Abstract
Ca2+ influx is an early signal initiating cytosolic immune responses to pathogen perception in plant cells; molecular components linking pathogen recognition to Ca2+ influx are not delineated. Work presented here provides insights into this biological system of non-self recognition and response activation. We have recently identified a cyclic nucleotide-activated ion channel as facilitating the Ca2+ flux that initiates immune signaling in the plant cell cytosol. Work in this report shows that elevation of cAMP is a key player in this signaling cascade. We show that cytosolic Ca2+ elevation, nitric oxide (NO) and reactive oxygen species generation, as well as immune signaling, lead to a hypersensitive response upon application of pathogens and/or conserved molecules that are components of microbes and are all dependent on cAMP generation. Exogenous cAMP leads to Ca2+ channel-dependent cytosolic Ca2+ elevation, NO generation, and defense response gene expression in the absence of the non-self pathogen signal. Inoculation of leaves with a bacterial pathogen leads to cAMP elevation coordinated with Ca2+ rise. cAMP acts as a secondary messenger in plants; however, no specific protein has been heretofore identified as activated by cAMP in a manner associated with a signaling cascade in plants, as we report here. Our linkage of cAMP elevation in pathogen-inoculated plant leaves to Ca2+ channels and immune signaling downstream from cytosolic Ca2+ elevation provides a model for how non-self detection can be transduced to initiate the cascade of events in the cell cytosol that orchestrate pathogen defense responses.
Keywords: calcium signaling, cyclic nucleotide gated channels, plant innate immunity
Plants are typically sessile creatures; when challenged with a stress, they cannot run or hide. In addition, vascular higher plants exist as multicellular organisms without a defense network of circulating mobile sentry cells equivalent to macrophages of the jawed vertebrate immune system. Therefore, cellular level recognition of pathogens as non-self by plants is a critical (and little understood) feature of system fitness. Non-self perception of pathogens initiates a signaling cascade leading to immune responses in plants. A key question underlying the rationale of the work reported here is the identification of components of the molecular mechanisms that allow for translation of non-self perception into a response signaling cascade in the plant cell; or as delineated by the Biblical-era scholar Hillel-the-Elder, “If I am not for myself, who will be for me?”
One component of the repertoire of plant immune defense responses to non-self perception of a pathogen invader is the hypersensitive response (HR). HR involves rapid programmed cell death in the local region surrounding an infection site, limiting growth of the invading (avirulent) pathogen and arresting progression of disease symptoms (1). For well over a decade, it has been known that Ca2+ is involved as an early signal in this defense response cascade (1). Much of the plant immune response characterization to date has involved application of pathogen cell extracts (or molecules purified from pathogens) to plant cell cultures (2–4; see refs. 5 and 6 for review). Specific evolutionarily conserved essential components of microbes, or pathogen associated molecular pattern (PAMP) molecules, elicit non-self perception and defense responses in plants under pathogenic attack. Early studies of pathogen-derived elicitor effects on cultured plant cells (7) identified an increase in cell-associated Ca2+ as occurring within minutes after exposure to the pathogen-derived elicitor. Patch clamp studies suggested that elicitor-dependent activation of a cell membrane inwardly conducting Ca2+-permeable ion channel contributed to the intracellular Ca2+ elevation; elicitors increased channel open-state probability (8). Subsequent work with cell cultures and intact leaves confirmed that influx of Ca2+ across the plasma membrane (PM) (and possibly efflux from intracellular stores as well) is an early event in plant immune signaling (6, 9–11). However, until only recently, no specific translation product of a plant ion channel gene was associated with Ca2+ conductance leading to pathogen response signaling (or, for that matter, any other signal transduction system involving cytosolic Ca2+ elevation in the plant cell).
The Arabidopsis dnd1 (“defense-no-death”) mutant lacks a functional cyclic nucleotide (cNMP) gated (non-selective) cation channel (CNGC2) and does not display an HR to avirulent pathogens (12). Prior work from this laboratory (13) has demonstrated that dnd1 cells lack a cAMP-activated inward PM Ca2+ current, and that lack of this current is associated with impaired nitric oxide (NO) generation and plant immune responses, including HR (exogenous NO complements the dnd1 phenotype). NO has been referred to as the downstream “concert master” of innate immunity (14); orchestrating a suite of responses that includes defense gene expression and HR.
Little is currently known about how PAMP perception is linked to cytosolic Ca2+ elevation during immune signaling in plant cells. The identification of a cAMP-activated, Ca2+-conducting channel as a key component of plant immune signaling led to the work reported here that links the gating properties of this channel to upstream components of this signal transduction pathway.
Results
cNMP, CNGCs, and Immune Signaling.
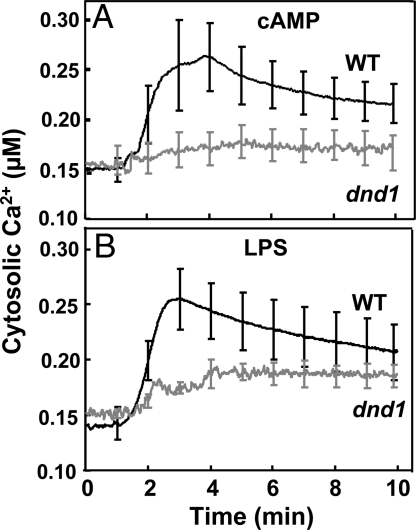
A number of studies have demonstrated CNGC channel involvement in plant signaling cascades responding to pathogens (12, 13, 15). Patch clamp analysis of plant CNGCs in native (Arabidopsis) PM as well as upon expression in heterologous systems (13, 16–19) indicates that they are activated by cNMPs (most of the work has used cAMP), and that they can conduct Ca2+, K+, and, in some cases, Na+. We extend knowledge of CNGC function in plants here by showing that application of cAMP results in CNGC2-dependent elevation of cytosolic Ca2+ in leaves (Fig. 1A). The transitory cytosolic Ca2+ elevation initiated by this CNGC channel activating ligand in intact leaf cells of wild type (WT) plants occurs within minutes, and is absent in leaves of dnd1 (CNGC2 mutant) plants. In a fashion similar to these studies with leaves of Arabidopsis plants expressing recombinant apoaequorin, Volotovski et al. (20) have shown that application of cNMPs to isolated tobacco (Nicotiana tabacum) protoplasts resulted in elevation of cytosolic Ca2+. In this prior work, however, the elevation in protoplast Ca2+ was not associated with a specific ion channel gene translation product, as we show here.
Fig. 1.
Effect of the cAMP and the PAMP LPS on cytosolic Ca2+ in leaves of WT (dark lines) and dnd1 (gray lines) plants measured using aequorin luminescence. In all cases, the luminescence signal was stable before addition of ligand (at time '0′). The signals shown are averages generated from replicate leaves (WT cAMP n = 6, dnd1 cAMP n = 4, WT LPS n = 5, dnd1 LPS n = 5). At 1-min time intervals, S.E. was calculated for each mean; S.E.s are portrayed as error bars. Aequorin-expressing WT and dnd1 plants responded similarly (i.e., with a strong cytosolic Ca2+ elevation) when challenged with a temporary cold temperature treatment; this control experiment indicated that aequorin was functional in both genotypes.
Perception of PAMPs by plant cells initiates basal-level, or “innate” immune responses (21). Lipopolysaccharide (LPS), a PAMP common to all Gram-negative bacteria (including Pseudomonas syringae), activates an inward cation current in Arabidopsis (and Vicia faba) protoplasts as well as CNGC2-, and inward Ca2+ conduction-dependent NO generation in leaf tissue (13). Here, we provide experimental evidence that PAMP (i.e., LPS) perception by cells in leaves results, within minutes, in a CNGC2-dependent, transient cytosolic Ca2+ elevation (Fig. 1B). Results presented in Fig. 1B provide direct evidence linking the cAMP gated channel to the pathogen-associated, early (i.e., within minutes of PAMP application) cytosolic Ca2+ elevation in plant leaves. When the same experimental system is used, the cytosolic Ca2+ “spike” induced by cAMP and the PAMP LPS appears to be similar (Fig. 1).
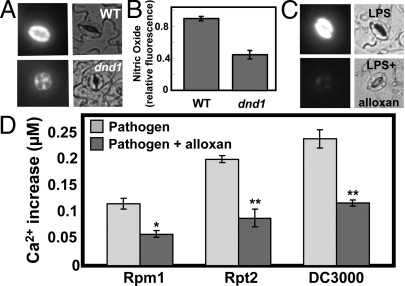
In prior work from this lab (13), LPS-dependent NO generation in leaf cells of dnd1 plants was impaired compared to WT. Here, we provide evidence that exogenous cAMP can evoke NO generation in the absence of a PAMP when added to the incubation solution of epidermal peels prepared from WT Arabidopsis plants (Fig. 2 A and B). As was the case with cAMP and PAMP (LPS) induction of a cytosolic Ca2+ spike (Fig. 1) and PAMP signaling to NO generation during innate immune response cascades (13), cAMP-dependent NO generation was also impaired in dnd1 cells compared to WT (Fig. 2 A and B).
Fig. 2.
Cyclic nucleotide involvement in pathogen response signaling. Effects of cAMP and LPS on NO generation in guard cells are shown in (A–C). Pathogen-induced cytosolic Ca2+ elevation is shown in (D). (A and B) Results are shown for cAMP addition to WT or dnd1 leaf tissue. In the absence of cAMP, no fluorescence is evident in guard cells (e.g., see “buffer” image in Fig. 4C). (C) Results are shown for LPS addition (in the presence and absence of the adenylyl cyclase inhibitor alloxan) to WT tissue. (A and C) Fluorescence (left panels) and bright field (right panels) images are shown at a point (≈5–10 min after addition of cAMP or LPS) of maximal fluorescence. (B) Quantitative analysis of the NO fluorescence signals recorded in WT and dnd1 tissue after addition of cAMP (treatments correspond to those shown in A) are shown as treatment means (n = 3) of relative fluorescence intensity ± SE. This experiment was repeated a total of three times with similar results. For other fluorescence experiments monitoring in vivo generation of NO and ROS, quantitative analyses are shown in Fig. S4; see Fig. S4A for quantitative analysis of the experiment shown here in Fig. 2C. (D) Analysis of the pathogen-induced cytosolic Ca2+ elevation in the presence and absence of alloxan. For the experiments shown in Fig. 3 and the corresponding replications, mean [Ca2+] during the Ca2+ spike was ascertained for leaves inoculated with Pst avrRpm1, Pst avrRpt2, and Pst without avr genes ('DC3000′) in the presence and absence of alloxan. Mean values are shown for each treatment ± SE. ANOVA analysis was undertaken comparing the mean [Ca2+] in the presence and absence of alloxan. Bars with a * above indicate a significant effect of alloxan at P < 0.05 and bars with a ** above indicate a significant effect of alloxan at P < 0.01.
PAMP Signaling to NO Mediated by Endogenous cNMP.
Genes encoding enzymes that synthesize (adenylyl cyclase) and break down (cNMP phosphodiesterase) cAMP in plant leaves have not yet been identified (22); translational arrest of a specific gene has not been associated with altered level of cAMP in plants. In consideration of this point, we took a pharmacological approach to manipulate endogenous cAMP. Dedioxyadenosine (DDA) and 1,3-diazinane-2,4,5,6-tetrone (alloxan) are well characterized inhibitors of animal adenylyl cyclase (23–25), and block cAMP-dependent signaling in plants (26–29). Results presented in Fig. 2C and Fig. S1A indicate that both of these adenylyl cyclase inhibitors block LPS-dependent generation of NO in leaf tissue from WT plants.
cNMP phosphodiesterase enzymatic activity is present in plant cells (22, 26, 30) and acts to catabolize cAMP and maintain homeostatic levels in the cytosol after changes that occur in the level of this secondary messenger during signaling cascades (4). We hypothesized that blocking the breakdown of endogenous cAMP by inhibition of cNMP phosphodiesterase activity could therefore affect NO generation in the plant cell. Animal and plant cNMP phosphodiesterase is sensitive to the inhibitor 1-methyl-3-(2-methylpropyl)-7H-purine-2,6-dione (IBMX) (31, 32). Application of IBMX to leaf epidermal peels from WT plants evokes NO generation in the absence of a pathogen signal (i.e., the PAMP LPS) (Fig. S1B). IBMX induction of NO generation is impaired in dnd1 cells (Fig. S1B), and blocked by chelation of extracellular Ca2+ using EGTA (Fig. S1C). These results, demonstrating that IBMX induction of NO generation is dependent on the presence of free extracellular Ca2+ and a functional (cAMP-activated) PM Ca2+-conducting channel (CNGC2) are consistent with the aforementioned assertion that IBMX acts through activating cNMP-dependent Ca2+ current to evoke NO generation.
HR and cNMPs In Planta.
Results presented in Figs. 1 and 2 and Fig. S1 provide evidence consistent with a link between cAMP, cytosolic Ca2+ elevation, and innate immune signaling in plant cells. Results presented in Figs. 2D and 3–5 extend this link to the plant HR to pathogens. We used the well-studied in planta interaction between Arabidopsis and the bacterial pathogen P. syringae p.v. tomato DC3000 (Pst) (33). Inoculation of WT Arabidopsis plants with Pst harboring the avirulence (avr) genes avrRpm1 or avrRpt2 results in HR, while in dnd1 mutant plants lacking a PM inwardly conducting Ca2+-permable channel this immune response is impaired (12, 13). Here, we focus on examining the possibility that cAMP acts as a signaling molecule to gate Ca2+ conductance through CNGCs during immune signaling leading to HR.
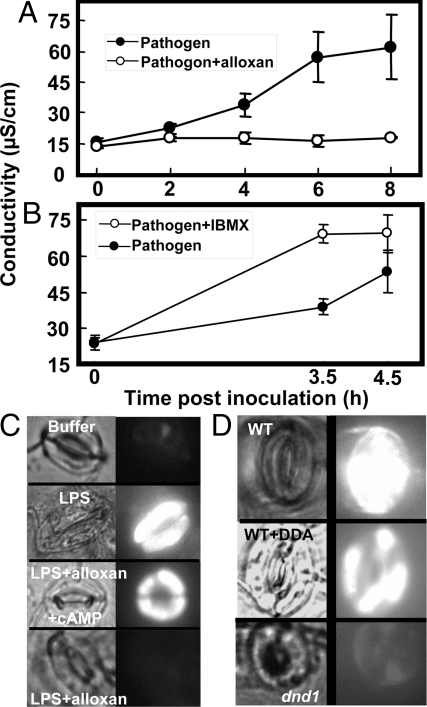
Fig. 3.
Effect of adenylyl cyclase inhibitor alloxan on pathogen-induced cytosolic Ca2+ elevation. Pathogen (Pst), pathogen with alloxan, or mock inoculum was syringe-injected into leaves of WT aequorin-expressing plants at time ‘0′. Pst avrRpm1 was used for (A and B), Pst avrRpt2 was used for (C), and Pst without avr genes (‘DC 3,000′) was used for (D). Cytosolic Ca2+ measurements shown in (B) are from the same leaf as is shown in (A); in (B), only the first 30 min of the recording is presented. The results shown in this figure are representative of 3–5 replications per treatment using leaves from different plants.
Fig. 4.
Effects of adenylyl cyclase or cNMP phosphodiesterase inhibitor on HR of plants to avirulent pathogen Pst avrRpt2 (A and B), and LPS-dependent NO (C) or ROS generation (D). For A and B, HR was monitored as ion leakage, and results are presented as means (n = 3) ± SE. These experiments were repeated at least two times. For C and D, bright field (Left) and fluorescence (Right) images are shown. (C) Effect of alloxan and exogenous cAMP on LPS-dependent NO generation in guard cells of leaf tissue from WT plants. For the images labeled as “buffer,” leaf tissue was incubated in loading buffer containing no LPS, cAMP, or alloxan. (D) LPS-dependent ROS generation in guard cells of leaf tissue from WT and dnd1 plants. In all cases, LPS was present in the loading buffer. Quantitative analyses of results presented in (C and D) are shown in Fig. S4.
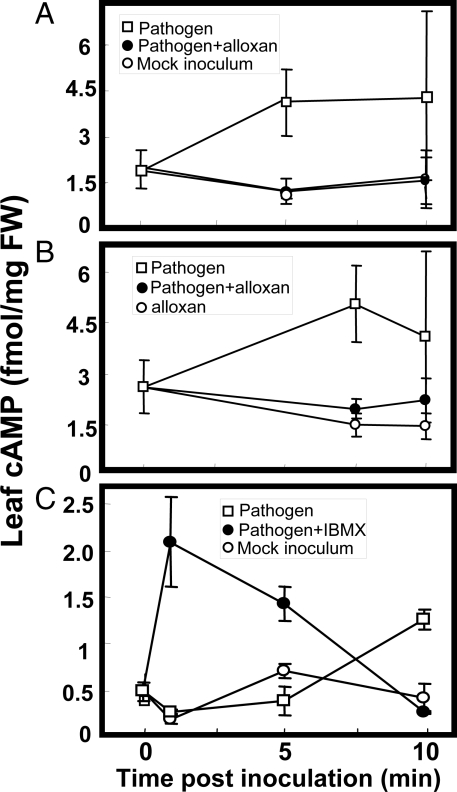
Fig. 5.
Effect of pathogen inoculation on cAMP levels in Arabidopsis leaves. In all cases, Pst avrRpm1 pathogen or mock inoculum was vacuum-infiltrated into leaves at time ‘0′. Results are presented as means (n = 3–4) ± SE. For the experiment shown in (C), all treatments included 0.1% (vol/vol) DMSO. ANOVA analysis comparing pooled values from the three experiments (A–C) for “pathogen” (unfilled square symbols) with “no pathogen” (unfilled circles) treatments at 10-min post-inoculation indicated that leaf [cAMP] in pathogen-treated leaves was significantly greater at P < 0.05.
The Ca2+-dependent luminescence of recombinant aequorin has been used to demonstrate pathogen-associated cytosolic Ca2+ elevations in leaves of Arabidopsis plants exposed to Pst (11). Grant et al. (11) demonstrated that inoculating Arabidopsis with Pst harboring avrRpm1 (but not avrRpt2) resulted in biphasic cytosolic Ca2+ elevations in leaf tissue; the first Ca2+ spike lasted approximately 15 min and peaked at approximately 8–10 min post-inoculation and a second, broader Ca2+ transient initiated at approximately 60 min post-inoculation and peaked at approximately 105 min (11). Grant et al. concluded that the first Ca2+ spike occurred in response to Pst whether or not the pathogen harbored an avr gene.
Here, we used a similar experimental system to examine whether coinfiltration of an adenylyl cyclase inhibitor with pathogen affected the pathogen-induced cytosolic Ca2+ elevation in Arabidopsis leaves (Fig. 3). A cytosolic Ca2+ elevation occurred between approximately 10 and 20 min post-inoculation of Arabidopsis leaves with Pst avrRpm1 (Fig. 3 A and B). However, we note only a modest, slow second rise in Ca2+ initiating an approximate 60-min post-inoculation that did not result in a discernable peak much above that occurring with mock inoculum (either by ≈105 min post-inoculation, or thereafter) (e.g., Fig. 3A). In our studies, inoculation of leaves with the pathogen Pst either with, or without avr genes led to a cytosolic Ca2+ elevation (with a peak at ≈10–15 min post-inoculation) above that observed in leaves treated with mock inoculum (Fig. 3 B–D); a result similar to that found previously (11). The objective of these experiments was to determine if adenylyl cyclase inhibitor affected pathogen-associated cytosolic Ca2+ elevation. As shown in Fig. 3, coinfiltration of pathogen with inhibitor reduced the cytosolic Ca2+ elevation; this occurred with Pst avrRpm1+ (Fig. 3 A and B), Pst avrRpt2+ (Fig. 3C), or Pst avrRpm1−, avrRpt2− (Fig. 3D); also see Fig. 2D.
The effect we find of the adenylyl cyclase inhibitor on plant response to pathogen cannot be attributed to a direct effect of the inhibitor on the bacterium. P. syringae growth on solid culture medium was unaffected by the adenylyl cyclase inhibitor (Fig. S2). Thus, we conclude that blocking cAMP synthesis during immune signaling in plants exposed to the bacterial pathogen P. syringae impairs pathogen-associated cytosolic Ca2+ elevation; specifically the initial Ca2+ spike occurring at approximately 10–15 min.
We also found that inhibition of the enzyme responsible for cAMP synthesis in plants had corresponding effects on HR as well as other steps in the immune signal transduction pathway (Fig. 4). Adenylyl cyclase inhibitor blocked HR in WT plants (Fig. 4A). In contrast to the effect of the adenylyl cyclase inhibitor, blocking the breakdown of cAMP had the converse effect on HR. Examination of leaves at an early time point post-inoculation indicated that coinfiltration of the cNMP phosphodiesterase inhibitor IBMX along with avirulent pathogen potentiated plant response, hastening onset of HR (Fig. 4B). In prior studies of Ca2+ signaling and HR from this lab (13), we monitored HR visually in ethanol-bleached leaves. In our current work, we note that development of HR can be ascertained at an earlier time (post-inoculation), and in a more sensitive and quantitative manner by monitoring ion leakage as was done in the work shown in Fig. 4 A and B. In other experiments similar to those shown in these figures, we found similar treatment effects using photography of ethanol-bleached leaves to monitor HR-related tissue necrosis. In these additional experiments, we found that treatment of leaves with the adenylyl cyclase inhibitor DDA blocked HR-related necrosis (black regions of ethanol-bleached leaves), and treatment of leaves with IBMX hastened onset of HR to avirulent pathogen.
Generation of reactive oxygen species (ROS) is known to occur downstream from cytosolic Ca2+ elevation in immune responses to pathogens. This point has been demonstrated with signaling cascades leading to HR of (Arabidopsis) plants inoculated with avr pathogens (6, 11), and also with ROS generation by cultured plant cells responding to pathogen elicitors/PAMPs (2, 34, 35). Results of the experiment shown in Fig. 4D provide genetic and pharmacological evidence that Ca2+ as well as cNMPs are upstream from PAMP-dependent ROS generation. Application of the PAMP LPS led to ROS generation in plant cells; this pathogen signaling cascade was inhibited either by addition of an adenylyl cyclase inhibitor or in the absence of a functional Ca2+-conducting CNGC2 channel in dnd1 cells (Fig. 4D).
Results presented here document effects of adenylyl cyclase inhibitors on a series of steps in immune signaling cascades; including those linking pathogen perception to cytosolic Ca2+ elevation, steps leading from PAMP recognition to NO and ROS generation, and plant HR to avirulent pathogens. The effects of the pharmacological agents (DDA and alloxan) on pathogen defense responses in plants could be due to a specific inhibition of cNMP synthesis, or potential nonspecific effects of these compounds on plant cells. Results presented in Fig. 4C are consistent with a specific inhibition of cAMP synthesis as mediating effects on immune signaling. If the pharmacological agent alloxan blocked the plant immune signaling cascade due to a specific effect on cAMP synthesis, then addition of exogenous cAMP should reverse the block. As shown in Fig. 4C, addition of cAMP did complement the effect of this pharmacological agent.
Results presented in Fig. 4B (and Fig. S1 B and C) are consistent with IBMX acting to alter pathogen signaling by activating (through inhibition of cAMP breakdown) PM Ca2+-conducting CNGC channels. IBMX potentiates the signaling cascade, leading to NO generation in the absence of a pathogen signal (Fig. S1B) and a hastening of HR in leaves inoculated with avr pathogen (Fig. 4B). Chelation of free extracellular Ca2+ in the assay medium prevents IBMX induction of NO generation (Fig. S1C). IBMX induction of NO generation is also impaired in dnd1 cells (Fig. S1B). Thus, these results suggest that IBMX acts through effects on cNMP mediated Ca2+ channel activation (as opposed to a nonspecific or unknown mechanism), and support a model of cAMP acting upstream from Ca2+ in the pathogen response signaling cascade. These results also are consistent with IBMX potentiation of HR in plants exposed to an avirulent pathogen (Fig. 4B) due to specific effects on these components of the pathogen response signaling cascade.
Further support for this model is presented in Fig. 5. In a series of experiments, cAMP levels in leaves during pathogen response signaling were monitored. During pathogen response signaling cascades we observe a cytosolic Ca2+ spike occurring approximately 10–20 min post-inoculation (Fig. 3). If this cytosolic Ca2+ elevation was caused by a pathogen-induced increase in cAMP, then the elevation in cAMP should occur in concert with, or before the onset of this influx of Ca2+ across the PM. As shown in Fig. 5 A–C, we observe a rise in leaf cAMP 5–10 min after inoculation with pathogen. This cAMP elevation above ambient levels did not occur in mock-inoculated leaves (Fig. 5 A and C). Coinfiltration of leaves with pathogen and adenylyl cyclase inhibitor abolished the pathogen-associated cAMP rise (Fig. 5 A and B), while addition of the adenylyl cyclase inhibitor to leaves in the absence of pathogen had no effect (Fig. 5B). As shown in Fig. 5C, the pathogen-associated cAMP elevation (occurring at 10 min in this experiment) was potentiated by coinfiltration of IBMX with pathogen; in the presence of IBMX the cAMP elevation occurred sooner and was increased.
Results in Fig. 5 support the aforementioned assertion that the pharmacological agents act through corresponding inhibition of cAMP synthesis or breakdown during the signaling cascade. In addition, results shown in Fig. 5 provide evidence documenting a rise in cAMP in pathogen-inoculated leaves during plant immune signaling, and provide evidence consistent with a central tenant of the model developed here; that is, that elevation of cytosolic cAMP is a key step in the transduction of pathogen perception to downstream defense signaling cascades in plants. Further support for this model is provided in Fig. S3. Application of exogenous cAMP to Arabidopsis plants led to the expression of PR1, a gene known to be induced during pathogen response signaling leading to plant defense responses (36). The PAMP LPS has been shown in this report to activate a number of CNGC2- (and cAMP-) dependent steps of the pathogen response signaling cascade, including cytosolic Ca2+ elevation (Fig. 1), NO generation (Fig. 2), and ROS production (Fig. 4); LPS application leads to PR1 expression in Arabidopsis as well (36).
Discussion
Work presented here provides evidence for a series of pathogen response signal transduction cascade steps involving cAMP elevation upstream from a CNGC-dependent PM Ca2+ current, cytosolic Ca2+ elevation leading to downstream NO generation and ROS production, and (in the case of an avirulent pathogen), HR. Although the body of evidence is equivocal, some prior work suggests that cNMPs may act in plant immune signaling pathways; these prior studies are consistent with the work here linking cytosolic cAMP elevation to Ca2+-dependent pathogen response signaling and HR in the plant. The prior work is limited to studies of cultured cell response to extracts of pathogenic fungi containing elicitors. Application of fungal extracts induced elevation of endogenous cAMP level in plant cell cultures (4, 37, 38). However, when cAMP elevation in response to fungal elicitor was monitored specifically in plants, no pathogen-induced changes were found (4). The level of pathogen-induced cAMP elevation we find in Arabidopsis leaves (≈3-fold increase over background; Fig. 5) was of a similar magnitude as that demonstrated in some of these previous studies with elicitor-induced elevations in cell cultures (4). The level of cAMP elevation we find here in response to pathogen was also of a similar magnitude as that found in prior studies associating cNMP signaling with plant response to abiotic (salinity and osmotic) stress (39).
In addition to the aforementioned prior studies linking pathogens to altered endogenous level of cNMP in cultured plant cells, other work indicates that application of exogenous cAMP can in some cases activate components of the pathogen response signaling cascade in cultured plant cells. Bindschedler et al. found that application of exogenous cAMP led to generation of H2O2 in French bean cells (3), but Davies et al. found no effect of exogenous cAMP on H2O2 generation in cultured Arabidopsis cells (2). The work here expands on these studies by linking pathogen perception to changes in cAMP and cAMP-dependent Ca2+ signaling during immune responses in plants.
At present, it is unclear what specific gene products encode the proteins responsible for synthesis and catabolism of cAMP in plants (22). Nonetheless, cAMP has been unequivocally documented to be present in plant cells (27, 40). Adenylyl cyclase activity as well as cAMP phosphodiesterase activity is present in plants (4, 27). Intriguingly, application of fungal extracts to plant cell cultures affects the level of extractable activities of cyclase (i.e., rising in the first few minutes) and phosphodiesterase (declining during this time) in a manner consistent with generation of transient cAMP elevations in a plant cell upon perception of a pathogen (4).
Within the context of work presented here, the molecular structure and functional properties of plant CNGCs provides a basis to refine our current model of immune signaling cascades. Plant CNGCs are PM localized and are ligand, as well as voltage gated (17–19). At the (inside negative) membrane potentials present across the plant cell PM, a rise in cytosolic cAMP would activate inwardly rectified cation current (17–19). CNGCs conduct monovalent cations and Ca2+, but in the presence of Ca2+ [i.e., at the millimolar concentrations likely present in the apoplast (41)], monovalent cation conductance through CNGCs is restricted (18). Cytosolic calmodulin (CaM) binds to CNGCs at a region of the protein overlapping with the cNMP binding region; CaM binding prevents cNMP activation of CNGC current (42). Cytosolic Ca2+ is required for CaM block of current (42). In the presence of an exogenous supply of cAMP, CNGC current is non-inactivating (17). However, sustained PAMP (LPS) activation of inward Ca2+ current through CNGCs only occurs in the presence of a CaM antagonist (13).
Experimental evidence is lacking, but threading quaternary models of plant CNGCs through known crystal structures of ion channels suggests they are tetramers; all native (animal) CNGCs are heterotetramers comprised of more than one CNGC gene product (43). Thus, the role CNGCs play in the pathogen response signal cascade can be envisioned as follows. Due to their likely heterotetrameric structure in native membranes, translational arrest of one plant CNGC gene alone could alter Ca2+ conductance and impair pathogen signaling leading to HR. Consistent with this point, loss-of-function mutants of either CNGC2 or CNGC4 (both are expressed in leaves) have impaired HR (12, 13, 16). Perhaps loss-of-function mutation of one CNGC gene (for example, CNGC2 in the dnd1 mutant) prevents assembly of the native channel complex but leads to formation of non-native (partially functional) channels in mutant plants. We find that Ca2+-dependent (see ref. 13) NO generation in response to either cAMP (Fig. 2A) or PAMPs (13) is impaired, but not completely inhibited in dnd1 cells; results consistent with this model of channel assembly.
Cytosolic cAMP rise mediated by PAMP binding to an (as-yet-unidentified) PAMP receptor (or a step downstream from this receptor) could initiate and/or amplify pathogen perception-mediated cytosolic immune signaling by activating inward CNGC Ca2+ current. Resultant cytosolic Ca2+ elevation could then impact downstream steps of the signal cascade leading to NO generation through increased binding of cytosolic Ca2+ to CaM (44). Elevated cytosolic Ca2+/CaM could also block sustained inward Ca2+ conduction through CNGCs, leading to a transitory cytosolic Ca2+ spike associated with pathogen/PAMP perception (Figs. 2D and 3 above, also see refs. 11 and 13). The initial cAMP-dependent activation of inward Ca2+ current and concomitant cytosolic Ca2+ spike (occurring minutes after pathogen recognition) can be thus envisioned to be temporally separated from the pathogen defense responses mediated through a rise in NO occurring over hours (44). The critical and early CNGC-mediated Ca2+ conductance occurring during plant immune signaling cascades could be initiated through PAMP perception and be similar during plant defense responses to virulent pathogens and HR invoked by plant recognition of avirulence factors in pathogens. (The question of what factors initiated by avr gene product recognition augment this “basal” Ca2+ signal to induce HR remains unanswered here). This model of CNGC-mediated Ca2+ signaling is informed by, and consistent with the experimental results presented here. This work elucidates an example of a plant signaling pathway involving cAMP and that identifies a specific protein target of this important secondary messenger.
Materials and Methods
For details, see SI Text.
Plant Material and Pathogen Inoculation.
Arabidopsis WT [Columbia (Col) ecotype], dnd1 (12), and aequorin-transformed plants of these genotypes were used for all experiments. Avirulent Pst (avrRpt2+ or avrRpm1+) and virulent (avrRpt2− and avrRpm1−) strains of Pst were used for syringe-injection or vacuum infiltration inoculation exactly as described in ref. 44.
Reagents.
Unless otherwise noted, all reagents were purchased from Sigma. The membrane permeable lipophilic cAMP analog dibutyryl-cAMP was used in all cases.
In Vivo NO and ROS Analysis.
NO and ROS generation were evaluated in guard cells of epidermal peels using fluorescent dyes as described (13, 35).
Evaluation of Tissue Necrosis and HR.
HR of pathogen inoculated leaves was evaluated as ion leakage associated with programmed cell death as described by Ma et al. (44) with modifications.
Leaf cAMP Extraction and Content Determination.
Detached leaves (from WT plants) were vacuum-infiltrated with pathogen. Leaves were ground in liquid N2 and frozen leaf powder was extracted with HClO4, neutralized with KOH, and the extract clarified by centrifugation. Supernatants were lyophilized and cAMP content was quantified by an enzyme-linked immunoassay kit (GE Healthcare).
Leaf Cytosolic Ca2+ Measurement Using Aequorin Luminometry.
Cytosolic Ca2+ elevation occurring in Arabidopsis leaves inoculated with pathogen or exposed to ligands (cAMP or LPS) was evaluated using aequorin-transformed plants. Details are provided in SI Text.
Supplementary Material
Acknowledgments.
This work was supported by National Science Foundation Award 0844715 (to G.A.B).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0905831106/DCSupplemental.
References
- 1.Dangl JL, Dietrich RA, Richberg MH. Death don't have no mercy: Cell death programs in plant-microbe interactions. Plant Cell. 1996;8:1793–1807. doi: 10.1105/tpc.8.10.1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davies DR, Bindschedler LV, Strickland TS, Bolwell GP. Production of reactive oxygen species in Arabidopsis thaliana cell suspension cultures in response to an elicitor from Fusarium oxysporum: Implications for basal resistance. J Exp Bot. 2006;57:1817–1827. doi: 10.1093/jxb/erj216. [DOI] [PubMed] [Google Scholar]
- 3.Bindschedler LV, et al. Early signaling events in the apoplastic oxidative burst in suspension cultured French bean cells involve cAMP and Ca2+ New Phytol. 2001;151:185–194. doi: 10.1046/j.1469-8137.2001.00170.x. [DOI] [PubMed] [Google Scholar]
- 4.Cooke CJ, Smith CJ, Walton TJ, Newton RP. Evidence that cyclic AMP is involved in the hypersensitive response of Medicago sativa to a fungal elicitor. Phytochemistry. 1994;35:889–895. [Google Scholar]
- 5.Garcia-Brugger A, et al. Early signaling events induced by elicitors of plant defenses. Mol Plant Microbe Interact. 2006;19:711–724. doi: 10.1094/MPMI-19-0711. [DOI] [PubMed] [Google Scholar]
- 6.Lecourieux D, Ranjeva R, Pugin A. Calcium in plant defence-signaling pathways. New Phytol. 2006;171:249–269. doi: 10.1111/j.1469-8137.2006.01777.x. [DOI] [PubMed] [Google Scholar]
- 7.Nürnberger T, et al. High affinity binding of a fungal oligopeptide elicitor to parsley plasma membranes triggers multiple defense responses. Cell. 1994;78:449–460. doi: 10.1016/0092-8674(94)90423-5. [DOI] [PubMed] [Google Scholar]
- 8.Zimmermann S, et al. Receptor-mediated activation of a plant Ca2+-permeable ion channel involved in pathogen defense. Proc Natl Acad Sci USA. 1997;94:2751–2755. doi: 10.1073/pnas.94.6.2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lamotte O, et al. Mechanisms of nitric-oxide-induced increase of free cytosolic Ca2+ concentration in Nicotiana plumbaginifolia cells. Free Rad Biol Med. 2006;40:1369–1376. doi: 10.1016/j.freeradbiomed.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 10.Blume B, Nürnberger T, Nass N, Scheel D. Receptor-mediated increase in cytoplasmic free calcium required for activation of pathogen defense in parsley. Plant Cell. 2000;12:1425–1440. doi: 10.1105/tpc.12.8.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grant M, et al. The RPM1 plant disease resistance gene facilitates a rapid and sustained increase in cytosolic calcium that is necessary for the oxidative burst and hypersensitive cell death. Plant J. 2000;23:441–450. doi: 10.1046/j.1365-313x.2000.00804.x. [DOI] [PubMed] [Google Scholar]
- 12.Clough SJ, et al. The Arabidopsis dnd1“defense, no death” gene encodes a mutated cyclic nucleotide-gated ion channel. Proc Natl Acad Sci USA. 2000;97:9323–9328. doi: 10.1073/pnas.150005697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ali R, et al. Death don't have no mercy and neither does calcium: Arabidopsis CYCLIC NUCLEOTIDE GATED CHANNEL2 and innate immunity. Plant Cell. 2007;19:1081–1095. doi: 10.1105/tpc.106.045096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dangl JL. Innate immunity: Plants just say NO to pathogens. Nature. 1998;394:525–527. doi: 10.1038/28958. [DOI] [PubMed] [Google Scholar]
- 15.Yoshioka K, et al. The chimeric Arabidopsis CYCLIC NUCLEOTIDE-GATED ION CHANNEL11/12 activates multiple pathogen resistance responses. Plant Cell. 2006;18:747–763. doi: 10.1105/tpc.105.038786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Balagué C, et al. HLM1, and essential signaling component in the hypersensitive response, is a member of the cyclic nucleotide-gated channel ion channel family. Plant Cell. 2003;15:365–379. doi: 10.1105/tpc.006999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lemtiri-Chlieh F, Berkowitz GA. Cyclic adenosine monophosphate regulates calcium channels in the plasma membrane of Arabidopsis leaf guard and mesophyll cells. J Biol Chem. 2004;279:35306–35312. doi: 10.1074/jbc.M400311200. [DOI] [PubMed] [Google Scholar]
- 18.Leng Q, Mercier RW, Hua BG, Fromm H, Berkowitz GA. Electrophysiological analysis of cloned cyclic nucleotide-gated ion channels. Plant Physiol. 2002;128:400–408. doi: 10.1104/pp.010832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leng Q, Mercier RW, Yao WZ, Berkowitz GA. Cloning and first functional characterization of a plant cyclic nucleotide-gated cation channel. Plant Physiol. 1999;121:753–761. doi: 10.1104/pp.121.3.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Volotovski ID, Sokolovsky SG, Molchan OV, Knight MR. Second messengers mediate increases in cytosolic calcium in tobacco protoplasts. Plant Physiol. 1998;117:1023–1030. doi: 10.1104/pp.117.3.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones JD, Dangl JL. The plant immune system. Nature. 2006;444:323–329. doi: 10.1038/nature05286. [DOI] [PubMed] [Google Scholar]
- 22.Martinez-Atienza J, Van Ingelgem C, Roef L, Maathuis FJM. Plant cyclic nucleotide signaling: Facts and fiction. Plant Signal Behav. 2007;2:540–543. doi: 10.4161/psb.2.6.4789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Monaghan TK, Mackenzie CJ, Plevin R, Lutz EM. PACAP-38 induces neuronal differentiation of human SH-SY5Y neuroblastoma cells via cAMP-mediated activation of ERK and p38 MAP kinases. J Neurochem. 2008;104:74–88. doi: 10.1111/j.1471-4159.2007.05018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brayden DJ, Pickles RJ, Cuthbert AW. Ion transport in cultured epithelia from human sweat glands: Comparison of normal and cystic fibrosis tissues. Brit J Pharmacol. 1991;102:7–64. doi: 10.1111/j.1476-5381.1991.tb12132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.May LG, Gay CV. Parathyroid hormone uses both adenylate cyclase and protein kinase C to regulate acid production in osteoclasts. J Cell Biochem. 1997;65:565–573. doi: 10.1002/(sici)1097-4644(19970615)65:4<565::aid-jcb11>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 26.Newton RP, Smith CJ. Cyclic nucleotides. Phytochemistry. 2004;65:2423–2437. doi: 10.1016/j.phytochem.2004.07.026. [DOI] [PubMed] [Google Scholar]
- 27.Witters E, Quanten L, Bloemen J, Valcke R, Van Onckelen H. Product identification and adenylyl cyclase activity in chloroplasts of Nicotiana tabacum. Rapid Commun Mass Spectrom. 2004;18:499–504. doi: 10.1002/rcm.1365. [DOI] [PubMed] [Google Scholar]
- 28.Moutinho A, Hussey PJ, Trewavas AJ, Malhó R. cAMP acts as a second messenger in pollen tube growth and reorientation. Proc Natl Acad Sci USA. 2001;98:10481–10486. doi: 10.1073/pnas.171104598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsuruhara A, Tezuka T. Relationship between the self-incompatibility and cAMP level in Lilium longiflorum. Plant Cell Physiol. 2001;42:1234–1238. doi: 10.1093/pcp/pce159. [DOI] [PubMed] [Google Scholar]
- 30.Brown EG, Edwards MJ, Newton RP, Smith CJ. The cyclic nucleotide phosphodiesterases of spinach chloroplasts and microsomes. Phytochemistry. 1980;19:23–30. [Google Scholar]
- 31.Skeberdis VA, et al. beta3-adrenergic receptor activation increases human atrial tissue contractility and stimulates the L-type Ca2+ current. J Clin Invest. 2008;118:3219–3227. doi: 10.1172/JCI32519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ishioka N, Tanimoto S. Involvement of cyclic AMP in adventitious bud initiation of Torenia stem segments. Plant Cell Physiol. 1990;31:91–97. [Google Scholar]
- 33.Katagiri F, Thilmony R, He SY. The Arabidopsis thaliana–Pseudomus Syringae interaction. In: Somerville CR, Meyerowitz EM, editors. The Arabidopsis Book. Rockville, MD: Am Soc Plant Biologists; 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Desaki Y, et al. Bacterial lipopolysaccharides induce defense responses associated with programmed cell death in rice cells. Plant Cell Physiol. 2006;47:1530–1540. doi: 10.1093/pcp/pcl019. [DOI] [PubMed] [Google Scholar]
- 35.Gerber IB, Zeidler D, Durner J, Dubery IA. Early perception responses of Nicotiana tabacum cells in response to lipopolysaccharides from Burkholderia cepacia. Planta. 2004;218:647–657. doi: 10.1007/s00425-003-1142-0. [DOI] [PubMed] [Google Scholar]
- 36.Zeidler D, et al. Innate immunity and Arabidopsis thaliana: Lipopolysaccharides activate nitric oxide synthase (NOS) and induce defense genes. Proc Natl Acad Sci USA. 2004;101:15811–15816. doi: 10.1073/pnas.0404536101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bolwell PG. A role for phosphorylation in the down-regulation of phenylalanine ammonia-lyase in suspension-cultured cells of French bean. Phytochemistry. 1992;31:4081–4086. [Google Scholar]
- 38.Kurosaki F, Tsurusawa Y, Nishi A. The elicitation of phytoalexins by Ca2+ and cyclic AMP in carrot cells. Phytochemistry. 1987;26:1919–1923. [Google Scholar]
- 39.Donaldson L, Ludidi N, Knight MR, Gehring C, Denby K. Salt and osmotic stress cause rapid increases in Arabidopsis thaliana cGMP levels. FEBS Lett. 2004;569:317–320. doi: 10.1016/j.febslet.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 40.Richards H, et al. Cyclic nucleotide content of tobacco BY-2 cells. Phytochemistry. 2002;61:531–537. doi: 10.1016/s0031-9422(02)00266-2. [DOI] [PubMed] [Google Scholar]
- 41.White PJ, Broadley MR. Calcium in plants. Ann Bot. 2003;92:487–511. doi: 10.1093/aob/mcg164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hua BG, Mercier RW, Zielinski RE, Berkowitz GA. Functional interaction of calmodulin with a plant cyclic nucleotide gated cation channels. Plant Physiol Biochem. 2003;41:945–954. [Google Scholar]
- 43.Zheng J, Zagotta WN. Stoichiometry and assembly of olfactory cyclic nucleotide-gated channels. Neuron. 2004;42:411–421. doi: 10.1016/s0896-6273(04)00253-3. [DOI] [PubMed] [Google Scholar]
- 44.Ma W, Smigel A, Tsai YC, Braam J, Berkowitz GA. Innate immunity signaling: Cytosolic Ca2+ elevation is linked to downstream nitric oxide generation through the action of calmodulin or a calmodulin-like protein. Plant Physiol. 2008;148:818–828. doi: 10.1104/pp.108.125104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.