Abstract
Hypertension is a well-known risk factor for various cardiovascular diseases. Recently, exercise has been recommended as a part of lifestyle modification for all hypertensive patients. However, the precise mechanisms of exercise training (ExT)-induced effects on the development of hypertension are poorly understood. Therefore, we hypothesized that chronic ExT would delay the progression of hypertension in young spontaneously hypertensive rats (SHR). In addition, we explored whether the beneficial effects of chronic ExT were mediated by reduced pro-inflammatory cytokines (PICs) and improved redox status. We also investigated the involvement of NF-κB in exercise-induced effects. To test our hypotheses, young normotensive (WKY) and spontaneously hypertensive rats (SHR) were given moderate-intensity ExT for 16 weeks. Blood pressure was determined by the tail-cuff method and cardiac function was assessed by echocardiography. Myocardial total reactive oxygen species (ROS) and superoxide (O2•−) production were measured by electron paramagnetic resonance spectroscopy; TNF-α, IL-1β, gp91phox and iNOS by real-time PCR, and NF-κB activity by EMSA. Chronic ExT in hypertensive rats resulted in significantly reduced blood pressure, reduced concentric hypertrophy and improved diastolic function. ExT significantly reduced PICs, iNOS, attenuated total ROS and O2•− production, and increased antioxidants in SHR. ExT also resulted in increased nitric oxide production and decreased NF-κB activity in SHR. In summary, chronic ExT delays the progression of hypertension and improves cardiac function in young SHR; these ExT-induced beneficial effects are mediated by reduced PICs and improved redox homeostasis via downregulation of NF-κB.
Keywords: Exercise training, pro-inflammatory cytokines, NF-κB, hypertension, oxidative stress
Introduction
Hypertension is a well-known risk factor for various cardiovascular diseases; currently, it is estimated that more than 72 million American adults have hypertension.1 One of the hallmarks of hypertension is chronic low-grade inflammation. Pro-inflammatory cytokines (PICs) such as tumor necrosis factor alpha (TNF-α),2 interleukin (IL)-1β,2, 3 and IL-63, 4, have been reported to increase with the severity of hypertension and are of prognostic significance. In addition to PICs, free radicals such as reactive oxygen species (ROS) and superoxide (O2•−), contribute to the pathogenesis of hypertension. More importantly, PICs have been found to activate ROS,5–7 which in turn can activate various intracellular signaling pathways, including that of nuclear factor-kappa B (NF-κB). Activation of NF-κB induces gene transcription of PICs, which leads to further increase in ROS production, fostering a positive feedback mechanism, and eventually leading to the progression of hypertension.
Exercise has recently been recommended as a part of lifestyle modification for all patients diagnosed with hypertension.8 Several previous studies investigated the effects of exercise on hypertension; however, most of the studies were performed on severely hypertensive patients or animal models by using short-term exercise protocols or exercise combined with dietary interventions.9–14 However, the effects of long-term exercise training (ExT) in the progression of hypertension remain unclear. More importantly, the mechanisms by which ExT exerts its effects are largely unknown. Various proposed mechanisms of the anti-hypertensive effects of ExT include: reduced cardiac output, reduced peripheral vascular resistance,11 alterations in autonomic nervous system activity,15 and attenuated sympathetic modulation14, 16 and hypothalamic-pituitary-adrenal axis responsiveness,17 but a complete understanding of the molecular mechanisms underlying the exercise-induced reduction of blood pressure is still lacking.
The purpose of the present study was to investigate the effects of regular long-term moderate-intensity ExT in young spontaneously hypertensive rats (SHR) and to elucidate the mechanisms behind these effects. We hypothesized that: 1) regular chronic moderate-intensity ExT would delay the progression of hypertension in young SHR; 2) the beneficial effects of chronic ExT in young SHR would be mediated by reduced myocardial PICs and NF-κB activity; and 3) chronic ExT would improve myocardial redox homeostasis and nitric oxide bioavailability. Results from these studies will help us to further understand the mechanism by which ExT ameliorates hypertension.
Materials and Methods
For expanded materials and methods, please see http://hyper.ahajournals.org. All procedures in this study were approved by the Louisiana State University Institutional Animal Care and Use Committee and were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Animals and Experimental Design
Seven week old male normotensive Wistar-Kyoto (WK) and spontaneously hypertensive (SHR) rats were randomly assigned either to the sedentary group (SHRsed; n=10 and WKsed; n=10) or to the exercise group (SHRex; n=10 and WKex; n=10). Exercise groups were subjected to moderate-intensity exercise on a motor-driven treadmill continuously for a period of 16 weeks. Animals were euthanized twenty-four hours after the last exercise session at the age of 23 weeks and left ventricle (LV) tissues were collected for later analyses. We performed the following experimental procedures: blood pressure measurements, echocardiographic analysis, real time RT-PCR, western blot analysis, electron paramagnetic resonance (EPR) studies, antioxidant assays, electrophoretic mobility shift assay (EMSA), reverse-phase high-performance liquid chromatography (HPLC), ELISA, and statistical analysis.
Results
Body Weight
Body weight (BW) did not differ among groups at the start of the experiment. At the end of the study period, BW was not significantly different between WKsed and SHRsed. Chronic ExT resulted in reduction in BW both in WK and SHR rats (Table 1).
Table 1.
Weights and blood pressure parameters at the end of the training period.
| Parameters | WKsed | WKex | SHRsed | SHRex |
|---|---|---|---|---|
| N | 10 | 10 | 10 | 10 |
| Body weight (g) | 365.7 ± 8.9 | 327.6 ± 9.7‡ | 387.9 ± 3.4 | 327.2 ± 9.7† |
| Heart weight (mg) | 1.01 ± 0.04 | 1.21 ± 0.04‡ | 1.44 ± 0.02* | 1.24 ± 0.05† |
| HW/BW (mg/kg) | 2.88 ± 0.04 | 3.18 ± 0.1‡ | 3.45 ± 0.06* | 3.68 ± 0.02 |
| SBP (mmHg) | 134 ± 3 | 140 ± 7 | 233 ± 2* | 202 ± 0.5† |
| DBP (mmHg) | 89 ± 2 | 98 ± 6 | 176 ± 2* | 152 ± 5† |
| MAP (mmHg) | 101 ± 2 | 112 ± 6 | 191 ± 0.5* | 168 ± 3† |
WK, Wistar Kyoto rats; SHR, spontaneously hypertensive rats; sed, sedentary; ex, exercise. SBP, systolic blood pressure; DBP, diastolic blood pressure; MAP, mean arterial pressure. Values are means±SE.
p<0.05 WKsed vs SHRsed;
p<0.05 SHRsed vs SHRex;
p<0.05 WKsed vs WKex.
ExT Reduces Blood Pressure in SHR
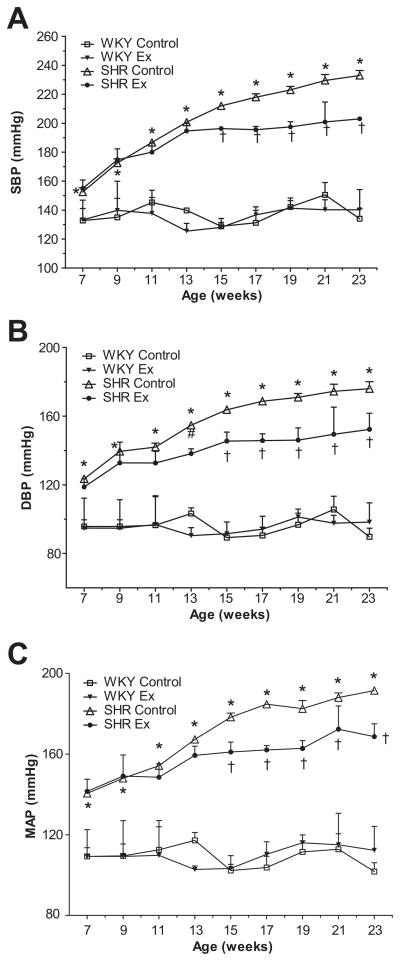
Systolic, diastolic, and mean arterial blood pressure (SBP, DBP, and MAP, respectively) were significantly higher in SHRsed than WKsed at the beginning of the experiment (at age 7 weeks) and remained increased for the duration of the study (Figure 1). At the end of the study, chronic ExT was found to have significantly reduced SBP, DBP and MAP in trained SHR compared to SHRsed (Table 1). Interestingly, BP in the SHRex group began to decrease significantly from 8 weeks of ExT; this trend remained until study end. ExT did not affect BP in WK rats (Figure 1).
Figure 1.
Time course of blood pressure (mmHg) in WK and SHR rats. a) Systolic blood pressure (SBP); b) diastolic blood pressure (DBP); c) mean arterial pressure (MAP). BP was significantly reduced in SHRex compared to SHRsed from 8 weeks of exercise (arrow). Values are means±SE. n=10 in each group. *p<0.05 WKsed vs SHRsed; †p<0.05 SHRsed vs SHRex.
ExT Reduces Pathological Cardiac Hypertrophy in SHR
At the end of the study period, SHRsed had higher heart weight (HW) and HW/BW ratio compared to WK rats (Table 1). Echocardiographic studies (Table 2) revealed that SHRsed rats had significantly higher interventricular septal thickness, posterior wall thickness, relative wall thickness (RWT), and left ventricular mass index (LVMI), without modification of LV chamber size, compared to WKsed. These echocardiographic changes suggest the presence of concentric hypertrophy in SHRsed. Chronic ExT significantly reduced interventricular septal thickness, posterior wall thickness, and RWT in hypertensive rats when compared to their sedentary controls, indicating reduced concentric hypertrophy with ExT. In addition, moderate ExT increased LV chamber size and decreased LVMI in these animals, though values did not reach statistical significance. However, ExT induced eccentric hypertrophy in WK rats as indicated by increased HW and HW/BW ratio without significant changes in septal and posterior wall thickness in WKex compared to WKsed.
Table 2.
Echocardiographic analysis of cardiac hypertrophy and function.
| Parameters | WKsed | WKex | SHRsed | SHRex |
|---|---|---|---|---|
| N | 10 | 10 | 10 | 10 |
| LVIDd, mm | 8.050 ± 0.14 | 8.320 ± 0.40 | 7.543 ± 0.20 | 8.009 ± 0.08 |
| LVIDs, mm | 4.933 ± 0.18 | 5.150 ± 0.38 | 5.143 ± 0.26 | 5.809 ± 0.09 |
| IVSd, mm | 1.550 ± 0.05 | 1.7 ± 0.05 | 2.071 ± 0.08* | 1.745 ± 0.06† |
| IVSs, mm | 2.367 ± 0.12 | 2.540 ± 0.04 | 2.843 ± 0.13* | 2.480 ± 0.05† |
| PWTd, mm | 1.525 ± 0.06 | 1.7 ± 0.07 | 1.925 ± 0.07* | 1.627 ± 0.04† |
| PWTs, mm | 2.225 ± 0.105 | 2.3 ± 0.13 | 2.683 ± 0.11* | 2.273 ± 0.05† |
| FS (%) | 36.85 ± 3.0 | 36 ± 2.8 | 29.80 ± 1.2 | 28.34 ± 0.4 |
| EF (%) | 73.23 ± 4.9 | 73.10 ± 4.1 | 65.12 ± 2.2 | 61 ± 1.5 |
| Tei index | 0.280 ± 0.04 | 0.345 ± 0.04 | 0.699 ± 0.04* | 0.534 ± 0.04† |
| LVMI | 2.00 ± 0.17 | 2.31 ± 0.25 | 2.67 ± 0.08* | 2.34 ± 0.16 |
| RWT | 0.380 ± 0.02 | 0.450 ± 0.02 | 0.530 ± 0.03* | 0.407 ± 0.01† |
| IVRT (ms) | 18.75 ± 1.4 | 19.27 ± 1.0 | 30.55 ± 4.5* | 23.96 ± 3.5† |
Values are means±SE. LVIDd and LVIDs indicate left ventricular internal diameter at diastole and systole, respectively; IVSd and IVSs, interventricular septal thickness at diastole and systole, respectively; PWTd and PWTs, posterior wall thickness at diastole and systole, respectively; FS, fractional shortening (%); EF (%), ejection fraction; LVMI, left ventricular mass index; RWT, relative wall thickness; IVRT, isovolumic relaxation time.
p<0.05 WKsed vs SHRsed;
p<0.05 SHRsed vs SHRex.
ExT Improves LV Diastolic Function in SHR
We evaluated the cardiac performance of all rats using M-mode and Doppler echocardiography (Table 2). LV systolic function was not altered in hypertensive rats, as indicated by the lack of significant changes in LV ejection fraction (EF) and fractional shortening (FS) in SHRsed compared to WKsed. However, diastolic function was severely impaired in SHR as indicated by significantly increased Tei index and increased isovolumic relaxation time (IVRT, an indicator of impaired LV relaxation) in SHRsed compared to WKsed. Chronic moderate-intensity ExT significantly reduced Tei index and IVRT in SHR, indicative of improved diastolic function. ExT did not alter systolic function in SHR or in WK rats.
ExT Reduces Myocardial and Circulating Pro-inflammatory Cytokines in SHR
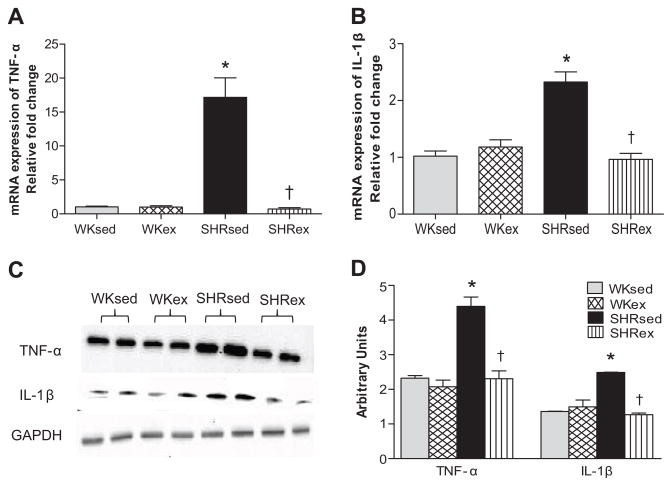
To determine whether the effects of chronic ExT were mediated by PICs, we examined TNF-α and IL-1β levels in the LV and plasma. SHRsed rats exhibited marked increases in expression of TNF-α and IL-1β in the LV as compared to WKsed (Figures 2 and S1). This upregulation of TNF-α and IL-1β was significantly attenuated by chronic ExT in SHR. However, ExT did not change PIC levels in WK rats.
Figure 2.
Effects of exercise training on LV pro-inflammatory cytokines (PICs) in WK and SHR rats. a) mRNA expression of TNF-α; b) mRNA expression of IL-1β; c) a representative western blot; d) densitometric analysis of protein expression. Values are means±SE. n=6–8 in each group. *p<0.05 WKsed vs SHRsed; †p<0.05 SHRsed vs SHRex.
ExT Improves Myocardial Redox Homeostasis in SHR
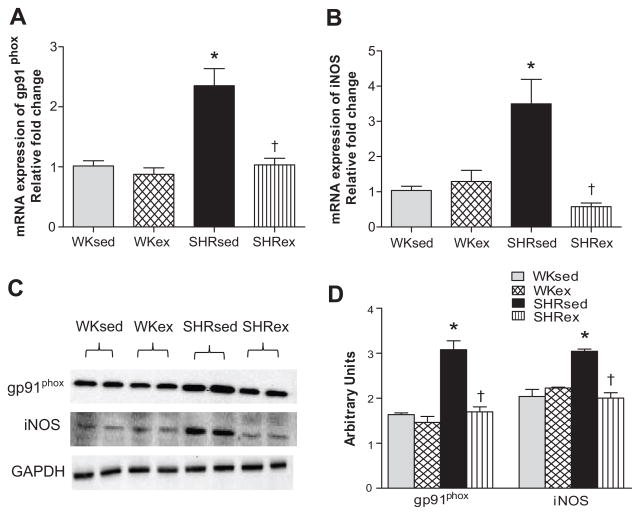
To elucidate the potential role of improved redox status in the beneficial effects of chronic ExT, we measured and quantified total ROS and O2•− production in the LV by EPR. We also examined the mRNA and protein levels of gp91phox by RT-PCR and western blotting. Sedentary SHR had significantly increased levels of total ROS (Figure 3a) and O2•− (Figure 3b) production compared to WKsed. Chronic ExT significantly attenuated total ROS and O2•− production in SHR. ExT did not affect free radical production in WK rats. Furthermore, gp91phox expression was markedly higher in SHRsed when compared to WKsed rats; this expression was significantly reduced by chronic ExT (Figure 4a, 4b, 4c).
Figure 3.
Effects of exercise training on LV free radical production in WK and SHR rats. a) Total reactive oxygen species; b) superoxide; c) peroxynitrite. Values are means±SE. n=10 in each group. *p<0.05 WKsed vs SHRsed; †p<0.05 SHRsed vs SHRex.
Figure 4.
Effects of exercise on gp91phox, and iNOS expression in the LV of WK and SHR rats. a) mRNA expression of gp91phox; b) a representative western blot; c) densitometric analysis of protein expression; d) mRNA expression of iNOS. Values are means±SE. n=6 in each group. *p<0.05 WKsed vs SHRsed; †p<0.05 SHRsed vs SHRex.
Because decreased local antioxidant protection is one of the potential sources of ROS formation,18 we analyzed various enzymatic and nonenzymatic antioxidant levels in LV tissue. We observed that SHRsed rats had no changes in myocardial superoxide dismutase (SOD; Figure S2) and reduced glutathione (GSH; Figure S3) concentrations when compared to WKsed. In addition, SHRsed exhibited significantly increased GSSG (oxidized disulfide glutathione; Figure S4) concentration, and reduced GSH/GSSG ratio (an important marker of cellular redox balance19, 20; Figure S5) in comparison to WKsed rats. Chronic ExT in SHR rats resulted in significantly increased GSH/GSSG ratio, decreased GSSG levels, and increased SOD activity, indicative of improvements in antioxidant defense by ExT. ExT did not affect antioxidant levels in WK rats.
ExT Reduces Myocardial Nitric Oxide Synthase and Peroxynitrite in SHR
Hypertensive rats showed significantly higher mRNA and protein expression of myocardial inducible-nitric oxide synthase (iNOS) when compared to WKsed. Chronic ExT resulted in significantly decreased iNOS expression in SHR. ExT did not affect iNOS expression in WK rats (Figures 4b, 4c, 4d). Direct measurement and quantification of peroxynitrite (OONO−) by EPR studies revealed that SHRsed rats had significantly increased myocardial production of OONO− in comparison to WKsed. Interestingly, chronic ExT in SHR resulted in significantly decreased OONO− production (Figure 3c).
ExT Normalizes Myocardial NO Level in SHR
Myocardial total nitrate/nitrite concentration, a marker of NO production, was significantly lower in SHRsed compared to WKsed. Chronic ExT normalized myocardial total nitrate/nitrite concentration in SHR (Figure S6).
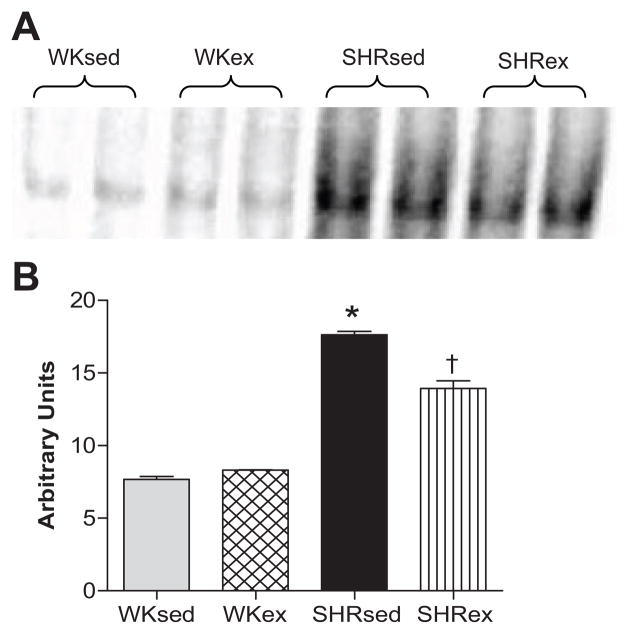
ExT Attenuates NF-κB Binding Activity in SHR
SHRsed had significantly higher myocardial NF-κB binding activity than WKsed. Chronic ExT resulted in a significant decrease in NF-κB binding activity in SHR. NF-κB binding activity was unaltered by ExT in WK rats (Figure 5).
Figure 5.
Effects of exercise on LV NF-κB binding activity in WK and SHR rats. a) Representative EMSA results of NF-κB binding activity; b) densitometric analysis of NF-κB binding intensity (mean±SE). Values are means±SE. n=6 in each group. *p<0.05 WKsed vs SHRsed; †p<0.05 SHRsed vs SHRex.
ExT Decreases Plasma NE Levels in SHR
At the end of the study, plasma NE levels were found to be significantly higher in SHRsed compared to WKsed. Chronic ExT resulted in significantly decreased plasma NE concentrations in SHR, but did not change plasma NE level in WK rats (Figure S7).
DISCUSSION
In this study, we investigated the effects of chronic moderate-intensity ExT and possible mechanisms of these effects on young spontaneously hypertensive rats (SHR), a genetic model of hypertension which shares many common features of human essential hypertension. The salient findings of this study are: 1) regular long-term moderate-intensity ExT delays the progression of hypertension, reduces cardiac hypertrophy and improves diastolic cardiac function in young SHR with developing hypertension; 2) training-induced beneficial effects in SHR rats are mediated by decreased myocardial and circulating TNF-α and IL-1β, and reduced myocardial NF-κB activity; and 3) chronic ExT exerts its effects via improved myocardial redox status and NO production in SHR rats. These findings provide evidence of involvement of PICs, redox homeostasis, and NF-κB in exercise-induced delayed progression of hypertension and cardiac improvements in SHR.
At the end of the study, we observed significant reductions in SBP, DBP and MAP in trained SHR compared to SHRsed, and saw no comparable changes in trained WK rats. The pressure-lowering effect of ExT was significant starting from 8 weeks of regular exercise, and continued until the end of the study, emphasizing the importance of long-term exercise in patients with hypertension. Previous reports suggest that exercise training did not reduce BP in severely hypertensive patients and rats.9, 13 The discrepancies in results could be due to low-intensity and/or shorter duration of exercise protocols employed in those studies. Also, most of the previous studies were done in severely hypertensive rats.9, 13, 14 Nonetheless, results of our study suggest that regular moderate-intensity ExT delays the progression of hypertension.
Our echocardiographic data showed that chronic ExT resulted in improved cardiac diastolic function in SHR as indicated by decreased IVRT. Evidence from previous studies indicates that the beneficial effects of ExT on diastolic function were blunted in rats with severe hypertension12, 21. To the best of our knowledge, this is the first animal study to report the effects of chronic ExT on diastolic function in young SHR with early hypertension. Our findings, together with previous reports, suggest that moderate-intensity ExT, when initiated in the early stages of hypertension, can maximize its own cardio-protective effects.
Furthermore, ExT did not alter cardiac function in WK rats as assessed by EF and FS, however, LV internal dimension was found to be slightly increased, though, changes did not reach statistical significance. This observation is in accordance with several previous studies.12, 22 Pluim et al., in their meta-analysis encompassing 59 studies and 1451 athletes, have reported normal cardiac function in endurance- and strength-trained athletes and concluded that there is no relationship between cardiac geometry and systolic function.22
In the present study, chronic ExT also resulted in reduced cardiac hypertrophy in SHR. This finding is significant from a clinical perspective, since pathological cardiac hypertrophy is known to lead to cardiac failure.23 Conversely, ExT in WK rats resulted in eccentric hypertrophy. Eccentric hypertrophy, also known as physiological hypertrophy, is mainly related to training-induced volume-overload,24 and considered as a cardiac adaptation of exercise training.22 These results were in accordance with previous studies.14
Recent evidence suggests that PICs play important roles in hypertension-induced cardiac hypertrophy. Various PICs such as TNF-α, IL-6, and IL-1β have reported to increase with the severity of hypertension.25 Few studies have documented the reduction in inflammatory markers by exercise training on obese individuals26, 27 or diabetic patients.28 Several randomized clinical trials have shown reduced plasma TNF-α and/or IL-6 levels by physical training with or without dietary interventions in patients with chronic heart failure29 and coronary heart disease30, accompanied by various degrees of treated hypertension. However, until now, no studies have examined the effect of chronic ExT on left ventricular PICs in hypertension. In our study, we found that chronic ExT resulted in decreased myocardial TNF-α and IL-1β in SHR, suggesting that the beneficial effects of chronic ExT in hypertensive rats are mediated by reduced myocardial and circulating PICs.
A growing body of evidence indicates that hypertension is also characterized by increased sympathetic activity.31, 32 The success of beta-blockers in reducing hypertension-induced cardiac hypertrophy suggests that sympathetic hyperactivity plays an important role in cardiac hypertrophy and cardiac damage in hypertension. In this study, the increases in circulating plasma NE (an indirect marker of sympathetic activity) seen in SHR compared to WK rats were found to be significantly decreased by chronic ExT. This finding was in agreement with recently published data showing significantly reduced cardiac NE concentrations in trained SHR.14 These findings, together with previous findings from our lab that TNF-α blockade attenuates sympathoexcitation in heart failure,33 provide strong evidence of an association between PICs and sympathetic hyperactivity and reinforce the idea that ExT causes a reduction in PICs by attenuating sympathetic activity in SHR. Therefore, it can be suggested that reduced sympathetic activity may contribute, at least in part, to exercise-induced reduced PICs in young SHR.
Previous studies have shown that oxidative stress plays a key role in the development of hypertension and cardiac hypertrophy.14 We previously reported that cytokines, and their transcription factor, NFκB, contribute to the induction of oxidative stress in heart failure34 and hypertension.35 Given the current finding that ExT reduces PICs in SHR, we further examined the effect of chronic ExT on redox balance in hypertensive animals. Our EPR studies revealed that myocardial total ROS and O2•− production rates were significantly higher in SHRsed as compared to WKsed; however, the antioxidant defense system was unaltered. These data suggest that an imbalance in redox homeostasis plays an important role in the progression of hypertension. More importantly, chronic ExT not only reduced myocardial total ROS and O2•− production rates, but also increased antioxidants leading to restoration of cellular redox homeostasis. Previous evidence that TNF-α is an important contributor to oxidative stress,34 and our finding that decreased oxidative stress is associated with decreased PICs in SHRex rats, raises the possibility that decreased PICs might be responsible for the exercise-induced decrease in oxidative stress in SHR. In addition, we observed that ExT resulted in attenuation of increased expression of gp91phox (a subunit of NAD(P)H oxidase, a major source of ROS) in SHR. Angiotensin II is a major regulator of NAD(P)H oxidase activity; therefore, the possible contribution of the renin-angiotensin system to exercise-mediated effects cannot be ignored. Nonetheless, our data lead us to conclude that pressure-lowering and anti-hypertrophic effects of regular long-term moderate-intensity ExT in unestablished hypertension are mediated by improved redox status in the body rather than the attenuation of oxidant production alone.
In last few years, iNOS has been documented to be associated with the development of hypertension.36, 37 The evidence that iNOS is predominantly induced by cytokines38, and our finding that ExT reduces PICs in SHR, led us to explore whether ExT results in decreased myocardial iNOS expression. We observed that mRNA and protein expression of myocardial iNOS was markedly higher in SHRsed compared to WKsed; these levels were significantly decreased by chronic ExT in SHR. Furthermore, reduced myocardial total nitrate/nitrite levels in SHRsed were normalized in SHRex rats, which is indicative of increased NO bioavailability by chronic ExT in SHR. The decrease in iNOS level by ExT suggests lowered NO production; however, the concomitant decrease in O2•− in SHRex rats seems responsible for attenuated O2•− mediated degradation of NO, leading to increased NO bioavailability. This was further supported byour finding that ExT significantly attenuated increased OONO− production in SHR. Therefore, the results of this study suggest that chronic moderate-intensity ExT not only decreases iNOS expression, but also decreases OONO− -induced tissue damage, and increases NO bioavailability in SHR. Also, in support of our results, recent studies have demonstrated that iNOS gene deletion reduces oxidative stress and preserves cardiac function in mice with hypertension.39
Recent work from our lab suggests that NF-κB blockade reduces cytosolic and mitochondrial oxidative stress and attenuates hypertension in SHR.35 PICs have also been shown to act via NF-κB -mediated signaling pathways. Therefore, one possible mechanism by which exercise exerts its beneficial effects could be via down regulation of NF-κB activity. Our present observation that NF-κB activity was increased in SHRsed compared to WKsed further supports this hypothesis. More importantly, chronic ExT resulted in downregulation of NF-κB activity in SHR. In our study, reduced NF-κB activity was also associated with reduced PICs and oxidative stress, suggesting that attenuation of NF-κB activity by ExT might be attributable to exercise-mediated reduced PICs and oxidative stress, which in turn leads to disruption of the detrimental positive feedback cycle involved in the progression of hypertension.
Perspectives
The findings of this study indicate that chronic regular moderate-intensity ExT delays the progression of hypertension, reduces cardiac hypertrophy, and improves diastolic function in rats with developing hypertension. More importantly, this study provides mechanistic evidence that the pressure-lowering and cardio-protective effects of chronic exercise are mediated by reduced PICs, improved cellular redox homeostasis, increased NO production, and downregulation of NF-κB activity. We also observed that decreases in PICs by ExT were associated with reduced plasma NE levels. Although a direct cause/effect relationship could not be established in this study, we can attribute the beneficial effects of ExT on hypertension to an altered interplay between sympathetic activity, PICs, and oxidative stress via NF-κB-mediated signaling pathways. Future studies could be directed towards providing more direct evidence to support the cause/effect relationship between various parameters. Furthermore, here, we chose a moderate-intensity exercise protocol to elucidate the mechanisms of the beneficial effects of exercise training in SHR rats. However, the comparison of different intensities of ExT with regard to parameters studies could certainly be an important perspective of this study.
Supplementary Material
Acknowledgments
Source of Funding
This study was supported by National Heart, Lung, and Blood Institute Grant HL-80544 to Joseph Francis.
Footnotes
Disclosures
None
References
- 1.Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, Ford E, Furie K, Go A, Greenlund K, Haase N, Hailpern S, Ho M, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott M, Meigs J, Mozaffarian D, Nichol G, O’Donnell C, Roger V, Rosamond W, Sacco R, Sorlie P, Stafford R, Steinberger J, Thom T, Wasserthiel-Smoller S, Wong N, Wylie-Rosett J, Hong Y. Heart disease and stroke statistics--2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:480–486. doi: 10.1161/CIRCULATIONAHA.108.191259. [DOI] [PubMed] [Google Scholar]
- 2.Dorffel Y, Latsch C, Stuhlmuller B, Schreiber S, Scholze S, Burmester GR, Scholze J. Preactivated peripheral blood monocytes in patients with essential hypertension. Hypertension. 1999;34:113–117. doi: 10.1161/01.hyp.34.1.113. [DOI] [PubMed] [Google Scholar]
- 3.Peeters AC, Netea MG, Janssen MC, Kullberg BJ, Van der Meer JW, Thien T. Pro-inflammatory cytokines in patients with essential hypertension. Eur J Clin Invest. 2001;31:31–36. doi: 10.1046/j.1365-2362.2001.00743.x. [DOI] [PubMed] [Google Scholar]
- 4.Chae CU, Lee RT, Rifai N, Ridker PM. Blood pressure and inflammation in apparently healthy men. Hypertension. 2001;38:399–403. doi: 10.1161/01.hyp.38.3.399. [DOI] [PubMed] [Google Scholar]
- 5.Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res. 2000;87:840–844. doi: 10.1161/01.res.87.10.840. [DOI] [PubMed] [Google Scholar]
- 6.Neri M, Cerretani D, Fiaschi AI, Laghi PF, Lazzerini PE, Maffione AB, Micheli L, Bruni G, Nencini C, Giorgi G, D’Errico S, Fiore C, Pomara C, Riezzo I, Turillazzi E, Fineschi V. Correlation between cardiac oxidative stress and myocardial pathology due to acute and chronic norepinephrine administration in rats. J Cell Mol Med. 2007;11:156–170. doi: 10.1111/j.1582-4934.2007.00009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mariappan N, Soorappan RN, Haque M, Sriramula S, Francis J. TNF-alpha-induced mitochondrial oxidative stress and cardiac dysfunction: restoration by superoxide dismutase mimetic Tempol. Am J Physiol Heart Circ Physiol. 2007;293:H2726–2737. doi: 10.1152/ajpheart.00376.2007. [DOI] [PubMed] [Google Scholar]
- 8.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 9.Chicco AJ, McCune SA, Emter CA, Sparagna GC, Rees ML, Bolden DA, Marshall KD, Murphy RC, Moore RL. Low-intensity exercise training delays heart failure and improves survival in female hypertensive heart failure rats. Hypertension. 2008;51:1096–1102. doi: 10.1161/HYPERTENSIONAHA.107.107078. [DOI] [PubMed] [Google Scholar]
- 10.Emter CA, McCune SA, Sparagna GC, Radin MJ, Moore RL. Low-intensity exercise training delays onset of decompensated heart failure in spontaneously hypertensive heart failure rats. Am J Physiol Heart Circ Physiol. 2005;289:H2030–2038. doi: 10.1152/ajpheart.00526.2005. [DOI] [PubMed] [Google Scholar]
- 11.Sun MW, Qian FL, Wang J, Tao T, Guo J, Wang L, Lu AY, Chen H. Low-intensity voluntary running lowers blood pressure with simultaneous improvement in endothelium-dependent vasodilatation and insulin sensitivity in aged spontaneously hypertensive rats. Hypertens Res. 2008;31:543–552. doi: 10.1291/hypres.31.543. [DOI] [PubMed] [Google Scholar]
- 12.Boissiere J, Eder V, Machet MC, Courteix D, Bonnet P. Moderate exercise training does not worsen left ventricle remodeling and function in untreated severe hypertensive rats. J Appl Physiol. 2008;104:321–327. doi: 10.1152/japplphysiol.00442.2007. [DOI] [PubMed] [Google Scholar]
- 13.Graham DA, Rush JW. Exercise training improves aortic endothelium-dependent vasorelaxation and determinants of nitric oxide bioavailability in spontaneously hypertensive rats. J Appl Physiol. 2004;96:2088–2096. doi: 10.1152/japplphysiol.01252.2003. [DOI] [PubMed] [Google Scholar]
- 14.Bertagnolli M, Schenkel PC, Campos C, Mostarda CT, Casarini DE, Bello-Klein A, Irigoyen MC, Rigatto K. Exercise training reduces sympathetic modulation on cardiovascular system and cardiac oxidative stress in spontaneously hypertensive rats. Am J Hypertens. 2008;21:1188–1193. doi: 10.1038/ajh.2008.270. [DOI] [PubMed] [Google Scholar]
- 15.Sigvardsson K, Svanfeldt E, Kilbom A. Role of the adrenergic nervous system in development of training-induced bradycardia. Acta Physiol Scand. 1977;101:481–488. doi: 10.1111/j.1748-1716.1977.tb06032.x. [DOI] [PubMed] [Google Scholar]
- 16.Mitchell JB, Flynn MG, Goldfarb AH, Ben-Ezra V, Copmann TL. The effect of training on the norepinephrine response at rest and during exercise in 5 degrees and 20 degrees C environments. J Sports Med Phys Fitness. 1990;30:235–240. [PubMed] [Google Scholar]
- 17.Johnson EO, Kamilaris TC, Chrousos GP, Gold PW. Mechanisms of stress: a dynamic overview of hormonal and behavioral homeostasis. Neurosci Biobehav Rev. 1992;16:115–130. doi: 10.1016/s0149-7634(05)80175-7. [DOI] [PubMed] [Google Scholar]
- 18.Kobayashi S, Inoue N, Azumi H, Seno T, Hirata K, Kawashima S, Hayashi Y, Itoh H, Yokozaki H, Yokoyama M. Expressional changes of the vascular antioxidant system in atherosclerotic coronary arteries. J Atheroscler Thromb. 2002;9:184–190. doi: 10.5551/jat.9.184. [DOI] [PubMed] [Google Scholar]
- 19.Powers SK, Jackson MJ. Exercise-induced oxidative stress: cellular mechanisms and impact on muscle force production. Physiol Rev. 2008;88:1243–1276. doi: 10.1152/physrev.00031.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lew H, Pyke S, Quintanilha A. Changes in the glutathione status of plasma, liver and muscle following exhaustive exercise in rats. FEBS Lett. 1985;185:262–266. doi: 10.1016/0014-5793(85)80919-4. [DOI] [PubMed] [Google Scholar]
- 21.Palmer BM, Lynch JM, Snyder SM, Moore RL. Renal hypertension prevents run training modification of cardiomyocyte diastolic Ca2+ regulation in male rats. J Appl Physiol. 2001;90:2063–2069. doi: 10.1152/jappl.2001.90.6.2063. [DOI] [PubMed] [Google Scholar]
- 22.Pluim BM, Zwinderman AH, van der Laarse A, van der Wall EE. The athlete’s heart. A meta-analysis of cardiac structure and function. Circulation. 2000;101:336–344. doi: 10.1161/01.cir.101.3.336. [DOI] [PubMed] [Google Scholar]
- 23.Rysa J, Leskinen H, Ilves M, Ruskoaho H. Distinct upregulation of extracellular matrix genes in transition from hypertrophy to hypertensive heart failure. Hypertension. 2005;45:927–933. doi: 10.1161/01.HYP.0000161873.27088.4c. [DOI] [PubMed] [Google Scholar]
- 24.Lorell BH, Carabello BA. Left ventricular hypertrophy: pathogenesis, detection, and prognosis. Circulation. 2000;102:470–479. doi: 10.1161/01.cir.102.4.470. [DOI] [PubMed] [Google Scholar]
- 25.Edwards KM, Ziegler MG, Mills PJ. The potential anti-inflammatory benefits of improving physical fitness in hypertension. J Hypertens. 2007;25:1533–1542. doi: 10.1097/HJH.0b013e328165ca67. [DOI] [PubMed] [Google Scholar]
- 26.Marfella R, Esposito K, Siniscalchi M, Cacciapuoti F, Giugliano F, Labriola D, Ciotola M, Di Palo C, Misso L, Giugliano D. Effect of weight loss on cardiac synchronization and proinflammatory cytokines in premenopausal obese women. Diabetes Care. 2004;27:47–52. doi: 10.2337/diacare.27.1.47. [DOI] [PubMed] [Google Scholar]
- 27.Esposito K, Pontillo A, Di Palo C, Giugliano G, Masella M, Marfella R, Giugliano D. Effect of weight loss and lifestyle changes on vascular inflammatory markers in obese women: a randomized trial. JAMA. 2003;289:1799–1804. doi: 10.1001/jama.289.14.1799. [DOI] [PubMed] [Google Scholar]
- 28.Giannopoulou I, Fernhall B, Carhart R, Weinstock RS, Baynard T, Figueroa A, Kanaley JA. Effects of diet and/or exercise on the adipocytokine and inflammatory cytokine levels of postmenopausal women with type 2 diabetes. Metabolism. 2005;54:866–875. doi: 10.1016/j.metabol.2005.01.033. [DOI] [PubMed] [Google Scholar]
- 29.Adamopoulos S, Parissis J, Karatzas D, Kroupis C, Georgiadis M, Karavolias G, Paraskevaidis J, Koniavitou K, Coats AJ, Kremastinos DT. Physical training modulates proinflammatory cytokines and the soluble Fas/soluble Fas ligand system in patients with chronic heart failure. J Am Coll Cardiol. 2002;39:653–663. doi: 10.1016/s0735-1097(01)01795-8. [DOI] [PubMed] [Google Scholar]
- 30.Goldhammer E, Tanchilevitch A, Maor I, Beniamini Y, Rosenschein U, Sagiv M. Exercise training modulates cytokines activity in coronary heart disease patients. Int J Cardiol. 2005;100:93–99. doi: 10.1016/j.ijcard.2004.08.073. [DOI] [PubMed] [Google Scholar]
- 31.Donohue SJ, Stitzel RE, Head RJ. Time course of changes in the norepinephrine content of tissues from spontaneously hypertensive and Wistar Kyoto rats. J Pharmacol Exp Ther. 1988;245:24–31. [PubMed] [Google Scholar]
- 32.Guyenet PG. The sympathetic control of blood pressure. Nat Rev Neurosci. 2006;7:335–346. doi: 10.1038/nrn1902. [DOI] [PubMed] [Google Scholar]
- 33.Guggilam A, Patel KP, Haque M, Ebenezer PJ, Kapusta DR, Francis J. Cytokine blockade attenuates sympathoexcitation in heart failure: cross-talk between nNOS, AT-1R and cytokines in the hypothalamic paraventricular nucleus. Eur J Heart Fail. 2008;10:625–634. doi: 10.1016/j.ejheart.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guggilam A, Haque M, Kerut EK, McIlwain E, Lucchesi P, Seghal I, Francis J. TNF- alpha blockade decreases oxidative stress in the paraventricular nucleus and attenuates sympathoexcitation in heart failure rats. Am J Physiol Heart Circ Physiol. 2007;293:H599–609. doi: 10.1152/ajpheart.00286.2007. [DOI] [PubMed] [Google Scholar]
- 35.Elks CM, Mariappan N, Haque M, Guggilam A, Majid DS, Francis J. Chronic NF-{kappa}B blockade reduces cytosolic and mitochondrial oxidative stress and attenuates renal injury and hypertension in SHR. Am J Physiol Renal Physiol. 2009;296:F298–305. doi: 10.1152/ajprenal.90628.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hong HJ, Loh SH, Yen MH. Suppression of the development of hypertension by the inhibitor of inducible nitric oxide synthase. Br J Pharmacol. 2000;131:631–637. doi: 10.1038/sj.bjp.0703603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Escames G, Khaldy H, Leon J, Gonzalez L, Acuna-Castroviejo D. Changes in iNOS activity, oxidative stress and melatonin levels in hypertensive patients treated with lacidipine. J Hypertens. 2004;22:629–635. doi: 10.1097/00004872-200403000-00027. [DOI] [PubMed] [Google Scholar]
- 38.Cotton JM, Kearney MT, Shah AM. Nitric oxide and myocardial function in heart failure: friend or foe? Heart. 2002;88:564–566. doi: 10.1136/heart.88.6.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sun Y, Carretero OA, Xu J, Rhaleb NE, Yang JJ, Pagano PJ, Yang XP. Deletion of inducible nitric oxide synthase provides cardioprotection in mice with 2-kidney, 1-clip hypertension. Hypertension. 2009;53:49–56. doi: 10.1161/HYPERTENSIONAHA.108.121822. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.