Abstract
Malaria kills over one million children each year, and there is little doubt that an effective vaccine would play a central role in preventing these deaths. However, the strategies that proved so successful in developing the vaccines we have today may simply not be adequate to confront complex, persistent infectious diseases, including malaria, AIDS and TB. We believe that the development of a highly effective vaccine will require a better understanding of several features of the immune response to malaria. At the top of the list is the complex and ancient relationships between the parasite that causes malaria and the immune system that enables the parasite to persist in an otherwise functional immune system. A close second is the antigenic targets in malaria and how to overcome the enormous polymorphism of these. Meeting these challenges represents a call to arms of basic immunologists to advance our knowledge of malaria immunity.
Why another review of malaria immunity
A recent PubMed search for malaria and immunity retrieved over 30 reviews on the topic, many excellent. What compelled us to write another? What we hope to accomplish here is to present to the immunology community the challenges of developing a malaria vaccine. We do so in an effort to recruit expert immunologists to bring their fresh perspective to this ancient and deadly disease. We point out the enormity of the problem, the current state of vaccine development, and what we will need to know about malaria and the human immune system to develop a highly effective vaccine. We invite members of the immunology community to consider what they see as gaps in our knowledge of malaria immunity and, better yet, to roll up their sleeves and help fill in those gaps.
We need a vaccine because malaria is a killer and unlikely to be controlled without one
Malaria is an infectious disease caused by obligate intracellular Apicomplexa parasitic protozoa that are members of the genus Plasmodium. The most deadly species, P. falciparum, prevails in Africa. The enormous impact of malaria on mortality in humans can readily be appreciated by considering the high prevalence in Africa of the hemoglobin S allele (Hb-S). Despite the fact the Hb-S homozygosity causes sickle-cell anaemia and death in children in West Africa, Hb-S is maintained at a gene frequency of nearly 18% because in the heterozygous state, Hb-SA confers protection against severe malaria (1). Calculations of the mortality from malaria that were required to maintain this high frequency of Hb-S in Africa indicate that nearly half of all deaths by any cause in Africa were due to P. falciparum infection (1), prior to the introduction of the inexpensive antimalarial drug, chloroquine. Thus, malaria is almost unimaginably deadly.
Can we control malaria without a vaccine? P. falciparum is transmitted from person to person by the bite of female Anopheles mosquitoes, A. gambiae and A. funestus in Africa. A. gambiae is a highly efficient, highly adapted vector that feeds nearly exclusively on humans and has a long lifespan, greater than 30 days. As a consequence, A. gambiae mosquitoes feeding on a single infected individual are able to transmit malaria to hundreds of others. Any means of limiting the ability of mosquitoes to transmit the parasite, limits the spread of P. falciparum, including the use of bednets and insecticides. P. falciparum is also sensitive to several drugs when in the human host. There is no question that today the artemisinin combination therapy is highly effective in reducing severe disease (2). However, insecticides and antimalarial drugs are predicted to be only partially effective in controlling disease in the long run due to a large part to the inevitable acquisition of resistance. The enormous impact of drug resistance on disease is illustrated by the coincidence of the appearance of chloroquine resistance in parasites over a three-year period in the 1980s in Africa with a huge increase in the malaria mortality rate from 4.8% to 15.3% in one hospital in Zaire (3). Difficulties with both the delivery and compliance in the use of bednets and antimalarial drugs also decrease the effectiveness of these as tools in disease control. Thus, the development of a malarial vaccine that could be delivered to infants along with other childhood vaccines has become a top public health priority. We believe that malaria will remain a shadow over African children taking more than one million young lives a year until an effective vaccine that confers protection from disease is available.
The special challenges of developing a malarial vaccine
There are a large number of successful vaccines in use for other diseases, raising the question: how is malaria different? The diseases for which we currently have effective vaccines, including polio, diphtheria, tetanus, measles and pertussis, are caused by pathogens or toxins of the pathogens to which exposure confers life-long sterile immunity. Indeed, it was possible to develop a vaccine for smallpox more than 200 years ago based only on the principle that exposure to the pustules of cowpox conferred resistance to smallpox, without any knowledge of either the organism that caused the disease or of the immune response to it. However, malaria is different. P. falciparum infections can persist for months and individuals are always susceptible to reinfection. P. falciparum appears to both evade and disable the immune system by a variety of mechanisms, that allows it to persist in the host. Suffice it to say that despite an enormous research effort and expense, there are still no vaccines available for ‘chronic’ infectious diseases, including malaria.
Several features of malaria and the immune response to it suggest that the parasite and the immune system interact through a myriad of complex mechanisms that ultimately result in the inability of an otherwise functional immune system to eliminate the parasite and resist subsequent infections. In considering the interplay of the parasite and the immune system, it is important to consider that P. falciparum is estimated to be over 100,000 years old (4), as old as humans, suggesting that the human immune system and the parasite co-evolved. The enormous selective pressure imposed by malaria mortality described above, predicts that any mutation in the human genome that conferred protection against severe disease and death would be rapidly selected for and fixed in the human population. Indeed, malaria has had more impact than any other pathogen in shaping the human genome (5, 6). It is entirely possible that malaria has shaped the immune mechanisms that come to play at the interface of the parasite and human immune system, permitting chronic and recurrent infections by the parasite. The parasite’s survival is absolutely dependent on its ability to persist in the human host and to be transmitted. In areas of seasonal transmission, as is the case for most of West Africa, the mosquitoes do not transmit during the six-month dry season, and so survival of the parasite depends on its ability to persist in the human host for months. It seems that co-adaptation of the parasite and the human host has ensured that the human immune system does not efficiently clear the parasite. Presumably, the persistence of the parasite has some advantage to the host, perhaps by reducing risk of disease (7, 8). Thus, an effective vaccine may need to disrupt the balance between the parasite and the human immune system that has evolved to benefit both.
The clinical picture of malaria in young children and the natural acquisition of resistance
Children are infected with P. falciparum sporozoites by the bite of an infected mosquito (Fig. 1). A small number of injected sporozoites enters the blood and travels to the liver where they invade and replicate within hepatocytes. There are no symptoms during this phase of the infection. The sporozoites in the liver expand enormously, up to 40,000 fold, and differentiate into merozoites. The merozoites are released from infected liver cells and enter the bloodstream, inundating the immune system with a huge bolus of parasites that infect red blood cells. The merozoites replicate in red cells and are released by rupture of the red cell. The released merozoites infect new red blood cells establishing a cycle of invasion, replication and rupture. Parasitemia often exceeds densities of 50,000 or more parasites per μl of blood. A number of parasite products are released upon red cell lysis and are linked to the symptoms of mild disease – headaches, chills, fever, and lethargy. In young children, under the age of five, the infection can progress to severe disease. Severe malaria, which accounts for the high mortality among young children, includes cerebral malaria (9), acidosis (10), and severe anemia combined with acidosis (11). For cerebral malaria the symptoms are associated with sequestration of the infected red blood cells in the brain (9) causing inflammation (Fig. 1). At present, the mechanisms by which parasite sequestration leads to severe malaria and death are only poorly understood. Clearly, understanding these mechanisms is an important priority in malaria immunology.
Fig. 1. The life cycle of P. falciparum.
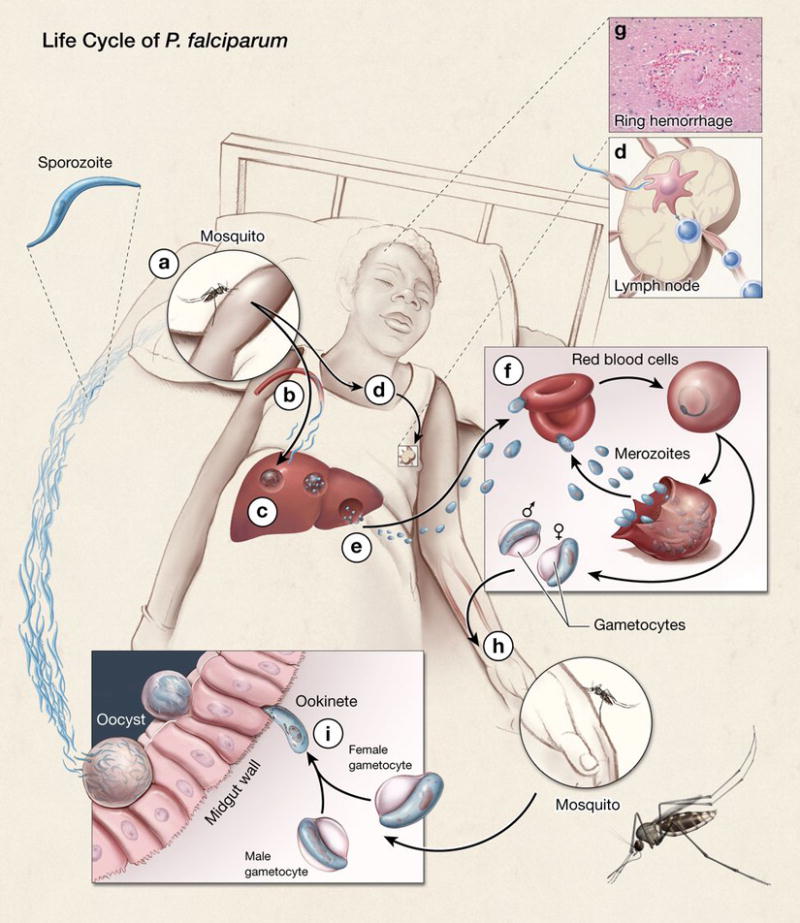
Humans are infected with P. falciparum haploid sporozoites by the bite of an infected female mosquito. The sporozoites, present in the infected mosquito’s salivary glands, are injected into the skin as the mosquito probes the skin preparing to take a blood meal (a). Only a small number of sporozoites are injected and these enter the blood stream (b) and travel to the liver where they invade hepatocytes (c). Sporozoites also enter the draining lymphatics and travel to the draining lymph node (d), where T-cell priming has been reported to occur. In a hepatocyte, the parasite replicates and differentiates giving rise to many thousands of merozoites that are released into the blood (e). Merozoites infect red blood cells and within 12 h remodel the red blood cell and its membrane, thereby facilitating growth of the parasites and allowing the infected red blood cell to bind to endothelium in different tissues, avoiding clearance in the spleen. In severe disease, parasitized red blood cells attach to endothelial cells in various tissues, for example, in the brain causing cerebral malaria (g). Shown is a ring hemorrhage around a small vessel in the brain. In the red blood cells, merozoites replicate giving rise to up to 24 new merozoites per red blood cell (f). The released merozoites infect new red blood cells and the cycle of red blood cell invasion, replication, rupture and reinvasion continues until treated with antimalarials or controlled by an immune response. At some point in a poorly understood process, a subset of merozoites differentiate into male and female gametocytes (h). These gametocytes, taken up in the blood meal by the mosquito, develop into gametes that fuse in the midgut of the mosquito to form a motile zygote, the ookinete, where meiosis occurs (i). The ookinete crosses the midgut wall and forms an oocyst that develops into sporozoites that enter the mosquito salivary gland, thereby completing the cycle.
What is the nature of naturally acquired resistance to disease in malaria endemic areas? Sero-epidemiological studies indicate that immunity to malaria is slow to develop, taking several years in sub-Saharan Africa, despite repeated bites from infected mosquitoes. Young children appear to have insufficient immunity to infection and to mild and severe disease. Children between the ages of five and ten develop an immunity to severe disease but still suffer from mild disease and it is not until adolescence that immunity to disease is established. Immunity to infection is never acquired and adults living in malaria endemic areas, although rarely sick from malaria, often carry the parasite albeit at lower levels than children. Collectively these observations suggest that immunity to severe disease, mild disease and infection may involve different immune mechanisms. In part, optimism that a malaria vaccine is possible comes from the fact that, although the human immune system does not prevent infection, immunity to both severe and mild disease is eventually acquired through continued natural exposure to the parasite. However, a vaccine might need to evoke protection against disease by mechanisms that do not have dominant roles in naturally acquired immunity. For example, natural immunity is not acquired to the pre-erythrocytic stage of infection (12); nonetheless, the leading vaccine candidate RTS,S, which is under development at Glaxo-SmithKline, targets the pre-erythrocytic stage (13). In this context, it will also be critical that RTS,S induces life-long immunity as presumably there will be no natural boosting from infection.
The P. falciparum life cycle and vaccine targets
Pre-erythrocytic vaccines
Individuals are initially infected by P. falciparum sporozoites that invade hepatocytes. A small number of sporozoites present in the salivary glands of infected mosquitoes are injected into the skin as the mosquito takes a blood meal. These cross the endothelium of blood capillaries, enter the blood and travel to the liver, where they invade hepatocytes. Recent evidence in mice shows that sporozoites also travel to the local draining lymph node, where T-cell priming occurs (14). In hepatocytes, each sporozoite undergoes extensive replication within a parasite-derived vacuole, essentially walling off the parasite from the liver-cell cytoplasm. A set of parasite proteins are selectively transported across the vacuolar membrane into the cytosol and, in the case of the circumsporozoite (CS) protein, into the nucleus, where the CS protein influences the expression of hundreds of liver cell genes, creating a hospitable environment for parasite growth (15). Immunity to the pre-erythrocytic stage of the infection is, for the most part, not acquired naturally (16), indicating that the small number of sporozoites and infected liver cells are not readily detected by the immune system. Nonetheless, several vaccine candidates including the leading vaccine candidate, RTS,S, target the pre-erythrocytic stage. Given that there is little natural immunity to the pre-erythrocyte stage, what is the evidence that this stage of malarial infection is a viable vaccine target? Landmark experiments reported in the late 1960s showed in mice (17) that inoculation with a large number of irradiated sporozoites resulted in complete protective immunity for six months against sporozoite challenge. Immunity appears to involve both CD4+ and CD8+ T cells and antibodies. Based to a large extent on these observations, several pre-erythrocyte stage vaccines have been tested both in nonimmune volunteers challenged with bites of P. falciparum infected mosquitoes (phase IIa trials) and in the field in malaria endemic areas (phase IIb trials). Thus far, several vaccines have failed in phase IIa and IIb trials, for example the DNA based vaccines that contained multiple T cell epitopes of pre-erythrocyte-stage antigens including CS (18). In phase IIa trials of the CS protein based RTS,S vaccine (19), the most advanced malaria vaccine candidate, protection was achieved in 30–50% of vaccinated volunteers. The results of phase IIb trials of RTS,S seem to be promising, showing a delayed time to infection in children lasting for 18 months (13) and some efficacy in infants. In another phase IIb trial there was a reduction in clinical malaria (20). Additional pre-erythrocytic vaccines are in development that have not yet been tested in human trials including one consisting of whole irradiated sporozoites.
Asexual erythrocytic vaccines
Merozoites released from infected liver cells enter the bloodstream and infect red blood cells. Parasite-specific antibodies have been shown to have a crucial role in controlling disease at this stage. The transfer of antibodies from immune adults to African children with severe clinical malaria and high parasitemia with malaria resulted in a significant decrease in parasite levels and disease (21). Based on these observations, several merozoite products (22) are under development as erythrocytic stage vaccines. Ideally the selection of candidate antigens for the development of an erythrocytic stage vaccine would be guided by knowledge of the nature of the antibodies that confer naturally acquired protection in immune adults. Unfortunately, at present, we simply do not know the specificity, affinity or isotype of antibodies that confer protection against disease. The P. falciparum genome encodes an estimated 5,600 proteins making the identification of the targets of protective immunity a daunting task. In the absence of such knowledge what are the rationale targets for protective antibodies? Blood-stage infection involves the attachment of the merozoite through various erythrocyte-binding proteins to any of several receptors on the surface of the red blood cell (23, 24). On the face of it, these receptors would seem to be good vaccine targets. However, P. falciparum uses many, often functionally redundant, receptors to invade red blood cells so that antibodies to only one receptor may not block blood stage infection. The only bona fide parasite receptor for red cells currently under development as a vaccine is based on the P. falciparum protein erythrocyte binding antigen 175 which is a receptor for glycophorin A on red blood cells (25). In addition, several recombinant merozoite membrane proteins of unknown function including merozoite surface proteins (MSPs) and apical membrane antigen 1 (AMA1) have been tested or plan to be tested alone and in combination in phase 2 clinical trials. To date, only one vaccine has shown some protection against the blood stage and it contains a combination of merozoite proteins (26). Three proteins released in large quantities from the lysed red blood cell, SERA5, GLURP and MSP3, are also under development as vaccines. The rationale for these vaccines is based, in part, on the observation that antigen-antibody complexes bound to monocyte Fc receptors trigger the release of as yet uncharacterized products that are inhibitory to parasite growth in infected red blood cells, a process termed antibody dependent cellular inhibition (27). Lysed red blood cells also result in the release of a variety of inflammation inducing products that result in the symptoms of malaria including the parasite toxin glycosylphosphatidylinositol (GPI) that has been proposed as a target of an anti-inflammatory vaccine for malaria (28).
In addition to antibody responses, the innate system and particularly inflammation plays a crucial role in controlling blood-stage malaria, but for the most part, the potential importance of this inflammatory process has not been taken into consideration in vaccine design. In mice, the initial malaria infection can be controlled by a strong pro-inflammatory response that is rapidly downregulated (29, 30), presumably to limit the pathology associated with inflammation. In humans, an important aspect of immunity to disease might be the ability to mount a pro-inflammatory response as well as the ability to rapidly control the inflammatory response. The first insights into the regulation of malaria-induced pro-inflammatory responses in humans come from clinical records of individuals infected with P. falciparum as a treatment for neurosyphilis. These records provide evidence that humans learn to control the inflammation quickly despite continued parasitemia (7). More recent studies have shown a complex relationship between inflammatory cytokines and severe disease in malaria (31, 32) and suggested a possible link between the initial innate response to infection and the outcome of disease (33). However, in no case has causality between disease outcome and cytokine production been proved. Certainly it will be essential to understand the role of the innate immune system and inflammation in severe disease in developing a malaria vaccine.
How P. falciparum evades and disables the immune response
Despite the large number of potential antigen targets, malaria vaccine development might be confounded by the immune-evasion strategies of the parasite. Whether this will be a serious problem depends to a large extent on the cellular and molecular mechanisms that underlie the effect of P. falciparum on the immune system and whether the mechanisms that P. falciparum uses to evade or disable the natural immune response will also disrupt immunity established by vaccination. At present we simply do not know enough about such mechanisms to evaluate their potential impact.
Immune evasion
P. falciparum uses both antigenic variation and polymorphism to avoid immune recognition, mainly in the blood stage of the life cycle. Rapid antigenic variation is achieved through the var genes. The var gene products, PfEMP1 proteins, are expressed on the surface of infected red blood cells and are essential for binding endothelium and sequestering the infected red blood cells to avoid their removal in the spleen (9). Each P. falciparum genome contains approximately 60 antigenically distinct var genes that individual parasites express one at a time. However, within a clonal parasite population there is a constant low level of switching between the expressed var genes (34). Antibodies elicited to the predominant var gene products that are expressed by the infecting parasite clone block sequestration, thereby decreasing the number of infected red blood cells and the parasite load. Immune elimination of these parasites allows the expansion of parasite clones that have switched to express different vars against which existing antibodies have little effect. Antibodies specific for the new dominant var variant are then induced, which eliminate the infected red blood cells and allow another switched parasite clone to expand (9). The education of the immune system to recognize all of the numerous var genes is a slow process that accounts, to a large extent, for the late acquisition of adaptive immunity to disease in endemic areas (35). The enormous antigenic variation of PfEMP1s makes them poor vaccine candidates at present unless, for example, a subset of var genes that causes cerebral malaria is identified.
There is, however, one var gene that may be a good vaccine candidate for pregnancy-associated malaria. Pregnancy-associated malaria is a devastating disease in which red blood cells are infected by parasites expressing one particular PfEMP1, VAR2CSA, that has the unique ability to bind to chondroitin sulphate A on placental syncytiotrophoblast cells (36, 37). VAR2CSA-expressing parasites sequester in the placenta where they cause inflammation and in many cases death of the mother and dangerously low birth weights in infants. During first pregnancies in endemic areas, women are highly susceptible to placental malaria, but they develop rapid protective immunity to VAR2CSA such that placental malaria is only problematic in women during their first or second pregnancy (38). The VAR2CSA protein is a highly attractive candidate for a vaccine for placental malaria and based on the epidemiological data, the first fully protective malaria vaccine developed might well be for placental malaria. At present, efforts to develop a placental malaria vaccine are still in the pre-clinical stages.
The 60 var genes, although a significant component of P. falciparum’s immune-evasion armory, are not the only parasite products against which immunity is acquired. Indeed, individuals are eventually able to control infections despite var gene switching. Unfortunately, these additional immune targets are themselves highly polymorphic and the immunity acquired to one parasite clone does not protect against infection with another clone. The parasite’s AMA1 is a good example. Immune responses to AMA1 can control disease in animal models (39), which makes AMA1 an important vaccine candidate. However, AMA1 is highly polymorphic with more than 150 haplotypes, all of which seem to be present in all malaria endemic regions (40). Antibody responses are generally haplotype specific, such that antibodies specific for one AMA1 haplotype that block invasion of red blood cells only partially block invasion by parasites expressing another haplotype. How can such high degrees of polymorphism be addressed in vaccine development? Recently, a detailed population structure analysis of the relationship between AMA1 haplotypes indicates that they can, in fact, be grouped into six subpopulations and that vaccination with one member of a subpopulation protects against parasites expressing other members of that subpopulation (40). So, it might be possible to combine as few as six AMA1 haplotypes into a vaccine that would be effective in protecting against all parasites. Other approaches are indicated by the structure of AMA1, which shows that most of the polymorphic residues are concentrated on one face of the protein, meaning that the other face is highly conserved. The highly conserved face appears to be a vaccine target as a monoclonal antibody that binds to this region blocks invasion by all parasites regardless of AMA1 haplotype (41). The conserved face might be immunogenic if, for example, the AMA1 could be engineered to eliminate the apparently immunodominant polymorphic residues on the other face and focus the response to the conserved face.
The very real challenge for vaccine design posed by the antigenic variation and polymorphism of many of the proteins of P. falciparum raises the question of whether there is an as of yet unidentified group of nonvariant antigens that would induce full protection. How would one identify such antigens? One approach is through ‘reverse vaccinology’ as pioneered for bacterial vaccines (42). Reverse vaccinology is an approach to develop vaccines starting from genomic information rather than growing the causative microorganism. In this approach criteria are set for potential vaccine candidates that can be discerned from genomic information alone (for example, membrane expression or time expressed in the life cycle) and candidates are tested for their ability to evoke protective responses. New vaccine candidates could also be discovered by taking advantage of the known P. falciparum genome in another way, namely to create protein microarrays (43) that contain most if not all P. falciparum gene products, which can then be used to screen sera from children in endemic areas to correlate the production of antibodies specific for particular gene products with the acquisition of protection from disease (44, 45). Clearly, the identification of new malaria vaccine candidates will benefit the vaccine development effort and might be crucial if current candidates fail to confer protective immunity.
Disabling immunity
As described above results from sero-epidemiology studies indicate that immunity to malaria is slow to develop and incomplete. For example, in young children, the process of acquiring a specific antibody response to a defined parasite antigen is slow and such responses, once acquired, can be short lived (in the order of days) and may not be recalled upon reinfection (46). This short half life of acquired immunity to parasite antigens is in stark contrast to many other infectious diseases. For example, the estimated half life of antibody responses to viruses ranges from 50 years for varicella-zoster virus to more than 200 years for the viruses that cause measles and mumps (47).
How might malaria interfere with immune function and the acquisition or maintenance of immunological memory? Hundreds of parasite products are released from lysed red blood cells (48, 49), most of which are of unknown function but could potentially interfere with the immune response. At least two of these products interact with the innate immune system’s Toll-like receptors (TLRs) and TLRs have been implicated in playing a role in the generation and maintenance of B cell memory (50). Red blood cell lysis results in the release of GPI, recognized by TLR2 and to some extent TLR4 (51). A second product that is released by ruptured parasitized red blood cells is hemozoin, a detoxified crystalline form of heme produced by the parasite that has been suggested to be a TLR9 agonist (52), although this is controversial (53). A possible mechanism by which immunological memory might be disabled was indicated by results of studies in a mouse model in which parasite-specific memory B cells generated by immunization with parasite proteins were observed to undergo apoptosis when the mouse was infected with a malaria parasite (54). Are there any clues from other chronic infections in humans as to how P. falciparum might interfere with immunity? For example, T cell exhaustion, or loss of function, is a common feature of many chronic human infections, including HIV and hepatitis B and C (55). An exhausted B-cell phenotype has also been described recently for memory B cells in HIV-infected individuals (56). The possibility that in malaria, the continued presence of the parasite drives parasite-specific T cells and B cells into an exhausted phenotype should be explored.
To better understand how malaria affects the immune system, it might be possible to mine the human genome for genetic traits that confer resistance to malaria infection and disease (5, 6). As described above, malaria has shaped the human genome to a greater extent than any other pathogen. Understanding how the human genome has adapted to co-exist with malaria parasites could provide new insights into the mechanisms of both the innate and adaptive immune systems that underlie resistance to severe disease and death. Whole genome-wide screens for malaria-resistance alleles by genetic association analysis now seem to be feasible (5). There are also well described differences between ethnic groups living sympatrically in malaria endemic areas in which members of one group, for example the Fulani in Mali and Burkina Faso, have significantly lower parasite rates compared to other ethnic groups (57). Several immune parameters have been compared between these groups but we still have an incomplete understanding of what differences are key to protection. In terms of candidate gene analyses, of particular interest are genes that confer susceptibility to the systemic autoimmune disease, especially systemic lupus erythematosus (SLE), a disease for which women of African descent are significantly at risk. It is possible that polymorphisms in genes of the immune system that confer resistance to disease and death caused by malaria might in nonendemic areas be susceptibility genes for autoimmunity. For example, a polymorphism in the inhibitory Fc receptor, FcγRIIB, which is a susceptibility gene in SLE, was recently shown to confer resistance to malaria in a mouse model (58). Conversely, malaria infections have been shown to suppress SLE-like autoimmune disease in mice (59). We predict that such an approach might be profitable in discovering mechanisms by which malaria influences the immune response.
Conclusion
Here we have attempted to make the case that we may not know enough about malaria to make an effective vaccine. If we agree that the development of a malaria vaccine would profit from a better understanding of the basic immunology of the human response to malaria, we then need to ask: have we engaged a sufficient number of immunologists to address the problem? At the moment, probably not. Relative to the magnitude of the global disease burden imposed by malaria, there are only a small number of scientists with the training and expertise in the human immune system who are committed to working at the molecular interface of the parasite and the immune system. We believe that this interface will provide exciting careers for young scientists meeting the challenges of controlling one of the world’s most deadly diseases. In the near future, we anticipate the commitment of significant additional resources from both private and public institutions for studies leading to the development of vaccines for malaria including the NIAID Malaria Research Program, the Bill and Melinda Gates Foundation, The Global Fund and the President’s Malaria Initiative. We feel that the recent call for eradication of malaria by the Bill and Melinda Gates Foundation (Bill and Melinda Gates Call for New Global Commitment to Chart a Course for Malaria Eradication) represents a call to arms of immunologists to use their knowledge of the intricate workings of the immune system to the fight to reduce the malaria death toll in African children.
References
- 1.Molineaux L. The impact of parasitic diseases and their control on mortality, with emphasis on malaria and Africa. In: Vallin J, Lopez A, editors. Health policy, social policy, and mortality prospects. Ordina Editions; Liege: 1985. pp. 13–44. [Google Scholar]
- 2.Bhattarai A, Ali AS, Kachur SP, Martensson A, Abbas AK, Khatib R, Al-Mafazy AW, Ramsan M, Rotllant G, Gerstenmaier JF, Molteni F, Abdulla S, Montgomery SM, Kaneko A, Bjorkman A. Impact of artemisinin-based combination therapy and insecticide-treated nets on malaria burden in Zanzibar. PLoS medicine. 2007;4:e309. doi: 10.1371/journal.pmed.0040309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Greenberg AE, Ntumbanzondo M, Ntula N, Mawa L, Howell J, Davachi F. Hospital-based surveillance of malaria-related paediatric morbidity and mortality in Kinshasa, Zaire. Bulletin of the World Health Organization. 1989;67:189–196. [PMC free article] [PubMed] [Google Scholar]
- 4.Mu J, Duan J, Makova KD, Joy DA, Huynh CQ, Branch OH, Li WH, Su XZ. Chromosome-wide SNPs reveal an ancient origin for Plasmodium falciparum. Nature. 2002;418:323–326. doi: 10.1038/nature00836. [DOI] [PubMed] [Google Scholar]
- 5.Ntoumi F, Kwiatkowski DP, Diakite M, Mutabingwa TK, Duffy PE. New interventions for malaria: mining the human and parasite genomes. Am J Trop Med Hyg. 2007;77:270–275. [PubMed] [Google Scholar]
- 6.A global network for investigating the genomic epidemiology of malaria. Nature. 2008;456:732–737. doi: 10.1038/nature07632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collins WE, Jeffery GM. A retrospective examination of sporozoite- and trophozoite-induced infections with Plasmodium falciparum: development of parasitologic and clinical immunity during primary infection. Am J Trop Med Hyg. 1999;61:4–19. doi: 10.4269/tropmed.1999.61-04. [DOI] [PubMed] [Google Scholar]
- 8.Crompton PD, Traore B, Kayentao K, Doumbo S, Ongoiba A, Diakite SA, Krause MA, Doumtabe D, Kone Y, Weiss G, Huang CY, Doumbia S, Guindo A, Fairhurst RM, Miller LH, Pierce SK, Doumbo OK. Sickle cell trait is associated with a delayed onset of malaria: implications for time-to-event analysis in clinical studies of malaria. J Infect Dis. 2008;198:1265–1275. doi: 10.1086/592224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller LH, Baruch DI, Marsh K, Doumbo OK. The pathogenic basis of malaria. Nature. 2002;415:673–679. doi: 10.1038/415673a. [DOI] [PubMed] [Google Scholar]
- 10.Marsh K, Forster D, Waruiru C, Mwangi I, Winstanley M, Marsh V, Newton C, Winstanley P, Warn P, Peshu N, et al. Indicators of life-threatening malaria in African children. The New England journal of medicine. 1995;332:1399–1404. doi: 10.1056/NEJM199505253322102. [DOI] [PubMed] [Google Scholar]
- 11.Lackritz EM, Campbell CC, Ruebush TK, 2nd, Hightower AW, Wakube W, Steketee RW, Were JB. Effect of blood transfusion on survival among children in a Kenyan hospital. Lancet. 1992;340:524–528. doi: 10.1016/0140-6736(92)91719-o. [DOI] [PubMed] [Google Scholar]
- 12.Hoffman SL, Oster CN, Plowe CV, Woollett GR, Beier JC, Chulay JD, Wirtz RA, Hollingdale MR, Mugambi M. Naturally acquired antibodies to sporozoites do not prevent malaria: vaccine development implications. Science. 1987;237:639–642. doi: 10.1126/science.3299709. [DOI] [PubMed] [Google Scholar]
- 13.Alonso PL, Sacarlal J, Aponte JJ, Leach A, Macete E, Aide P, Sigauque B, Milman J, Mandomando I, Bassat Q, Guinovart C, Espasa M, Corachan S, Lievens M, Navia MM, Dubois MC, Menendez C, Dubovsky F, Cohen J, Thompson R, Ballou WR. Duration of protection with RTS,S/AS02A malaria vaccine in prevention of Plasmodium falciparum disease in Mozambican children: single-blind extended follow-up of a randomised controlled trial. Lancet. 2005;366:2012–2018. doi: 10.1016/S0140-6736(05)67669-6. [DOI] [PubMed] [Google Scholar]
- 14.Chakravarty S, I, Cockburn A, Kuk S, Overstreet MG, Sacci JB, Zavala F. CD8+ T lymphocytes protective against malaria liver stages are primed in skin-draining lymph nodes. Nat Med. 2007;13:1035–1041. doi: 10.1038/nm1628. [DOI] [PubMed] [Google Scholar]
- 15.Singh AP, Buscaglia CA, Wang Q, Levay A, Nussenzweig DR, Walker JR, Winzeler EA, Fujii H, Fontoura BM, Nussenzweig V. Plasmodium circumsporozoite protein promotes the development of the liver stages of the parasite. Cell. 2007;131:492–504. doi: 10.1016/j.cell.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 16.Beadle C, McElroy PD, Oster CN, Beier JC, Oloo AJ, Onyango FK, Chumo DK, Bales JD, Sherwood JA, Hoffman SL. Impact of transmission intensity and age on Plasmodium falciparum density and associated fever: Implications for malaria vaccine trial design. J Infect Dis. 1975;172:1047–1054. doi: 10.1093/infdis/172.4.1047. [DOI] [PubMed] [Google Scholar]
- 17.Nussenzweig RS, Vanderberg J, Most H, Orton C. Protective immunity produced by the injection of X-irradiated sporozoites of Plasmodium berghei. Nature. 1967;216:160–162. doi: 10.1038/216160a0. [DOI] [PubMed] [Google Scholar]
- 18.Wang R, Richie TL, Baraceros MF, Rahardjo N, Gay T, Banania JG, Charoenvit Y, Epstein JE, Luke T, Freilich DA, Norman J, Hoffman SL. Boosting of DNA vaccine-elicited gamma interferon responses in humans by exposure to malaria parasites. Infection and immunity. 2005;73:2863–2872. doi: 10.1128/IAI.73.5.2863-2872.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stoute JA, Slaoui M, Heppner DG, Momin P, Kester KE, Desmons P, Wellde BT, Garcon N, Krzych U, Marchand M. A preliminary evaluation of a recombinant circumsporozoite protein vaccine against Plasmodium falciparum malaria. RTS,S Malaria Vaccine Evaluation Group. N Engl J Med. 1997;336:86–91. doi: 10.1056/NEJM199701093360202. [DOI] [PubMed] [Google Scholar]
- 20.Bejon P, Lusingu J, Olotu A, Leach A, Lievens M, Vekemans J, Mshamu S, Lang T, Gould J, Dubois MC, Demoitie MA, Stallaert JF, Vansadia P, Carter T, Njuguna P, Awuondo KO, Malabeja A, Abdul O, Gesase S, Mturi N, Drakeley CJ, Savarese B, Villafana T, Ballou WR, Cohen J, Riley EM, Lemnge MM, Marsh K, von Seidlein L. Efficacy of RTS,S/AS01E vaccine against malaria in children 5 to 17 months of age. The New England journal of medicine. 2008;359:2521–2532. doi: 10.1056/NEJMoa0807381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cohen S, I, McGregor A, Carrington S. Gamma-globulin and acquired immunity to human malaria. Nature. 1961;192:733–737. doi: 10.1038/192733a0. [DOI] [PubMed] [Google Scholar]
- 22.Maher B. Malaria: the end of the beginning. Nature. 2008;451:1042–1046. doi: 10.1038/4511042a. [DOI] [PubMed] [Google Scholar]
- 23.Gaur D, Mayer DC, Miller LH. Parasite ligand-host receptor interactions during invasion of erythrocytes by Plasmodium merozoites. Int J Parasitol. 2004;34:1413–1429. doi: 10.1016/j.ijpara.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 24.Cowman AF, Crabb BS. Invasion of red blood cells by malaria parasites. Cell. 2006;124:755–766. doi: 10.1016/j.cell.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 25.Sim BK, Chitnis CE, Wasniowska K, Hadley TJ, Miller LH. Receptor and ligand domains for invasion of erythrocytes by Plasmodium falciparum. Science. 1994;264:1941–1944. doi: 10.1126/science.8009226. [DOI] [PubMed] [Google Scholar]
- 26.Genton B, Betuela I, Felger I, Al-Yaman F, Anders RF, Saul A, Rare L, Baisor M, Lorry K, Brown GV, Pye D, Irving DO, Smith TA, Beck HP, Alpers MP. A recombinant blood-stage malaria vaccine reduces Plasmodium falciparum density and exerts selective pressure on parasite populations in a phase 1-2b trial in Papua New Guinea. J Infect Dis. 2002;185:820–827. doi: 10.1086/339342. [DOI] [PubMed] [Google Scholar]
- 27.Bouharoun-Tayoun H, Oeuvray C, Lunel F, Druilhe P. Mechanisms underlying the monocyte-mediated antibody-dependent killing of Plasmodium falciparum asexual blood stages. J Exp Med. 1995;182:409–418. doi: 10.1084/jem.182.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schofield L, Hewitt MC, Evans K, Siomos MA, Seeberger PH. Synthetic GPI as a candidate anti-toxic vaccine in a model of malaria. Nature. 2002;418:785–789. doi: 10.1038/nature00937. [DOI] [PubMed] [Google Scholar]
- 29.Weidanz WP, Batchelder JM, Flaherty P, LaFleur G, Wong C, van der Heyde HC. Plasmodium chabaudi adami: use of the B-cell-deficient mouse to define possible mechanisms modulating parasitemia of chronic malaria. Exp Parasitol. 2005;111:97–104. doi: 10.1016/j.exppara.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 30.Perry JA, Olver CS, Burnett RC, Avery AC. Cutting edge: the acquisition of TLR tolerance during malaria infection impacts T cell activation. J Immunol. 2005;174:5921–5925. doi: 10.4049/jimmunol.174.10.5921. [DOI] [PubMed] [Google Scholar]
- 31.Kwiatkowski D. Tumour necrosis factor, fever and fatality in falciparum malaria. Immunology Letters. 1990;25:213–216. doi: 10.1016/0165-2478(90)90117-9. [DOI] [PubMed] [Google Scholar]
- 32.Lyke KE, Burges R, Cissoko Y, Sangare L, Dao M, Diarra I, Kone A, Harley R, Plowe CV, Doumbo OK, Sztein MB. Serum levels of the proinflammatory cytokines interleukin-1 beta (IL-1beta), IL-6, IL-8, IL-10, tumor necrosis factor alpha, and IL-12(p70) in Malian children with severe Plasmodium falciparum malaria and matched uncomplicated malaria or healthy controls. Infection and immunity. 2004;72:5630–5637. doi: 10.1128/IAI.72.10.5630-5637.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walther M, Woodruff J, Edele F, Jeffries D, Tongren JE, King E, Andrews L, Bejon P, Gilbert SC, De Souza JB, Sinden R, Hill AV, Riley EM. Innate immune responses to human malaria: heterogeneous cytokine responses to blood-stage Plasmodium falciparum correlate with parasitological and clinical outcomes. J Immunol. 2006;177:5736–5745. doi: 10.4049/jimmunol.177.8.5736. [DOI] [PubMed] [Google Scholar]
- 34.Roberts DJ, Craig AG, Berendt AR, Pinches R, Nash G, Marsh K, Newbold CI. Rapid switching to multiple antigenic and adhesive phenotypes in malaria. Nature. 1992;357:689–692. doi: 10.1038/357689a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bull PC, Lowe BS, Kortok M, Molyneux CS, Newbold CI, Marsh K. Parasite antigens on the infected red cell surface are targets for naturally acquired immunity to malaria. Nat Med. 1998;4:358–360. doi: 10.1038/nm0398-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Salanti A, Staalsoe T, Lavstsen T, Jensen AT, Sowa MP, Arnot DE, Hviid L, Theander TG. Selective upregulation of a single distinctly structured var gene in chondroitin sulphate A-adhering Plasmodium falciparum involved in pregnancy-associated malaria. Mol Microbiol. 2003;49:179–191. doi: 10.1046/j.1365-2958.2003.03570.x. [DOI] [PubMed] [Google Scholar]
- 37.Fried M, Duffy PE. Adherence of Plasmodium falciparum to chondroitin sulfate A in the human placenta. Science. 1996;272:1502–1504. doi: 10.1126/science.272.5267.1502. [DOI] [PubMed] [Google Scholar]
- 38.Fried M, Nosten F, Brockman A, Brabin BJ, Duffy PE. Maternal antibodies block malaria. Nature. 1998;395:851–852. doi: 10.1038/27570. [DOI] [PubMed] [Google Scholar]
- 39.Stowers AW, Kennedy MC, Keegan BP, Saul A, Long CA, Miller LH. Vaccination of monkeys with recombinant Plasmodium falciparum apical membrane antigen 1 confers protection against blood-stage malaria. Infect Immun. 2002;70:6961–6967. doi: 10.1128/IAI.70.12.6961-6967.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Duan J, Mu J, Thera MA, Joy D, Kosakovsky Pond SL, Diemert D, Long C, Zhou H, Miura K, Ouattara A, Dolo A, Doumbo O, Su X, Miller L. Population structure of the genes encoding the polymorphic P. falciparum apical membrane antigen1: implications for vaccine design. Proc Natl Acad Sci U S A. 2008 doi: 10.1073/pnas.0802328105. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Collins CR, Withers-Martinez C, Bentley GA, Batchelor AH, Thomas AW, Blackman MJ. Fine mapping of an epitope recognized by an invasion-inhibitory monoclonal antibody on the malaria vaccine candidate apical membrane antigen 1. J Biol Chem. 2007;282:7431–7441. doi: 10.1074/jbc.M610562200. [DOI] [PubMed] [Google Scholar]
- 42.Serruto D, Rappuoli R. Post-genomic vaccine development. FEBS Lett. 2006;580:2985–2992. doi: 10.1016/j.febslet.2006.04.084. [DOI] [PubMed] [Google Scholar]
- 43.Davies DH, Liang X, Hernandez JE, Randall A, Hirst S, Mu Y, Romero KM, Nguyen TT, Kalantari-Dehaghi M, Crotty S, Baldi P, Villarreal LP, Felgner PL. Profiling the humoral immune response to infection by using proteome microarrays: high-throughput vaccine and diagnostic antigen discovery. Proc Natl Acad Sci U S A. 2005;102:547–552. doi: 10.1073/pnas.0408782102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gray JC, Corran PH, Mangia E, Gaunt MW, Li Q, Tetteh KK, Polley SD, Conway DJ, Holder AA, Bacarese-Hamilton T, Riley EM, Crisanti A. Profiling the antibody immune response against blood stage malaria vaccine candidates. Clin Chem. 2007;53:1244–1253. doi: 10.1373/clinchem.2006.081695. [DOI] [PubMed] [Google Scholar]
- 45.Doolan DL, Mu Y, Unal B, Sundaresh S, Hirst S, Valdez C, Randall A, Molina D, Liang X, Freilich DA, Oloo JA, Blair PL, Aguiar JC, Baldi P, Davies DH, Felgner PL. Profiling humoral immune responses to P. falciparum infection with protein microarrays. Proteomics. 2008;8:4680–4694. doi: 10.1002/pmic.200800194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kinyanjui SM, Conway DJ, Lanar DE, Marsh K. IgG antibody responses to Plasmodium falciparum merozoite antigens in Kenyan children have a short half-life. Malar J. 2007;6:82. doi: 10.1186/1475-2875-6-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Amanna IJ, Carlson NE, Slifka MK. Duration of humoral immunity to common viral and vaccine antigens. N Engl J Med. 2007;357:1903–1915. doi: 10.1056/NEJMoa066092. [DOI] [PubMed] [Google Scholar]
- 48.Hiller NL, Bhattacharjee S, van Ooij C, Liolios K, Harrison T, Lopez-Estrano C, Haldar K. A host-targeting signal in virulence proteins reveals a secretome in malarial infection. Science. 2004;306:1934–1937. doi: 10.1126/science.1102737. [DOI] [PubMed] [Google Scholar]
- 49.Marti M, Good RT, Rug M, Knuepfer E, Cowman AF. Targeting malaria virulence and remodeling proteins to the host erythrocyte. Science. 2004;306:1930–1933. doi: 10.1126/science.1102452. [DOI] [PubMed] [Google Scholar]
- 50.Bernasconi NL, Onai N, Lanzavecchia A. A role for Toll-like receptors in acquired immunity: up-regulation of TLR9 by BCR triggering in naive B cells and constitutive expression in memory B cells. Blood. 2003;101:4500–4504. doi: 10.1182/blood-2002-11-3569. [DOI] [PubMed] [Google Scholar]
- 51.Nebl T, De Veer MJ, Schofield L. Stimulation of innate immune responses by malarial glycosylphosphatidylinositol via pattern recognition receptors. Parasitology. 2005;130(Suppl):S45–62. doi: 10.1017/S0031182005008152. [DOI] [PubMed] [Google Scholar]
- 52.Coban C, Ishii KJ, Kawai T, Hemmi H, Sato S, Uematsu S, Yamamoto M, Takeuchi O, Itagaki S, Kumar N, Horii T, Akira S. Toll-like receptor 9 mediates innate immune activation by the malaria pigment hemozoin. J Exp Med. 2005;201:19–25. doi: 10.1084/jem.20041836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Parroche P, Lauw FN, Goutagny N, Latz E, Monks BG, Visintin A, Halmen KA, Lamphier M, Olivier M, Bartholomeu DC, Gazzinelli RT, Golenbock DT. Malaria hemozoin is immunologically inert but radically enhances innate responses by presenting malaria DNA to Toll-like receptor 9. Proc Natl Acad Sci U S A. 2007;104:1919–1924. doi: 10.1073/pnas.0608745104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wykes MN, Zhou YH, Liu XQ, Good MF. Plasmodium yoelii can ablate vaccine-induced long-term protection in mice. J Immunol. 2005;175:2510–2516. doi: 10.4049/jimmunol.175.4.2510. [DOI] [PubMed] [Google Scholar]
- 55.Shin H, Wherry EJ. CD8 T cell dysfunction during chronic viral infection. Curr Opin Immunol. 2007;19:408–415. doi: 10.1016/j.coi.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 56.Moir S, Ho J, Malaspina A, Wang W, DiPoto AC, O’Shea MA, Roby G, Kottilil S, Arthos J, Proschan MA, Chun T-W, Fauci AS. Evidence for HIV-associated B-cell exhaustion in a dysfunctional memory B-cell compartment in HIV-infected viremic individuals. J Exp Med. 2008 doi: 10.1084/jem.20072683. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Modiano D, Petrarca V, Sirima BS, Nebie I, Diallo D, Esposito F, Coluzzi M. Different response to Plasmodium falciparum malaria in west African sympatric ethnic groups. Proc Natl Acad Sci U S A. 1996;93:13206–13211. doi: 10.1073/pnas.93.23.13206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Clatworthy MR, Willcocks L, Urban B, Langhorne J, Williams TN, Peshu N, Watkins NA, Floto RA, Smith KG. Systemic lupus erythematosus-associated defects in the inhibitory receptor FcγRIIb reduce susceptibility to malaria. Proc Natl Acad Sci U S A. 2007;104:7169–7174. doi: 10.1073/pnas.0608889104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Greenwood BM, Herrick EM, Voller A. Suppression of autoimmune disease in NZB and (NZB × NZW) F1 hybrid mice by infection with malaria. Nature. 1970;226:266–267. doi: 10.1038/226266a0. [DOI] [PubMed] [Google Scholar]


