Abstract
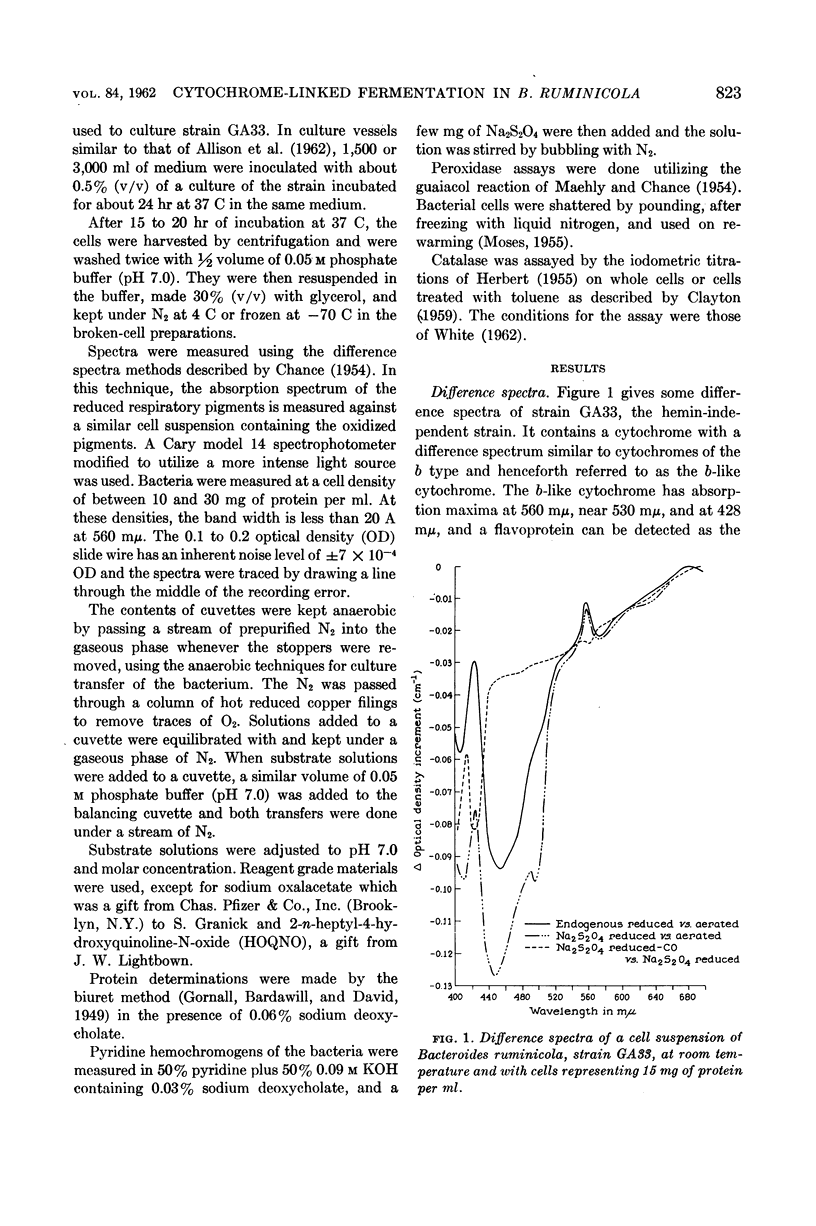
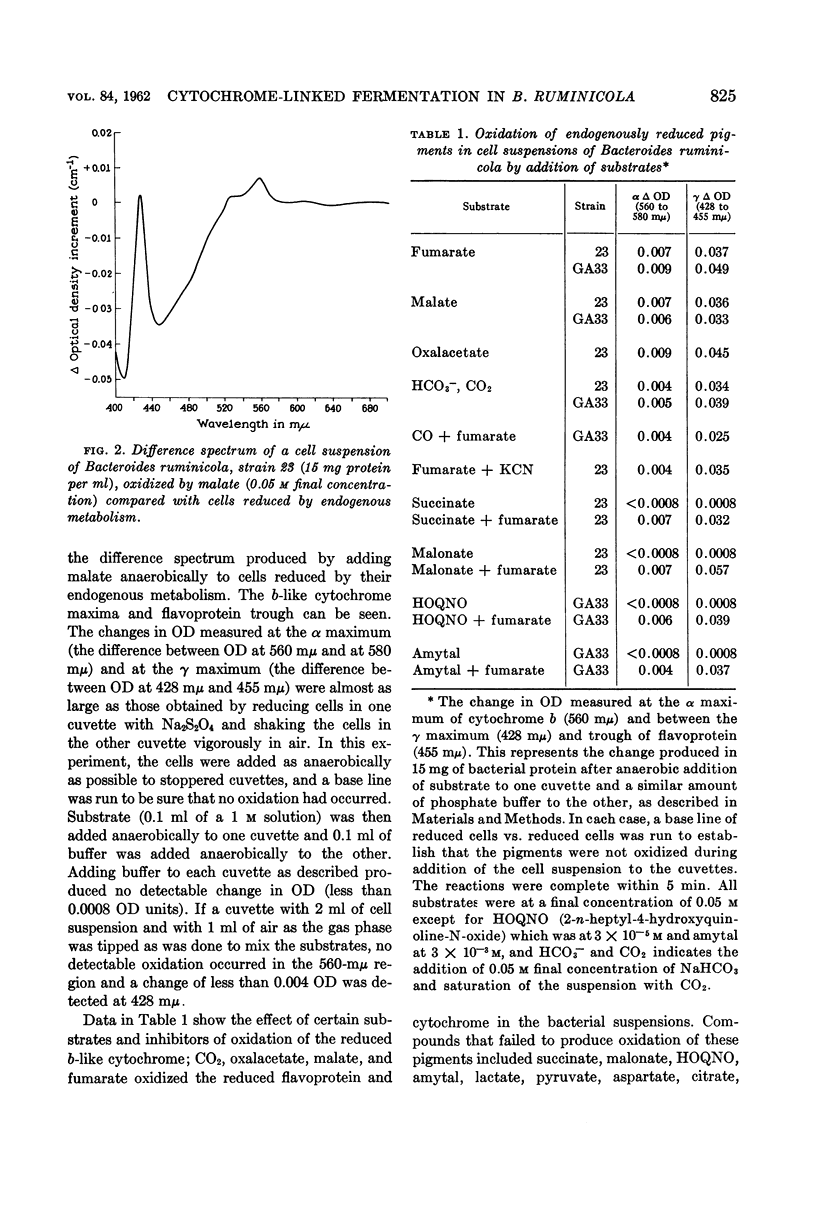
White, D. C. (Rockefeller Institute, New York, N.Y.), M. P. Bryant, and D. R. Caldwell. Cytochrome-linked fermentation in Bacteroides ruminicola. J. Bacteriol. 84:822–828. 1962—Previous studies showed that Bacteroides ruminicola, an anaerobic, saccharolytic, ruminal bacterium, ferments glucose with the production of succinic, acetic, and formic acids, requires a large amount of CO2, and most strains require heme for growth. Difference spectra of cell suspensions of both heme-requiring strain 23, B. ruminicola subsp. ruminicola, and heme-independent strain GA33, B. ruminicola subsp. brevis, showed the presence of a cytochrome (absorption maxima at 560 mμ, near 530 mμ, and 428 mμ) similar to cytochrome b. This cytochrome and flavoprotein (trough at 450 mμ) in the cells, reduced by endogenous metabolism, were oxidized on addition of air, CO2, oxalacetate, malate, or fumarate but no oxidation occurred in the presence of succinate, malonate, lactate, pyruvate, aspartate, citrate, NO3−, SO4=, 2-n-heptyl or hydroxyquinoline-N-oxide (HOQNO), amytal or azide. The oxidation of these cellular pigments by fumarate was not inhibited by CN−, CO, malonate, succinate, amytal, or HOQNO. Glucose and reduced diphosphopyridine nucleotide (DPNH), but not succinate, reduced the pigments in frozen-thawed cells previously exposed to air for 4 hr at room temperature. The results suggest that this cytochrome and flavoprotein form an electron transport system for fumarate reduction to succinate by DPNH generated by glycolysis, and that succinate is produced via CO2 condensation with pyruvate or phosphoenolpyruvate and with oxalacetate, malate, and fumarate as intermediates. A pigment similar to cytochrome o (absorption maxima at 570, 555, and 416 mμ) was observed when reduced cells were treated with CO and compared to reduced cells, but there was no detectable cytochrome oxidase activity. The function of this pigment is obscure. No peroxidase or catalase activity was detected in either strain. Pyridine hemochromogens of both strains indicate one major heme, a protoheme-like pigment, with absorption in the α region maximum at 556 mμ. As B. ruminicola is one of the most numerous of rumen bacteria and ferments a wide variety of carbohydrates of importance in ruminant rations, cytochrome must be of importance in electron transport in rumen contents, a highly anaerobic environment.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLISON M. J., BRYANT M. P., KATZ I., KEENEY M. Metabolic function of branched-chain volatile fatty acids, growth factors for ruminococci. II. Biosynthesis of higher branched-chain fatty acids and aldehydes. J Bacteriol. 1962 May;83:1084–1093. doi: 10.1128/jb.83.5.1084-1093.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ANDERSON R. L., ORDAL E. J. CO2-dependent fermentation of glucose by Cytophaga succinicans. J Bacteriol. 1961 Jan;81:139–146. doi: 10.1128/jb.81.1.139-146.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ANDERSON R. L., ORDAL E. J. Cytophaga succinicans sp. n., a factaltatively anaerobic, aquatic myxobacterium. J Bacteriol. 1961 Jan;81:130–138. doi: 10.1128/jb.81.1.130-138.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRYANT M. P. Bacterial species of the rumen. Bacteriol Rev. 1959 Sep;23(3):125–153. doi: 10.1128/br.23.3.125-153.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRYANT M. P., SMALL N., BOUMA C., CHU H. Bacteroides ruminicola n. sp. and Succinimonas amylolytica; the new genus and species; species of succinic acid-producing anaerobic bacteria of the bovine rumen. J Bacteriol. 1958 Jul;76(1):15–23. doi: 10.1128/jb.76.1.15-23.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant M. P., Robinson I. M. Some Nutritional Requirements of the Genus Ruminococcus. Appl Microbiol. 1961 Mar;9(2):91–95. doi: 10.1128/am.9.2.91-95.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CASTOR L. N., CHANCE B. Photochemical determinations of the oxidases of bacteria. J Biol Chem. 1959 Jun;234(6):1587–1592. [PubMed] [Google Scholar]
- CHANCE B. Cellular oxygen requirements. Fed Proc. 1957 Sep;16(3):671–680. [PubMed] [Google Scholar]
- CHANCE B. Spectrophotometry of intracellular respiratory pigments. Science. 1954 Nov 12;120(3124):767–775. doi: 10.1126/science.120.3124.767. [DOI] [PubMed] [Google Scholar]
- CLAYTON R. K. Permeability barriers and the assay of catalase in intact cells. Biochim Biophys Acta. 1959 Nov;36:35–39. doi: 10.1016/0006-3002(59)90066-6. [DOI] [PubMed] [Google Scholar]
- LIGHTBOWN J. W., JACKSON F. L. Inhibition of cytochrome systems of heart muscle and certain bacteria by the antagonists of dihydrostreptomycin: 2-alkyl-4-hydroxyquinoline N-oxides. Biochem J. 1956 May;63(1):130–137. doi: 10.1042/bj0630130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAEHLY A. C., CHANCE B. The assay of catalases and peroxidases. Methods Biochem Anal. 1954;1:357–424. doi: 10.1002/9780470110171.ch14. [DOI] [PubMed] [Google Scholar]
- MOSES V. Tricarboxylic acid cycle reactions in the fungus Zygorrhynchus moelleri. J Gen Microbiol. 1955 Oct;13(2):235–251. doi: 10.1099/00221287-13-2-235. [DOI] [PubMed] [Google Scholar]
- PECK H. D., Jr, SMITH O. H., GEST H. Comparative biochemistry of the biological reduction of fumaric acid. Biochim Biophys Acta. 1957 Jul;25(1):142–147. doi: 10.1016/0006-3002(57)90431-6. [DOI] [PubMed] [Google Scholar]
- WARRINGA M. G., GIUDITTA A. Studies on succinic dehydrogenase. IX. Characterization of the enzyme from Micrococcus lactilyticus. J Biol Chem. 1958 Jan;230(1):111–123. [PubMed] [Google Scholar]
- WHITE D. C. Cytochrome and catalase patterns during growth of Haemophilus parainfluenzae. J Bacteriol. 1962 Apr;83:851–859. doi: 10.1128/jb.83.4.851-859.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITE D. C., SMITH L. Hematin enzymes of Hemophilus parainfluenzae. J Biol Chem. 1962 Apr;237:1332–1336. [PubMed] [Google Scholar]


