 1332 (77.3) | .83 | 457 (77.5) | 1360 (77.4) | .98 |
| Exercise counselling | 267 (42.8) | 868 (50.4) | .001 | 252 (42.7) | 883 (50.3) | .01 |
| Lipid assessment | 67 (10.7) | 269 (15.6) | .01 | 74 (12.5) | 262 (14.9) | .16 |
| Smoking cessation | 199 (31.9) | 480 (27.9) | .06 | 204 (34.6) | 475 (27.0) | <.001 |
| Clinical site information | ||||||
| QOC: Number of eligible indicators | 5.1±1.4 | 5.2±1.3 | .45 | 5.1±1.3 | 5.2±1.3 | .20 |
| QOC: Percent of eligible indicators received | 85.3±19.0 | 88.2±17.0 | <.001 | 86.6±18.1 | 87.7±17.4 | .18 |
| Depressive symptoms, No. (%) | ||||||
| Depression present baseline (PHQ ≥10) | 444 (71.2) | 80 (4.6) | <.001 | 455 (77.1) | 69 (3.9) | <.001 |
Depression dimensions were defined by the highest score quartile for somatic and for cognitive depressive symptoms.
Plus-minus values are means ± standard deviation.
Abbreviations: AMI, acute myocardial infarction; BMI, body mass index (kilograms/meters2); CABG, coronary artery bypass grafting; CAD, coronary artery disease; PCI, percutaneous coronary interventions; PHQ, patient health questionnaire; QOC, quality of care; STEMI, ST-segment elevation.
Mortality
We first validated the association between formal depression and mortality in the cohort and found that the rates of all cause mortality were higher among the 524 (22.3%) patients with a PHQ-9 score ≥10 (24.2% vs. 16.3%; adjusted Hazard Ratio (HR), 1.41; 95% CI: 1.12–1.76; p=0.01) (Supplemental material).
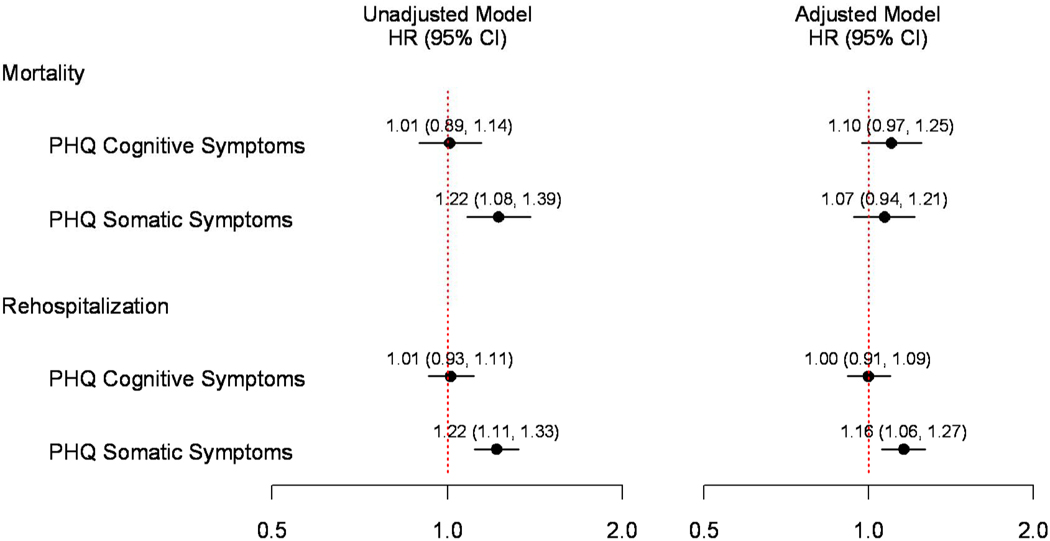
Next, we assessed the association between significant somatic and cognitive depressive symptoms and mortality. Event rates for patients with no depressive symptoms, patients with significant somatic but no cognitive symptoms, patients with significant cognitive but no somatic symptoms, and patients in the highest quartiles for both somatic and cognitive symptoms are presented in Table 3. The unadjusted mortality risk for patients with and without significant cognitive depressive symptoms was similar (unadjusted HR per SD increase=1.01; 95%CI, 0.89–1.14) (Table 3 and Figure 3). In contrast, compared to patients without significant somatic depressive symptoms, patients with significant somatic depressive symptoms had a higher unadjusted mortality risk (unadjusted HR per SD increase=1.22; 95%CI, 1.08–1.39). After multivariable adjustment, the association between somatic symptoms and mortality was attenuated (adjusted HR per SD increase=1.07; 95% CI, 0.94–1.21; p=0.30) (Figure 3). There was no evidence of non-linearity (p-value >0.25).
Table 3.
Event Rates by Depressive Symptom Group for 4-year Mortality and 1-year Rehospitalization.*
| Prognosis | ||||||
|---|---|---|---|---|---|---|
| 4-year Mortality | 1-year Rehospitalization | |||||
| Depressive symptoms | n | % | P Value | n | % | P Value |
| No depressive symptoms | 244/1508 | 16.2 | <.001 | 478/1426 | 33.5 | <.001 |
| Cognitive symptoms | 36/215 | 16.7 | 69/193 | 35.8 | ||
| Somatic symptoms | 52/249 | 20.9 | 100/235 | 42.6 | ||
| Somatic and cognitive symptoms | 92/375 | 24.5 | 151/337 | 44.8 | ||
Depressive symptom dimensions were defined by the highest score quartile for somatic and for cognitive depressive symptoms.
Figure 3. Model Estimates of Risk for 4-Year Mortality and 1-Year Rehospitalization For Somatic and Cognitive Depressive Symptoms.
Abbreviations: CI, confidence interval; HR, hazard ratio; PHQ, patient health questionnaire. Multivariable models adjusted for demographic (age, sex, race), clinical (diabetes mellitus, prior coronary artery disease, stroke, chronic renal failure, chronic lung disease, chronic heart failure, non-skin cancer, current smoking, body mass index) socioeconomic (marital status, education, insurance status and working status), AMI severity (ST elevation AMI, left ventricular ejection fraction <40%, heart rate), and treatment (angiography, revascularization, percent and number of quality of care indicators received) variables.
Rehospitalization
We also validated that formal depression (PHQ-9 ≥10) in this cohort was associated with a higher risk for rehospitalization (42.7% vs. 34.7%; adjusted HR, 1.23; 95% CI: 1.04–1.46; p=0.02) (Supplemental material). When examined by depressive symptom domain, patients with and without significant cognitive depressive symptoms had similar rates of rehospitalization during follow-up (unadjusted HR per SD increase=1.01, 95%CI, 0.93–1.11) (Table 3 and Figure 3). In contrast, compared to patients without significant somatic depressive symptoms, patients with significant somatic depressive symptoms had higher unadjusted rates of rehospitalization during follow-up (unadjusted HR per SD increase=1.22; 95%CI 1.11–1.33), an association which persisted after multivariable adjustment for numerous potential confounders (adjusted HR per SD increase=1.16; 95%CI, 1.06–1.27, p=0.01) (Figure 3). There was no evidence of non-linearity (p-value >0.25).
DISCUSSION
In this study, we found that 7 out of 10 patients with significant depressive symptoms were not recognized during the care and management of their AMI; despite accumulating evidence that depression is associated with higher morbidity and mortality. Among those patients with clinically recognized depression, prominent cognitive depressive symptoms (such as sadness, pessimism, and loss of interest) were more likely to facilitate the recognition of depression, while predominantly somatic symptoms (such as fatigue, loss of energy, and sleep difficulties) were not independently associated with depression recognition. While cognitive symptoms were associated with recognition of depression, they were not independently associated with a higher risk for rehospitalization or death. In contrast, somatic depressive symptoms were associated with a higher risk for mortality and for rehospitalization, although the association with mortality was attenuated after adjustment for clinical variables. These findings highlight an important dissonance in the current paradigm of care. While recognition of depression is associated with manifestations of cognitive symptoms, prognosis after AMI is associated with somatic depressive symptoms.
To our knowledge, this is the first study to jointly examine the relationship between somatic and cognitive depressive symptoms with depression recognition and prognosis among hospitalized AMI patients. These findings extend the findings of prior studies. Several studies in primary care patients have previously reported that the diagnosis of depression in patients with primarily somatic symptoms is particularly challenging because they resemble and are often attributed to the patient’s underlying illness.17, 18, 37 While primary care investigators have long recognized that presentation with somatic symptoms creates barriers to depression recognition and treatment, this issue has not been assessed in depressed AMI patients. In contrast to the recognition of depression, preliminary studies have suggested that somatic, and not cognitive, depressive symptoms are associated with worse prognosis.20–23 These few studies, however, have largely evaluated intermediate outcomes such as the metabolic syndrome21 and heart rate variability.20 Moreover, the extent to which these prognostic studies have been able to comprehensively control for potential confounders of somatic and cognitive depressive symptoms, such as socioeconomic factors (marital status, educational level, and insurance security), severity of the index AMI (ST-elevation AMI, left ventricular ejection fraction), and AMI treatment (diagnostic cardiac catheterization, PCI or CABG, and quality of care indicators), has been limited.20–22 In this study, we were able to control for these potential confounders of somatic and cognitive depressive symptoms and were able to identify a discordance as to which symptom dimension was associated with recognition and prognosis.
There is evidence to suggest that the cognitive symptoms of depression may be mediated by alterations in serotonin metabolism, whereas the somatic symptoms of depression are affected by decreased basal ganglia dopamine activity.38 Biochemical studies also suggest that selective serotonin-reuptake inhibitors (SSRIs), which increase serotonin levels, primarily improve cognitive depressive symptoms.38 Thus our study findings may help explain why prior pharmacologic and behavioral interventions of depression have not resulted in lower rates of mortality or rehospitalization.12, 19 These trials examined interventions (e.g., SSRI medications, cognitive-behavioral therapy or interpersonal therapy) which primarily target the cognitive features of depression, and their inability to demonstrate reductions in cardiovascular morbidity and mortality may, in part, be due to undertreatment of somatic depressive symptoms. While treating the cognitive symptoms of depressed AMI patients is of unquestioned importance, it may not be sufficient to improve cardiovascular prognosis. Given the effect of exercise training on somatic depressive symptoms39, 40 and the established effects of cardiac rehabilitation in decreasing morbidity and mortality in patients with coronary artery disease,41–43 future clinical trials of depression after AMI may wish to consider a more comprehensive treatment approach that targets both somatic and cognitive depressive symptoms.
Despite accumulating and consistent evidence that depression after AMI is associated with a worse prognosis, and despite efforts to increase its awareness and screening in cardiac patients 6, 7, our results, in this geographically diverse, multi-site, contemporary, ‘real-world’ registry, suggest that depression remains unrecognized in most patients hospitalized for an AMI. While therapeutic strategies to modify morbidity and mortality risk for patients with depression after AMI continue to be an active area of investigation, the PHQ-9 instrument remains an important tool in identifying high-risk patients who may benefit from closer monitoring or more aggressive medical therapy. In addition, close collaboration with specialists involved in treating depression will be essential in formulating individualized treatment plans aimed at both reducing patients’ depressive symptom burden and facilitating their recovery following AMI.
Our findings should be considered in light of several potential limitations. First, we assessed depressive symptoms with a self-report questionnaire during patients’ hospitalization and did not use a formal psychiatric interview. However, the PHQ-9 has been shown to have high concordance with psychiatric interviews and its ease of use allows for broader dissemination than a Structured Diagnostic Interview.28 Second, depression recognition was determined from data abstraction from patients’ hospital charts. As such, we cannot rule out the possibility that clinicians recognized but did not document their diagnosis of depression in the medical record, which would have underestimated the rates of recognized depression in this study. It is also important to note that thresholds for clinically relevant somatic and cognitive depressive symptoms have not been validated and require further study. Finally, a concern common to all observational studies, is the possibility of residual confounding, despite our efforts to adjust for a broad and detailed spectrum of socioeconomic, medical comorbidity, disease severity, and treatment characteristics. More specifically, the presence of somatic symptoms may be overlapping with unmeasured cardiac symptoms or factors related to cardiovascular fitness for which we could not adjust for in the current study.
In conclusion, by discriminating between somatic and cognitive depressive symptoms, we were able to identify a discrepancy between the relative association of these symptoms for depression recognition and AMI outcomes. Although cognitive depressive symptoms were associated with recognition of depression, somatic depressive symptoms were associated with long-term outcomes. Opportunities for active screening and comprehensive treatment programs that address both the somatic and cognitive manifestations of depression need to be explored as they may be needed to more effectively treat depression after AMI.
Bullet-Point Summary
“What We Know”
Depression in patients with acute myocardial infarction (AMI) is frequently under-recognized and is associated with worse cardiovascular outcomes and higher mortality.
“What This Article Adds”
Despite the expanding literature on the prevalence of depression in patients with AMI, depression went unrecognized in 7 of 10 patients in this contemporary AMI registry.
Cognitive depressive symptoms (such as sadness, pessimism, and loss of interest) facilitated the recognition of depression, while predominantly somatic symptoms (such as fatigue, loss of energy, and sleep difficulties) were not associated with its recognition.
Although cognitive depressive symptoms were associated with recognition of depression, somatic symptoms were more consistently associated with long-term outcomes, such as mortality and rehospitalization.
To our knowledge, this is the first study to jointly examine the relationship between somatic and cognitive depressive symptoms with depression recognition and prognosis in AMI patients. Our findings suggest that comprehensive screening and treatment of both somatic and cognitive symptoms may be necessary to optimize depression recognition and treatment in AMI patients.
Acknowledgments
Funding Sources
Grant support was received from the National Heart, Lung, and Blood Institute Specialized Center of Clinically Oriented Research in Cardiac Dysfunction and Disease (grant no. P50 HL077113) and CV Therapeutics, Palo Alto, California and from the Netherlands Organization for Scientific Research, The Hague, The Netherlands (VICI grant 453-04-004).
Footnotes
Subject codes: [4], [117]
Conflict of Interest Disclosures
None.
References
- 1.Ruo B, Rumsfeld JS, Hlatky MA, Liu H, Browner WS, Whooley MA. Depressive symptoms and health-related quality of life: the Heart and Soul Study. JAMA. 2003;290:215–221. doi: 10.1001/jama.290.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Frasure-Smith N, Lesperance F. Recent evidence linking coronary heart disease and depression. Can J Psychiatry. 2006;51:730–737. doi: 10.1177/070674370605101202. [DOI] [PubMed] [Google Scholar]
- 3.Barth J, Schumacher M, Herrmann-Lingen C. Depression as a risk factor for mortality in patients with coronary heart disease: a meta-analysis. Psychosom Med. 2004;66:802–813. doi: 10.1097/01.psy.0000146332.53619.b2. [DOI] [PubMed] [Google Scholar]
- 4.Carney RM, Blumenthal JA, Catellier D, Freedland KE, Berkman LF, Watkins LL, Czajkowski SM, Hayeno J, Jaffe AS. Depression as a risk factor for mortality after acute myocardial infarction. Am J Cardiol. 2003;92:1277–1281. doi: 10.1016/j.amjcard.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 5.Parashar S, Rumsfeld JS, Spertus JA, Reid KJ, Wenger NK, Krumholz HM, Amin A, Weintraub WS, Lichtman J, Dawood N, Vaccarino V. Time course of depression and outcome of myocardial infarction. Arch Intern Med. 2006;166:2035–2043. doi: 10.1001/archinte.166.18.2035. [DOI] [PubMed] [Google Scholar]
- 6.Lichtman JH, Bigger JT, Jr, Blumenthal JA, Frasure-Smith N, Kaufman PG, Lesperance F, Mark DB, Sheps DS, Taylor CB, Froelicher ES. Depression and Coronary Heart Disease. Recommendations for Screening, Referral, and Treatment. A Science Advisory From the American Heart Association Prevention Committee of the Council on Cardiovascular Nursing, Council on Clinical Cardiology, Council on Epidemiology and Prevention, and Interdisciplinary Council on Quality of Care and Outcomes Research. Circulation. 2008;118:1768–1775. doi: 10.1161/CIRCULATIONAHA.108.190769. [DOI] [PubMed] [Google Scholar]
- 7.Davidson KW, Kupfer DJ, Bigger JT, Califf RM, Carney RM, Coyne JC, Czajkowski SM, Frank E, Frasure-Smith N, Freedland KE, Froelicher ES, Glassman AH, Katon WJ, Kaufmann PG, Kessler RC, Kraemer HC, Krishnan KR, Lesperance F, Rieckmann N, Sheps DS, Suls JM. Assessment and treatment of depression in patients with cardiovascular disease: National Heart, Lung, and Blood Institute Working Group Report. Psychosom Med. 2006;68:645–650. doi: 10.1097/01.psy.0000233233.48738.22. [DOI] [PubMed] [Google Scholar]
- 8.Rumsfeld JS, Ho PM. Depression and cardiovascular disease: a call for recognition. Circulation. 2005;111:250–253. doi: 10.1161/01.CIR.0000154573.62822.89. [DOI] [PubMed] [Google Scholar]
- 9.Ziegelstein RC, Kim SY, Kao D, Fauerbach JA, Thombs BD, McCann U, Colburn J, Bush DE. Can doctors and nurses recognize depression in patients hospitalized with an acute myocardial infarction in the absence of formal screening? Psychosom Med. 2005;67:393–397. doi: 10.1097/01.psy.0000160475.38930.8d. [DOI] [PubMed] [Google Scholar]
- 10.Amin AA, Jones AM, Nugent K, Rumsfeld JS, Spertus JA. The prevalence of unrecognized depression in patients with acute coronary syndrome. Am Heart J. 2006;152:928–934. doi: 10.1016/j.ahj.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 11.Huffman JC, Smith FA, Blais MA, Beiser ME, Januzzi JL, Fricchione GL. Recognition and treatment of depression and anxiety in patients with acute myocardial infarction. Am J Cardiol. 2006;98:319–324. doi: 10.1016/j.amjcard.2006.02.033. [DOI] [PubMed] [Google Scholar]
- 12.Joynt KE, O'Connor CM. Lessons from SADHART, ENRICHD, and other trials. Psychosom Med. 2005 May–Jun;67 Suppl 1:S63–S66. doi: 10.1097/01.psy.0000163454.25036.fc. [DOI] [PubMed] [Google Scholar]
- 13.Belmaker RH, Agam G. Major depressive disorder. N Engl J Med. 2008;358:55–68. doi: 10.1056/NEJMra073096. [DOI] [PubMed] [Google Scholar]
- 14.van Praag HM. Kraepelin, biological psychiatry, and beyond. Eur Arch Psychiatry Clin Neurosci. 2008;258 Suppl 2:29–32. doi: 10.1007/s00406-008-2006-1. [DOI] [PubMed] [Google Scholar]
- 15.Lecrubier Y. Physical components of depression and psychomotor retardation. J Clin Psychiatry. 2006;67 Suppl 6:23–26. [PubMed] [Google Scholar]
- 16.Tylee A, Gandhi P. The importance of somatic symptoms in depression in primary care. Prim Care Companion J Clin Psychiatry. 2005;7:167–176. doi: 10.4088/pcc.v07n0405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Menchetti M, Murri B, Bertakis K, Bortolotti B, Berardi D. Recognition and treatment of depression in primary care: effect of patients’ presentation and frequency of consultation. J Psychosom Res. 2009:335–341. doi: 10.1016/j.jpsychores.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 18.Barkow K, Heun R, Ustun TB, Berger M, Bermejo I, Gaebel W, Harter M, Schneider F, Stieglitz RD, Maier W. Identification of somatic and anxiety symptoms which contribute to the detection of depression in primary health care. Eur Psychiatry. 2004;19:250–257. doi: 10.1016/j.eurpsy.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 19.Lesperance F, Frasure-Smith N, Koszycki D, Laliberte MA, van Zyl LT, Baker B, Swenson JR, Ghatavi K, Abramson BL, Dorian P, Guertin MC. Effects of citalopram and interpersonal psychotherapy on depression in patients with coronary artery disease: the Canadian Cardiac Randomized Evaluation of Antidepressant and Psychotherapy Efficacy (CREATE) trial. JAMA. 2007;297:367–379. doi: 10.1001/jama.297.4.367. [DOI] [PubMed] [Google Scholar]
- 20.de Jonge P, Mangano D, Whooley MA. Differential association of cognitive and somatic depressive symptoms with heart rate variability in patients with stable coronary heart disease: findings from the Heart and Soul Study. Psychosom Med. 2007;69:735–739. doi: 10.1097/PSY.0b013e31815743ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Capuron L, Su S, Miller AH, Bremner JD, Goldberg J, Vogt GJ, Maisano C, Jones L, Murrah NV, Vaccarino V. Depressive symptoms and metabolic syndrome: is inflammation the underlying link? Biol Psychiatry. 2008;64:896–900. doi: 10.1016/j.biopsych.2008.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Jonge P, Ormel J, van den Brink RH, van Melle JP, Spijkerman TA, Kuijper A, van Veldhuisen DJ, van den Berg MP, Honig A, Crijns HJ, Schene AH. Symptom dimensions of depression following myocardial infarction and their relationship with somatic health status and cardiovascular prognosis. Am J Psychiatry. 2006;163:138–144. doi: 10.1176/appi.ajp.163.1.138. [DOI] [PubMed] [Google Scholar]
- 23.Schiffer A, Pelle A, Smith O, Widdershoven JW, Hendriks EH, Pedersen SS. Somatic versus cognitive symptoms of depression as predictors of all-cause mortality and health status in chronic heart failure. J Clin Psychiatry. doi: 10.4088/JCP.08m04609. (In press.) [DOI] [PubMed] [Google Scholar]
- 24.Spertus JA, Peterson E, Rumsfeld JS, Jones PG, Decker C, Krumholz H. The Prospective Registry Evaluating Myocardial Infarction: Events and Recovery (PREMIER)--evaluating the impact of myocardial infarction on patient outcomes. Am Heart J. 2006;151:589–597. doi: 10.1016/j.ahj.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 25. [Accessed March 26, 2009];Joint Commission on Accreditation of Healthcare Organizations; Specifications Manual for National Hospital Quality Measures, version 2.0. http://www.jointcommission.org/PerformanceMeasurement/PerformanceMeasurement/Historical+NHQM+manuals.htm.
- 26.Spitzer RL, Williams JB, Kroenke K, Linzer M, deGruy FV, 3rd, Hahn SR, Brody D, Johnson JG. Utility of a new procedure for diagnosing mental disorders in primary care. The PRIME-MD 1000 study. JAMA. 1994;272:1749–1756. [PubMed] [Google Scholar]
- 27.Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 28.Gilbody S, Richards D, Brealey S, Hewitt C. Screening for depression in medical settings with the Patient Health Questionnaire (PHQ): a diagnostic meta-analysis. J Gen Intern Med. 2007;22:1596–1602. doi: 10.1007/s11606-007-0333-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. JAMA. 1999;282:1737–1744. doi: 10.1001/jama.282.18.1737. [DOI] [PubMed] [Google Scholar]
- 30.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harrell FE. Regression Modeling Strategies With Applications to Linear Models, Logistic Regression and Survival Analysis. New York: Springer-Verlag; 2001. [Google Scholar]
- 32.Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159:702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 33.Whooley MA, de Jonge P, Vittinghoff E, Otte C, Moos R, Carney RM, Ali S, Dowray S, Na B, Feldman MD, Schiller NB, Browner WS. Depressive symptoms, health behaviors, and risk of cardiovascular events in patients with coronary heart disease. JAMA. 2008;300:2379–2388. doi: 10.1001/jama.2008.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raghunathan TE, Solenberger PW, Van Hoeyk J. IVEware: Imputation and Variance Estimation Software - User Guide. Michigan: Survey Research Center, Institute for Social Research University of Michigan; 2002. [Google Scholar]
- 35.Lunceford JK, Davidian M. Stratification and weighting via the propensity score in estimation of causal treatment effects: a comparative study. Stat Med. 2004;23:2937–2960. doi: 10.1002/sim.1903. [DOI] [PubMed] [Google Scholar]
- 36.R Development Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2006. [Google Scholar]
- 37.Kirmayer LJ, Robbins JM, Dworkind M, Yaffe MJ. Somatization and the recognition of depression and anxiety in primary care. Am J Psychiatry. 1993;150:734–741. doi: 10.1176/ajp.150.5.734. [DOI] [PubMed] [Google Scholar]
- 38.Capuron L, Miller AH. Cytokines and psychopathology: lessons from interferon-alpha. Biol Psychiatry. 2004;56:819–824. doi: 10.1016/j.biopsych.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 39.Blumenthal JA, Babyak MA, Moore KA, Craighead WE, Herman S, Khatri P, Waugh R, Napolitano MA, Forman LM, Appelbaum M, Doraiswamy PM, Krishnan KR. Effects of exercise training on older patients with major depression. Arch Intern Med. 1999;159:2349–2356. doi: 10.1001/archinte.159.19.2349. [DOI] [PubMed] [Google Scholar]
- 40.Blumenthal JA, Babyak MA, Doraiswamy PM, Watkins L, Hoffman BM, Barbour KA, Herman S, Craighead WE, Brosse AL, Waugh R, Hinderliter A, Sherwood A. Exercise and pharmacotherapy in the treatment of major depressive disorder. Psychosom Med. 2007;69:587–596. doi: 10.1097/PSY.0b013e318148c19a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Walther C, Mobius-Winkler S, Linke A, Bruegel M, Thiery J, Schuler G, Halbrecht R. Regular exercise training compared with percutaneous intervention leads to a reduction of inflammatory markers and cardiovascular events in patients with coronary artery disease. Eur J Cardiovasc Prev Rehabil. 2008;15:107–112. doi: 10.1097/HJR.0b013e3282f29aa6. [DOI] [PubMed] [Google Scholar]
- 42.Milani RV, Lavie CJ. Impact of cardiac rehabilitation on depression and its associated mortality. Am J Med. 2007;120:799–806. doi: 10.1016/j.amjmed.2007.03.026. [DOI] [PubMed] [Google Scholar]
- 43.Witt BJ, Jacobsen SJ, Weston SA, Killian JM, Meverden RA, Allison TG, Reeder GS, Roger VL. Cardiac rehabilitation after myocardial infarction in the community. J Am Coll Cardiol. 2004;44:988–996. doi: 10.1016/j.jacc.2004.05.062. [DOI] [PubMed] [Google Scholar]